Naohidemyces vaccinii sporulates on wild species of ground flora in Finnish Norway spruce seed orchards but Thekopsora areolata does not on other species than Prunus
Kaitera J., Aarnio L., Ylioja T., Karhu J. (2021). Naohidemyces vaccinii sporulates on wild species of ground flora in Finnish Norway spruce seed orchards but Thekopsora areolata does not on other species than Prunus. Silva Fennica vol. 55 no. 5 article id 10568. https://doi.org/10.14214/sf.10568
Highlights
- Cherry-spruce rust, Thekopsora areolata, was not found on any of the common species of ground vegetation in Finnish Norway spruce seed orchards
- Blueberry rust, Naohidemyces vaccinii, was common on Vaccinium myrtillus and occasional on V. vitis-idaea in all seed orchards
- Thekopsora areolata occurs only on Prunus in Finnish Norway spruce seed orchards.
Abstract
Thekopsora areolata (Fr.) Magnus is a serious cone pathogen that reduces seed crop of Picea abies (L.) Karst. and other Picea spp. Natural sporulation of T. areolata was investigated in nine Norway spruce seed orchards suffering from severe successive T. areolata epidemics in Finland. Habitats occupied by Vaccinium myrtillus L., V. vitis-idaea L., Empetrum nigrum L. and Calluna vulgaris (L.) Hull, and a number of other wild species belonging to ground flora were investigated for Thekopsora areolata uredinia 9–10 times in May–September 2018–2019. Occurrence of Thekopsora uredinia was estimated in current-year leaves of the plants in ca. 25 sample plots of 1 m2 in each seed orchard. A sample of plant leaves with rust uredinia or necrotic pustules were collected from each plot. No rust fruiting stages of T. areolata were found on any of the test species of ground flora. However, rust uredinia were observed regularly on leaves of V. myrtillus and V. vitis-idaea in all seed orchards between mid-July and the end of September. Rust sporulation started on V. myrtillus in July and on V. vitis-idaea in August. Based on symptoms, uredinia and spore morphology, the rust on both V. myrtillus and V. vitis-idaea was identified as blueberry rust, Naohidemyces vaccinii (Jørst.) S. Sato, Katsuya & Y. Hirats. ex Vanderwegen & Fraiture. The uredinial stage of the rust on Vaccinium spp. were described. No evidence of natural sporulation of T. areolata on wild plant species other than Prunus was observed in Finnish Norway spruce seed orchards.
Keywords
Picea abies;
cherry-spruce rust;
epidemics;
Prunus;
blueberry rust;
Naohidemyces vaccinii
-
Kaitera,
Natural Resources Institute Finland (Luke), Natural Resources and Bioproduction, FI-90570 Oulu, Finland
E-mail
juha.kaitera@luke.fi

- Aarnio, Natural Resources Institute Finland (Luke), Natural Resources and Bioproduction, FI-00790 Helsinki, Finland E-mail leena.aarnio@luke.fi
- Ylioja, Natural Resources Institute Finland (Luke), Natural Resources and Bioproduction, FI-00790 Helsinki, Finland E-mail tiina.ylioja@luke.fi
- Karhu, Natural Resources Institute Finland (Luke), Natural Resources and Bioproduction, FI-90570 Oulu, Finland E-mail jouni.karhu@luke.fi
Received 17 May 2021 Accepted 18 October 2021 Published 20 October 2021
Views 14313
Available at https://doi.org/10.14214/sf.10568 | Download PDF

1 Introduction
Thekopsora areolata (Fr.) Magnus is a pathogen that causes cherry-spruce rust in Norway spruce [Picea abies (L.) Karst.] cones. The rust highly reduces Norway spruce seed crop in seed orchards in southern Finland (Savonen 2001; Kaitera 2013) and in seed tree stands in northern Finland (Nikula and Jalkanen 1990; Kaitera 2013). The rust reduces the number of seeds in infected cones and seed viability (Kaitera and Tillman-Sutela 2014). Recently, the heteroecious nature of the rust was confirmed by molecular (Capador et al. 2020) and inoculation studies (Kaitera et al. 2019). In addition to the cones of P. abies, the rust sporulates also on other spruce species, such as P. engelmannii Parry ex Engelm., P. glauca (Moench) Voss and P. omorika (Panĉić) Purk. that are non-native in Finland (Kaitera et al. 2009, 2014, 2017) as well as in shoots of P. abies and P. engelmannii (Roll-Hansen 1947; Hietala et al. 2008).
