Wood-decaying fungi in old-growth boreal forest fragments: extinctions and colonizations over 20 years
Komonen A., Puumala I., Várkonyi G., Penttilä R. (2021). Wood-decaying fungi in old-growth boreal forest fragments: extinctions and colonizations over 20 years. Silva Fennica vol. 55 no. 1 article id 10491. https://doi.org/10.14214/sf.10491
Highlights
- Rare fungi can persist for decades in isolated old-growth forest fragments
- The remaining old-growth forest fragments should be urgently protected, even if they are isolated from larger expanses of natural biotopes.
Abstract
According to ecology theory, isolated habitat fragments cannot maintain populations of specialized species. Yet, empirical evidence based on monitoring of the same fragments over time is still limited. We studied the colonization–extinction dynamics of eight wood-decaying fungal species in 16 old-growth forest fragments (<14 ha) over a 20-year period (1997–2017). We observed 19 extinctions and 5 colonizations; yet, the distribution of extinctions and colonizations did not differ from the one expected by chance for any of the species. Twenty-six percent of the extinctions took place in two natural fragments amid large forest–peatland complexes. Phellinus nigrolimitatus (Romell) Bourdot and Galzin decreased and Phellinus ferrugineofuscus (P. Karst.) Bourdot increased in abundance (number of logs occupied). The volume of living spruce trees in the forest fragments correlated positively with the number of logs inhabited in five of the study species. Because fragment characteristics did not affect species turnover, it seems that stochastic processes governed colonizations and extinctions. Although the least abundant species in 1997 had declined, and the most abundant species had become more abundant, it appears that specialized wood-decaying fungi can persist for decades in isolated old-growth forest fragments, if suitable dead wood is continuously available.
Keywords
polypores;
fragmentation;
extinction debt;
habitat loss;
spruce forest
-
Komonen,
University of Jyväskylä, Department of Biological and Environmental Science, School of Resource Wisdom, P.O. Box 35, FI-40014 University of Jyväskylä, Finland
E-mail
atte.komonen@jyu.fi

- Puumala, University of Jyväskylä, Department of Biological and Environmental Science, School of Resource Wisdom, P.O. Box 35, FI-40014 University of Jyväskylä, Finland E-mail ilkka.puumala1@outlook.com
- Várkonyi, Finnish Environment Institute, Friendship Park Research Centre, Lentiirantie 342 B, FI-88900 Kuhmo, Finland E-mail gergely.varkonyi@ymparisto.fi
- Penttilä, Natural Resources Institute Finland, Natural resources, Latokartanonkaari 9, FI-00790 Helsinki, Finland E-mail reijo.penttila@luke.fi
Received 3 December 2020 Accepted 25 January 2021 Published 27 January 2021
Views 48503
Available at https://doi.org/10.14214/sf.10491 | Download PDF

Supplementary Files
1 Introduction
Habitat loss and fragmentation are among the biggest threats to global biodiversity (Fischer and Lindenmayer 2007). In many regions, the proportion of primary or old-growth forest to all forest area has declined greatly (Schmitt et al. 2009). As a result, large and continuous populations have become small and isolated, and are more vulnerable to local extinction than large populations. Particularly vulnerable are specialist species at higher trophic levels (Roslin et al. 2014), such as wood-decaying fungi (Komonen et al. 2000; Penttilä et al. 2006). Wood-decaying fungi (Aphyllophorales) are dependent on dead wood, although a few species utilize living trees. Many species specialize in a particular tree species and require certain minimum log size or decay stage (Junninen and Komonen 2011).
