Genetic patterns in range-edge populations of Vaccinium species from the central Balkans: implications on conservation prospects and sustainable usage
Bjedov I., Obratov–Petković D., Mišić D., Šiler B., Aleksic J. M. (2015). Genetic patterns in range-edge populations of Vaccinium species from the central Balkans: implications on conservation prospects and sustainable usage. Silva Fennica vol. 49 no. 4 article id 1283. https://doi.org/10.14214/sf.1283
Highlights
- We studied fragmentary distributed range-edge populations of Vaccinium myrtillus, Vaccinium uliginosum and Vaccinium vitis-idaea from the Balkans using RAPDs
- Low genetic diversities and high genetic differentiation were found in all species
- The prevalence of clonal individuals was not observed
- Past interspecific hybridization among V. vitis-idaea and the other two species was detected
- Guidelines for conservation and sustainable usage were provided.
Abstract
Vaccinium myrtillus L., Vaccinium uliginosum L. and Vaccinium vitis-idaea L. are perennial, cold-adapted clonal shrubs distributed throughout Europe, northern Asia and North America. Due to their usage in food (berries) and pharmaceutical industry (berries and leaves), their natural populations are exposed to anthropogenic and other impacts that affect their genetic make-up. We analyzed 14 fragmentary distributed and small-sized peripheral populations of these species from the Balkans, which represents the southeastern-European marginal area of their wide European distributions, using RAPD molecular markers. The contemporary genetic patterns in all three species within the Balkans were generally similar, and in comparison to previous reports on populations of these species found in northward Europe, where they have a more continuous distribution, the levels of genetic diversity were more or less halved, genetic differentiation was several times higher, gene flow exceptionally low, and the expected prevalence of clonal individuals was lacking. The population dynamics of all three species within the Balkans was complex and distinct, and was characterized by a past admixture of individuals from discrete populations of the same species and interspecific hybridisation not only between V. myrtillus and V. vitis-idaea but also between V. uliginosum and V. vitis-idaea, the latter not being reported to date. Conservation measures suitable for preservation of presumably genetically distinct portions of the Balkans’ gene pools of studied species have been suggested, while the utility of interspecific hybrids in breeding programs and/ or in food/pharmaceutical industry is yet to be assessed.
Keywords
marginal populations;
genetic structure;
Vaccinium sp.;
the Balkans;
RAPD markers;
genetic profiles;
interspecific hybridization
- Bjedov, University of Belgrade, Faculty of Forestry, Kneza Višeslava 1, 11000 Belgrade, Serbia E-mail ivana.bjedov@sfb.bg.ac.rs
- Obratov–Petković, University of Belgrade, Faculty of Forestry, Kneza Višeslava 1, 11000 Belgrade, Serbia E-mail dragica.obratov-petkovic@sfb.bg.ac.rs
- Mišić, University of Belgrade, Institute for Biological Research “Siniša Stanković”, Boulevard Despota Stefana 142, 11000 Belgrade, Serbia E-mail dmisic@ibiss.bg.ac.rs
- Šiler, University of Belgrade, Institute for Biological Research “Siniša Stanković”, Boulevard Despota Stefana 142, 11000 Belgrade, Serbia E-mail branislav.siler@ibiss.bg.ac.rs
-
Aleksic,
University of Belgrade, Institute of Molecular Genetics and Genetic Engineering, Vojvode Stepe 444a, P.O. Box 23, 11010 Belgrade, Serbia
E-mail
aleksic_jelena@yahoo.com.au

Received 23 November 2014 Accepted 1 June 2015 Published 22 June 2015
Views 238886
Available at https://doi.org/10.14214/sf.1283 | Download PDF

1 Introduction
Vaccinium myrtillus L., Vaccinium uliginosum L. and Vaccinium vitis-idaea L. (Vaccinium, Ericaceae) are perennial, evergreen or deciduous cold-adapted clonal shrubs which have wide natural ranges that cover more or less continuously northern and central Europe, northern Asia and North America (Popova 1972; Jacquemart 1996; Gustavsson 2001; Persson and Gustavsson 2001). Available genetic studies in these three Vaccinium species involved mainly their natural populations from central and northern Europe and were based predominantly on variability of RAPD markers employed alone (e.g. Persson and Gustavsson 2001; Albert et al. 2004, 2005; Garkava-Gustavsson et al. 2005) or along with AFLP markers (Albert et al. 2003), but also on allozyme (Alsos et al. 2002), chloroplast (cpDNA) (Alsos et al. 2005) and combined nuclear (nrDNA) and cpDNA variability (Eidesen et al. 2007). However, populations of these species from the Balkans, which represents southeastern marginal edge of their European range distributions, have not been studied genetically to date with one exception only – Eidesen et al. (2007) carried out a comprehensive study of V. uliginosum covering the entire range distribution of this species, including populations from southern Europe and the Balkans (one population from Bulgaria represented by six individuals). Eidesen et al. (2007) found that populations of this species from southern Europe differ from the remaining populations at the ploidy level, and are also genetically distinct at the nrDNA and cpDNA levels. Furthermore, they detected a sub-structure within this region, with three well-differentiated sub-groups confined to western southern Europe, eastern southern Europe (the Balkans) and Caucasus, and found that these southward populations have not expanded northwards from these areas.
Within the Balkans, natural populations of V. myrtillus, V. uliginosum and V. vitis-idaea are highly fragmentary distributed and their relatively small-sized populations are scattered at higher elevations (above 800 m a.s.l.) of numerous mountain chains of this region. It is well-known that fragmentary distributed and smaller peripheral plant populations found at the limits of the species’ distribution are generally subjected to different regimes of natural selection as compared to central populations (Lesica and Allendorf 1995; Lenormand 2002; Eckert et al. 2008), and are exposed to the progressive loss of within-population genetic diversity and increase in among-population genetic differentiation owing to the smaller effective population sizes, more severe effects of random genetic drift and reduced inter-population gene flow due to isolation (Ellstrand and Elam 1993; Scalfi et al. 2009 and references therein).
The decrease in levels of genetic diversity at the range-edge may also be promoted by the prevalence of the clonal growth in so-called clonal plant species characterized by mixed sexual and clonal reproduction (e.g. Beatty et al. 2008). V. myrtillus, V. uliginosum and V. vitis-idaea are clonal plant species which reproduce generatively (predominantly by outcrossing) and vegetativelly (through rhizomes). In such species, an increase in levels of genetic diversity depends on the efficiency of the production of out-crossed seeds and mobilization of generatively reproduced plants (Eriksson 1989; Eriksson and Fröborg 1996; Persson and Gustavsson 2001; Albert et al. 2004), while clonal propagation reduces levels of out-crossing, the formation of new genetic combinations (Charpentier 2002; Honnay and Bossuyt 2005) and ultimately levels of genetic diversity through the prevalence and domination of more competitive genotypes (genets) which are more efficient in producing ramets (Eriksson 1989, 1993; Jacquemart et al. 1994; Pornon et al. 2000; Honnay and Bossuyt 2005; Clark-Tapia et al. 2006). Although lower levels of genetic diversity are generally expected in clonal plant species (Hamrick and Godt 1989; Bartish et al. 1999; Nybom and Bartish 2000), several studies revealed as much genetic diversity in clonal as in non-clonal plants (Ellstrand and Roose 1987), and this holds also for Vaccinium species (e.g. Stewart and Excoffier 1996; Kreher et al. 2000; Persson and Gustavsson 2001; Albert et al. 2003, 2004, 2005; Garkava-Gustavsson et al. 2005; Eidesen et al. 2007; Debnath 2007; Yakimowski and Eckert 2008; Žukauskienė et al. 2009; Hirai et al. 2010).
On the other hand, V. myrtillus, V. uliginosum and V. vitis-idaea are used in pharmaceutical industry and traditional medicine owing to the medicinal properties of their leaves and fruits (Sarić 1989; Bown 1995; Chevallier 1996). In addition, two of these species, V. myrtillus and V. vitis-idaea, are amongst economically the most important wild berry species worldwide (Kardell 1980; Salo 1995; Saastamoinen et al. 2000; Kangas 2001). Their berries, used in fresh or processed form for household consumption and for sale, are still excessively picked from plants from natural populations (Katsube et al. 2003; Zhao et al. 2004; Åkerström 2010; Garkava-Gustavsson et al. 2005) despite the increasing commercial production and cultivation of improved cultivars produced in breeding programs conducted worldwide (e.g. Garkava-Gustavsson et al. 2005; Martinussen et al. 2008). Over-exploitation of natural populations of all three Vaccinium species, however, poses a severe problem especially in developing countries such as those from the Balkans, because the trade of berries represents an important source of income (Tomićević et al. 2011; Bjedov 2012). Peripheral populations of Vaccinium species from the Balkans are additionally threatened by habitat losses due to the ongoing suppression by a more competitive Juniperus communis subsp. alpina (Bjedov 2012).
