Both nursery and field performance determine suitable nitrogen supply of nursery-grown, exponentially fertilized Chinese pine
Wang J., Li G., Pinto J. R., Liu J., Shi W., Liu Y. (2015). Both nursery and field performance determine suitable nitrogen supply of nursery-grown, exponentially fertilized Chinese pine. Silva Fennica vol. 49 no. 3 article id 1295. https://doi.org/10.14214/sf.1295
Highlights
- Increasing exponential fertilization rates in the nursery increased seedling biomass, N content, and N concentration for Chinese pine seedlings
- Second year seedling survival illustrated a curvilinear response to seedling fertilization rates rather than a linear one
- Considering both nursery responses to fertilization and field performance after two years yielded a recommended nitrogen supply rate of 80 mg N seedling–1.
Abstract
Optimum fertilization levels are often determined solely from nursery growth responses. However, it is the performance of the seedling on the outplanting site that is the most important. For Pinus species seedlings, little information is known about the field performance of plants cultured with different nutrient rates, especially with exponential fertilization. In this study, Chinese pine (Pinus tabulaeformis Carr.) seedlings grown in 187 ml containers were fertilized exponentially in 6 treatments ranging from 10 to 120 mg N seedling–1 for 25 weeks before outplanting. Dry mass and N content were measured at planting. Survival and field growth were monitored for two growing seasons. In the nursery, our data showed no difference in dry mass among the 40, 80, 100, and 120 mg N seedling–1 fertilizer treatments; collectively, these treatments were significantly greater than at 10 and 20 mg N seedling–1 treatments. Seedling N content was greatest for the 100 and 120 mg N seedling–1 rates. These data suggested that nursery optimum N fertilization rate was no less than 100 mg N seedling–1. Outplanting height and root-collar diameter growth characteristics were not significantly different after two years, whereas maximum mean survival was best for seedlings nursery-fertilized at 80 mg N seedling–1. In consideration of both nursery and field performance metrics, our data suggest that exponentially fertilizing Chinese pine seedlings at 80 mg N seedling–1 maximizes both nursery biomass accumulation and outplanting survival.
Keywords
water stress;
exponential fertilization;
nursery response;
field performance
- Wang, Key Laboratory for Silviculture and Conservation, Ministry of Education, Beijing Forestry University, 35 East Qinghua Road, Haidian District, Beijing 100083, China E-mail wjx198979@163.com
-
Li,
Key Laboratory for Silviculture and Conservation, Ministry of Education, Beijing Forestry University, 35 East Qinghua Road, Haidian District, Beijing 100083, China
E-mail
glli226@163.com

- Pinto, US Department of Agriculture, Forest Service, Rocky Mountain Research Station, 1221 South Main Street, Moscow, ID 83843, USA E-mail jpinto@fs.fed.us
- Liu, Key Laboratory for Silviculture and Conservation, Ministry of Education, Beijing Forestry University, 35 East Qinghua Road, Haidian District, Beijing 100083, China E-mail 1044902638@qq.com
- Shi, Key Laboratory for Silviculture and Conservation, Ministry of Education, Beijing Forestry University, 35 East Qinghua Road, Haidian District, Beijing 100083, China E-mail shiwenhui2008@163.com
- Liu, Key Laboratory for Silviculture and Conservation, Ministry of Education, Beijing Forestry University, 35 East Qinghua Road, Haidian District, Beijing 100083, China E-mail lyong@bjfu.edu.cn
Received 19 December 2014 Accepted 27 April 2015 Published 21 May 2015
Views 158913
Available at https://doi.org/10.14214/sf.1295 | Download PDF

1 Introduction
Due to poor root-soil contact, newly planted seedlings have limited access to soil nutrients and are mainly dependent on their internal nutrient resources (van den Driessche 1985). Providing proper mineral nutrition to seedlings in the nursery is, therefore, essential for nutrient storage and subsequent field performance (Villar-Salvador et al. 2012; Oliet et al. 2013). One method of increasing nutrient reserves in the nursery is through exponential fertilization. Compared to conventional fertilization, exponentially increasing fertilizer over the course of the growing season has proven to be advantageous in building seedling N reserves and improving subsequent field performance (Ingestad 1979; Malik and Timmer 1996; McAlister and Timmer 1998; Dumroese et al. 2005; Close et al. 2005; Way et al. 2007). This technique has been demonstrated with a variety of tree species including: Pinus spp. (Timmer and Armstrong 1987; Miller and Timmer 1994; Dumroese et al. 2005), Picea spp. (Quoreshi and Timmer 2000; Salifu and Timmer 2003a), Quercus spp. (Salifu and Jacobs 2006; Birge et al. 2006; Salifu et al. 2009), Larix spp. (Qu et al. 2003), and Tsuga heterophylla (Raf.) Sarg. (Hawkins et al. 2005).
