Relation of tree-ring width and earlywood vessel size of alien Quercus rubra L. with climatic factors in Latvia
Matisons R., Jansons J., Katrevičs J., Jansons Ā. (2015). Relation of tree-ring width and earlywood vessel size of alien Quercus rubra L. with climatic factors in Latvia. Silva Fennica vol. 49 no. 4 article id 1391. https://doi.org/10.14214/sf.1391
Highlights
- Climate-growth relationships of red oak from three sites in Latvia were studied
- Tree-ring width was mainly affected by temperature and precipitation in late summer
- Vessel size was correlated with temperature parameters in autumn–spring
- Sets of climatic factors significant for growth of red oak differed between sites
- Changes in climate-growth relationships occurred during 20th century.
Abstract
The effect of climatic factors on wood anatomy of the alien red oak (Quercus rubra L.) growing in three experimental plantations in Latvia was assessed by classical dendrochronological techniques. Two tree-ring proxies – tree-ring width (TRW) and mean area of earlywood vessel lumen (VLA) – were studied on 33 trees. Annual variation of TRW amongst trees was similar (mean r = 0.46), but there was more individuality in VLA (mean r = 0.26); nevertheless, chronologies of both proxies had rather synchronous variation amongst the sites. Annual variation of TRW was affected by factors related to water deficit in late summer, as suggested by the negative effect of temperature and positive effect of precipitation that have intensified during the 20th century, likely due to warming. Although weather conditions during the dormant period did not directly affect TRW, temperature during the autumn-spring period has been the main climatic determinant of VLA likely via influence on overwintering and hence vigour of tree. This suggests that conductive properties of wood and hence the susceptibility to water deficit have been affected by weather conditions before the formation of tree rings. During the 20th century, sensitivity of VLA has shifted from temperature in winter to temperature in autumn likely due to climate change. Still, the positive effect of these factors suggests that warming of climate would increase VLA and hence the risk of embolism and xylem disfunction. Therefore, the importance of availability of water for growth of red oak in Latvia is increasing.
Keywords
dendroclimatology;
climate-growth relationships;
introduced species;
earlywood vessel lumen area;
wood anatomy;
shifting sensitivity
-
Matisons,
LSFRI “Silava”, Rigas str. 111, Salaspils, Latvia, LV2169
E-mail
robism@inbox.lv

- Jansons, Latvian Forest Competence Centre, Dzērbenes str. 27, Riga, Latvia, LV 1006 E-mail janis.jansons@silava.lv
- Katrevičs, LSFRI “Silava”, Rigas str. 111, Salaspils, Latvia, LV2169 E-mail juris.katrevics@silava.lv
- Jansons, LSFRI “Silava”, Rigas str. 111, Salaspils, Latvia, LV2169 E-mail aris.jansons@silava.lv
Received 18 May 2015 Accepted 2 July 2015 Published 22 July 2015
Views 173472
Available at https://doi.org/10.14214/sf.1391 | Download PDF

1 Introduction
Climate change causes shifts in distribution of species and northward migration of nemoral species has been forecasted in Europe during the 21st century (Hickler et al. 2012; Maiorano et al. 2013). Such shifts inevitably have ecological and economic impact (Walther et al. 2002; Hanewinkel et al. 2012), therefore strategies for adaptation and mitigation of consequences of climate changes need to be evolved (Parry et al. 2007; Bright et al. 2014). In forestry, the use of species and provenances, which are robust against climatic factors, has been advised to sustain productivity of commercial stands under changing climate (Ledig and Kitzmiller 1992; Burton 2011). Forest distribution models (Hickler et al. 2012; Maiorano et al. 2013) predict that Latvia might be in a nemoral forest zone by the end of the 21st century. Under such a scenario, oaks might increase their commercial potential; however, the intensification of hazards for native European oaks (both pedunculate and sessile) has also been linked to climatic changes (Brassier 1996; Thomas et al. 2002). The alien red oak (Quercus rubra L.), which has been shown to be less affected by environmental factors than pedunculate oak (Quercus robur L.) (Jones 1959; Saliņš 1971), might be considered as an alternative in Latvia. Therefore, information about climate-growth relationships of red oak is necessary for better assessment of its potential in forestry.
Climate-growth relationships have been widely studied via retrospective analysis of tree-ring parameters (Fritts 2001). Tree-ring width (TRW), which is the most commonly used proxy, contains information about many environmental factors, thus the effect of a certain factor might be difficult to distinguish (Fritts 2001; Speer 2010). For this reason, trees growing close to their distribution limits, where a particular environmental factor is limiting growth, are advantageous for climate-growth studies (Speer 2010). Anatomical proxies of tree-ring, such as mean area of earlywood vessel lumen (VLA) for ring porous species (i.e. oaks), have been shown to contain climatic information, which might not be detectible in TRW (Fonti et al. 2010; Matisons et al. 2012). Earlywood vessels in ring porous species are the main water conductors (Carlquist 2001; Tyree and Zimmermann 2002); therefore, the analysis of VLA might be useful for a deeper understanding of the effect of climatic factors on tree-water relations (Tyree and Cochard 1996; Pallardy 2008; Gonzalez-Gonzalez et al. 2014).
The aim of this study was to assess the effect of climatic factors on high-frequency variation of TRW and VLA of the alien red oak growing in three experimental plantations in Latvia. We hypothesised that the sets of climatic factors significant for wood increment of red oak in Latvia have shifted during the 20th century due to changes in climate. Considering rise of mean temperature, we assumed that the effect of temperature during the dormant period and spring has weakened, but the effect of factors related to water deficit has intensified.
2 Material and methods
2.1 Study area
Red oaks growing in three experimental plantations were sampled; two sites were located in the western part of Latvia near Šķēde (57°14´N, 22°41´E) (SKD) and Auce (56°31´N, 22°56´E) (AUC) and one site was located in the eastern part of Latvia near Aglona (56°10´N, 27°2´E) (AGL). Studied sites belong to the hemiboreal forest zone (Hytteborn et al. 2005). The absolute elevation of sites ranged from 80 to 150 m a.s.l., the relief was flat and soils were loamy and fertile. The plantations were established around 1900, but no additional information (i.e. origin of planted material, management history or initial spacing) was available.