In order to preserve the overall genetic diversity of V. myrtillus, V. uliginosum and V. vitis-idaea, enhance breeding programs and enable sustainable usage of these species, the knowledge on contemporary genetic patterns and genealogical histories of their populations throughout their natural ranges is required (Frankham et al. 2002; Hampe and Petit 2005). In the frame of our study, we focus on genetically understudied peripheral populations of these three Vaccinium species from the Balkans which, at least in case of V. uliginosum (Eidesen et al. 2007), comprises a genetically distinct portion of the species overall gene pool. The aim of this study is: (i) to shed more light on levels of genetic diversity and genetic structuring of peripheral populations of V. myrtillus, V. uliginosum and V. vitis-idaea found within the central Balkans; (ii) to infer whether generative or clonal propagation prevails in highly fragmented and isolated populations of all three species within this region; and (iii) to provide insights into the population dynamics of these species within the Balkans. We utilized RAPD molecular markers which have commonly been used in molecular genetic studies in Vaccinium sp. (e.g. Stewart and Excoffier 1996; Kreher et al. 2000; Persson and Gustavsson 2001; Albert et al. 2003, 2004, 2005; Garkava-Gustavsson et al. 2005; Debnath 2007; Žukauskienė 2009), and provide guidelines for conservation and sustainable usage of these species.
2 Material and methods
2.1 Study species
Vaccinium myrtillus is a subboreal-circumpolar species whose European range spans from the polar circle to southern European mountains and along this latitudinal gradient, alters from a more continuous to a more fragmented form. Within the Balkans, V. myrtillus is found in mountainous and alpine regions at elevations from 800 to 2500 m a.s.l. (Bjedov 2012). Throughout the range, populations of this species are usually found on acid soils, from mountainous mineral heath to organic forest soils and old peat bogs (Ritchie 1956). V. uliginosum and V. vitis-idaea are boreal-circumpolar species, widely distributed in northern temperate, boreal and subarctic regions. They thrive in bogs, heathland, peaty woodland, mountainous heats and in tundra (Jacquemart 1996; Gustavsson 2001; Albert et al. 2005). Similarly as in V. myrtillus, their European range distributions fragment towards the southern Europe, and within the Balkans, populations of these species occur in subalpine and alpine regions at elevations from 1300 to 2500 m a.s.l. (Bjedov 2012).
The evergreen V. vitis-idaea and deciduous V. myrtillus and V. uliginosum are long-lived woody shrubs sensitive to changes of abiotic factors such as nitrogen (Percival et al. 2003; Percival and Sanderson 2004), light availability (Martinussen et al. 2009) and temperature (Raatikainen and Vänninen 1988). They are all insect-pollinated, and their seeds are dispersed by birds (Ritche 1955, 1956; Jacquemart 1996; Jacquemart and Thompson 1996; Nuortila et al. 2002). They are clonal plant species which reproduce sexually (outcrossing predominates) and vegetativelly through rhizomes. Although they are partially self-compatible, the capacity for selfing is rather poor in the absence of pollinators (Jacquemart and Thompson 1996).
2.2 Sampling and DNA extraction
During the spring 2010, young leaves from five to 14 individuals were collected from each of the five populations of V. myrtillus (56 individuals in total), five populations of V. uliginosum (51 individuals) and four populations of V. vitis-idaea (32 individuals) distributed within the central Balkans (Figs. 1 and 2, Table 1). Due to the potential occurrence of clonal individuals within populations of all species, the distances between sampled individuals within each population were at least 30 m, with one exception only. This was population Vvi1 of V. vitis-idaea at the locality Pajino preslo (Mt Kopaonik) which occupies on area of 40 m2 only (0.004 ha, Table 1) and is the smallest population used in the present study. We sampled 14 individuals from this population, and distances among them were c. 5 m. The fresh leaves used for RAPD analyses were submerged into the liquid nitrogen in the field, and stored at –80 °C until DNA extraction.
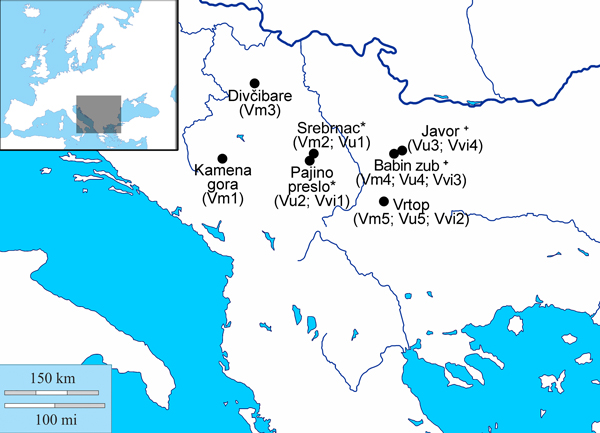
Fig. 1. Sites within the central Balkans from which populations of V. myrtillus, V. uliginosum and V. vitis-idaea were sampled. The list of populations sampled from each site is given in brackets; * - sites found at Mt Kopaonik; + - sites found at Mt Stara planina.
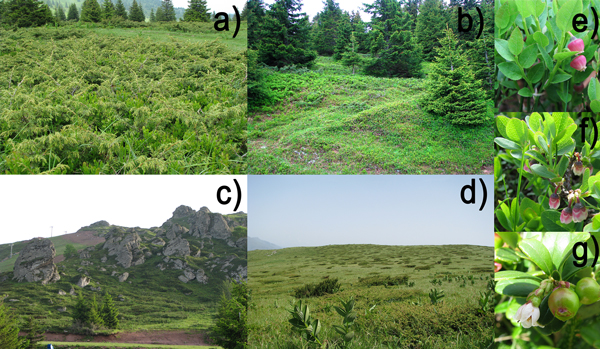
Fig. 2. Localities at Mts Kopaonik (a and b) and Stara planina (c and d) at which populations of Vaccinium myrtillus (e), Vaccinium uliginosum (f) and Vaccinium vitis-idaea (g) are found; a) locality Srebrnac, with populations Vm2 and Vu1; b) locality Pajino preslo, with populations Vu2 and Vvi1; c) locality Babin zub, with populations Vm4, Vu4 and Vvi3; d) locality Javor, with populations Vu3 and Vvi4.
| Table 1. Sampling localities of populations of three Vaccinium species from the Balkans, sample sizes, and parameters of genetic diversity at the population and at the species level. View in new window/tab. |
DNA was extracted from young leaves using a modified cetyltrimethylammonium-bromide (CTAB) procedure of Doyle and Doyle (1990), modified by Albert et al. (2003). The DNA yield and purity of DNA isolates were assessed by measuring absorbance with a spectrophotometer (Agilent 8453, Agilent Technologies, Waldbronn, Germany).
2.3 RAPD-PCR procedure and data interpretation
Initially, 15 decamer RAPD primers were tested using RAPD-PCR procedure of Albert et al. (2003) and Skorić et al. (2012), with modifications involving optimisation of the reaction mixture composition and of the temperatures used for PCR amplifications. However, based on the reproducibility of bands and profile quality, only nine primers were further used (Table 2). PCR reactions were carried out in 25 µl volumes containing 10 ng DNA, 2.5 µl of 10 × Taq NH4+ buffer (Thermo Scientific, Fermentas, Vilnius, Lithuania), 2.5 µl of 25 mM MgCl2, 0.5 µl of 10 mM dNTP (Thermo Scientific, Fermentas, Vilnius, Lithuania), 5.1 µl of ddH2O, 1.5 µl of a primer, and 2 U of Taq DNA polymerase (Thermo Scientific, Fermentas, Vilnius, Lithuania). PCR amplifications were performed using a peqSTAR 96 Universal Gradient thermal cycler (Peqlab, Biotechnologie GmbH, Erlangen, Germany) with the following profile: initial denaturation at 94 °C for 4 min, followed by 45 cycles of denaturation at 94 °C for 1 min, annealing at 36 °C for 2 min, elongation at 72 °C for 2 min, and final extension at 72 °C for 4 min. PCR products were separated by 1% agarose gel electrophoresis in 1 × TBE buffer with 5.5 V cm–1 during 2 h. Gels were stained with ethidium bromide, and banding pattern was visualized under the UV light using ST4 3026-WL/26M transilluminator (Vilber Lourmat, Torcy, France).
| Table 2. Characteristics of nine decamer primers used for RAPD-PCR analyses in three Vaccinium species from the Balkans. | ||||
| PIC values | ||||
| Primer name | Primer sequence (5’–3’) | V. myrtillus | V. uliginosum | V. vitis – idaea |
| OPA-05 | AGGGGTCTTG | 0.291 | 0.283 | 0.285 |
| OPA-07 | GAAACGGGTG | 0.246 | 0.338 | 0.292 |
| OPA-09 | GGGTAACGCC | 0.264 | 0.314 | 0.319 |
| OPA-10 | CTGATCGCAG | 0.224 | 0.282 | 0.311 |
| OPA-11 | CAATCGCCGT | 0.277 | 0.261 | 0.307 |
| OPA-13 | CAGCACCCAC | 0.342 | 0.320 | 0.323 |
| OPA-15 | TTCCGAACCC | 0.280 | 0.298 | 0.287 |
| OPO 7 | CAGCACTGAC | 0.302 | 0.303 | 0.303 |
| OPO 15 | TGGCGTCCTT | 0.227 | 0.303 | 0.303 |
| Average | 0.273 | 0.300 | 0.303 | |
RAPD-PCR amplification products, treated as described by Lynch and Milligan (1994), were scored automatically as 1 for presence and 0for absence of bands using the software TotalLab TL120 1Dv2009 (Nonlinear Dynamics Ltd., Newcastle, Great Britain), and their sizes were determined using 1 kb DNA ruler (Thermo Scientific, Fermentas, Vilnius, Lithuania). Only clear, distinct bands were scored (Fig. 3), the variation in intensity of bands was not taken into consideration, and a revision of band scoring and matching was performed manually. The banding patterns of all nine RAPD primers were combined into multilocus RAPD phenotypes for all individuals. Individuals showing an identical RAPD multilocus phenotype were treated as clonal individuals and they were included in the AMOVA analyses only (see later). To assess the informativeness of RAPD primers, a total number of bands, the number of polymorphic bands and Polymorphism Information Content (PIC) values were calculated for each RAPD primer and at the species level following Nagy et al. (2012).
