The demand of hybrid aspen (Populus tremula × P. tremuloides) on site conditions for a successful establishment on forest land
Hjelm K., Rytter L. (2018). The demand of hybrid aspen (Populus tremula × P. tremuloides) on site conditions for a successful establishment on forest land. Silva Fennica vol. 52 no. 5 article id 10036. https://doi.org/10.14214/sf.10036
Highlights
- Low pH (below 3.5) reduced growth, but not survival, in a greenhouse study
- Site preparation methods did not affect survival in field, but differences were found for growth
- Mounding had generally the best effect on growth
- Clonal differences were found that could be useful for improving establishment and growth.
Abstract
Hybrid aspen (Populus tremula L. × P. tremuloides Michx.) is a deciduous tree species suitable for producing large amounts of renewable biomass during short rotations. Its potential under North European conditions could be largely extended if not only agricultural land but also forest land was used for cultivation. Unfortunately, the knowledge of appropriate forest site conditions and effects of site preparation methods on hybrid aspen establishment is limited. In this paper, two studies that explore these questions are presented. In the first study, the sensitivity to acid soils was tested under greenhouse conditions in two type of soils: a) peat soil limed to certain pH levels (3.4–5.7) and b) collected forest soils where pH varied from 3.9 to 5.3. The lowest pH level resulted in reduced growth, elsewhere no significant differences were found. The second study was applied in the field and investigated the effect of four site preparation methods on survival and growth. The methods were: 1) control with no site preparation, 2) patch scarification, 3) mounding and 4) soil inversion. While no differences were found for survival, mounding was generally the method with the highest growth and patch scarification was least successful. The result was probably an effect of good soil aeration and less competition from vegetation after mounding. The field study also revealed clonal differences in growth performance, which stresses the importance of clone selection prior to planting. The results of these studies indicate that hybrid aspen is less sensitive to variation in pH and site preparation methods compared with other poplar species, as have been found in similar studies.
Keywords
regeneration;
site preparation;
clone effects;
poplars;
soil acidity
-
Hjelm,
Skogforsk, Ekebo 2250, SE-268 90 Svalöv, Sweden
E-mail
karin.hjelm@skogforsk.se

-
Rytter,
Skogforsk, Ekebo 2250, SE-268 90 Svalöv, Sweden
 http://orcid.org/0000-0001-6183-4832
E-mail
lars.rytter@skogforsk.se
http://orcid.org/0000-0001-6183-4832
E-mail
lars.rytter@skogforsk.se
Received 22 August 2018 Accepted 11 December 2018 Published 12 December 2018
Views 92472
Available at https://doi.org/10.14214/sf.10036 | Download PDF

1 Introduction
Biomass from forests is a major component in mitigating effects of climate change in Sweden. The energy use in the country is currently based on renewable sources to over 53% and biomass is the dominating source of the renewable supply (Swedish Government 2017). The biofuel part has shown a steady increase during the last 30 years (Swedish Energy Agency 2017). Products from the forest can act as carbon neutral energy sources with possibilities to substitute fossil energy but also the forest itself sequester large amounts of carbon in trees and soil. Investigations on future possibilities to increase production of wood biomass (Larsson et al. 2009) showed that 300 000–500 000 ha of abandoned agricultural land are available for afforestation, but the forest land area where fast-growing trees species could be used is much larger.
Fast-growing tree species for biomass-oriented cultivation, like hybrid aspen (Populus tremula L. × P. tremuloides Michx.) and poplar (Populus spp.), have so far mainly been used on agricultural land in northern Europe and North America, where much knowledge and information have been gathered (Christersson 2008; Tullus et al. 2012; Truax et al. 2014; Persson et al. 2015; Hjelm et al. 2018). Hybrid aspen, the crossing between European aspen and trembling aspen, has been tested for long time and used commercially during the latest decades. Results reveal a high biomass productivity during short rotations on former agricultural land (Tullus et al. 2012). The planted generation produces around 20 m3 of stemwood ha–1 yr–1, corresponding to 7 Mg DM of stemwood ha–1 yr–1, with genetic material selected during the 1980s (Rytter and Stener 2014). With the present material this figure is expected to raise to about 25 m3 (Stener and Karlsson 2004). The productivity is probably even higher in root sucker generations. Levels of 10 Mg DM ha–1 yr–1 within just a few years after harvest have been reported (Mc Carthy and Rytter 2015; Rytter and Rytter 2017). In addition to productivity, we also know rather well how stands should be established on agricultural land (Rytter et al. 2011; Persson et al. 2015).
However, information on stand establishment of fast-growing tree species on forest land is limited. An investigation on establishment success on forest land for Populus species showed that the success rate was much lower than on agricultural land, and that knowledge of necessary measures was highly demanded (Engerup 2011). Suggested reasons for the reduced success of hybrid aspen establishment on forest land were postponed planting resulting in presence of competing vegetation, and annual fluctuations where early summer drought was a major factor (Anander 2017). Another important aspect is browsing by ungulates. Aspen is a highly preferred species where a high browsing pressure leads to a longer transition to ungulate safe height (Edenius and Ericsson 2015), thus also prolonging the sensitive establishment phase.
Most existing information on soil requirement of Populus species is delivered for poplars from the Tacamahaca section of the genus, often P. trichocarpa Torr. & A. Gray and its hybrids (Boysen and Strobl 1991; Stanturf and van Oosten 2014; Böhlenius and Övergaard 2016; Böhlenius et al. 2016a; Hjelm and Rytter 2016). Existing information on hybrid aspen indicates that it is less sensitive to acid soil conditions than P. trichocarpa, which was tested in the same study (Böhlenius et al. 2016b). However, the performance of hybrid aspen on forest land needs to be further tested.