Long-lived species may survive in resource patches and habitat fragments for long periods. In fungi, fruiting bodies and mycelia may persist in an individual log for years and in an old-growth forest fragment for decades after landscape transformation (Berglund et al. 2005; Penttilä et al. 2006; Berglund and Jonsson 2008; Jönsson et al. 2008; Bässler and Müller 2010). However, the evidence is mostly based on observations of species in small and isolated fragments, and chronosequence studies, rather than monitoring the same logs and sites over years (Berglund and Jonsson 2005; Penttilä et al. 2006; but see Berglund et al. 2005; Jönsson et al. 2008). The time delay in species response to environmental change has been conceptualized as extinction debt (Tilman et al. 1994; Krauss et al. 2010). Although species local turnover happens in natural landscapes, higher number of extinctions than colonizations after fragmentation indicates paying of extinction debt (Berglund and Jonsson 2005).
Our aim was to study extinction-colonization dynamics of wood-decaying fungi in old-growth forest fragments, by resampling fragments which had been sampled 20 years ago. We surveyed fruiting bodies of eight spruce-associated species, of which seven are old-growth forest indicators and one is a common species. Our research questions were:
1. Has the abundance or occurrence of species changed over the past 20 years? We predicted that the abundance and occurrence of the most specialized species decline, whereas those of the common species remain virtually unchanged.
2. Do fragment characteristics, specifically area, isolation time, tree age and volume of living spruce, affect the abundance or occupancy of species? We predicted greater population declines and more extinctions in smaller and more isolated fragments that contained the lowest amount of dead wood.
2 Material and methods
2.1 Study area
The study was conducted in Kuhmo, eastern Finland, which belongs to the middle boreal zone (Ahti et al. 1968). The large‐scale forestry with clear-cuttings started after World War II and accelerated in the 1950s and 1960s (Gu et al. 2002); yet, it reached some areas as late as in the 1980s and 1990s (Sigurdsson 1999). The percentage of forests older than 140 years was 9.7% in 1997 (Tomppo et al. 2003), and the percentage of natural and seminatural old‐growth was 2.9% (Virkkala et al. 2000). The average volume of dead wood in forests was 6.2 m3 ha−1 (Tomppo et al. 2003).
We selected 16 old-growth forest fragments that had been inventoried for wood-decaying fungi in 1997 (Penttilä et al. (2006) and Supplementary file S1 for the map). In 2017, these fragments had been isolated from the surrounding old-growth forests for 22–45 years at least (Table 1; see Suppl. file S1 for measuring the isolation time). We could not resample all the original fragments (n = 18), because two fragments had been clear-cut. Although the Vepsä fragment had been partially (ca. 1/3) clear-cut, this was included. The mean area of fragments was 7 ha (min–max: 3.3–13.8 ha; Table 1). The mean age of spruces in the dominant-canopy layer was 164 yrs. The mean volume of spruce dead wood was 17 m3 ha–1 and that of coarse spruce logs was 7 m3 ha–1. We did not repeat the measurements of fragment characteristics in 2017. Study fragments were surrounded by managed forests (n = 13) or open peatland within larger protected areas (n = 3); the latter served as a control for natural turnover rates in stochastic equilibrium.
