Increased forest biomass production in the Nordic and Baltic countries – a review on current and future opportunities
Rytter L., Ingerslev M., Kilpeläinen A., Torssonen P., Lazdina D., Löf M., Madsen P., Muiste P., Stener L.-G. (2016). Increased forest biomass production in the Nordic and Baltic countries – a review on current and future opportunities. Silva Fennica vol. 50 no. 5 article id 1660. https://doi.org/10.14214/sf.1660
Highlights
- Annual growth is 287 million m3 in the forests of the Nordic and Baltic countries
- Growth can be increased by new tree species, tree breeding, high-productive management systems, fertilization and afforestation of abandoned agricultural land
- We predict a forest growth increment of 50–100% is possible at the stand scale
- 65% of annual growth is harvested today.
Abstract
The Nordic and Baltic countries are in the frontline of replacing fossil fuel with renewables. An important question is how forest management of the productive parts of this region can support a sustainable development of our societies in reaching low or carbon neutral conditions by 2050. This may involve a 70% increased consumption of biomass and waste to meet the goals. The present review concludes that a 50–100% increase of forest growth at the stand scale, relative to today’s common level of forest productivity, is a realistic estimate within a stand rotation (~70 years). Change of tree species, including the use of non-native species, tree breeding, introduction of high-productive systems with the opportunity to use nurse crops, fertilization and afforestation are powerful elements in an implementation and utilization of the potential. The productive forests of the Nordic and Baltic countries cover in total 63 million hectares, which corresponds to an average 51% land cover. The annual growth is 287 million m3 and the annual average harvest is 189 million m3 (65% of the growth). A short-term increase of wood-based bioenergy by utilizing more of the growth is estimated to be between 236 and 416 TWh depending on legislative and operational restrictions. Balanced priorities of forest functions and management aims such as nature conservation, biodiversity, recreation, game management, ground water protection etc. all need consideration. We believe that these aims may be combined at the landscape level in ways that do not conflict with the goals of reaching higher forest productivity and biomass production.
Keywords
fertilization;
tree breeding;
tree species;
coppice;
cultivation areas;
growth increment;
nurse crops
-
Rytter,
The Forestry Research Institute of Sweden (Skogforsk), Ekebo 2250, SE-26890 Svalöv, Sweden
E-mail
lars.rytter@skogforsk.se

- Ingerslev, Copenhagen University, Department of Geosciences and Natural Resource Management, Rolighedsvej 23, DK-1958, Frederiksberg C, Denmark E-mail moi@ign.ku.dk
- Kilpeläinen, Finnish Environment Institute, Joensuu Office, P.O. Box 111, FI-80101 Joensuu, Finland; University of Eastern Finland, Faculty of Science and Forestry, School of Forest Sciences, P.O. Box 111, FI-80101 Joensuu, Finland E-mail antti.kilpelainen@ymparisto.fi
- Torssonen, University of Eastern Finland, Faculty of Science and Forestry, School of Forest Sciences, P.O. Box 111, FI-80101 Joensuu, Finland E-mail Piritta.Torssonen@uef.fi
- Lazdina, Latvian State Forest Research Institute “Silava”, 111 Riga str, Salaspils, LV 2169 Latvia E-mail Dagnija.Lazdina@silava.lv
- Löf, Swedish University of Agricultural Sciences, Southern Swedish Forest Research Centre, Box 49 SE-230 53 Alnarp, Sweden E-mail magnus.lof@slu.se
- Madsen, Copenhagen University, Department of Geosciences and Natural Resource Management, Rolighedsvej 23, DK-1958, Frederiksberg C, Denmark E-mail pam@ign.ku.dk
- Muiste, Estonian University of Life Sciences, Institute of Forestry and Rural Engineering, Dept. Forest Industry, Kreutzwaldi 5, Tartu 51014, Estonia E-mail Peeter.Muiste@emu.ee
- Stener, The Forestry Research Institute of Sweden (Skogforsk), Ekebo 2250, SE-26890 Svalöv, Sweden E-mail Lars-Goran.Stener@skogforsk.se
Received 8 June 2016 Accepted 18 October 2016 Published 27 October 2016
Views 472294
Available at https://doi.org/10.14214/sf.1660 | Download PDF

1 Introduction
The focus of this review is on the potential role of forests and forest management, which will contribute to make Nordic energy systems carbon neutral by 2050. Biomass and renewable waste are the main sources of renewable energy in the Nordic and Baltic countries, here including Denmark, Estonia, Finland, Latvia, Lithuania, Norway, and Sweden. The share is ranging from 50% of renewables in Denmark to 97% in Estonia (Eurostat 2013). The exception is Norway where hydropower dominates. Forests cover more than 4.5 times the area of agricultural land in the region (FAO 2014a) and woody biomass is by far the largest component of the bioenergy supply (Mantau et al. 2010).
Wood products from well-managed forests complies with the ‘Brundtland’ sustainability declaration, i.e. by contributing to social, environmental, and economic well-being for the present as well as future generations (United Nations 1987; Lippke et al. 2011). Thereby the forest management may not only represent a sustainable management of the forest itself but equally important, it should support the sustainable development of society. This is an issue now rapidly climbing on the political agenda (FAO 2012) based on increasing evidence of rapid climate change caused by the extensive use of fossil fuels (IPCC 2014).
The most productive and intensively managed forests of the world are by the FAO categorized as planted forests (FAO 2016a). This concept includes forests predominantly established through planting or direct seeding. Globally, the planted forests occupied 271 million ha in 2005, which corresponds to 7% of the total forest or 2% of the total land area (FAO 2010). The productivity of planted forests is, however, so high that their potential capacity is expected to cover two-thirds of the global consumption of industrial wood. Moreover, by expanding areas through forest landscape restoration efforts (Jacobs et al. 2005; Stanturf 2015; Stanturf et al. 2014, 2015) in combination with continued improvements of management, site-adapted species selection and genetic improvement, the production potential is expected to expand even further (Carle et al. 2009).
The productive planted forests in the Nordic and Baltic countries become highly relevant in the climate change mitigation strategies with regard to forest management and forest landscape restoration. These forests already cover a relatively high proportion of today’s production of industrial wood. They are often intensively managed, which is important for the potential to further increase the forest productivity. The planted forests of the Nordic and Baltic region belong today predominantly to the semi-natural forest types, whereas the plantations including e.g. non-native tree species – except in Denmark – only cover small proportions of the forest area.
The highest proportion of planted forests, but also the smallest forest area, is found in Denmark with an estimated 66% share of productive planted forests (FAO 2014b). Second and third are, however, the two largest countries, Sweden and Finland, with planted forest shares of 43 and 26%, respectively.
The productive planted forests and particularly plantation forests reach levels of productivity that are comparable, or higher, with those of agricultural energy crops, but with generally less need for intensive management such as use of fertilizer, frequent and intensive harvests, soil preparation and drainage work (e.g. Christersson and Sennerby-Forsse 1994; Christersson 2010). Compared to agricultural residues and short rotation energy crops, forestry produces timber and biomass that may have multiple uses and options for recycling before final use as energy. In addition, the woody biomass typically has a lower mineral content and higher energy density than non-woody biomass (e.g. McKendry 2002). These characteristics underline the low energy use needed for production, harvesting and transport and the low environmental impact of forestry (United Nations 1987; Gustavsson et al. 2007, 2011).
Increased harvest levels and use of growth promoting measures will affect stands and landscapes. The influence can be both positive and negative depending on the factors studied and the prior conditions (e.g. Hartley 2002). However, it is not the aim of this review to examine the environmental effects.
The aim is to inform about the potentials, limitations and processes of sustainable forestry in the Nordic and Baltic region to support the development of competitive, efficient and renewable energy systems. This information is valuable since the political decision of making the Nordic energy systems carbon neutral by 2050 (Nordiska Ministerrådet 2009; IEA 2013) has recently been adopted. The main focus of our study is thus to illuminate the potential of the forests – particularly the intensively managed planted forests – to contribute cost-efficiently and with high impact to a sustainable development of our modern societies in the light of climate change.
2 Current situation on biomass availability
The Nordic and the Baltic countries are generally rich in forests (Table 1; Rytter et al. 2014a, 2015). The majority of the forests are located in the boreal and temperate continental zones where the land-use of the low-lands has not widely and permanently been changed into farmland. About 51% of the land area is defined as productive forest land, and an additional 21% are low-productive lands of different kinds (Table 1). Sweden and Finland have the highest shares of productive forest land (57 and 67%, respectively). Agricultural lands are dominating only in Denmark and Lithuania, where forest land is only 14 and 34%, respectively, of the land area. A comparably small share of forest land in Norway (38%) is mainly explained by large areas of mountains and plateaus. Considering only altitudes below the tree limit Norway has almost 80% forest cover.
| Table 1. Land areas in 1000 ha, distributed on land use classes in Nordic and Baltic countries, but not including Iceland. | ||||||
| Country | Productive forest land | Other wooded land, poorly productive forest land, high altitude mountains, plateaus etc., naturally colonized and sparsely wooded land | Barren land, unproductive land, marsh/wetlands, wetlands, dunes etc., other land | Other land, agriculture, build-up areas, artificial, agricultural areas etc., agriculture land | Total land area | Productive forest land of total land area (%) |
| Denmark | 608 | 45 | 295 | 3361 | 4310 | 14.1 |
| Finland | 20 259 | 2518 | 3196 | 4442 | 30 414 | 66.6 |
| Norway | 11 622 | 15 638 | 1765 | 1400 | 30 425 | 38.2 |
| Sweden | 23 171 | 7245 | 4968 | 5346 | 40 729 | 56,9 |
| Estonia | 2212 | 79 | 604 | 1374 | 4269 | 51.8 |
| Latvia | 2974 1) | 113 2) | 946 2) | 2403 2) | 6448 2) | 46.1 |
| Lithuania | 2220 | 106 | 735 | 3467 | 6528 | 34.0 |
| Total | 63 066 | 25 744 | 12 509 | 21 793 | 123 123 | 51.2 |
| Sources: Danmarks Statistik (2012), Johannsen et al. (2013), Estonian Environment Information Centre (2012), Finnish Forest Research Institute (2012a), 1) mean value from Latvian State Forest Service (2012) and 2) Latvian State Land Service (2012), Lithuanian Ministry of Environment (2015), Statistics Norway (2011) and Swedish Forest Agency (2014) | ||||||
Not all productive forest areas are however available for wood supply due to different kinds of legislation and protection, but there is still about 85% of the forest land area accessible where harvest operations are allowed and can be carried out. The range in the different countries is 75–92% (Rytter et al. 2015).
There is a trend in the region that fertile forest soils are less protected than less fertile ones, which means that a reduction of forest land area will be of less importance for available biomass than the area figures suggest (Rytter et al. 2014a). Accordingly, it has been reported that 89% of the growing stock is available for wood supply within a sustainable forest management context (Forest Europe 2011). The range was 85–93% among the Nordic and Baltic countries. The difference between annual increment in the area (287 million m3 of stem wood over bark) and annual fellings (189 million m3) shows that on average about 65% of the annual growth is used for wood supply (Forest Europe 2011; Rytter et al. 2015). The range is 35–84% among the countries.
The total potentially available forest fuel of the region amounts to between 236 and 416 TWh yr–1 (854–1508 PJ) depending on restriction levels (Table 2). At the national level the Danish potential of forest fuel supply is 5–12 TWh (Graudal et al. 2013). For Finland, prognoses by Kärkkäinen et al. (2008) of the theoretical potential indicate 22–35 million tons of logging residues yr–1 (117–186 TWh yr–1) in a sustainable cutting scenario with the current climate. Norwegian estimates of the current forest fuel potential include three levels, from all possible wood to technical and biological restrictions (Gjølsjø and Hobbelstad 2009). The annual harvesting potential was found to be 3.8–5.1 million tons dry mass (DM) yr–1 and represents 20–27 TWh yr–1 for energy purposes. Swedish calculations for the period 2010–2019 showed a potential of 11–29 million tons DM yr–1, which was translated as 53–143 TWh yr–1, with three levels involved (Swedish Forest Agency 2008). In Estonia around 6.2 million m3 yr–1 (c. 13.5 TWh yr-1) has been considered available according to the moderate wood supply scenario, while in Latvia the technologically available forest fuel amount has been estimated to almost 24 TWh yr–1 (Gruduls et al. 2013). With data from FAO (2016b) the available forest fuel potential in Lithuania was estimated to 1.4 million tons DM yr–1 (c. 7.4 TWh yr–1).
| Table 2. Annual current harvest potential of forest fuels in the Nordic and Baltic countries. Table revised from Rytter et al. (2015) with Lithuanian data from FAO (2016b). | ||||||
| Country | Potential with lowest level of restrictions | Potential with highest level of restrictions | ||||
| Mton DM | TWh | PJ | Mton DM | TWh | PJ | |
| Denmark | 2.3 | 11.5 | 42 | 1.0 | 5.1 | 18 |
| Finland | 35 | 186 | 670 | 22 | 117 | 420 |
| Norway | 5.1 | 27.1 | 98 | 3.8 | 20.4 | 74 |
| Sweden | 29.3 | 143.4 | 522 | 10.9 | 53.1 | 194 |
| Estonia1) | 3.2 | 16.8 | 62 | 1.7 | 9.1 | 33 |
| Latvia | 4.5 2) | 23.9 | 87 | n.a.3) | n.a.3) | n.a.3) |
| Lithuania | 1.4 4) | 7.4 | 27 | n.a.3) | n.a.3) | n.a.3) |
| Summary | 80.9 | 416 | 1508 | 45.4 | 236 | 854 |
| 1) = estimation according to scenarios of the Forestry Development Programme for the period 2011–2020. 2) = calculated backwards, 3) = the same value was used for lowest and highest restriction levels in the Summary row, n.a. = not available, 4) = calculated from FAO information. | ||||||
The primary supply of biomass and waste in 2010 was according to Eurostat (2012) 26 TWh in Denmark, 102 TWh in Finland, 15 TWh in Norway, 124 TWh in Sweden, 12 TWh in Estonia, 22 TWh in Latvia and 14 TWh in Lithuania (Table 3). However, values are not easily comparable between scenarios and real supply. The real supply includes waste and it should be noted that forest biomass is one part in the supply. Nevertheless, the figures indicate that substantially more forest fuels can be utilized with the current forestry than are used today.
| Table 3. Primary production of renewable energy (TWh) in 2013. | ||||||
| Country | Total primary supply | Solar energy | Biomass and waste | Geothermic energy | Hydropower energy | Wind energy |
| Denmark | 37.7 | 0.8 | 25.7 | 0.1 | 0 | 11.1 |
| Finland | 115.5 | 0 | 101.9 | 0 | 12.8 | 0.8 |
| Norway | 144.9 | 0 | 14.5 | 0 | 128.5 | 1.9 |
| Sweden | 195.0 | 0.2 | 123.7 | 0 | 61.4 | 9.8 |
| Estonia | 13.0 | 0 | 12.4 | 0 | 0 | 0.5 |
| Latvia | 24.9 | 0 | 21.8 | 0 | 2.9 | 0.1 |
| Lithuania | 15.0 | 0 | 13.8 | 0 | 0.5 | 0.6 |
| Total | 546.0 | 1.0 | 313.8 | 0.1 | 206.1 | 24.8 |
| Source: Eurostat (2013): http://ec.europa.eu/eurostat/statistics-explained/index.php/File:Primary_production_of_renewable_energy,_2003_and_2013_YB15.png | ||||||
3 Effect of climate change on productivity
3.1 Background
The rise of carbon dioxide (CO2) concentration in the atmosphere has been estimated to increase global mean temperatures on average up to 4 °C by the end of the century (IPCC 2013). In recent climate estimates, CO2 concentration is expected to increase linearly from 389 to 538 ppm under the RCP4.5 scenario, and under the RCP 8.5 scenario from 389 to 935 ppm by the end of 2100 (Taylor et al. 2012). In the RCP4.5 pathways, the global temperature may increase 2.4 °C relative to the pre-industrial level by the end of the century. In a business-as-usual scenario the global mean temperature may increase 4.3 °C. The rise in temperature will also have impacts on global precipitation and evaporation, and the magnitude of the projected changes in climatic conditions varies around the world. A larger warming may occur in high latitudes and the highest increases in precipitation are foreseen in mid and high latitudes (IPCC 2013). However, projected changes in precipitation vary greatly among climate models.