The climatic conditions of the region are mild due to dominant western winds, which bring cool and moist air masses from the Atlantic Ocean and the Baltic Sea. Continentality increases eastwards (Klavins and Rodinov 2010). Mean annual temperature is about +6 °C and +4.5 °C and mean monthly temperature ranges from –3.6 to –6.4 °C in January and from +15.2 to +16.9 °C in July in the western and eastern regions of Latvia, respectively. Vegetation period extends from mid-April to mid-October. Mean annual precipitation sum in the studied sites is ca. 600 mm. Temporal distribution of precipitation is uneven and most of the annual precipitation falls during May–September period. Summer precipitation tends to be higher in the eastern region. Climatic changes in Latvia are reflected as an increase of mean temperature (especially in April) (Lizuma et al. 2007) and an extension of the growing period (Ahas et al. 2000). Annual precipitation has a non-significant trend (Briede and Lizuma 2007), but the distribution of summer precipitation is becoming more heterogeneous (Avotniece et al. 2010).
Red oak is native to North America where its distribution reaches the 50th parallel of northern latitude and where it is commercially important species (Sander 1990). In Latvia, it is an alien species, which is mainly planted as urban landscaping elements (Laiviņš et al. 2009). According to data from the National Forest Inventory, plantations of red oak cover ca. 90 ha, particularly in the western part of Latvia. Considering the climatic requirements of red oak of minimum annual temperature of 4 °C and sum of annual precipitation ca. 700 mm (Sander 1990), Latvian climate matches with the northern, but not the marginal regions of the natural distribution area of the species. This explains slightly higher robustness of red oak against weather fluctuations compared to native pedunculate oak described by Saliņš (1971) and its productivity as described by Dreimanis and Šulcs (2006).
2.2 Sampling and measurements
Canopy oaks with healthy crowns were selected for sampling within each site. The number of sampled trees was 7, 16 and 10 in SKD, AUC and AGL sites, respectively. From each tree, two samples (increment cores) from opposite sides of the stem were collected with a Pressler increment borer at ca. 1.3 m stem height. In the laboratory, increment cores were air-dried, fixed and gradually grinded using sandpaper of four roughness grits (100, 150, 240 and 400 grains per inch) by vibration sanding machine. Sanded surface of samples was then rubbed with chalk to increase the contrast between early- and latewood and to highlight earlywood vessels. It was ensured that chalk had remained only in vessel lumina.
Tree-ring width was measured with the precision of 0.01 mm using measurement system LINTAB 5 (RinnTECH, Germany). Width of earlywood and latewood was not measured as these parameters provide little additional information for oak (Zhang 1997). Measurements of VLA were made using WinCELL 2007a software (Regent Instruments, Canada). Sample images, with 24-bit colour depth and 1200 dpi resolution, were acquired using EPSON GT 15000 scanner. For the measurements of VLA, sample images were manually cut into earlywood images of each tree-ring, ensuring that they contained at least 10 of the larger vessels from the initial two rows, which express the strongest environmental signal (Garcia-Gonzalez and Fonti 2006). Pixel classification was based on grey levels and threshold values were set manually (intensity ranged from 220 to 238). Before launching the batch function for measurements, correct recognition of earlywood vessel was ensured by inspection of manual analysis of 10–12 randomly selected earlywood images per sample; slight adjustments of parameters were made if necessary. Data filter, removing any objects with size outside the empirically determined range of 9500–70000 μm2, was used to exclude chalk debris, small and merged vessels.
2.3 Data analysis
All measured time series were cross-dated and their quality was checked by both graphical inspection and statistically, using program COFECHA (Grissino-Mayer 2001). For the assessment of yearly variation of TRW and VLA, residual chronologies were produced for each site based on cross-dated time series of trees using the program ARSTAN (Cook and Holmes 1986). Double detrending (by negative exponential curve followed by cubic spline with rigidity of 64 years and 50% frequency cut-off) and autoregressive modelling were applied. Mean sensitivity, first order autocorrelation, inter-series correlation (Grissino-Mayer 2001), Gleichläufigkeit (GLK) and expressed population signal (EPS) (Wigley et al. 1984) indices for description of the datasets were calculated in program R (R Core Team, 2014) using library “dplR” (Bunn 2008) (interseries correlation and EPS were calculated based on detrended series).
Climatic data (mean monthly temperature and precipitation sums) were obtained from the Climatic Research Unit high-resolution gridded dataset repository (Harris et al. 2014) for grid centres located at < 30 km distance from the studied sites. Relationships between the residual chronologies of TRW and VLA and climatic factors were assessed by bootstrapped Pearson correlation analysis using the DendroClim2002 program (Biondi and Waikul 2004). The analysis was conducted for the whole period (1910–2010) and for overlapping 50-year moving intervals within it. Climatic data were divided into time windows from the previous June to the current September. Such division of climatic data has been sufficient for pedunculate oak (Matisons and Brūmelis 2012).