The alternate hosts of T. areolata are known to belong to the genus Prunus (Gäumann 1959; Kaitera et al. 2014, 2017, 2019). Seed orchard managers try to control and remove Prunus padus L. populations from within and the vicinity of orchards. However, the infection rate can be high even in seed orchards where P. padus appears non-existing (Kaitera et al. 2009, 2021b). This can be either due to efficient long-distance spore dispersal or there exist other alternate hosts than Prunus within the seed orchards.
Rust genera Thekopsora and Pucciniastrum have been considered to belong to the same genus, Pucciniastrum sensu lato, although teliospores of Thekopsora are produced in plant epidermis and those of Pucciniastrum in mesophyll (Cummins and Hiratsuka 1983). Yet, the phylogeny of these two genera is still unclear (Maier et al. 2003). Recently, however, their phylogenetic relationship was reviewed (Aime and McTaggert 2021). In general, the rusts in these genera spread via species of Rosaceae, Ericaceae, Rubinaceae, Asteraceae, Hydrangeaceae and Boraginaceae (Gäumann 1959). Other Thekopsora and Pucciniastrum rusts have been described on e.g. Vaccinium spp., Arctostaphylos spp., Erica spp., Calluna spp. and Galium spp. (Gäumann 1959; CABI 2019).
Epidemics of T. areolata have been reported in remote locations (Nikula and Jalkanen 1990) and seed orchards (Kaitera et al. 2009), where Prunus species have either low prevalence or are missing, suggesting that other species than Prunus might serve as alternate hosts for the rust. The aim of the study was to clarify, if other plant species than Prunus can spread T. areolata under natural conditions in Finnish seed orchards, and therefore, to investigate and describe the natural sporulation of T. areolata on species of Ericaceae and some other species belonging commonly to the ground flora in Norway spruce seed orchards in Finland. Recently, we tested by artificial inoculation the susceptibility of a number of species to T. areolata (Kaitera et al. 2019, 2021a), the current monitoring of natural sporulation of rusts on various plant species providing supplementary evidence. It was also aimed to describe another rust found commonly on Vaccinium in seed orchards.
2 Materials and methods
2.1 Rust estimation and sampling of plants
Plant communities of Vaccinium myrtillus L., V. vitis-idaea L., Empetrum nigrum L. and Calluna vulgaris (L.) Hull growing within seed orchards, were investigated for rust uredinia in nine Norway spruce seed orchards in southern Finland (Fig. 1; Nikkanen et al. 1999) in May–September 2018 (nine times) and 2019 (eight times). The sampling dates are presented in Table 1. Young leaves of the plants growing in homogenous habitats of ca. 1 m2 were checked visually in the field. The plant communities covered various areas within the seed orchards, and some plants were either missing or with very low frequency in some orchards. In each seed orchard ca. 25 habitats per species were randomly selected for the investigation. In addition, other common plant species of the ground flora were also checked visually for rust fruiting stages during some of the sampling dates (Table 1) at the same time, when T. areolata sporulated on Prunus padus L. in the seed orchards (Kaitera et al. 2021b). When leaves with rust symptoms including violet pustules with whitish to yellowish uredinia (Kaitera et al. 2019) were observed, a sample of ca. 25 leaves per sampling time were collected to paper bags and transported into laboratory for rust checking.
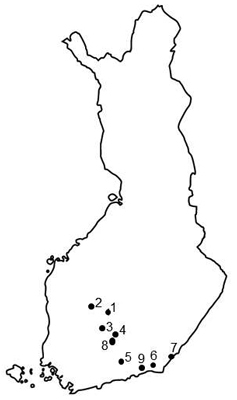
Fig. 1. Locations of investigated Norway spruce seed orchards in Finland. 1 = Heinämäki 170 (62°13´N, 25°24´E), 2 = Metsä-Ihala 176 (62°12´N, 24°07´E), 3 = Riihimäki 169 (61°53´N, 24°52´E), 4 = Paronen 1 365 (61°39´N, 26°17´E), 5 = Sillanpää 235 (60°55´N, 26°13´E), 6 = Taavetti 428 (60°56´N, 27°35´E), 7 = Imatra 374 (61°09´N, 28°47´E), 8 = Paronen 2 366 (61°39´N, 26°17´E), 9 = Palvaanjärvi 172 (60°48´N, 27°29´E).