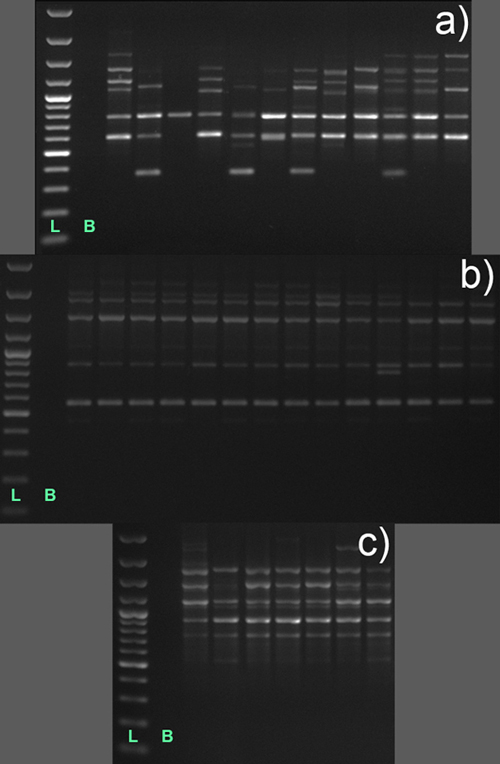
Fig. 3. RAPD banding patterns on 1% agarose gels obtained upon PCR amplification with two decamer primers, OPA 07 (a) and OPA 15 (b and c), in individuals of three Vaccinium species; a – Vaccinium myrtillus, population Vm2 represented by 12 individuals; b – Vaccinium uliginosum, Vu1, 14 individuals; and c – Vaccinium vitis-idaea, Vvi3, 7 individuals; each lane represents a banding pattern in different individual; L – 1 kb DNA ladder; B – blank.
2.4 Genetic diversity and genetic differentiation
The parameters of genetic diversity, Nei’s gene diversity, h, (Nei 1973) and Shannon’s information index, I, (Lewontin 1972), were calculated at the population and the species levels using the software POPGENE ver. 1.31 (Yeh et al. 1997). Parameters of genetic differentiation of pairs of populations and at the species levels (FST), and gene flow (Nm) estimated according to the equation: Nm = 0.5 (1 - FST)/FST were assessed with the software Arlequin ver. 3.5.1.2 (Excoffier 2010) with 1000 permutations. The same software was used to perform an Analysis of Molecular Variance (AMOVA, Excoffier et al. 1992) using a matrix of squared Euclidean distances among all genotypes in all three species. This analyses were performed at the ramet level (i.e. including all individuals, clonal and non-clonal) and at the genet level (i.e. excluding clonal individuals from calculations) for all studied species in order to estimate the relative contribution of vegetative and generative propagation to the population composition. The statistical significance was evaluated using 1000 permutations.
2.5 Cluster analyses
To further study genetic differentiation of populations in all three species, we applied three approaches. Firstly, we calculated the Jaccard’s similarity coefficients (Jsc) to generate pairwise similarity matrices for each species used for constructing dendrograms with unweighted pair-group method (UPGMA) in the software PAST (PAleontologicalSTatistics, ver. 1.89, Hammer et al. 2009). Statistical support was obtained with 100 bootstrap (BS) replicates. Secondly, we used a model-based Bayesian clustering method in STRUCTURE 2.2 (Pritchard et al. 2000). RAPD multilocus phenotypes were treated as diploid multilocus genotypes with the unknown alleles as missing values, and run under allele frequencies correlated and admixture models. Iteration parameters were set to a burn-in period of 1000000 iterations followed by 5000000 iterations. We run the analysis for each species independently setting the number of genetic groups, K, from 1 to 6 for V. myrtillus and V. uliginosum, and from 1 to 5 for V. vitis-idaea, and performed ten independent runs for each of the assumed K. To determine the optimal K value for each species, we used ΔK method of Evanno et al. (2005) which can detect higher levels of hierarchical structure. Thirdly, we conducted a Principal Component Analysis (PCA) based on the matrix of pairwise population Jaccard’s similarity coefficients using the software PAST.
2.6 Spatial distribution of genetic diversity and genealogical relations of individuals
To explore the spatial distribution of genetic variation and possible isolation-by-distance (IBD) pattern, we used Mantel test (Mantel 1967) in Arlequin ver. 3.5.1.2 to test for correlations between the genetic distances and geographical distances. We used a matrix of pairwise population Jaccard’s similarity coefficients, and obtained statistical support with 5000 permutations.
We constructed Minimum Spanning Trees (MST) in PAST software and inferred genealogical relations among individuals belonging to discrete populations at the genus and the species levels. MST trees were presented within PCA graphs generated previously, and the congruence between inferred genealogical lineages and their spatial distribution was inferred.
3 Results
3.1 Characteristics of RAPD primers
The total number of bands and the number of polymorphic bands in V. myrtillus, V. uliginosum and V. vitis-idaea were 182 and 175, 174 and 165, and 194 and 191, respectively. The PIC values of individual RAPD primers at the species level are presented in Table 2. They ranged from 0.224 for OPA-10 in V. myrtillus to 0.338 for OPA-7 in V. uliginosum and averaged to 0.273, 0.300 and 0.303 in these three species, respectively. These values were in the range of those reported for Vaccinium species (see references in Discussion referring to the levels of genetic diversity in Vaccinium species).
3.2 Genetic diversity and genetic differentiation
Individuals characterized by identical multilocus RAPD phenotypes, treated as clonal individuals, were observed in 10 out of 14 populations (Table 1) and were used for AMOVA analyses only (see later). The total number of non-clonal individuals used for other analyses was 109, of which 47 belonged to V. myrtillus, 44 to V. uliginosum and 18 to V. vitis-idaea.
The values of Nei’s gene diversity and Shannon’s information index at the population and at the species levels for all three species are presented in Table 1. The values of the former parameter were slightly lower and revealed relatively low levels of genetic diversity in populations and at the species levels in all three species (average in V. myrtillus, V. uliginosum and V. vitis-idaea of 0.138, 0.158 and 0.186, respectively), while the latter estimate, which is more suitable and widely used for dominant molecular markers, revealed low to moderate levels of genetic diversities at the population level and at the species levels (average of 0.221, 0.244 and 0.292 in these three species, respectively).
The values of pairwise population differentiation parameters (FST) at the species level are presented in Table 3. They ranged from 0.392 between Vm1 and Vm2 in V. myrtillus to 0.696 between Vu1 and Vu5 in V. uliginosum, and all were significant at 95% level. Average genetic differentiation among populations (FST) in all three species was rather high, 0.430 in V. myrtillus, 0.584 in V. uliginosum and 0.520 in V. vitis-idaea. Accordingly, the values of the effective gene flow between populations (Nm) were below 1.00 in all three species (0.740, 0.371 and 0.321, respectively).