3 Results
3.1 Measurements and chronologies
The agreement of measured time series of TRW among trees within a site was good (mean r > 0.40, GLK > 0.66) and, after crossdating and quality check, only a few series were omitted from further analysis (Table 1). Age trend was present in the crossdated time series of both proxies (Fig. 1 A, C) – TRW showed reduction while VLA increased with age. Mean TRW was higher and mean VLA was lower in AUC site compared to other two sites (Table 1). Time series of TRW showed better agreement among trees in a site compared to VLA, as indicated by higher inter-series correlation and synchrony (mean r of 0.46 and 0.26 and mean GLK of 0.69 and 0.61, respectively) (Table 1). The EPS values of TRW datasets exceeded 0.85 in all sites, but they ranged only from 0.69 to 0.83 for datasets of VLA. Mean sensitivity was higher for TRW compared to VLA (0.21 and 0.17, respectively), likely due to greater amplitude of high-frequency variation (Fig. 1 A, C). Autocorrelation was slightly higher for VLA compared to TRW (0.77 and 0.74, respectively).
| Table 1. Periods covered by crossdated measurement time series of TRW and VLA and statistics of datasets for the studied sites for the common period of 1910–2010: number of crossdated trees and samples; range, mean and standard deviation (St. dev.) of measurements; mean sensitivity (SENS), autocorrelation (AC), inter-series correlation (IC), Gleichläufigkeit (GLK) and expressed population signal (EPS) of cross-dated time series (IC and EPS were calculated for detrended series). | ||||||
| TRW, 10 mm | VLA, 104 μm2 | |||||
| SKD | AUC | AGL | SKD | AUC | AGL | |
| Period covered by measurements | 1902–2013 | 1907–2013 | 1906–2013 | 1902–2013 | 1910–2013 | 1909–2013 |
| Number of crossdated trees (samples) | 7 (13) | 16 (29) | 10 (18) | 6 (12) | 15 (25) | 10 (20) |
| Min | 0.42 | 0.23 | 0.15 | 0.99 | 0.96 | 0.97 |
| Max | 5.30 | 7.96 | 6.99 | 7.35 | 8.40 | 8.88 |
| Mean | 2.26 | 2.93 | 2.09 | 3.41 | 2.71 | 3.10 |
| St. dev. | 0.93 | 1.01 | 0.81 | 1.00 | 0.92 | 0.99 |
| SENS | 0.18 | 0.22 | 0.22 | 0.17 | 0.16 | 0.17 |
| AC | 0.83 | 0.64 | 0.74 | 0.74 | 0.80 | 0.76 |
| IC | 0.48 | 0.43 | 0.46 | 0.27 | 0.22 | 0.29 |
| EPS | 0.86 | 0.89 | 0.87 | 0.69 | 0.75 | 0.83 |
| GLK | 0.68 | 0.69 | 0.69 | 0.62 | 0.61 | 0.60 |
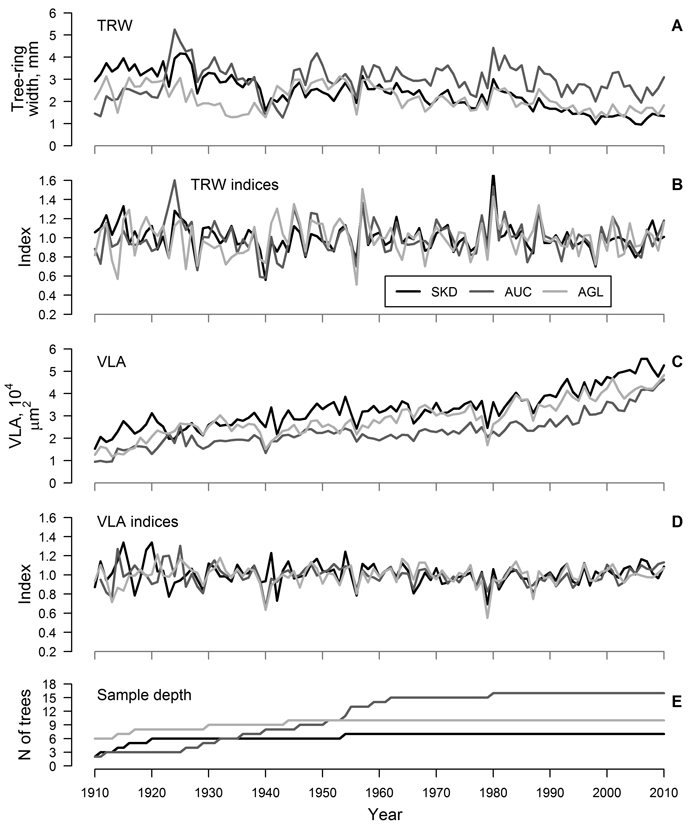
Fig. 1. Mean time series (A and C) and residual chronologies (B and D) of tree-ring width (TRW) and mean area of earlywood vessel lumen (VLA) of studied sites and sample depth of the datasets (E) for sites near Šķēde (SKD), Auce (AUC) and Aglona (AGL). Curves have been produced based on crossdated mean time series of trees.
For each site, residual chronologies of both proxies were successfully produced (Fig. 1 B, D). The range of chronology index values was higher for TRW than for VLA (Fig. 1 A, D), but it was similar between the sites. Still, during a few recent decades, a decrease in the range of index values was observed, particularly for VLA. The agreement of chronologies of TRW was good (mean GLK = 0.67) and correlation between chronologies of TRW was intermediate (mean r = 0.55), suggesting similarity of the growth patterns among the sites. Chronologies of VLA also showed good agreement (mean GLK = 0.69); however, correlation between chronologies was weaker (mean r = 0.39), suggesting stronger influence of local factors on variation of VLA. Nevertheless, common signatures such as decreased VLA in 1940 (except SKD), 1956 and 1979, decreased TRW in 1928, 1940, 1956 and 1998 and increased TRW in 1957 and 1980 were observed.
3.2 The effect of climatic factors on TRW
Amongst the tested 32 climatic factors, seven showed significant correlation with chronologies of TRW (Fig. 2). The absolute value of coefficients did not exceed 0.41, suggesting that none of the tested climatic factors had been strictly limiting. Temperature in previous July-August, apparently, was the main climatic factor affecting the high-frequency variation of TRW in all sites, as suggested by the highest values of correlation coefficients. Nevertheless, some local characteristics in the sets of significant climatic factors were observed. In SKD site, precipitation in previous November and current August showed significant correlation with TRW. In AUC site, temperature in July and precipitation in previous November and current June had a positive effect on TRW; however, the values of coefficients were lower than observed for temperature in previous summer. In AUC site, TRW was affected only by temperature in previous summer.
