Effects of logging on the threatened epiphytic lichen Usnea longissima: an experimental approach
Storaunet K. O., Rolstad J., Rolstad E. (2014). Effects of logging on the threatened epiphytic lichen Usnea longissima: an experimental approach. Silva Fennica vol. 48 no. 1 article id 949. https://doi.org/10.14214/sf.949
Highlights
- A re-inventory of the threatened lichen Usnea longissima in ten Norway spruce forest stands where experimental selective loggings had been conducted 5 to 8 years before revealed that the number of lichen thalli had increased with 34%
- The number of thalli increased more where the forest was open whether or not the low tree density was caused by the loggings.
Abstract
Usnea longissima Ach. is a circumboreal epiphytic lichen draping tree canopies in moist coastal and mountainous forests. It is extinct from many European and North-American localities, presumably due to industrial forestry and air pollution, but still has a stronghold in parts of Scandinavia and U.S. and Canadian Pacific Northwest. In 2005/06 we used a comparative and retrospective approach to evaluate how present and historic tree and stand characteristics influenced the occurrence and abundance of the lichen (Storaunet et al. 2008). In 2012, we re-inventoried ten Norway spruce forest stands with 401 U. longissima-bearing trees and recorded changes in the number of U. longissima thalli. Seven of the stands had been experimentally, selectively logged 5–8 years before, where the lichen-bearing trees had been marked in the field and were avoided during the logging operation. Total number of lichen-bearing trees decreased slightly (2.9%), whereas the number of thalli had increased with 34%. Number of thalli increased more where the forest was open (low basal area, m2ha-1) whether or not the low tree density was caused by the logging events. At high tree densities the change in number of thalli was negligible. We suggest that selective logging, securing lichen-bearing trees, may be a viable management option to keep tree density from becoming too dense, thereby enhancing growth and establishment of U. longissima.
Keywords
boreal forest;
Picea abies;
selective logging;
Usnea longissima;
threatened lichen
-
Storaunet,
Norwegian Forest and Landscape Institute, P.O. Box 115, NO-1431 Ås, Norway
E-mail
stk@skogoglandskap.no

- Rolstad, Norwegian Forest and Landscape Institute, P.O. Box 115, NO-1431 Ås, Norway E-mail roj@skogoglandskap.no
- Rolstad, Skogfaglig Rådgivning, Holmsida 126, NO-1488 Hakadal, Norway E-mail roe@skogoglandskap.no
Received 21 June 2013 Accepted 23 January 2014 Published 11 February 2014
Views 124716
Available at https://doi.org/10.14214/sf.949 | Download PDF

1 Introduction
Industrial forestry profoundly alters natural stand structure and landscape mosaics, influencing a variety of organisms inhabiting forest ecosystems. In particular, clearcutting practice poses a threat to a range of species inhabiting late seral stages (e.g. Esseen et al. 1997; Siitonen 2001; Gjerde et al. 2010). During later years, stronger regulations regarding how forests are harvested and managed have emerged. In turn, this has led forest managers to request scientific advices to guide forest planning and operational procedures (e.g. Kohm and Franklin 1997; Rametsteiner and Simula 2003).
Due to their arboreal life, epiphytic lichens are vulnerable to changes in tree density and canopy closure (Dettki and Esseen 1998; Sillett and Goslin 1999). In particular, Usnea longissima Ach. has received attention because it seems to lose ground in many regions of former high abundance, probably due to modern forestry and air pollution (Esseen et al. 1981; Olsen and Gauslaa 1991; Rolstad and Rolstad 1996; Tønsberg et al. 1996; Josefsson et al. 2005). It is redlisted in Norway, Sweden, and Finland (Gärdenfors 2010; Kålås et al. 2010; Rassi et al. 2010), but despite being one of the most studied lichen species in Fennoscandia, relatively little is known about its tolerance to logging operations (Esseen et al. 1981; Rolstad and Rolstad 1999; Keon and Muir 2002; Josefsson et al. 2005; Gauslaa et al. 2007).