| Table 1. Wood-decaying fungal species in fragments of old growth boreal forest. Study site characteristics. Tree volume data are from 1997, whereas time-since-isolation and mean tree age represent the situation in 2017. | |||||||||||
| Site | Coordinates (N, E) a | Area (ha) | Time since isolation (yrs) | All trees | Spruce | ||||||
| Living (m3 ha–1) | Dead (m3 ha–1) | Living (m3 ha–1) | Dead (m3 ha–1) | Coarse b logs (m3 ha–1) | Mean age (yrs) | ||||||
| Puntari | 70904:36165 | 5.9 | 22 | 216 | 43 | 152 | 26 | 11.1 | 178 | ||
| Lauvuskylä | 70891:36265 | 5.5 | 22 | 234 | 53 | 195 | 27 | 7.1 | 168 | ||
| Jonkeri | 70929:36364 | 8.4 | 22 | 197 | 50 | 134 | 34 | 14.6 | 193 | ||
| Vepsä c | 70917:36154 | 7.7 | 22 | 231 | 24 | 118 | 7 | 2 | 133 | ||
| Kujanki | 70869:36179 | 4.9 | 32 | 253 | 42 | 162 | 26 | 9.5 | 181 | ||
| Näveri | 71042:35997 | 4.1 | 35 | 343 | 59 | 177 | 14 | 4.4 | 144 | ||
| Luhtavaara | 71525:36104 | 13.8 | 32 | 245 | 29 | 161 | 10 | 3.9 | 164 | ||
| Pitkävaara | 71622:36171 | 9.6 | 32 | 259 | 40 | 126 | 13 | 4.6 | 159 | ||
| Ypykkävaara | 71584:36228 | 9.2 | 22 | 217 | 25 | 151 | 9 | 4.2 | 168 | ||
| Iso-Matojärvi | 70867:35907 | 4.3 | 35 | 260 | 64 | 162 | 35 | 18.3 | 178 | ||
| Luvesuo | 71640:36213 | 7.5 | 35 | 220 | 47 | 125 | 13 | 4.2 | 164 | ||
| Pellinkangas | 71354:35946 | 3.3 | 35 | 126 | 27 | 70 | 16 | 8.2 | 107 | ||
| Kuivikkovaara | 71256:36541 | 3.6 | 32 | 145 | 50 | 82 | 13 | 7.3 | 224 | ||
| Elimyssalo d | 71293:36674 | 6.4 | >100 | 205 | 46 | 61 | 5 | 1.4 | 144 | ||
| Juortanansalo d | 71678:36359 | 6.2 | >100 | 198 | 14 | 128 | 5 | 1.3 | 155 | ||
| Tuli-Varpusuo d | 71646:36167 | 10.8 | >100 | 145 | 37 | 109 | 19 | 9.8 | 167 | ||
| a Based on Finnish YKJ coordinate system; b Diameter at breast height ≥ 20 cm; c One third of this area was clearcut between 1997 and 2017; d Natural forest fragments surrounded by open peatlands. | |||||||||||
2.2 Study species
We studied eight spruce-dwelling aphyllophoroid fungal species (Table 2). Phlebia centrifuga is a corticoid and the rest are polypores. Seven species are old-growth forest indicators in Finland, whereas Fomitopsis pinicola is a common species as it can utilize living and dead wood of many tree species (Niemelä 2016). Species were selected so that they would predominantly occur on coarse logs and would be easy to identify in the field. Nomenclature follows Kotiranta et al. (2009).
| Table 2. Studied wood-decaying fungal species and their abundance (number of logs occupied) in fragments of old growth boreal forest in 2017. | |||||
| Study species | IUCN-status 2001/2019 a | Ecology b | Mean ± SD | Min/Max | Total |
| Amylocystis lapponica (Romell) Singer | VU/NT | Annual, brown rot | 7.7 ± 8.3 | 0/27 | 123 |
| Fomitopsis rosea (Alb. and Schwein.: Fr.) P. Karst. | NT/NT | Perennial, brown rot | 7.8 ± 9.0 | 0/32 | 124 |
| Phellinus ferrugineofuscus (P. Karst.) Bourdot | NT/LC | Perennial, white rot | 16.3 ± 16.7 | 0/53 | 260 |
| Phellinus viticola (Schwein.: Fr.) Donk | LC/LC | Perennial, white rot | 49.5 ± 35.2 | 7/113 | 792 |
| Leptoporus mollis (Pers.: Fr.) Quél. | LC/LC | Annual, brown rot | 0.8 ± 1.0 | 0/4 | 13 |
| Phlebia centrifuga P. Karst. | VU/LC | Annual, white rot | 1.6 ± 3.3 | 0/12 | 25 |
| Phellinus nigrolimitatus (Romell) Bourdot and Galzin | LC/LC | Perennial, white rot | 1.9 ± 3.0 | 0/12 | 31 |
| Fomitopsis pinicola (Sw.: Fr.) P. Karst. | LC/LC | Perennial, brown rot | 48.6 ± 39.0 | 3/131 | 777 |
| a Rassi et al. (2001) and Hyvärinen et al. (2019); VU = vulnerable, NT = near-threatened, LC = least concern; b Niemelä (2016) | |||||
2.3 Sampling
We surveyed fungi between 4 October and 12 November 2017. All lying spruce logs (diameter at breast height, dbh > 10 cm) were searched for fruit bodies. Fully moss-covered logs were not inspected. For each species, we counted the number of occupied logs. Species were identified in the field and if in doubt identification was confirmed in the laboratory. It took 4 to 10 hours to survey each fragment. See Suppl. file S1 for description of the 1997 survey.