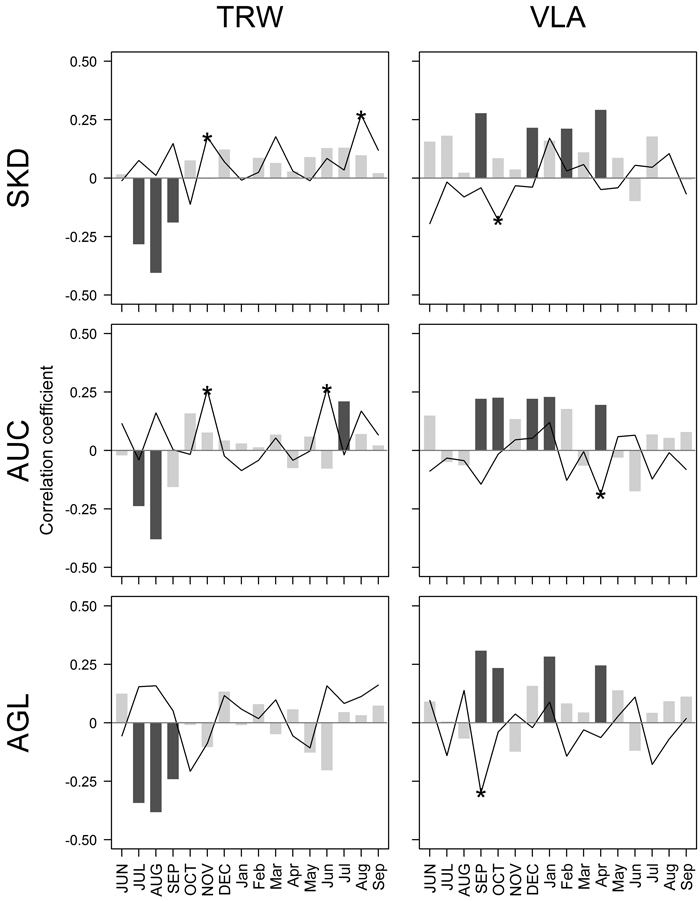
Fig. 2. Pearson correlation coefficients calculated between climatic factors – mean monthly temperature (bars) and precipitation sums (lines), and residual chronologies of tree-ring width (TRW) and mean area of earlywood vessel lumen (VLA) for sites near Šķēde (SKD), Auce (AUC) and Aglona (AGL) for the period from 1910 to 2010. Significant coefficients at α = 0.05 are in dark grey (bars) or indicated by asterisks (lines). Months of the year prior to tree-ring formation are in uppercase.
The relationships between TRW and climatic factors have changed during the 20th century, as shown by correlation analysis conducted for moving intervals (Fig. 3). Correlations with temperature in previous August have been significant during most of the analysed period (1910–2010) in all sites (Fig. 3), but the strength of such relationships has been fluctuating. A slight weakening of the effect of this factor was observed in sites in the western part of Latvia, while slight increase occurred in AGL site. The effect of temperature in previous July has become significant and intensified in SKD and AGL site in the intervals after about 1925–1975 (Fig. 3). In AUC site, the effect of temperature in previous September has lost its significance in intervals after 1934–1984 while the effect of temperature in current June has been significant in several intervals in mid- and later part of the analysed period. The effect of precipitation in current June or August in sites in western Latvia (AUC and SKD) and the effect of precipitation in previous August in eastern site (AGL) have become significant in intervals after about 1930–1980 (Fig. 3). In AGL site, precipitation in current September has been significant in several intervals in the mid part of the 20th century.
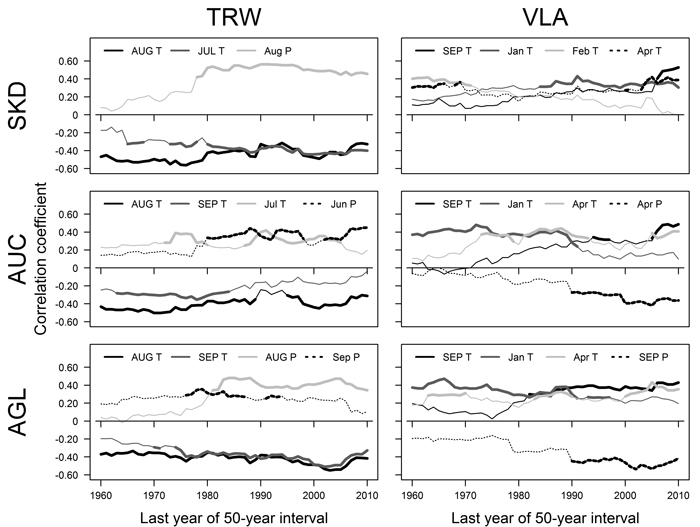
Fig. 3. Pearson correlation coefficients calculated between mean monthly temperature (T), precipitation sums (P) and residual chronologies of tree-ring width (TRW) and mean area of earlywood vessel lumen (VLA) for 50-year moving intervals for sites near Šķēde (SKD), Auce (AUC) and Aglona (AGL). Significant correlations are indicated by bold lines. Climatic factors that had significant correlations in > 15 intervals are shown. The dates represent the last year of moving intervals. Months of the year prior to tree-ring formation are in uppercase. Note that the plotted climatic factors, their sequence and encoding differ. View larger in new window/tab.
3.3 The effect of climatic factors on VLA
Chronologies of VLA correlated significantly with nine climatic factors, but the values of coefficients were lower compared to TRW (|r| < 0.31) (Fig. 2). The effect of tested climatic factors on VLA differed among the sites; it was the weakest in the AUC site compared to the other two sites, as shown by lower values of correlation coefficients. In general, temperature in previous autumn, some months in the dormant period and spring had positive effect on VLA, while the significant correlations with precipitation were negative. Temperature in previous September and current April showed significant effect on VLA in all studied sites. Still, the effect of temperature in previous October and December and in current January was significant in two of three studied sites. Significant effect of February temperature and precipitation in previous October was observed in SKD site; the effect of precipitation in April and previous September was observed in AUC and AGL site, respectively.