In 2005 we were invited by forest managers in Lillehammer, Norway, to survey and monitor populations of U. longissima as part of a long-term logging experiment. In 2005/06 we conducted a comparative study of U. longissima abundance related to the historic and present-day forest structure (Storaunet et al. 2008). In 2012 (five to eight years after logging) we revisited a selection of the same forest stands to see how U. longissima had responded to the logging experiments. Here, we report the outcome of this experiment.
2 Material and methods
2.1 Study area
The study area is located in the valley Saksumdalen in the municipality of Lillehammer, in south-central Norway (61°05´N, 10°17´E). Elevation ranges from 350 to 700 m a.s.l. and the area has mean annual precipitation of 800 mm and mean annual temperature of 2 °C. July is the warmest month of the year and reaches a mean temperature of 14 °C, whereas January is the coldest with an average of –10 °C (Moen 1999). The bedrock is primarily sandstone with schist and feldspar, and the soils are thick unsorted glacial deposits in the hillsides but sparse in top areas. The region is in the middle boreal vegetation zone (Moen 1999), and Norway spruce (Picea abies (L.) Karst.) is the almost totally dominating tree species. Other tree species, like birch (Betula pubescens Ehrh.), rowan (Sorbus aucuparia L.), and willow (Salix caprea L.) occur very sparsely in the area, and they probably do not influence the U. longissima population. The predominating vegetation type in the study area are characterized by bilberry (Vaccinium myrtillus L.), however, also types characterized by small-ferns (e.g., Gymnocarpium dryopteris (L.) Newman, Phegopteris connectilis (Michx.) Watt) are common. The forest history of the area is outlined in Storaunet et al. (2008).
Environmental surveys were conducted in 2002–2003 in Lillehammer municipality to upgrade forest planning databases (Mjøsen Skogeierforening 2005; Gjerde et al. 2007). A special search was launched to identify locations of U. longissima, based on previous knowledge of this species in the area (Gaarder 1997). A total of 82 sites with presence of U. longissima were found, of which 76 were protected as woodland key habitats (Mjøsen Skogeierforening 2005). The present study was conducted within a landscape section of ca. 30 km2, on both sides of the Saksumdalen valley. Old Norway spruce forest covered 40% of the forested part of the landscape, with individual stands varying in size from < 1 ha to ca. 25 ha.
2.2 Stand selection and logging operations
In 2005/06 twenty-one forest stands harboring U. longissima and three comparable stands without the lichen were selected for comparisons of historical logging activity and present and historic stand structure (see Storaunet et al. 2008). Detailed historical stand reconstructions were performed within small study plots (0.1 ha) centered around trees with the highest abundance of the lichen, whereas all trees with the lichen present in the forest stands were recorded. In seven of the forest stands experimental selective logging operations were carried out during 2005–2007. The logging operators reported that between 41 and 53% of the growing stock were cut, resulting in a remaining standing volume of 100 to 170 m3ha–1 (Vestad 2008) (Table 1). Before logging, trees with U. longissima had been marked in the field, and the logging operators were carefully instructed to avoid cutting these trees. Most of the loggings were carried out as single-tree selections, however, in some sites with no lichen-bearing trees, small groups of trees or strip cuttings were performed. This implied that individual lichen-bearing trees were influenced by the loggings to a varying degree.
| Table 1. Number of trees in different categories (Usnea longissima present, newly established, disappeared, or if the tree had fallen to the ground), in 2012 compared to 2005/06, within study sites and whether the trees were influenced by the experimental selective loggings. The five last columns show forest stand level information. Sites with no logging are shaded. View in new window/tab. |
In addition, three undisturbed stands were selected among those from the 2005/06-study that were located closest to the logged stands. The 10 stands did not differ from the other stands in the 2005/06 survey, neither in basal area nor in number of U. longissima thalli per tree (t < 0.7, p > 0.50 in both cases). Number of trees with U. longissima present was however markedly higher in the stands that were re-inventoried (401 trees in 10 selected stands vs. 154 trees in 11 remaining stands). This was mainly because the stands selected for the experimental loggings were among those that had the highest number of lichen-bearing trees.
