Combined effects of provenance and slow-release fertilizer on nursery and field performance of yellowhorn seedlings
Ao Y., Hirst P. M., Li G., Miao Y., Zhang R. (2018). Combined effects of provenance and slow-release fertilizer on nursery and field performance of yellowhorn seedlings. Silva Fennica vol. 52 no. 5 article id 10034. https://doi.org/10.14214/sf.10034
Highlights
- Combining slow-release fertilizer (SRF) and provenance in the nursery has large effects on most seedling characteristics in yellowhorn
- Stem and root P contents in the nursery, and height at the end of the second growing season (T3) in the field were mainly affected by provenance
- Higher rates of SRF tended to increase root N, stem and root P contents in the nursery, diameter, and biomass at T3
- The combination of AQ provenance with 120–200 mg N seedling–1 SRF yielding better nursery and field performance was recommended.
Abstract
Yellowhorn (Xanthoceras sorbifolium Bunge) has been widely planted for biodiesel production in China, but has frequently shown poor field performance. Container-grown yellowhorn seedlings originating from three Chinese provenances, Wengniute Qi (WQ), Alukeerqin Qi (AQ), and Shanxian (SX), were fertilized with slow-release fertilizer (SRF) at 40, 80, 120, 160 or 200 mg N seedling–1. Tree growth, survival and nutrient content were measured after one year’s growth in a greenhouse followed by two years in a field site. Plants from AQ and SX tended to have higher stem and root P contents in the nursery. Higher rates of SRF increased root N, and stem and root P contents. After one year in the nursery, there were a number of interactions between provenance and SRF for plant growth responses and nutrient content in the nursery, however after two years of additional growth in the field, plants from the different provenances generally responded similarly to applied SRF in the nursery, with few interactions. Final plant height was approximately 10% lower in trees from provenance SX but was not affected by application of SRF. Conversely, final trunk diameter and stem and root biomass were unaffected by provenance but increased with higher rates of applied SRF. Our results indicate that application of SRF may be a useful tool to nutrient load yellowhorn in the nursery and facilitate transplanting performance in the field. Overall, optimal nursery and field performance of yellowhorn were observed in provenance AQ at 120–200 mg N seedling–1 SRF. We suggest that growers consider a wider range of yellowhorn provenances and SRF rates (above 200 mg N seedling–1) to yield even better growth response.
Keywords
nursery response;
field performance;
Xanthoceras sorbifolium;
plant nutrition;
short rotation plantation
-
Ao,
Key Laboratory for Silviculture and Conservation, Ministry of Education, Beijing Forestry University, 35 East Qinghua Road, Haidian District, Beijing 100083, China
E-mail
aoyan316@163.com

- Hirst, Department of Horticulture and Landscape Architecture, Purdue University, West Lafayette, IN 47907, USA E-mail hirst@purdue.edu
- Li, Key Laboratory for Silviculture and Conservation, Ministry of Education, Beijing Forestry University, 35 East Qinghua Road, Haidian District, Beijing 100083, China E-mail glli226@163.com
- Miao, Key Laboratory for Silviculture and Conservation, Ministry of Education, Beijing Forestry University, 35 East Qinghua Road, Haidian District, Beijing 100083, China E-mail 372902610@qq.com
- Zhang, College of Forestry, Beijing Forestry University, 35 East Qinghua Road, Haidian District, Beijing 100083, China E-mail 793755837@qq.com
Received 31 August 2018 Accepted 14 December 2018 Published 18 December 2018
Views 107995
Available at https://doi.org/10.14214/sf.10034 | Download PDF

1 Introduction
Reducing greenhouse gas emissions and providing fuel security have drawn attention to the use of oil-rich seed plants as raw materials for biodiesel production. Yellowhorn (Xanthoceras sorbifolium Bunge), belonging to the Sapindaceae family, is a high-quality feedstock for biodiesel production, with oil concentration of 55–66% in seed kernel (Lee et al. 2015; Shao and Chu 2008). This species is widely distributed in northern China and adapts well to drought, low temperature, high salinity and alkali soils. Yellowhorn seedlings typically start bearing fruit after 4–5 years growing in the field and higher yield is expected as plants age increased. Yellowhorn plantation for biodiesel production has increased significantly during the past few years (Shen et al. 2018) . However the species often exhibits slow growth and poor field performance (the average seed yield of mature trees is 670 kg ha–1, and 0.798 kg plant–1), particularly in areas with low nutrient availability (Ao 2016). Low nutrient availability is believed to be a major factor limiting yellowhorn plantation success (Zhu et al. 2009).
The use of slow-release fertilizer (SRF) is characterized by a moderate but consistent nutrient supply over a long period, which improves nutrient-use efficiency, and reduces leaching losses (Li et al. 2017). Incorporation of SRF directly into the nursery growing medium has the potential to improve nutrient loading for field growing. Nutrient loaded seedlings have a greater capability to overcome planting stresses especially on harsh sites and high level of competition (Grossnickle 2012). SRF has been considered as an important tool to improve seedling establishment and to meet forestation objectives (Halmschlager and Katzensteiner 2017; Seletkovic et al. 2009; Seletkovic et al. 2011; Teixeira et al. 2009). In addition to SRF, provenance (PRO) is believed to influence survival and growth performance because adaptation to different climatic conditions leads to ecotype evolution (Jones and Hayes 2001). Plants from different provenances exhibit different responses to stressful conditions, such as low temperature or drought, which is considered to be under strong genetic control (Andivia et al. 2012). Based on the literatures, the effect of combined influence of nursey fertilization × provenance on nutrient status and growth response produced a wide range of results. Some showed provenances demonstrated similar responses to fertilizer and high fertilization increased nutrient concentration and growth (Andivia et al. 2012), others suggested that fertilization had different effects in different provenances (Gleason et al. 1990; Villar-Salvador et al. 2008). These varied results reflect species-specific responses or may be due to the wide range of experimental conditions differing among studies. Among these reports, there has not been enough attention toward the combined effect of SRF and PRO. Previous study of yellowhorn seedlings subjected to different nitrogen application rates, resulted in improved plant quality and performance as fertilization applied compared to control and the rate of 230 mg N seedling–1 was recommended. However, the role of nursery SRF and PRO on yellowhorn plant quality and subsequent field performance is still unknown, this research has practical implications for successful forestation.
This study was designed to evaluate the performance of three provenances of yellowhorn seedlings while five rates of SRF incorporated into the growing medium. We hypothesized that both SRF and PRO would significantly affect seedling nutrient status and growth at the end of the nursery period. Also, we postulated that incorporating SRF would produce seedlings with greater nutrient reserves, which would in turn improve field performance of all provenance. The current study focused on early plant growth and establishment in the absence of fruit production. The effects of SRF and PRO on seed yield and oil characteristics will be reported in a subsequent manuscript.