Site preparation on forest land is different from agricultural land where ploughing, harrowing, and herbicide treatments are frequently carried out. The most common site preparation methods prior to planting on forest land are patch scarification, disc trenching, mounding and soil inversion (Nilsson et al. 2010). Knowledge about the performance of fast-growing hardwood species after these methods is insufficient. Previous studies on the effect of site preparation on forest land in the Nordic countries are almost exclusively directed to conifer plants (Nilsson et al. 2010). Results show that soil scarification has positive effects on survival and early growth of plants (Nilsson et al. 2010; Bilodeau-Gauthier et al. 2011; Johansson et al. 2013). Main reasons for these effects are increased soil temperature and reduced competition from weeds. In addition, clonal differences in establishment performance have been reported for Norway spruce by Johansson et al. (2005). It is of great importance to check if fast-growing hardwood plants react in the same way as conifers.
In summary, our knowledge of soil conditions and site preparation on forest land, necessary for successful establishment and growth of hybrid aspen, is limited. To receive more information, two parallel studies were initiated. One of the studies concentrated on the effect of soil acidity on establishment and early growth. This study was carried out in a greenhouse. The other study investigated the effect of different site preparation methods on establishment success on forest land. Similar studies have previously been carried out and published for poplars (Hjelm and Rytter 2016; Mc Carthy et al. 2017).
Our hypotheses were:
a) soil acidity influences establishment of hybrid aspen with less success at lower pH;
b) different site preparation methods will give varying establishment results;
c) clones of hybrid aspen react differently to site preparation.
2 Materials and methods
The study consisted of two parts, one that investigated the effect of different pH-levels on hybrid aspen establishment in a greenhouse experiment, and one that investigated effects of soil preparation on establishment on forest land.
2.1 Greenhouse study
The greenhouse study was divided in two parts: A) a study where limestone was added to peat to achieve specified pH levels and B) a study where forest soils collected in southern Sweden were used.
In study A, sphagnum peat without any supplementary fertilizer or lime (supplied from Hasselfors Garden, Örebro, Sweden) was used. The peat was given a basic nutrient supply of 1.1 kg NPK micro (Multimix, NPK 14-7-15) per m3 according to the recommendations for deciduous trees and bushes given by the peat manufacturer. Thereafter, the peat was limed (Limestone Cresco Vital) to five different pH-levels. The peat, fertilizer and lime were blended together in a concrete mixer. Samples were taken from the liming treatments for analyses and checks of pH levels and nutrient conditions (Table 1).
| Table 1. Soil characteristics in the five pH treatments of the greenhouse study A. Total C and N (% of dry matter) was for respective treatment, while P, K, Ca, Mg and Al were analysed for plant-available content (mg g–1 dry matter) with the ammonium lactate (AL) method. na = not available. | |||||||||
| Limestone (kg m–3 peat) | pH level | CTot | NTot | PAL | KAL | CaAL | MgAL | AlAL | |
| (distilled H2O) | (0.01M CaCl2) | ||||||||
| 6 | 5.7 | 5.1 | 44.0 | 0.82 | 73 | 251 | 1524 | 142 | 15 |
| 4 | 4.9 | 4.5 | na | na | na | na | na | na | na |
| 3 | 4.6 | 4.1 | 49.4 | 0.91 | 74 | 265 | 817 | 138 | 11 |
| 2 | 4.2 | 3.7 | 49.4 | 0.94 | 83 | 287 | 647 | 140 | 11 |
| 0 | 3.4 | 2.9 | 50.6 | 0.96 | 81 | 291 | 66 | 111 | 9 |
In study B, mineral soil was collected from five forest experimental sites planted with hybrid aspen and poplars in southern Sweden (Table 2). Norway spruce had been grown on all sites during the previous rotation. The soil was collected from 10 spots per site, from below the organic layer and down to about 20 cm depth. The collecting procedure was adjusted to the experimental layout of the present experiments with regards to factors such as blocking. The soil from each site was pooled prior to planting and analysed for nutrient contents (Table 2).
| Table 2. Information on the forest soils used in study B. Total C and N content (% of dry matter) was given for each site, while P, K, Ca, Mg and Al were analysed for plant-available content (mg kg–1 dry matter) with the ammonium lactate method. | |||||||||
| Site | pH level | CTot | NTot | PAL | KAL | CaAL | MgAL | AlAL | |
| (distilled H2O) | (0.01M CaCl2) | ||||||||
| Duveholm | 5.3 | 4.6 | 1.5 | 0.12 | 21 | 70 | 1138 | 162 | 213 |
| Brattön | 4.7 | 3.8 | 4.3 | 0.32 | 26 | 62 | 145 | 44 | 1368 |
| Dimbo | 4.5 | 3.9 | 1.6 | 0.09 | 11 | 9 | 85 | 11 | 393 |
| Toftaholm | 4.4 | 4.0 | 1.5 | 0.09 | 22 | 12 | 65 | 6 | 722 |
| Matteröd | 3.9 | 3.4 | 4.0 | 0.17 | 12 | 12 | 52 | 21 | 940 |
Measurements of pH in the greenhouse study were performed according to Karltun (1996) to ensure that a well-defined pH level had been reached. For peat 2 g of soil were used per sample and for forest soil 5 g were used. However, the ratio between soil sample and liquid solution has rarely any substantial influence on the pH values (Van Lierop 1981; Karltun 1996). Three samples were prepared per peat treatment and forest soil in both 0.01M CaCl2-solution and distilled H2O. Both methods for determining pH were used since distilled water is the most common method used while CaCl2-solution is theoretically more stable (Karltun 1996). Previous studies of pH methodology have shown that the difference between the methods is usually 0.3–0.6 units with distilled water showing the highest value (Davies 1971; Stanek 1973; Van Lierop 1981). The differences between the methods were mainly within the expected range. Halfway through the experimental period and at the end, soil samples were collected to assure that the pH-levels were relatively constant. A slight drop of pH was seen at the end of the experimental period.
