Modelling ecological niches of Sclerocarya birrea subspecies in Tanzania under the current and future climates
Munna A. H., Amuri N. A., Hieronimo P., Woiso D. A. (2023). Modelling ecological niches of Sclerocarya birrea subspecies in Tanzania under the current and future climates. Silva Fennica vol. 57 no. 3 article id 23009. https://doi.org/10.14214/sf.23009
Highlights
- Tanzania harbors ecological niches of Sclerocarya birrea (S. birrea) subsp. caffra, multifoliata and birrea in the eastern, southern-central-northern, and northeastern part of the country, covering 184 814 km2, 139 918 km2 and 28 446 km2 of Tanzania’s land area, respectively
- Ecological niches will contract under future warming climates
- Currently, significant parts of ecological niches for Sclerocarya birrea subspecies are beyond Tanzania’s protected areas network.
Abstract
The information on ecological niches of the Marula tree, Sclerocarya birrea (A. Rich.) Horchst. subspecies are needed for sustainable management of this tree, considering its nutritional, economic, and ecological benefits. However, despite Tanzania being regarded as a global genetic center of diversity of S. birrea, information on the subspecies ecological niches is lacking. We aimed to model ecological niches of S. birrea subspecies in Tanzania under the current and future climates. Ecological niches under the current climate were modelled by using ecological niche models in MaxEnt using climatic, edaphic, and topographical variables, and subspecies occurrence data. The Hadley Climate Center and National Center for Atmospheric Research's Earth System Models were used to predict ecological niches under the medium and high greenhouse gases emission scenarios for the years 2050 and 2080. Area under the curves (AUCs) were used to assess the accuracy of the models. The results show that the models were robust, with AUCs of 0.85–0.95. Annual and seasonal precipitation, elevation, and soil cation exchange capacity are the key environmental factors that define the ecological niches of the S. birrea subspecies. Ecological niches of subsp. caffra, multifoliata, and birrea are currently found in 30, 22, and 21 regions, and occupy 184 814 km2, 139 918 km2, and 28 446 km2 of Tanzania's land area respectively, which will contract by 0.4–44% due to climate change. Currently, 31–51% of ecological niches are under Tanzania’s protected areas network. The findings are important in guiding the development of conservation and domestication strategies for the S. birrea subspecies in Tanzania.
Keywords
climate change;
conservation;
GIS;
agroforestry;
domestication;
MaxEnt;
protected areas network
-
Munna,
Department of Soil and Geological Sciences, Sokoine University of Agriculture, P.O. Box 3008, Morogoro, Tanzania
 https://orcid.org/0000-0001-8858-0457
E-mail
amabmunna81@gmail.com
https://orcid.org/0000-0001-8858-0457
E-mail
amabmunna81@gmail.com

-
Amuri,
Department of Soil and Geological Sciences, Sokoine University of Agriculture, P.O. Box 3008, Morogoro, Tanzania
 https://orcid.org/0000-0003-3092-3458
E-mail
namuri@sua.ac.tz
https://orcid.org/0000-0003-3092-3458
E-mail
namuri@sua.ac.tz
-
Hieronimo,
Department of Agricultural Engineering, Sokoine University of Agriculture, P.O. Box 3003 Morogoro, Tanzania
 https://orcid.org/0000-0002-4450-5073
E-mail
phmusigula@gmail.com
https://orcid.org/0000-0002-4450-5073
E-mail
phmusigula@gmail.com
- Woiso, Department of Biosciences, Sokoine University of Agriculture, P.O. Box 3038 Morogoro, Tanzania E-mail dino@sua.ac.tz
Received 22 February 2023 Accepted 5 December 2023 Published 14 December 2023
Views 46631
Available at https://doi.org/10.14214/sf.23009 | Download PDF

Supplementary Files
1 Introduction
Marula tree, Sclerocarya birrea (A. Rich.) Horchst. is a member of the Anacardiaceae family, believed to originate in the savanna biome of Africa, as the tree is not found in other tropical regions (Hall et al. 2002). The S. birrea is prevalent in dryland savanna ecosystems in Africa, and it has three subspecies which are subspecies birrea (A. Rich.) Horchst., subspecies caffra (Sond.) Kokwaro and subspecies multifoliata (Engl.) Kokwaro (Hall et al. 2002; Kadu et al. 2006). All three subspecies are found in Tanzania and subsp. multifoliata is endemic to Tanzania (Hall et al. 2002).
Sclerocarya birrea is an economically, nutritionally, and ecologically important fruit tree in African drylands (Hall et al. 2002). Economically, marula fruits are processed to produce various products ranging from alcoholic and non-alcoholic beverages, and food products to cosmetic oil (Mng’omba et al. 2012), and it is of nutritional, food security, and medicinal importance (Hall et al. 2002; Mariod and Abdelwahab 2012; Leakey et al. 2015; Leakey et al. 2022). Ecologically, S. birrea provides food and habitats for wild animals (Cook et al. 2019) including elephants (Gadd 2002). However, despite its abundance and diversity in Tanzania the tree is nutritionally and commercially underutilized, and its population is currently declining in Tanzania and across its native range in Africa due to agricultural expansion, overgrazing, and climatic conditions (Munna 2015; Machani et al. 2017), destruction by elephants, and poor regeneration (Cook et al. 2017). Moreover, the S. birrea seeds are semi-recalcitrant (Machani et al. 2007) and can remain viable in the seed bank in a short period (< 3 to 4 years) (Hall et al. 2002; Machani et al. 2007). Therefore, the species needs urgent conservation measures for both ecological benefits and potential sustainable exploitation for economic and nutrition benefits in agroforestry systems.
Some initiatives on S. birrea in Africa by ICRAF/World Agroforestry Centre focused on collecting and devising their management including through establishment of field genebanks as a first step in domesticating and conserving it (Machani et al. 2017). Sclerocarya birrea is also among of the trees which constitute the African Orphan Crops Consortium initiative (Hendre et al. 2019). The initiative involves genomic studies to breed tree varieties with high yields, nutritive quality, and that can adapt to changing climates (Hendre et al. 2019) as a strategy to enhance its conservation through domestication and utilization (Jama et al. 2008; Leakey et al. 2022). However, the success of these initiatives requires information on ecological niches to guide where to plant it (Jama et al. 2008). However, although Tanzania is regarded to be a global center of genetic diversity for S. birrea and should be the focus of research, development, and conservation activities, information on ecological niche of S. birrea subspecies is currently lacking.
Climate change due to global warming has been documented to have an impact on the distribution and phenology of species on the Earth’s surface, with tree species being one example (Noce et al. 2017; Prevéy et al. 2020; Rodriguez et al. 2020). This necessitates the identification of suitable habitats and an understanding of how the environment affects declining taxa, and reliable methods should be used to detect species range shifts and contraction in order to establish conservation priorities (Farnsworth et al. 2006; Rovzar et al. 2016).
The Ecological Niche Modelling (ENM) approach is widely used to map species ecology and predict the patterns of changes in biodiversity under the current and future climates (Martinez-Meyer et al. 2006; Kulhanek et al. 2011; Ganglo et al. 2017; Djotan et al. 2018; Mothes et al. 2019). Among several species distribution models (Segurado et al. 2004; Beaumont et al. 2005; Byeon et al. 2018), CLIMEX models estimate how environmental attributes and climate change influence species distribution and ecosystems. Other ENM approaches frequently used in predicting species ecological niches include the Genetic Algorithm for Rule Set Production (GARP), and maximum entropy modeling (MaxEnt) which discover habitats (Çoban et al. 2020) and predict species distribution accurately even with small samples (Tarkesh et al. 2012; Heidari 2019).
This study aimed to (i) document environmental variables that define ecological niches of S. birrea subspecies in Tanzania; (ii) predict and quantify ecological niches of S. birrea subspecies under the current and future climates; and, (iii) determine portions of S. birrea subspecies ecological niches which are under Tanzania’s protected areas network. The findings can be used to guide field inventories and development of domestication and conservation plans to improve conservation and domestication and consequently utilization of S. birrea subspecies in Tanzania.
2 Materials and methods
2.1 Description of the study area
The study was conducted in four districts of Tanzania, Rombo, Morogoro Municipal, Iringa and Serengeti districts (Fig. 1). Rombo district is located on the eastern slope of Mount Kilimanjaro between latitudes 3° 01’ and 3° 09’ S and longitudes 37° 28’ and 37° 33’ E, and altitude of 800 to 5895 meters above sea level. The mean annual rainfall range between 500 to 1000 mm and the daily temperature ranges from 18 to 28 degrees celsius. Morogoro municipal lies between latitude 5° 58’ and 10° 0’ S and longitude 35° 25’ and 35° 30’ E, with the annual rainfall ranges from 600 mm in low lands to 1200 mm in the highland plateau. The average monthly temperature varies between 17.48 °C in the mountains to 31.31 °C in river valleys. Iringa district lies between latitudes 7° 0’ and 8° 30’ S and longitudes 34° 0’ and 37° 0’ E, and altitude of 800 to 1800 meters above sea level. The district receives rainfall of between 600 and 1000 mm annually with mean temperature ranging from 15 °C to 20 °C. Serengeti district lies between latitude 1° 30’ S and 2° 40’ S, and 34° 15’ E and 35° 30’ E, and an altitude ranging from 1000 to 2300 m. The district receives bi-modal rainfall with annual mean rainfall ranging from 900 to 1000 mm. Temperatures in Serengeti district generally range from 15 °C in April to 29 °C in July (Serengeti District Council 2018). The regions were selected in reference to Woiso (2011) and Woiso (2014) who confirmed subspecies birrea, caffra and multifoliata to exist in Rombo district, Morogoro municipal and Iringa district respectively. Therefore, the sites were considered to have sufficient environmental information and suitable sites to serve as study areas for this study. However, we further sought additional subspecies occurrence data across Tanzania from the GBIF database to avoid misrepresentation of environmental variables across the countries where subspecies have been reported to occur.