2.4 Statistical analyses
Statistical analyses were conducted with non-parametric statistics, because model assumptions of parametric tests were not adequately met. Changes in fungal species abundance (number of occupied logs) between 1997 and 2017 were analyzed with the Wilcoxon sign test for paired samples. Changes in fungal species occurrence (presence or absence in the fragments) between 1997 and 2017 were analyzed with the McNemar test for paired samples. We also compared fragment characteristics in those fragments that experienced extinctions to the fragments where no extinctions took place, using the Mann-Whitney U-test or the chi-squared test. Correlations of fungal abundances between 1997 and 2017, and among the study species, were analyzed with Pearson’s correlation coefficient. The relationship between dead wood volume and the total fungal abundance was tested with linear regression.
3 Results
Altogether we recorded 2145 spruce logs occupied by the study species (mean per fragment = 134, SD = 27). Phellinus viticola was the most abundant species and Leptoporus mollis the least abundant species (Table 2). Six forest fragments hosted all the study species, whereas two natural fragments surrounded by open peatland hosted only half of the study species.
3.1 Occupancy
In most fragments, the studied species had persisted between 1997 and 2017. We documented 19 extinctions and five colonizations (Fig. 1), but the distribution of extinctions and colonizations did not differ from the one expected by chance only for any of the species (McNemar’s test: χ2 < 3.3, n = 16, p > 0.063). Extinction–colonization balance was negative (i.e. below one) for the four rarest species and for the rather common P. ferrugineofuscus. The two most abundant species in 1997, F. pinicola and P. viticola, occupied all fragments also in 2017.
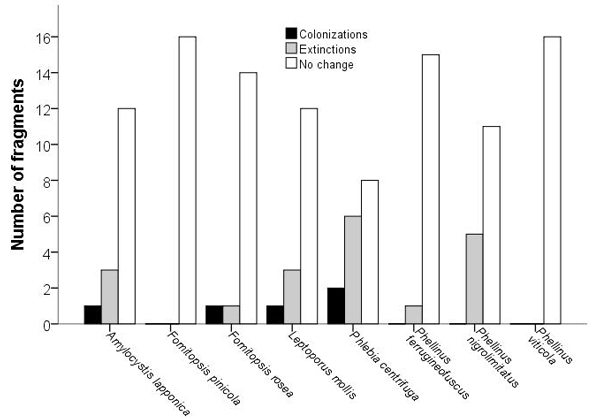
Fig. 1. Occurrence of wood-decaying fungi in fragments (n = 16) of old growth boreal forest. Changes in the number of occupied fragments between 1997 and 2017. Full author references of fungal taxa are shown in Table 2.
The fragments in which extinctions were documented had less spruce dead wood and coarse spruce logs in 1997 than fragments without extinctions (Mann-Whitney: U = 9, n = 16, p = 0.022 and U = 10, n = 16, p = 0.030, respectively), whereas fragment area, volume of living spruce or mean tree age did not differ significantly. Extinctions were documented in 40% and 75% of the fragments isolated at least for 22 and 33–45 years, respectively, but the difference was not significant (Fisher’s exact test: χ2 = 1.6, df = 1, p = 0.29). For Phlebia centrifuga and Phellinus nigrolimitatus, the fragments which faced extinctions or remained unoccupied, had similar characteristics than fragments which were colonized or remained occupied (U < 34, n = 16, p > 0.14); the small number of extinctions did not warrant analyzing the other species.