The estimated temperature increase in the Nordic region is similar to the global mean in the southern and western parts but almost double in the north and east. The increase will be largest during wintertime and in areas with a continental climate. Precipitation is projected to increase in the Fennoscandian countries (Finland, Sweden, Norway), especially during winter. However, summer precipitation may decrease in the southern part of the region. More and heavier extreme precipitation events are also expected (IPCC 2013). Thus, according to new climate scenarios, climate change may increase both mean annual temperature and precipitation. In northern Europe (53.75°–71.25°N and 3.75°– 41.25°E), this means on average 2.5 °C warmer during summer and 3.5 °C warmer in winter by the end of the 21st century. The average figures on increased precipitation in northern Europe are 3 and 13%, respectively (Räisänen and Ylhäisi 2014).
The rate of change in climatic conditions will affect current biomes and causes concerns on their capability to adapt to the changing conditions (Walther et al. 2002). In addition, the forest biomes may be under extended focus with respect to climate change mitigation as they have many roles in this context (Canadell and Raupach 2008).
3.2 Effects of climate change and forest management on growth and biomass production in Nordic boreal conditions
Climate change will probably enhance growth of forests in northern Europe directly through physiological response to elevated CO2 and temperature (Bergh et al. 2003; Briceño-Elizondo et al. 2006; Kirilenko and Sedjo 2007). A longer growing season and enhanced mineralization of nitrogen may add to increased forest growth (Saxe et al. 2001; Kellomäki et al. 2005, 2008; Hyvönen et al. 2007; Alam et al. 2010). The concurrent elevation of mean annual temperature and atmospheric CO2, together with changes in precipitation, are thus expected to greatly affect the functioning and dynamics of boreal forests and the biomass production and carbon sequestration of forest ecosystems (Garcia-Gonzalo et al. 2007a, 2007b; Kellomäki et al. 2008; Alam et al. 2010; Poudel et al. 2011, 2012).
Forest growth is expected to be higher in absolute terms in southern Finland and Sweden than in northern parts of these countries, but in relative terms, the growth will probably be higher in the north (Kellomäki et al. 2008; Swedish Forest Agency 2008; Alam et al. 2010). The growth of forests in Finland, with current forest management practices, may be 29% higher by 2050 and 44% higher by 2100 compared to today (Kellomäki et al. 2008). The Swedish Forest Agency (2008) predicted a 25% increase in annual stem wood production in Swedish forests due to the direct effects of climate change by the end of the century. For north-central Swedish conditions it has been estimated that an increase in CO2 and an increase of temperature by 4 °C will lead to an increase in forest growth of 33%, and thereby a potential cutting increase of 32% by the end of the century (Poudel et al. 2011).
The changes in forest growth may be species-specific and depend on site fertility and environmental conditions at the stand level (Kellomäki et al. 2008; Ge et al. 2013a, 2013b; Granda et al. 2013; Torssonen et al. 2015). For example, the growth of Scots pine (Pinus sylvestris L.) has been estimated to increase in boreal Finland and Sweden. Deciduous trees will probably also benefit from a changed climate in the northern boreal zone (Kellomäki et al. 2008; Poudel et al. 2012). In contrast, the productivity of Norway spruce (Picea abies (L.) Karst.) has been estimated to decrease in some sites in Finland as the conditions will be suboptimal with a gradual climate change. A negative growth effect may be strongest on dry sites where the performance of Norway spruce is already low due to water limitations (Kellomäki et al. 2005). A simulation study in north-central Sweden showed that the biomass growth of Norway spruce was not affected by changed climatic conditions under varying intensity of forest management (Poudel et al. 2012). In order to adapt to future changed climatic conditions, proper site-specific cultivation of different tree species may ensure sufficient water and nutrient availability for tree growth under boreal conditions (Kolström et al. 2011; Torssonen et al. 2015).
The forests on a landscape level constitute mosaics of single stands with varying site fertility, tree species composition, age structure and biomass stocking that further affect the biomass potential (e.g. Routa et al. 2012). Forest management practices, such as thinning, are thus affecting biomass production and carbon sequestration together with changed climatic conditions (Briceño-Elizondo et al. 2006; Garcia-Gonzalo et al. 2007a, 2007b; Matala et al. 2009). They both influence growth and development of the tree stand by redistributing the extra available resources to the remaining trees. Thus, the management of the forest stands can modify biomass harvesting both for energy biomass and timber purposes.
The mean annual carbon stock and carbon sequestration of forests may be increased over a rotation by maintaining a high stocking, which results in lower harvesting frequency and somewhat lower timber production than in the business-as-usual management (Garcia-Gonzalo et al. 2007a, 2007b; Nunery and Keeton 2010; Alam et al. 2010; Torssonen et al. 2016). In Finland, for example, maintaining high stocking of stands after thinning enhanced carbon stocks and energy wood production at final felling compared with business-as-usual thinning over a 90-year period (2010–2100) according to Alam et al. (2010). Maintaining high stocking increased productivity also under changed climatic conditions, while maintaining low stocking decreased the growth and timber production over the whole country (Alam et al. 2008, 2010). Alam et al. (2010) also found that climate change and age structure effects in Finnish forests were interactive, and that forest age structure affected the future growth of the forests more than did the climate change over a 90-year simulation period. Torssonen et al. (2016) reported an increase in carbon stocks, but a reduction in economic profitability in Norway spruce stands over an 80-year rotation period when stocking was 20% higher than current recommendations in Finland. However, the calculated net climate impacts for higher stocking were improved compared to the business-as-usual management (Torssonen et al. 2016). The use of nitrogen fertilization may also improve climate impacts of energy biomass utilization and economic profitability of management, both under current and changing climatic conditions (Pyörälä et al. 2014; Torssonen et al. 2016). The efficiency of climate change mitigation by utilizing energy biomass was, however, found to be lower under changing climate compared to under the current climate (Torssonen et al. 2016).
4 Possibilities to improve a sustainable biomass supply
4.1 The status of tree breeding and its potential for improving biomass production
4.1.1 Background
Forest tree breeding is considered an effective and environmentally friendly option to increase sustainable biomass production in our forests. The effect of using genetically improved forest plant material is, at the stand level, similar to increasing the site index. The forest grows faster, the harvest comes earlier and the rotation time can be shorter, while effects on the environment are small. Improved material has better overall quality and survival, of which the latter is of great importance in northern areas with harsh climate.
4.1.2 Tree breeding procedures
Traditional selection breeding is dominantly used in Scandinavia and includes field testing and selection of the best individuals for future breeding and for mass propagation of improved material for commercial deployment. Molecular and biotech methods have so far had little impact on the actual breeding process. However, there is an on-going progress in the development of gene sequencing techniques (Meuwissen 2009). Thus, selection by using genetic markers for traits affected by few genes, such as resistance to pathogens, may be applied in the near future.
The breeding is focused on traits of economic value for production of timber and pulp such as growth, survival, stem quality and vitality. Vitality is a complex trait that encompasses climatic adaptation, resistance to pathogens and robustness over a wide range of environments.
4.1.3 Status and genetic gains
Intensive and long-term breeding in Sweden and Finland is in progress for Scots pine, Norway spruce and to some extent birch (Betula spp.), while other species are worked with intermittently. In Norway the interest in breeding has now resumed after a drop in activities for some years, resulting in a new breeding program for Norway spruce. The aim of the programs is to combine intensive breeding, gene conservation and preparedness for future climatic changes (Danell 1993; Edvardsen et al. 2010; Ruotsalainen and Persson 2013; Westin and Haapanen 2013). The present resources directed to breeding in Denmark are small, partly due to a major change in silviculture management towards nature-based forest management in the publicly owned forests.
Seed from seed orchards is the dominant way to supply forestry with genetically improved plant material. The total number of plants of Norway spruce and Scots pine originating from seed orchards currently amounts to around 350, 170 and 30 million yr–1 in Sweden, Finland and Norway respectively, and corresponds to 94–99% of the total number of plants used. Denmark is in this respect a small country where around 10 million plants are used annually, of which roughly 50% is oak (Quercus robur L.) and beech (Fagus sylvatica L.). The average extra gain in yield obtained by using material from existing seed orchards is currently 10–15% compared to local unimproved material. In 2050 it is estimated that this gain will be raised to 20–25% and together with increased use of improved plants, this will result in a substantial increase in forest productivity.
The time from establishment of a seed orchard to the first seed harvest is often long (10–20 years) and delays the realisation of the genetic improvement efforts. An alternative is to use plants obtained from vegetative propagation of genetically well-performing seed sources or individuals, which makes it possible to capture the progress from breeding immediately. Utilisation of clones is also a way to reduce the consequences of limited amounts of seed from seed orchards, which for instance is the case for Norway spruce in southern Sweden. Using clonal material for Norway spruce can immediately deliver a gain of around 25–35% in yield, and by 2050 this figure may be increased to 40% (Haapanen et al. 2015). It should be noted, however, that the use of clonal material can be limited by forest regulations.
There are also other species than Norway spruce and Scots pine that are genetically improved (see Breeding and climatic change below), but their contribution to the overall increase in production is small so far due to their limited use in forestry.
4.1.4 Breeding and climate change
Trees will adapt to a changing climate by natural selection in the long-run, but any adaptation occurs in response to current conditions. Thus, adaptation lags behind since it happens after any change in climate. Breeding, on the other hand, provides the opportunity to adapt trees to future climate in a faster and more efficient way. The Multiple Population Breeding Strategy (MPBS) for example, is designed to provide preparedness for future climatic changes by dividing the breeding population into different sub-populations (Danell 1993), each of which is bred for different adaptation targets defined by temperature and photo period. On a long-term basis, each sub-population will gradually adapt to the climate profile for which it is designed. In the short-term it will be possible to use material from sub-populations adapted to southerly climates in northerly areas.
Another way to be prepared for an unknown future is to increase the plasticity and adaptability of existing populations (Haapanen et al. 2015). This is achieved by establishing field tests at several sites covering a wide range of climate conditions, and selecting trees that perform well at all sites. Genotypes performing well across a range of sites can be expected to be more robust, which is desirable when dealing with varied growth conditions and an uncertain future climate.
4.2 Effects of changing tree species
The main part of forest resources in northern Europe consists of native tree species, which are well adapted to the current climatic and edaphic conditions. There are also exotic (or non-native) species, which grow successfully under these conditions (Table 4).
| Table 4. Growing stocks and areas of native and exotic tree species in the Nordic and Baltic countries. Table revised from Rytter et al. (2013) now including Lithuanian data. | ||
| Tree species | Growing stock, (million m3) | Area as dominant tree species (ha) |
| Native species | ||
| Norway spruce | >2800 | c. 19 million |
| Scots pine | c. 3500 | >30 million |
| Silver and downy birch | c. 1550 | c. 8 million |
| Black and grey alder | c. 380 | – |
| Aspen | >180 | – |
| Oak | >80 | – |
| Beech | >40 | – |
| Exotic species | ||
| Lodgepole pine | c. 30 | c. 600 000 |
| Sitka spruce | – | c. 85 000 |
| Douglas fir | – | >6000 |
| Grand fir | – | c. 3000 |
| Hybrid larch | c. 1.4 | – |
| Siberian larch | – | c. 30 000 |
| Populus (excl. P. tremula) | – | c. 5000 |
Future climate will probably change the growing conditions in a way that makes forestry with exotic species more attractive or possibly necessary (Rosvall 2011; Kjaer et al. 2014). Using several species instead of just a few is one way to spread risks of an unknown future. Several exotic species with a potential to grow in Scandinavia are already in field tests aimed at selecting genotypes for commercial use. However, there is a need for analysis of environmental consequences, political decisions and general acceptance by the environmental certification systems before these species could be introduced for commercial use on a broader scale (Haapanen et al. 2015). In Denmark, however, several exotic conifers and broadleaved species have been an integrated part of the forestry for more than 100 years and they cover 50% of the forest area today.
The most abundant native species are Norway spruce, Scots pine, and silver and downy birch (Betula pendula Roth and B. pubescens Ehrh.). Furthermore, aspen (Populus tremula L.) is a common species but grows seldom in pure stands. Black alder (Alnus glutinosa (L.) Gaertn.) and grey alder (Alnus incana (L.) Moench.) are also widespread in the Nordic and Baltic countries, while the hardwoods common in Central Europe (e.g. oak and beech) are only growing in the southern part of the region.