The hybrid aspen plants were purchased from a commercial nursery and consisted of a clone mix, which means that the individual clone identity was unknown. Hybrid aspens were delivered as container plants and the original peat was carefully removed before planting to avoid influences from pre-study conditions. Each plant was planted in a 2 l plastic pot filled with prepared soil according to the treatments in study A or with soil from one of the forest sites of study B. The pots had been watered to field capacity and checked with a TDR (time domain reflectometer) before planting to assure a non-limiting water availability.
Each treatment (pH level) was replicated eight times in study A and five times in study B.
A set of pots including all treatments was a block. The pots were randomly arranged within the block.
The experiment was carried out in 2014 in a ventilated greenhouse at the research station Ekebo of the Forestry Research Institute of Sweden, Skogforsk, outside Svalöv. The duration of study A lasted from May 14 to July 29 and of study B from May 13 to August 18. Water and nutrients (Gröna PlantTM NPK 9-1-6) were continuously supplied to study A plants to avoid limitations in availability of these elements. If conductivity in the peat soil became less than 1 mS m–1 nutrients were added in liquid form with the irrigation water up to values between 2 and 3 mS m–1. The soil conductivity level was checked weekly. In study B, plants were watered as in study A but had to rely on the initial nutrient resource since the original nutrient composition of the forest soils should stay unaffected.
Height of plants (cm) was recorded at planting. In study A, height was repeatedly recorded four times before harvest, which was conducted after about 14 weeks. In study B, height was recorded five times before harvest that took place after about 17 weeks. At harvest the stem was cut off at the soil surface level for each plant and the leaves were separated from the stem. The soil was removed from the roots by rinsing in water. Stems, leaves and roots were dried at 70 °C to constant weight and thereafter dry masses were recorded for each plant part.
2.2 Forest site study
This part of the study was established on relatively fertile forest land at three different sites in southern Sweden (Table 3). The sites were previous Norway spruce stands clear-felled during the winter 2012–2013.
| Table 3. Site characteristics from where the forest study was established. Annual precipitation (Prec.), annual mean temperature (Temp), and annual vegetation length (Veg.) are averages for the period 1961–1990 from the Swedish Meteorological and Hydrological Institute (SMHI). Soil moisture was given according to the classification used at the Swedish National Forest Inventory. | |||
| Variable | Site | ||
| Brattön | Dimbo | Duveholm | |
| Coordinates (WGS84) | 58°35´N, 11°50´E | 59°07´N, 15°43´E | 58°58´N, 16°09´E |
| Elevation (m a.s.l.) | 135 | 90 | 55 |
| Soil structure | Clay-silt moraine | Sandy moraine & surged gravel | Glacial clay & silty moraine |
| Soil moisture | Mesic-moist | Mesic | Mesic |
| Prec. (mm) | 900 | 500 | 500 |
| Temp. (°C) | 6 | 6 | 6 |
| Veg. (days) | 190 | 190 | 180 |
The Brattön site was divided into four blocks and the other two sites were divided into five blocks (each 50 × 30 m) based on differences in topography and soil moisture conditions. The blocks contained four plots of site preparation treatments; (1) control without site preparation, (2) patch scarification, (3) mounding and (4) soil inversion. All site preparation treatments were performed with an excavator and were randomly applied within the blocks. With patch scarification, the humus layer was removed in patches leaving undisturbed areas in between. Mounding consisted of elevated planting spots with the soil turned upside-down and soil inversion was planting spots with an inverted humus layer at the same level as the surrounding ground. For more information about the site preparation methods used, see Mc Carthy et al. (2017).
Site preparation and fencing, to avoid browsing damage, were done before planting took place in late May and early June 2013. One-year old containerized hybrid aspen plants of three different clones (named 60, 61 and 62) were planted randomly within the different treatments. The spacing was approximately 2 × 2 m but differed somewhat depending on suitable planting spots.
All plants were measured for total height (from the soil surface to the base of the highest living bud) at planting and after each growing season during three consecutive years. Damage was recorded simultaneously after each season, and the plants were assigned to one of four classes: undamaged, lightly damaged, severely damaged (damage with a negative effect on plant height between years), and dying or dead. Additionally, the cause of damage was noted (e.g., browsing by moose, competing vegetation or damage by voles). If the cause was difficult to define, it was registered as unknown.
2.3 Statistical analyses
Treatment effects on height, total biomass, shoot to root ratio (S:R-ratio) and dry mass of plant parts were analysed in the greenhouse study. The S:R-ratio was calculated as leaf and stem mass divided by root mass. Data was analysed as a complete randomised block design with the procedure Proc Mixed in SAS 9.4 (SAS Institute, Cary, NC, USA) using the following statistical model:
![]()
where µ = the general mean, αi = fixed effect of treatment, i.e. pH or site (i = 1–5), Bj = random effect of blocks (j = 1–8 or 1–5) and eij = error term of the model. Pair-wise comparisons were performed when significant effects were found. The tests utilised differences of least square means with p-values adjusted according to Tukey-Kramer.
In the forest site study, all living trees were used when analysing tree height (cm). The height followed a normal distribution with equal variances and was analysed with a mixed model implemented in Proc Mixed in SAS 9.4:
![]()
where µ is the general mean, αi is the fixed effect of site (i = 1–3), bij is the random effect of block (j = 1–4, 5) within site, γk is the fixed effect of site preparation treatment (k = 1–4) and δl is the fixed effect of clone (l = 1–3). The interactions between site, site preparation treatment and clone were included in the model as fixed effects.
When analysing effects of the explanatory variables on the proportion (η) of damaged plants after three growing seasons, the response variable followed a binomial distribution. Therefore, a generalised linear mixed model implemented in Proc Glimmix in SAS 9.4 (SAS Institute, Cary, NC, USA) was applied:
![]()
where the explanatory variables were the same as in Eq. 2. Satterthwaite approximation was used to determine the appropriate degrees of freedom in all analyses. When significant differences between treatment means were detected, they were separated using least square means with p-values adjusted according to Tukey-Kramer. An α-level of 0.05 was used in all analyses.