Exponential fertilization is not only a technique used to provide adequate nutrients for nursery seedlings but has also been used to promote nutrient loading. Timmer (1996) proposed a conceptual model that relates plant nutrition, plant growth, and nutrient supply to rationalize exponential fertilization regimes and nutrient loading in nursery culture. Nutrient loading induces luxury nutrient uptake in excess of growth demand and is more compatible with exponential than conventional fertilization (Salifu and Jacobs 2006). Optimum exponential fertilization maximizes both seedling dry mass and tissue N content (Ingestad 1979; McAlister and Timmer 1998; Salifu 2003; Salifu and Timmer 2003a; Birge et al. 2006). Determining optimum nutrient supplies requires an assay of plant growth and nutrition responses to a broad spectrum of nutrient supply ranging from nutrient deficiency to toxicity (Ingestad 1979; Ingestad and Kähr 1985); this optimum is generally both species and stocktype specific. Thus far, optimum exponential fertilization rates have been determined for Picea mariana (Mill.) BSP container seedlings (64 mg N seedling–1; Salifu and Timmer 2003b), Quercus rubra L. container ƒs (100 mg N seedling–1; Salifu and Jacobs 2006), and Q. rubra and Quercus alba L. bareroot seedlings (1680 mg N seedling–1; Birge et al. 2006). While Pinus species are distributed worldwide, surprisingly little work has been published on optimum nutrient supplies (Timmer and Armstrong 1987; Miller and Timmer 1994; Hawkins et al. 2005; Dumroese et al. 2005). Furthermore, field data to support the optimum nursery fertilization rate is also scarce.
Seedling field performance compared with nursery responses to exponential fertilization can be varied depending on the environment they are tested in. Under controlled condition in the greenhouse, higher internal nutrient reserves acquired from exponential fertilization has been shown to stimulate nitrogen (N) remobilization and facilitate growth performance (Oliet et al. 2009). However, studies conducted on outplanting sites using Q. rubra, Q. alba (Salifu et al. 2009), and T. heterophylla (Hawkins et al. 2005) have shown that there were no differences in growth among seedlings from the greenhouse nutrient supply treatments. This may imply that seedling nutrient requirements should not be based on nursery response alone but that specific conditions of a given site should be considered. To our best knowledge, little information is available on the field performance of Pinus species subjected to exponential fertilization.
Chinese pine (Pinus tabulaeformis Carr.) is planted widely in northern China because of its resistance to drought and tolerance of low fertility soils (Xu 1993). Along with planting methods (Shi et al. 2011; Zhang and Chen 2013), intensive nursery techniques for promoting seedling quality have been well documented including: choice of growing media (Lu et al. 2000); inoculating ectomycorrhizal fungi (Yao and Yan 2005; Yu et al. 2008); incorporating fertilizers into growing media prior to sowing (Huang et al. 2004); and use of fall fertilization by top-dressing (Zou et al. 2012). Despite the application of these practices, few Pinus species studies have connected two of the most important steps in forest establishment and regeneration, namely, growing quality seedlings and testing subsequent field performance. In this study, Chinese pine container seedlings were grown in the nursery, exponentially fertilized across a broad range of rates, and subsequently outplanted for two growing seasons. Our objective was to determine the rational nursery N supply for Chinese pine comprehensively considering both nursery and field performance. Specifically, we wanted to: (1) characterize dry mass, N storage and optimum N rate in response to exponential fertilization nursery treatments; (2) evaluate outplanting survival and growth as a result of nursery N treatment.
2 Material and methods
2.1 Nursery experiment
On 21 February 2010, Chinese pine seeds from a local seed orchard (National Seed Orchard for Chinese Pine, Qigou Forest Farm, 41°00´N, 118°27´E, 526 m a. s. l.) were sown into plastic containers (8 cm diameter × 8 cm deep, 187 ml capacity, Huifeng Plastic Co., Hebei, China) filled with a 3:1 (v:v) peat (Pindstrup Seeding, pH 6.0, Screening 0–6 mm): perlite (5 mm diameter, Xinyang Jinhualan Mining Co., Henan, China) mixture. Seedlings were grown in a greenhouse at the Chinese Academy of Forestry Sciences in Beijing (40°40´N, 116°14´E). Thirty containers were randomly assigned to a tray (53 cm long × 44.5 cm wide), providing a density of 127 seedlings m–2. Fertilization treatments began one week after sowing and continued for 25 weeks (from 1 March to 22 August). N treatments were 10, 20, 40, 80, 100 and 120 mg N seedling–1; supply rates were applied following the exponential function (Ingastad and Lund 1986; Timmer and Armstrong 1987; McAlister and Timmer 1998):
![]()
where NT (10, 20, 40, 80, 100, and 120 mg) was the desired amount of N to be added weekly over the number of fertilizer applications, t (in this case, 25). Ns is the initial N content in each seed, and r (8.0, 10.5, 13.1, 15.8, 16.7, and 17.4%) was the relative addition rate required to increase Ns to final N content (NT + NS). Using the composite sample method of Salifu and Jabobs (2006), Ns was determined at the time of sowing using four replicates, each comprising 15 seeds that were oven-dried (48 h at 65 °C), measured for dry mass, ground and wet-digested in a block digester using the KMnO4-Fe-H2SO4 method modified to recover NO3 (Bremner and Mulvaney 1982). Subsequently, N concentration was measured by a standard Kjeldahl digestion with a distillation unit (UDK-152, Velp Scientifica, USA). Ns was calculated to be 1.58 mg per seed.
The quantity of N to apply on a specific week (Nt) was calculated with the following equation (Ingastad and Lund 1986; Timmer and Armstrong 1987):
where Nt–1 is the cumulative amount of N added up to and including the previous application.