Introduced exotic coniferous and deciduous species have generally high growth potentials but their roles in supplying energy biomass is still small (Table 4). Exotic conifers include Sitka spruce (Picea sitchensis (Bong) Carrière), Douglas fir (Pseudotsuga menziesii (Mirb.) Franco), grand fir (Abies grandis (Douglas ex D.Don)) and hybrid larch (Larix ×eurolepis Henry) in the southern parts of the Nordic and Baltic area. In the north, lodgepole pine (Pinus contorta Douglas ex Loudon) and also Siberian larch (Larix sibirica Ledeb.) have been successfully established. Exotic deciduous species include several poplars (Populus spp.), hybrid aspen (P. tremula L. × P. tremuloides Michx.) and willows (Salix spp.), the latter only used on agricultural land. Table 5 shows productivity and wood density for relevant tree species.
| Table 5. Estimated productivity of stem wood of selected trees species representing natural populations and present genetic gain representing populations originating from genetically improved trees. The values represent populations on suitable sites in the southern and central parts of the Nordic and Baltic region. Data taken from Rytter et al. (2013) and Haapanen et al. (2015). | |||||
| Tree species | MAI for “natural” stands (m3 ha–1 yr–1) | Present genetic gain (%) | MAI for improved plant material 4) (m3 ha–1 yr–1) | MAI for improved plant material 3) (Mg ha–1 yr–1) | Basic wood density (kg m–3) |
| Norway spruce | 4–18 | 8–25 | 5–19 | 2–7 | 350 |
| Scots pine | 2–7 | 0–15 | 2–7 | 1–3 | 440 |
| Silver birch | 7–8 | 10–25 | c. 9 | c. 4 | 480 |
| Black alder | 9 | 10 | 10 | 4 | 370 |
| Grey alder | 10–15 | 18 3) | 12–18 | 4–6 | 360 |
| Aspen | 7–10 | n.a. | 3–4 | 380 | |
| Oak | 4–6 | 0–10 | 4–7 | 2–4 | 575 |
| Beech | 6–10 | 6–10 | 7–11 | 4–6 | 580 |
| Poplar | 20–25 2) | n.a. | 20–25 | 8–9 | 345 |
| Hybrid aspen | 16 | 40 | 22 | 8 | 360 |
| Lodgepole pine 1) | 5–7 | 10 | 5–8 | 2–3 | 430 |
| Hybrid larch | 12–13 | 30–60 | 16–21 | 6–9 | 411 |
| Siberian larch 1) | 4–6 | 10 | 4–6 | 3–4 | 600 5) |
| Sitka spruce | 12–18 | 0–40 | 12–24 | 4–9 | 360 |
| Douglas fir | 15–17 | 8 | 16–18 | 7–8 | 450 |
| Grand fir | c. 21 | 0–20 | 21–25 | 7–9 | 350 |
| MAI is the mean annual increment; n.a. is not available. 1) = In the central and northern part of the region; 2) = Result obtained with the OP42 clone; 3) = Estimated in Rytter and Rytter (2016); 4) = Where improved material was not available, the figures were based on the productivity in natural stands; 5) = Density based on volume of 5% moisture content, and thus resulting in an overestimation of the productivity in terms of mass. | |||||
The most important and fast-growing native and non-native tree species are presented in the following two sections.
4.2.1 Common native species
Conifers
Norway spruce is a dominant species in northern Europe. It occupies 19 million hectares of forest land and the total growing stock is 2800 million m3 (Keskkonnateabe Keskus 2010; Bekeris 2011; Danmarks Statistik 2012; Directorate General of State Forests 2012; Finnish Forest Institute 2012a; Statistics Norway 2013; Swedish Forest Agency 2013; Lithuanian Ministry of Environment 2015). Norway spruce is also the most planted tree species in the region, with an annual production of more than 350 million plants (Finnish Forest Research Institute 2012a; Swedish Forest Agency 2013). The species is native throughout the Nordic and Baltic countries, except Denmark (Hultén 1950), and is best suited to mesic and nutrient-rich sites (Seppä et al. 2009). It is shade-tolerant with a comparatively low initial growth rate but with faster growth later on, and can grow with high stand density without losing vigour. The productivity of Norway spruce on fertile forest sites is commonly 10–14 m3 ha–1 yr–1 for stands of unimproved plant material (Eriksson 1976) and rotation time is generally over 55 years. Harvest residues from Norway spruce are an important source of forest fuels in Finland and Sweden (Brunberg 2011; Parviainen and Västilä 2011).
Scots pine forests are widely distributed and cover over 30 million hectares in the region. It is competitive on poor sites, with an important ability to tolerate water shortage. The total growing stock in the region is around 3500 million m3. Scots pine is native to all Nordic and Baltic countries, except Denmark (Hultén 1950). The production level is generally around 7 m3 ha–1 yr–1 on fertile sites (Persson 1992), while on sites of medium fertility it is reduced to 3–5 m3 ha–1 yr–1. The rotation period is usually 70–100 years depending on site conditions (e.g. Persson 1992). Scots pine yields less harvest residues than Norway spruce per unit area (cf. Marklund 1988) due to lower production and shorter life span of branches and foliage.
Deciduous species
Silver and downy birches are the dominant deciduous tree species in the Nordic and Baltic countries. Their total growing stock is about 1550 million m3. Birch often grows mixed with Scots pine and Norway spruce, but birch-dominated stands cover almost 8 million hectares. Both species produce best on nutrient-rich sites with sufficient availability of water. Silver birch is more successful on dryer sites while downy birch grows better on nutrient rich peatlands drained for forests (Hynynen et al. 2010; Rytter et al. 2014b) and both species tolerate pH levels below 4 (Cameron 1996), making them useful on most forest and agricultural sites. The birches are pioneer species and native in all Nordic and Baltic countries. In the southern parts of the Nordic countries, average growth of silver birch could be 9–10 m3 ha–1 yr–1 on fertile sites (Niemistö 1996; Rytter 2004) over a 40–50-year rotation. In northern regions growth is reduced to 5–8 m3 ha–1 yr–1. Birch wood is heavier than for most conifers (Table 5). Birches have rarely been planted for energy purposes alone due high establishing costs. In general, the forest fuel based on birches is a by-product from naturally regenerated young stands thinned in conventional forestry operations.
Black alder is common in Denmark, southern Finland and Sweden and along the southern coast of Norway. Grey alder has a more northerly distribution and is not native to Denmark. Both alder species are common in the Baltic countries, where they account for a growing stock of 260 million m3 (Latvia Forest Industry Federation 2008; Keskkonateabe Keskus 2010; Directorate General of State Forests 2012; Lithuanian Ministry of Environment 2015). The total growing stock of alders in Sweden and Finland is 120 million m3 (Finnish Forest Institute 2012a; Swedish Forest Agency 2013). Black alder grows best on nutrient- and mull-rich soils with generous water supply (Claessens et al. 2010). Grey alder prefers similar sites but it is more tolerant to limited availability of nutrients and water (e.g. Rytter 1996). Both alders fix atmospheric nitrogen up to 100 kg N ha–1 yr–1 in symbiosis with the Frankia bacteria (Binkley 1981; Rytter 1996), which is favourable for maintaining site productivity after harvest of nutrient-rich tree residues. The mean growth of black and grey alders may be over 15 m3 ha–1 yr–1 in dense young stands used in short-rotation forestry, but is generally around 10 m3 ha–1 yr–1 in conventional forestry (Rytter 2004; Aosaar et al. 2012; Rytter and Rytter 2016). A rotation of less than 30 years may be used in biomass-oriented cultivation, while a rotation period of 40–50 years is common for black alder in conventional forestry. The red colour of the wood means low attraction for pulping use (Rytter 1998), and therefore small-dimensioned stems may primarily be used as forest fuels. Black alder mainly regenerates from stump sprouts and grey alder produces mostly root suckers (Rytter et al. 2000). This behaviour could potentially be developed when cultivating grey alder for biomass production.
Aspen is common throughout the Nordic and the Baltic countries (Hultén 1950). It grows mainly in mixture with other species, thus making it difficult to estimate the coverage of aspen. Stener (1998), for example, reported that almost 60% of the aspen stock was growing mixed with Norway spruce and Scots pine in Sweden. This is also a reason why aspen is not considered an important biomass species. The total growing stock of aspen in the region is over 180 million m3 and the production level is generally 7–10 m3 ha–1 yr–1 on suitable high fertility sites (Rytter 2004).
Oak grows in Denmark, southern Sweden, along the southwest coast of Norway, in the Baltic countries and in southern Finland (Hultén 1950). Beech is of economic importance only in Denmark and in the southernmost parts of Sweden. The total growing stock is 80 million m3 of oak and 40 million m3 of beech. The productivity of oak on fertile sites is commonly 4–6 m3 ha-1 yr–1 and for beech it is 6–10 m3 ha–1 yr–1 (e.g. Löf et al. 2015). The basic density of wood is high for both species (Table 5) and they are grown for high-value timber, but low grade parts are commonly used as an energy source.
4.2.2 Non-native species
Conifers
Lodgepole pine is the most common non-native tree species in the region and is native to north-western North America. Lodgepole pine was introduced in northern Sweden on a large scale in the 1970s. Today plantations cover almost 600 000 ha with 30 million m3 (Elfving et al. 2001; Swedish Forest Agency 2013). It has been less used in other Nordic countries, and in Finland for example, plantations cover only 9000 ha (Finnish Forest Research Institute 2012b). The productivity is 36–50% larger than in Scots pine regardless of site fertility, while wood density is about 3% lower (Elfving et al. 2001). Lodgepole pine grows successfully over a wide range of sites, but is not so competitive on moist and highly fertile sites. It is less sensitive to low temperatures than Scots pine, which positively affects establishment, and it is browsed less by moose and suffers less from snow blight (Phacidium infestans) and twist rust (Melampsora pinitorqua). However, lodgepole pine is more sensitive to wind and snow damage, and to Scleroderris (Gremmeniella abietina) canker (Elfving et al. 2001).
Hybrid larch is a cross between the European (L. decidua Mill.) and Japanese (L. kaempferi (Lamb.) Carriére) larches. The hybrid accounts for the main part of the 1.4 million m3 of larches found in Sweden (Swedish Forest Agency 2013). Annual growth is estimated to 12–13 m3 ha–1 yr–1 on fertile sites over a 35–40-year rotation (Ekö et al. 2004; Haapanen et al. 2015). Hybrid larch is sensitive to root rot (Rönnberg and Vollbrecht 1999) and vulnerable to browsing animals (Frisk 2011). Siberian larch is only marginally used in the forestry of the region and the growth on fertile sites is reported to reach 10 m3 ha–1 yr–1 during a fairly long rotation (Karlman 2010; Per-Magnus Ekö and Ulf Johansson, SLU, pers. comm. in 2016). Hybrid larch is used as a nurse crop species in Denmark to provide early income of fuelwood and shelter more frost intolerant species in regenerations.
Sitka spruce is native in western North America and is likely best used in the maritime parts of the Nordic and Baltic countries. Sitka spruce is most common in Denmark, with 34 000 ha (Danmarks Statistik 2012), and Norway, where it has been planted on c. 50 000 ha (Øyen 2005; Vadla 2007). It grows up to 40% faster than Norway spruce. In western Norway growth will peak at an age of 70–115 years at a level of 20–33 m3 ha–1 yr–1 (Øyen 2005). Under similar conditions the growth of Norway spruce is 12–24 m3 ha–1 yr–1. Sitka spruce resembles Norway spruce in many respects and the wood is used for similar purposes.
Douglas fir is divided into two main subspecies, the costal and the interior. The coastal Douglas fir is found in northern British Columbia and along the Rocky Mountains in California. The interior Douglas fir is native to the eastern Rocky Mountains through Montana down to Mexico. Although the interior Douglas fir is preferable, the coastal Douglas fir is more widely used in the region (Svensson 2011), with frost damages as a consequence. Douglas fir plantations cover only 500 ha in Finland (Metla 2011), and account for around 1% (~5000 ha) of the forested area in Denmark (Nord-Larsen et al. 2009). The growth of Douglas fir is superior to that of Norway spruce and in Denmark its average annual growth is expected to reach 20 m3 ha–1 yr–1 (Henriksen 1988). Douglas fir is usually cultivated for production of high quality timber, but tops and branches is used for energy.
Grand fir has seldom been used in Northern Europe, and productivity is therefore poorly known across the region. Grand fir covers c. 3000 ha of forest land in Denmark (Bergstedt and Jørgensen 1992) where annual mean growth is estimated to 25–30 m3 ha–1 yr–1 (Bergstedt 2005) after 50 years. The yield can thus be 65–70% higher than for Norway spruce. However, Swedish permanent research plots have so far produced 12–18 m3 ha–1 yr–1, which is considerably lower (Per-Magnus Ekö and Ulf Johansson, SLU, pers. comm. in 2016). Establishment may be difficult because plants are sensitive to handling, frost and browsing. Frost damage may be a reason for the low productivity on the Swedish plots, but the use of shelter, e.g. nurse crops may counteract this problem. Grand fir seems to be less sensitive to root rot (Heterobasidion spp.) than Norway spruce (Swedjemark and Stenlid 1995) and can grow on a wide range of site conditions. It is a secondary species with relatively high light demands and capable of growing in multi-layered stands.
Deciduous species
The use of Populus species in forestry is a relatively recent phenomenon in the Nordic and Baltic countries. Poplars belonging to the section balsam poplars (Tacamahaca) seem best suited to Nordic conditions. At present, about 5000 ha of land has been planted with poplars and hybrid aspen together (Rytter et al. 2011; Tullus et al. 2012). All selected poplars are fast-growing and preferably used on fertile sites. For example, planted hybrid aspen is forecasted to produce over 20 m3 ha–1 yr–1 in 20–25-year-rotations (Rytter and Stener 2005, 2014; Tullus et al. 2012). There is less information available on other poplars, but the production level of selected poplars will probably be somewhat higher than for hybrid aspen (e.g. Stener 2010; Rytter et al. 2011). Hybrid aspen is a well-adapted and promising candidate for effective supply of forest fuels and produces root suckers after the final felling, whereas other poplars mainly regenerate via stump sprouts. Root sucker stands of hybrid aspen quickly produce large amounts of biomass, which may reach 10 Mg ha–1 yr–1 in a few years (about 30 m3 ha–1 yr–1) (Rytter 2006; Tullus et al. 2012; Mc Carthy and Rytter 2015). Concerning stump sprouts in poplar regeneration, some clones sprout willingly while others do not (Mc Carthy et al. 2014). Natural regeneration of poplar by using stump sprouts is thus an unreliable way to establish new plantations until clonal performances of sprouting are better known.
4.3 Fertilization and biomass production
4.3.1 Conventional fertilization and the potential to increase biomass production
Fertilization is a common tool for improving growth in advanced thinning forest stands in Sweden and Finland and has been so since the mid-sixties. In Norway, Denmark and Baltic countries fertilization has only been conducted on a small scale, often as research activities. The fertilization in Sweden peaked in 1979, when c. 190 000 ha (0.8% of the forested area) were fertilized annually. Fertilization in Finland reached a peak a few years earlier on a level of c. 240 000 ha yr–1. After that, fertilization decreased substantially in both countries to low levels in the nineties and the first years of the new millennium. Since then it has fluctuated (Lindkvist et al. 2011), but was in 2013 carried out on only 24 000 ha in Sweden (Swedish Forest Agency 2014) and about 65 000 ha of forest land in Finland in 2010.