| Table 3. Pairwise population FST values (below diagonals), average FST values at the species level, and geographic distances among populations of three Vaccinium species from the Balkans given in km (above diagonals). | ||||||
| Vaccinium myrtillus | ||||||
| Vm1 | Vm2 | Vm3 | Vm4 | Vm5 | ||
| Vm1 | 100.65 | 95.30 | 243.40 | 230.73 | ||
| Vm2 | 0.392 | 110.49 | 143.15 | 142.25 | ||
| Vm3 | 0.349 | 0.258 | 226.03 | 240.03 | ||
| Vm4 | 0.465 | 0.467 | 0.347 | 69.50 | ||
| Vm5 | 0.549 | 0.510 | 0.465 | 0.502 | ||
| Average FST: 0.430 | ||||||
| Vaccinium uliginosum | ||||||
| Vu1 | Vu2 | Vu3 | Vu4 | Vu5 | ||
| Vu1 | 4.72 | 172.65 | 143.32 | 138.07 | ||
| Vu2 | 0.602 | 173.75 | 145.39 | 137.19 | ||
| Vu3 | 0.557 | 0.517 | 35.77 | 65.05 | ||
| Vu4 | 0.624 | 0.591 | 0.496 | 69.50 | ||
| Vu5 | 0.696 | 0.686 | 0.495 | 0.577 | ||
| Average FST: 0.584 | ||||||
| Vaccinium vitis-idaea | ||||||
| Vvi1 | Vvi2 | Vvi3 | Vvi4 | |||
| Vvi1 | 137.19 | 145.39 | 173.75 | |||
| Vvi2 | 0.552 | 69.50 | 65.05 | |||
| Vvi3 | 0.550 | 0.492 | 35.77 | |||
| Vvi4 | 0.524 | 0.498 | 0.505 | |||
| Average FST: 0.520 | ||||||
| FST values significant at 95% level are presented in bold | ||||||
The outcomes of AMOVA analyses at the ramet and the genet levels in all three species are presented in Table 4. In V. myrtillus, slightly larger amount of molecular variance was assigned to variation within populations then to among-population variation at both the ramet (53.39% vs. 46.61%) and the genet level (56.81% vs. 43.19%), while the opposite trend was obtained in V. uliginosum and V. vitis-idaea (Table 4). The difference in the proportion of within population molecular variance at the ramet and the genet level was relatively low in V. myrtillus (3.42%) and V. uliginosum (2.75%), but relatively high in V. vitis-idaea (21.31%), revealing that relatively high contribution of clonal growth in genetic diversity and genetic structuring was present in V. vitis-idaea only. At the genet level, the difference in proportion of molecular variance assigned to among and within population variation was 13.62% in V. myrtillus, 15.24% in V. uliginosum and 2.4% in V. vitis-idaea.
| Table 4. Outcomes of AMOVA analyses in three Vaccinium species at the ramet and at the genet level. View in new window/tab. |
3.3 Cluster analyses
A dendrogram obtained with UPGMA clustering method using Jaccard’s similarity coefficients (Jsc) is presented in Fig. 4a. Within a dendrogram, each species formed a separate cluster supported with 100% BS (V. myrtillus and V. uliginosum) and 83% BS (V. vitis-idaea), and the values of Jsc between pairs of species were below 0.15. V. myrtillus was characterized as the most homogeneous species (Jsc between individuals ranging from 0.35 to 0.88), and V. vitis-idaea as the most heterogeneous species (Jsc between individuals ranging from 0.22 to 0.75). At the species level, individuals belonging to distinct populations of the same species formed relatively well-supported separate subclusters with one exception only – in V. myrtillus, individual M24 from population Vm3 formed a separate, sixth subcluster. Within each species, differential relations among populations and among individuals within each population were assessed and they were generally concordant with outcomes of other clustering methods (see later).
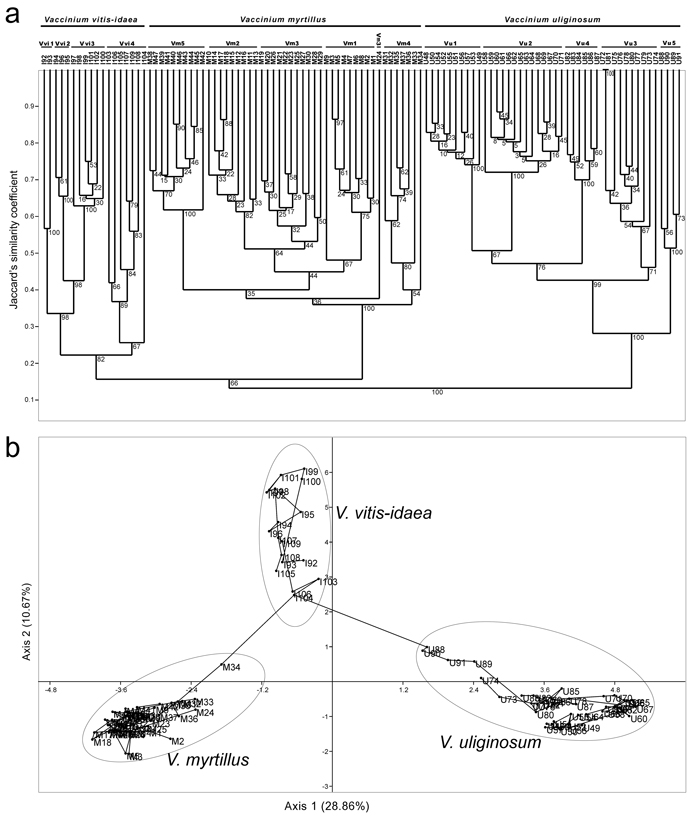
Fig. 4. Dendrogram generated with UPGMA method based on Jacquard’s similarity coefficients among 109 RAPD multilocus phenotypes of three Vaccinium species (a), and outcomes of PCA analysis based on pairwise population Jaccard’s similarity coefficients (b). View larger in new window/tab.
The outcomes of Bayesian clustering at the species level are presented in Fig. 5a (V. myrtillus), Fig. 5c (V. uliginosum) and Fig. 5f (V. vitis-idaea). Based on ΔK method of Evanno et al. (2005), the optimal number of genetic groups, K, in V. myrtillus and V. uliginosum corresponded to the number of studied populations (five for both species), while it was two in V. vitis-idaea (Fig. 5e). In the latter species, one gene pool comprised individuals from populations Vvi1, Vvi2 and Vvi3, and the second gene pool comprised individuals from a single population, Vvi4. However, upon exclusion of Vvi4 from analyses, the remaining three populations were distinguished as independent gene pools at K = 3 and K = 4 in an additional analysis (Fig. 5g). Clustering of populations at lower and higher K than the optimal value in V. myrtillus (Fig. 5a) and V. uliginosum (Fig. 5c) performed in order to assess genetic affinities of populations and potential sub-structuring, were generally concordant with outcomes of other clustering analyses (see later).
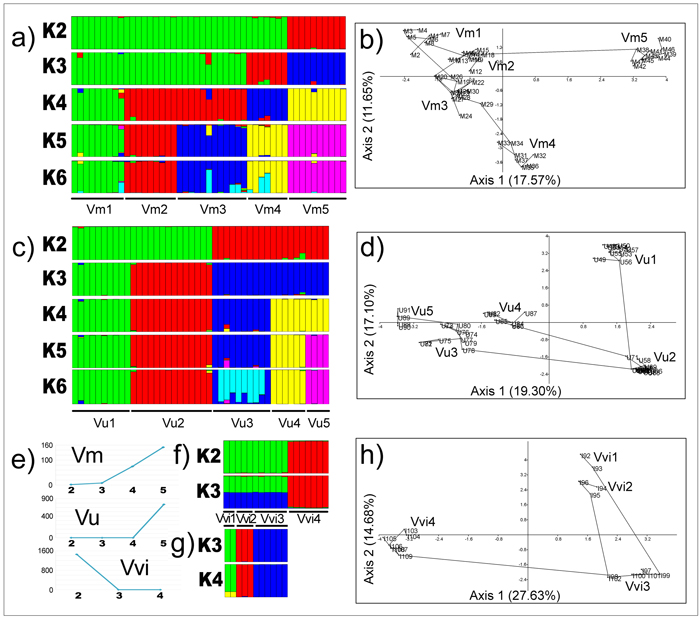
Fig. 5. Outcomes of Bayesian clustering of populations (a – V. myrtillus; c – V. uliginosum; f – V. vitis-idaea represented by all populations; g – V. vitis-idaea represented by populations Vvi1, Vvi2 and Vvi3), the optimal number of genetic groups in all species as inferred by Evanno’s method (e), and outcomes of PCA analyses based on pairwise population Jaccard’s similarity coefficients at the species level (b – V. myrtillus; d – V. uliginosum; h – V. vitis-idaea). View larger in new window/tab.
3.4 Spatial distribution of genetic diversity and genealogical relations of individuals
Isolation by distance pattern was detected in V. vitis-idaea only (r2 = 0.670, P = 0.040), while in the other two species, the correlation coefficients between genetic and geographic distances were low and insignificant (V. uliginosum, r2 = 0.077, P = 0.212), or marginally significant at the 95% level (V. myrtillus, r2 = 0.130, P = 0.070).
The outcomes of PCA analyses and MST trees reconstructed at the genus level are presented in Fig. 4b, and at the species levels in Fig. 5 (3b – V. myrtillus, 3d – V. uliginosum and 3h – V. vitis-idaea). The PCA analysis with all three species included (Fig. 4b) revealed that the scores of individuals belonging to each out of three species were grouped together and scattered within different parts of the two-dimensional PCA space defined by the first two axes which explained jointly 39.53% of variation (28.86% for the first axis, 10.67% for the second axis). The least homogeneous species was V. vitis-idaea, as observed also in UPGMA analysis (Fig. 4a). Interestingly, the scores of all four individuals from population Vu5 of V. uliginosum (U88, U89, U90 and U91) were shifted towards the scores of individuals of V. vitis-idaea, implying past hybridization between these two species and a hybrid origin of all individuals from population Vu5 of V. uliginosum. The same holds for individuals U73 and U74 from population Vu3 of V. uliginosum, while the score of an individual M34 from population Vm3 of V. myrtillus, which was shifted towards the scores of individuals of V. vitis-idaea, implied a hybrid origin of this individual as well. Given the position of scores of individuals I103, I104 and I106 from population Vvi4 of V. vitis-idaea, which were shifted towards the scores of individuals of the other two species, and genealogical relations of individuals from this species to hybrid individuals of the other two species, the likely source of V. vitis-idaea gene-pool in both V. myrtillus and V. uliginosum were ancestors of individuals from population Vvi4 from locality Javor, Stara planina. This population, on the other hand, most likely comprised a portion of gene-pools of both V. myrtillus and V. uliginosum. It is worth mentioning that the observed hybrid origin of individuals from population Vvi4 of V. vitis-idaea may account for the lower bootstrap support for a cluster representing this species in UPGMA analysis (Fig. 4a) and increased heterogeneity of this species observed in all analyses.