2 Materials and methods
2.1 Plant materials
Yellowhorn seeds were collected from mature plantations in three provenances in China: Wengniute Qi, Inner Mongolia province (WQ), Alukeerqin Qi, Inner Mongolia province (AQ), and Shanxian, Henan province (SX). The three provenances cover the distribution of yellowhorn from northern to southern part of China, with different geographical and environmental conditions (Table 1). Undamaged seeds were collected from mature, seeds producing trees (approx. 50 years old). Those trees had been established by local farmers from the seeds collected locally from wild sources. Therefore, they represented the wild germplasm in each provenance. Approximately 4000 seeds were sampled from 30 trees with 30–40 m apart from each other in July (SX) and August (WQ and AQ) 2013. Seeds from each location were pooled, so the genetic variability was only considered at the provenance level. Seeds were stored in partially sealed Kraft paper bags (permeable to carbon dioxide and oxygen yet largely impermeable to moisture) at 2 °C until the experiment began the following April.
| Table 1. Geographical location and environmental factors for three provenances of yellowhorn. | |||||||||
| PRO | Longitude, latitude | Altitude (m) | Mean annual temperature (°C) | January mean temperature (°C) | July mean temperature (°C) | Annual total accumulative temperature above 10 °C | Annual rainfall (mm) | Annual sunshine hours (h) | Frost-free period (d) |
| WQ | 42°57´N, 119°00´E | 623 | 5.8 | –12.5 | 22.5 | 2640 | 320 | 2923 | 130 |
| AQ | 44°13´N, 119°18´E | 550 | 5.5 | –11.5 | 22.9 | 2800 | 340 | 2900 | 120 |
| SX | 34°63´N, 111°62´E | 1026 | 13.9 | –0.7 | 27.0 | 4276 | 650 | 2354 | 219 |
2.2 Nursery experiment
The nursery experiment was conducted in a greenhouse at Beijing Forestry University Forest Science Company, Ltd (40°00´N, 116°34´E). Our experiment investigated the main and interacting effects of five rates of SRF (40, 80, 120, 160 and 200 mg N seedling–1) and three provenances (WQ, AQ, and SX). Pre-germination was performed to reduce germination time. Seeds were sown on 2 April 2014 at a depth of 1~2 cm in 1050 ml containers (8 cm diameter × 20 cm deep) commonly used in China that were filled with a 3:1 (v:v) peat: vermiculite mixture (Li et al. 2014). Commercial SRF Osmocote Exact Standard (5–6 months, O.M. Scotts Co., Marysville, OH, USA), comprising 8.4% NH4-N +6.6% NO3-N, 3.9% P, 9.1% K, 2% MgO, 0.45% Fe, 0.06% Mg, 0.05% Cu, 0.015% Zn, 0.03% B, and 0.02% Mo, was incorporated into the growth media prior to sowing. Germination completed within 2 weeks after sowing. Germinated seedlings were thinned to one per container. For each provenance, there were 900 seedlings (one per container) and 180 seedlings were randomly assigned to each SRF treatment. Fifteen treatments (3 provenances and 5 rates of SRF) were arranged in a completely randomized design with four replications (i.e. a total of 2700 seedlings; 45 seedlings per replication × 4 replications × 5 rates of SRF × 3 provenances). Fifteen containers were randomly assigned to each of 180 trays (44.5 cm long × 26.5 cm wide) (3 trays per replication). Trays of seedlings were put onto raised rolling benches in the greenhouse and rotated weekly to minimize any microsite effects (Li et al. 2014). The seedlings were irrigated as needed, about 2 times per week. From September, irrigation was reduced to initiate hardening, an interval of approximately 5 days. When most of the seedlings formed terminal buds, all seedlings were moved outdoors on 24 October 2014 to hasten hardening. Temperature was measured with a JL-18 Series thermometer (Huayan Instrument and Equipment Co., Shanghai, China) at 15-min intervals throughout nursery culture. Greenhouse temperatures averaged 24 ⁚ 21 °C (day ⁚ night), and day length in the greenhouse averaged 13 h 28 min. Once outside, seedlings were exposed to natural light conditions with temperatures averaging 13 ⁚ 8 °C (day ⁚ night). After 28 November 2014, seedlings were stored under plastic cover during winter to avoid frost injury. Monthly average temperatures during the winter were 6.2, –0.6, –0.2, and 1.6 °C, respectively from November 2014 through February 2015.
2.3 Field trial
Seedlings were transplanted to a field site on 4 April 2015. The site was located in Yanqing County, Beijing, China (40°46´N, 115°97´E) and it was previously used for agriculture. The soil texture was sandy loam with 19% clay, 21% silt, 60% sand with a pH of 7.2 and soil organic carbon of 0.7%. Soil depth varied between 45 and 60 cm. Soil was sampled from five locations across the field, and three samples were collected from each location. The top 2 cm od the soil was discarded and soil was then sampled at depths of 2–20 cm. The soil samples were oven-dried at 70 °C for 24 h and crushed gently to pass a 2-mm sieve before analyses. Soil total N, P and K were determined according to Official Methods of Chinese Academy of Forestry Research Institute (Chinese Academy of Forestry Research Institute 1987a, 1987b, 1999). Briefly, N was determined using semi-micro Kjeldahl distillation, and P and K were measured by a Mo-Sb colorimetric method and a flame photometry method, respectively. The average total N, P, and K of the surface soil (2–20 cm) were 1239, 1459, and 6186 mg kg–1, respectively. The soil was considered to have low soil fertility according to the macronutrient classified criteria (Bao 2000).
The area is a temperate continental monsoon climate and characterized by dry winter and spring seasons. During the experiment, annual precipitation was 471 mm in 2015 and 491 mm in 2016 and mean annual air temperature was 11.1 °C and 10.3 °C, respectively. The average monthly precipitation and air temperature was typically 8.6 mm and 2.4 °C in the cold and dry season from October to April; and 87.8 mm and 21.0 °C in the hot and moist season from May to September. The field experiment was arranged as a randomized complete block experimental design with four replicates. Each block measured 1.5 m × 25 m and was separated from adjacent blocks. From each replicate in the greenhouse, 15 seedlings were randomly selected and planted in a single row within each block, resulting in a total of 900 seedlings planted in the field site. Rows were spaced at 0.5 m with seedlings 0.5 m apart within the rows. Each seedling was placed in a hole that was refilled with the excavated soil by hand. Weeds were removed as necessary and seedlings were irrigated using an overhead automatic irrigation system evenly across the experiment as needed.
2.4 Sampling and measurements
Seedlings were sampled prior to transplanting to measure plant biomass and nutritional status. Eight seedlings were randomly sampled from each replicate, resulting in a total of 32 seedlings per nursery fertilization treatment. Seedlings were washed gently to free it of growing medium and were then cut at the root collar, and separated into stems (without leaves due to defoliation) and roots. Each plant tissue type was oven-dried at 70 °C for 48 h, and then weighed to determine dry mass (Tsakaldimi 2006).
After stem and root dry mass measurement, organ (stem and root) N, P and K contents were determined from composite samples of 8 seedlings for each treatment. Samples were ground, sieved through a 0.25 mm screen, and approximately 0.2 g of each of the subsamples was wet-digested in a heating block at 250 °C with a mixture of H2SO4 and H2O2 (Soon and Kalra 1995). We determined total N content by using semi-micro Kjeldahl distillation by a distillation unit (UDK-152, Velp Scientifica, USA), P content by using a UV-visible spectrophotometer (Agilent 8453, USA) and K content by using atomic emission spectrophotometry (SpectrAA 220 Atomic Absorption Spectrometer, Varian Inc., USA). Approximately 0.1 g of each subsample was extracted with 80% ethanol at 80 °C for carbohydrate analysis (Li et al. 2000). Soluble sugar content was determined by the anthrone colorimetry method (Spiro 1966).