Each exponential fertilization treatment was applied to 4 trays (120 seedlings per treatment), resulting in a total of 24 trays. N was supplied as urea (Xilong Chemical Co., China), an N source commonly used in Chinese nurseries. Elemental P and K were applied as KH2PO4 (Guangdong Guanghua Sci-Tech Co., Ltd. China); applications were weekly and were evenly split for a total of 26.2 mg P and 33.0 mg K. The desired amounts of N in addition to P and K were dissolved in water so that 20 ml of solution, applied by hand to each seedling, delivered the target amount of nutrients. No micronutrients were applied during the experiment. Foliage was rinsed after each application to avoid foliar fertilizer burn. Seedlings were watered to field capacity, approximately 2 times each week (State Forestry Administration 2013). Temperature was measured with a JL-18 Series thermometer (Huayan Instrument and Equipment Co., Shanghai, China) at 15-min intervals throughout nursery culture. From sowing (21 February) to the end of the nursery fertilization treatments (22 August), ambient temperature averaged 25:18 °C (day:night), and day length in the greenhouse averaged 13 h 28 min. Starting September 13, irrigation was reduced to initiate hardening, an interval of approximately 4 days. On 29 November, seedlings were removed from the greenhouse and stored outdoors under snow cover during winter. From 23 August to 29 November, greenhouse temperatures averaged 19:14 °C (day:night), and day length averaged 11 h 42 min. Trays were completely randomized on raised benches and their position rotated every 2 weeks to minimize edge effect.
2.2 Outplanting trial
On 1 May 2011, seedlings from the 6 N treatments were transported and outplanted. The site was previously used for agriculture and was located at the Beijing Forestry University Northern Experimental Base at Pingquan, Hebei province (41°13´N, 118°40´E). The site had a < 2% slope and averaged 765 m in elevation. Soil depth varied between 45 and 60 cm. The surface soil (0–20 cm) was 73% sand, 11% silt, and 16% clay (a sandy clay loam) with a pH of 6.2 and soil organic carbon of 0.7%. Average total N, available P, and available K were 628.7, 139.5, and 113.5 mg kg–1, respectively. The soil sample was measured and the soil was considered to have moderate fertility according to the macronutrient classified criteria (Bao 2000). The area is a temperate continental monsoon climate and characterized by dry winter and spring seasons. Based on the weather data between 1981 and 2000, mean annual air temperature was 7.7 °C and an average annual precipitation was 517 mm. During the experiments, precipitation and temperature data were recorded with an on-site weather station. Annual precipitation was 486 mm in 2011 and 626 mm in 2012 and mean annual air temperature was 7.4 and 6.8 °C, respectively (Fig. 1).
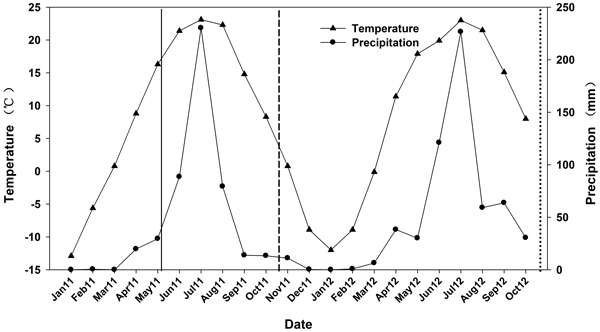
Fig. 1. Mean monthly temperature and precipitation data from January 2011 to October 2012. Time of outplanting is represented by a solid line. First outplanting performance measurement is represented by a short dash line. Second outplanting performance measurement is represented by a dotted line.
The seedlings were outplanted in a randomized complete block design replicated in 4 blocks. Each block measured 7.5 × 11 m and was separated from adjacent blocks by 1 m buffers. Twelve seedlings for each treatment were planted in single parallel rows within each block, resulting in a total of 288 seedlings planted. Seedlings were planted in 0.40 × 0.40 × 0.40 m manually dug planting holes with 1 × 1.5 m spacing. Each seedling was placed in a hole that was refilled with the excavated soil by hand. In early September 2010, the site was ploughed to reduce competing vegetation. Weeds were removed by hand during the growing seasons.
2.3 Plant sampling, chemical, and statistical analyses
Seedlings were sampled prior to planting to evaluate N storage and growth response to nursery fertilization. Eight seedlings were sampled randomly from each tray, resulting in a total of 32 seedlings per nursery fertilization treatment. Seedlings were washed gently free of growing medium and were separated into foliage, stems, and roots. Each plant tissue type was oven-dried at 65 °C for 48 h to determine dry mass. N values were determined from a composite sample of each treatment tray combination.
Outplanting survival percentages were calculated as the number of saplings of the original 12 remaining alive for each nursery-treatment block at the time of each annual measurement. Height (root-collar to the tip of the terminal bud) and root-collar diameter (hereafter RCD) were measured immediately after planting (T1), and on 28 October of the first (T2) and second years (T3; when growth ceases on this site). Net increment from T1 to T2 (or from T2 to T3) for height or RCD was calculated with the following equation.
where i = 2, 3.