3 Results
3.1 Greenhouse study
No significant differences in height was found for hybrid aspen plants grown in soil collected from the forest sites (p = 0.9344) (Fig. 1). In the peat soil, significant differences in height were found at the end of the experimental period (p = 0.002). Around 50 days after planting, plants in the treatment with the lowest pH (pH 3.5) had a slower growth and their height was significantly shorter than plants grown at higher pH levels, except for the highest pH (pH 6.0). All seedlings survived in all peat soil treatments and forest soils.
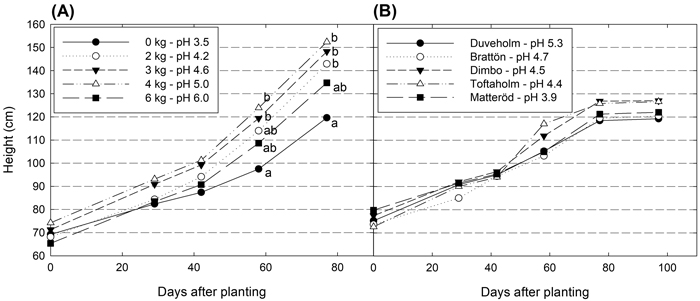
Fig. 1. Height development (cm) for hybrid aspen plants grown in in peat with different pH-levels (A) and in soil collected at five different forest sites (B). Different letters show significant differences among treatments on the measurement occasion (p < 0.05).
Dry mass of plant parts and total biomass did not differ between plants in forest soils, but between those in peat soil treatments (Table 4). As for height, the lowest values were found in the treatment with the lowest pH (pH 3.5). Also, in the treatment with the highest pH (pH 6.0), dry masses were constantly lower than in treatments with pH ranging from 4.2–5.0, but they were not always significantly different. The only variable that did not differ significantly among treatments was the S:R ratio.
| Table 4. Dry mass (g) of leaves, stem, roots and total biomass for plants grown in the different peat soil treatments. Values followed by different letters are significantly different column wise at p < 0.05. The p-value for the analyses of each response variable is also shown. | |||||
| Treatment | Leaves | Stem | Roots | Biomass | S:R ratio |
| pH 3.5 | 9.81 a | 13.94 a | 12.84 a | 36.59 a | 1.9 a |
| pH 4.2 | 19.27 b | 26.10 b | 22.76 b | 68.12 b | 2.1 a |
| pH 4.6 | 18.47 bc | 28.26 b | 24.65 b | 71.38 b | 1.9 a |
| pH 5.0 | 18.10 bc | 27.63 b | 25.05 b | 70.78 b | 1.9 a |
| pH 6.0 | 14.74 c | 20.75 ab | 21.25 ab | 56.74 b | 1.8 a |
| p-value | 0.0001 | 0.0001 | 0.0052 | 0.0001 | 0.7282 |
3.2 Forest site study
Survival was high in the forest site experiment and only 2% of the plants died. Although survival was high, a large amount of the plants was damaged and significant effects between clones were found (Table 5). Three years after planting, 29% of the plants of clone 62 had been damaged since planting, which was significantly lower than for clone 60 (39%) and clone 61 (40%). There was also a significant interaction effect of site and site preparation treatment, showing that plants in patch scarification at Dimbo were less damaged than plants in other site preparation treatments and in comparison with the two other sites (Table 6). The cause of damage also differed between sites (Table 7). At Brattön, plants generally suffered from competition by surrounding vegetation and climate. At Duveholm, voles caused damage by bark gnaw and there were also some damage caused by insects. At Dimbo, the fence broke during the second year due to a wind-felled tree and browsing by moose was therefore a common cause of damage. A lower number of plants were browsed in the patch scarification treatment, which is part of the interaction effect in the level of damage between site and site preparation (Table 5).
| Table 5. Results of the statistical analyses on damage, height and diameter three years after planting. | |||
| Effect | p-value | ||
| Damage | Height | Diameter | |
| Site | 0.1288 | 0.3002 | 0.0347 |
| Site prep | 0.1561 | <0.0001 | <0.0001 |
| Site × Site prep | 0.0001 | <0.0001 | 0.0004 |
| Clone | 0.0008 | <0.0001 | <0.0001 |
| Site × Clone | 0.0633 | 0.5334 | 0.3175 |
| Site prep × Clone | 0.2321 | 0.0070 | 0.0296 |
| Site × Site prep × Clone | 0.1623 | 0.8482 | 0.8086 |
| Table 6. Percent damaged plants (Dam, %), plant height (Height, cm) and diameter at 50 cm (Diam, mm) three years after planting in the different site preparation methods at each site. Values followed by different letters are significantly different column wise at p < 0.05. | |||||||||
| Treatment | Brattön | Dimbo | Duveholm | ||||||
| Dam | Height | Diam | Dam | Height | Diam | Dam | Height | Diam | |
| No site prep | 35 a | 147 ab | 11.2 a | 39 a | 160 a | 10.6 a | 36 a | 176 ab | 6.9 a |
| Patch | 31 a | 144 ab | 6.6 b | 18 b | 113 b | 5.5 b | 42 a | 159 a | 3.4 b |
| Mounding | 24 a | 174 a | 13.6 a | 43 a | 184 a | 11.7 a | 40 a | 174 ab | 7.4 a |
| Inversion | 22 a | 136 b | 6.5 b | 42 a | 167 a | 10.5 a | 51 a | 191 b | 6.2 a |
| Table 7. Cause of damage (%) at different sites during the experimental period. Veg. = competing vegetation and Mech. = mechanical damage and leaning stems. | |||||||
| Site | Unknown | Insects | Voles | Moose | Veg. | Climate | Mech. |
| Brattön | 31 | 4 | 1 | 0 | 26 | 12 | 26 |
| Dimbo | 22 | 0 | 1 | 56 | 0 | 1 | 20 |
| Duveholm | 43 | 17 | 31 | 0 | 0 | 0 | 9 |
Both height and diameter differed significantly between both site preparation treatments and clones three years after planting (Table 5). Clone 60 achieved the greatest height, on average about 50 cm higher than both clone 61 and 62 (Fig. 2). Overall, mounding resulted in the tallest plants and patch scarification led to the shortest. Significant interactions between site preparation and clone showed that clone 60 responded differently to site preparation treatments with greater differences in height. For clone 60, the greatest difference was 66 cm, while it was 17 cm for clone 61 and 34 cm for clone 62, respectively. For clone 60 and 62 these differences were statistically significant, but for clone 61, no significant differences between site preparation treatments were found.