Seedling height, diameter at ground level, and survival have been used to measure seedling quality in relation to field performance potential (Pinto et al. 2011; Tsakaldimi et al. 2013). Immediately after transplanting on 4 April 2015 (T1) seedling height and diameter at ground level was determined (8 seedlings per treatment per replicate were sampled randomly). When seedlings stopped growing in late October 2015 (T2) and 2016 (T3), survival, height and ground diameter were recorded. Net increment of height or ground diameter was defined as the difference observed between T2 and T1, and between T3 and T2. At T3, three seedlings in the middle of each block were excavated (12 seedlings per treatment) for determination of stem and root dry mass.
2.5 Statistical analysis
Statistical analyses were performed using SPSS 16.0 (Chicago, Illinois, USA). The explore function was used to examine data for normality and homogeneity prior to analyses. Survival was arcsine transformed in order to fulfill the normality and homogeneity requirements of ANOVA (Li et al. 2016). Untransformed values are presented here.
Effects of SRF, provenance, and their interaction on morphological and nutritional attributes were assessed using a two-way ANOVA. When a significant interaction occurred between SRF and provenance, one-way ANOVA was used to examine specific significant differences among the 15 treatment combinations. Duncan’s test was carried out for multiple comparisons among treatments (α = 0.05)(Li et al. 2014). Field performance was analyzed separately for each sampling period.
3 Results
3.1 Nursery response
Stem N content was significantly influenced by the interaction of PRO and SRF (Table 2, Table 3). In provenances AQ and SX, high level of SRF significantly enhanced the stem N content compared with low level of SRF, with the maximum stem N content being observed at 200 mg N seedling–1 for both provenances. In provenance WQ, stem N content peaked at 80 mg N seedling–1. Overall, there was no difference among provenances on root N content, with WQ being slightly low but not statistically significant. SRF rate had an effect at 160 mg N seedling–1 (Table 2, Fig. 1). From 40 to 160 mg N seedling–1, root N content increased with the rate of SRF and slightly declined at 200 mg N seedling–1 (Fig. 1). Compared with 40 mg N seedling–1, root N content was increased by 107% at 160 mg N seedling–1 treatment.
| Table 2. Effects of provenance (PRO), slow-release fertilizer (SRF) and their interaction (PRO × SRF) on N, P, K and soluble sugar content of yellowhorn seedling prior to transplanting. | ||||||||
| Treatment | N content | P content | K content | Soluble sugar content | ||||
| Stem | Root | Stem | Root | Stem | Root | Stem | Root | |
| PRO | NS | NS | * | * | NS | NS | NS | NS |
| SRF | ** | ** | *** | * | NS | NS | *** | NS |
| PRO × SRF | * | NS | NS | NS | NS | NS | ** | *** |
| NS, *, **, *** Non-significant; and significant at p = 0.05, 0.01, 0.001, respectively. | ||||||||
| Table 3. Means ± SE of stem N content, stem and root soluble sugar content of yellowhorn seedling prior to transplanting. | ||||
| Provenance | SRF rate (mg N seedling-1) | Stem N content (mg) | Stem soluble sugar content (mg) | Root soluble sugar content (mg) |
| AQ | 40 | 6.5 ± 1.2 ab | 22.2 ± 1.6 bcd | 36.4 ± 1.1 cde |
| 80 | 8.6 ± 2.7 abc | 24.3 ± 1.4 cde | 43.6 ± 2.7 de | |
| 120 | 18.3 ± 6.6 cde | 12.0 ± 4.5 ab | 38.6 ± 7.6 cde | |
| 160 | 16.8 ± 4.4 bcde | 27.1 ± 1.4 de | 29.2 ± 1.0 bcd | |
| 200 | 24.9 ± 2.1 e | 22.5 ± 3.6 bcd | 30.8 ± 3.0 bcd | |
| SX | 40 | 8.2 ± 1.3 abc | 6.2 ± 1.3 a | 25.1 ± 2.3 abc |
| 80 | 7.3 ± 1.2 ab | 26.6 ± 8.4 de | 46.2 ± 6.8 e | |
| 120 | 12.7 ± 2.9 abcd | 19.0 ± 1.4 bcd | 40.3 ± 4.4 de | |
| 160 | 9.8 ± 1.4 abc | 14.6 ± 2.4 abc | 20.3 ± 8.0 ab | |
| 200 | 20.3 ± 6.7 ade | 23.9 ± 2.7 cde | 32.5 ± 7.0b cde | |
| WQ | 40 | 11.4 ± 1.2 abcd | 14.8 ± 3.0 abc | 41.8 ± 2.6 de |
| 80 | 13.7 ± 2.2 abcd | 33.5 ± 1.6 e | 15.1 ± 0.5 a | |
| 120 | 5.4 ± 0.6 a | 18.8 ± 4.0 bcd | 30.1 ± 4.1 bcd | |
| 160 | 12.2 ± 2.0 abcd | 28.1 ± 0.9 de | 31.7 ± 4.3 bcde | |
| 200 | 9.5 ± 1.4 abc | 18.0 ± 1.3 bcd | 42.8 ± 2.6 de | |
| Values marked with different letters are statistically different according to Duncan’s test (α = 0.05). | ||||
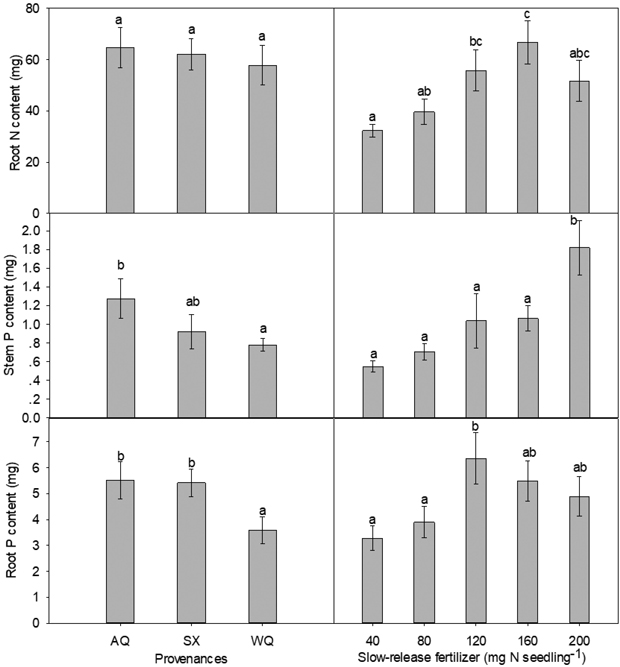
Fig. 1. Main effects of provenance and slow-release fertilizer on nursery stage root N content, stem P content and root P content (mean±SE) of yellowhorn seedlings prior to transplanting. Bars marked with different letters differ significantly for each treatment according to Duncan’s test α = 0.05.
PRO and SRF, but not their interaction, had significant impact on the amount of P in stem and root (Table 2). The trends for stem and root P contents among the three provenances were similar (Fig. 1). Compared with WQ seedlings, stem and root P content was increased by 63% and 54% in AQ, respectively. High level of SRF (200 mg N seedling–1) significantly enhanced stem P content relative to low level of SRF. The root P content was maximal at 120 mg N seedling–1.
Provenance, SRF and their interaction did not significantly affect stem or root K content (Table 2). The mean values (±SE) for all the provenances and fertilization treatments as a whole were 13.22 ± 8.48 mg for stem K content and 43.72 ± 19.95 mg for root K content.