| Table 1. Plant species investigated for uredinia of Thekopsora areolata and Naohidemyces vaccinii in nine Finnish seed orchards in 2018–2019. Numbers refer to sampling dates of the plants in each seed orchard. The sampling dates were: 1 = May 21–24, 2 = June 11–13, 3 = July 2–5, 4 = July 16–18, 5 = July 30 – Aug 1, 6 = Aug 13–15, 7 = Aug 27–29, 8 = Sept 10–12 and 9 = Sept 24–27 in 2018, and 1 = May 20–22, 2 = June 11–13, 3 = June 24–27, 4 = July 8–10, 5 = July 22–25, 6 = Aug 5–7, 7 = Aug 19–21 and 8 = Sept 2–5 in 2019. For the locations of the seed orchards, see Fig. 1. | |||||||||
| Test species | Seed Orchard | ||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| Sampling dates | |||||||||
| 2018 | |||||||||
| Vaccinium myrtillus L. | 2–9 | 1–9 | 1–9 | 1–9 | 2–9 | 1–9 | 1–9 | 1–9 | 2–9 |
| Vaccinium vitis-idaea L. | 2–9 | 2–9 | 9 | 1–9 | 2–9 | 1–9 | 1–9 | 1–9 | 2–9 |
| Calluna vulgaris (L.) Hull | 0 | 1–9 | 9 | 1–9 | 0 | 2–9 | 1–9 | 1–9 | 2–9 |
| Empetrum nigrum L. | 0 | 1–9 | 9 | 1–9 | 0 | 0 | 0 | 0 | 0 |
| Salix sp. | - | 2 | - | - | - | - | - | - | - |
| Lysimachia europaea (L.) U.Manns & Anderb. | - | 2,4,6 | - | - | - | - | - | - | - |
| Maianthemum bifolium (L.) F.W. Schmidt | - | 2,4 | - | - | - | - | - | - | - |
| Chamaenerion angustifolium (L.) Scop. | 2,4 | 6 | - | 4 | 4 | 6 | 4,6,7 | - | - |
| Lupinus polyphyllus Lindl. | 2,4 | - | - | - | - | - | - | - | - |
| Tanacetum vulgare L. | 4 | - | - | - | - | - | - | - | - |
| Rubus idaeus L. | 4 | - | - | - | - | - | - | - | - |
| Anthriscus sylvestris (L.) Hoffmann | 4 | - | - | 4 | 4 | - | - | - | - |
| Sorbus aucuparia L. | - | - | 4 | - | 4 | - | - | - | 4 |
| Filipendula ulmaria (L.) Maxim. | 4 | - | - | - | - | - | - | - | - |
| Populus tremula L. | - | - | - | - | - | - | 4 | - | - |
| Betula pubescens Ehrh. | - | - | - | - | - | - | 6 | - | - |
| 2019 | |||||||||
| Vaccinum myrtillus L. | 1–8 | 1–8 | 1–8 | 1–8 | 1–8 | 1–8 | 1–8 | 1–8 | 1–8 |
| Vaccinum vitis-idaea L. | 1–8 | 1–8 | 1–8 | 1–8 | 1–8 | 1–8 | 1–8 | 1–8 | 1–8 |
| Calluna vulgaris (L.) Hull | 1–8 | 1–8 | 1–8 | 1–8 | 1–8 | 1–8 | 1–8 | 1–8 | 1–8 |
| Empetrum nigrum L. | 1–8 | 1–8 | 1–8 | 1–8 | 1–8 | 1–8 | 1–8 | 1–8 | 1–8 |
| Salix sp. | 1,2,4 | - | 2 | - | - | 2 | 2 | - | - |
| Lysimachia europaea (L.) U.Manns & Anderb. | - | 2,4 | - | 3 | - | - | - | - | - |
| Maianthemum bifolium (L.) F.W. Schmidt | - | - | - | - | - | - | - | 4 | - |
| Chamaenerion angustifolium (L.) Scop. | 2,4 | - | 2 | 2,4 | 2 | 2 | 2 | - | - |
| Lupinus polyphyllus Lindl. | 1,2,4 | - | - | 4 | - | - | 2 | - | - |
| Rubus idaeus L. | 2 | - | - | - | 2 | 2 | 2 | - | - |
| Anthriscus sylvestris (L.) Hoffmann | 2 | - | - | 2 | 2 | - | 2 | - | - |
| Sorbus aucuparia L. | 1,2,4 | - | - | - | - | - | 2 | - | 2 |
| Filipendula ulmaria (L.) Maxim. | - | - | - | 3 | 1 | - | - | - | - |
| Populus tremula L. | - | 2 | - | - | - | - | 2 | - | - |
| Betula pubescens Ehrh. | - | 2 | - | - | - | - | - | - | 2 |
| Fragaria vesca L. | 1,2 | - | - | - | - | - | - | - | - |