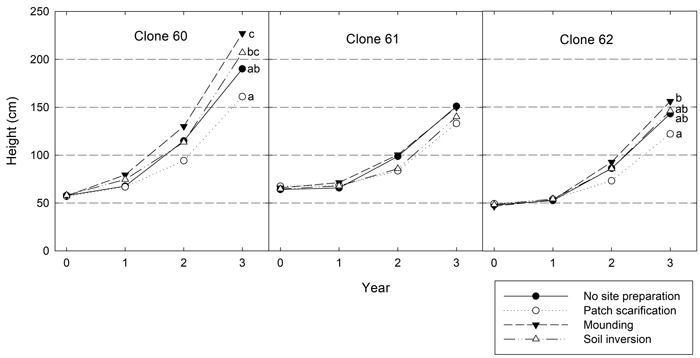
Fig. 2. Height development (cm) for the three clones in the different site preparation treatments. Different letters show significant differences among treatments within the clone after three years (p < 0.05).
Significant interactions also occurred between site and site preparation treatment (Table 5). At Brattön, inversion resulted in the shortest height and mounding the tallest, at Dimbo patching resulted in the shortest height despite lower levels of browsing in this treatment. At Duveholm patch also generated the shortest plants but they were only significantly shorter than plants grown in inversion.
Plant diameter (at 50 cm) three years after planting differed between clones and, as for height, clone 60 had a significantly larger mean value (10.2 mm) compared with clone 61 (7.4 mm) and clone 62 (7.3 mm). Mounding resulted in the greatest diameter for all clones and patch in the smallest (Fig. 3). There was a greater variation between site preparation treatments for clone 60. Significant interactions between site and site preparation showed that, as for height, the inversion treatment gave different results at different sites (Table 5).
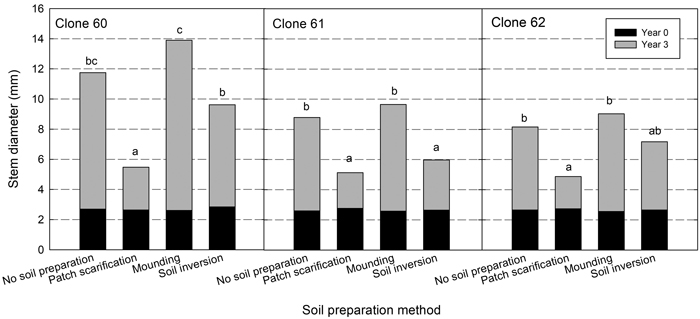
Fig. 3. Diameter (mm) at planting (at the stem base, black bars) and after three years (at 50 cm, black bars plus grey bars) for each clone in the different site preparation treatments. Different letters show significant differences among soil treatments for respective clone after three years (p < 0.05).
4 Discussion
This study showed that the pH level affected growth development of hybrid aspen although survival was unaffected. In the limed peat study in the greenhouse study, with similar nutrient availability for all tested pH levels, a pH of 3.5 significantly reduced height (Fig. 1) and biomass development (Table 4). On the other hand, the smaller span of pH (3.9–5.3) in the forest soil study revealed no differences among sites. As a comparison, Hjelm and Rytter (2016) tested 12 poplar clones in a similar study and found that a pH level of about 4.5 was needed for a satisfying growth development. This indicates less tolerance to low pH in poplars compared with hybrid aspen. In accordance, Böhlenius et al. (2016b) found the optimum pH levels to be 4.0–7.0 for hybrid aspen while the simultaneously tested Populus trichocarpa had a narrower optimum of 5.5–6.5. In our study, height growth decreased at pH around 6, indicating that there probably is an upper limit for optimal growth. This has also been found by Lutter et al. (2017) where growth of hybrid aspen on previous agricultural land was faster at sites with a pH level around 5.4–5.6 and slower at a site with pH 6.5. In addition, Zhang et al. (2013) tested tolerance for high pH for seedlings of trembling aspen, white spruce and tamarack. A pH level of 7.0 and higher affected growth negatively for trembling aspen and tamarack. The reason was considered to be water stress due to changes in root water transport causing a disturbed water balance. In a comparison of pH effects on growth of small plants Ericsson and Lindsjö (1981) found that poplar and willows were more sensitive to the pH situation than silver birch and grey alder. While birch and alder grew equally well at pH 3.8–6.7, willow and poplar needed a pH of at least 5.0 to perform well and at pH 6.7 growth declined again.
As the research show, species respond differently to pH and our conclusion is that hybrid aspen is less sensitive for pH variation in the soil than poplar and can therefore more easily be used on acid forest soils. It should be kept in mind though, that pH should be looked at as an indicator of growing conditions. The true physiological reason for varying behaviour at different pH levels is still not fully understood (Zhang et al. 2013), although it is known that plant availability of essential and deleterious elements varies with soil acidity (Bidwell 1979; Mengel and Kirkby 1987). For example, Lu and Sucoff (2001) showed that for small trembling aspen seedlings, the presence of calcium could counteract the negative effect of low pH on growth.