During the 20th century, the relationships between climatic factors and VLA have changed, as suggested by correlation analysis conducted for moving intervals (Fig. 3). The effect of temperature in previous September became significant in the second part of the analysed period in all sites, but this occurred earlier in the eastern site (AGL). In AUC and AGL sites, the effect of temperature in January has become non-significant in moving intervals after 1945–1995, but the opposite was observed in SKD site, where this factor became significant in moving intervals ending after 1984. The effect of temperature in April has been significant for VLA in AUC and AGL sites in scattered intervals, while in SKD site it has been significant in the beginning and ending of the analysed period. The effect of precipitation in current April and previous September has become significant in intervals after 1940–1990 in AUC and AGL sites, respectively. In SKD site, the effect of February temperature has been significant in intervals ending before 1970s.
4 Discussion
4.1 Variation of measurement time series
Similarity of variation of TRW is known for oaks growing within the same stand, suggesting explicit effect of certain environmental factors at local scales (Bailie and Pilcher 1973; Matisons and Brūmelis 2012). In this study, the agreement of time series of TRW of red oak was good (inter-series correlation was about 0.46) and EPS values exceeded 0.85 (Table 1) suggesting that environmental signals have been clearly reflected in the datasets (Wigley et al. 1984). The agreement of time series of VLA was lower (mean inter-series correlation was 0.26) and the EPS values of datasets were below 0.85 (Table 1), suggesting stronger individuality in variation of vessel size (Wigley et al. 1984) as previously observed for pedunculate oak (Matisons and Brūmelis 2012). This might be also related to sampling technique. Although 5-mm increment cores have been described as sufficient for vessel analysis (Fonti et al. 2009a), apparently, they were not completely representing the variability of vessel size within a tree-ring. Nevertheless, the synchrony of time series of VLA within a stand (mean GLK was 0.61) (Table 1) and the common signature years (i.e. in 1940 and 1956) (Fig. 1 C, D) suggested the validity of the datasets (Table 1). Matisons and Brūmelis (2012) also showed that strong environmental signals could be extracted from VLA datasets with EPS values within the range of 0.60–0.80.
Autocorrelation of TRW and VLA of red oak (Table 1) was higher compared to native pedunculate oak (Matisons and Brūmelis 2012) (mean autocorrelation was 0.73 vs 0.69 and 0.77 vs 0.44, respectively), suggesting stronger dependency of wood formation on previous growth and on nutrient reserves (Pallardy 2008; Speer 2010), particularly for earlywood. Correlations between the chronologies of TRW and VLA of red oak were weaker, compared to that of pedunculate oak (Matisons and Brūmelis 2012) (mean r was 0.55 vs 0.60 and 0.39 vs 0.64, respectively) thus accordingly variation of radial increment, and particularly earlywood vessels size, of red oak has been more influenced by local factors. Though mean sensitivity of TRW time series was higher compared to VLA for red oak (0.21 and 0.17, respectively) (Table 1), it was similar to that of pedunculate oak (0.21 and 0.19, respectively) (Matisons and Brūmelis 2012), suggesting comparable effect of environmental factors on tree-ring formation of both species. Mean values and the range of TRW and VLA of red oak (Table 1) were similar to pedunculate oak (Matisons and Brūmelis 2012), but the number of sampled red oak stands is too low for such comparison.
4.2 Climatic forcing of TRW and VLA
Although climatic conditions during the 20th century in Latvia have been comparable with that of the northern range of natural distribution of red oak (Sander 1990), TRW of red oak in Latvia has been affected by water deficit in summer, as suggested by the negative effect temperature in summer months (Figs. 2, 3). Late summer is the period when latewood and nutrient reserves, which affect growth in following year, are formed (Barbaroux and Breda 2002; Morecroft et al. 2003). The negative effect of temperature at that time can be explained by the intensification of evapotranspiration (Traykovic 2005) leading to drought (Epron and Dreyer 1993) and/or by heat stress (Haldimann and Feller 2004) and inhibited assimilation. The observed positive correlation between TRW of red oak and July temperature in AUC site (Fig. 2) is difficult to explain. Probably, it might be related to years with moist summers, when increased temperature can facilitate assimilation (Jurik et al. 1988). The correlations with summer temperature observed for red oak (Fig. 2) were stronger than for pedunculate oak (Matisons et al. 2012), but in contrast to pedunculate oak (Matisons and Brūmelis 2012), red oak did not show sensitivity to temperature in autumn-spring period. The role of water deficit in variation of TRW has been supported also by the positive correlation with summer precipitation (Figs. 2, 3), but the time lag of the significant precipitation variables suggests differences in tree response. In western Latvia, TRW was affected by the precipitation in the current year (Fig. 2), suggesting that red oak was able to adjust growth to varying conditions quite quickly. In the eastern site, TRW was affected by precipitation in the preceding summer (Fig. 3) suggesting that growth was more affected by nutrient reserves (Barbaroux and Breda 2002). The absence of reaction to current precipitation in the site in eastern Latvia in recent decades might be explained by embolization of vessels (Tyree and Cochard 1996; Tyree and Zimmermann 2002), when trees might not utilize the available moisture due to limited transport capability. Similar climate-growth relationships have been common for oaks growing under the Mediterranean climate (Rozas 2005; Fonti and Garcia-Gonzalez 2008).