The 10 stands were located in gentle slopes or flat terrain, mainly with a northeastern exposure. Tree diameters showed a large variation both between and within stands (Table 1), and the diameter-height relationship established from the study area shows that trees reach heights of < 30 meters. More details on site characteristics (e.g. diameter and age distribution) before logging and the historical development of standing volume can be found in Storaunet et al. (2008).
2.3 Field surveys
To survey U. longissima in 2005/06, all trees within the stands were carefully checked from the ground using binoculars. As an index of lichen abundance, all visible pendant threads of U. longissima thalli were counted, irrespective of the length of the threads. All trees with the lichen were numbered and marked in the field, and the basal area (m2ha–1) around these trees was measured using a relascope (Storaunet et al. 2008).
In 2012 (five to eight years after logging), we revisited the ten stands and conducted a new search and recording of U. longissima thalli along with new measurements of basal area around the lichen-bearing trees, following the same methodology as in 2005/06. The vicinity of the previously recorded lichen trees was carefully searched for possible new establishments of U. longissima. Only a part of stand E8 was surveyed, since a proportion of this stand had been exposed to wind throws and quite a few trees had died of drought and lost its bark, implying that the marking from 2005/06 had disappeared.
2.4 Analyses and statistics
A total of 401 trees were re-inventoried (Table 1), of which ten trees from 2005/06 were not relocated, 183 trees were not influenced by the selective loggings (i.e., no reduction in basal area), whereas 208 trees had been exposed to a varying degree of logging influence. Ten trees had died and fallen to the ground since 2005/06. These were included in the analyses at forest stand level, but removed from the single-tree analyses. Stands A1, E9, and K3 were intact control stands, and in stands E4, E8, K1, and K2 all trees were influenced by the loggings (Table 1). Logging impact was measured as the difference in basal area around a tree between 2005/06 and 2012, calculated as the percentage of the basal area removed. To achieve normality and homoscedasticity, the change in number of U. longissima thalli on single trees was measured as
diffLog = Log(thalliafter + 1) – Log(thallibefore + 1)
where thalliafter was number of thalli in 2012 and thallibefore was number of thalli in 2005/06. The ‘+1’ in the formula was added to include trees with new establishment of the lichen (no thalli in 2005/06) and trees where U. longissima had disappeared in 2012 (no thalli in 2012). A diffLog-value of 0implies no change in number of thalli, 0.25 implies approximately 100% increase, 0.5 ≈ 300% increase, −0.25 ≈ 50% decrease, −0.5 ≈ 75% decrease, etc.
In 2005/06, 20–25 trees in each stand were age determined (increment cored and tree-ring measured) and several growth and tree-ring derived explanatory variables were constructed (see Storaunet et al. 2008 for details). In 2012, 75 of these trees were among the re-inventoried ones, and we used these variables to explore possible relationships with the change in number of U. longissima thalli on the trees.
We used a t-test to check for differences between trees influenced by logging and those that were not, and Spearman rank correlations (rs) to check for relationships between diffLog (change in number of thalli on individual trees from 2005/06 to 2012) and the growth and tree-ring derived variables. In an ANCOVA model, we treated diffLog as the dependent variable and present tree density (basal area, m2ha–1) and logging influence (categorical) as independents. Statistical analyses were calculated using the StatView 5.0 software package (SAS Institute Incorporated, Cary, North Carolina).