In the greenhouse study, height growth was slower in the forest soil than in the peat soil in the later stage of the experimental period (Fig. 1). A main reason is probably that the peat soil received nutrients continuously while the plants in the forest soil had to rely on the initial nutrient pool. It could also be that the pots started to be too small after 80 days with decreased possibility for the root systems to expand. Although there were variations in plant-available nutrient content among the forest soils (Table 2), the development and probably nutrient availability decreased in a similar way. It is well known that nutrient addition increases growth rate as long as free access to nutrients is limited (Tamm and Aronsson 1982; Ingestad 1991), but in this study the focus was to keep similar nutrient and water conditions for different pH treatments and soils. Differences in nutrient availability usually alters the S:R ratio (Ericsson 1995) and that was not seen in the greenhouse studies (Table 4).
Site preparation method did not affect survival of hybrid aspen in field but had an effect on plant growth. Patch scarification was the least successful method, even in comparison with no site preparation, and showed the slowest development of both height and stem diameter. Mounding, on the other hand, was one of the best methods. The result is probably a combined effect of higher soil temperature (Örlander et al. 1990; Vapaavuori et al. 1992), less risk for waterlogged conditions (Mc Carthy et al. 2017) and reduced competition from vegetation (Nilsson and Örlander 1995; Bilodeau-Gauthier et al. 2011; Johansson et al. 2013). The low growth in the patch scarification was considered an effect of planting spots below soil surface. At times standing water resulted in poor oxygen levels. The soil inversion method gave the best establishment result at Duveholm, which was less moist than the other two sites. Earlier studies have shown that soil inversion can be an effective method where soil conditions are suitable, i.e. on podzolic soils with medium coarse texture (Hallsby and Örlander 2004; Wallertz et al. 2018). In our study, no site preparation also resulted in decent growth. Here it should be noted that all forest sites at the time of planting were fresh clear cuts with quite low initial amounts of competing vegetation. If plants are to be planted at older clear cuts, the amount of vegetation will increase significantly and the need of a site preparation method that reduce the amount of competing vegetation will increase (Nilsson and Örlander 1999).
Damage was observed on many plants with 18 to 51% of the total number of plants affected (Table 6) but it had little effect on survival. The cause and degree of damage varied among sites and site preparation methods (Table 7). Due to a damaged fence at Dimbo, moose did some harm. Since aspen is an attractive food source for ungulates (Edenius et al. 2011; Myking et al. 2011; Bergqvist et al. 2014) fencing is recommended at establishment (Rytter et al. 2011; Persson et al. 2015). At Duveholm, voles caused a lot of damage (Table 7), and the plants at Brattön were exposed to competition from surrounding vegetation. Mechanical damage was observed on all sites. However, in many cases it was not possible to identify the primary reason of damage. An intact fence is a prerequisite to avoid browsing from ungulates while other types of damage are more difficult to avoid. If the amount of competing vegetation can be kept low, both the competition effect and the presence of voles could be decreased (Hytönen and Jylhä 2005; DesRochers and Sigouin 2014).
One of the three clones tested in the forest site study showed a faster height and diameter development than the others. This clone also had a greater difference in the response to site preparation. Since no differences in damage levels and preferences were seen the effect was probably genetically based. It has been shown that there is a large potential for genetic breeding of hybrid aspen (Stener and Karlsson 2004; Stener and Westin 2017), and resistance to pests is included in the breeding work.
In summary, this study showed that hybrid aspen growth is affected by pH levels commonly found in forest soils in the Nordic countries, but that the differences between growth at low and high pH levels are less dramatic than for other poplar species. From the forest site study, it seems like mounding is a successful way to establish hybrid aspen on forest land, while patch scarification should be avoided, at least on mesic to moist sites. A successful establishment could also be achieved without site preparation on fresh clear cuts, however plant damage can be reduced by removing competing vegetation with site preparation, which also lowers the risk for vole attacks. Clonal differences in performance were observed, which stresses the importance of the ongoing clone selection work. The best performing clone in this study showed the greatest response to site preparation with significantly higher growth after mounding.
Acknowledgements
This study was jointly supported by the Royal Swedish Academy of Agriculture and Forestry (KSLA, from the foundation Stiftelsen Erik och Ellen Sökjer-Petersens stipendiefond), the Swedish Energy Agency and the frame program of Skogforsk (the Forestry Research Institute of Sweden). We would like to thank, Mihály Czimbalmos, Johan Karlsson, Michael Krook, Johan Malm, Eva Persson, Vera Rytter and Lars Wremert for their careful and patient work with measurements, management and processing of plants and soil both in the greenhouse study and out on the forest sites. A special thanks to Rebecka Mc Carthy, who was highly involved in the forest site study, and to the land owners of the forest sites, Skogsällskapets Fastighet AB and Holmen Skog AB.
References
Anander E. (2017). Hybridaspens utveckling på skogsmark – en studie av bestånd efter stormen Gudrun. [Development of hybrid aspen on forest land – a study of stands after the storm Gudrun]. SLU, Institutionen för Sydsvensk skogsvetenskap, Alnarp, Examensarbete nr 278. 40 p. [In Swedish].
Bergqvist G., Bergström R., Wallgren M. (2014). Recent browsing damage by moose on Scots pine, birch and aspen in young commercial forests – effects of forage availability, moose population density and site productivity. Silva Fennica 48(1) article 1077. https://doi.org/10.14214/sf.1077.
Bidwell R.G.S. (1979). Plant physiology. 2nd ed. Macmillan Publishing Co., New York. 726 p.
Bilodeau-Gauthier S., Paré D., Messier C., Bélanger N. (2011). Juvenile growth of hybrid poplars on acidic boreal soil determined by environmental effects of site preparation, vegetation control, and fertilization. Forest Ecology and Management 261(3): 620–629. https://doi.org/10.1016/j.foreco.2010.11.016.