The size of earlywood vessels in red oak, which influence water-conducting properties of tree-rings and susceptibility to embolism (Tyree and Zimmermann 2002), was significantly affected by climatic factors (Fig. 2), suggesting that susceptibility of tree-rings to xylem disfunction is also influenced by weather. The effect of climatic factors on VLA (Fig. 2) was slightly stronger in site in the eastern part of Latvia where climate is harsher, still the relationships between VLA of red oak and climatic factors were weaker than for pedunculate oak (Matisons et al. 2012). Annual variation of VLA of red oak was mainly affected by weather conditions prior to xylogenesis (Fig. 2) that might be explained by the dependence of earlywood formation on stored assimilates (Barbaroux and Breda 2002; Pallardy 2008). Correlation between VLA of red oak and temperature in September of the preceding year (Fig. 2) can be explained by an extension of vegetation season (Ahas et al. 2000) and additional assimilation (White et al. 1999), increasing vigour of the trees and hence vessel size (Fonti et al. 2009b). The negative effect of precipitation in the preceding September (Fig. 2) might be related to a lower amount of solar radiation in precipitation-rich autumns. The negative effect of April precipitation, which has been observed also in southern region of distribution of oak (Fonti and Garcia-Gonzalez 2008), is difficult to explain. The positive correlation between VLA and temperature in the dormant period (Fig. 2) might be explained by decreased cold damage in warmer winters (Pearce 2001). Correlation with April temperature (Fig. 2) might be related to direct influence of environmental conditions on xylogenesis (Fonti and Garcia-Gonzalez 2008). Nevertheless, this relationship was weaker than observed for pedunculate oak (Matisons et al. 2012), suggesting red oak to be more robust against varying weather conditions in spring.
The effect of climatic factors on TRW and VLA has shifted during the 20th century, likely due to changes of the climate (Briede and Lizuma 2007; Lizuma et al. 2007) as suggested by the analysis of moving intervals (Fig. 3). The correlations between TRW and summer precipitation (Fig. 3) had become significant in all sites in the mid-part of the 20th century suggesting that limiting effect of water deficit has intensified, likely due to increasing temperature (Traykovic 2005). Such effect also seems to be facilitated by the enlargement of VLA, which occurred during recent decades (Fig. 1 D), suggesting that the risk of summer embolization and hence to physiological drought has increased (Tyree and Cochard 1996). The intensity of the effect of late summer (August) temperature that has been significant throughout the analysed period also has changed (Fig. 3). In the sites in western Latvia, where the climate is milder, the weakening of signatures of late summer (August) temperature in the TRW (Fig. 3) might be explained by the adaptation of the trees to increased temperature (Petit et al. 2004). Under harsher climate in the site in eastern Latvia, the effect of late summer temperature has been intensifying (Fig. 3) suggesting that the favourable temperature regime is being exceeded.
The correlations between temperature during the dormant period and VLA (Fig. 3) mainly have become non-significant during the analysed period that can be explained by warming of climate (Lizuma et al. 2007). In contrast, temperature in the dormant period has been significant for pedunculate oak during most of the 20th century (Matisons and Brūmelis 2012). The effect of January temperature has remained significant in SKD (Fig. 3) that is difficult to explain. Generally, the climatic limitation of VLA has shifted to temperature in previous September (Fig. 3) likely in response to extension of vegetation season (Ahas et al. 2000). Apparently, the observed negative correlations between TRW and September (late summer) temperature (Fig. 2) might be related to increased possibility of embolization caused by larger VLA. Considering that negative effect of precipitation on VLA of oak has been observed in Central Europe (Fonti and Garcia-Gonzalez 2008), the intensification of negative correlations with precipitation in AUC and AGL sites in the later part of the 20th century (Fig. 3) might be explained by temperature exceeding a certain threshold. The observed positive relationships between temperature and VLA (Fig. 3) suggested that the warming of climate apparently will continue to increase the susceptibility of red oak to embolization and hence to water deficit (Tyree and Zimmermann 2002), explaining the effect of water deficit related factors on radial increment (Fig. 3). Still, vessel size is physiologically determined (Tyree and Zimmermann 2002), therefore limiting increase of VLA. Alternatively, considering that at the beginning of the analysed period studied trees were young, changes in climatic sensitivity might be also age-related (Carrer and Urbinati 2004).
5 Conclusions
Climatic factors significantly affected TRW and VLA of the alien red oak in Latvia, but these relationships were weaker than for the native pedunculate oak, suggesting red oak to be more robust against weather conditions. Red oak has been losing sensitivity to temperature in the dormant period, hence at present this factor appears non-limiting for growth. Nevertheless, climate-TRW relationships suggested that wood increment of red oak was mainly affected by factors related to water deficit in late summer and that this effect has intensified. Temperature during the autumn-spring period has significantly affected VLA, suggesting that susceptibility to water deficit and xylem disfunction has been climatically predetermined. Considering the positive correlations between VLA and autumn-spring temperature, warming of the climate in Latvia might increase susceptibility of red oak to water deficit via altered wood anatomy increasing the significance of availability of water for growth.
Acknowledgements
The study was supported by Forest Competence Centre (ERAF) project “Methods and technologies for increasing forest capital value” (No. L-KC-11-0004).We acknowledge University of Latvia, Faculty of Biology and Didzis Elferts, which provided access to software for vessels measurements and helped with arrangements of climatic data. Thanks are extended to Juris Kalniņš and Oskars Krišāns, who helped during the sampling and Sabina J. Khan for proof reading and language editing. Revisions of two anonymous reviewers helped to improve the manuscript.
References
Ahas R., Jaagus J., Aasa A. (2000). The phenological calendar of Estonia and its correlation with mean air temperature. International Journal of Biometeorology 44: 159–166. http://dx.doi.org/10.1007/s004840000069.
Avotniece Z., Rodinov V., Lizuma L., Briede A., Kļaviņš M. (2010). Trends in frequency of extreme climate events in Latvia. Baltica 23: 135–148.
Bailie M.G.L., Pilcher J.R. (1973). A simple program for tree-ring research. Tree-Ring Bulletin 33: 7–14.
Barbaroux C., Breda N. (2002). Contrasting distribution and seasonal dynamics of carbohydrate reserves in stem wood of adult ring-porous sessile oak and diffuse porous beech trees. Tree Physiology 22: 1201–1210. http://dx.doi.org/10.1093/treephys/22.17.1201.
Biondi F., Waikul K. (2004). DENDROCLIM2002: A C++ program for statistical calibration of climate signals in tree ring chronologies. Computers and Geosciences 30: 303–311. http://dx.doi.org/10.1016/j.cageo.2003.11.004.