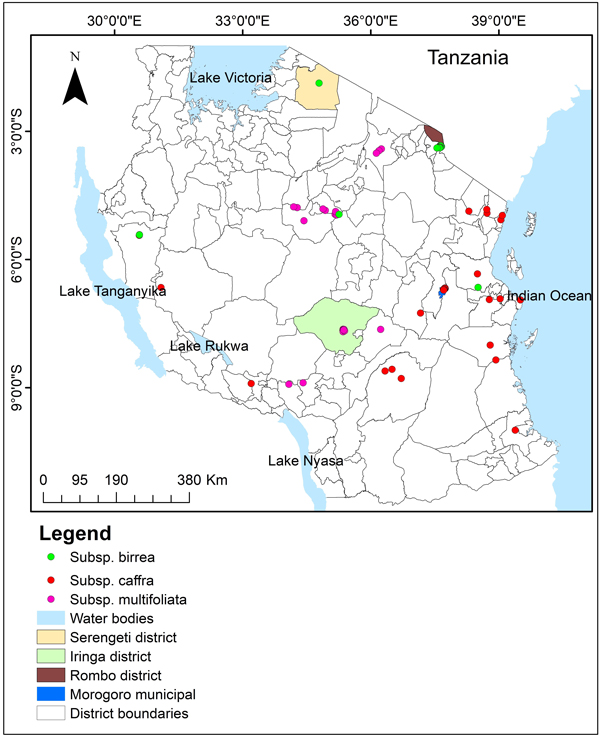
Fig. 1. Tanzania map showing study sites and the distribution of spatially-rarefied occurrence data for Marula tree (Sclerocarya birrea) subsp. birrea (green), subsp. caffra (red) and subsp. multifoliata (pink) in Tanzania.
2.2 Input data
2.2.1 Occurrence data
The subspecies occurrence points (GPS coordinates) where the subspecies occur were collected during field surveys in Holili village in Rombo district, Kiegea village in Morogoro municipal, Malinzanga village in Iringa Rural district and Bonchugu in Serengeti district in Tanzania in 100 × 100-m plots along the transects at a distance of 250-m between plots. The transects were 2-km long and were separated by 500-m distance between transects. The number of transects was 7, 8, and 12 in Kiegea, Holili and Malinzanga villages respectively. Occurrence points consisting of GPS coordinates at points where subspecies were found, were collected by using a handheld Global Positioning System (GPS) Garmin eTrex version 3.5. We collected a total of 362 occurrence points, 100 for subsp. birrea, 49 for subsp. caffra and 226 for subsp. multifoliata. Additional occurrence points were sourced from Global Biodiversity Information Facility (GBIF) website, https://www.gbif.org/: 8 for subsp. birrea, 28 for subsp. caffra and 16 for subsp. multifoliata, resulting into 108, 77 and 242 occurrence points for subspecies birrea, caffra and multifoliata, respectively. To prevent models over-fit due to spatial auto-correlation and sampling bias, occurrence data were spatially-rarefied at 1-km by using SDMToolbox software version 2.4 (Brown 2014) and retain 19, 29 and 23 occurrence points for subsp. birrea, caffra and multifoliata respectively.
2.2.2 Environmental variables
The study used three categories of environmental variables, namely bioclimatic, edaphic, and topographical-Digital Elevation Model (DEM) raster layers (Supplementary file S1: Table S1). Nineteen bioclimatic and potential evapotranspiration variables with a spatial resolution of 30-arc second (~1 km) for the years 1970–2000 was obtained from the WorldClim–climate data version 2.1, https://www.worldclim.org (Fick and Hijmans 2017). The soil texture, cation exchange capacity, electrical conductivity, soil organic carbon concentration, soil pH, sand, clay and silt fractions of the soil, and water holding capacity raster layers were sourced from Africa Soil Profiles Database, https://www.isric.org/projects/africa-soil-profiles-database-afsp (Leenaars et al. 2014). The DEM for Tanzania was obtained from https://cgiarcsi.community/data/srtm-90m-digital-elevation-database-v4.1 (Jarvis et al. 2008). The slope, aspect, and hillshade was derived from DEM. Cross-correlation between environmental variables was assessed by using ENMTools software version 1.4.3 (Warren et al. 2010) by calculating Pearson’s Correlation Coefficient (r). A variable from a pair of variables with a cross-correlation coefficient value > 0.7 was excluded (Heidari 2019). We retained variables (Table 1a-c) with higher predictive power for a set of highly correlated variables during preliminary models run, as described by Dormann et al. (2013). Prediction of S. birrea subspecies ecological niches under future climates were done under two concentration pathways (RCPs) with different greenhouse gas (GHG) emissions scenarios (Thomson et al. 2011), the RCP4.5 and RCP8.5, for the year 2050 (2040–2069) and 2080 (2070–2099) time horizons. Future predictions were done by using The Hadley Climate Centre Global Environmental Model 2-Earth System (HadGEM2-ES model) and The Community Climate System Model, Version 4 (CCSM4) climate data from the Consultative Group on International Agricultural Research (CGIAR)’s Program on Climate Change, Agriculture, and Food Security (CCAFS) data archive (https://www.ccafs-climate.org/) (Navarro-Racines et al. 2020). The use of these global climate models in this study was in reference to use of similar models in earlier similar studies by Bogawski et al. (2019), Kogo et al. (2019), Abrha et al. (2018), and Zarei et al. (2021) in eastern African region and other parts of Africa. Future predictions were done by using bioclimatic variables that significantly contributed by at least 3% in defining the subspecies ecological niches under the current environmental conditions.
2.3 Modelling and mapping
Modelling of S. birrea subspecies ecological niches was done by using Maximum Entropy software version 3.4.1(Phillips et al. 2006). MaxEnt is a machine learning program which uses environmental factors and presence-only species occurrence data to develop an ecological niche model, which is capable of predicting suitable habitat where a species can thrive (Phillips et al. 2006). Phillips et al. (2006) provide more details on how MaxEnt executes predictions. The subspecies ecological niche models (ENMs) were developed by using 10 000 background data, a regulation multiplier of 2.5, a maximum of 5000 iterations, and 15 replications of the models before the models were run. Other model settings were maintained as a default as detailed by Phillips et al. (2006). Due to the small sample size of our data, we used Pearson et al. (2007) method, which employs the jackknife (leave-one-out) strategy at employing P values to calculate the prediction success rate by using pValueCompute.exe software. The accuracy of models was further assessed by using Receiver Operating Characteristics (ROC)’s Area under the Curves (AUCs) threshold (Phillips et al. 2006). The AUCs for S. birrea subspecies models were classified according to Thuiller et al. (2008) AUC threshold: AUC > = 0.90 (excellent), AUC = 0.8–0.9 (good), AUC = 0.7–0.80 (acceptable), AUC = 0.6–0.70 (bad) and AUC = 0.5–0.60 (invalid). The ArcGIS 10.5 was used to develop final suitability maps and quantify the size of ecological niches based on four suitability classes: 0.00–0.25 (unsuitable), 0.25–0.50 (moderately suitable), 0.50–0.75 (suitable) and 0.75–1.00 (highly suitable) using natural breaks option (Kogo et al. 2019) in ArcGIS 10.5. Ecological niches which fall under protected areas network and other land uses were estimated by overlaying the subspecies ecological niche maps with the current Tanzania protected areas shape file retrieved from the World Database on Protected Areas, https://www.protectedplanet.net/ (UNEP-WCMC and IUCN 2021).
3 Results
3.1 Models performance and variables contribution
The subspecies ENMs were robust with mean AUCs of 0.85 for subsp. caffra, 0.92 for subsp. multifoliata, and 0.95 for subsp. birrea, (Suppl. file S1: Figure S1). Our subspecies ENMs were also statistically significant, with q = 0.81 and P-value (p) = 0.002 for subsp. birrea, q = 0.93 and p = 0.014 for subsp. caffra, and q = 0.93 and p = 0.001 for subsp. multifoliata. Results, (Table 1a-c), show contribution of each predictor variable to the ENMs of S. birrea subspecies. The results show that precipitation of the driest month and elevation are the major environmental factors contributed the most to the ENM of subsp. caffra respectively, each contributing 47.0% and 20.5% to the model (Table 1a). The Jackknife results support the findings that elevation and clay soil had the greatest effects on the ecological niche of subsp. caffra (Suppl. file S1: Figure S2).
| Table 1. The contribution of environmental factors that define the ecological niches of the Marula tree (Sclerocarya birrea) subspecies in Tanzania under the current environmental conditions. | |||||
| Subspecies | Code | Environmental variables | Percent contribution | Cumulative contribution | Permutation importance |
| (a) Subsp. caffra | Bio14 | Precipitation of Driest Month | 47.0 | 47.0 | 3.2 |
| ELV | Elevation | 20.5 | 67.5 | 31.7 | |
| CLY | Clay soil fraction | 11.4 | 78.9 | 23.8 | |
| SLT | Silt soil fraction | 9.7 | 88.6 | 4.4 | |
| Bio19 | Precipitation of Coldest Quarter | 4.3 | 92.9 | 10.1 | |
| Bio08 | Mean Temperature of Wettest Quarter | 3.6 | 96.5 | 9.9 | |
| SLP | Slope | 0.7 | 97.2 | 6.5 | |
| ELC | Soil electrical conductivity | 0.7 | 97.9 | 6.1 | |
| ASP | Aspect | 0.7 | 98.6 | 2.5 | |
| Bio18 | Precipitation of Warmest Quarter | 0.5 | 99.1 | 0.5 | |
| AWC | Available water holding capacity | 0.4 | 99.5 | 0.0 | |
| Bio01 | Annual Mean Temperature | 0.3 | 99.8 | 0.4 | |
| pH | Soil pH | 0.1 | 99.9 | 0.9 | |
| (b) Subsp. multifoliata | Bio19 | Precipitation of Coldest Quarter | 38.6 | 38.6 | 5.3 |
| Bio12 | Annual Precipitation | 16.1 | 54.7 | 27.3 | |
| PET | Potential Evapotranspiration | 13.2 | 67.9 | 39.9 | |
| SOC | Soil organic carbon concentration | 10.8 | 78.7 | 0.3 | |
| Bio15 | Precipitation Seasonality | 10.4 | 89.1 | 14 | |
| CLY | Clay soil fraction | 4.0 | 93.1 | 2.6 | |
| Bio18 | Precipitation of Warmest Quarter | 3.0 | 96.1 | 4.0 | |
| SLP | Slope | 2.5 | 98.6 | 3.0 | |
| ASP | Aspect | 0.9 | 99.5 | 0.0 | |
| Bio07 | Temperature Annual Range | 0.3 | 99.8 | 1.3 | |
| ELC | Soil electrical conductivity | 0.1 | 99.9 | 1.6 | |
| (c) Subsp. birrea | CEC | Soil cation exchange capacity | 47.2 | 47.2 | 3.7 |
| Bio14 | Precipitation of Driest Month | 16.9 | 64.1 | 32.7 | |
| SLP | Slope | 8.9 | 73.0 | 39.8 | |
| PET | Potential evapotranspiration | 6.3 | 79.3 | 0.5 | |
| Bio18 | Precipitation of Warmest Quarter | 5.3 | 84.6 | 17.4 | |
| Bio19 | Precipitation of Coldest Quarter | 4.6 | 89.2 | 3.6 | |
| pH | Soil pH | 4.5 | 93.7 | 0.0 | |
| SOC | Soil organic carbon concentration | 3.9 | 97.6 | 0.5 | |
| SLT | Silt soil fraction | 2.2 | 99.8 | 1.6 | |
| ASP | Aspect | 0.1 | 99.9 | 0.2 | |
Precipitation of the coldest quarter and annual precipitation, which each contributed 38.6% and 16.1% to the ENM (Table 1b), respectively, are the main environmental factors contributed the most to the ENM of subsp. multifoliata. The jackknife tests result for subsp. multifoliata revealed that annual precipitation and precipitation of coldest quarter increased the model training gain when used alone, and potential evapotranspiration reduced model training gain when excluded (Suppl. file S1: Figure S3). This implies that ecological niche of this subspecies is mainly influenced by these environmental variables. The results for subsp. birrea reveal that soil CEC and precipitation of the driest month are the two environmental factors that contributed the most to the ENM of subsp. birrea, each accounting for 47.2% and 16.9% of the model, respectively (Table 1c). The Jackknife test results indicate that the CEC is the environmental variable which increases the model training gain the most when used alone and model’s training gain drops when slope is excluded (Suppl. file S1: Figure S4), making them the key environmental variables defining ecological niche of subsp. birrea.