Böhlenius H., Övergaard R. (2016). Impact of seedling type on early growth of poplar plantations on forest and agricultural land. Scandinavian Journal of Forest Research 31: 733–741. https://doi.org/10.1080/02827581.2016.1167239.
Böhlenius H., Övergaard R., Jämtgård S. (2016a). Influence of soil types on establishment and early growth of Populus trichocarpa. Open Journal of Forestry 6(5): 361–372. http://dx.doi.org/10.4236/ojf.2016.65029.
Böhlenius H., Övergaard R., Asp H. (2016b). Growth response of hybrid aspen (Populus × wettsteinii) and Populus trichocarpa to different pH levels and nutrient availabilities. Canadian Journal of Forest Research 46(11): 1367–1374. https://doi.org/10.1139/cjfr-2016-0146.
Boysen B., Strobl S. (1991). A grower’s guide to hybrid poplar. Ministry of Natural Resources, Ontario. 148 p.
Christersson L. (2008). Poplar plantations for paper in the south of Sweden. Biomass and Bioenergy 32(11): 997–1000. https://doi.org/10.1016/j.biombioe.2007.12.018.
Davies B.E. (1971). A statistical comparison of pH values of some English soils after measurement in both water and 0.01M calcium chloride. Soil Science Society of America Proceedings 35(4): 551–552. https://doi.org/10.2136/sssaj1971.03615995003500040022x.
DesRochers A., Sigouin M.-E. (2014). Effect of soil mounding and mechanical weed control on hybrid poplar early growth and vole damage. Écoscience 21(3–4): 278–285. https://doi.org/10.2980/21-(3-4)-3645.
Edenius L., Ericsson G. (2015). Effects of ungulate browsing on recruitment of aspen and rowan: a demographic approach. Scandinavian Journal of Forest Research 30(4): 283–288. https://doi.org/10.1080/02827581.2014.999823.
Edenius L., Ericsson G., Kempe G., Bergström R., Danell K. (2011). The effects of changing land use and browsing on aspen abundance and regeneration: a 50-year perspective from Sweden. Journal of Applied Ecology 48(2): 301–309. https://doi.org/10.1111/j.1365-2664.2010.01923.x.
Engerup P.-O. (2011). Föryngring med poppel och hybridasp på skogs- och åkermark. [Regeneration with poplar and hybrid aspen on forest and farm land]. SLU, Institutionen för Sydsvensk skogsvetenskap, Alnarp, Examensarbete nr 167. 99 p. [In Swedish].
Ericsson T. (1995). Growth and shoot:root ratio of seedlings in relation to nutrient availability. Plant and Soil 168(1): 205–214. https://doi.org/10.1007/BF00029330.
Ericsson T., Lindsjö I. (1981). Tillväxtens pH-beroende hos några energiskogsarter. [The influence of pH on growth and nutrition of some energy forest tree species]. Swedish University of Agricultural Sciences, Energy Forestry Project (EFP), Technical Report No 11. 7 p. [In Swedish].
Hallsby G., Örlander G. (2004). A comparison of mounding and inverting to establish Norway spruce on podzolic soils in Sweden. Forestry 77(2): 107–117. https://doi.org/10.1093/forestry/77.2.107.
Hjelm K., Rytter L. (2016). The influence of soil conditions, with focus on soil acidity, on the establishment of poplar (Populus spp.). New Forests 47(5): 731–750. https://doi.org/10.1007/s11056-016-9541-9.
Hjelm K., Mc Carthy R., Rytter L. (2018). Establishment strategies for poplars, including mulch and plant types, on agricultural land in Sweden. New Forests 49(6): 737–755. https://doi.org/10.1007/s11056-018-9652-6.
Hytönen J., Jylhä P. (2005). Effects of competing vegetation and post-planting weed control on the mortality, growth and vole damages to Betula pubescens planted on former agricultural land. Silva Fennica 39(3): 365–380. https://doi.org/10.14214/sf.374.
Ingestad T. (1991). Nutrition and growth of forest trees. Tappi Journal 74: 55–62.
Johansson K., Söderbergh I., Nilsson U., Allen H.L. (2005). Effects of scarification and mulch on establishment and growth of six different clones of Picea abies. Scandinavian Journal of Forest Research 20(5): 421–430. https://doi.org/10.1080/02827580500292121.
Johansson K., Nilsson U., Örlander G. (2013). A comparison of long-term effects of scarification methods on the establishment of Norway spruce. Forestry 86(1): 91–98. https://doi.org/10.1093/forestry/cps062.
Karltun E. (1996). Markkemiska analyser inom ståndortskarteringen. Metodbeskrivningar. [Soil chemical analyzes within the site mapping program. Methodological descriptions]. SLU, Ståndortskarteringen, Rapport. 17 p. [In Swedish].
Larsson S., Lundmark T., Ståhl G. (2009). Möjligheter till intensivodling av skog. [Opportunities for intensive cultivation of forests]. SLU, Slutrapport från regeringsuppdrag Jo 2008/1885. 138 p. [In Swedish].
Lu E.-Y., Sucoff E.I. (2001). Responses of Populus tremuloides seedlings to solution pH and calcium. Journal of Plant Nutrition 24(1): 15–28. https://doi.org/10.1081/PLN-100000309.
Lutter R., Tullus A., Kanal A., Tullus T., Tullus H. (2017). Above-ground growth and temporal plant–soil relations in midterm hybrid aspen (Populus tremula L. × P. tremuloides Michx.) plantations on former arable lands in hemiboreal Estonia. Scandinavian Journal of Forest Research 32(8): 688–699. https://doi.org/10.1080/02827581.2017.1278784.
Mc Carthy R., Rytter L. (2015). Productivity and thinning effects in hybrid aspen root sucker stands. Forest Ecology and Management 354: 215–223. https://doi.org/10.1016/j.foreco.2015.06.015.