Brassier C.M. (1996). Phytophthora cinnamomi and oak decline in southern Europe. Environmental constraints including climate change. Annals of Forest Science 53: 347–358. http://dx.doi.org/10.1051/forest:19960217.
Briede A., Lizuma L. (2007). Long-term variability of precipitation in the territory of Latvia. In: Kļaviņš M. (ed.). Climate change in Latvia. University of Latvia, Rīga, Latvia. p. 35–45.
Bright R.M., Antón-Fernández C., Astrup R., Cherubini F., Kvalevåg M., Strømman A.H. (2014). Climate change implications of shifting forest management strategy in a boreal forest ecosystem of Norway. Global Change Biology 20: 607–621. http://dx.doi.org/10.1111/gcb.12451.
Bunn A.G. (2008). A dendrochronology program library in R (dplR). Dendrochronologia 26: 115–124. http://dx.doi.org/10.1016/j.dendro.2008.01.002.
Burton L.D. (2011). Introduction to forestry science, 3rd ed. Delmar, Clifton Park. 544 p.
Carlquist S.J. (2001). Comparative wood anatomy: systematic, ecological and evolutionary aspects of dicotyledon wood. Springer-Verlag, Berlin Heidelberg. 448 p.
Carrer M., Urbinati C. (2004). Age-dependent tree-ring growth responses to climate in Larix decidua and Pinus cembra. Ecology 85: 730–740. http://dx.doi.org/10.1890/02-0478.
Cook E.R., Holmes R.L. (1986). Guide for computer program ARSTAN. In: Holmes R.L., Adams R.K., Fritts H.C. (eds.). Tree-ring chronologies of western North America: California, eastern Oregon and northern Great Basin. University of Arizona Press, Tucson, Arizona. p. 50–65.
Dreimanis A., Šulcs V. (2006). Stands of red oak Quercus rubra L. in Skede forest district. Proceeding of Latvia University of Agriculture 17: 78–87. [In Latvian].
Epron D., Dreyer E. (1993). Long-term effects of drought on photosynthesis of adult oak trees [Quercus petraea (Matt.) Liebl., Quercus robur L.] in a natural stand. New Phytologist 125: 381–389. http://dx.doi.org/10.1111/j.1469-8137.1993.tb03890.x.
Fonti P., Garcia-Gonzalez I. (2008). Earlywood vessel size of oak as a potential proxy for spring precipitation in mesic sites. Journal of Biogeography 35: 2249–2257. http://dx.doi.org/10.1111/j.1365-2699.2008.01961.x.
Fonti P., Eilmann B., García-González I., von Arx G. (2009a). Expeditious building of ring-porous earlywood vessel chronologies without loosing signal information. Trees–Structure and Function 23: 665–671. http://dx.doi.org/10.1007/s00468-008-0310-z.
Fonti P., Treydte K., Osenstetter S., Frank D., Esper J. (2009b). Frequency dependent signals in multi-centennial oak vessel data. Palaeogeography, Palaeoclimatology, Palaeoecology 275: 92–99. http://dx.doi.org/10.1016/j.palaeo.2009.02.021.
Fonti P., von Arx G., García-González I., Elimann B., Sass-Klaassen U., Gärtner H., Eckstein D. (2010). Studying global changes through investigation on the plastic responses of xylem anatomy in tree-rings. New Phytologist 185: 42–63. http://dx.doi.org/10.1111/j.1469-8137.2009.03030.x.
Fritts H.C. (2001). Tree-rings and climate. The Blackburn Press, Caldwell. 582 p.
Garcia-Gonzalez I., Fonti P. (2006). Selecting earlywood vessels to maximize their environmental signal. Tree Physiology 26: 1289–1296. http://dx.doi.org/10.1093/treephys/26.10.1289.
Gonzalez-Gonzalez B.D., Rozas V., Garcia-Gonzalez I. (2014). Earlywood vessels of the sub-Mediterranean oak Quercus pyrenaica have greater plasticity and sensitivity than those of the temperate Q. petraea at the Atlantic–Mediterranean boundary. Trees-Structure and Function 28: 237–252. http://dx.doi.org/10.1007/s00468-013-0945-2.
Grissino-Mayer H.D. (2001). Evaluating crossdating accuracy: a manual and tutorial for the computer program COFECHA. Tree-Ring Research 57: 205–221.
Haldimann P., Feller U. (2004). Inhibition of photosynthesis by high temperature in oak (Quercus pubescens L.) leaves grown under natural conditions closely correlates with a reversible heat dependent reduction of the activation state of ribulose-1,5-bisphosphate carboxylase/oxygenase. Plant Cell and Environment 27: 1169–1183. http://dx.doi.org/10.1111/j.1365-3040.2004.01222.x.
Hanewinkel M., Cullmann D.A., Schelhaas M.J., Nabuurs G.J. (2012). Climate change may cause severe loss in the economic value of European forest land. Nature Climate Change 3: 203–207. http://dx.doi.org/10.1038/nclimate1687.
Harris I., Jones P.D., Osborn T.J., Lister D.H. (2014). Updated high-resolution grids of monthly climatic observations – the CRU TS3.10 Dataset. International Journal of Climatology 34: 623–642. http://dx.doi.org/10.1002/joc.3711.
Hickler T., Vohland K., Feehan J., Miller P.A., Smith B., Costa L., Giesecke T., Fronzek S., Carter T.R., Cramer W., Kuhn I., Sykes M.T. (2012). Projecting the future distribution of European potential natural vegetation zones with a generalized, tree species-based dynamic vegetation model. Global Ecology and Biogeography 21: 50–63. http://dx.doi.org/10.1111/j.1466-8238.2010.00613.x.
Hytteborn H., Maslov A.A., Nazimova D.I., Rysin L.P. (2005). Boreal Forests of Eurasia. In: Anddersson F. (ed.). Coniferous forests, ecosystems of the world. Sixth ed. Elsevier, Amsterdam, Netherlands. p. 23–99.