Fertilization practice in Finland and Sweden is generally performed with a nitrogen rich fertilizer, commonly based on ammonium nitrate, with an N supply of c. 150 kg N ha–1 at one or more occasions (Ingerslev et al. 2001; Nohrstedt 2001; Saarsalmi and Mälkönen 2001). Forest land suitable for fertilization is characterized by sandy-silty moraine, mesic soil moisture, moderate soil fertility and a deep soil layer. Application of fertilizer should only be carried out in stands without high nature values. It is important to avoid shallow soils and soils with high fertility. It is also recommended to avoid fertilization with N on peatland and areas where N deposition is high. If all these considerations are included and the recommendations/regulations by Swedish Forest Agency are followed (Swedish Forestry Agency 2011), c. 50% of the total forest land area in Sweden is suitable for fertilization (Fig. 1). The largest areas for fertilization are seen in northern Sweden, while only small areas are found in the southern part. Conditions and amounts of forest land suitable for fertilization in northern Sweden might be similar for the situation in Finland.
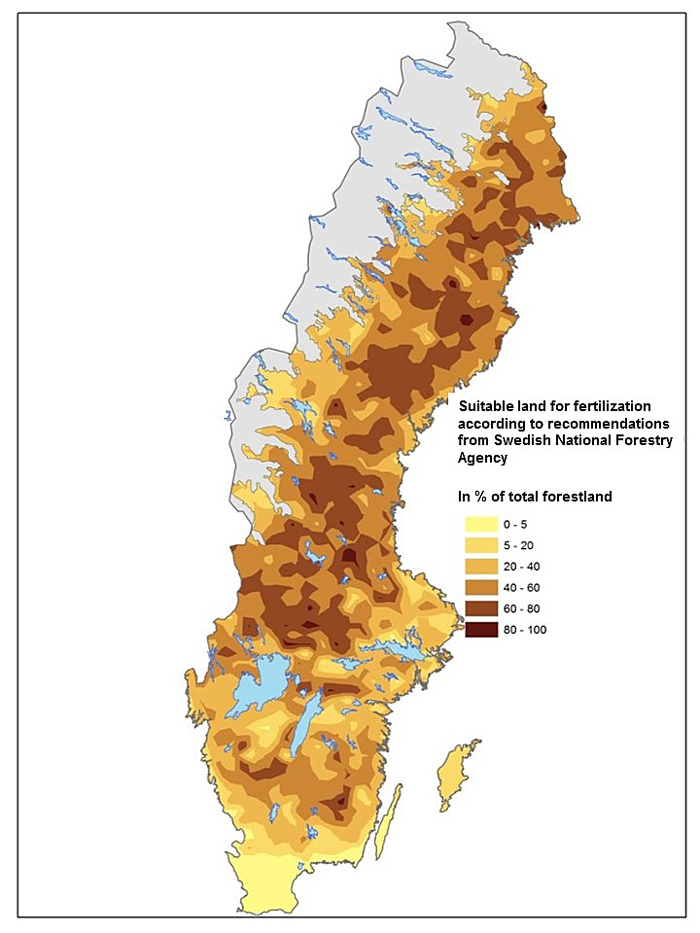
Fig. 1. Forest land suitable for fertilization according to guidelines (30§ SVL) by the Swedish Forest Agency. Data from the Swedish forest inventory.
A majority of the Scandinavian fertilization experiments have been conducted in middle-aged or older conifer stands. Findings from these experiments have been compiled in several national reviews (Denmark: Vejre et al. 2001; Finland: Saarsalmi and Mälkönen 2001; Iceland: Óskarsson and Sigurgeirsson 2001; Norway: Nilsen 2001; and Sweden: Nohrstedt 2001) published in 2001 and summarized by Ingerslev et al. (2001). Furthermore, Hedwall et al. (2014) reviewed fertilization experiments in northern forests with emphasis on the Scandinavian countries when using fertilisation to increase the biomass production. These reviews concluded that a single application of 150 kg N ha−1 increases the growth of stem wood by approximately 30% in mature Norway spruce and Scots pine stands during a 10-year period in areas with a low deposition of anthropogenic nitrogen.
A Swedish forestry impact analysis (Swedish Forest Agency 2008) indicated that an annual fertilization on maximum 1.5% (400 000 ha) of the total forest area in Sweden can be considered long-term sustainable. In productivity terms this means an additional growth of c. 6 million m3 during a 10-year period. It is possible to increase the level of biomass production by fertilization in Norway, Estonia and Latvia but it is difficult to estimate the potential for the future. However, in some areas of Scandinavia experiments have shown that fertilization may not beneficially increase the biomass production in conifer stands. An example of this is western Denmark, where other factors were suggested to restrict fertilization effects. They were high N-deposition and climatic stress of trees growing outside their natural distribution range (Dralle and Larsen 1995; Ingerslev 1998; Vejre et al. 2001).
4.3.2 Potential to increase biomass production by fertilization – balanced supply of nutrients in young forests
Fertilization has a potential to substantially increase biomass production, especially when applied as frequent doses with a balanced supply of essential nutrients in young forests. There are fundamental differences between this kind of nutrient supply and conventional fertilization as described above. Frequent fertilization in young stands may give a large reduction in rotation length compared with conventional management. The rotation periods may be shortened by 10 to 30 years in the southern part of Scandinavia and 30 to 60 years in the north (Bergh et al. 2005). The first fertilization is suggested to be performed at 2–4-meter tree height and will be repeated frequently until canopy closure. Experiments with balanced fertilization show that fertilization of Norway spruce may be performed every second year and still maintain the same level of production as fertilization each year (Bergh et al. 2008), which is advantageously for the operation economy. After canopy closure, fertilization is conducted every 7th to every 10th year in a similar way as conventional fertilization. Fertilization needs to be repeated 1 to 3 times after first thinning in closed and mature stands. Last fertilization should be carried out no later than 10 years before final felling to fully utilize the effects of the fertilization before harvest. The total amount of nitrogen during a whole rotation will be 800 to 1500 kg N ha–1 (lower amounts in southern parts of Scandinavia and higher in the north), of which about 75% is supplied before canopy closure.
Estimations of useful land for balanced nutrient supply (Larsson et al. 2009) show that 5.5 million ha of forest land in Sweden is suitable and 2.6 million ha will become available within 50 years. This means it will take 50 years before this management method can be applied on 10% of the forest land in Sweden.
The SKA08 analysis in Sweden (Swedish Forest Agency 2008) indicated that a balanced supply of nutrients on 5% (c. 1.3 million ha) of Sweden’s forest area would imply increased growth by 7–9 million m3 yr–1 for the next 100 years. In terms of energy the increased stem-wood growth corresponds to about 15 TWh yr–1. It should be noted that these prognoses are based on stands with Norway spruce. New experiments with other fast-growing species have since then been established.
4.4 High productive silvicultural systems for the future
There are basically two ways of “new” high-productive silviculture systems which are currently not commonly used: 1) fast-growing nurse tree crops mixed with high-productive shade-tolerant species, and 2) intensively managed short rotation crops in dense stands of highly-productive species, hybrids and clones exemplified by root sucker stands of hybrid aspen and naturally regenerated birch after harvest. The use of genetically improved material, fertilizers, efficient regeneration techniques and pesticides may be involved depending on national legislation, certification standards, management goals etc.
4.4.1 Two-storied mixed plantations
Mixed forest plantations can be designed and managed to meet a variety of social, economic, and environmental objectives, and can provide key ecosystem services, help preserve remaining primary forests, and sequester an important proportion of the atmospheric carbon released by human activities (Paquette and Messier 2010). Mixed forests may be a better option than monocultures to meet multiple land use objectives (Knoke et al. 2005). In theory, mixed-species ecosystems could be more productive than single-species ecosystems (e.g. Hector et al.1999; Forrester and Pretzsch 2015; Pretzsch et al. 2015). However, an over-yielding (higher production of individual tree species in the mixture compared to the monoculture) may be difficult to utilize in forestry because forest industry is mainly interested in the total stand production for pulp and timber uses and because e.g. timber quality may be more difficult to control in mixed stands. With increased awareness of wood production for bioenergy, the whole tree harvest – sometimes including stumps – gains attention. Thus, high-productive mixed stands become more relevant for forestry where high growth rate, high carbon sequestration and resilience is combined with other important goals (Hulvey et al. 2013). For example, two-storied mixed plantations combining late successional and shade tolerant tree species valuable for high quality timber and for environmental purposes in long rotations with fast-growing tree species in short rotations for biomass production goals is an interesting concept to develop further (Fig. 2).

Fig. 2. Two-storied forest structure following afforestation of agricultural land in Denmark. The nurse crop is 11 years old and is a mixture of black alder and hybrid poplar (clone OP42), with directly sown beech below.
Photo: Palle Madsen.
Fast growing nurse trees have a potential for rapidly building new forest structures and simultaneously increase productivity, which might be a cost-effective strategy for raising new forests (Stark et al. 2015). Nurse trees can reduce competing vegetation, protect against late spring frost, facilitate establishment and improve stem form of slow growing and often shade tolerant target tree species (Gardiner et al. 2004; Löf et al. 2014). However, the nurse crop may, a few years after it has supported the early establishment of the target species, compete strongly and thereby potentially damage the target species if not thinned or removed. Delayed planting of target tree species relative to the nurse crop is a means to allow shelter to develop before the shade tolerant species are introduced and may offer methods that are easier managed. The first thinning to open up the canopy and introduce the target species can wait until the first profitable thinning of the nurse crops. Additionally, the thinning intensity can then be adapted to match the demands of the target species. There are several species combinations described in the literature that may be suitable such as poplar and oak (Gardiner et al. 2004), birch, larch or grey alder under-planted with beech, oak or Norway spruce (Löf et al. 2014). Other candidate species for this concept include high productive target species such as Douglas fir, grand fir or western red cedar (Thuja plicata Donn ex D.Don.), which are known from Danish forestry to benefit from shelter in the regeneration phase (Nord-Larsen and Meilby 2016). The many species combinations that are relevant for these systems make it possible to adapt to a wide range of site conditions.
The overall yield is expected to increase, as have been shown for several combinations such as naturally regenerated mixed stands of for example birch and Norway spruce with 10–20% transgressive over-yielding (Tham 1994; Mård 1996; Bergqvist 1999) or trembling aspen (Populus tremuloides Michx.) and white spruce (Picea glauca (Moench.) Voss), also with 20 % transgressive over-yielding (Kabzems et al. 2007; Comeau 2014). Additional gain is expected by combining this approach with e.g. genetically improved material.
The nurse crop system needs further development to identify appropriate thinning regimes or canopy densities of the nurse crops that allow various main species, with different management objectives, a successful establishment. A transgressive over-yielding of 10%, depending on product and rotation length, can offset increased costs associated with planting and managing mixed-species stands (Nichols et al. 2006). The role of high productive mixed forests is, however, unclear regarding protection of biodiversity and therefore this aspect needs to be included in the overall development of these novel silvicultural systems.
4.4.2 Coppice
Comparisons of production among different tree species in coppice are difficult due to the lack of comparable experiments. Except for willow and hybrid aspen there are only few experiments available. In addition, fertilization will most often lead to a substantial increase of productivity in coppice stands (e.g. Alriksson et al. 1997; Aronsson et al. 2014; Konstantinavičienė et al. 2014). Information about interesting tree species for coppice in the Nordic countries is gathered in Table 6. Coppice stands probably produce at least on the same levels as ordinary managed stands, but the result depends on the number of stems regenerated per hectare and the sustainability of the system (Harmer 2003). Except for willow there is little information available of recommended rotation periods. For temperate broadleaved tree species in England, 25–35-year rotation has been advocated, including ash (Fraxinus excelsior L.), lime (Tilia cordata Mill.) and oak (Harmer 2003). These stands have often been managed as coppice with standards (i.e. combination of coppice with individual trees that are managed as high forests) and the levels of production could probably be higher in pure coppice stands. Similar periods were recommended for silver birch in northern Finland (Hytönen and Issakainen 2001), while Johansson (2008) recommended rotation lengths of 10–15 years for birch in Sweden. Aspen and alder seem to be highly productive also with short rotations. Different rotation lengths have been tested for root sucker stands of hybrid aspen (Mc Carthy and Rytter 2015; Rytter and Mc Carthy 2016) and results indicate that the rotation may be varied if an adequate thinning is performed.
| Table 6. Published information of wood production during coppice in some tree species. Mean production have been calculated using basic density and by including tops and twigs, and formulas for energy in wood (Ebenhard et al. 2013). Year is the rotation period; S = Southern; C = Central; N = Northern. | |||||||
| Tree species | Year | Mean production (tons dry mass ha–1 yr–1) | Mean production (MWh ha–1 yr–1) | Site | Soil type | Comments | Reference |
| Willow | 4 | 10 | 49 | S. Sweden | Agricultural | Often fertilized | Mola-Yudego and Aronsson 2008 |
| Hybrid aspen | 2 | 10 | 47 | S. Sweden | Agricultural | Rytter 2006 | |
| 12 | 10 | 47 | S. Sweden | Agricultural | Mc Carthy and Rytter 2015 | ||
| Aspen | 5 | 4 | 18 | S. Germany | Agricultural | Liesebach et al. 1999 | |
| 35 | 5 | 26 | Finland | Rich soil | Vuokila 1977 | ||
| Black alder | 2 | 3–10 | 17–49 | Kansas, USA | Agricultural | Geyer 2006 | |
| 5 | 13 | 62 | Kentucky, USA | Fine texture | Wittwer and Stringer 1985 | ||
| 20 | 2 | 10 | England | Moist soil | With standards | Harmer 2003 | |
| Grey alder | 3 | 4–5 | 18–23 | C. Sweden | Forest soil | Rytter et al. 2000 | |
| 8 | 5 | 25 | Finland | Forest soil | Saarsalmi et al. 1991 | ||
| 15 | 5.5 | 29 | N. Europe | Various soils | Rytter and Rytter 2016 | ||
| Silver birch | 8 | 3 | 13 | C. Finland | Peat | Hytönen and Issakainen 2001 | |
| 16 | 2 | 10 | N. Finland | Peat | Hytönen and Issakainen 2001 | ||
| Downy birch | 21 | 3 | 21 | S. Finland | Peat | Downy and Silver birch | Hytönen and Aro 2012 |
| 31 | 3 | 16 | England | Dry soil | With standards | Begley and Coates 1961 | |
| Ash | 32 | 2 | 10 | England | Loamy clay | With standards | Harmer 2003 |
| Oak | 37 | 3 | 13 | England | Forest soil | With standards | Harmer 2003 |
| 20–45 | 6–3 | 30–13 | England & Wales | - | - | Crockford and Savill 1991 | |
| Lime | 12 | 2 | 9 | England | Clay soil | With standards | Harmer 2003 |
There is some information available saying that the first generation of coppice tends to produce more than the original stand, whereas the next generations tend to produce somewhat less (Geyer 2006). Different tree species probably produce different results, but a plausible explanation is that repeated coppice may not be fully sustainable concerning soil nutrient availability (Buckley 1992).