3.2 Abundance
The median change in the number of logs occupied among the fragments ranged from 0to 3.5 for the rarer species, and from 11 to 16 for the common P. viticola and F. pinicola, respectively. Even for the rarer species, notable (≥ 10 logs) increases or decreases were documented: Amylocystis lapponica and Fomitopsis rosea in three fragments, P. ferrugineofuscus in four, and P. nigrolimitatus in two fragments. The number of logs occupied by P. ferrugineofuscus increased from 1997 to 2017 (Wilcoxon’s test: z = 2.62, n = 16, p = 0.009; Fig. 2) and that of P. nigrolimitatus decreased (z = –2.55, n = 16, p = 0.011). There were no significant changes for other species (ǀzǀ < 1.55, n = 16, p > 0.12). In the fragment that had been partially clearcut after 1997, five species occupied more logs and two species fewer logs in 2017 than in 1997.
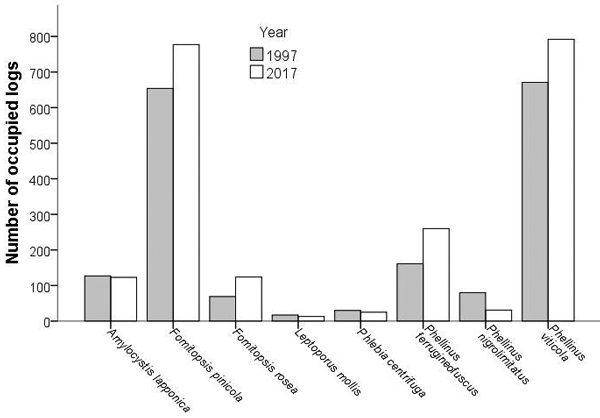
Fig. 2. Abundance of wood-decaying fungi in fragments of old growth boreal forest. Changes in species abundance (number of logs occupied) between 1997 and 2017. Full author references of fungal taxa are shown in Table 2.
The number of logs occupied correlated between 1997 and 2017 for all species (rp > 0.52, n = 16, p < 0.05), except for L. mollis, which had very low abundance in both years. Moreover, species abundances (number of logs occupied) correlated with each other both in 1997 (rp > 0.51, n = 16, p < 0.05; L. mollis being the only exception), and in 2017 (rp > 0.57, n = 16, p < 0.05; L. mollis and the correlation between Phl. centrifuga and P. nigrolimitatus being the exceptions). The total abundance of the seven indicator species correlated significantly between 1997 and 2017 (rp = 0.79, n = 16, p < 0.001). In both years, the total volume of dead spruces in 1997 was linked with the total indicator abundance (linear regression; 1997: r2 = 0.61, r = 0.78, n = 16, p < 0.001; 2017: r2 = 0.41, r = 0.64, n = 16, p < 0.01).
4 Discussion
We documented colonization–extinction dynamics of eight wood-decaying fungal species in old-growth boreal forest fragments between 1997 and 2017. Fewer colonizations than extinctions were observed, but the population turnover did not differ from random for any of the species. Most fragments had maintained populations over the 20 years, but there were – sometimes notable – changes in abundance.
A higher number of extinctions than colonizations was expected, since most fragments were already occupied by the studied species in 1997. Species requiring larger or more decayed logs experienced more extinctions and declined more often than species preferring fresh dead wood (Jönsson et al. 2008). Thus, it is possible that the amount of fresh and well-decayed dead wood had changed accordingly. Because we did not record the number of logs in 2017, we cannot analyze changes in the proportion of logs occupied; however, the number of occupied logs reflects resource availability and true population size and is thus more important from the conservation point of view.