3.2 Ecological Niches of Sclerocarya birrea subspecies under the current and future climates
Under the current environmental conditions, model results reveal subsp. caffra ecological niche to occur in 30 regions across Tanzania (Fig. 2a), and occupy an estimated area of 184 814 km2 of Tanzania terrestrial area (Table 2a), including protected areas network and other land uses (Fig. 2b and Table 2a). Under future warming climates, the CCSM4 and HadGEM2-ES models predict the current ecological niche to contract by an average of 0.4 to 6% in both emission scenarios and time horizons (Fig. 2c–f and 3a–d and Table 3a).
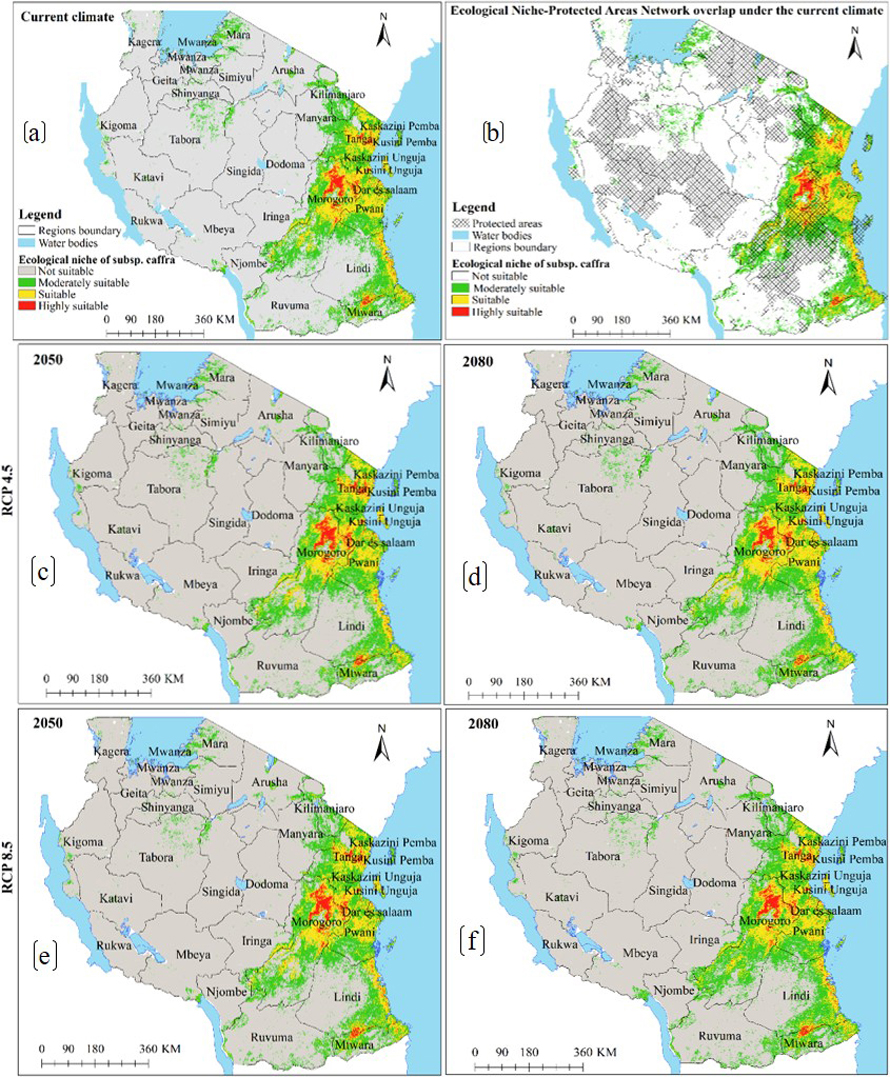
Fig. 2. Ecological Niches of Marula tree (Sclerocarya birrea) subsp. caffra in Tanzania: (a) ecological niche under the current environmental conditions, (b) ecological niche-protected areas network overlap under the current environmental conditions and, (c)–(f) are ecological niches under RCP4.5 and RCP8.5 for the years 2050 and 2080 predicted by using CCSM4 model. Note: “RCP” is representative concentration pathway and “CCSM4” is Community Climate System Model version 4.
| Table 2. Ecological niches of Marula tree (Sclerocarya birrea) (a) subsp. caffra (b) subsp. multifoliata and (c)subsp. birrea in Tanzania under the current environmental conditions and future climates as predicted by CCSM4 and HadGEM2-ES models under RCP4.5 and RCP8.5 for the year 2050 and 2080. Note: “RCP” is representative concentration pathway; “CCSM4” is Community Climate System Model version 4, and “HadGEM2-ES” is Hadley Centre Global Environmental Model 2-Earth System. | ||||||||||
| Subspecies name | Ecological niche class of suitability | Area (km2) under the current climate | Area (km2) under RCP4.5 | Area (km2) under RCP8.5 | ||||||
| CCSM4 | HadGEM2-ES | CCSM4 | HadGEM2-ES | |||||||
| 2050 | 2080 | 2050 | 2080 | 2050 | 2080 | 2050 | 2080 | |||
| (a) Subsp. caffra | Not suitable | 694 327 | 708 571 | 701 428 | 705 702 | 696 233 | 724 908 | 695 131 | 709 449 | 695 147 |
| Moderately suitable | 114 307 | 103 717 | 105 710 | 106 031 | 114 073 | 110 633 | 116 027 | 102 684 | 112 284 | |
| Suitable | 62 368 | 57 890 | 64 397 | 58 913 | 60 838 | 59 531 | 60 179 | 57 830 | 64 125 | |
| Highly suitable | 8140 | 8963 | 7605 | 8495 | 7998 | 9123 | 7804 | 9177 | 7584 | |
| Total suitable area | 184 814 | 170 570 | 177 713 | 173 438 | 182 908 | 179 287 | 184 009 | 169 691 | 183 994 | |
| Protected areas | 58 306 | 53 419 | 56 573 | 54 965 | 59 303 | 54 497 | 59 245 | 53 393 | 59 335 | |
| Other land uses | 126 508 | 117 151 | 121 139 | 118 473 | 123 605 | 124 790 | 124 764 | 116 299 | 124 658 | |
| (b) Subsp. multifoliata | Not suitable | 757 251 | 763 077 | 768 075 | 762 276 | 762 870 | 769 740 | 769 613 | 769 225 | 770 232 |
| Moderately suitable | 98 726 | 95 129 | 88 563 | 94 965 | 94 413 | 90 310 | 89 795 | 89 443 | 90 048 | |
| Suitable | 35 303 | 33 099 | 34 024 | 33 865 | 33 706 | 30 678 | 32 200 | 31 683 | 30 657 | |
| Highly suitable | 5889 | 5865 | 6507 | 6063 | 6180 | 6441 | 5561 | 6819 | 6232 | |
| Total suitable area | 139 918 | 134 093 | 129 094 | 134 894 | 134 299 | 127 429 | 127 556 | 127 945 | 126 937 | |
| Protected areas | 47 566 | 44 413 | 41 849 | 47 074 | 46 285 | 41 832 | 41 660 | 44 990 | 42 943 | |
| Other land uses | 92 352 | 89 680 | 87 246 | 87 819 | 88 014 | 85 597 | 85 897 | 82 955 | 83 994 | |
| (c) Subsp. birrea | Not suitable | 851 201 | 868 169 | 863 270 | 863 902 | 862 936 | 864 283 | 861 796 | 863 310 | 863 379 |
| Moderately suitable | 23 749 | 24 150 | 13 333 | 12 649 | 13 448 | 12 440 | 14 510 | 13 283 | 13 432 | |
| Suitable | 4024 | 4137 | 2500 | 2544 | 2675 | 2372 | 2783 | 2520 | 2293 | |
| Highly suitable | 672 | 713 | 544 | 552 | 588 | 551 | 557 | 534 | 543 | |
| Total suitable area | 28 446 | 29 000 | 16 377 | 15 745 | 16 711 | 15 364 | 17 851 | 16 337 | 16 268 | |
| Protected areas | 14 550 | 14 532 | 9439 | 8794 | 9408 | 8264 | 10 237 | 9124 | 8601 | |
| Other land uses | 13 895 | 14 468 | 6937 | 6950 | 7303 | 7100 | 7614 | 7213 | 7667 | |
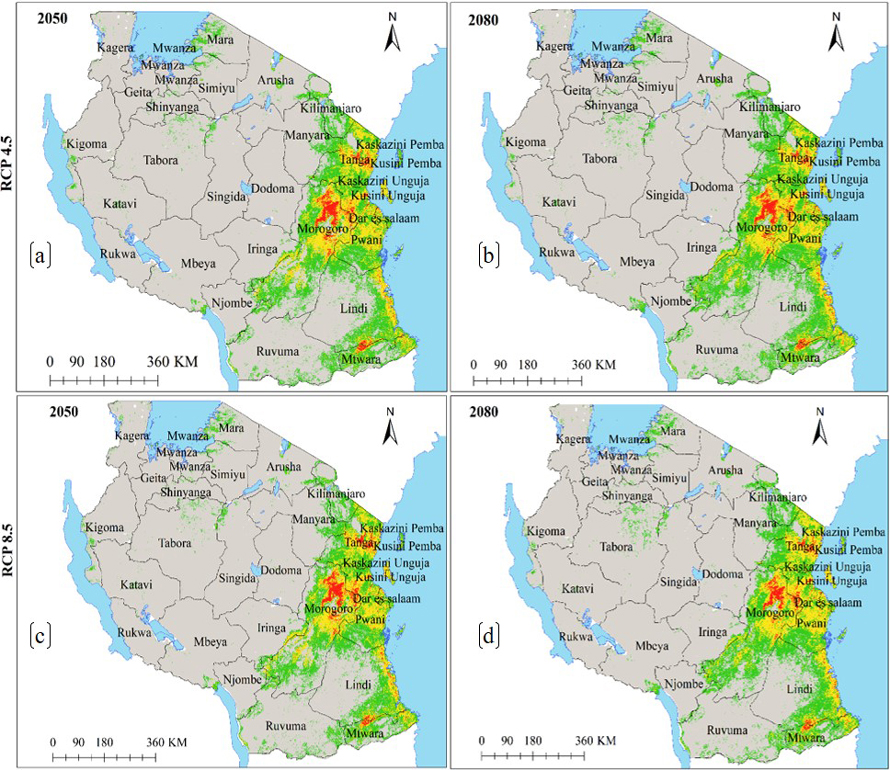
Fig. 3. Future Ecological Niches of Marula tree (Sclerocarya birrea) subsp. caffra in Tanzania under RCP4.5 and RCP8.5 for the years 2050 and 2080 predicted by using HadGEM2-ES model. Note: “RCP” is representative concentration pathway and “HadGEM2-ES” is Hadley Centre Global Environmental Model 2-Earth System.