Jones E.W. (1959). Quercus L. Journal of Ecology 47: 169–222.
Jurik T.W., Weber J.A., Gates D.M. (1988). Effects of temperature and light on photosynthesis of dominant species of a northern hardwood forest. Botanical Gazette 149: 203–208.
Klavins M., Rodinov V. (2010). Influence of large-scale atmospheric circulation on climate in Latvia. Boreal Environment Research 15: 533–543.
Laiviņš M., Bice M., Krampis I., Knape D., Šmite D., Šulcs V. (2009). Atlas of Latvian woody plants. Apgāds Mantojums, Rīga, Latvia. 606 p. [In Latvian].
Ledig F.T., Kitzmiller J.H. (1992). Genetic strategies for reforestation in the face of global climate change. Forest Ecology and Management 50: 153–169. http://dx.doi.org/10.1016/0378-1127(92)90321-Y.
Lizuma L., Kļaviņš M., Briede A., Rodinovs V. (2007). Long-term changes of air temperature in Latvia. In: Kļaviņš M. (ed.). Climate change in Latvia. University of Latvia, Riga, Latvia. p. 11–20.
Maiorano L., Cheddadi R., et al. (2013). Building the niche through time: using 13,000 years of data to predict the effects of climate change on three tree species in Europe. Global Ecology and Biogeography 22: 302–317. http://dx.doi.org/10.1111/j.1466-8238.2012.00767.x.
Matisons R., Brūmelis G. (2012). Influence of climate on tree-ring and earlywood vessel formation in Quercus robur in Latvia. Trees-Structure and Function 26: 1251–1266. http://dx.doi.org/10.1007/s00468-012-0701-z.
Matisons R., Elferts D., Brūmelis G. (2012). Changes in climatic signals of English oak tree-ring width and cross-section area of earlywood vessels in Latvia during the period 1900–2009. Forest Ecology and Management 279: 34–44. http://dx.doi.org/10.1016/j.foreco.2012.05.029.
Morecroft M.D., Stokes V.L., Morison J.I.L. (2003). Seasonal changes in the photosynthetic capacity of canopy oak (Quercus robur) leaves: the impact of slow development on annual carbon uptake. International Journal of Biometeorology 47: 221–226. http://dx.doi.org/10.1007/s00484-003-0173-3.
Pallardy S.G. (2008). Physiology of woody plants. Third ed. Elsevier, London, UK. 464 p.
Parry M., Canziani O., Palutikof J., van der Linden P., Hanson C. (eds.). (2007). Climate change 2007: impacts, adaption and vulnerability. Cambridge University Press, Cambridge, UK. 976 p.
Pearce R.S. (2001). Plant freezing and damage. Annals of Botany 87: 417–424. http://dx.doi.org/10.1006/anbo.2000.1352.
Petit R.J., Bialozyt R., Garnier-Gere P., Hampe A. (2004). Ecology and genetics of tree invasions: from recent introductions to quaternary migrations. Forest Ecology and Management 197: 117–137. http://dx.doi.org/10.1016/j.foreco.2004.05.009.
R Core Team (2014). R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/. [Cited 1 January 2014].
Rozas V. (2005). Dendrochronology of pedunculate oak (Quercus robur L.) in an oldgrowth pollarded woodland in northern Spain: tree-ring growth responses to climate. Annals of Forest Science 62: 209–218. http://dx.doi.org/10.1051/forest:2005012.
Saliņš S. (1971). Establishment of foreign tree species. In: Bušs M., Mangalis I. (eds.). Forest cultures. Zvaigzne, Rīga, Latvia. p. 382–409. [In Latvian].
Sander I.L. (1990). Quercus rubra L. Northern red oak. In: Burns R.M., Honkala B.H. (eds.). Silvics of North America, Vol. 2, Hardwoods. Forest Service United States Department of Agriculture, Washington, DC. p. 727–733.
Speer J.H. (2010). Fundamentals of tree-ring research. The University of Arizona Press, Tucson, Arizona. 333 p.
Thomas F.M., Blank R., Hartmann G. (2002). Abiotic and biotic factors and their interactions as causes of oak decline in Central Europe. Forest Pathology 32: 277–307. http://dx.doi.org/10.1046/j.1439-0329.2002.00291.x.
Traykovic S. (2005). Temperature-based approaches for estimating reference evapotranspiration. Journal of Irrigation and Drainage Engineering-ASCE 131: 316–323. http://dx.doi.org/10.1061/(ASCE)0733-9437(2005)131:4(316).
Tyree M.T., Cochard H. (1996). Summer and winter embolism in oak: impact of water relations. Annals of Forest Science 53: 173–180. http://dx.doi.org/10.1051/forest:19960201.
Tyree M.T., Zimmermann M.H. (2002). Xylem structure and ascent of sap. Springer, Berlin, Germany. 283 p.
Walther G.R., Post E., Convey P., Menzel A., Parmesan C., Beebee T.J.C., Fromentin J.M., Hoegh-Guldberg O., Bairlein F. (2002). Ecological response to recent climate change. Nature 416: 389–395. http://dx.doi.org/10.1038/416389a.
White M.A., Running S.W., Thornton P.E. (1999). The impact of growing-season length variability on carbon assimilation and evapotranspiration over 88 years in the eastern US deciduous forest. International Journal of Biometeorology 42: 139–145. http://dx.doi.org/10.1007/s004840050097.
Wigley T.M.L., Briffa K.R., Jones P.D. (1984). On the average value of correlated time series, with applications in dendroclimatology and hydrometeorology. Journal of Climate and Applied Meteorology 23: 201–213. http://dx.doi.org/10.1175/1520-0450(1984)023<0201:OTAVOC>2.0.CO;2.
Zhang S.Y. (1997). Variations and correlations of various ring width and ring density features in European oak: implications in dendroclimatology. Wood Science and Technology 31: 63–72. http://dx.doi.org/10.1007/BF00705701.
Total of 55 references