| Viola tricolor L. | - | - | - | - | 1 | - | - | - | - |
| Convallaria majalis L. | 2 | - | 2 | 2 | 2 | - | - | - | 2 |
| Geranium sylvaticum L. | 2,4 | - | 2 | - | 2 | - | 2 | - | - |
| Melampyrum sylvaticum L. | 2 | - | - | - | 2 | - | - | - | 2 |
| Rubus saxatilis L. | - | 2 | 2 | - | - | - | - | - | 2 |
| Betula pendula Roth | 4 | - | - | - | - | 2 | 2 | - | 2 |
| Salix caprea L. | - | - | - | - | - | - | - | - | 2 |
| Alnus incana (L.) Moench | - | - | - | - | - | - | 2 | - | - |
| Quercus robur L. | - | - | - | - | - | - | 2 | - | - |
| Melampyrum pratense L. | - | 2 | 2 | - | - | - | - | - | - |
| Veronica chamaedrys L. | 2 | - | - | - | - | - | - | - | - |
| Ranunculus sp. | 2 | - | - | - | - | - | - | - | - |
| Pteridium aquilinum (L.) Kuhn | 2,4 | - | - | 4 | - | - | 2 | - | - |
| Cirsium helenioides (L.) Hill. | 2 | - | - | - | - | - | 2 | - | - |
| Ribes rubrum L. | 2,4 | - | - | - | - | - | - | - | - |
| Malus sylvestris (L.) Mill. | 2,4,5 | - | - | - | - | - | - | - | - |
| Silene dioica (L.) Clairv. | 2,4 | - | - | 2 | - | - | - | - | - |
| Paris quadrifolia L. | 2 | - | - | - | - | - | - | - | - |
| 0 = No leaves observed. - = Not sampled. | |||||||||
2.2 Morphological identification of the rust fungi
The morphology of rust pustules on the sampled plant leaves was described. The size (length and width) of ca. 150 rust pustules carrying uredinia were measured. The diameters (length and width) of ca. 450 uredinia were measured. In addition, the diameters (length and width) of 100 urediniospores on both Vaccinium myrtillus and V. vitis-idaea were measured. The diameters were compared to species descriptions in the literature (Gäumann 1959; Sato et al. 1993). Typical rust pustules, uredinia and urediniospores were photographed. In case uredinia of T. areolata were found, their morphology was compared to reference material from inoculations with T. areolata on Prunus (Kaitera et al. 2019). The measurements were done using stereo (Meiji Techno RZ, Meiji Techno Co., ltd, Japan) and light microscopy (Meiji MX, Meiji Techno Co., ltd, Japan) with magnification up to 600 x.
2.3 Statistical analysis
The average diameters of rust pustules, uredinia and urediniospores were counted and compared with t-test using SAS software (SAS Institute Inc., version 9.4) between V. myrtillus and V. vitis-idaea. The diameters of uredinia and pustules of the rust on V. myrtillus were compared between the collection times (2018 and 2019), and between seed orchards (observations from different sampling times pooled together) with Tukey’s test of one-way ANOVA.
3 Results
3.1 Sporulation of Thekopsora areolata and an unspecified rust on wild plants in the sample plots in seed orchards
No uredinia of T. areolata were observed on V. myrtillus, V. vitis-idaea, E. nigrum or C. vulgaris in the sample plots in any of the seed orchards either in 2018 or 2019. Neither were T. areolata uredinia observed on species of ground vegetation other than Prunus in any of the seed orchards during the investigation.