Under the current environmental conditions, the ENM for subsp. multifoliata (Fig. 4a) predict ecological niche of this subspecies to exists in 21 regions which account 139 918 km2 of Tanzania’s land area including protected areas and other land use categories (Fig. 4b and Table 2b). Under future climates, although the areas classified as highly suitable will slightly increase in future for all greenhouse gases emission scenarios and time horizons, both HadGEM2-ES and CCSM4 models predict ecological niche of subsp. multifoliata to retract by an average of 3.9–9.0% under RCP4.5 and RCP8.5 in 2050 and 2080 (Fig. 4a–f and 5a–d and Table 3b).
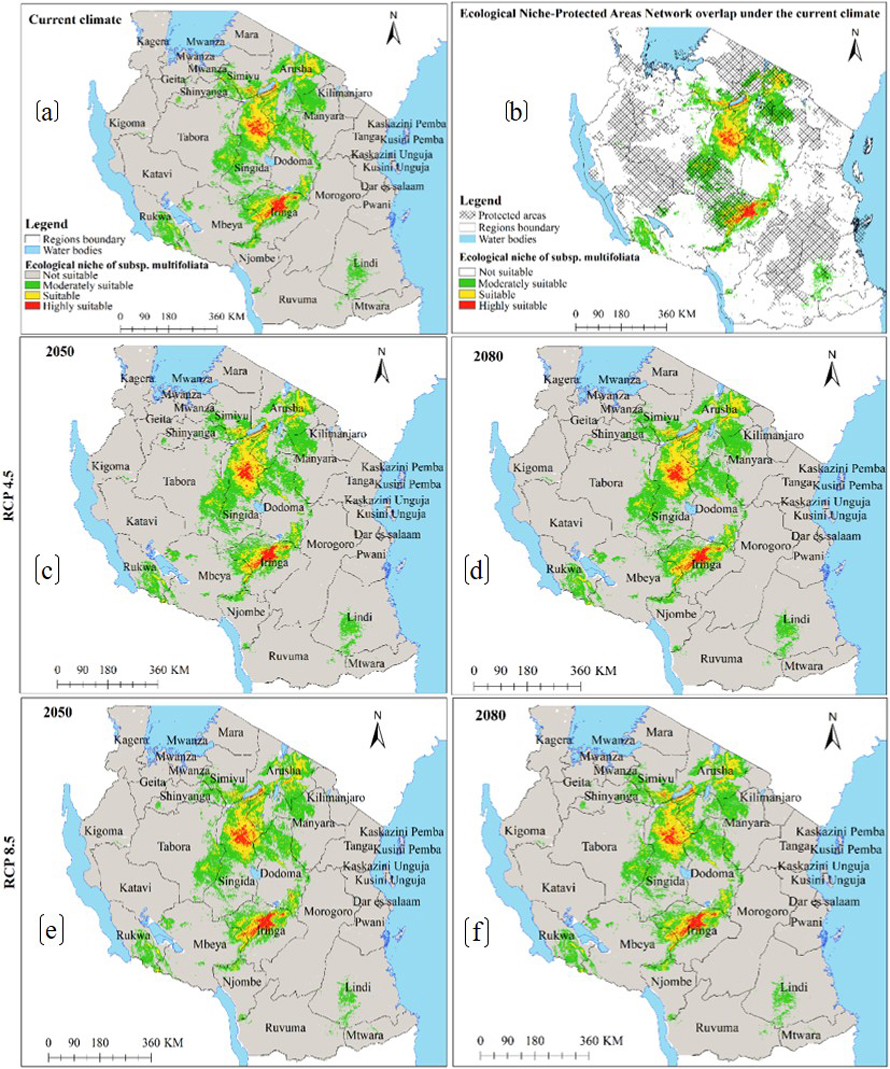
Fig. 4. Ecological Niches of Marula tree (Sclerocarya birrea) subsp. multifoliata in Tanzania: (a) ecological niche under the current environmental conditions, (b) ecological niche-protected areas network overlap under the current environmental conditions and, (c)–(f) are ecological niches under RCP4.5 and RCP8.5 for the years 2050 and 2080 predicted by using CCSM4 model. Note: “RCP” is representative concentration pathway and “CCSM4” is Community Climate System Model version 4.
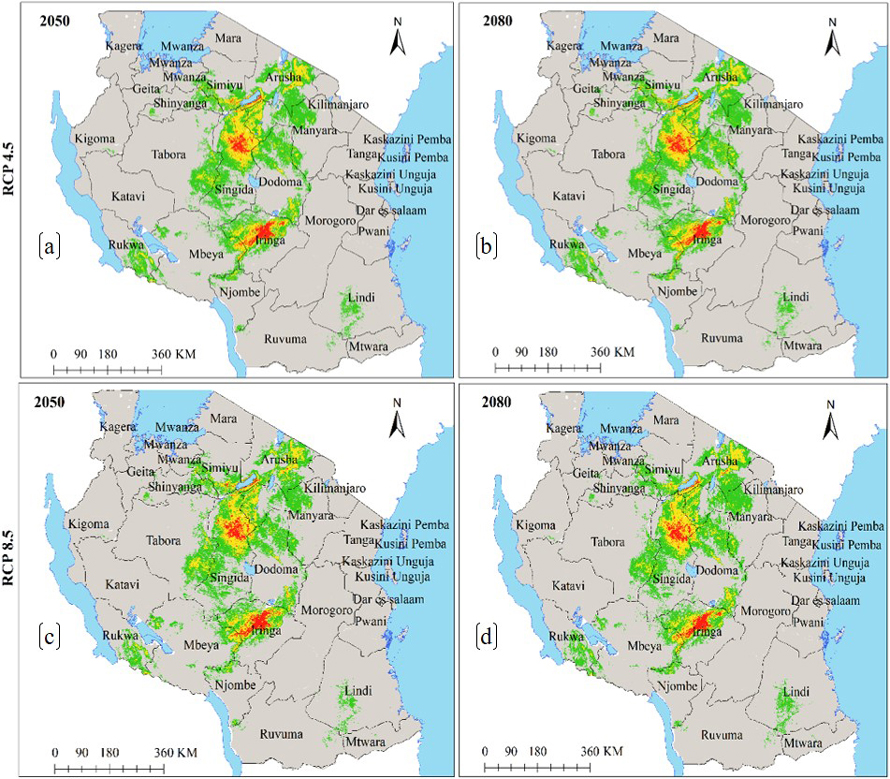
Fig. 5. Future Ecological Niche of Marula tree (Sclerocarya birrea) subsp. multifoliata in Tanzania under RCP4.5 and RCP8.5 for the years 2050 and 2080 predicted by using HadGEM2-ES model. Note: “RCP” is representative concentration pathway and “HadGEM2-ES” is Hadley Centre Global Environmental Model 2-Earth System.
Moreover, under the current environmental conditions, subsp. birrea ecological niche map (Fig. 6a) shows that 22 regions across Tanzania harbour ecological niche for this subspecies and occupy 28 446 km2 of Tanzania land area including areas classified as protected areas and other land uses (Fig. 6b and Table 2c). However, HadGEM2-ES and CCSM4 models predict the current ecological niche of this subspecies to contract by about 21–44% under future warming climates in all greenhouse gases emission scenarios and time horizons (Fig. 6c–f and 7a–d and Table 3c).