Neither stem nor root soluble sugar content was influenced by provenance (Table 2). However both stem and root soluble sugar content was affected by the PRO × SRF interaction (Table 2, Table 3). Soluble sugar content of stem and root was highly variable, and there did not appear to be consistent patterns across provenances or rates of application. Interestingly, provenance WQ combined with 80 mg N seedling–1 yielded among the highest stem soluble sugar content and the lowest root soluble sugar content.
During the nursery phase (initial), the interaction of PRO × SRF had significant impacts on stem and root biomass (Table 4, Table 5). The combination of AQ provenance and 160 mg N seedling–1 SRF yielded the maximum initial stem biomass. The maximum initial root biomass occurred in SX provenance with 40 mg N seedling–1 SRF.
| Table 4. Effects of provenance (PRO), slow-release fertilizer (SRF) and their interaction (PRO × SRF) on yellowhorn seedling stem and root biomass prior to transplanting and at the end of the second growing season (2016, T3). | ||||
| Treatment | Stem biomass | Root biomass | T3 stem biomass | T3 root biomass |
| PRO | NS | ** | NS | NS |
| SRF | ** | NS | ** | *** |
| PRO × SRF | ** | * | NS | NS |
| NS, *, **, *** Non-significant; and significant at p = 0.05, 0.01, 0.001, respectively. | ||||
| Table 5. Means ± SE of stem and root biomass of yellowhorn seedling prior to transplanting. | |||
| Provenance | SRF rate (mg N seedling-1) | Stem biomass (g) | Root biomass (g) |
| AQ | 40 | 0.37 ± 0.04 a | 2.06 ± 0.22 a |
| 80 | 0.54 ± 0.04 bcd | 3.02 ± 0.27 cd | |
| 120 | 0.56 ± 0.07 cde | 2.86 ± 0.34 bcd | |
| 160 | 0.71 ± 0.07 e | 2.06 ± 0.21 a | |
| 200 | 0.58 ± 0.07 cde | 2.11 ± 0.25 ab | |
| SX | 40 | 0.59 ± 0.04 cde | 3.23 ± 0.27 d |
| 80 | 0.39 ± 0.03 ab | 2.42 ± 0.20 abc | |
| 120 | 0.48 ± 0.04 abcd | 2.98 ± 0.26 cd | |
| 160 | 0.60 ± 0.04 cde | 2.78 ± 0.22 abcd | |
| 200 | 0.64 ± 0.06 de | 2.80 ± 0.22 abcd | |
| WQ | 40 | 0.44 ± 0.04 abc | 2.13 ± 0.17 ab |
| 80 | 0.49 ± 0.04 abcd | 2.33 ± 0.23 abc | |
| 120 | 0.57 ± 0.06 cde | 2.27 ± 0.20 abc | |
| 160 | 0.50 ± 0.04 abcd | 2.39 ± 0.21 abc | |
| 200 | 0.48 ± 0.05 abcd | 2.34 ± 0.29 abc | |
| Values marked with different letters are statistically different according to Duncan’s test (α = 0.05). | |||
3.2 Field performance
Seedling height and diameter at the time of transplanting (initial) and at the end of the first growing season (2015, T2) were affected by PRO × SRF (Table 6, Table 7). The SX seedlings with 200 mg N seedling–1 SRF were the tallest and were among the largest in term of initial diameters (Table 7). At the end of the first growing season, for provenance AQ and SX, seedling height and diameter trends were similar to the trends observed during the nursery phase. At the end of the second growing season (2016, T3), height was significantly influenced by provenance whereas diameter was affected by SRF rate (Fig. 2). AQ and WQ seedlings were significantly higher than SX seedlings. Compared with 40 mg N seedling–1 treatment, 120, 160, 200 mg N seedling–1 treatments increased seedling diameter by 15%, 14%, and 26%, respectively.
| Table 6. Effects of provenance (PRO), slow-release fertilizer (SRF) and their interaction (PRO × SRF) on yellowhorn seedling height and diameter at T1, T2, and T3, and survival at T2 and T3. | ||||||||
| Treatment | T1 height | T1 diameter | T2 height | T2 diameter | T3 height | T3 diameter | T2 survival | T3 survival |
| PRO | *** | *** | ** | *** | * | NS | NS | NS |
| SRF | *** | *** | * | *** | NS | ** | NS | NS |
| PRO × SRF | *** | *** | ** | *** | NS | NS | * | ** |
| NS, *, **, *** Non-significant; and significant at p = 0.05, 0.01, 0.001, respectively. T1 means immediately after transplanting. T2 and T3 means at the end of the first (2015) and the second (2016) growing season after transplanting, respectively. | ||||||||
| Table 7. Means ± SE of height and diameter at T1 and T2, and survival at T2 and T3 of yellowhorn seedling. | |||||||
| PRO | SRF rate (mg N seedling-1) | T1 height (cm) | T1 diameter (mm) | T2 height (cm) | T2 diameter (mm) | T2 survival (%) | T3 survival (%) |
| AQ | 40 | 21.9 ± 0.3 bc | 2.9 ± 0.1 a | 32.4 ± 2.7 a | 4.0 ± 0.1 abc | 86.5 ± 9.1 a | 84.6 ± 8.3 abc |
| 80 | 20.3 ± 0.9 b | 2.9 ± 0.0 a | 32.9 ± 2.3 a | 3.9 ± 0.1 ab | 100.0 ± 0.0 b | 87.9 ± 5.4 abc | |
| 120 | 27.4 ± 0.6 e | 3.4 ± 0.1 cd | 41.2 ± 1.5 ab | 4.5 ± 0.0 efg | 100.0 ± 0.0 b | 97.7 ± 2.3 c | |
| 160 | 25.1 ± 0.4 d | 3.2 ± 0.1 b | 34.5 ± 2.3 a | 4.3 ± 0.1 cdef | 100.0 ± 0.0 b | 88.1 ± 4.2 abc | |
| 200 | 18.2 ± 0.2 a | 2.9 ± 0.0 a | 29.4 ± 4.6 a | 4.1 ± 0.2 bcd | 97.7 ± 2.3 ab | 70.5 ± 9.4 a | |
| SX | 40 | 28.7 ± 0.5 e | 3.2 ± 0.1 b | 40.7 ± 2.4 ab | 4.4 ± 0.1 def | 98.3 ± 1.7 ab | 76.7 ± 6.9 a |
| 80 | 21.4 ± 0.9 b | 3.2 ± 0.0 bc | 31.6 ± 2.1 a | 4.2 ± 0.1 bcde | 100.0 ± 0.0 b | 89.6 ± 4.3 abc | |
| 120 | 23.7 ± 0.4 cd | 3.5 ± 0.0 ef | 33.5 ± 0.6 a | 4.8 ± 0.1 g | 90.0 ± 6.4 ab | 75.0 ± 6.3 a | |
| 160 | 28.1 ± 0.6 e | 3.4 ± 0.1 cde | 35.0 ± 2.3 a | 4.6 ± 0.1 fg | 98.2 ± 1.8 ab | 94.8 ± 3.4 bc | |
| 200 | 33.6 ± 0.9 f | 3.6 ± 0.1 f | 49.0 ± 6.1 bc | 4.8 ± 0.1 g | 100.0 ± 0.0 b | 96.5 ± 2.0 bc | |
| WQ | 40 | 21.3 ± 0.7 b | 2.8 ± 0.0 a | 31.0 ± 1.9 a | 3.9 ± 0.1 ab | 100.0 ± 0.0 b | 82.7 ± 7.3 ab |
| 80 | 24.9 ± 0.6 d | 3.1 ± 0.0 b | 37.8 ± 2.9 ab | 4.1 ± 0.1 bcd | 98.5 ± 1.5 ab | 81.5 ± 5.0 ab | |
| 120 | 20.2 ± 0.7 b | 2.9 ± 0.1 a | 41.8 ± 2.4 ab | 3.7 ± 0.2 a | 100.0 ± 0.0 b | 81.7 ± 4.0 ab | |
| 160 | 27.8 ± 0.7 e | 3.6 ± 0.1 f | 48.1 ± 8.7 bc | 4.5 ± 0.0 efg | 100.0 ± 0.0 b | 91.7 ± 3.2 abc | |
| 200 | 26.9 ± 0.3 e | 3.4 ± 0.0 de | 55.7 ± 5.5 c | 4.8 ± 0.0 g | 95.0 ± 2.9 ab | 80.2 ± 5.9 ab | |