In V. myrtillus, within the PCA plot defined by the first two axes which explained jointly 29.22% of variation (17.57% for the first axis, 11.65% for the second axis, Fig. 5b), the scores of individuals belonging to each out of five populations were clustered together with the exception of an individual M9 from Vm1 whose score was plotted jointly with scores of individuals from populations Vm2 indicating that this individual is an immigrant in Vm1 originating from Vm2. The scores of individuals from populations Vm1, Vm2 and Vm3 were clustered together but occupied relatively large area within the two-dimensional PCA space. They were closely genealogically related as well, and MST revealed that Vm3 is likely the source-population of individuals from extant population Vm4, while Vm2 is likely the source-population of individuals from extant population Vm5. The score of an individual M24 from Vm3 was shifted towards scores of individuals from Vm4, and the scores of individuals M33 and M34 from Vm4 were shifted towards the scores of individuals from Vm3, revealing that in both Vm3 and Vm4, admixed individuals which shared portions of gene-pools from both currently discrete populations were present.
In V. uliginosum, within the PCA plot defined by the first two axes which explained jointly 36.40% of variation (19.30% for the first axis, 17.10% for the second axis, Fig. 5d), the scores of individuals belonging to each out of five populations were clustered together, and the scores of individuals from Vu3, Vu4 and Vu5 were grouped together and separated from scores of individuals from populations Vu1 and Vu2 along axis 1. The latter two populations were distinguished from each other along axis 2. However, the MST tree revealed that population Vu2 was a source-population of individuals from extant populations Vu1, Vu3 and Vu4, while population Vu3 was as a source-population of individuals from extant population Vu5. A similar pattern was obtained in V. vitis-idaea as well (Fig. 5h), with one source-population (Vvi3, locality Babin zub, Stara planina) and three derivative extant populations (Vvi1, Vvi2 and Vvi4) scattered within the PCA plot defined by the first two axes which explained jointly 42.31% of variation (27.63% for the first axis, 14.68% for the second axis). The most distinct population in this species was Vvi4, separated from the remaining populations along axis 1.
The above listed individuals from populations from all three species, representing intraspecific hybrids harboring portions of gene-pools of currently well-differentiated populations and those delineated as interspecific hybrids in PCA analyses were observed also in Bayesian analyses (Figs. 5a, 3c and 3f). In UPGMA analyses at the species level, these individuals were generally first to diverge, at the lowest Jsc levels, among individuals from each population (Fig. 4a).
4 Discussion
4.1 Genetic profiles of Vaccinium sp. populations within the Balkans
In accordance with expectations on decreased levels of genetic diversities in fragmented range-edge populations of plant species (Ellstrand and Elam 1993; Scalfi et al. 2009 and references therein), the observed levels of genetic diversities in populations of V. myrtillus, V. uliginosum and V. vitis-idaea from the Balkans were more or less half of the values of those found in more continuously distributed populations of these species from the central and northern Europe estimated using the same or alternative markers and estimates of genetic diversity.
Albert et al. (2003) reported a value of 0.546 of the Shannon’s information index (I) in one population of V. myrtillus from the Belgium based on RAPD molecular markers. Using the same type of molecular markers, Albert et al. (2004) obtained the value of the Simpson’s diversity index of 0.472 in an extended set of six populations of the same species from the same area. Based on RAPD markers, I of 0.647 was found in two natural populations of V. uliginosum from the Belgium (Albert et al. 2005), while Alsos et al. (2002) observed a rather low allozyme diversity in two marginal populations of this species from Svalbard (Arctic Archipelago). Using 105 AFLP markers, Eidesen et al. (2007) reported a significantly lower intra-population diversity D in the south Europe as compared to other regions inhabited by this species, a pattern concordant with our results on decreased levels of genetic diversity in range-edge populations of V. uliginosum from the Balkans. In V. vitis-idaea, Persson and Gustavsson (2001) reported I of 0.568 in four Swedish populations based on RAPD markers, and Garkava-Gustavsson et al. (2005) found a slightly lower value of 0.431 in 15 populations of this species from Sweden, Finland, Norway, Estonia and Russia based on the same type of molecular markers. The estimates of genetic diversities in other Vaccinium species assessed by various molecular markers are provided elsewhere (e.g. Kreher et al. 2000; Persson and Gustavsson 2001; Albert et al. 2003, 2004, 2005; Garkava-Gustavsson et al. 2005; Yakimowski and Eckert 2008; Žukauskienė et al. 2009; Hirai et al. 2010).
However, the comparison of levels of genetic diversities per population and population sizes of all three species (Table 1) revealed that the expected correlation between these two parameters (Leimu et al. 2006; Honnay and Jacquemyn 2007) was apparently lacking. This is because increased levels of genetic diversities were observed in both relatively large populations (e.g. Vu3 - V. uliginosum; Vvi4 - V. vitis-idaea) and rather small populations (e.g. Vm3 and Vm4 - V. myrtillus) and vice versa, i.e. reduced levels of genetic diversity were found in small (e.g. Vu4 - V. uliginosum; Vvi1 - V. vitis-idaea) and relatively large populations (e.g. Vm2 and Vm5 - V. myrtillus; Vu1 - V. uliginosum). Nonetheless, it is possible that the increase in sample sizes would most likely contribute towards the increase in levels of genetic diversity at least in those large populations which were represented by a relatively small number of individuals (e.g. Vu5 - V. uliginosum; Vvi2 - V. vitis-idaea). Interestingly, we observed that increased levels of genetic diversities in populations of all three Vaccinium species regardless of their sizes resulted mainly from the presence of immigrants in populations (e.g. individual M9 from Vm1 - V. myrtillus), admixed individuals (e.g. individuals M24 and M29 from Vm3, and individual M33 from Vm4 of V. myrtillus, which mutually share gene-pools of both currently well differentiated populations), and individuals representing inter-specific hybrids (e.g. individual M34 from Vm4 - V. myrtillus; U74 from Vu3, and all four individuals from Vu5 - V. uliginosum; individuals I103, I104 and I106 from Vvi4 - V. vitis-idaea). It is worth mentioning that interspecific hybridisation between V. myrtillus and V. vitis-idaea has been observed previously (Hokkanen et al. 2009), while hybridisation between V. uliginosum and V. vitis-idaea has not been reported to date. The latter hybridisation and potential production of a fertile offspring, however, would not be hampered by ploidy levels since both V. uliginosum from the Balkans (Eidesen et al. 2007) and V. vitis-idaea (cf. Garkava-Gustavsson et al. 2005) are diploid (2n = 24), and their flowering periods overlap (Janković 1972).
On the other hand, AMOVA analysis revealed that an increased proportion of clonal individuals in populations, which is expected in range-edge populations of clonal plant species (e.g. Beatty et al. 2008), was present in V. vitis-idaea only. However, upon exclusion of a single population of V. vitis-idaea from calculations (Vvi1, occupying an area of 40 m2 and comprising 11 clonal individuals out of 13 analysed individuals), the contribution of the clonal propagation to the genetic diversity and genetic structuring in V. vitis-idaea decreased as well (data not shown). It is worth mentioning that the large number of clonal individuals in this population was found because contrary to the sampling scheme applied to other populations, we sampled individuals from this population at every 5 m. In case that the same sampling strategy as that applied in other populations was used in this population as well, only two individuals would be sampled from Vvi1. Thus, it appears that despite the large number of clonal individuals detected in this population, the ratio between clonal and non-clonal individuals with regard to the area occupied by a population may be similar in this and in other populations. We observed also that a rather large and genetically diverse populations comprised clonal individuals (e.g. Vu3 in V. uliginosum), and that clonal individuals were lacking in smaller and less diverse populations (e.g. Vm1 in V. myrtillus). This would imply that the trends with regard to the representation of clonal individuals in populations of various sizes and levels of genetic diversities were lacking.