Statistical analyses were performed by using the SPSS 10.6 statistical package (Chicago, Illinois, USA). One-way ANOVA was used to evaluate the effect of nursery N treatments on morphological and nutritional attributes at the end of the nursery phase (completely random design) and field performance after planting (randomized complete block design). The explore function of SPSS was used to examine data for normality and homogeneity prior to analyses. Variables N concentration (%) and survival were arcsine transformed in order to fulfill normality and homogeneity requirements of ANOVA. When the ANOVA results showed a significant effect, the Duncan test was carried out for multiple comparisons between treatments at α = 0.05. Field performance was analyzed separately for each sampling period.
3 Results
3.1 Nursery response
Nursery fertilization had an effect on seedling dry mass (Fig. 2). Both stem tissue (p < 0.001) and whole plant (p = 0.024) dry mass were significantly different among treatments. In general, as fertilizer increased, so did whole plant dry mass. Both the 10 and 20 mg N seedling–1 rates were significantly lower than others, and the dry mass peaked at 100 mg N seedling–1. Multiple comparison testing showed the lowest N rate (10 mg) consistently yielded lower stem and whole plant dry mass compared to the two highest N rates (100 and 120 mg). Foliage (p = 0.482) and root (p = 0.142) tissues were not significantly different among any of the fertilizer treatments.
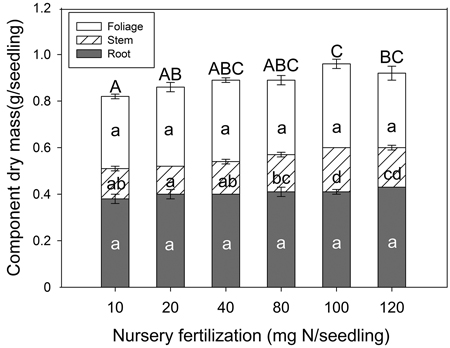
Fig. 2. Chinese pine seedling tissue dry mass (mean ± SE, n = 32) in relation to nursery fertilization prior to planting. Treatments marked with different lower-case and capital letters differ statistically for each tissue and whole plant according to Duncan`s test α = 0.05, respectively.
Nursery fertilization affected plant tissue N concentration (p ≤ 0.003) (Fig. 3). Foliage reached maximum N concentration when nursery fertilization was 120 mg N seedling–1. Stem N concentration increased when seedlings were fertilized from 10 to 20 mg N seedling–1; no differences were observed among the 20–120 mg N seedling–1 treatments. Root and whole plant N concentration also increased with fertilization; maximum N concentrations were observed at the 120 mg N seedling–1 rate.
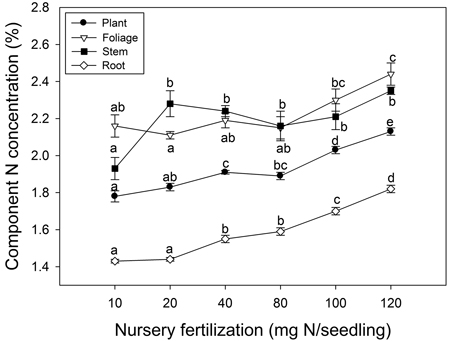
Fig. 3. Chinese pine seedling tissue N concentration (mean ± SE, n = 4) in relation to nursery fertilization prior to planting. Treatments marked with different lower-case letters differ statistically for each tissue and whole plant according to Duncan`s test α = 0.05.
Seedling N content varied among fertilizer treatments but depended on tissue type (Fig. 4). Foliage N content was not significantly different among treatments (p = 0.258); however, stem tissue (p < 0.001), root tissue (p < 0.001), and whole plant (p < 0.001) variables all increased with fertilization. Maximum N content for both stem and whole plant variables were reached at the 100 mg seedling–1 fertilization rate. For root tissue, maximum N content was observed at the 120 mg N seedling–1 rate.
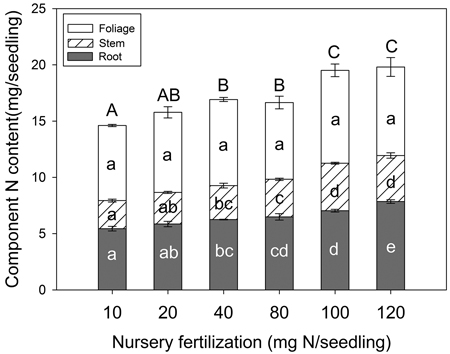
Fig. 4. Chinese pine seedling tissue N content (mean ± SE, n = 4) in relation to nursery fertilization prior to planting. Treatments marked with different lower-case and capital letters differ statistically for each tissue and whole plant according to Duncan`s test α = 0.05, respectively.
3.2 Field performance
At the end of the first growing season, outplanting survival was high (79–96%), but not significantly different (p = 0.134) among nursery fertilization treatments (Table 1). At the end of the second growing season, survival declined and significant differences were observed among nursery fertilization treatments (p = 0.029). Seedlings fertilized at the 10 mg N rate showed the lowest mean survival (54%), while seedlings from the 80 mg N rate showed the highest mean survival (92%) and was significantly greater than other treatments.
| Table 1. Chinese pine seedling field survival (mean ± SE, n = 4) in relation to nursery fertilization at the end of first and second growing seasons. Treatments marked with different letters in a column differ statistically according to Duncan`s test α = 0.05. | ||
| Treatments (mg N seedling–1) | The first year growing season | The second year growing season |
| 10 | 79 (0.04) a | 54 (0.04) a |
| 20 | 82 (0.06) a | 75 (0.06) b |
| 40 | 88 (0.07) a | 75 (0.10) b |
| 80 | 96 (0.04) a | 92 (0.08) c |
| 100 | 93 (0.04) a | 78 (0.03) b |
| 120 | 92 (0.05) a | 75 (0.11) b |
Nursery fertilization had no significant impact on total height at planting or at the end of the first and second growing season (p = 0.062–0.843) (Fig. 5). RCD did vary among treatments at planting (p = 0.003), but the differences vanished by the end of the first growing season (p = 0.138). Consistently, height (p = 0.266–0.809) and RCD (p = 0.238–0.930) increments were not significant for any growing season.