| Values marked with different letters are statistically different according to Duncan’s test (α = 0.05). T1 means immediately after transplanting. T2 and T3 means at the end of the first (2015) and the second (2016) growing season after transplanting, respectively. | |||||||
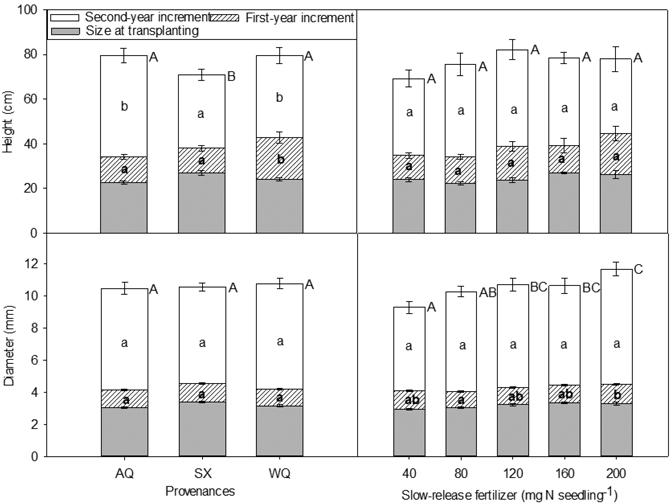
Fig. 2. Height, diameter and their increments in relation to provenance and slow-release fertilizer of yellowhorn seedling at transplanting (T1), at the end of the first (2015, T2) and the second (2016, T3) growing season after transplanting. Error bars represent SE of increments at the end of the first (2015, T2) and the second (2016, T3) growing seasons, and of size at transplanting. Bars marked with different letters differ significantly for total height or diameter (upper case letters) and growth increment (lower case) according to Duncan’s test (α = 0.05), respectively. Comparison letters for height and diameter at T1 and T2 are not presented owing to an occurring interaction.
There were significant differences in the first and second year height increments among provenances, with the greatest increment occurring in WQ (the first year), and AQ and WQ (the second year), respectively (Fig. 2). For SX seedlings, despite of being the tallest at the time of transplanting, this difference disappeared in the following two growing seasons, with minimum increments (Fig. 2).
The first year diameter increment was significantly affected by SRF with seedlings receiving 200 mg N seedling–1 increasing their diameter more than those grown with 80 mg N seedling–1 by 24% (Fig. 2). Diameter increment in the second year was not affected by SRF, provenance or their interaction. Seedlings receiving higher rates of SRF tended to have greater diameter at the end of the experiment (Fig. 2).
At the end of the second growing season, both stem and root biomass, and biomass increment, were affected only by SRF rate (Fig. 3). In general, increased rates of SRF resulted in plants with larger stem and root biomass and biomass increment.
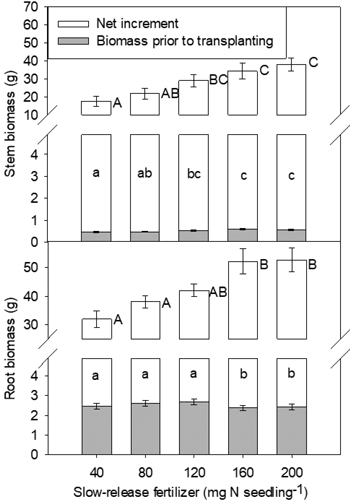
Fig. 3. Yellowhorn seedling stem biomass, root biomass and their increments in relation to slow-release fertilizer (SRF) prior to transplanting and at the end of the second (2016, T3) growing season after transplanting. Error bars represent SE of biomass prior to transplanting and increments at the end of the second growing season (2016, T3). Bars marked with different letters differ significantly for stem or root biomass (upper case letters) and their increments (lower case) according to Duncan’s test (α = 0.05), respectively. Comparison letters for stem and root biomass prior to transplanting are not presented owing to an occurring interaction.
Survival after the first and the second growing season depended on the SRF × PRO interaction (Table 6, Table 7). The mean values of survival for all the provenances and fertilization treatments as a whole were 97.6% and 85.3% for the first and second growing season, respectively. Furthermore, at the end of the second growing season, survival of most treatments decreased compared with the previous year, with the maximum difference observed in the combination of provenance AQ and 200 mg N seedling–1 SRF.
4 Discussion
4.1 Nursery growth performance
Generally, nitrogen fertilization application increases seedling growth and nutritional status of forest species (Broncano et al. 1998; Oliet et al. 2009; Salifu and Jacobs 2006). As a result, fertilization has been considered a beneficial nursery practice for a long time (Grossnickle and MacDonald 2018). In the present study, our hypothesis that SRF would significantly affect seedling nutrient status was shown to be partially correct. Among all nutrient parameters, only root N and P contents and stem P content were significantly increased by SRF rate in three provenances (Fig. 1), and SRF did not significantly influence stem and root K contents. It is reported that high rate of SRF increased the amount of N, P, and K in shoots and roots of Viburnum opulus L., and provenances differed significantly for the high fertilization level in N, P, and K contents (Bohne et al. 2011), while based on our results, provenance difference only occurred in stem and root P contents. N content is useful in forecasting seedling field performance, because it reflects differences in both initial seedling size and nutrient status (Cuesta et al. 2010; Puértolas et al. 2003; Quoreshi and Timmer 2000). The high level of N and P contents in yellowhorn seedlings can be remobilized after transplanting to improve seedling establishment when nutrient-uptake is impaired, and this was later proved by seedling growth in our experiment.
Currently, we cannot provide a definitive conclusion on the effect of PRO and SRF rates on non-structural carbohydrate content (NSC), since seedling starch content was not measured in this study. In terms of soluble sugar content, both stem and root soluble sugar contents depended on the PRO × SRF interaction (Tables 2, 3). Furthermore, we did not find consistent patterns in terms of effects of provenances or SRF rates. To a certain extent, this finding is similar with Chinese pine seedlings (Fu et al. 2017), but differs from studies with Picea abies (L.) H. Karst. and Pinus pinea L. seedlings, demonstrating fertilization rate has both positive and negative effects on soluble sugar content (Kaakinen et al. 2004; Villar-Salvador et al. 2013). Interestingly, provenance SX showed the highest stem soluble sugar content (22.64 g), but the lowest root soluble sugar content (32.29 g) among three provenances based on our findings. The relationship between nursery fertilization treatments and yellowhorn seedling NSC warrants further investigation.