4.5 Afforestation
Increasing land areas may be available for afforestation and biomass production in the future. There is a general trend of decreasing areas for agriculture in Europe (Rounsevell et al. 2005). A political goal in Denmark is to obtain 20–25% more forest land, which means that 250 000–470 000 ha will be afforested. The total area of fallows and uncultivated arable land was estimated to nearly 280 000 ha in Finland in 2011 (Ministry of Agriculture and Forestry in Finland 2012). Almost 200 000 ha of coastal heathland and unused agriculture land is found in Norway (Granhus et al. 2012; SSB 2012), and in Sweden (e.g. Larsson et al. 2009) 300 000–500 000 ha have been estimated available for afforestation. In Estonia almost 300 000 ha (Landresource 2007) and in Latvia about 260 000 ha (Rural Support Service of Latvia 2012) agricultural land are potentially available for forest fuel production. In Lithuania there is around 600 000 ha of abandoned land and the willingness to afforest those areas is reported to be 30%, giving 180 000 ha of available land area (Mizaraite and Mizaras 2006). Thus, there is 1.8–2.6 million ha of abandoned agricultural land which is available for afforestation to mitigate climate change by increased wood production.
However, to afforest the abandoned agricultural areas some efforts are often needed, e.g. draining, weed control and often fencing. We need better knowledge on how to treat these areas and what are the relevant tree species to establish. The afforestation rate also indicates that it will take time before the potential is fully utilized. For example, the annual afforestation rate was estimated to 1600–3600 ha–1 yr–1 in Finland during the period 2006–2013 (Ministry of Agriculture and Forestry 2014). In Sweden, the afforestation of agricultural land amounted to 5000 ha–1 yr–1 during the period 1990–2003 (Jordbruksverket 2008).
5 Discussion
Information of biomass potentials from the sections above shows that the availabilities of increased wood supply are comprehensive. Current harvest of biomass is not exceeding growth, which is thus producing climate benefits, and it is possible to use more biomass than has hitherto been exploited. There are three approaches to increase the sustainable use of biomass from our forests: 1) we can use more of today’s stock and growth with limited negative effects on other values of the forests, 2) we can use growth improving measures like breeding, changes of tree species, fertilization and implementation of high productive silviculture systems, and 3) we can use surplus agriculture land for afforestation. In addition, the changing climate itself is expected to increase forest growth in the northern regions of Europe.
The Nordic and Baltic countries contain a large forest area with 63 million ha of productive forest land, which is more than 51% of the total land area (Table 1). The growing stock has been estimated to 7.9 billion m3 (Rytter et al. 2015; Lithuanian Ministry of Environment 2015) of which 89% is available for wood supply within a sustainable forest management context according to Forest Europe (2011), and 35% of the annual growth of 287 million m3 is not harvested.
Estimates on harvest potentials of forest fuels have been made for the Nordic and Baltic region. These estimates include harvesting of low grade woody biomass of slash, stumps and small-sized trees. The estimate with a low restriction level is 416 TWh (Table 2). The primary supply of biomass and waste in 2010 was 314 TWh. Since this assortment contains both biomass and an unknown but considerable part of waste, it is difficult to show the real current potential of forest fuel. However, the conclusion is that both total harvest and the share of forest residues can potentially be increased in a short-term perspective (~0–20 years) relative to today’s utilization without compromising the sustainability and other ecosystem services.
Nevertheless, the largest potential to increase availability of biomass is represented by the different measures to enhance future forest growth. Some measures take long time before they show impact because they depend on the regeneration of the present forests. However, in a time perspective to 2050 and further their potential to increase forest growth is considerable.
Fertilization is the measure that will show positive effect on tree growth in a short time. The traditional fertilization regime is performed on medium fertile forest land in stands close to final harvest. The effect of a single fertilizer dose is estimated to about 30% growth increase during a 10-year period (Ingerslev et al. 2001). Today only small areas are fertilized but according to available studies about 50% of the forest area in Sweden is suitable for fertilization (Fig. 1). There is also an option with using a balance supply of nutrients in young forests (e.g. Bergh et al. 2008). This concept is new and it is difficult to estimate its true potential. It should be noticed that although fertilization can be applied immediately it can only be used on a small share of forest stands at a time, and thus it will take several decades before it has been applied on larger areas (cf. Larsson et al. 2009).
Breeding of forest tree species is the improving measure that will alter the apparent and visible forest structure and landscape the least and thereby probably given acceptance from society. It is also the measure which will be most well-distributed over forest lands since planting of improved plant material is a common procedure applied on most forest sites already today. The average gain in yield obtained by using material from seed orchards is estimated to be 20–25% in 2050, and together with further increased use of improved plants, this will result in a substantial increase in productivity. Thus, even if the estimated gain is not the highest among measures it will most probably have the best effect on increasing biomass availability since it the most widely used tool.
A change of tree species has in many cases strong effect. Recently, species and hybrids within the genus Populus have been introduced and planted for productivity reasons. By using poplar and hybrid aspen the areal growth is often doubled compared to the forest stand growing before (Table 5). Exotic conifers like hybrid larch, Sitka spruce, grand fir and Douglas fir may also contribute extensively to increased forest growth. Using foreign tree species is, however, often restricted by existing forest legislation or certification standards (cf. Rytter et al. 2015) and areas used so far are often small, except for lodgepole pine and in Denmark. The regulations are not perdurable and may change with time. Nevertheless, breeding of native trees for higher vitality and growth is an important measure along with new species, and indigenous species like grey alder and birch show fast initial growth.
It is also possible to improve forest annual increment by using species with a fast initial growth rate. There are two distinctively different ways of doing this. 1) We can use slow starting and shade tolerant species, which have a high productivity at a later stage of the rotation time and complement them with nurse crop species that will deliver early biomass harvests and income. Poplar and black alder serving the nurse crop function for beech appears from Fig. 2, and another example of this strategy is the use of birch as a nurse crop for Norway spruce (Löf et al. 2014). 2) The other way is to use coppice, which is dense stands of high-producing pioneer species during short rotations. An example is the root sucker generations of hybrid aspen (Mc Carthy and Rytter 2015) or stump sprouts of willows (e.g. Mola-Yudego and Aronsson 2008). The main hypothesis behind these management systems is to quickly reach a high productivity level and maintain it over a long period of time.
The various methods and systems to increase productivity should not be viewed in isolation but considered as tools that could be combined to reach even higher growth levels. For example, breeding work can be carried out on exotic species which are then used as nurse trees in a fertilized stand. The combinations are not suitable everywhere but offer many applicable alternative systems on large areas for increased forest growth, and yet still leave part of the forest areas to support other important functions and ecosystem services.
The forest areas in the region are large but it is still possibly to increase them by afforestation on abandoned agricultural land. These kind of land resources are available in all Nordic and Baltic countries and are estimated in total to 1.8–2.6 million ha (Rytter et al. 2015). This means an increase of the current productive forest area of 3–4%. However, this kind of land is generally more fertile than average forest land and thereby it can contribute with more biomass than the areas suggest.
There is no doubt that human civilization has contributed to increased CO2 content in the atmosphere (IPCC 2013). This will have effects on forest growth worldwide. Although we can change the future development of the CO2 content, the current increase is expected to continue long into the future. For the Nordic and Baltic conditions, the increased CO2 level is predicted to give an increased forest growth in the order of 30% (e.g. Kellomäki et al. 2008; Swedish Forest Agency 2008; Alam et al. 2010; Poudel et al. 2011). The effects of increased atmospheric CO2 concentration and consequent changes in temperature and precipitation will also be species and site type specific. Therefore, implementation of adaptive forest management measures are urgently needed for forests to mitigate climate change and support society in its transformation to a more sustainable development.
By using breeding, ”new” tree species, fertilization and “new” intensive management systems this review suggest that the growth can be increased by 50–100% in certain stands, but it is difficult to suggest an average level of increase in the region. The growth increase will anyway give large quantities of biomass available for the development of a sustainable society. A changing climate and afforestation of surplus agricultural land will add to the potential. It is also possible to use more biomass already today by increasing the harvest of forest fuel.
As the use of renewable energy is already high in the region (e.g. Eurostat 2012), the vision of independence of fossil energy by 2050 in the Nordic countries can be approached with confidence and energy from forest fuels will most likely be of great importance in the future in all Nordic and Baltic countries.
Acknowledgements
This review work was financially supported by the Nordic Energy Research in its research funding programme Sustainable Energy Systems 2050 and carried out in the project Wood based energy systems from Nordic and Baltic forests (ENERWOODS). The authors also want to thank all colleagues in the project for their contribution with material and for pleasurable cooperation.
References
Alam A., Kilpeläinen A., Kellomäki S. (2008). Impacts of thinning on growth, timber production and carbon stocks in Finland under changing climate. Scandinavian Journal of Forest Research 23: 501–512. http://dx.doi.org/10.1080/02827580802545564.
Alam A., Kilpeläinen A., Kellomäki S. (2010). Potential energy wood production with implications to timber recovery and carbon stocks under varying thinning and climate scenarios in Finland. Bioenergy Research 3: 362–372. http://dx.doi.org/10.1007/s12155-010-9095-1.
Alriksson B., Ledin S., Seeger P. (1997). Effect of nitrogen fertilization on growth in a Salix viminalis stand using a response surface experimental design. Scandinavian Journal of Forest Research 12: 321–327. http://dx.doi.org/10.1080/02827589709355418.
Aosaar J., Varik M., Uri V. (2012). Biomass production potential of grey alder (Alnus incana (L.) Moench.) in Scandinavia and Eastern Europe: a review. Biomass and Bioenergy 45: 11–26. http://dx.doi.org/10.1016/j.biombioe.2012.05.013.
Aronsson P., Rosenqvist H., Dimitriou I. (2014). Impact of nitrogen fertilization to short-rotation willow coppice plantations grown in Sweden on yield and economy. Bioenergy Research 7: 993–1001. http://dx.doi.org/10.1007/s12155-014-9435-7.
Begley C.D., Coates A.E. (1961). Estimating yield of hardwood coppice for pulpwood growing. Forestry Commission Report on Forest Research 1959/60: 189–196. HMSO, London.
Bekeris P. (2011). Latvia’s forest during 20 years of independence. BALTI Group, Riga. 46 p. https://www.zm.gov.lv/public/ck/files/ZM/mezhi/buklets/MN_20_EN.pdf.
Bergh J., Freeman M., Sigurdsson B., Kellomäki S., Laitinen K., Niinistö S., Peltola H., Linder S. (2003). Modelling short-term effects of climate change on the productivity of selected tree species in Nordic countries. Forest Ecology and Management 183: 327–340. http://dx.doi.org/10.1016/S0378-1127(03)00117-8.
Bergh J., Linder S., Bergström J. (2005). Potential production of Norway spruce in Sweden. Forest Ecology and Management 204: 1–10. http://dx.doi.org/10.1016/j.foreco.2004.07.075.
Bergh J., Nilsson U., Grip H., Hedwall P.O., Lundmark T. (2008). Effects of frequency of fertilisation on production, foliar chemistry and nutrient leaching in young Norway spruce stands in Sweden. Silva Fennica 42(5): 721–733. http://dx.doi.org/10.14214/sf.225.
Bergqvist G. (1999). Wood volume yield and stand structure in Norway spruce understorey depending on birch shelterwood density. Forest Ecology and Management 122: 221–229. http://dx.doi.org/10.1016/S0378-1127(99)00008-0.
Bergstedt A. (2005). Træarternas anvendelse og produktionspotentiale. [The use and production potential of tree species]. Dansk Skovbrugs Tidsskrift 1–2: 342–360.
Bergstedt A., Jørgensen B.B. (1992). Hugstforsøg i Abies grandis. [Thining trials in Abies grandis]. Videnblande Skovbrug 5.6-2. http://videntjenesten.ku.dk/skov_og_natur/bevoksningspleje/hugstbehandling/videnblad_05.06-02/.
Binkley D. (1981). Nodule biomass and acetylene reduction rates of red alder and sitka alder on Vancouver Island, B.C. Canadian Journal of Forest Research 11: 281–286. http://dx.doi.org/10.1139/x81-037.
Briceño-Elizondo E., Garcia-Gonzalo J., Peltola H., Matala J., Kellomäki S. (2006). Sensitivity of growth of Scots pine, Norway spruce and silver birch to climate change and forest management in boreal conditions. Forest Ecology and Management 232(1–3): 152–167. http://dx.doi.org/10.1016/j.foreco.2006.05.062.
Brunberg T. (2011). Forest fuel: assortments, methods and costs 2010. Uppsala, Skogforsk, Resultat no. 8. 2 p. [In Swedish with English summary]. http://www.skogforsk.se/contentassets/3a849981dd414d25b0c21c97a583aa6d/resultat12-10_lowres.pdf.
Buckley G.P. (ed.) (1992). Ecology and management of coppice woodlands. Chapman & Hall, London. 339 p. ISBN 978-94-011-2362-4. http://dx.doi.org/10.1007/978-94-011-2362-4.
Cameron A.D. (1996). Managing birch woodlands for the production of quality timber. Forestry 69: 357–371. http://dx.doi.org/10.1093/forestry/69.4.357.
Canadell J.G., Raupach M.R. (2008). Managing forests for climate change mitigation. Science 320(5882): 1456–1457. http://dx.doi.org/10.1126/science.1155458.
Carle J.B., Ball J.B., Del Lungo A. (2009). The global thematic study of planted forests. In: Evans J. (ed.). Planted forests – uses, impacts and sustainability. FAO, CABI Head Office, Wallingford, UK. p. 33–46. ISBN 9781845935641. http://dx.doi.org/10.1079/9781845935641.0000.
Christersson L. (2010). Wood production potential in poplar plantations in Sweden. Biomass and Bioenergy 34: 1289–1299. http://dx.doi.org/10.1016/j.biombioe.2010.03.021.
Christersson L., Sennerby-Forsse L. (1994). The Swedish programme for intensive short-rotation forests. Biomass and Bioenergy 6: 145–149. http://dx.doi.org/10.1016/0961-9534(94)90094-9.
Claessens H., Oosterbaan A., Savill P., Rondeux J. (2010). A review of the characteristics of black alder (Alnus glutinosa (L.) Gaertn.) and their implications for silvicultural practices. Forestry 83: 163–175. http://dx.doi.org/10.1093/forestry/cpp038.
Comeau P. (2014). Effects of aerial strip spraying on mixedwood stand structure and tree growth. Forestry Chronicle 90: 479–485. http://dx.doi.org/10.5558/tfc2014-097.