The observed extinctions and declines suggest that the populations of the rarest species might have experienced extinction debt (Penttilä et al. 2006). Our results corroborate Berglund and Jonsson (2008) who suggested extinction debt for A. lapponica, Phl. centrifuga and L. mollis in similar old-growth forest fragments using a chronosequence approach. However, it is practically impossible to evaluate if a local extinction represents paying off extinction debt or stochasticity not linked with fragmentation. Ideally, verifying extinction debt requires species occurrence data prior to fragmentation.
Extinction frequency was similar in recently isolated and older fragments, despite tendency for being higher in older fragments. Yet, small sample size does not warrant definitive conclusions considering the effect of isolation time, especially since the older fragments were also of poorer quality. It is notable that 26% of the extinctions occurred in two natural fragments amid large forest–peatland conservation areas. Extinctions and colonizations in natural fragments are assumed to be in stochastic equilibrium and thus not related to recent fragmentation. These fragments had clearly the smallest amount of coarse spruce logs; hence, species turnover in these fragments can overestimate the baseline turnover in the other fragments.
Under natural gap disturbance dynamics, higher site productivity leads into larger amounts of dead wood, and, consequently, higher fungal diversity and abundance (Gjerde et al. 2005). The fragments in which extinctions were documented had less spruce dead wood and coarse spruce logs in 1997 than the fragments without extinctions. Fragment characteristics did not affect population turnover of Phl. centrifuga and P. nigrolimitatus in the fragments. The weak effect of fragment characteristics is not unexpected, because fragments were originally selected such that their forest characteristics would be similar. Although we did not measure forest characteristics in 2017, we presume that they did not change markedly as key characteristics result from site productivity and thus tend to remain without stand-replacing disturbances (Jönsson et al. 2017).
Fruiting bodies (not necessarily the same brackets) can persist in a single log at least for eight years, and the fruiting bodies of perennial species persist longer than those of annual species (Berglund et al. 2005). This means more uncertainty in documenting the presence or absence of annual than perennial species (Halme and Kotiaho 2012). Because fungi persist as mycelia, the occupancy based on fruiting bodies is an underestimate of the true abundance but does indicate the reproductive population size at the given moment. We had a different sampling protocol than the original survey in 1997. Because we focused on a few, relatively conspicuous species, and surveyed all downed spruce logs, it is likely that at least extinctions were documented reliably. Furthermore, the notable (≥ 10 logs) increases or decreases in abundance in many fragments, even for rarer species, indicates that the general results obtained are not due to differences in sampling.
Some conservation-relevant conclusions can be drawn. First of all, wood-decaying fungi can persist in small old-growth forest fragments for decades, and thus the importance of small fragments should be acknowledged. Our species-specific results regarding extinctions, colonizations and declines are consistent with the studies of the same species in Sweden (Berglund and Jonsson 2008; Jönsson et al. 2008; Ruete et al. 2016; Jönsson et al. 2017). Taken together, all these studies underscore that rare species generally seem to decline following fragmentation, but abundant species become more abundant. One possible explanation is that wind disturbance creates dead wood in fragment edges, which are utilized by common fungi (Ruete et al. 2016). Another explanation is that common species benefit from clear-cuttings and invade the old-growth fragments from the matrix (Laaksonen et al. 2008).
Because fungal spores can disperse large distances (Komonen and Müller 2018), fragment level turnover was influenced by landscape changes between 1997 and 2017. In the past decades, the amount of old-growth in Kuhmo has decreased but not markedly. Kuhmo still has more old-growth forest than southern Finland generally. It is situated near the large old-growth forest areas in Russia (Laaksonen et al. 2008), and possibly receives spore dispersal from there. Thus, the documented extinction–colonization dynamics cannot be readily transferred to other landscapes with longer history of intensive human impact.
5 Conclusions
Our study suggests that wood-decaying fungi can survive in small old-growth fragments for a few decades if the fragments are of good quality. From the conservation point of view, it is notable that the least abundant species in 1997 had declined, whereas the most abundant species had become more abundant. The remaining small old-growth forest fragments should be urgently protected, even if they are isolated from larger expanses of natural biotopes.