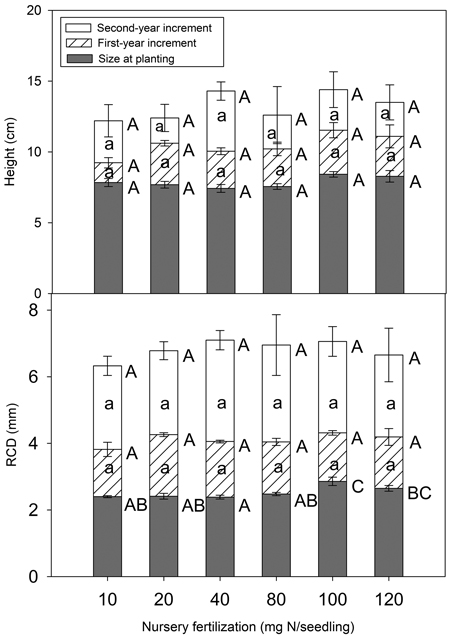
Fig. 5. Chinese pine seedlings total height, root-collar diameter (RCD) and their increments (mean ± SE) in relation to nursery fertilization at planting, at the end of first and second growing seasons. Treatments marked with different capital and lower-case letters differ statistically for total height, RCD, and its increment according to Duncan`s test α = 0.05, respectively.
4 Discussion
4.1 Nursery growth and N storage
Exponential fertilization in the nursery illustrated a curvilinear relationship with increased fertilization for seedling dry mass and N content (McAlister and Timmer 1998; Salifu 2003; Salifu and Timmer 2003a; Birge et al. 2006). As described in the conceptual model by Timmer (1996), N concentration was increased in Chinese pine seedlings with increasing fertilization. According to our data, a rate of 40 mg N seedling–1 or greater, was sufficient enough to maximize seedling dry mass; concomitantly, a rate of 100 mg N seedling–1 or greater, maximized N content. Ideally, optimum exponential fertilization would maximize both seedling dry mass and tissue N content before nutrient toxicity elicits a decline in seedling dry mass (Landis et al. 1989). The absence of a sharp decline in dry mass related to tissue nutrient concentration or content in our study, however, indicated that nutrient toxicity did not occur. We therefore were unable to develop a full understanding of luxury consumption limits. In other Pinus species, such as Pinus resinosa Ait. (Timmer and Armstrong 1987; Miller and Timmer 1994), and Pinus monticola Douglas ex D. Don (Dumroese et al. 2005), no more than three fertilization rates were used; consequently, optimum fertilization of Pinus species was also not determined. Conversely, optimum N levels have been comprehensively determined for Picea and Quercus species (64 and 100 mg N seedling–1, respectively); although, these recommendations were for much smaller and much larger container sizes (40 ml and 2800 ml, respectively; Salifu and Timmer 2003a; Salifu and Jacobs 2006). Despite the fact that toxicity levels were not achieved in our study, dry mass and N content responses to fertilization suggest that optimum N application with exponential fertilization of Chinese pine seedlings is no less than 100 mg N seedling–1.
4.2 Field growth performance
In this study, RCD differences at planting vanished after the first growing season indicating that nursery fertilization may have had a transitory effect on field growth performance. This finding is shared by Picea abies (L.) Karsten, Quercus suber L.,Q. rubra and Q. alba (Heiskanen et al. 2009; Salifu et al. 2009; Trubat et al. 2010), but differs from Pinus canariensis C. Sm., whose RCD dominance at planting was retained for three years (Luis et al. 2009). In these instances, direct nutrient uptake from soil and variations in soil fertility likely contributed to inconsistencies in growth over time (Hawkins et al. 2005). Xu (1987) suggest that Chinese pine seedlings undergo slow growth during the first 5 years after outplanting and attribute some of this to soil nutrient availability and soil depth. In some instances, the influence of high field fertility may outweigh the effect of nursery fertilization (Hawkins et al. 2005; Heiskanen et al. 2009). Based on the site classified criteria by Bao (2000), the moderate soil fertility in our study may have contributed to the field performance, but we also think other factors, such as soil moisture, had an influence on our results. Other unpublished data suggest Chinese pine undergo a slow establishment period indicating the possibility of planting stress sensitivity, before resuming vigorous growth after several years. Other species, including spruce and pine, have also shown sensitivity to planting stress (also called, growth check), lasting several years after outplanting (citations from Grossnickle 2005; Heiskanen et al. 2009). Because literature is lacking on the outplanting performance of Chinese pine, it is difficult to know how widespread this problem is.