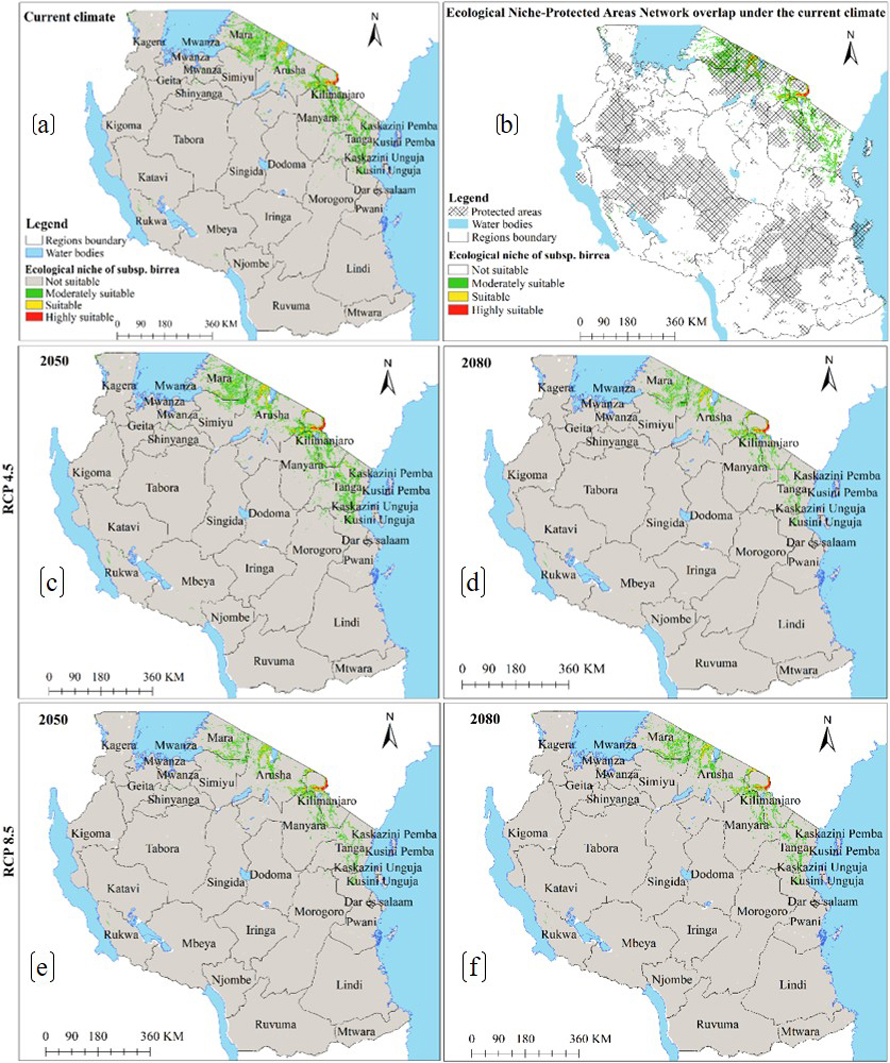
Fig. 6. Ecological Niches of Marula tree (Sclerocarya birrea) subsp. birrea in Tanzania: (a) ecological niche under the current environmental conditions, (b) ecological niche-protected areas network overlap under the current environmental conditions and, (c)–(f) are ecological niches under RCP4.5 and RCP8.5 for the years 2050 and 2080 predicted by using CCSM4 model. Note: “RCP” is representative concentration pathway and “CCSM4” is Community Climate System Model version 4.
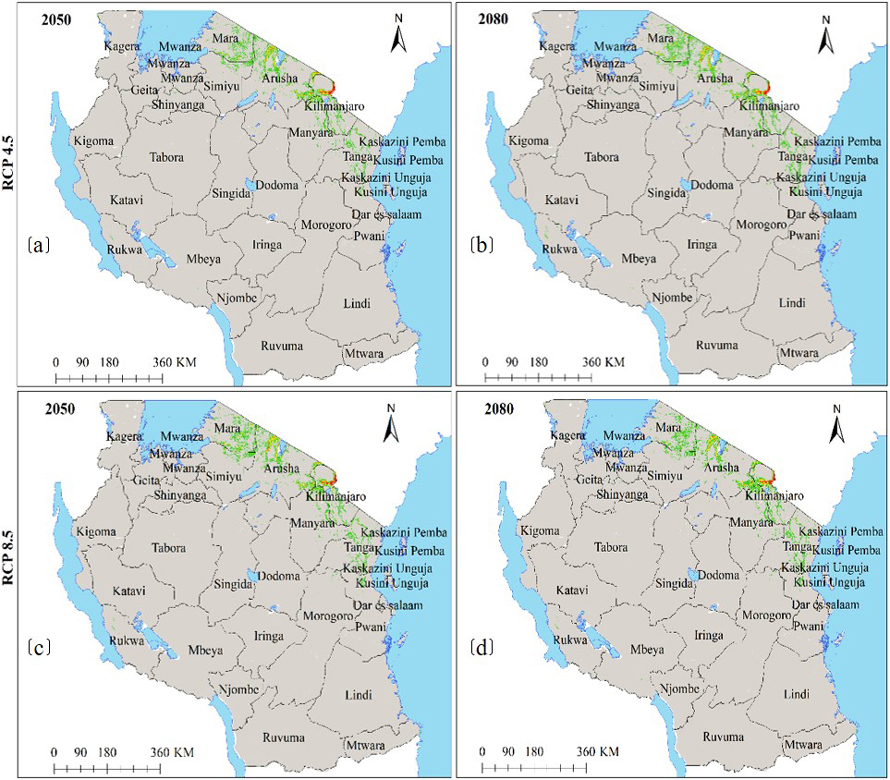
Fig. 7. Future Ecological Niche of Marula tree (Sclerocarya birrea) subsp. birrea in Tanzania under RCP4.5 and RCP8.5 for the years 2050 and 2080 predicted by using HadGEM2-ES model. Note: “RCP” is representative concentration pathway and “HadGEM2-ES” is Hadley Centre Global Environmental Model 2-Earth System.
| Table 3. Mean Area Change in km2 of ecological niches of Marula tree (Sclerocarya birrea) (a) subsp. caffra (b) subsp. multifoliata and (c) subsp. birrea in Tanzania under future climates as predicted by CCSM4 and HadGEM2-ES models under RCP4.5 and RCP8.5 for the years 2050 and 2080. Note: “RCP” is a representative concentration pathway; “CCSM4” is Community Climate System Model version 4; “HadGEM2-ES” is Hadley Centre Global Environmental Model 2-Earth System; figures with “–” sign indicate a decrease/negative change and that with “+” indicates an increase/positive change in predicted mean area, and “%” implies mean area change in percentage. | |||||||||
| Subspecies name | Ecological niche suitability class | RCP4.5 | RCP8.5 | ||||||
| 2050 | 2080 | 2050 | 2080 | ||||||
| Area change | % | Area change | % | Area change | % | Area change | % | ||
| (a) Subsp. caffra | Not suitable | +12 810 | +1.8 | +4504 | +0.6 | +22 852 | +3.3 | +813 | +0.1 |
| Moderately suitable | –9432 | –8.3 | –4415 | –3.9 | –7648 | –6.7 | –151 | –0.1 | |
| Suitable | –3967 | –6.4 | +250 | +0.4 | –3687 | –5.9 | –216 | –0.3 | |
| Highly suitable | +589 | +7.2 | –338 | –4.2 | +1011 | +12.4 | –446 | –5.5 | |
| Total suitable area | –12 810 | –6.9 | –4504 | –2.4 | –10 325 | –5.6 | –813 | –0.4 | |
| Protected areas | –4114 | –7.1 | –367 | –0.6 | –4361 | –7.5 | +985 | +1.7 | |
| Other land uses | –8696 | –6.9 | –4136 | –3.3 | –5964 | –4.7 | –1797 | –1.4 | |
| (b) Subsp. multifoliata | Not suitable | +5425 | +0.7 | +5722 | +0.8 | +12 231 | +1.6 | +12 671 | +1.7 |
| Moderately suitable | –3679 | –3.7 | –3955 | –4.0 | –8849 | –9.0 | –8804 | –8.9 | |
| Suitable | –1821 | –5.2 | –1900 | –5.4 | –4122 | –11.7 | –3875 | –11.0 | |
| Highly suitable | +75 | +1.3 | +133 | +2.3 | +741 | +12.6 | +7 | +0.1 | |
| Total suitable area | –5425 | –3.9 | –5722 | –4.1 | –12 231 | –8.7 | –12 671 | –9.1 | |
| Protected areas | –1822 | –3.8 | –3499 | –7.4 | –4155 | –8.7 | –5265 | –11.1 | |
| Other land uses | –3602 | –3.9 | –4722 | –5.1 | –8076 | –8.7 | –7407 | –8.0 | |
| (c) Subsp. birrea | Not suitable | +14 835 | +1.7 | +11 902 | +1.4 | +12 595 | +1.5 | +11 386 | +1.3 |
| Moderately suitable | –5350 | –22.5 | –10 359 | –43.6 | –10 888 | –45.8 | –9778 | –41.2 | |
| Suitable | –684 | –17.0 | –1437 | –35.7 | –1578 | –39.2 | –1486 | –36.9 | |
| Highly suitable | –39 | –5.8 | –106 | –15.7 | –130 | –19.3 | –122 | –18.1 | |
| Total suitable area | –6073 | –21.4 | –11 902 | –41.8 | –12 595 | –44.3 | –11 386 | –40.0 | |
| Protected areas | –2887 | –19.8 | –5126 | –35.2 | –5856 | –40.2 | –5131 | –35.3 | |
| Other land uses | –3186 | –22.9 | –6775 | –48.8 | –6739 | –48.5 | –6255 | –45.0 | |
4 Discussion
The ecological niche modeling approach was applied for the first time to predict ecological niches of S. birrea subspecies in Tanzania. The ENMs of the S. birrea subspecies had significant p-values similar to those reported by Gbètoho et al. (2017) and Hernández-Baz et al. (2016). The AUCs for subspecies’ ENMs ranged from 0.85 to 0.95, which is ranked as good and excellent, respectively. The AUCs of our ENMs were greater than the 0.79 for S. birrea reported by Smith et al. (2012) in South Africa. This study is also the first attempt to provide a general overview and advance our knowledge of how effectively Tanzania’s protected areas network contribute in conserving and safeguarding of ecological niches of S. birrea subspecies.