Crockford K.J., Savill P.S. (1991). Preliminary yield tables for oak coppice. Forestry 64: 29–50. http://dx.doi.org/10.1093/forestry/64.1.29.
Danell Ö. (1993). Breeding programmes in Sweden. General approach. In: Lee S.J. (ed.). Progeny testing and breeding strategies. Proceedings from a meeting with the Nordic group for tree breeding, Oct 1993, Edinburgh. Forestry Commission.
Danmarks Statistik (2012). Statistisk Årbog 2012. [Statistical Yearbook 2012]. Danmarks Statistik, Copenhagen. 523 p. [In Danish]. http://www.dst.dk/da/Statistik/Publikationer/VisPub?cid=16252.
Directorate General of State Forests (2012). Forest resources. Directorate General of State Forests at the Ministry of Environment Republic of Lithuania, Vilnius. http://www.gmu.lt.
Dralle K., Larsen J.B. (1995). Growth response to different types of NPK-fertilizer in Norway spruce plantations in Western Denmark. Plant and Soil 168–169: 501–504. http://dx.doi.org/10.1007/BF00029362.
Ebenhard T., Dahlström A., Emanuelsson U., Lennartsson T., Löf M., Palme U. (2013). Lågskogsbruk – biobränsleproduktion i samklang med miljömål. CBM, Sveriges Lantbruksuniversitet, Uppsala. 115 p. [In Swedish].
Ekö P.M., Larsson-Stern M., Albrektson A. (2004). Growth and yield of hybrid larch (Larix × eurolepis A. Henry) in southern Sweden. Scandinavian Journal of Forest Research 19: 320–328. http://dx.doi.org/10.1080/02827580410024151.
Edvardsen O.M., Steffenrem A., Johnskås O.R., Johnsen Ø., Myking T., Kvaalen H. (2010). Strategi for skogplanteforedling 2010–2040 (høringsutkast). Stiftelsen Det norske Skogfrøverk. The Norwegian Forest Seed Center, Hamar. [In Norwegian]. http://www.skogplanteforedling.no/Dokumenter/Skogfroverket_strategi.pdf.
Elfving B., Ericsson T., Rosvall O. (2001). The introduction of lodgepole pine for wood production in Sweden – a review. Forest Ecology and Management 141: 15–29. http://dx.doi.org/10.1016/S0378-1127(00)00485-0.
Eriksson H. (1976). Yield of Norway spruce in Sweden. Royal College of Forestry, Dept. Forest Yield Research, Stockholm, Research Notes No 41. 291 p.
Estonian Environmental Information Centre (2012). Yearbook Forest 2010. Keskkonnateabe Keskus, Tartu. 226 p. [In Estonian with English translations].
Eurostat (2012). The contribution of renewable energy up to 12.4% of energy comsumption in the EU27 in 2010. Eurostat Newsrelease 94. 2 p. http://ec.europa.eu/eurostat/en/web/products-press-releases/-/8-18062012-AP.
Eurostat (2013). Primary production of renewable energy, 2003 and 2013. http://ec.europa.eu/eurostat/statistics-explained/index.php/File:Primary_production_of_renewable_energy,_2003_and_2013_YB15.png.
FAO (2010). Global forest resource assessment 2010. FAO, Rome. 378 p. http://www.fao.org/docrep/013/i1757e/i1757e.pdf.
FAO (2012). Supporting sustainable forest management through the global forest resources assessment: long-term strategy (2012–2030). FAO, Rome. 9 p. http://www.fao.org/forest-resources-assessment/en/.
FAO (2014a). Statistical yearbook 2014: Europe and Central Asia food and agriculture. Food and Agriculture Organization of the United Nations, Regional Office for Europe and Central Asia, Budapest. 130 p. http://www.fao.org/economic/ess/ess-publications/ess-yearbook/en/#.Vzrt2mxf2Um.
FAO (2014b). Global forest eesource assessment 2015 – country report: Denmark. FAO, Rome. 83 p. http://www.fao.org/3/a-az199e.pdf.
FAO (2016a). Planted forests. http://www.fao.org/forestry/plantedforests/en/.
FAO (2016b). Forestry in Lithuania. http://www.fao.org/docrep/w3722e/w3722e22.htm.
Finnish Forest Research Institute (2012a). Finnish statistical yearbook of forestry. Finnish Forest Research Institute (Metla), Vantaa. 452 p. http://www.metla.fi/tiedotteet/2012/2012-12-13-statistical-yearbook.htm.
Finnish Forest Research Institute (2012b). State of Finland’s forests 2012: criterion 4 biological diversity. Introduced tree species (4.4). Finnish Forest Research Institute (Metla), Vantaa. http://www.metla.fi/metinfo/sustainability/c4-introduced-tree.htm.
Forest Europe (2011). State of Europe’s forests 2011. Forest Europe Liaision Unit Oslo, UNECE and FAO. 337 p. http://www.foresteurope.org/documentos/State_of_Europes_Forests_2011_Report_Revised_November_2011.pdf.
Forrester D.I., Pretzsch H. (2015). On the strength of evidence when comparing ecosystem functions of mixtures with monocultures. Forest Ecology and Management 356: 41–53. http://dx.doi.org/10.1016/j.foreco.2015.08.016.
Frisk A. (2011). Grazing damages on larch seedlings. Swedish University of Agricultural Sciences, School for Forest Management, Skogsmästarprogrammet Examensarbete 12, Skinnskatteberg. 37 p. [In Swedish with English abstract]. http://stud.epsilon.slu.se/2836/1/Frisk_A_110616.pdf.
Garcia-Gonzalo J., Peltola H., Gerendiain A.Z., Kellomäki S. (2007a). Impacts of forest landscape structure and management on timber production and carbon stocks in the boreal forest ecosystem under changing climate. Forest Ecology and Management 241: 243–257. http://dx.doi.org/10.1016/j.foreco.2007.01.008.
Garcia-Gonzalo J., Peltola H., Briceno-Elizondo E., Kellomäki S. (2007b). Changed thinning regimes may increase carbon stock under climate change: a case study from a Finnish boreal forest. Climatic Change 81: 431–454. http://dx.doi.org/10.1007/s10584-006-9149-8.
Gardiner E.S., Stanturf J.A., Schweitzer C.J. (2004). An afforestation system for restoring bottomland hardwood forests: biomass accumulation of nuttall oak seedlings interplanted beneath eastern cottonwood. Restoration Ecology 12: 525–532. http://www.treesearch.fs.fed.us/pubs/7876.
Ge Z.M., Kellomäki S., Peltola H., Zhou X., Väisänen H., Strandman H. (2013a). Impacts of climate change on primary production and carbon sequestration of boreal Norway spruce forests: Finland as a model. Climatic Change 118: 259–273. http://dx.doi.org/10.1007/s10584-012-0607-1.
Ge Z.M., Kellomäki S., Peltola H., Zhou X., Väisänen H. (2013b). Adaptive management to climate change for Norway spruce forests along a regional gradient in Finland. Climatic Change 118: 275–289. http://dx.doi.org/10.1007/s10584-012-0656-5.
Geyer W.A. (2006). Biomass production in the central great plains USA under various coppice regimes. Biomass and Bioenergy 30: 778–783. http://dx.doi.org/10.1016/j.biombioe.2005.08.002.
Gjølsjø S., Hobbelstad K (2009). Energy potential in Norwegian forest. Norwegian Forest and Landscape Institute, Report 09. 11 p. [In Norwegian].
Granda E., Camarero J.J., Gimeno T.E., Martínez-Fernández J.,Valladares F. (2013). Intensity and timing of warming and drought differentially affect growth patterns of co-occurring Mediterranean tree species. European Journal of Forest Research 132(3): 469–480. http://dx.doi.org/10.1007/s10342-013-0687-0.
Granhus A., Hylen G., Nilsen J.-E.Ø. (2012). Skogen i Norge. Statistikk over skogforhold og skogressurser i Norge registrert i perioden 2005–2009. [Statistics of Forest Conditions and Resources in Norway]. Ressursoversikt fra Skog og landskap 03. 85 p. [In Norwegian with English Introduction]. http://www.skogoglandskap.no/publikasjon/skogen_i_norge_statistikk_over_skogforhold_og_skogressurser_i_norge_registrert_i_perioden_2005_2009/publication_view.
Graudal L., Nielsen U.B., Schou E., Thorsen B.J., Hansen J.K., Bentzen N.S., Johannsen V.K. (2013). Muligheder for bæredygtig udvidelse af dansk produceret vedmasse 2010–2100. [Options for sustainable expansion of Danish-produced wood biomass 2010–2100]. University of Copenhagen, Skov og Landskab. 87 p. [In Danish]. http://forskning.ku.dk/find-en-forsker/?pure=da/publications/uuid(acc0f235-ded2-42ff-907c-ca4d8cfba650).html.
Gruduls K., Bārdulis A., Lazdiņš A., Makovskis K. (2013). Forest biomass resource assessment on the base of National Forest Inventory in Latvia. Central Baltic Interreg IV A Programme 2007–2013, The Development of the Bioenergy and Industrial Charcoal (Biocoal) Production, Report of BalBiC – project cb46. 63 p.
Gustavsson L., Holmberg J., Dornburg V., Sathre R., Eggers T., Mahapatra K., Marland G. (2007). Using biomass for climate change mitigation and oil use reduction. Energy Policy 35: 5671–5691. http://dx.doi.org/10.1016/j.enpol.2007.05.023.
Gustavsson L., Eriksson L., Sathre R. (2011). Costs and CO2 benefits of recovering, refining and transporting logging residues for fossil fuel replacement. Applied Energy 88: 192–197. http://dx.doi.org/10.1016/j.apenergy.2010.07.026.
Haapanen M., Jansson G., Nielsen U.B., Steffenrem A., Stener L.G. (2015). The status of tree breeding and its potential for improving biomass production – a review of breeding activities and genetic gains in Scandinavia and Finland. Skogforsk, Uppsala. 56 p. http://www.skogforsk.se/contentassets/9d9c6eeaef374a2283b2716edd8d552e/the-status-of-tree-breeding-low.pdf.
Harmer R. (2003). The silviculture and management of coppice woodlands. Forestry Commission, Edinburgh. 88 p.
Hartley M.J. (2002). Rationale and methods for conserving biodiversity in plantation forests. Forest Ecology and Management 155: 81–95. http://dx.doi.org/10.1016/S0378-1127(01)00549-7.
Hector A., Schmid B., Beierkuhnlein C., Caldeira M.C., Diemer M., Dimitrakopoulos P.G., Finn J.A., Freitas H., Giller P.S., Good J., Harris R., Högberg P., Huss-Danell K., Joshi J., Jumpponen A., Körner C., Leadley P.W., Loreau M., Minns A., Mulder C.P.H., O’Donovan G., Otway S.J., Pereira J.S., Prinz A., Read D.J., Scherer-Lorenzen M., Schulze E.-D., Siamantziouras A.-S.D., Spehn E.M., Terry A.C., Troumbis A.Y., Woodward F.I., Yachi S., Lawton J.H. (1999). Plant diversity and productivity experiments in European grasslands. Science 286: 1123–1127. http://dx.doi.org/10.1126/science.286.5442.1123.
Hedwall P.-O., Gong P., Ingerslev M., Bergh J. (2014). Fertilization in northern forests – biological, economic and environmental constraints and possibilities. Scandinavian Journal of Forest Research 29: 301–311. http://dx.doi.org/10.1080/02827581.2014.926096.
Henriksen H.A. (1988). Skoven og dens dyrkning. [The forest and its cultivation]. Dansk Skovforening/Nyt Nordisk Forlag Arnold Busck, København. 664 p. [In Danish]. ISBN 9788717059375.
Hultén E. (1950). Atlas of the distribution of vascular plants in N.W. Europe. Stockholm, Generalstabens Litografiska Anstalts Förlag. 512 p.
Hulvey K.B., Hobbs R.J., Standish R.J., Lindenmayer D.B., Lach L., Perring P. (2013). Benefits of tree mixtures in carbon plantings. Nature Climate Change 3: 869–874. http://dx.doi.org/10.1038/nclimate1862.
Hynynen J., Niemistö P., Viherä-Aarnio A., Brunner A., Hein S., Velling P. (2010). Silviculture of birch (Betula pendula Roth and Betula pubescens Ehrh.) in northern Europe. Forestry 83: 103–119. http://dx.doi.org/10.1093/forestry/cpp035.
Hytönen J., Aro L. (2012). Biomass and nutrition of naturally regenerated and coppiced birch on cutaway peatland during 37 years. Silva Fennica 46(3): 377–394. http://dx.doi.org/10.14214/sf.48.
Hytönen J., Issakainen J. (2001). Effect of repeated harvesting on biomass production and sprouting of Betula pubescens. Biomass and Bioenergy 20: 237–245. http://dx.doi.org/10.1016/S0961-9534(00)00083-0.
Hyvönen R., Ågren G.I., Linder S., Persson T., Cotrufo M.F., Ekblad A., Freeman M., Grelle A., Janssens I.A., Jarvis P.G., Kellomäki S., Lindroth A., Loustau D., Lundmark T., Norby R.J., Oren R., Pilegaard K., Ryan M.G., Sigurdsson B.D., Strömgren M., van Oijen M., Wallin G. (2007). The likely impact of elevated [CO2], nitrogen deposition, increased temperature and management on carbon sequestration in temperate and boreal forest ecosystems: a literature review. New Phytologist 173: 463–480. http://dx.doi.org/10.1111/j.1469-8137.2007.01967.x.
IEA (2013). Nordic energy technology perspectives. International Energy Agency & Nordic Energy Research, IEA/ETP Publications, Paris and Oslo. 204 p. https://www.iea.org/publications/freepublications/publication/NETP.pdf.
Ingerslev M. (1998). Vitalization of mature Norway spruce stands by fertilization and liming [PhD thesis]. University of Copenhagen (previously in 1998 the Royal Veterinary and Agricultural University). Forest and Landscape, Copenhagen, Denmark, The Research Series no. 23. 126 p.
Ingerslev M., Mälkönen E., Nilsen P., Nohrstedt H.-Ö., Óskarsson H., Raulund-Rasmussen K. (2001). Main findings and future challenges in forest nutritional research and management in the Nordic countries. Scandinavian Journal of Forest Research 16: 488–501. http://dx.doi.org/10.1080/02827580152699330.
IPCC 2013. Climate Change 2013. The physical science basis. In: Stocker T.F., Qin D., Plattner G.K. et al. (eds.). Contribution of WG I to the 5th Assessment report of the IPCC on Climate Change, IPCC, Geneva. http://www.ipcc.ch/report/ar5/wg1/.