4.3 Field survival
Similar to results observed in Q. rubra and Q. alba (Salifu et al. 2009), nursery fertilization rates did not affect outplanting survival at the end of the first growing season. While our first year survival data was not significant, the data illustrated a curvilinear pattern with nursery fertilizer rates; mean survival peaked at 80 mg N seedling–1. At the end of the second growing season, survival shared the curvilinear pattern and was significant; the greatest outplanting survival occurred at a nursery fertilization rate of 80 mg N seedling–1. Our curvilinear pattern is different than the linear ones reported in published papers; these studies show positive or negative survival responses to nursery fertilization (Villar-Salvador et al. 2004; Trubat et al. 2008; Luis et al. 2009; Cuesta et al. 2010). In the Mediterranean, the positive effects of nursery fertilization on plantation survival have been demonstrated in both conifers and hardwood species during two consecutive outplanting seasons (Villar-Salvador et al. 2004; Luis et al. 2009). Rich pre-planted nutritional status (mainly of N) could confer nutrient remobilization capacity, high photosynthesis, and plant water potential and thus promote outplanting survival (Puértolas et al. 2003; Villar-Salvador et al. 2004, 2012; Luis et al. 2009; Cuesta et al. 2010). Conversely, several studies in the same environments have also argued that N deficient seedlings tended to show higher survival than those subjected to standard nursery fertilization (Trubat et al. 2008, 2010). This may be attributed to the reduced shoot size that decreases the demand for water and thereby has a greater ability to tolerate drought (Poorter and Nagel 2000; Rubio et al. 2003). Positively or negatively linear responses of survival to nursery fertilization could result from the fact that there were as few as two or three fertilization treatments in these studies. These conflicted views in this and other studies suggest that the relation between field performance and nursery fertilization should be verified by a series of fertilization rates. Additional studies on other trees are needed to explain why maximum survival is asynchronous with optimum nursery N rate from physiological perspectives, including N remobilization, non-structural carbohydrate content, water potential, and photosynthesis.
Seedling survival at the end of the second growing season (54%–92%) declined dramatically relative to the first growing season (79%–96%). This may be attributed to the water stress that outplanted seedlings were exposed to during the two growing seasons. The model of seedling establishment, proposed by Burdett (1990), highlights the importance of quality seedlings and their physiological processes in the presence of favorable outplanting conditions. One major limiting factor to seedling establishment, however, is soil moisture (Burdett 1990; Grossnickle 2005). Water stress in transplanted seedlings limits the physiological functioning required to grow new roots thereby contributing to a negative feedback cycle and lack of establishment (Grossnickle 2005). Conditions in our study were likely limiting because of the prolonged dry winter (according to precipitation data; Figure 1) preceding outplanting. Despite this, a short monsoon rain season allowed seedlings a brief growing period before dry conditions returned. However, outplanting conditions following the first year’s measurement remained dry and became cold for 6 months. We suspect that winter and spring desiccation likely contributed to the decline in seedling survival measured at the end of the second growing season. Water stress often causes the imbalance of water uptake and transpiration and therefore is a significant factor of seedling mortality (Walker et al. 1981; Archer 1989; Crow et al. 1994; Grossnickle 2005).
5 Conclusions
In the nursery, N content was significantly higher in the seedlings exponentially fertilized at 100 and 120 mg N seedling–1 than the other four treatments. Dry mass at 100 mg N seedling–1 was significantly greater than at 10 and 20 mg N seedling–1 but slightly higher than 40, 80 and 120 mg N seedling–1. Response of field survival during two consecutive growing seasons to nursery fertilization generally exhibited a non-linear pattern. Maximum mean survival occurred in the seedlings nursery-fertilized at 80 mg N seedling–1. Thus, comprehensive consideration of both nursery and field performance determine rational nitrogen supply for Chinese pine in the nursery. An application rate of 80 mg N seedling–1 yields optimum field survival in the given site condition as well as satisfactory nursery growth for the particular container type we used.
Acknowledgements
The study was funded by the Fundamental Research Funds for the Central Universities (Contract No. TD2011-08). We gratefully acknowledge the anonymous reviewers for their insightful comments of the manuscript and thank Meteorological Bureau of Pingquan County for assistance with providing weather data.
References
Archer S. (1989). Have southern Texas savannas been converted to woodlands in recent history? American Naturalist 134: 545–561.
Bao S.D. (2000). Soil and agricultural chemistry analysis. Chinese Agriculture Press, Beijing. p. 39–114.
Birge Z.K.D., Salifu K.F., Jacobs D.F. (2006). Modified exponential nitrogen loading to promote morphological quality and nutrient storage of bareroot-cultured Quercus rubra and Quercus alba seedlings. Scandinavian Journal of Forest Research 21: 306–316. http://dx.doi.org/10.1080/02827580600761611.
Bremner J.M., Mulvaney C.S. (1982). Nitrogen-Total. In: Page AL (ed.). Methods of soil analysis. American Society of Agronomy, Madison, WI. p. 595–624.
Burdett A.N. (1990). Physiological processes in plantation establishment and the development of specifications for forest planting stock. Canadian Journal of Forest Research 20: 415–427. http://dx.doi.org/10.1139/x90-059.
Close D.C., Bail I., Hunter S., Beadle C.L. (2005). Effects of exponential nutrient-loading on morphological and nitrogen characteristics and on after-planting performance of Eucalyptus globulus seedlings. Forest Ecology and Management 205: 397–403. http://dx.doi.org/10. 1016/j. forcco. 2004.10.041.