Mc Carthy R., Rytter L., Hjelm K. (2017). Effects of soil preparation methods and plant types on the establishment of poplars on forest land. Annals of Forest Science 74(47): 1–12. https://doi.org/10.1007/s13595-017-0647-9.
Mengel K., Kirkby E.A. (1987). Principles of plant nutrition. 4th ed. International Potash Institute, Worblaufen-Bern, Switzerland. 687 p.
Myking T., Bøhler F., Austrheim G., Solberg E.J. (2011). Life history strategies of aspen (Populus tremula L.) and browsing effects: a literature review. Forestry 84(1): 61–71. https://doi.org/10.1093/forestry/cpq044.
Nilsson U., Örlander G. (1995). Effects of regeneration methods on drought damage to newly planted Norway spruce seedlings. Canadian Journal of Forest Research 25(5): 790–802. https://doi.org/10.1139/x95-086.
Nilsson U., Örlander G. (1999). Vegetation management on grass-dominated clearcuts planted with Norway spruce in southern Sweden. Canadian Journal of Forest Research 29(7): 1015–1026. https://doi.org/10.1139/x99-071.
Nilsson U., Luoranen J., Kolström T., Örlander G., Puttonen P. (2010). Reforestation with planting in northern Europe. Scandinavian Journal of Forest Research 25(4): 283–294. https://doi.org/10.1080/02827581.2010.498384.
Örlander G., Gemmel P., Hunt J. (1990). Site preparation: a Swedish overview. Canada-BC Forest Resource Development and B.C. Ministry of Forests, FRDA Report 105. 61 p.
Persson P.-O., Rytter L., Johansson T., Hjelm B. (2015). Handbok för odlare av Poppel och Hybridasp. [Manual for poplar and hybrid aspen growers]. Jordbruksverket, Jönköping. 24 p. [In Swedish].
Rytter L., Rytter R.-M. (2017). Productivity and sustainability of hybrid aspen (Populus tremula L. × P. tremuloides Michx.) root sucker stands with varying management strategies. Forest Ecology and Management 401: 223–232. https://doi.org/10.1016/j.foreco.2017.07.020.
Rytter L., Stener L.-G. (2014). Growth and thinning effects during a rotation period of hybrid aspen in southern Sweden. Scandinavian Journal of Forest Research 29(8): 747–756. https://doi.org/10.1080/02827581.2014.968202.
Rytter L., Stener L.-G., Övergaard R. (2011). Odling av hybridasp och poppel. [Cultivation of hybrid aspen and poplar]. Skogforsk, Uppsala, Handledning. 40 p. [In Swedish].
Stanek W. (1973). Comparisons of methods of pH determination for organic terrain surveys. Canadian Journal of Soil Science 53(2): 177–183. https://doi.org/10.4141/cjss73-028.
Stanturf J.A., van Oosten C. (2014). Operational poplar and willow culture. In: Isebrands J.G., Richardson J. (eds.). Poplars and willows: trees for society and the environment. FAO and CABI, Rome and Boston. p. 200–257. https://doi.org/10.1079/9781780641089.0200.
Stener L.-G., Karlsson B. (2004). Improvement of Populus tremula × P. tremuloides by phenotypic selection and clonal testing. Forest Genetics 11: 13–27.
Stener L.-G., Westin J. (2017). Early growth and phenology of hybrid aspen and poplar in clonal field tests in Scandinavia. Silva Fennica 51(2) article 5656. https://doi.org/10.14214/sf.5656.
Swedish Energy Agency (2017). Energy in Sweden 2017. The Swedish Energy Agency, Eskilstuna, Report ET 2017:33. 90 p.
Swedish Government (2017). Sveriges fjärde rapport om utvecklingen av förnybar energi enligt artikel 22 i Direktiv 2009/28/EG. [Sweden’s fourth report on the development of renewable energy according to Article 22 of Directive 2009/28/EC]. Regeringskansliet, Stockholm. 74 p. [In Swedish].
Tamm C.O., Aronsson A. (1982). Optimum nutrition of some non-food plants. In: Optimizing yields – the role of fertilizers. Proceedings of the 12th Congress of International Potash Institute, Berne, Switzerland. p. 181–196.
Truax B., Gagnon D., Fortier J., Lambert F. (2014). Biomass and volume yield in mature hybrid poplar plantations on temperate abandoned farmland. Forests 5(12): 3107–3130. https://doi.org/10.3390/f5123107.
Tullus A., Rytter L., Tullus T., Weih M., Tullus H. (2012). Short-rotation forestry with hybrid aspen (Populus tremula L. × P. tremuloides Michx.) in Northern Europe. Scandinavian Journal of Forest Research 27(1): 10–29. https://doi.org/10.1080/02827581.2011.628949.
Van Lierop W. (1981). Conversion of organic soil pH values measured in water, 0.01M CaCl2 or 1N KCl. Canadian Journal of Soil Science 61(4): 577–579. https://doi.org/10.4141/cjss81-067.
Vapaavuori E.M., Rikala R., Ryyppö A. (1992). Effects if root temperature on growth and photosynthesis in conifer seedlings during shoot elongation. Tree Physiology 10(3): 217–230. https://doi.org/10.1093/treephys/10.3.217.
Wallertz K., Björklund N., Hjelm K., Petersson M., Sundblad L.-G. (2018). Comparison of different site preparation techniques: quality of planting spots, seedling growth and pine weevil damage. New Forests 49(6): 705–722. https://doi.org/10.1007/s11056-018-9634-8.
Zhang W., Calvo-Polanco M., Chen Z.C., Zwiazek J.J. (2013). Growth and physiological responses of trembling aspen (Populus tremuloides), white spruce (Picea glauca) and tamarack (Larix laricina) seedlings to root zone pH. Plant and Soil 373(1–2): 775–786. https://doi.org/10.1007/s11104-013-1843-5.
Total of 52 references.