Unspecified rust pustules were not observed on other species of ground vegetation except on Vaccinium in any of the seed orchards regardless of the sampling date and year of investigation. However, orange-yellowish uredinia of an unspecified rust were observed on V. myrtillus and orange-yellowish-brownish uredinia on V. vitis-idaea. Uredinia of the rust occurred commonly on V. myrtillus in nine seed orchards in 2018 and in four seed orchards in 2019 (Table 2). Urediniospores were formed from mid-July to the end of September in 2018. In 2019, sporulation was observed in the second week of July, which continued until early September. On V. vitis-idaea, uredinia were detected in three seed orchards both in 2018 and 2019. Sporulation occurred in early September in 2018 and late August in 2019 (Table 2).
| Table 2. Number of sample leaves of Vaccinium myrtillus and V. vitis-idaea bearing uredinia of Naohidemyces vaccinii during the growing seasons in 2018–2019 in nine Norway spruce seed orchards in Finland. For the names of the seed orchards, see Fig. 1. | |||||||||
| Time/Plant species | Seed orchard | ||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| 2018 | |||||||||
| Vaccinium myrtillus | |||||||||
| 21.–23.5. | - | 0 | 0 | 0 | - | 0 | 0 | 0 | 0 |
| 11.–13.6. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2.–5.7. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 16.–18.7. | 0 | 0 | 10 | 0 | 0 | 0 | 0 | 0 | 0 |
| 30.7.–2.8. | 0 | 2 | 3 | 1 | 7 | 1 | 0 | 1 | 2 |
| 13.–15.8. | 0 | 0 | 4 | 0 | 1 | 0 | 1 | 0 | 2 |
| 27.–29.8. | 0 | 0 | 1 | 4 | 3 | 0 | 5 | 0 | 0 |
| 10.–11.9. | 0 | 0 | 1 | 1 | 8 | 0 | 0 | 0 | 0 |
| 24.–27.9. | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 2 |
| Vaccinium vitis-idaea | |||||||||
| 21.–23.5. | - | - | 0 | 0 | - | 0 | 0 | 0 | 0 |
| 11.–13.6. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2.–5.7. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 16.–18.7. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 30.7.–2.8. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 13.–15.8. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 27.–29.8. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 10.–11.9. | 0 | 2 | 2 | 1 | 0 | 0 | 0 | 0 | 0 |
| 24.–27.9. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2019 | |||||||||
| Vaccinum myrtillus | |||||||||
| 20.–23.5. | - | - | - | - | - | - | - | - | - |
| 11.–14.6. | - | - | - | - | - | - | - | - | - |
| 24.–26.6. | 0 | - | 0 | 0 | - | 0 | 0 | 0 | - |
| 8.–10.7. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| 22.–24.7. | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| 5.–8.8. | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 4 | 4 |
| 19.–21.8. | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 5 | 0 |
| 2.–4.9. | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Vaccinum vitis-idaea | |||||||||
| 20.–23.5. | - | - | - | - | - | - | - | - | - |
| 11.–14.6. | - | - | - | - | - | - | - | - | - |
| 24.–26.6. | 0 | - | 0 | 0 | - | 0 | 0 | 0 | - |
| 8.–10.7. | - | - | - | - | 0 | 0 | 0 | 0 | 0 |
| 22.–24.7. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 5.–8.8. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 19.–21.8. | 0 | 3 | 1 | 0 | 0 | 0 | 0 | 1 | 0 |
| 2.–4.9. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| - = No leaves were observed. | |||||||||
3.2 Morphology of an unspecified rust on sample leaves