Climatic, edaphic, and topographical conditions define ecological niches of S. birrea in distinct ways. This is in line with what has been documented by Hall et al. (2002). Sclerocarya birrea is generally a mesophytic plant species (Hall et al. 2002) hence, well adapted to low-nutrient soil or to both, the lack of water and poor soils (Rivera et al. 2017). However, there are variations among subspecies. The subsp. birrea is a dry savanna species, semi-sclerophyllous with mesic and marginally xerophytic characteristics (Hall et al. 2002), implying that it can adaptably strive under both xeric and mesic environmental conditions. In addition, based on Thornthwaite climate index categories, subsp. birrea can survive in sub-humid, semi-arid, and dry situations, while subsp. caffra and multifoliata can mostly inhabit sub-humid and semi-arid habitats (Hall et al. 2002). Drought-resistant plants often have xeromorphic structures including, small or strongly lobulated leaves (Fang et al. 2015; Rivera et al. 2017; Simioni et al. 2018). The leaflets size varies among S. birrea subspecies (Hall et al. 2002). The subsp. multifoliata has the smallest leaflets, followed by subsp. birrea and subsp. caffra (Hall et al. 2002). This suggests that subspecies birrea and multifoliata are likely more drought-tolerant than subsp. caffra and will therefore thrive under high future temperatures. This is probably due to their morphological, anatomical and physiological variations. Moreover, S. birrea is reported to preferably occur in low elevation areas, mostly <1600 m (Hall et al. 2002). However, subsp. caffra have been reported at 1700–1800 m, subsp. birrea at around 1700 m and, subsp. multifoliata at 1500 m. The subsp. birrea and subsp. caffra are also present in low altitude coastal areas and, subsp. multifoliata above 800 m (Hall et al. 2002). Topography affects microclimates. Therefore, varied ability of S. birrea subspecies to inhabit different topographical gradients suggests that topography affect their ecological niches differently.
Regarding soil types where S. birrea occurs, Hall et al. (2002) reported that all S. birrea subspecies mainly occur in sandy soil, sandy loam and loam. However, subsp. caffra and subsp. birrea are reported to occur in clayey basaltic soils in southern Africa. The subsp. birrea have been reported to grow successfully in halomorphic soils but also in relatively fertile cambisols to erosion-prone regosols and leptosols and, acrisols, arenosols and ferralsols soils that is of very low nutrient status, especially in nitrogen and phosphorus. The subsp. caffra is regarded as an indicator of fertile soils and, was reported in Tanzania to associate with soils that are more alkaline (Hall et al. 2002).
Precipitation of the driest month and elevation were the main environmental variables which contributed the most to the ENM of subsp. caffra (Table 1a), similar to another study in Tanzania by John et al. (2020). Elsewhere, Chhetri et al. (2018) and Tesfamariam et al. (2022) reported that elevation was the most important predictive variable for tree species in Nepalese Himalayas Mountains and in Ethiopia, respectively. Elevation modifies atmospheric pressure, solar radiation, precipitation, and cloud cover, hence indirectly affect some physiology of plants (Oke et al. 2015) In contrast to our study, Jinga et al. (2021) reported temperature to be a climatic variable that mostly determine the distribution of subsp. caffra in Sub-Sahara Africa. The Ecological niche of this subspecies is widespread in Tanzania, mostly in coastal regions along the Indian Ocean, and around some inland water bodies (Fig. 2 and 3). The current study reveals existence of ecological niche of this subspecies in other areas across Tanzania beyond what has previously been reported by Hall et al. (2002), Woiso (2011), and GBIF (2019).
Furthermore, precipitation of the coldest quarter and annual precipitation were the main environmental factors which contributed the most to the ENM of subsp. multifoliata (Table 1b). While exploring the potential distribution of bamboo plants (Oxyte- nanthera abyssinica) in Ethiopia, Gebrewahid et al. (2020) similarly reported that the distribution of this plant species was mostly defined by precipitation of the coldest quarter. Our findings are also corroborated by Fer et al. (2017) who pointed out that, eastern Africa vegetation is influenced by seasonal rainfall. Our findings on the influence of annual precipitation conform to that of Jinga et al. (2021) while modelling the potential distribution of S. birrea and its three subspecies across Africa continent. The subsp. multifoliata ENM results (Fig. 4 and 5) revealed that subsp. multifoliata has a relatively extensive ecological niche that predominantly stretches from the southern highlands through the central zone to the northern part of the country. This implies that suitable areas for this subspecies was previously underreported.
Moreover, model results show that soil CEC and precipitation of the driest month ranked high among environmental parameters that contributed the most to the ENM of subsp. birrea (Table 1c). Our findings on the influence of cation exchange are corroborated by Hall et al. (2002) that reported cation exchange capacity and water holding capacity to be higher under the canopies of subsp. birrea than away from the canopies. Moreover, our findings concur with Velazco et al. (2017) that, soil plays a significant role in determining plant ecology. Similarly, John et al. (2020) and Tesfamariam et al. (2022) reported that precipitation of the driest month and the driest quarter significantly contributed in defining habitat suitability for forest types in Tanzania and in Ethiopia. Our study findings however differed with Jinga et al. (2021), who reported precipitation as the most important variable determining distribution of subsp. birrea, while in our study, precipitation was the second most important variable. The model results (Fig. 6 and 7) also consistently show that ecological niche of this subspecies is very narrow, mainly concentrated in the northeastern part of the country as previously reported by Hall et al. (2002), Woiso (2011), and GBIF (2019).
The ecological niche of subsp. caffra exist in 30 regions across Tanzania mostly in coastal areas along the Indian Ocean, and around some inland water bodies. The ENM for subsp. multifoliata predict this subspecies to occur in 21 regions, and its ecological niche predominantly extend from the southern highlands through central zone to the northern part of the country, and subsp. birrea occurs in 22 regions mainly in northeastern part of the country. Our findings on the location of ecological niche of subsp. caffra are consistent with Hall et al. (2002), Woiso (2011), and Jinga et al. (2022), for subspecies birrea and multifoliata.
HadGEM2ES model outperformed other models in the models’ comparison analysis conducted in Africa (Brands et al. 2013). However, McAvaney et al. (2001) emphasized the need to take results from multiple models into account because, in most circumstances, one model cannot be flawless. The position of ecological niches of S. birrea subspecies under future climates will remain relatively similar to the position under the current environmental conditions. However, the CCSM4 and HadGEM2ES models predict ecological niches of subsp. caffra, subsp. multifoliata, and subsp. birrea to generally retract under future climates in all emissions scenarios and time horizons, and the magnitude of change will vary among subspecies (Fig. 3, 5 and 7 and Tables 2a–c and 3a–c). Our findings generally agree with John et al. (2020) on Tanzania’s forest habitat suitability under future climates.
The S. birrea subspecies ENMs however predict all subspecies to strive under future climates. The S. birrea tree was classified as “arido-active” by Seghieri et al. (1995), which refers to plant species that continue to be metabolically active during the dry season (Hall et al. 2002). However, comparing all three subspecies, the study findings suggests that subsp. birrea is more vulnerable to warming climates, contrary to Jinga et al. (2021) that predicted distribution of subsp. birrea to expand under future climates. However, our study findings concur with other studies elsewhere in Africa, which reported that S. birrea is a mesophytic plant species and, drought is the major threat to its populations (Hall et al. 2002). Studies in western Africa reported that during the drought periods between 1979–1985, the subsp. birrea populations suffered more mortality than other woody species and experienced 11–15% loss of standing subsp. birrea trees (Hall et al. 2002). Furthermore, despite prediction variations in the magnitude of change, our findings regarding the ecological niches of subsp. multifoliata under future climates are consistent with those of Jinga et al. (2021), that ecological niche of this subspecies will decline by 98% under future warming climates.
Ecological niches of subspecies caffra and multifoliata are predicted to contract slightly under future climates compared to subsp. birrea, which suggest these subspecies probably have anatomical and physiological characteristics that make them more drought-tolerant compared to subsp. birrea. Based on moisture index, subsp. birrea is considered to be a dryland savanna species (Hall et al. 2002). However, based on the habitat score, subsp. birrea appears to inhabit mesic rather than exceptionally dry environments (Hall et al. 2002). In fact, this is true because subsp. birrea occurs in some areas, such as the Mara region in Tanzania, where rainfall is bimodal, as compared to the semi-arid areas such as Iringa and Singida regions where subsp. multifoliata occurs, where there are unimodal rains. Our study findings on contraction of ecological niches of S. birrea subspecies under future climates are in accordance with other studies (Djotan et al. 2018; Bogawski et al. 2019; Gebrewahid et al. 2020) and Wani et al. (2021). Despite having physiological traits that make them drought-tolerant according to Hall et al. (2002) and Jinga et al. (2021), the current ecological niches of the S. birrea subspecies will probably shift but are unlikely to expand under future warming climates.
The ENMs for S. birrea subspecies further revealed that, apart from climate change, all subspecies are currently threatened by anthropogenic activities because significant portions of subspecies’ ecological niches are outside Tanzania’s protected areas network (Fig. 2b, 4b and 6b). Only about 31% of the ecological niche of subsp. caffra, 33% of subsp. multifoliata, and 51% of subsp. birrea are currently harbored by Tanzania’s protected areas network (Table 2a–c). Comparable findings were reported by Urbina-Cardona et al. (2008), Meminvegni et al. (2018), Kakpo et al. (2021), and Srinivasulu et al. (2021).
5 Conclusion
Ecological niche of subsp. caffra is mainly defined by precipitation of the driest month and elevation; subsp. multifoliata by precipitation of the coldest quarter and annual precipitation, and subsp. birrea by soil CEC and precipitation of the driest month. Ecological niche of subsp. caffra is primarily restricted to coastal regions along the Indian Ocean, as well as around some inland water bodies, and occupies a significant part of Tanzania’s terrestrial area compared to subspecies multifoliata and birrea. Ecological niche of subsp. multifoliata mainly extends from the southern highlands through central zone to the northern part of the country, while that of subsp. birrea is confined in the northeastern part of the country. Under future climates, ecological niches of S. birrea subspecies will contract, albeit the severity of climate change impacts will vary among subspecies. Ecological niche of subsp. birrea will be impacted the most by future climates compared to subspecies caffra and multifoliata. Due to the limited range of subsp. birrea in Tanzania, conservation measures are needed to prevent it from becoming extinct in the near future. Significant parts of S. birrea subspecies ecological niches are currently beyond the current Tanzania’s protected areas network. Therefore, the use of S. birrea subspecies for agroforestry and landscape restoration programmes in small-scale farming systems and communal lands in drylands agroecosystems while taking into account climate variability will enhance their conservation beyond Tanzania’s protected areas network. However, to avoid amplifying water insecurity in dryland agroecological systems in and beyond Tanzania, prior knowledge on the rate of water consumption and rooting depths where subspecies extract water from the ground will be invaluable.