Authors’ contributions
All authors contributed to the conception of research questions and design of the work. IP collected the 2017 fungal data and RP led the collection of the 1997 data. IP and AK analysed the data. All authors participated in the interpretation of data and results, and writing.
Acknowledgements
Miia Kokkonen and Panu Halme helped with the species identification. We thank all the people involved in the Biodiversity in Boreal Forests project in the late 1990s, especially Mariko Lindgren for collecting fungal data.
Funding
Study was funded by Suomen Biologian Seura Vanamo (research grant to IP).
References
Ahti T, Hämet-Ahti L, Jalas J (1968) Vegetation zones and their sections in northwestern Europe. Ann Bot Fenn 5: 169–211. https://www.jstor.org/stable/23724233.
Bässler C, Müller J (2010) Importance of natural disturbance for recovery of the rare polypore Antrodiella citrinella Niemelä and Ryvarden. Fungal Biol 114: 129–133. https://doi.org/10.1016/j.funbio.2009.11.001.
Berglund H, Jonsson BG (2005) Verifying an extinction debt among lichens and fungi in northern Swedish boreal forests. Conserv Biol 19: 338–348. https://doi.org/10.1111/j.1523-1739.2005.00550.x.
Berglund H, Jonsson BG (2008) Assessing the extinction vulnerability of wood-inhabiting fungal species in fragmented northern Swedish boreal forests. Biol Conserv 141: 3029–3039. https://doi.org/10.1016/j.biocon.2008.09.007.
Berglund H, Edman M, Ericson L (2005) Temporal variation of wood-fungi diversity in boreal old-growth forests: implications for monitoring. Ecol Appl 15: 970–982. https://doi.org/10.1890/04-0628.
Fischer J, Lindenmayer D (2007) Landscape modification and habitat fragmentation: a synthesis. Global Ecol Biogeogr 16: 265–280. https://doi.org/10.1111/j.1466-8238.2007.00287.x.
Gjerde I, Sætersdal M, Rolstad J, Storaunet KO, Blom HH, Gundersen V, Heegaard E (2005) Productivity‐diversity relationships for plants, bryophytes, lichens, and polypore fungi in six northern forest landscapes. Ecography 28: 705–720. https://doi.org/10.1111/j.2005.0906-7590.04249.x.
Gu WD, Heikkilä R, Hanski I (2002) Estimating the consequences of habitat fragmentation on extinction risk in dynamic landscapes. Landsc Ecol 17: 699–710. https://doi.org/10.1023/A:1022993317717.
Halme P, Kotiaho JS (2012) The importance of timing and number of surveys in fungal biodiversity research. Biodivers Conserv 21: 205–219. https://doi.org/10.1007/s10531-011-0176-z.
Hyvärinen E, Juslén A, Kemppainen E, Uddström A, Liukko U-M (2019) Suomen lajien uhanalaisuus 2019 – Punainen kirja. [The 2019 Red List of Finnish species]. Ympäristöministeriö and Suomen ympäristökeskus, Helsinki. http://hdl.handle.net/10138/299501.
Jönsson MT, Edman M, Jonsson BG (2008) Colonization and extinction patterns of wood–decaying fungi in a boreal old–growth Picea abies forest. J Ecol 96: 1065–1075. https://doi.org/10.1111/j.1365-2745.2008.01411.x.
Jönsson MT, Ruete A, Kellner O, Gunnarsson U, Snäll T (2017) Will forest conservation areas protect functionally important diversity of fungi and lichens over time. Biodivers Conserv 26: 2547–2567. https://doi.org/10.1007/s10531-015-1035-0.
Junninen K, Komonen A (2011) Conservation ecology of boreal polypores: a review. Biol Conserv 144: 11–20. https://doi.org/10.1016/j.biocon.2010.07.010.