Optimum N levels have been comprehensively determined for some woody species , such as Picea (64 mg N seedling–1) and Quercus (100 mg N seedling–1) (Salifu and Jacobs 2006; Salifu and Timmer 2003). In one study on yellowhorn seedlings application of fertilization 230 mg N seedling–1 was suggested, based on the nursery nutrient status and growth (Yang et al. 2014). A statistically significant decline in dry mass or tissue nutrient content was not observed in current study (Figs. 1, 3), which indicated that nutrient toxicity did not occur. As a result, we were unable to develop a full understanding of luxury consumption limits.
4.2 Field growth performance
In recent years, the effects of nursery fertilization on field performance have been studied on a wide range of forest species across various environments (Girardi et al. 2005; Griffiths et al. 2013; Haase et al. 2006; Hawkins et al. 2005). Nutrient loaded plants are assumed to perform better after out-planting compared to non-loaded ones (Grossnickle and MacDonald 2018). However, some inconsistent results have been reported, suggesting that field performance may be species- and provenance-specific (Li et al. 2016). In our experiment, after transplanting, seedling height increment was significantly influenced by provenance in the first and the second year (Fig. 2), indicating that provenance had a relatively long-term effect on height. The duration of a positive influence of high fertilization on out-planting performance differed, for instance, one year for Malus ‘Sutyzam’ (Lloyd et al. 2006) and 4 years for Acacia salicina Lindl. (Oliet et al. 2005). In our experiment, the effects persisted for diameter, stem and root biomass in the second year after out-planting (Figs. 2, 3). This was also consistent with our hypothesis that, incorporating SRF improves field performance. Plant growth after out-planting, especially on nutrient poor soils, mainly depends on nutrient reserves (Andivia et al. 2011). In the present experiment, the out-planting site soil condition was characterized by nutrient unavailability, with a low level of N and K concentrations. Therefore, when the external nutrient source was unable to meet the nutrient demand for growth, nutrient remobilization provided yellowhorn seedlings the capability to overcome planting stress.
Both seedling height and diameter increments in the first year after being transplanted were less than those observed in the second year, indicating that yellowhorn seedlings underwent slow growth during the first year after transplanting. As previously reported, it is possible that nursery grown stock might have not been able to absorb enough water and nutrients from the field soil and the root system might have been damaged (Burdett and Brand 1990). Similar results were found in Chinese pine and Pinus tabulaeformis seedlings (Li et al. 2016; Wang et al. 2015), but different results were reported in Douglas-fir of improved first-year growth and in Norway spruce (Heiskanen et al. 2009; Margolis and Waring 1986).
Field performance of seedlings may be closely affected by pre-planting nutrient status (Quoreshi and Timmer 2000; Timmer 1997). Interpretations of plant response to fertilization are often based on dry mass, because biomass would seem to be a more complete measure of plant size and may be more closely related to potential for future growth (Grossnickle 2000; Marschner and Rimmington 1988). In the nursery, SRF rates of 120–200 mg N seedling–1 promoted root nutritional status (N and P contents) (Fig. 1). Correspondingly, at the end of the second growing season, there was a positive relationship between fertilizer application rate and root biomass, with the maximum value being observed at SRF rate of 160–200 mg N seedling–1 (Fig. 3). The result indicated that seedlings with optimal nutrient reserves had increased root egress after out-planting and the ability to absorb more nutrients and water, then outcompeted other vegetation (Grossnickle and MacDonald 2018; Malik and Timmer 1996). According to previous study in yellowhorn, 180 and 230 mg N seedling–1 treatments increased plant biomass effectively (Yang et al. 2014). The rate of 280 mg N seedling–1 slightly decreased biomass. Based on the result, it can be inferred that the optimum SRF rate may be above 200 mg N seedling–1. To achieve higher biomass, further research is suggested to increase SRF rate to 300 mg N seedling–1.
4.3 Field survival
Seedlings are hardly ever irrigated in the field in forest restoration programs. However, to improve survival, growth and seed production, it’s suggested that yellowhorn be planted in ideal conditions with adequate illumination and irrigation conditions. Consistent with a previous study on Chines pine (Wang et al. 2015), yellowhorn seedling survival at the end of the second growing season declined dramatically, relative to the first growing season (70–98% and 87–100% survival, respectively). This may be attributed to the similar climatic conditions under which both studies were conducted. The two sites are proximately located, and both are temperate arid climate regions and characterized by low annual precipitation, which was related to the decline in seedling survival (Burdett 1990; Crow et al. 1994; Grossnickle 2005). Another reason may be that survival of newly planted seedlings improves when forest restoration site conditions dictate the amount of root growth required to overcome planting stress and ensure survival (Grossnickle 2012), however the soil property in our experiment might not meet the demands of seedling. Furthermore, in the present study, there was no consistent survival pattern across provenances or rates of SRF application (Table 7), which differs from some previous literatures that showed positive or negative survival responses to nursery fertilization (Bárbara et al. 2010; Trubat et al. 2008; Villar-Salvador et al. 2004). Therefore, survival may be affected by comprehensive factors, such as genetic, geographic, and climatic differences.
In conclusion, this study indicated the potential benefits of nursery application of SRF into the substrate of container-grown yellowhorn seedling. Nursery SRF treatment and PRO, either alone or as interaction term, influenced seedling growth. Higher rates of SRF tended to result in better seedling nutritional status. Considering overall nursery and field performance, we currently suggest that combining provenance AQ with 120–200 mg N seedling–1 SRF may be preferred for yellowhorn seedling. However, further study is needed to monitor seedling growth dynamic and SRF nutrient release patterns to determine the optimal nutrient release pattern that synchronizes best with the growth rhythm of yellowhorn seedlings. In addition, higher SRF rates (above 200 mg N seedling–1) are suggested to quantify the optimum target rate for effective nutrient loading of yellowhorn.
Acknowledgments
The study was funded by National Natural Science Foundation of China (No.31600241) and the Fundamental Research Funds for the Central Universities (2015ZCQ-LX-02). We thank Bruce Bordelon, Jules Janick and Khalil Jahed for editing this manuscript.
References
Andivia E., Fernández M., Vázquez-Piqué J. (2011). Autumn fertilization of Quercus ilex ssp. ballota (Desf.) Samp. nursery seedlings: effects on morpho-physiology and field performance. Annals of Forest Science 68: 543–553. https://doi.org/10.1007/s13595-011-0048-4.
Andivia E., Fernández M., Alejano R. (2012). Two provenances of Quercus ilex ssp. ballota (Desf) Samp. nursery seedlings have different response to frost tolerance and autumn fertilization. European Journal of Forest Research 131(4): 1091–1101. https://doi.org/10.1007/s10342-011-0578-1.
Ao Y. (2016). Comparison of floral ontogeny between wild-type Xanthoceras sorbifolia Bunge and its double-flowered mutant. Bangladesh Journal of Botany 45: 367–375.
Bao S.D. (2000). Soil and agricultural chemistry analysis. Chinese Agriculture Press, Beijing. p. 39–114. [In Chinese].