Authors’ contributions
A.H.M., conceptualization, methodology, software, data curation and analysis, writing the first draft, and project administration; P.H., N.A.A and A.D.W., methodology, validation, and interpretation of results; reviewing and editing of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research has not received any external funding.
Acknowledgments
Special thanks to Mr. Richard Msigala of Malinzanga village, Mr. Yohana of Kiegea village and Mr. Muli of Holili village for their support during field data collection, Mr. Yahya Abeid a botanist who helped in subspecies identification during field data collection, Mr. Kayeye of Sokoine University of Agriculture for assisting in transects establishment, Mr. John Reuben for his assistance on GIS-related work at early stages of this study. Mr. Roy E. Gereau, Assistant Curator and Tanzania Program Director, Africa and Madagascar Department, Missouri Botanical Garden, U.S.A is highly appreciated for sharing subspecies occurrence data from Missouri Botanical Garden. The editor and two anonymous reviewers are thanked for their constructive comments. The study was granted a research permit by Directorate of Research and Post-Graduate Studies of Sokoine University of Agriculture, Tanzania.
Data availability
The data generated during the current study are available from the corresponding author on reasonable request.
Conflicts of interest
The authors have no conflicts of interest.
References
Abrha H, Birhane E, Hagos H, Manaye A (2018) Predicting suitable habitats of endangered Juniperus procera tree under climate change in Northern Ethiopia. J Sust Forest 37: 842–853. https://doi.org/10.1080/10549811.2018.1494000.
Beaumont L, Hughes L, Poulsen M (2005) Predicting species distributions: use of climatic parameters in BIOCLIM and its impact on predictions of species’ current and future distributions. Ecol Modell 186: 251–270. https://doi.org/10.1016/j.ecolmodel.2005.01.030.
Bogawski P, Damen T, Nowak MM, Pędziwiatr K, Wilkin P, Mwachala G, Pierzchalska J, Wiland‐Szymańska J (2019) Current and future potential distributions of three Dracaena Vand. ex L. species under two contrasting climate change scenarios in Africa. Ecol Evol 9: 6833–6848. https://doi.org/10.1002/ece3.5251.
Brands S, Herrera S, Fernández J, Gutiérrez JM (2013) How well do CMIP5 earth system models simulate present climate conditions in Europe and Africa? Clim Dyn 41: 803–817. https://doi.org/10.1007/s00382-013-1742-8.
Brown J (2014) SDM toolbox: a python‐based GIS toolkit for landscape genetic, biogeographic and species distribution model analyses. Methods Ecol Evol 5: 694–700. https://doi.org/10.1111/2041-210X.12200.
Byeon DH, Jung S, Lee WH (2018) Review of CLIMEX and MaxEnt for studying species distribution in South Korea. J Asia Pac Biodivers 11: 325–333. https://doi.org/10.1016/j.japb.2018.06.002.
Chhetri P, Gaddis K, Cairns D (2018) Predicting the suitable habitat of treeline species in the Nepalese Himalayas under climate change. Mount Res Dev 38: 153–163. https://doi.org/10.1659/MRD-JOURNAL-D-17-00071.1.
Çoban H, Örücü Ö, Arslan E (2020) MaxEnt modeling for predicting the current and future potential geographical distribution of Quercus libani Olivier. Sustainability 12, article id 2671. https://doi.org/10.3390/su12072671.
Cook R, Henley M (2019) Complexities associated with elephant impact on Sclerocarya birrea subsp. caffra in the Greater Kruger National Park. S Afr J Bot 121: 543–548. https://doi.org/10.1016/j.sajb.2019.01.016.
Cook R, Witkowski E, Helm C, Henley M, Parrini F (2017) Recent exposure to African elephants after a century of exclusion: rapid accumulation of marula tree impact and mortality, and poor regeneration. For Ecol Manag 401: 107–116. https://doi.org/10.1016/j.foreco.2017.07.006.
Djotan A, Aoudji A, Tessi D, Kakpo S, Gbetoho A, Kourouma K, Ganglo J (2018) Vulnerability of Khaya senegalensis Desr & Juss to climate change and to the invasion of Hypsipyla robusta Moore in Benin (West Africa). Inter J Biol Chem Sci 12: 24–42. https://doi.org/10.4314/ijbcs.v12i1.3.
Dormann C, Elith J, Bacher S, Buchmann C, Carl G, Carré G, Marquéz J, Gruber B, Lafourcade B, Leitao P (2013) Collinearity: a review of methods to deal with it and a simulation study evaluating their performance. Ecography 36: 27–46. https://doi.org/10.1111/j.1600-0587.2012.07348.x.
Fang Y, Xiong L (2015) General mechanisms of drought response and their application in drought resistance improvement in plants. Cell Mol Life Sci 72: 673–689. https://doi.org/10.1007/s00018-014-1767-0.
Farnsworth E, Ogurcak D (2006) Biogeography and decline of rare plants in New England: historical evidence and contemporary monitoring. Ecol Appl 16: 1327–1337. https://doi.org/10.1890/1051-0761(2006)016[1327:BADORP]2.0.CO;2.
Fer I, Tietjen B, Jeltsch F, Wolff C (2017) The influence of El Niño–Southern Oscillation regimes on eastern African vegetation and its future implications under the RCP8. 5 warming scenario. Biogeosciences 14: 4355–4374. https://doi.org/10.5194/bg-14-4355-2017.
Gadd M (2002) The impact of elephants on the marula tree Sclerocarya birrea. Afr J Ecol 40: 328–336. https://doi.org/10.1046/j.1365-2028.2002.00385.x.
Ganglo J, Djotan G, Gbètoho JA, Kakpo S, Aoudji A, Koura K, Tessi R (2017) Ecological niche modeling and strategies for the conservation of Dialium guineense Willd. (Black velvet) in West Africa. Inter J Biodivers Conserv 9: 373–388. https://doi.org/10.5897/IJBC2017.1151.
Gbètoho A, Aoudji A, Roxburgh L, Ganglo J (2017) Assessing the suitability of pioneer species for secondary forest restoration in Benin in the context of global climate change. Bois et Forets des Trop 332: 43–55. https://doi.org/10.19182/bft2017.332.a31332.
GBIF (2019) The global biodiversity information facility. https://www.gbif.org. Accessed 1 January 2019.
Gebrewahid Y, Abrehe S, Meresa E, Eyasu G, Abay K, Gebreab G, Kidanemariam K, Adissu G, Abreha G, Darcha G (2020) Current and future predicting potential areas of Oxytenanthera abyssinica (A. Richard) using MaxEnt model under climate change in Northern Ethiopia. Ecol Process 9, article id 6. https://doi.org/10.1186/s13717-019-0210-8.
Hall J, O’brien E, Sinclair F (2002) Sclerocarya birrea: a monograph. School of Agriculture and Forest Science Publication 19, University of Wales, Bangor, UK.
Heidari N (2019) Ecological niche differentiation between Acanthodactylus micropholis and A. khamirensis (Sauria: Lacertidae) in southern Iran. Zoologia 36, article id e27357. https://doi.org/10.3897/zoologia.36.e27357.
Hendre PS, Muthemba S, Kariba R, Muchugi A, Fu Y, Chang Y, Song B, Liu H, Liu M, Liao X (2019) African Orphan Crops Consortium (AOCC): status of developing genomic resources for African orphan crops. Planta 250: 989–1003. https://doi.org/10.1007/s00425-019-03156-9.
Hernández-Baz F, Romo H, González J, Hernández M, Pastrana R (2016) Maximum entropy niche-based modeling (Maxent) of potential geographical distribution of Coreura albicosta (Lepidoptera: Erebidae: Ctenuchina) in Mexico. Fla Entomol 99: 376–380. https://doi.org/10.1653/024.099.0306.
Fick SE, Hijmans RJ (2017) WorldClim 2: new 1km spatial resolution climate surfaces for global land areas. Int J Climatol 37: 4302–4315. https://doi.org/10.1002/joc.5086.
Jama B, Mohamed A, Mulatya J, Njui A (2008) Comparing the “Big Five”: a framework for the sustainable management of indigenous fruit trees in the drylands of East and Central Africa. Ecol Indic 8: 170–179. https://doi.org/10.1016/j.ecolind.2006.11.009.
Jarvis A, Reuter HI, Nelson A, Guevara E (2008) Hole-filled SRTM for the globe version 4, available from the CGIAR-CSI SRTM 90m Database. http://srtm.csi.cgiar.org/. Accessed 20 April 2019.
Jinga P, Liao Z, Nobis M (2021) Species distribution modeling that overlooks intraspecific variation is inadequate for proper conservation of marula (Sclerocarya birrea, Anacardiaceae). Glob Ecol Conserv 32, article id e01908. https://doi.org/10.1016/j.gecco.2021.e01908.
Jinga P, Zingoni E, Bobo ED, Munosiyei P (2022) Marula (Sclerocarya birrea subsp. caffra, Anacardiaceae) thrives under climate change in sub‐Saharan Africa. Afr J Ecol 60: 736–749. https://doi.org/10.1111/aje.12943.
John E, Bunting P, Hardy A, Roberts O, Giliba R, Silayo DS (2020) Modelling the impact of climate change on Tanzanian forests. Divers Distrib 26: 1663–1686. https://doi.org/10.1111/ddi.13152.
Kadu C, Imbuga, M, Jamnadass R, Dawson I (2006) Genetic management of indigenous fruit trees in southern Africa: a case study of Sclerocarya birrea based on nuclear and chloroplast variation. S Afr J Bot 72: 421–427. https://doi.org/10.1016/j.sajb.2005.12.007.
Kakpo S, Aoudji A, Gnanguènon-Guéssè D, Gbètoho A, Koura K, Djotan, G, Ganglo J (2021) Spatial distribution and impacts of climate change on Milicia excelsa in Benin, West Africa. J For Res 32: 143–150. https://doi.org/10.1007/s11676-019-01069-7.