Violet to brownish pustules were typical for unspecified rust infection on leaves of V. myrtillus (Fig. 2A). Such patches were missing on V. vitis-idaea. The average length and width of these pustules was 1367 µm (N = 142, std = 741 µm, min-max = 375-4750 µm) and 828 µm (N = 142, std = 492 µm, min-max = 125-3750 µm), respectively, on V. myrtillus, while on V. vitis-idaea the average length and width was 2406 µm (N = 4, std = 1891 µm, min-max = 625-5000 µm) and 1562 µm (N = 4, std = 1048 µm, min-max = 500-3000 µm), respectively. Uredinia were orange-yellowish on V. myrtillus (Fig. 2B) and orange-yellowish-brownish on V. vitis-idaea (Fig. 3A), and they occurred on lower leaf surface of the leaves. There was a central pore at the top of the uredinium. On V. myrtillus their average length and width was 170 µm (N = 407, std = 38 µm, min-max = 75-300 µm) and 143 µm (N = 407, std = 31 µm, min-max = 50-250 µm), respectively. On V. vitis-idaea the average length and width of uredinia was 243 µm (N = 44, std = 59 µm, min-max = 125-375 µm) and 207 µm (N = 44, std = 59 µm, min-max = 100-325 µm ), respectively. The urediniospores were orange-yellowish, subglobose and echinulate, with an average length of 21.11 µm (N = 100, std = 2.77 µm, min-max = 16.13-29.03 µm) and average width of 16.52 µm (N = 100, std = 2.06 µm, min-max = 11.29-22.58 µm) on V. myrtillus (Fig. 2C), while on V. vitis-idaea their average length and width was 24.39 µm (N = 100, std = 2.89 µm, min-max = 16.13-32.26 µm) and 16.81 µm (N = 100, std = 1.81 µm, min-max = 12.90-20.97 µm; Fig. 3B), respectively. The average length (t = 2.62, DF = 144, p < 0.01) and width (t = –2.84, DF = 144, p < 0.01) of pustules, length (t = –11.24, DF = 449, p < 0.001) and width (t = –11.51, DF = 449, p < 0.001) of uredinia and length of urediniospores (t = –8.17, DF = 198, p < 0.001) were significantly larger on V. vitis-idaea than on V. myrtillus. The width of urediniospores was similar (t = –1.06, DF = 198, p = 0.291) between V. myrtillus and V. vitis-idaea. Based on rust symptoms, and the size of pustules, uredinia and urediniospores on V. myrtillus and V. vitis-idaea leaves, the rust was identified as Naohidemyces vaccinii (Jørst.) S. Sato, Katsuya & Y. Hirats. ex Vanderwegen & Fraiture.

Fig. 2. A. Violet pustules (black arrows), B. uredinia (black arrows) and C. urediniospores (black arrows) of blueberry rust (Naohidemyces vaccinii) with sharp edges on leaf of Vaccinium myrtillus.
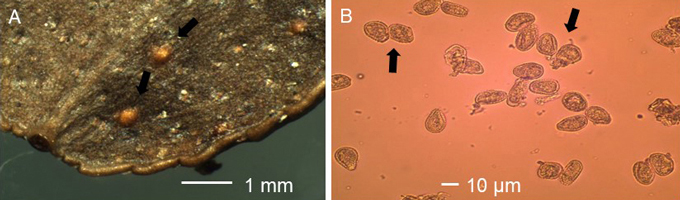
Fig. 3. A. Uredinia (black arrows) and B. urediospores (black arrows) of blueberry rust Naohidemyces vaccinii on leaf of Vaccinium vitis-idaea.
The average length (F = 11.93, p < 0.001) and width (F = 7.05, p < 0.001) of uredinia, collected in the third (July 16–18), fourth (July 30.7.–2.8.) and seventh (Aug 27–29) time were significantly lower than when collected in the eighth (Sept 10–12) time in 2018. In 2019, neither length (F = 0.03, p = 0.995) nor width (F = 2.22, p = 0.088) of uredinia differed significantly between the sampling times. Neither did the length (F = 1.31, p = 0.274) nor width (F = 1.54, p = 0.196) of pustules differ significantly between sampling times in 2018 or 2019 (F = 0.18, p = 0.833 and F = 0.49, p = 0.616).
The average length (F = 4.63, p < 0.001) and width (F = 4.25, p < 0.001) of uredinia from seed orchards 2 and 3 were significantly higher than those from seed orchard 7. The length (F = 1.70, p = 0.126) and width (F = 2.29, p = 0.039) of pustules did not differ significantly between seed orchards.
4 Discussion
In this study, no uredinia of T. areolata were observed on any of the species of Ericaceae or other species of ground flora in the Finnish Norway spruce seed orchards. This was evident regardless of the time of investigation within growing seasons among years. At the same time T. areolata sporulated regularly in May–September on P. padus in the investigated seed orchards (Kaitera et al. 2021b). This suggests that the only wild species that acts as alternate host for T. areolata in the seed orchards is P. padus. This is also supported by recent pathogenicity tests of T. areolata on Prunus and Picea (Kaitera et al. 2019). In addition, common ground flora species at the seed orchards, other than Prunus, were also inoculated recently using T. areolata, with negative results (Kaitera et al. 2021a). Therefore, no wild plant species other than Prunus were found to be susceptible to T. areolata either under artificial or natural conditions in Finnish Norway spruce seed orchards.