IPCC 2014. Climate Change 2014. Synthesis report. Intergovernmental Panel of Climate Change, Geneva, Switzerland. 151 p. http://www.ipcc.ch/report/ar5/syr/.
Jacobs D.F., Oliet J.A., Aronson J., Bolte A., Bullock J.M., Donoso P.J., Landhäusser S.M., Madsen P., Peng S., Rey-Benayas J.M., Weber J.C. (2015). Restoring forests: what constitutes success in the twenty-first century? New Forests 46: 601–614. http://dx.doi.org/10.1007/s11056-015-9513-5.
Johannsen V.K., Nord-Larsen T., Riis-Nielsen T., Suadicani K., Jørgensen B.B. (2013). Skove og plantager 2012. [Forests and forest plantations in 2012]. University of Copenhagen, Skov og Landskab, Frederiksberg. 189 p. [In Danish]. http://ign.ku.dk/samarbejde-raadgivning/myndighedsbetjening/skovovervaagning/skove-og-plantager-2012.pdf.
Johansson T. (2008). Sprouting ability and biomass production of downy and silver birch stumps of different diameters. Biomass and Bioenergy 32: 944–951. http://dx.doi.org/10.1016/j.biombioe.2008.01.009.
Jordbruksverket (2008). Kartläggning av mark som tagits ur produktion. [Mapping of land taken out of production]. Jordbruksverket, Jönköping. 46 p. http://www2.jordbruksverket.se/webdav/files/SJV/trycksaker/Pdf_rapporter/ra08_7.pdf.
Kabzems R., Nemec A.L., Farnden C. (2007) Growing trembling aspen and white spruce intimate mixtures: early results (13–17 years) and future projections. BC Journal and Ecosystems and Management 8: 1–14. http://www.forrex.org/publications/jem/ISS39/vol8_no1_art1.pdf.
Kärkkäinen L., Matala J., Harkonen K., Kellomäki S., Nuutinen T. (2008). Potential recovery of industrial wood and energy wood raw material in different cutting and climate scenarios for Finland. Biomass and Bioenergy 32: 934–943. http://dx.doi.org/10.1016/j.biombioe.2008.01.008.
Karlman L. (2010). Genetic variation in frost tolerance, juvenile growth and timber production in Russian larches (Larix Mill.) – implications for use in Sweden. Doctoral Thesis. Acta Universitatis Agriculturae Sueciae 30, Umeå. 91 p. http://pub.epsilon.slu.se/2264/.
Kellomäki S., Strandman H., Nuutinen T., Peltola H., Korhonen K.T., Väisänen H. (2005). Adaptation of forest ecosystems, forest and forestry to climate change. FINADAPT. Working Paper 4. Finnish Environment Institute Mimeographs 334, Helsinki. https://helda.helsinki.fi/handle/10138/41042.
Kellomäki S., Peltola H., Nuutinen T., Korhonen K.T.,Strandman H. (2008). Sensitivity of managed boreal forests in Finland to climate change, with implications for adaptive management. Philosophical Transactions of the Royal Society of London Series B-Biological Sciences 363: 2341–2351. http://dx.doi.org/10.1098/rstb.2007.2204.
Keskkonnateabe Keskus (2010). Yearbook Forest 2009. Environmental Information Center, Tartu. 217 p. [In Estonian with English translations]. http://www.keskkonnainfo.ee/main/index.php/en/publications.
Kirilenko A.P., Sedjo R.A. (2007). Climate change impacts on forestry. Proceedings of the National Academy of Sciences of the United States of America 104: 19697–19702. http://dx.doi.org/10.1073/pnas.0701424104.
Kjaer E.D, Lobo A., Myking T. (2014). The role of exotic tree species in Nordic forestry. Scandinavian Journal of Forest Research 29: 323–332. http://dx.doi.org/10.1080/02827581.2014.926098.
Kolström M., Lindner M., Vilén T., Maroschek M., Seidl R., Lexer M.J., Netherer S., Kremer A., Delzon S., Barbati A., Marchetti M., Corona P. (2011). Reviewing the science and implementation of climate change adaptation measures in European forestry. Forests 2: 961–982. http://dx.doi.org/10.3390/f2040961.
Knoke T., Stimm B., Ammer C., Moog M. (2005). Mixed forests reconsidered: a forest economics contribution on an ecological concept. Forest Ecology and Management 213: 102–116. http://dx.doi.org/10.1016/j.foreco.2005.03.043.
Konstantinavičienė J., Škėma M., Stakėnas V., Šilinskas B. (2014). Gluosniu Salix viminalis L. energetiniu plantaciju antžeminės biomasės produktyvumas Lietuvoje. [Aboveground biomass productivity of energy willow (Salix viminalis L.) plantations in Lithuania]. Miškininkystė 76: 29–37.
Landresource (2007). Final report of the study financed by the Estonian Rural Development Foundation. 88 p. [In Estonian with English Summary].
Larsson S., Lundmark T., Ståhl G. (2009). Möjligheter till intensivodling av skog. [Opportunities for intensive cultivation of forests]. Slutrapport från regeringsuppdrag Jo 2008/1885, SLU. 136 p. [In Swedish].
Latvia Forest Industry Federation (2008). Forest sector in Latvia 2008. Latvia Forest Industry Federation, Riga. 32 p. https://www.zm.gov.lv/public/ck/files/ZM/mezhi/buklets/MN_2008_EN.pdf.
Latvian State Forest Service (2012). State register of forests (SRF) Latvia. CD Forest Statistics. http://www.vmd.gov.lv.
Latvian State Land Service (2012). Land report of the Republic of Latvia. The State Land Service, Riga. 20 p. http://www.vzd.gov.lv.
Liesebach M., von Wuehlisch G., Muhs H.-J. (1999). Aspen for short rotation coppice plantation on agricultural sites in Germany: effects of spacing and rotation time on growth and biomass production of aspen progenies. Forest Ecology and Management 121: 25–39. http://dx.doi.org/10.1016/S0378-1127(98)00554-4.
Lindkvist A., Kardell Ö., Nordlund C. (2011). Intensive forestry as progress or decay? An analysis of the debate about forest fertilization in Sweden, 1960–2010. Forests 2: 112–146. http://dx.doi.org/10.3390/f2010112.
Lippke B., Oneil E., Harrison R., Skog K., Gustavsson L., Sathre R. (2011). Life cycle impacts of forest management and wood utilization on carbon mitigation: knowns and unknowns. Carbon Management 2: 303–333. http://www.treesearch.fs.fed.us/pubs/38598.
Lithuanian Ministry of Environment (2015). Lithuanian statistical yearbook of forestry 2015. [Miškų ūkio statistika 2015]. Ministry of Environment, State Forest Service, Kaunas. http://www.amvmt.lt/index.php/leidiniai/misku-ukio-statistika/2015.
Löf M., Bolte A., Jacobs D.F., Jensen A.M. (2014). Nurse trees as a forest restoration tool for mixed plantations: effects on competing vegetation and performance in target tree species. Restoration Ecology 22: 758–765. http://dx.doi.org/10.1111/rec.12136.
Löf M., Møller Madsen E., Rytter L. (2015). Skötsel av ädellövskog. [Management of noble hardwoods]. Skogsstyrelsens förlag, Skogsskötselserien, Jönköping. 71 p. http://www.skogsstyrelsen.se/skogsskotselserien. [In Swedish].
Mantau U., Saal U., Prins K., Steierer F., Lindner M., Verkerk H., Eggers J., Leek N., Oldenburger J., Asikainen A., Anttila P. (2010). EUwood – real potential for changes in growth and use of EU forests. Final report. Hamburg, Germany. 160 p. http://www.egger.com/downloads/bildarchiv/187000/1_187099_DV_Real-potential-changes-growth_EN.pdf.
Mård H. (1996) The influence of a birch shelter (Betula spp) on the growth of young stands of Picea abies. Scandinavian Journal of Forest Research 11: 343–350. http://dx.doi.org/10.1080/02827589609382945.
Marklund L.G. (1988). Biomass functions for pine, spruce and birch in Sweden. Swedish University of Agricultural Sciences, Dept. Forest Survey, Umeå, Report no. 45. 73 p. [In Swedish with English summary].
Matala J., Kärkkäinen L., Härkönen K., Kellomäki S., Nuutinen T. (2009). Carbon sequestration in the growing stock of trees in Finland under different cutting and climate scenarios. European Journal of Forest Research 128: 493–504. http://dx.doi.org/10.1007/s10342-009-0299-x.
Mc Carthy R., Rytter L. (2015). Productivity and thinning effects in hybrid aspen root sucker stands. Forest Ecology and Management 354: 215–223. http://dx.doi.org/10.1016/j.foreco.2015.06.015.
Mc Carthy R., Ekö P.M., Rytter L. (2014). Reliability of stump sprouting as a regeneration method for poplars: clonal behaviour in survival, sprout straightness and growth. Silva Fennica 48(3) article 1126. http://dx.doi.org/10.14214/sf.1126.
McKendry P. (2002). Energy production from biomass (part 1): overview of biomass. Bioresource Technology 83: 37–46. http://dx.doi.org/10.1016/S0960-8524(01)00118-3.
Metla (2011). Främmande trädslag – en möjlighet eller ett hot? [Foreign tree species – a possibility or a threat?]. The Finnish Forest Research Institute (Metla), Vantaa. http://www.metla.fi/uutiskirje/rannikkometsat/2011-02/uutinen-3.html. [In Swedish].
Meuwissen T.H.E. (2009). Accuracy of breeding values of ‘unrelated’ individuals predicted by dense SNP genotyping. Genetics Selection Evolution 41(35). 9 p. http://dx.doi.org/10.1186/1297-9686-41-35.
Ministry of Agriculture and Forestry in Finland (2012). Yearbook of farm statistics. Information Centre of the Ministry of Agriculture and Forestry. 269 p. http://stat.luke.fi/en/yearbook-farm-statistics-2003-2013_en.
Ministry of Agriculture and Forestry in Finland (2014). Yearbook of farm statistics 2014. Information Centre of the Ministry of Agriculture and Forestry. 328 p. http://stat.luke.fi/sites/default/files/maatilatilastollinen_vuosikirja_2014.pdf.
Mizaraite D., Mizaras S. (2006). Afforestation of agriculture land as a tool of rural forestry development in Lithuania. In: Wall S. (ed.). Small-scale forestry and rural development: the intersection of ecosystems, economics and society. Proceedings from the Small.scale Forestry and Rural Development Conference, Gallway, Ireland, 18–23 June. p. 305–311. http://www.fao.org/fileadmin/user_upload/rome2007/docs/Afforestation_agriculture_land_Lithuania.pdf.
Mola-Yudego B., Aronsson P. (2008). Yield models for commercial willow biomass plantations in Sweden. Biomass and Bioenergy 32: 829–837. http://dx.doi.org/10.1016/j.biombioe.2008.01.002.
Nichols J.D., Bristow M., Vanclay J.K. (2006). Mixed-species plantations: prospects and challenges. Forest Ecology and Management 233: 383–390. http://dx.doi.org/10.1016/j.foreco.2006.07.018.
Niemistö P. (1996). Yield and quality of planted silver birch (Betula pendula) in Finland – preliminary review. Norwegian Journal of Agricultural Sciences 24: 51–59.
Nilsen P. (2001). Fertilization experiments on forest mineral soils: a review of the Norwegian results. Scandinavian Journal of Forest Research 16(6): 541–554. http://dx.doi.org/10.1080/02827580152699376.
Nohrstedt H.-Ö. (2001). Response of coniferous forest ecosystems on mineral soils to nutrient additions: a review of Swedish experiences. Scandinavian Journal of Forest Research 16: 555–573. http://dx.doi.org/10.1080/02827580152699385.
Nordiska Ministerrådet (2009). Hållbar utveckling – en ny kurs för Norden. Nordiska Ministerrådet, Copenhagen, Denmark. Report ANP 2009:726. 40 p. http://norden.diva-portal.org/smash/get/diva2:701331/FULLTEXT01.pdf.
Nord-Larsen T., Meilby H. (2016). Effects of nurse trees, spacing, and tree species on biomass production in mixed forest plantations. Scandinavian Journal of Forest Research 31: 592–601. http://dx.doi.org/10.1080/02827581.2015.1131845.
Nord-Larsen T., Meilby H., Skovsgaard J.P. (2009). Site-specific height growth models for six common tree species in Denmark. Scandinavian Journal of Forest Research 24: 194–204. http://dx.doi.org/10.1080/02827580902795036.
Nunery J.S., Keeton W.S. (2010). Forest carbon storage in the northeastern United States: net effects of harvesting frequency, post-harvest retention, and wood products. Forest Ecology and Management 259: 1363–1375. http://dx.doi.org/10.1016/j.foreco.2009.12.029.
Óskarsson H., Sigurgeirsson A. (2001). Fertilization in Icelandic afforestation: evaluation of results. Scandinavian Journal of Forest Research 16(6): 536–540. http://dx.doi.org/10.1080/02827580152699367.
Øyen B.H. (2005). Growth and yield in stands of Sitka spruce (Picea sitchensis (Bong.) Carr.) in Norway. Rapport fra skogforskningen 4/05. 46 p. [In Norwegian with English summary].
Paquette A., Messier C. (2010). The role of plantations in managing the world’s forests in the Anthropocene. Frontiers in Ecology and the Environment 8(1): 27–34. http://dx.doi.org/10.1890/080116.
Parviainen J., Västilä S. (2011). State of Finland’s forests 2011. Ministry of Agriculture and Forestry & Finnish Forest Research Institute (Metla), Publication no 5a. 95 p. ISBN 978-952-453-660-8. http://www.metla.fi/metinfo/sustainability/doc/state-of-finlands-forests-2011.pdf.
Persson O. (1992). A growth simulator for Scots pine (Pinus sylvestris L.) in Sweden. Swedish University of Agricultural Sciences, Dept. Forest Yield Research, Garpenberg, Report no. 31. 206 p. [In Swedish with English summary].
Poudel B.C., Sathre R., Gustavsson L., Bergh J., Lundström A., Hyvönen R. (2011). Effects of climate change on biomass production and substitution in north-central Sweden. Biomass and Bioenergy 35: 4340–4355. http://dx.doi.org/10.1016/j.biombioe.2011.08.005.
Poudel B.C., Sathre R., Bergh J., Gustavsson L., Lundström A., Hyvönen R. (2012). Potential effects of intensive forestry on biomass production and total carbon balance in north-central Sweden. Environmental Science and Policy 15: 106–124. http://dx.doi.org/10.1016/j.envsci.2011.09.005.