Kogo B, Kumar L, Koech R, Kariyawasam C (2019) Modelling climate suitability for rainfed Maize cultivation in Kenya using a Maximum Entropy (MaxENT) approach. Agronomy 9, article id 727. https://doi.org/10.3390/agronomy9110727.
Kulhanek S, Leung B, Ricciardi A (2011) Using ecological niche models to predict the abundance and impact of invasive species: application to the common carp. Ecol Appl 21: 203–213. https://doi.org/10.1890/09-1639.1.
Leakey RRB (2015) The role of trees in agroecology and sustainable agriculture in the tropics. Annu Rev Phytopathol 52: 113–33. https://doi.org/10.1146/annurev-phyto-102313-045838.
Leakey RRB, Tientcheu Avana M-L, Princely Awazi N, Assogbadjo AE, Mabhaudhi T, Hendre PS, Degrande A, Hlahla S, Manda L (2022) The future of food: domestication and commercialization of indigenous food crops in Africa over the third decade (2012–2021). Sustainability 14, article id 2355. https://doi.org/10.3390/su14042355.
Leenaars J, Van Oostrum A, Ruiperez G (2014) Africa soil profiles database, Version 1.2. A compilation of georeferenced and standardised legacy soil profile data for Sub-Saharan Africa (with dataset). ISRIC Report 2014/01. Africa Soil Information Service (AfSIS) project and ISRIC – World Soil Information, Wageningen, the Netherlands. https://edepot.wur.nl/481288. Accessed 14 March 2019.
Machani FG, Alice M, Piero N, Ramni J, George O (2017) Molecular characterization of Sclerocarya birrea ICRAF field genebank collections. J Phylogenetics Evol Biol 5, article id 190. https://doi.org/10.4172/2329-9002.1000190.
Mariod A, Abdelwahab S (2012) Sclerocarya birrea (Marula), an African tree of nutritional and medicinal uses: a review. Food Rev Int 28: 375–388. https://doi.org/10.1080/87559129.2012.660716.
Martinez-Meyer PA, Servín J, Kiff L (2006) Ecological niche modelling and prioritizing areas for species reintroductions. Oryx 40: 411–418. https://doi.org/10.1017/S0030605306001360.
McAvaney BJ, Covey C, Joussaume S, Kattsov V, Kitoh A, Ogana W, Pitman A, Weaver A, Wood R, Zhao ZC (2001) Model evaluation. In: Climate change 2001: the scientific basis. Contribution of Working Group I to the Third Assessment Report of the Intergovernmental Panel on Climate Change. Houghton JT, Ding Y, Griggs DJ, Noguer M, van der Linden PJ, Dai X, Maskell K, Johnson CA (eds) Cambridge University Press, pp. 471–523. http://hdl.handle.net/11295/49071.
Meminvegni Landry GG, Azihou F, Okhimamhe AA, Sinsin B, Usman BS, Adet L (2018) Examining the effectiveness of a protected areas network in the conservation of Kigelia africana under climate change by 2050 in Benin. Open Access Libr 5, article id e4326. https://doi.org/10.4236/oalib.1104326.
Mng’omba S, Sileshi G, Jamnadass R, Akinnifesi F, Mhango J (2012) Scion and stock diameter size effect on growth and fruit production of Sclerocarya birrea (Marula) trees. J Hortic For 4: 153–160.
Mothes C, Stroud J, Clements S, Searcy C (2019) Evaluating ecological niche model accuracy in predicting biotic invasions using South Florida’s exotic lizard community. J Biogeogr 46: 432–441. https://doi.org/10.1111/jbi.13511.
Munna A (2015) Value chain analysis of Sclerocarya birrea products in Tanzania: case studies of Uyui and Kilosa districts. Dissertation for Award of MSc Degree, Sokoine University of Agriculture, Morogoro.
Navarro-Racines C, Tarapues J, Thornton P, Jarvis A, Ramirez-Villegas J (2020) High-resolution and bias-corrected CMIP5 projections for climate change impact assessments. Sci Data 7, article id 7. https://doi.org/10.1038/s41597-019-0343-8.
Noce S, Collalti A, Santini M (2017) Likelihood of changes in forest species suitability, distribution, and diversity under future climate: the case of Southern Europe. Ecol Evol 7: 9358–9375. https://doi.org/10.1002/ece3.3427.
Oke O, Thompson K (2015) Distribution models for mountain plant species: the value of elevation. Ecol Modell 301: 72–77. https://doi.org/10.1016/j.ecolmodel.2015.01.019.
Pearson R, Raxworthy C, Nakamura M, Townsend P (2007) Predicting species distributions from small numbers of occurrence records: a test case using cryptic geckos in Madagascar. J Biogeogr 34: 102–117. https://doi.org/10.1111/j.1365-2699.2006.01594.x.
Phillips S, Anderson R, Schapire R (2006) Maximum entropy modeling of species geographic distributions. Ecol Modell 190: 231–259. https://doi.org/10.1016/j.ecolmodel.2005.03.026.
Prevéy JS, Parker LE, Harrington C (2020) Projected impacts of climate change on the range and phenology of three culturally-important shrub species. PLoS One 15, article id e0232537. https://doi.org/10.1371/journal.pone.0232537.
Rivera P, Villaseñor JL, Terrazas T (2017) Meso-or xeromorphic? Foliar characters of Asteraceae in a xeric scrub of Mexico. Bot Stud 58, article id 12. https://doi.org/10.1186/s40529-017-0166-x.
Rodriguez L, García JJ, Tuya F, Martínez B (2020) Environmental factors driving the distribution of the tropical coral Pavona varians: predictions under a climate change scenario. Mar Ecol 41: 1–12. https://doi.org/10.1111/maec.12590.
Rovzar C, Gillespie T, Kawelo K (2016) Landscape to site variations in species distribution models for endangered plants. For Ecol Manag 369: 20–28. https://doi.org/10.1016/j.foreco.2016.03.030.
Seghieri J, Floret C, Pontanier R (1995) Plant phenology in relation to water availability: herbaceous and woody species in the savannas of northern Cameroon. J Trop Ecol 11: 237–254. https://doi.org/10.1017/S0266467400008713.
Segurado P, Araujo M (2004) An evaluation of methods for modelling species distributions. J Biogeogr 31: 1555–1568. https://doi.org/10.1111/j.1365-2699.2004.01076.x.
Serengeti District Council (2018) The Serengeti district land use framework plan.
Simioni PF, Pessoa MG, Cardoso MA, Cabral FF, Teixeira SO, Silva IV (2018) Leaf anatomy of Xylopia aromatica (Lam.) Mart.(Annonaceae) occurring in a rocky savannah in the Brazilian Amazonian. Acta Sci Biol Sci 40, article id e37334. https://doi.org/10.4025/actascibiolsci.v40i1.37334.
Smith A, Page B, Duffy K, Slotow R (2012) Using Maximum Entropy modeling to predict the potential distributions of large trees for conservation planning. Ecosphere 3: 1–21. https://doi.org/10.1890/ES12-00053.1.
Srinivasulu A, Srinivasulu B, Srinivasulu C (2021) Ecological niche modelling for the conservation of endemic threatened squamates (lizards and snakes) in the Western Ghats. Glob Ecol Conserv 28, article id e01700. https://doi.org/10.1016/j.gecco.2021.e01700.
Tarkesh M, Jetschke G (2012) Comparison of six correlative models in predictive vegetation mapping on a local scale. Environ Ecol Stat 19: 437–457. https://doi.org/10.1007/s10651-012-0194-3.
Tesfamariam B, Gessesse B, Melgani F (2022) MaxEnt-based modeling of suitable habitat for rehabilitation of Podocarpus forest at landscape-scale. Environ Syst Res 11, article id 4. https://doi.org/10.1186/s40068-022-00248-6.
Thomson A, Calvin K, Smith S, Kyle GP, Volke A, Patel P, Delgado-Arias S, Bond-Lamberty B, Wise M, Clarke L (2011) RCP4. 5: a pathway for stabilization of radiative forcing by 2100. Clim Change 109: 77–94. https://doi.org/10.1007/s10584-011-0151-4.
Thuiller W, Albert C, Araujo M, Berry M, Cabeza M, Guisan A, Hickler T, Midgley G, Paterson J, Schurr F (2008) Predicting global change impacts on plant species’ distributions: future challenges. Perspect Plant Ecol. Evol Syst 9: 137–152. https://doi.org/10.1016/j.ppees.2007.09.004.
UNEP-WCMC, IUCN (2021) Protected planet: the world database on protected areas (WDPA) and world database on other effective area-based conservation measures (WD-OECM). UNEP-WCMC and IUCN, Cambridge, UK. https://www.protectedplanet.net/. Accessed 14 September 2020.
Urbina-Cardona JN, Loyola R (2008) Applying niche-based models to predict endangered-hylid potential distributions: are neotropical protected areas effective enough? Trop Conserv Sci 1: 417–445. https://doi.org/10.1177/194008290800100408.
Velazco S, Galvao F, Villalobos F, De Marco JP (2017) Using worldwide edaphic data to model plant species niches: an assessment at a continental extent. PLoS One 12, article id e0186025. https://doi.org/10.1371/journal.pone.0186025.
Wani I, Verma S, Kumari P, Charles B, Hashim M, El-Serehy H (2021) Ecological assessment and environmental niche modelling of Himalayan rhubarb (Rheum webbianum Royle) in northwest Himalaya. PLoS One 16, article id e0259345. https://doi.org/10.1371/journal.pone.0259345.
Warren D, Glor R, Turelli M (2010) ENMTools: a toolbox for comparative studies of environmental niche models. Ecography 33: 607–611. https://doi.org/10.1111/j.1600-0587.2009.06142.x.
Woiso AD (2011) A comparative study of the subspecies of Sclerocarya birrea: their potential for domestication in Tanzania. Thesis for Award of PhD Degree at Bangor University, University of Wales, Bangor.
Zarei A, Chemura A, Gleixner S, Hoff H (2021) Evaluating the grassland NPP dynamics in response to climate change in Tanzania. Ecol Indic 125, article id 107600. https://doi.org/10.1016/j.ecolind.2021.107600.
Total of 71 references.