Other rust species were observed on Vaccinium species in the seed orchards. The rust on both V. myrtillus and V. vitis-idaea was identified as Naohidemyces vaccinii. This species is common on Vaccinium spp. in Norway (Aamlid 2001). Based on our results, the rust was also common on V. myrtillus but less frequent on V. vitis-idaea in the seed orchards in southern Finland, where the rust sporulated from mid-July to September. The sporulation occurred earlier on V. myrtillus in the seed orchards, which is probably due to overwintering of the rust as mycelium on V. myrtillus (Gäumann 1959) that allows the rust to form uredinia rapidly on blueberry leaves in early summer. Symptoms and spore morphology of the rust agrees with descriptions in the literature (Gäumann 1959; Sato et al. 1993; Aamlid 2001). The rust had bigger uredinia and longer urediniospores and it sporulated later on V. vitis-idaea than on V. myrtillus. However, this variation in spore morphology agrees with morphological comparison between T. myrtillina P. Karst. on V. myrtillus and T. vaccinii (Jørst.) Hirats on V. vitis-idaea (Gäumann 1959). In a recent review, Naohidemyces was grouped to Milesinaceae, Thekopsora to Coleosporaceae and Pucciniastrum to Pucciniastraceae (Aime and McTaggert 2021). Longer and wider uredinia on V. myrtillus at the latest time of collection in September compared to collections earlier in July and August is explained by the development of uredinia to their full size during the growing season.
In conclusion, Thekopsora areolata did not fruit and sporulate on wild species of Ericaceae or any other common species of ground vegetation in Finnish Norway spruce seed orchards. As neither the inoculation studies nor inventories on wild plants species during rust epidemics in 2018–2019 revealed any other species that were susceptible to the rust and able to spread it besides P. padus, we conclude that Prunus spp. are the only susceptible species capable of spreading the rust in Finnish Norway spruce seed orchards. Therefore, control of T. areolata should be concentrated only on Prunus species in Norway spruce seed orchards. Blueberry rust is common on Vaccinium myrtillus and occasional on V. vitis-idaea in southern Finland, where it may cause early withering of leaves of Vaccinium spp.
Acknowledgements
This study was part of the MESIKE (https://www.luke.fi/wp-content/uploads/2018/11/Yleisesittely.pdf) and SITKE (https://www.luke.fi/projektit/sitke) projects financed by the Finnish Ministry of Agriculture and Forestry. We thank Siemen Forelia Oy and Tapio Palvelut Oy for the use of their seed orchards for the study.
Declaration of openness of research materials and data
The research materials and data are available on request from the authors.
Authors’ contribution
Kaitera: Planning of the study, writing of the manuscript, field inventory and data collection, laboratory work, microscopy and photographing of the samples.
Aarnio: Field inventory and data collection.
Ylioja: Organising the field work and reviewing the manuscript.
Karhu: Statistical analysis of the data.
References
Aamlid D (2000) Infections of Valdensinia heterodoxa and Pucciniastrum vaccinia on bilberry (Vaccinium myrtillus). Implications for monitoring ground vegetation. For Pathol 30: 135–139. https://doi.org/10.1046/j.1439-0329.2000.00194.x.
Aime MC, McTaggert AR (2021) A higher-rank classification for rust fungi, with notes on genera. Fungal Syst Evol 7: 21–47. https://doi.org/10.3114/fuse.2021.07.02.
CABI (2019) Datasheet Thekopsora areolata (cherry spruce rust). Invasive species compendium. https://www.cabi.org/isc/datasheet/45892.
Capador H, Samils B, Kaitera J, Olson Å (2020) Genetic evidence for sexual reproduction and multiple infections of Norway spruce cones by the rust fungus Thekopsora areolata. Ecol Evol 10: 7389–7403. https://doi.org/10.1002/ece3.6466.
Cummins GB, Hiratsuka Y (1983) Illustrated genera of rust fungi. The American Phytopathological Society, St. Paul, Minnesota.
Gäumann E (1959) Die Rostpilze Mitteleuropas. [Rust fungi of Central Europe]. Beiträge zur Kryptogamenflora der Schweiz 12: 53–57.