Bárbara C., Pedro V.S., Jaime P., Douglassf J., Josém R.B. (2010). Why do large, nitrogen rich seedlings better resist stressful transplanting conditions? A physiological analysis in two functionally contrasting Mediterranean forest species. Forest Ecology and Management 260(1): 71–78. https://doi.org/10.1016/j.foreco.2010.04.002.
Bohne H., Salomon L., Gerhard D. (2011). Influence of provenance and fertilization in the tree nursery on outplanting performance and N-, P-, and K-content of Viburnum opulus L. in shoots and roots. Journal of Environment Horticultue 29(3):137–142.
Broncano M.J., Riba M., Retana J. (1998). Seed germination and seedling performance of two Mediterranean tree species, holm oak (Quercus ilex L.) and Aleppo pine (Pinus halepensis Mill.): a multifactor experimental approach. Plant Ecology 138(1): 17–26. https://doi.org/10.1023/A:1009784215900.
Burdett A.N., Brand D.G. (1990). Physiological processes in plantation establishment and the development of specifications for forest planting stock Canadian Journal of Forest Research 20(4): 415–427. https://doi.org/10.1139/x90-059.
Chinese Academy of Forestry Research Institute (1987a). Chinese national standard GB 7852-1987. Determination of total phosphorus in forest soil. [In Chinese].
Chinese Academy of Forestry Research Institute (1987b). Chinese national standard GB 7854- 1987. Determination of total potassium in forest soil. [In Chinese].
Chinese Academy of Forestry Research Institute (1999). Chinese national standard GB 7854-1987. Determination of total Nitrogen in forest soil. [In Chinese].
Crow T.R., Johnson W.C., Adkisson C.S. (1994). Fire and recruitment of quercus in a postagricultural field. American Midland Naturalist 131(1): 84–97. https://doi.org/10.2307/2426611.
Cuesta B., Villarsalvador P., Puértolas J., Jacobs D.F., Rey Benayas J.M. (2010). Why do large, nitrogen rich seedlings better resist stressful transplanting conditions? A physiological analysis in two functionally contrasting mediterranean forest species. Forest Ecology and Management 260(1): 71–78. https://doi.org/10.1016/j.foreco.2010.04.002.
Fu Y., Oliet J.A., Li G., Wang J. (2017). Effect of controlled release fertilizer type and rate on mineral nutrients, non-structural carbohydrates, and field performance of Chinese pine container-grown seedlings. Silva Fennica 51(2) article 1607. https://doi.org/10.14214/sf.1607.
Girardi E.A., Mourão Filho F.A.A., Graf C.C.D., Olic F.B. (2005). Influence of soluble and slow-release fertilizers on vegetative growth of containerized citrus nursery trees. Journal of Plant Nutrition 28(9): 1465–1480. https://doi.org/10.1080/01904160500201337.
Gleason J.F., Duryea M., Rose R., Atkinson M. (1990). Nursery and field fertilization of 2 + 0 ponderosa pine seedlings: the effect on morphology, physiology, and field performance. Canadian Journal of Forest Research 20(11): 1766–1772. https://doi.org/10.1139/x90-235.
Griffiths E., Stevens J.C., Griffiths E. (2013). Managing nutrient regimes improves seedling root-growth potential of framework banksia-woodland species. Australian Journal of Botany 61(8): 600–610. https://doi.org/10.1071/BT13181.
Grossnickle S.C. (2000). Ecophysiology of northern spruce species. National Research Council, Ottawa. 407 p.
Grossnickle S.C. (2005). Importance of root growth in overcoming planting stress. New Forests 30(2–3): 273–294. https://doi.org/10.1007/s11056-004-8303-2.
Grossnickle S.C. (2012). Why seedlings survive: influence of plant attributes. New Forests 43(5–6): 711–738. https://doi.org/10.1007/s11056-012-9336-6.
Grossnickle S.C., MacDonald J.E. (2018). Why seedlings grow: influence of plant attributes. New Forests 49(1): 1–34. https://doi.org/10.1007/s11056-017-9606-4.
Haase D.L., Rose R., Trobaugh J. (2006). Field performance of three stock sizes of douglas-fir container seedlings grown with slow-release fertilizer in the nursery growing medium. New Forests 31(1): 1–24. https://doi.org/10.1007/s11056-004-5396-6.
Halmschlager E., Katzensteiner K. (2017). Vitality fertilization balanced tree nutrition and mitigated severity of Sirococcus shoot blight on mature Norway spruce. Forest Ecology & Management 389: 96–104. https://doi.org/10.1016/j.foreco.2016.12.019.
Hawkins B.J., Burgess D., Mitchell A.K. (2005). Growth and nutrient dynamics of western hemlock with conventional or exponential greenhouse fertilization and planting in different fertility conditions. Canadian Journal of Forest Research 35(4): 1002–1016. https://doi.org/10.1139/x05-026.
Heiskanen J., Lahti M., Luoranen J., Rikala R. (2009). Nutrient loading has a transitory effect on the nitrogen status and growth of outplanted Norway spruce seedlings. Silva Fennica 43(2): 249–260. https://doi.org/10.14214/sf.210.
Jones A.T., Hayes M.J. (2001). The effect of provenance on the performance of Crataegus monogyna in Hedges. Journal of Applied Ecology 38(5): 952–962. https://doi.org/10.1046/j.1365-2664.2001.00650.x.
Kaakinen S., Jolkkonen A., Iivonen S., Vapaavuori E. (2004). Growth, allocation and tissue chemistry of Picea abies seedlings affected by nutrient supply during the second growing season. Tree Physiology 24(6): 707–719. https://doi.org/10.1093/treephys/24.6.707.
Lee H., Yi J., An C., Kim M., Lee J. (2015). Component characteristics of Xanthoceras sorbifolium seeds for bioenergy plant utilization. Journal of Forest and Environmental Science 31(4): 272–279. https://doi.org/10.7747/JFES.2015.31.4.272.
Li G., Zhu Y., Liu Y., Wang J., Liu J., Dumroese R.K. (2014). Combined effects of pre-hardening and fall fertilization on nitrogen translocation and storage in Quercus variabilis seedlings. European Journal of Forest Research 133(6): 983–992. https://doi.org/10.1007/s10342-014-0816-4.
Li G., Wang J., Oliet J.A., Jacobs D.F. (2016). Combined pre-hardening and fall fertilization facilitates N storage and field performance of Pinus tabulaeformis seedlings. iForest – Biogeosciences and Forestry 9: 483–489. https://doi.org/10.3832/ifor1708-008.
Li H., Sun Q., Zhao S., Zhang W. (2000). Principles and techniques of plant physiological biochemical experiment. Higher Education, Beijing. 300 p. [In Chinese].
Li Y.M., Sun Y.X., Liao S.Q., Zou G.Y., Zhao T.K., Chen Y.H., Yang J.G., Zhang L. (2017). Effects of two slow-release nitrogen fertilizers and irrigation on yield, quality, and water-fertilizer productivity of greenhouse tomato. Agricultural Water Management 186: 139–146. https://doi.org/10.1016/j.agwat.2017.02.006.
Lloyd J.E., Herms D.A., Rose M.A., Wagoner J.V. (2006). Fertilization rate and irrigation scheduling in the nursery influence growth, insect performance, and stress tolerance of ‘Sutyzam’ Crab apple in the landscape. Hortscience 41: 442–445.
Malik V., Timmer V.R. (1996). Growth, nutrient dynamics, and interspecific competition of nutrient-loaded black spruce seedlings on a boreal mixedwood site. Canadian Journal of Forest Research 26(9): 1651–1659. https://doi.org/10.1139/x26-186.