Due to the potentially high dispersal of both pollen and seeds in V. myrtillus, V. uliginosum and V. vitis-idaea (Ritchie 1955, 1956; Jacquemart 1996; Jacquemart and Thompson 1996; Albert et al. 2004), low genetic differentiation of their populations (ranging from c. 0.04% to c. 20%) and larger proportion of molecular variance assigned to within-population than to among-population variation have been reported to date in all three Vaccinium species (Persson and Gustavsson 2001; Albert et al. 2004, 2005; Garkava-Gustavsson et al. 2005; Eidesen et al. 2007; Balsdon et al. 2011). Interestingly, the highest percentage of variation assigned to among-population variation has been observed among V. uliginosum populations from south European group (39.8%, Eidesen et al. 2007), while we detected even higher values within the Balkans not only among V. uliginosum populations (57.62%) but also among populations of V. myrtillus (43.19%) and V. vitis-idaea (51.20%). Concordantly, average FST values in all three species were high as well (0.584, 0.430 and 0.520, respectively). Furthermore, all population pairwise FST values were high and significant at 95% level even among populations distant less than 5 km, as it was the case with populations Vu1 and Vu2 of V. uliginosum (FST of 0.601, P ≤ 0.05). Thus, the values of the effective gene flow between populations (Nm) were below 1.00 in all three species (0.740, 0.371 and 0.321, respectively). Although our estimates of gene flow have to be taken with caution because Nm values based on FST values reflect historical rather than contemporary gene flow (Bossart and Powell 1998; Sork et al. 1999), rather limited gene flow and connectivity of populations are common in fragmented landscapes (Young et al. 1996).
4.2 Relations of Vaccinium sp. populations within the Balkans
V. myrtillus may be characterized as the most homogeneous and the least differentiated and diverse species out of all three Vaccinium species from the Balkan region. Among five studied populations of this species, Vm1, Vm2 and Vm3 were genetically well differentiated but more closely related among themselves than to other populations, as inferred from all clustering analyses (UPGMA, Fig. 4a; Bayesian analysis at K = 3, Fig. 5a; and PCA analysis, Fig. 5b). However, UPGMA and Bayesian analyses were inconclusive in delineating genetically the most distinct population (Vm4 - based on UPGMA analysis; Vm5 - based on Bayesian analysis). Nonetheless, as evident from the MST reconstruction (Fig. 5b), these two populations were actually founded with genotypes from Vm3 (Vm4) and Vm2 (Vm5), and this would imply that the partial incongruence of clustering methods may be caused by their different methodologies. At present, populations Vm4 and Vm5 are genetically well-differentiated (FST = 0.502, P ≤ 0.05) but geographically rather close (70 km distant), while more closely related populations Vm1, Vm2 and Vm3 are distant c. 100 km on average (Table 3). Thus, it is not surprising that the IBD pattern was marginally significant in V. myrtillus (r2 = 0.130, P = 0.070). On the other hand, based on inferred genealogical relations among individuals from all populations of V. myrtillus, it is very likely that more ancient populations of this species are currently found within western central Balkans, while relatively recently founded populations are confined to the eastern central Balkans where, in addition, past hybridisation with V. vitis-idaea must have had occurred.
UPGMA analysis revealed that out of five currently well-differentiated populations of V. uliginosum, geographically closest populations Vu1 and Vu2 (distant less than 5 km) were also genetically closest, while Vu5 was delineated as genetically the most distinct population (Fig. 4a). Although the profound genetic distinctiveness of Vu5 was not that apparent in PCA analysis at the species level (Fig. 5d) nor in Bayesian analysis (Fig. 5c), the hybrid origin of all individuals from this population was detected in PCA analysis at the genus level (Fig. 4b). Taking into account these findings and inferred genealogical relations of individuals from distinct populations, it is possible that the western central Balkans is inhabited by more ancient populations of V. uliginosum, while within the eastern Balkans, where an ancient hybridisation with V. vitis-idaea took place, more recently founded populations of this species are currently present. Due to the current spatial distribution of eastward populations of V. uliginosum, i.e. relatively low geographic distances (35 to 70 km) between genetically well differentiated populations including the genetically most distinct population Vu5, it is not surprising that the IBD pattern was lacking in this species.
Out of all three studied Vaccinium species, V. vitis-idaea from the Balkans appears to be the least coherent and the most heterogeneous species, as inferred from UPGMA and PCA analyses (Fig. 4a and 2b, respectively). It also displays the highest level of genetic diversity at the species level (I = 0.291) and high genetic differentiation (FST = 0.520). Bayesian analysis revealed two gene pools in this species, one comprised of individuals from individuals from the three populations (Vvi1, Vvi2 and Vvi3), and the other comprised of individuals from a single population Vvi4 (Fig. 5f). The underlying reason for a marked genetic distinctiveness of Vvi4 was inferred from PCA analysis at the genus level (Fig. 4b), which revealed that individuals from this populations most likely harbour portions of gene-pools of both V. myrtillus and V. uliginosum. Given the current geographical positioning of this population, past inter-specific hybridisations most likely have had occurred in south-eastern central Balkans. In addition, this region is most likely inhabited by more anciently founded populations, while those more recently founded are currently confined to the western central Balkans. The IBD pattern observed in V. vitis-idaea (r2 = 0.670, P = 0.040) further supports past recolonization of the western central Balkans by this species.
4.3 Conservation prospects and sustainable usage
Recently, Eidesen et al. (2007) demonstrated that V. uliginosum, which displays a considerable morphological and ploidy level variation (Hultén 1970; Young 1970; Vander Kloet 1988; Jacquemart et al. 1996; Alsos 2003), is characterized by a rather complex genetic pattern throughout its range distribution. The authors reported the prevalence of diploid V. uliginosum individuals in southern Europe indicating genetic distinctiveness of this part of the species range, and observed high haplotype diversity and private cpDNA haplotypes and low nrDNA diversity within this region. Nonetheless, based on nrDNA data, they were able to depict a sub-structure within southern Europe, with three well-differentiated sub-groups confined to western southern Europe, eastern southern Europe (the Balkans) and Caucasus. They concluded that in southern Europe, populations of V. uliginosum probably survived the last glaciation in several smaller peripheral refugia in these three regions, and have not expanded northwards from these areas. These findings are in accordance also with the postulated low migration in trees below 45 ° N latitude (Petit et al. 2005), and support the view that populations not only of V. uliginosum but also of V. myrtillus and V. vitis-idaea from the Balkans may represent an important and a distinct portion of overall genetic diversity of these species that requires conservation.
Given the rather low levels of genetic diversity and exceptionally high genetic differentiation of all extant populations of V. myrtillus, V. uliginosum and V. vitis-idaea within the Balkans, the most suitable conservation measure for these range-edge populations of all three species would be in situ conservation along with the monitoring of exploitation which must be limited and sustainable. Such a measure, however, cannot prevent the loss of habitats by the advancing Juniperus communis subsp. alpina currently affecting populations of all three Vaccinium species especially at Mts Kopaonik and Stara planina (Bjedov 2012). The most threatened populations at present are Vvi1 of V. vitis-idaea, currently occupying an area of 40 m2 only, and Vu4 of V. uliginosum, whose size decreased severely over the past few years not only due to the suppression by J. communis subsp. alpina but also due to the establishment of the ski resort. For these populations, immediate ex situ conservation is required along with the prevention of further exploitation through implementation of suitable legislation.
5 Conclusions
According to expectations on genetic patterns in peripheral populations of plant species (Ellstrand and Elam 1993; Scalfi et al. 2009 and references therein), decreased levels of genetic diversities and increased genetic differentiation among populations was observed in range-edge populations of V. myrtillus, V. uliginosum and V. vitis-idaea from the central Balkans. However, the expected prevalence of clonal individuals in populations of all three species was lacking. Assuming that populations of all three Vaccinium species from the Balkans represent a distinct portion of the overall gene pools of these species, their sustainable usage and conservation are required. On the other hand, a particularly important outcome of our work is detection of inter-specific hybrids in populations Vu3 and Vu5 of V. uliginosum and Vvi4 of V. vitis-idaea. Although the economic value of these hybrids is yet to be assessed as well as their potential commercial exploitation, they may represent a promising sources for future breeding efforts and food and/or pharmaceutical industry. Therefore, further studies in various research areas in all three Vaccinium species from the Balkans are required. However, those focusing on variability at the molecular level should take into account the effects of possible inter-specific hybridisation among all three species which most likely took place within this region in the past as demonstrated in the present study.
Acknowledgement
This work was financially supported by the Ministry of Education, Science and Technological Development of the Republic of Serbia, Research Grants III 43007 and OI 173030.
References
Åkerström A. (2010). Factors affecting the anthocyanidin concentration in fruits of Vaccinium myrtillus L.. PhD Thesis, Swedish University of Agricultural Sciences, Faculty of Natural Resources and Agricultural Sciences, Department of Agricultural Research for Northern Sweden, SLU, Umeå, Sweden.
Albert T., Raspé O., Jacquemart A.L. (2003). Clonal structure in Vaccinium myrtillus L. revealed by RAPD and AFLP markers. International Journal of Plant Sciences 164: 649–655.
Albert T., Raspé O., Jacquemart A.L. (2004). Clonal diversity and genetic structure in Vaccinium myrtillus populations from different habitats. Belgian Journal of Botany 137: 155–162.
Albert T., Raspé O., Jacquemart A.L. (2005). Diversity and spatial structure of clones in Vaccinium uliginosum populations. Canadian Journal of Botany 83: 211–218. http://dx.doi.org/10.1139/B04-164.