Crow T.R., Johnson W.C., Adkisson C.S. (1994). Fire and recruitment of Quercus in a post agricultural field. American Midland Naturalist 131: 84–97.
Cuesta B., Pedro V.S., Jaime P., Douglass F.J., José M.R.B. (2010). Why do large, nitrogen rich seedlings better resist stressful transplanting conditions? A physiological analysis in two functionally contrasting Mediterranean forest species. Forest Ecology and Management 260: 71–78. http://dx.doi.org/10.1016/j.foreco.2010.04.002.
Dumroese R.K., Page-Dumroese D.S., Salifu K.F., Jacobs D.F. (2005). Exponential fertilization of Pinus monticola seedlings: nutrient uptake efficiency, leaching fractions, and early outplanting performance. Canadian Journal of Forest Research 35: 2961–2967. http://dx.doi.org/10.1139/X05-226.
Grossnickle S.C. (2000). Ecophysiology of Northern spruce species: The performance of planted seedlings. NRC Research Press, Ottawa, Ontario, Canada. 409 p.
Grossnickle S.C. (2005). Importance of root growth in overcoming planting stress. New Forests 30: 273–294. http://dx.doi.org/10.1007/s11056-004-8303-2.
Hawkins B.J., Burgess D., Mitchell A.K. (2005). Growth and nutrient dynamics of western hemlock with conventional or exponential greenhouse fertilization and planting in different fertility conditions. Canadian Journal of Forest Research 35: 1002–1016. http://dx.doi.org/10.1139/X05-026.
Heiskanen J., Lahti M., Luoranen J., Rikala R. (2009). Nutrient loading has a transitory effect on the nitrogen status and growth of outplanted Norway spruce seedlings. Silva Fennica 43: 249–260. http://dx.doi.org/10.14214/sf.210.
Huang G.Y., Dong L.F., Qi F.M. (2004). Fertilization effect and application of diagnosis and recommendation integrated system in container system seedling raising of Pinus tabulaeformis. Journal of West China Forestry Science 33(3): 26–29.
Landis T.D., Tinus R.W., McDonald S.E., Barnett J.P. (1989). Seedling nutrition and irrigation, Vol. 4, The container tree nursery manual. Agricultural Handbook 674. US Department of Agriculture, Forest Service, Washington, DC. 119 p.
Lu M., Li Y.Y., Ge T.K. (2000). Studies on the medium of container seedling of Chinese pine. Acta Agriculturae Boreali-Sinica 15 (supp): 167–174.
Luis V.C., Puértolas J., Climent J., Peters J., González-Rodriguez A., Morales D., Jiménez M. (2009). Nursery fertilization enhances survival and physiological status in Canary Island pine (Pinus canariensis) seedlings planted in a semiarid environment. European Journal of Forest Research 128: 221–229. http://dx.doi.org/10.1007/s10342-009-0257-7.
Malik V., Timmer V.R. (1996). Growth, nutrient dynamics, and interspecific competition of nutrient-loaded black spruce seedlings on a boreal mixedwood site. Canadian Journal of Forest Research 26: 1651–1659. http://dx.doi.org/10.1139/x26-186.
McAlister J.A., Timmer V.R. (1998). Nutrient enrichment of white spruce seedlings during nursery culture and initial plantation establishment. Tree Physiology 18: 195–202.
Miller B.D., Timmer V.R. (1994). Steady-state nutrition of Pinus resinosa seedlings: response to nutrient loading, irrigation and hardening regimes. Tree Physiology 14: 1327–1338.
Oliet J.A., Tejada M., Salifu K.F., Collazos A., Jacobs D.F. (2009). Performance and nutrient dynamics of holm oak (Quercus ilex L.) seedlings in relation to nursery nutrient loading and post-transplant fertility. European Journal of Forest Research 128: 253–263. http://dx.doi.org/10.1007/s10342-009-0261-y.
Poorter H., Nagel O. (2000). The role of biomass allocation in the growth response of plants to different levels of light, CO2, nutrients and water: a quantitative review. Australian Journal of Plant Physiology 27: 595–607. http://dx.doi.org/10.1071/pp99173.
Puértolas J., Gil L., Pardos J.A. (2003). Effects of nutritional status and seedling size on field performance of Pinus halepensis planted on former arable land in the Mediterranean basin. Forestry 76: 159–168. http://dx.doi.org/10.1093/forestry/76.2.159.
Qu L.Y., Ali M., Quoreshi A.M., Koike T. (2003). Root growth characteristics, biomass and nutrient dynamics of seedlings of two larch species raised under different fertilization regimes. Plant and Soil 255: 293–302. http://dx.doi.org/10.1023/A:1026159709246.
Quoreshi A.M., Timmer V.R. (2000). Early outplanting performance of nutrient-loaded containerized black spruce seedlings inoculated with Laccaria bicolor: a bioassay study. Canadian Journal of Forest Research 30: 744–752. http://dx.doi.org/10.1139/x00-003.
Rubio G., Zhu J.M., Lynch J.P. (2003). A critical test of the two prevailing theories of plant response to nutrient availability. American Journal of Botany 90: 143–152. http://dx.doi.org/10.3732/ajb.90.1.143.