3 Results
3.1 Effects at the level of forest stands
At the level of forest stands, total number of trees inhabited by U. longissima ranged 7–89 in 2005/06 and 5–74 in 2012, whereas total number of thalli ranged 45–751 in 2005/06 and 49–1226 in 2012 (Fig. 1). Although the total number of U. longissima trees in the ten locations decreased slightly (2.9%, from 340 to 330), total number of thalli increased markedly with 34% (from 2 582 to 3 449). At 51 trees U. longissima was not relocated and possibly had disappeared, whereas at 51 others we found new establishments (Table 1).
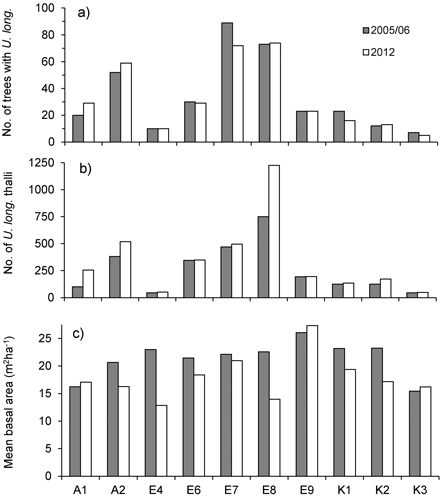
Fig. 1. a) Total number of trees with Usnea longissima, b) total number of U. longissima thalli, and c) mean basal area (m2ha–1) around the trees with U. longissima, within study sites in 2005/06 (gray bars) and in 2012 (white bars).
Basal area, averaged over all lichen-bearing trees within each study site, was 22 m2ha–1 (range 16–26 m2ha–1) before logging. After the selective loggings (in 2012) the average basal area was 18.3 m2ha–1 (range 13 to 27) (Fig. 1c). In the logged stands, mean basal area removed around trees varied from 4.3% (site E7) to 44% (E4) (Fig. 2a). To see if there was a relationship between harvest level and relative change in the number of lichen thalli at the level of forest stands, we ranked the stands according to logging impact (Fig. 2). With all sites included, a rank test did not reveal any statistical relationship (rs = 0.36, p = 0.30, n = 10). However, site A1, which was not logged, showed a marked increase in number of thalli due to a group of trees where U. longissima recently had established. Excluding this site from the test disclosed a positive correlation (rs = 0.74, p = 0.02, n = 9).
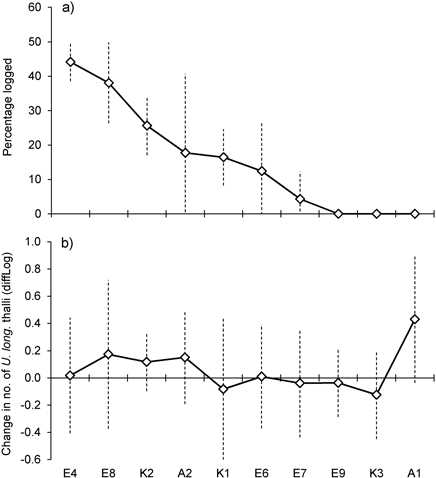
Fig. 2. a) Percent of basal area logged within study sites, sorted from the highest to the lowest amount of logging. b) Mean change in number of Usnea longissima thalli within study sites. Note that the y-axes show diffLog values (0 = no change, 0.25 ≈ 2x, 0.5 ≈ 4x, –0.25 ≈ ½x, –0.5 ≈ ¼x, etc., see Methods for further details). Vertical bars show ± 1 SD.
3.2 Effects at the level of single trees
Within stands, the logging impact and the subsequent change in number of U. longissima thalli varied strongly (Fig. 2). However, overall mean change (diffLog) on trees influenced by logging was 0.17 (corresponding to a relative increase of ~50%), whereas mean change on trees not influenced by the logging was 0.04 (increase ca. 10%, not statistically different from 0). The difference between logging-influenced and undisturbed trees was statistically highly significant (t = 2.97, p = 0.003, n = 381).