Alsos I.G. (2003). Conservation biology of the most thermophilous plant species in the Arctic. Genetic variation, recruitment and phylogeography in a changing climate. Unpublished Dr. Scient., University of Tromsø.
Alsos I.G., Engelskjøn T., Brochmann C. (2002). Conservation genetics and population history of Betula nana, Vaccinium uliginosum, and Campanula rotundifolia in the Arctic Archipelago of Svalbard. Arctic, Antarctic and Alpine Research 34: 408–418.
Alsos I.G., Engelskjøn T., Gielly L., Taberlet P., Brochmann C. (2005). Impact of ice ages on circumpolar molecular diversity: insights from an ecological key species. Molecular Ecology 14: 2739–2753. http://dx.doi.org/10.1111/j.1365-294X.2005.02621.x.
Balsdon J.L., Smith T.W., Lundholm J.T. (2011). Phenotypic and genotypic differentiation of Vaccinium vitis-idaea between coastal barrens and forests in Nova Scotia, Canada. Botany 89: 147–155. http://dx.doi.org/10.1139/B11-003.
Bartish I.V., Jeppson N., Nybom H. (1999). Population genetic structure in the dioecious pioneer plant species Hippophae rhamnoides investigated by random amplified polymorphic DNA (RAPD) markers. Molecular Ecology 8: 791–802. http://dx.doi.org/10.1046/j.1365-294X.1999.00631.x.
Beatty G.E., McEvoy P.M., Sweeney O., Provan J. (2008). Range-edge effects promote clonal growth in peripheral populations of the one-sided wintergreen Orthilia secunda. Diversity and Distributions 14: 546–555. http://dx.doi.org/10.1111/j.1472-4642.2008.00469.x.
Bjedov I. (2012). Taxonomic and ecological investigation of Vaccinium L. genus in Serbia. PhD Thesis, University of Belgrade, Faculty of forestry, Belgrade, Serbia.
Bossart J.L., Prowell D.P. (1998). Genetic estimates of population structure and gene flow: limitations, lessons and new directions. Trends in Ecology and Evolution 13: 202–206. http://dx.doi.org/10.1016/S0169-5347(97)01284-6.
Bown D. (1995). Encyclopedia of Herbs and their Uses. Dorling Kindersley, London.
Charpentier A. (2002). Consequences of clonal growth for plant mating. Evolutionary Ecology 15: 521–530.
Chevallier A. (1996). The Encyclopedia of Medicinal Plants. Dorling Kindersley Publishing, Australia.
Clark-Tapia R., Alfonso-Corrado C., Mandujano M.C., Molina-Freaner F. (2006). Reproductive consequences of clonal growth in Stenocereus eruca, a rare clonal cactus of the Sonoran desert. Evolutionary Ecology 20: 131–142. http://dx.doi.org/10.1007/s10682-005-5379-x.
Debnath S.C. (2007). An assessment of the genetic diversity within a collection of wild cranberry (Vaccinium macrocarpon Ait.) clones with RAPD-PCR. Genet Resources and Crop Evolution 54: 509–517. http://dx.doi.org/10.1007/s10722-006-0007-3.
Doyle J.J., Doyle J.L. (1990). Isolation of plant DNA from fresh tissue. Focus 12: 13–15.
Eckert C.G., Samis K.E., Lougheed S.C. (2008). Genetic variation across species’ geographical ranges: the central-marginal hypothesis and beyond. Molecular Ecology 17: 1170–1188. http://dx.doi.org/10.1111/j.1365-294X.2007.03659.x.
Eidesen P.B., Alsos I.G., Popp M., Stensrud Ø., Suda V., Brochmann C. (2007). Nuclear vs. plastid data: complex Pleistocene history of a circumpolar key species. Molecular Ecology 16: 3902–3925. http://dx.doi.org/10.1111/j.1365-294X.2007.03425.x.
Ellstrand N.C., Roose L. (1987). Patterns of genotypic diversity in clonal plant species. American Journal of Botany 74: 123–131. http://dx.doi.org/10.2307/2444338.
Ellstrand N.C., Elam D.R. (1993). Population genetic consequences of small population size: implications for plant conservation. Annual Review of Ecology and Systematics 24: 217–242. http://dx.doi.org/10.1146/annurev.es.24.110193.001245.
Eriksson O. (1989). Seedling dynamics and life histories in clonal plants. Oikos 55: 231–238.
Eriksson O. (1993). Dynamics of genets in clonal plants. Trends in Ecology and Evolution 8: 313–316. http://dx.doi.org/10.1016/0169-5347(93)90237-J.
Eriksson O., Fröborg H. (1996). “Windows of opportunity” for recruitment in long-lived clonal plants: experimental studies of seedling establishment in Vaccinium shrubs. Canadian Journal of Botany 74: 1369–1374. http://dx.doi.org/10.1139/b96-166.
Evanno G., Regnaut S., Goudet J. (2005). Detecting the number of clusters of individuals using the software Structure: a simulation study. Molecular Ecology 14: 2611–2620. http://dx.doi.org/10.1111/j.1365-294X.2005.02553.x.
Excoffier L. (2010). Arlequin ver 3.5 CMPG. Institute of Ecology and Evolution, University of Bern.
Excoffier L., Smouse P.E., Quattro J.M. (1992). Analysis of molecular variance inferred from metric distances among DNA haplotypes: application to human mitochondrial DNA restriction data. Genetics 131: 479–491.
Frankham R., Ballou J., Briscoe D. (2002). Introduction to conservation genetics. Cambridge University Press, New York.
Garkava-Gustavsson K., Persson H.A., Nybom H., Rumpunen K., Gustavsson B.A., Bartish I.V. (2005). RAPD-based analysis of genetic diversity and selection of lingonberry (Vaccinium vitis-idaea L.) material for ex situ conservation. Genet Resources and Crop Evolution 52: 723–735. http://dx.doi.org/10.1007/s10722-003-6123-4.
Gustavsson B. (2001). Genetic variation in horticularally important traits of fifteen wild lingonberry Vaccinium vitis-idaea L. populations. Euphytica 120: 173–182. http://dx.doi.org/10.1023/A:1017550609218.
Hammer O., Harper D.A.T., Ryan P.D. (2009). PAST – palaeontological statistics, ver. 1.89. http://folk.uio.no/ohammer/past. [Cited 7 November 2013].
Hampe A., Petit R.J. (2005). Conserving biodiversity under climate change: the rear edge matters. Ecology Letters 8: 461–467. http://dx.doi.org/10.1111/j.1461-0248.2005.00739.x.
Hamrick J.L., Godt J.W. (1989). Allozyme diversity in plant species. In: Brown A.H.D., Clegg M.T., Kahler A.L., Weir B.S. (eds.). Plant population genetics, breeding and genetic resources. Sinauer, Sunderland, Massachusetts, USA. p. 43–63.
Hirai M., Yoshimura S., Ohsako T., Kubo N. (2010). Genetic diversity and phylogenetic relationships of the endangered species Vaccinium sieboldii and Vaccinium ciliatum (Ericaceae). Plant Systematics and Evolution 287: 75–84. http://dx.doi.org/10.1007/s00606-010-0291-4.
Hokkanen J., Mattila S., Jaakola L., Pirttila A.M., Tolonen A. (2009). Identification of phenolic compounds from lingonberry (Vaccinium vitis-idaea L.), bilberry (Vaccinium myrtillus L.) and hybrid bilberry (Vaccinium intermedium Ruthe L.) leaves. Journal of Agricultural and Food Chemistry 57: 9437–9447. http://dx.doi.org/10.1021/jf9022542.
Honnay O., Bossuyt B. (2005). Prolonged clonal growth: escape route or route to extinction? Oikos 108: 427–432. http://dx.doi.org/10.1111/j.0030-1299.2005.13569.x.
Honnay O., Jacquemyn H. (2007). Susceptibility of common and rare plant species to the genetic consequences of habitat fragmentation. Conservation Biology 21: 823–831. http://dx.doi.org/10.1111/j.1523-1739.2006.00646.x.
Hultén E. (1970). The circumpolar plants II, Dicotyledons. Kungliga Svenska Vetenskaps-Akademiens Handlingar 13: 1–463.
Jacquemart A.L. (1996). Vaccinium uliginosum L. Journal of Ecology 84: 771–785.
Jacquemart A.L., Mahy G., Raspé O., De Sloover J.R. (1994). An isozyme study in bilberry (Vaccinium myrtillus). Mating system and genetic structure. Belgian Journal of Botany 127: 105–114.
Jacquemart A.L., Thompson J.D. (1996). Floral and pollination biology of three sympatric Vaccinium (Ericaceae) species in the Upper Ardenne, Belgium. Canadian Journal of Botany 74: 210–221. http://dx.doi.org/10.1139/b96-025.
Janković M. (1972). Fam. Vacciniaceae Lindl. In: Josifović M. (ed.). Flora SR Srbije III. Srpska akademija nauka i umetnosti, Beograd. p. 465–469.