Salifu K.F. (2003). Nitrogen retranslocation of young Picea mariana to varied nitrogen supply and plant nutrient reserves. PhD Thesis, University of Toronto, Canada.
Salifu K.F., Jacobs D.F. (2006). Characterizing fertility targets and multi-element interactions in nursery culture of Quercus rubra seedlings. Annals of Forest Science 63: 231–237. http://dx.doi.org/10.1051/forest:2006001.
Salifu K.F., Timmer V.R. (2003a). Optimizing nitrogen loading of Picea mariana seedlings during nursery culture. Canadian Journal of Forest Research 33: 1287–1294. http://dx.doi.org/10.1139/x03-057.
Salifu K.F., Timmer V.R. (2003b). Nitrogen retranslocation response of young Picea mariana to nitrogen-15 supply. Soil Science Society of America Journal 67: 309–317. .
http://dx.doi.org/10.2136/sssaj2003.3090.
Salifu K.F., Jacobs D.F., Birge Z.K.D. (2009). Nursery nitrogen loading improves field performance of bareroot oak seedlings planted on abandoned mine lands. Restoration Ecology 17: 339–349. http://dx.doi.org/10.1111/j.1526-100X.2008.00373.x.
Shi S.J., Sun X.L., Zhang Y.Z., Zhang K., Liu B.Y. (2011). Preliminary study on a method of improving survival of Chinese pine container seedling. Science and Technology of West China 10(14): 30, 48.
State Forestry Administration. (2013). Technical regulations of containerized seedlings. Chinese Standard Press, Beijing. 3 p.
Timmer V.R. (1996). Exponential nutrient loading: a new fertilization technique to improve seedling performance on competitive sites. New Forests 13: 275–295. http://dx.doi.org/10.1023/A:1006502830067.
Timmer V.R., Armstrong G. (1987). Growth and nutrition of containerized Pinus resinosa at exponentially increasing nutrient additions. Canadian Journal of Forest Research 17: 644–647. http://dx.doi.org/10.1139/x87-105.
Trubat R., Cortina J., Vilagrosa A. (2008). Short-term nitrogen deprivation increases field performance in nursery seedlings of Mediterranean woody species. Journal of Arid Environments 72: 879–890. http://dx.doi.org/10.1016/j.jaridenv.2007.11.005.
Trubat R., Cortina J., Vilagrosa A. (2010). Nursery fertilization affects seedling traits but not field performance. Journal of Arid Environments 74: 491–497. http://dx.doi.org/10.1016/j.jaridenv.2009.10.007.
van den Driessche R. (1985). Late season fertilization, mineral nutrient reserves, and translocation in planted Douglas-fir (Pseudotsuga menziesii (Mirb.) Franco) seedlings. Forest Science 31: 485–496.
Villar-Salvador P., Planelles R., Enríquez E., PenuelasRubira J. (2004). Nursery cultivation regimes, plant functional attributes, and field performance relationships in the Mediterranean oak Quercus ilex L. Forest Ecology and Management 196: 257–266. http://dx.doi.org/10.1016/j.foreco.2004.02.061.
Villar-Salvador P., Puértolas J., Cuesta B., Peñuelas J.L., Uscola M., Heredia-Guerrero N., Rey Benayas J.M. (2012). Increase in size and nitrogen concentration enhances seedling survival in Mediterranean plantations. Insights from an ecophysiological conceptual model of plant survival. New Forests 43: 755–770. http://dx.doi.org/10.1007/s11056-012-9328-6.
Walker B.H.D., Ludwig C.S., Holling, Peterman R.M. (1981). Stability of semiarid savanna grazing systems. Journal of Ecology 69: 473–498. http://dx.doi.org/10.2307/2259679.
Way D.A., Seegobin S.D., Sage R.F. (2007). The effect of carbon and nutrient loading during nursery culture on the growth of black spruce seedlings: a six-year field study. New Forests 34: 307–312. http://dx.doi.org/10.1007/s11056-007-9053-8.
Xu H.C. (1987). A preliminary study on the adaptability of provenances of Pinus tabulaeformis. Journal of Beijing Forestry University 9(1): 111–123.
Xu H.C. (1993). Pinus tabulaeformis. Chinese Forestry Press, Beijing. p. 480.
Yao Q.Z., Yan W. (2005). Effect of ectomycorrhizal inoculation on seelings growth of Pinus tabulaeformis Carr. in nursery condition. Journal of Arid Land Resources and Environment 19(3): 185–188.
Yu X.F., Bai Z., Wang J., Xue H.X. (2008). Application of Chinese pine container seedlings inoculated with ectomycorrhizal fungi in arid environment of Baotou. Inner Mongolia Forestry Investigation and Design 31(5): 65, 67.
Zhang G.X., Chen H.X. (2013). Effect of different planting methods of Pinus tabulaeformis container seedling on forestation survival rate. Science and Technology of Modern Agriculture 1: 150, 153.
Zou S.Q., Li G.L., Liu Y., Zhu Y., Pang W., Jiang L., Shi W.H. (2012). The effect of fall fertilization on Pinus tabulaeformis container seedlings growth, nitrogen uptake and cold tolerance. Journal of Anhui Agricultural Science 40: 11710–11714, 11740.
Total of 48 references