Hietala A M, Solheim H, Fossdal C G (2008) Real-time PCR-based monitoring of DNA pools in the tri-trophic interaction between Norway spruce, the rust Thekopsora areolata, and an opportunistic ascomycetous Phomopsis sp. Phytopathology 98: 51–58. https://doi.org/10.1094/PHYTO-98-1-0051.
Kaitera J (2013) Thekopsora and Chrysomyxa cone rusts damage Norway spruce cones after a good cone crop in Finland. Scand J For Res 28: 217–222. https://doi.org/10.1080/02827581.2012.727024.
Kaitera J, Tillman-Sutela E (2014) Germination capacity of Thekopsora areolata aeciospores and the effect of cone rusts on seeds of Picea abies. Scand J For Res 29: 22–26. https://doi.org/10.1080/02827581.2013.844851.
Kaitera J, Tillman-Sutela E, Kauppi A (2009) Seasonal fruiting and sporulation of Thekopsora and Chrysomyxa cone rusts in Norway spruce cones and alternate hosts in Finland. Canad J For Res 39: 1630–1646. https://doi.org/doi 10.1139/X09-070.
Kaitera J, Hiltunen R, Kauppila T, Pitkäranta M, Hantula J (2014) Fruiting and sporulation of Thekopsora and Chrysomyxa cone rusts in Picea cones and Prunus leaves. For Pathol 44: 387–395. https://doi.org/10.1111/efp.12114.
Kaitera J, Kauppila T, Hantula J (2017) New Picea hosts for Chrysomyxa ledi and Thekopsora areolata. For Pathol 47, article id e12365. https://doi.org/10.1111/efp.12365.
Kaitera J, Kauppila T, Hantula J (2019) Pathogenicity of Thekopsora areolata from seed orchards in Finland on Prunus spp. and Picea abies. For Pathol 49, article id e12567. https://doi.org/10.1111/efp.12567.
Kaitera J, Kauppila T, Hantula J (2021a) Assessment of the potential of Norway-spruce-seed-orchard associated plants to serve as alternate hosts of Thekopsora areolata. Silva Fenn 55, article id 10446. https://doi.org/10.14214/sf.10446.
Kaitera J, Aarnio L, Karhu J, Ylioja T (2021b) Temporal sporulation of Thekopsora areolata and Chrysomyxa spp. in Finnish Norway spruce seed orchards. For Ecol Manag 499, article id 119557. https://doi.org/10.1016/j.foreco.2021.119557.
Maier W, Begeron D, Weiß M, Oberwinkler W (2003) Phylogeny of the rust fungi: an approach using nuclear large subunit ribosomal DNA sequences. Canad J Bot 81: 12–23. https://doi.org/10.1139/b02-113.
Nikkanen T, Karvinen K, Koski V, Rusanen M, Yrjänä-Ketola L (1999) Kuusen ja männyn siemenviljelykset ja niiden käyttöalueet. [Seed orchards of Norway spruce and Scots pine and the areas of use of their seeds]. Metsäntutkimuslaitoksen tiedonantoja 730: 1–203. http://urn.fi/URN:ISBN:951-40-1677-7.
Nikula A, Jalkanen R (1990) Kuusen käpytuholaisten ja -tautien esiintyminen Pohjois-Suomessa kesällä 1989. [Occurrence of spruce cone insects and pathogens in northern Finland in summer 1989]. In: Varmola M, Katermaa T (eds) Metsänparannus: Metsäntutkimuspäivät Rovaniemellä 1990. Metsäntutkimuslaitoksen tiedonantoja 362: 83–89. http://urn.fi/URN:ISBN:951-40-1116-3.
Roll-Hansen F (1947) Nytt om lokkrusten (Pucciniastrum padi). [New information of cherry-spruce rust]. Medd Norske Skogforsøksv 9: 503–510.
Sato S, Katsuya K, Hiratsuka Y (1993) Morphology, taxonomy and nomenclature of Tsuga-Ericaceae rusts. Trans Mycol Soc Japan 34: 47–62.
Savonen E-M (2001) Kuusella hyvä käpyvuosi, mutta runsaasti tuhoja. [Norway spruce had a good cone year but abundant damages]. In: Poteri M (ed) Taimiuutiset 1: 10–13. http://urn.fi/URN:NBN:fi-metla-201211066680.
Total of 21 references.