Kangas K. (2001). Wild berry utilisation and markets in Finland. PhD thesis, Faculty of Forestry, University of Joensuu at Joensuu, Finland.
Kardell L. (1980). Occurrence and production of bilberry, lingonberry and raspberry in Sweden’s forests. Forest Ecology and Management 2: 285–298. http://dx.doi.org/10.1016/0378-1127(79)90055-0.
Katsube N., Iwashita K., Tsushida T., Yamaki K., Kobori M. (2003). Induction of apoptosis in cancer cells by bilberry (Vaccinium myrtillus) and the anthocyanins. Journal of Agricultural Food and Chemistry 51: 68–75. http://dx.doi.org/10.1021/jf025781x.
Kreher S.A., Fore S.A., Collons B.S. (2000). Genetic variation within and among patches of the clonal species, Vaccinium stamineum L. Molecular Biology 9: 1247–1252. http://dx.doi.org/10.1046/j.1365-294x.2000.01002.x.
Leimu R., Mutikainen P., Koricheva J., Fisher M. (2006). How general are positive relationships between plant population size, fitness and genetic variation? Journal of Ecology 94: 942–952. http://dx.doi.org/10.1111/j.1365-2745.2006.01150.x.
Lenormand T. (2002). Gene flow and the limits to natural selection. Trends in Ecology and Evolution 17: 183–189. http://dx.doi.org/10.1016/S0169-5347(02)02497-7.
Lesica P., Allendorf F.W. (1995). When peripheral populations valuable for conservation? Conservation Biology 9: 753–760. http://dx.doi.org/10.1046/j.1523-1739.1995.09040753.x.
Lewontin R.C. (1972). The apportionment of human diversity. Evolutionary Biology 6: 381–394.
Lynch M., Milligan B.G. (1994). Analysis of population genetic structure with RAPD markers. Molecular Ecology 3: 91–99.
Mantel N. (1967) The detection of disease clustering and a generalized regression approach. Cancer Research 27: 209–220.
Martinussen I., Nestby R., Nes A. (2008). Potential of the European blueberry (Vaccinium myrtillus) for cultivation and industrial exploitation in Norway. Acta Horticulturae 810: 211–5.
Martinussen I., Rohloff J., Uleberg E., Junttila O., Hohtola A., Jaakola L., Häggman H. (2009). Climatic effects on the production and quality of bilberries (Vaccinium myrtillus). Agronomijas Vēstis 12: 71–74.
Nagy S., Poczai P., Cernák I., Gorji A.M., Hegedűs G., Taller J. (2012). PICcalc: an online program to calculate polymorphic information content for molecular genetic studies. Biochemical Genetics 50: 670–672. http://dx.doi.org/10.1007/s10528-012-9509-1.
Nei M. (1973). Analysis of gene diversity in subdivided populations. Proceedings of the National Academy of Sciences (USA) 70: 3321–3323.
Nuortila C., Tuomi J., Laine K. (2002). Inter-parent distance affects reproductive success in two clonal dwarf shrubs, Vaccinium myrtillus and Vaccinium vitis-idaea (Ericaceae). Canadian Journal of Botany 80: 875–884. http://dx.doi.org/10.1139/b02-079.
Nybom H., Bartish I.V. (2000). Effects of life history traits and sampling strategies on genetic diversity estimates obtained with RAPD markers in plants. Perspectives in Plant Ecology 300: 93–114. http://dx.doi.org/10.1078/1433-8319-00006.
Percival D.C., Sanderson K. (2004). Main and interactive effects of vegetative - year applications of nitrogen, phosphorus and potassium fertilizers on the wild Blueberry. Small Fruits Review 3: 105–121. http://dx.doi.org/10.1300/J301v03n01_11.
Percival D.C., Janem D.E., Stevens D.E., Sanderson K. (2003). Impact of multiple fertilizer applications on plant growth, development, and yield of wild lowbush blueberry (Vaccinium angustifolia Aiton). Acta Horticulturae 626: 415–421.
Persson H.A., Gustavsson B.A. (2001). The extent of clonality and genetic diversity in lingonberry (Vaccinium vitis-idaea L.) revealed by RAPDs and leaf-shape analysis. Molecular Ecology 10: 1385–1397. http://dx.doi.org/10.1046/j.1365-294X.2001.01280.x.
Petit R.J., Hampe A., Cheddadi R. (2005). Climate changes and tree phylogeography in the Mediterranean. Taxon 54: 877–885.
Pornon A., Escaravage N., Thomas P., Taberlet P. (2000). Dynamics of genotypic structure in clonal Rhododendron ferrugineum (Ericaceae) populations. Molecular Ecology 9: 1099–1111. http://dx.doi.org/10.1046/j.1365-294x.2000.00976.x.
Popova T.N. (1972) Vaccinium L. In: Tutin TG et al. (eds.). Flora Europaea, Vol. 3. Cambridge University Press, Cambridge.
Pritchard J.K., Stephens M., Donnelly P. (2000). Inference of population structure using multilocus genotype data. Genetics 155: 945–959.
Raatikainen M., Vänninen I. (1988). The effects of the 1984–1985 cold winter on the bilberry and lingonberry yield in Finland. Acta Botanica Fennica 136: 43–47.
Ritchie J.C. (1955). Vaccinium vitis-idaea. Journal of Ecology 43: 701–708.
Ritchie J.C. (1956). Vaccinium myrtillus L. Journal of Ecology 44: 291–299.
Saastamoinen O., Kangas K., Aho H. (2000). The picking of wild berries in Finland in 1997 and 1998. Scandinavian Journal of Forest Research 15: 645–650. http://dx.doi.org/10.1080/02827580050216897.
Salo K. (1995). Non-timber forest products and their utilization. In: Hytönen M (ed.). Multiple-use forestry in the Nordic countries. The Finnish Forest Research Institute. p. 117–155.
Sarić M. (ed.) (1989). Lekovite biljke SR Srbije. Srpska akademija nauka i umetnosti, Beograd. [Aromatic plants in the SR Serbia]. Serbian Academy of Sciences and Arts, Belgrade. [In Serbian].
Scalfi M., Piotti A., Rossi M., Piovani P. (2009). Genetic variability of Italian southern Scots pine (Pinus sylvestris L.) populations: the rear edge of the range. European Journal of Forest Research 128: 377–386. http://dx.doi.org/10.1007/s10342-009-0273-7.
Sork V.L., Nason J., Campbell D.R., Fernandez J.F. (1999). Landscape approaches to historical and contemporary gene flow in plants. Trends in Ecology and Evolution 14: 219–224. http://dx.doi.org/10.1016/S0169-5347(98)01585-7.
Skorić M., Šiler B., Banjanac T., Živković-Nestorović J., Dmitrović S., Mišić D., Grubišić D. (2012). The reproducibility of RAPD profiles: effects of PCR components on RAPD analysis of four Centaurium species. Archives of Biological Sciences 64: 191–199. http://dx.doi.org/10.2298/ABS1201191S.
Stewart C.N.Jr., Excoffier L. (1996). Assessing population genetic structure and variability with RAPD data: application to Vaccinium macrocarpon (American Cranberry). Journal of Evolutionary Biology 9: 153–171. http://dx.doi.org/10.1046/j.1420-9101.1996.9020153.x.
Tomićević J., Bjedov I., Obratov-Petković D., Milovanović M. (2011). Exploring the park–people relation: collection of Vaccinium myrtillus L. by local people from Kopaonik National Park in Serbia. Environmental Management 48: 835–846. http://dx.doi.org/10.1007/s00267-011-9725-1.
Vander Kloet S.P. (1988). The genus Vaccinium in North America. Ottawa. Canadian Government Publishing Centre.
Yakimowski S.B., Eckert C. (2007). Threatened peripheral populations in context: geographical variation in population frequency and size and sexual reproduction in a clonal woody shrub. Conservation Biology 21: 811–822. http://dx.doi.org/10.1111/j.1523-1739.2007.00684.x.
Yeh F.C., Yang R.C., Boyle T. (1997). POPGENE, version 1.21: software microsoft window-based freeware for population genetic analysis. University of Alberta, Edmonton.
Young S.B. (1970). On the taxonomy and distribution of Vaccinium uliginosum. Rhodora 72: 439–459.
Young A., Boyle T., Brown T. (1996). The population genetic consequences of habitat fragmentation for plants. Trends in Ecology and Evolution 11: 413–418. http://dx.doi.org/10.1016/0169-5347(96)10045-8.
Zhao C., Giusti M.M., Malik M., Moyer M.P., Magnuson B.A. (2004). Effects of commercial anthocyanin-rich extracts on colonic cancer and nontumorigenic colonic cell growth. Journal of Agricultural and Food Chemistry 52: 6122–6128. http://dx.doi.org/10.1021/jf049517a.
Žukauskienė J., Paulauskas A., Èesonienė L., Daubaras R. (2009). Genetic structure of isolated Vaccinium oxycoccus populations in Lithuania. Proceedings of the Latvian Academy of Sciences, Section B. Natural, Exact, and Applied Sciences 63: 33–36. http://dx.doi.org/10.2478/v10046-009-0018-5.
Total of 84 references