Margolis H., Waring R. (1986). Carbon and nitrogen allocation patterns of Douglas-fir seedlings fertilized with nitrogen in autumn. II. Field performance. Canadian Journal of Forest Research 16(5): 903–909. https://doi.org/10.1139/x86-161.
Marschner H., Rimmington G. (1988). Mineral nutrition of higher plants. Plant Cell Environment 11(2): 147–148. https://doi.org/10.1111/1365-3040.ep11604921.
Oliet J.A., Planelles R., Artero F., Jacobs D.F. (2005). Fall fertilization of Holm oak affects N and P dynamics, root growth potential, and post-planting phenology and growth. Annals of Forest Science 68(3): 647–656. https://doi.org/10.1007/s13595-011-0060-8.
Oliet J.A., Tejada M., Salifu K.F., Collazos A., Jacobs D.F. (2009). Performance and nutrient dynamics of holm oak (Quercus ilex L.) seedlings in relation to nursery nutrient loading and post-transplant fertility. European Journal of Forest Research 128(3): 253–263. https://doi.org/10.1007/s10342-009-0261-y.
Pinto J.R., Marshall J.D., Dumroese R.K., Davis A.S., Cobos D.R. (2011). Establishment and growth of container seedlings for reforestation: a function of stocktype and edaphic conditions. Forest Ecology and Management 261(11): 1876–1884. https://doi.org/10.1016/j.foreco.2011.02.010.
Puértolas J., Gil L., Pardos J.A. (2003). Effects of nutritional status and seedling size on field performance of Pinus halepensis planted on former arable land in the Mediterranean basin. Forestry 76(2): 159–168. https://doi.org/10.1093/forestry/76.2.159.
Quoreshi A.M., Timmer V.R. (2000). Early outplanting performance of nutrient-loaded containerized black spruce seedlings inoculated with Laccaria bicolor: a bioassay study. Revue Canadienne De Recherche Forestière 30(5): 744–752. https://doi.org/10.1139/cjfr-30-5-744.
Salifu K.F., Jacobs D.F. (2006). Characterizing fertility targets and multi-element interactions in nursery culture of Quercus rubra seedlings. Annals of Forest Science 63(6): 231–237. https://doi.org/10.1051/forest:2006001.
Salifu K.F., Timmer V.R. (2003). Optimizing nitrogen loading of Picea mariana seedlings during nursery culture. Canadian Journal of Forest Research 3(7): 1287–1294(1288). https://doi.org/10.1139/x03-057.
Seletkovic I., Potocic N., Jazbec A., Cosic T., Jakovljevic T. (2009). The influence of various growing substrates and slow-release fertilizers on the growth and physiological parameters of common beech (Fagus Sylvatica L.) seedlings in a nursery and following planting in the field. Sumarski List 133: 469–481. [In Croatian].
Seletkovic I., Potocic N., Topic V., Butorac L., Jelic G., Jazbec A. (2011). Influence of various container types and slow-release fertilizer doses on growth and physiological parameters of Black pine (Pinus Nigra Arn.) seedlings. Sumarski List 135: 90–102. [In Croatian].
Shao H., Chu L. (2008). Resource evaluation of typical energy plants and possible functional zone planning in China. Biomass and Bioenergy 32(4): 283–288. https://doi.org/10.1016/j.biombioe.2007.10.001.
Shen Z., Zhang K., Ao Y., Ma L., Duan J. (2018). Evaluation of biodiesel from Xanthoceras sorbifolia Bunge seed kernel oil from 13 areas in China. Journal of Forestry Research. 9 p. https://doi.org/10.1007/s11676-018-0683-9.
Soon Y., Kalra Y. (1995). A comparison of plant tissue digestion methods for nitrogen and phosphorus analyses. Canadian Journal of Soil Science 75(2): 243–245. https://doi.org/10.4141/cjss95-034.
Spiro R. (1966). Determination of neutral sugars. In: Neufeld E.F., Ginsberg V. (eds.). Methods in enzymology. Academic Press, New York. p. 4–5.
Teixeira P.C., Rodrigues H.S., Lima W.A.A., Rocha R.N.C., Cunha R.N.V., Lopes R. (2009). Container distribution and slow release fertilizers application along the pre-nursery influencing oil palm seedlings growth. Ciencia Florestal 19: 157–168. [In Portuguese].
Timmer V.R. (1997). Exponential nutrient loading: a new fertilization technique to improve seedling performance on competitive sites. New Forests 13(1–3): 279–299. https://doi.org/10.1023/A:1006502830067.
Trubat R., Cortina J., Vilagrosa A. (2008). Short-term nitrogen deprivation increases field performance in nursery seedlings of Mediterranean woody species. Journal of Arid Environments 72(6): 879–890. https://doi.org/10.1016/j.jaridenv.2007.11.005.
Tsakaldimi M. (2006). Kenaf (Hibiscus cannabinus L.) core and rice hulls as components of container media for growing Pinus halepensis M. seedlings. Bioresource Technology 97(14): 1631–1639. https://doi.org/10.1016/j.biortech.2005.07.027.
Tsakaldimi M., Ganatsas P., Jacobs D.F. (2013). Prediction of planted seedling survival of five Mediterranean species based on initial seedling morphology. New Forests 44(3): 327–339. https://doi.org/10.1007/s11056-012-9339-3.
Villar-Salvador P., Planelles R., Enrı́Quez E., Rubira J.P. (2004). Nursery cultivation regimes, plant functional attributes, and field performance relationships in the Mediterranean oak Quercus ilex L. Forest Ecology and Management 196(2–3): 257–266. https://doi.org/10.1016/j.foreco.2004.02.061.
Villar-Salvador P., Valladares F., Domcnguez-Lerena S., Ruiz-Dcez B., Fernclndez-Pascual M., Delgado A., Pecluelas J.L. (2008). Functional traits related to seedling performance in the Mediterranean leguminous shrub Retama sphaerocarpa: Insights from a provenance, fertilization, and rhizobial inoculation study Environmental and Experimental Botany 64(2): 145–154. https://doi.org/10.1016/j.envexpbot.2008.04.005.
Villar-Salvador P., Peñuelas J.L., Jacobs D.F. (2013). Nitrogen nutrition and drought hardening exert opposite effects on the stress tolerance of Pinus pinea L. seedlings. Tree Physiology 33(2): 221–232. https://doi.org/10.1093/treephys/tps133.
Wang J., Li G., Pinto J.R., Liu J., Shi W., Liu Y. (2015). Both nursery and field performance determine suitable nitrogen supply of nursery-grown, exponentially fertilized Chinese pine. Silva Fennica 49(3) article 1295. https://doi.org/10.14214/sf.1295.
Yang T., Duan J., Ma L.Y., Jia L.M., Peng Z.D., Chen C., Chen J. (2014). Effects of N application rates on growth nutrient accumulation and translocation of Xanthoceras sorbifolia. Journal of Beijing Forestry University 36(3): 57–62. [In Chinese].
Zhu L., Zhang X., Long Z., Pang H., Li X., Yang Y. (2009). Effect of fertilizing nitrogen on seedling quality of Xanthoceras sorbifolia Bge. Forest By-Product and Speciality in China (100): 29–31. [In Chinese].
Total of 60 references.