Komonen A, Müller J (2018) Dispersal ecology of deadwood organisms and connectivity conservation. Conserv Biol 32: 535–545. https://doi.org/10.1111/cobi.13087.
Komonen A, Penttilä R, Lindgren M, Hanski I (2000) Forest fragmentation truncates a food chain based on an old–growth forest bracket fungus. Oikos 90: 119–126. https://doi.org/10.1034/j.1600-0706.2000.900112.x.
Kotiranta H, Saarenoksa R, Kytövuori I (2009) Aphyllophoroid fungi of Finland. A check-list with ecology, distribution, and threat categories. Norrlinia 19.
Krauss J, Bommarco R, Guardiola M, Heikkinen RK, Helm A, Kuussaari M, Lindborg R, Öckinger E, Pärtel M, Pino J, Pöyry J (2010) Habitat fragmentation causes immediate and time‐delayed biodiversity loss at different trophic levels. Ecol Lett 13: 597–605. https://doi.org/10.1111/j.1461-0248.2010.01457.x.
Laaksonen M, Peuhu E, Várkonyi G, Siitonen J (2008) Effects of habitat quality and landscape structure on saproxylic species dwelling in boreal spruce-swamp forests. Oikos 117: 1098–1110. https://doi.org/10.1111/j.0030-1299.2008.16620.x.
Niemelä T (2016) Suomen käävät. [Finnish polypores]. Norrlinia 31.
Penttilä R, Lindgren M, Miettinen O, Rita H, Hanski I (2006) Consequences of forest fragmentation for polyporous fungi at two spatial scales. Oikos 114: 225–240. https://doi.org/10.1111/j.2006.0030-1299.14349.x.
Rassi P, Alanen A, Kanerva T, Mannerkoski I (2001) Suomen lajien uhanalaisuus 2000 – Punainen kirja. [The 2000 Red List of Finnish species]. Ympäristöministeriö and Suomen ympäristökeskus, Helsinki.
Roslin T, Várkonyi G, Koponen M, Vikberg V, Nieminen M (2014) Species–area relationships across four trophic levels – decreasing island size truncates food chains. Ecography 37: 443–453. https://doi.org/10.1111/j.1600-0587.2013.00218.x.
Ruete A, Snäll T, Jönsson M (2016) Dynamic anthropogenic edge effects on the distribution and diversity of fungi in fragmented old-growth forests. Ecol Appl 26: 1475–1485. https://doi.org/10.1890/15-1271.
Schmitt CB, Burgess ND, Coad L, Belokurov A, Besançon C, Boisrobert L, Campbell A, Fish L, Gliddon D, Humphries K, Kapos V, Loucks C, Lysenko I, Miles L, Mills C, Minnemeyer S, Pistorius T, Ravilious C, Steininger M, Winkel G (2009) Global analysis of the protection status of the world’s forests. Biol Conserv 142: 2122–2130. https://doi.org/10.1016/j.biocon.2009.04.012.
Sigurdsson A (1999) Landscape ecological changes in the Kuhmo border area after 1940. Finnish Environment 275.
Tilman D, May R, Lehman C, Nowak M (1994) Habitat destruction and the extinction debt. Nature 371: 65–66. https://doi.org/10.1038/371065a0.
Tomppo E, Tuomainen T, Henttonen H, Ihalainen A, Tonteri T (2003) Kainuun metsäkeskuksen alueen metsävarat 1969–2001. [Forest resources in the administrative area of Kainuu Forest Centre 1969–2001]. Metsätieteen aikakauskirja 2B/2003: 169–256. https://doi.org/10.14214/ma.5787.
Virkkala R, Korhonen KT, Haapanen R, Aapala K (2000) Metsien ja soiden suojelutilanne metsä- ja suokasvillisuusvyöhykkeittäin valtakunnan metsien 8. inventoinnin perusteella. [Conservation status of forests and peatlands in different vegetation zones, based on the 8th national forest inventory]. Suomen ympäristö 395: 1–52.
Total of 28 references.