Pretzsch H., del Río M., Ammer C., Avdagic A., Barbeito I., Bielak K., Brazaitis G., Coll L., Dirnberger G., Drössler L., Fabrika M., Forrester D.I., Godvod K., Heym M., Hurt V., Kurylyak V., Löf M., Lombardi F., Matović B., Mohren F., Motta R., den Ouden J., Pach M., Ponette Q., Schütze G., Schweig J., Skrzyszewski J., Sramek V., Sterba H., Stojanović D., Svoboda M., Vanhellemont M., Verheyen K., Wellhausen K., Zlatanov T., Bravo-Oviedo A. (2015). Growth and yield of mixed versus pure stands of Scots pine (Pinus sylvestris L.) and European beech (Fagus sylvatica L.) analysed along a productivity gradient through Europe. European Journal of Forest Research 134: 927–947. http://dx.doi.org/10.1007/s10342-015-0900-4.
Pyörälä P., Peltola H., Strandman H., Kilpeläinen A., Asikainen A., Jylhä K., Kellomäki S. (2014). Effects of management on economic profitability of forest biomass production and carbon neutrality of bioenergy use in Norway spruce stands under the changing climate. Bioenergy Research 7: 279–294. http://dx.doi.org/10.1007/s12155-013-9372-x.
Räisänen J., Ylhäisi J. (2015). CO2-induced climate change in northern Europe: CMIP2 versus CMIP3 versus CMIP5. Climate Dynamics 45: 1877–1897. http://dx.doi.org/10.1007/s00382-014-2440-x.
Rönnberg J., Vollbrecht G. (1999). Early infection by Heterobasidion annosum in Larix × eurolepis seedlings planted on infested sites. European Journal of Forest Pathology 29(1): 81–86. http://dx.doi.org/10.1046/j.1439-0329.1999.00134.x.
Rosvall O. (ed.) (2011). Review of the Swedish tree breeding program. Skogforsk, Uppsala, Sweden. 84 p. ISBN: 978-91-977649-6-4.
Rounsevell M.D.A., Ewert F., Reginster I., Leemans R., Carter T.R. (2005). Future scenarios of European agricultural land use. II. Projecting changes in cropland and grassland. Agriculture, Ecosystems and Environment 107: 117–135. http://dx.doi.org/10.1016/j.agee.2004.12.002.
Routa J, Kellomäki S., Peltola H. (2012). Impacts of intensive management and landscape structure on timber and energy wood production and net CO2 emissions from energy wood use of Norway Spruce. Bioenergy Research 5: 106–123. http://dx.doi.org/10.1007/s12155-011-9115-9.
Ruotsalainen S., Persson T. (2013). Scots pine – Pinus sylvestris L. In: Mullin T.J., Lee S. (eds.). Best practice for tree breeding in Europe. Skogforsk, Uppsala. p. 49–64. http://www.skogforsk.se/english/news/2014/best-practice-for-tree-breeding/.
Rural Support Service of Latvia (2012). http://www.lad.gov.lv.
Rytter L. (1996). Grey alder in forestry: a review. Norwegian Journal of Agricultural Sciences 24: 61–78.
Rytter L. (1998). Pure and mixed deciduous forest – ecology and silviculture. SkogForsk, Uppsala, Redogörelse no. 8. 62 p. [In Swedish with English summary].
Rytter L. (2004). Production potentials of aspen, birch and alder, Skogforsk, Uppsala, Redogörelse nr 4. 62 p. [In Swedish with English summary].
Rytter L. (2006). A management regime for hybrid aspen stands combining conventional forestry techniques with early biomass harvests to exploit their rapid early growth. Forest Ecology and Management 236: 422–426. http://dx.doi.org/10.1016/j.foreco.2006.09.055.
Rytter L., Mc Carthy R. (2016) Sustainable production of hybrid aspen after harvest. Skogforsk, Uppsala, Arbetsrapport no 898. 26 p. http://www.skogforsk.se/contentassets/56a8ec9abc074199a32db5d5cba2c78d/uthallig-produktion-av-hybridasp-efter-skord-slutrapport-2016-for-energimyndighetens-projekt-30346-arbetsrapport-898-2016.pdf.
Rytter L., Rytter R.-M. (2016). Growth and carbon capture of grey alder (Alnus incana (L.) Moench.) under north European conditions – estimates based on reported research. Forest Ecology and Management 373: 56–65. http://dx.doi.org/10.1016/j.foreco.2016.04.034.
Rytter L., Stener L-G. (2005). Productivity and thinning effects in hybrid aspen (Populus tremula L. × P. tremuloides Michx.) stands in southern Sweden. Forestry 78: 285–295. http://dx.doi.org/10.1093/forestry/cpi026.
Rytter L., Stener L.-G. (2014). Growth and thinning effects during a rotation period of hybrid aspen in southern Sweden. Scandinavian Journal of Forest Research 29: 747–756. http://dx.doi.org/10.1080/02827581.2014.968202.
Rytter L., Sennerby-Forsse L., Alriksson A. (2000). Natural regeneration of grey alder (Alnus incana (L.) Moench.) stands after harvest. Journal of Sustainable Forestry 10: 287–294. http://dx.doi.org/10.1300/J091v10n03_11.
Rytter L., Johansson T., Karačić A., Weih M. (2011). Investigation for a Swedish research program on the genus Populus. Skogforsk, Uppsala, Arbetsrapport no. 733. 148 p. [In Swedish with English summary].
Rytter L., Johansson K., Karlsson B., Stener L.-G. (2013). Tree species, genetics and regeneration for bioenergy feedstock in northern Europe. In: Kellomäki S., Kilpeläinen A., Alam A., (eds.). Forest bioenergy production. Springer Science+Business Media, New York. p. 7–37. http://dx.doi.org/10.1007/978-1-4614-8391-5_2.
Rytter L., Andreassen K., Bergh J., Ekö P.-M., Kilpeläinen A., Lazdina D., Muiste P., Nord-Larsen T. (2014a). Land areas and biomass production for current and future use in the Nordic and Baltic countries. Nordic Energy Research, Oslo. 44 p. http://www.nordicenergy.net/publications/.
Rytter L., Karlsson A., Karlsson M., Stener L.-G. (2014b). Skötsel av björk, al och asp. [Management of birch, alder and aspen]. Skogsstyrelsens förlag, Jönköping, Skogsskötsel-serien no. 9. 131 p. http://www.skogsstyrelsen.se/skogsskotselserien. [In Swedish].
Rytter L., Andreassen K., Bergh J., Ekö P.-M., Kilpeläinen A., Lazdina D., Muiste P., Nord-Larsen T. (2015). Availability of biomass for energy purposes in Nordic and Baltic countries – land areas and biomass amounts. Baltic Forestry 21: 375–390.
Saarsalmi A., Mälkönen E. (2001). Forest fertilization research in Finland: a literature review. Scandinavian Journal of Forest Research 16: 514–535. http://dx.doi.org/10.1080/02827580152699358.
Saarsalmi A., Palmgren K., Levula T. (1991). Biomass production and nutrient consumption of the sprouts of Alnus incana. Folia Forestalia 768. 25 p. ISBN 951-40-1143-0. [In Finnish with English summary].
Saxe H, Cannell M.G.R., Johnsen O., Ryan M.G., Vourlitis G. (2001). Tree and forest functioning in response to global warming. New Phytologist 149: 369–400. http://dx.doi.org/10.1046/j.1469-8137.2001.00057.x.
Seppä H., Alenius T., Bradshaw R.H.W., Giesecke T., Heikkilä M., Muukkonen P. (2009). Invasion of Norway spruce (Picea abies) and the rise of the boreal ecosystem in Fennoscandia. Journal of Ecology 97: 629–640. http://dx.doi.org/10.1111/j.1365-2745.2009.01505.x.
SSB (2012). Statistical yearbook. Statistisk sentralbyrå (SSB), Statistics Norway. 400 p. ISBN 978-82-537-8456-4. [In Norwegian].
Stanturf J.A. (2015). Future landscapes: opportunities and challenges. New Forests 46: 615–644. http://dx.doi.org/10.1007/s11056-015-9500-x.
Stanturf J.A., Palik B.J., Dumroese R.K. (2014). Contemporary forest restoration: a review emphasizing function. Forest Ecology and Management 331: 292–323. http://dx.doi.org/10.1016/j.foreco.2014.07.029.
Stanturf J.A., Kant P., Lillesø J.-P.-B., Mansourian S., Kleine M., Graudal L., Madsen P. (2015). Forest landscape restoration as a key component of climate change mitigation and adaptation. IUFRO World Series 34, Vienna. 72 p. ISBN 978-3-902762-50-4. http://www.iufro.org/publications/series/world-series/article/2015/12/01/world-series-vol-34-forest-landscape-restoration-as-a-key-component-of-climate-change-mitigation/.
Stark H., Nothdurft A., Block J., Bauhus J. (2015) Forest restoration with Betula ssp. And Populus ssp. Nurse crops increases productivity and soil fertility. Forest Ecology and Management 339: 57–70. http://dx.doi.org/10.1016/j.foreco.2014.12.003.
Statistics Norway (2011). The national forest inventory. Statistics Norway, Oslo.
Statistics Norway (2013). Statistical yearbook of Norway. Statistics Norway, Oslo. http://www.ssb.no/english/subjects/10/04/20/.
Stener L.-G. (1998). Provincial statistics on the forest land area and growing stock of broadleaved trees in Sweden. SkogForsk, Uppsala, Redogörelse no. 4. 61 p. [In Swedish with English summary].
Stener L.-G. (2010). Tillväxt, vitalitet och densitet för kloner av hybridasp och poppel i sydsvenska fältförsök. [Growth, vitality and density of clones of hybrid aspen and poplar in South-Swedish field trials]. Skogforsk, Uppsala, Arbetsrapport no. 717. 45 p. [In Swedish].
Svensson J. (2011). Survival and growth of Douglas fir in southern Sweden. Swedish University of Agricultural Sciences, School for Forest Management, Skinnskatteberg, Skogsmästarprogrammet Examensarbete 24. 47 p. [In Swedish with English abstract]. http://stud.epsilon.slu.se/3741/.
Swedjemark G., Stenlid J. (1995). Susceptibility of conifer and broadleaf seedlings to Swedish S and P strains of Heterobasidion annosum. Plant Pathology 44: 73–79. http://dx.doi.org/10.1111/j.1365-3059.1995.tb02717.x.
Swedish Forest Agency (2008). Skogliga konsekvensanalyser – SKA-VB 08. [Consequence analyses for forests – SKA-VB 08]. Skogsstyrelsen, Jönköping, Rapport 25. 146 p. [In Swedish]. http://shop.skogsstyrelsen.se/sv/publikationer/rapporter/skogliga-konsekvensanalyser-2008-ska-vb-08-rapport-2008-25.html.
Swedish Forest Agency (2011). Skogsstyrelsens föreskrifter och allmänna råd till Skogsvårdslagen. [The Swedish Forest Agency’s regulations and general advices to the Forestry Act]. Skogsstyrelsens författningssamling SKSFS7, Jönköping. 45 p. [In Swedish]. http://www.skogsstyrelsen.se/Global/myndigheten/f%C3%B6rfattningar/SKSFS%202011-7%20omtryck%20140813.pdf.
Swedish Forest Agency (2013). Swedish statistical yearbook of forestry. Swedish Forest Agency, Jönköping. 373 p. http://www.skogsstyrelsen.se/en/AUTHORITY/News/News-Archive/Printed-Swedish-Statistical-Yearbook-of-Forestry/.
Swedish Forest Agency (2014). Swedish statistical yearbook of forestry. Swedish Forest Agency, Jönköping. 370 p. http://www.skogsstyrelsen.se/en/AUTHORITY/Statistics/Statistical-Yearbook-/Statistical-Yearbooks-of-Forestry/.
Taylor K.E., Stouffer R.J., Meehl G.A. (2012). An overview of CMIP5 and the experiment design. Bulletin of the American Meteorological Society 93: 485–498. http://dx.doi.org/10.1175/BAMS-D-11-00094.1.
Tham Å. (1994). Crop plans and yield predictions for Norway spruce (Picea abies (L.) Karst.) and birch (Betula pendula Roth & Betula pubescens Ehrh.) mixtures. Studia Forestalia Suecica 195. 21 p. http://pub.epsilon.slu.se/3663/.
Torssonen P., Strandman H., Kellomäki S., Kilpeläinen A., Jylhä K., Asikainen A., Peltola H. (2015). Do we need to adapt the choice of main boreal tree species in forest regeneration under the projected climate change? Forestry 88: 564–572. http://dx.doi.org/10.1093/forestry/cpv023.
Torssonen P., Kilpeläinen A., Strandman H., Kellomäki S., Asikainen A., Jylhä K., Peltola H. (2016). Effects of climate change and management on net climate impacts of production and utilization of energy biomass in Norway spruce with stable age-class distribution. Global Change Biology Bioenergy 8: 419–427. http://dx.doi.org/10.1111/gcbb.12258.
Tullus A., Rytter L., Tullus T., Weih M., Tullus H. (2012). Short-rotation forestry with hybrid aspen (Populus tremula × P. tremuloides Michx.) in Northern Europe. Scandinavian Journal of Forest Research 27: 10–29. http://dx.doi.org/10.1080/02827581.2011.628949.
United Nations (1987). Our common future. Report of the World Commission on Environment and Development. 300 p. http://www.un-documents.net/our-common-future.pdf.
Vadla K. (2007). Sitkagran – utbredelse, egenskaper og anvendelse. [Sitka spruce – distribution, properties and use]. Viten fra Skog og landskap 2: 27–31. [In Norwegian]. http://www.skogoglandskap.no/filearchive/vadlaviten-02-2007-4.pdf.
Vejre H., Ingerslev M., Raulund-Rasmussen K. (2001). Fertilization of Danish forests: a review of experiments. Scandinavian Journal of Forest Research 16: 502–513. http://dx.doi.org/10.1080/02827580152699349.
Vuokila Y. (1977). On the growth capacity of aspen stands on good sites. Folia Forestalia 299. 11 p. [In Finnish with English summary]. https://jukuri.luke.fi/handle/10024/521882.
Walther G.-R., Post E., Convey P., Menzel A., Parmesan C., Beebee T.J.C., Fromentin J.-M., Hoegh-Guldberg O., Bairlein F. (2002). Ecological responses to recent climate change. Nature 416: 389–395. http://dx.doi.org/10.1038/416389a.
Westin J., Haapanen M. (2013). Norway spruce – Picea abies (L.) Karst. In: Mullin T.J., Lee S. (eds.). Best practice for tree breeding in Europe. Skogforsk, Uppsala. p. 29–49. http://www.skogforsk.se/english/news/2014/best-practice-for-tree-breeding/.
Wittwer R.F., Stringer J.W. (1985). Biomassproduction and nutrient accumulation in seedling and coppice hardwood plantations. Forest Ecology and Management 13: 223–233. http://dx.doi.org/10.1016/0378-1127(85)90036-2.
Total of 186 references.