For the trees influenced by logging, the change in number of U. longissima thalli was positively correlated to the percentage of basal area removed (R2 = 0.06, F = 11.6, p = 0.001, n = 202) (Fig. 3), implying a higher increase in number of thalli as more of the neighboring trees had been cut.
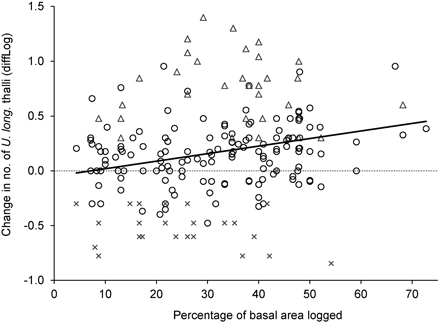
Fig. 3. The relationship between logging influence around trees (measured as the percentage of basal area removed), and the change in number of Usnea longissima thalli (diffLog). Circles show trees where the lichen was present both in 2005/06 and 2012, triangles show trees with new establishment, and crosses show trees where the lichen had disappeared.
Mean basal area around all trees inhabited by U. longissima before logging (in 2005/06) was 21.7 m2ha–1. The selective loggings were geared towards the denser parts of the forest stands. Thus, basal area before logging was higher around those trees that were influenced by the experimental loggings compared to those that were not (23.7 and 19.6 m2ha–1, respectively). After logging (in 2012), this had reversed to 16.3 m2ha–1 around the trees influenced by the loggings whereas the basal area had increased with ~5% around the control trees (20.6 m2ha–1).
Was the change in number of U. longissima thalli due to the relative amount of logging or to the general openness of the forest stands? This was checked with an ANCOVA, which showed that the change in number of thalli per tree was similar for the two groups, control and logged (Fig. 4, Fig. 5, Table 2), implying that U. longissima responded positively to the openness of the stands (low basal area) irrespective of the logging impact.
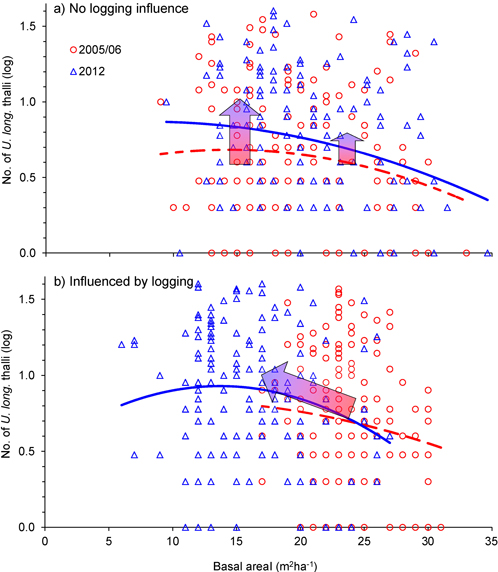
Fig. 4. Relationship between tree density (basal area, m2ha–1) and number of Usnea longissima thalli in 2005/06 (red) and in 2012 (blue), for single trees that were a) not influenced by logging, and b) influenced by the selective logging events. The blue 2nd-order polynomial regression lines were statistically significant (p = 0.01 in upper and p = 0.05 in lower panel), whereas the dotted, red lines were not. The wide red/blue arrows illustrate how mean values changed from 2005/06 to 2012. Note that the y-axes are logarithmically scaled.
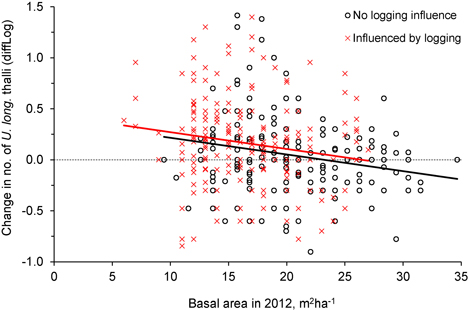
Fig. 5. The relationship between tree density (basal area, m2ha–1) after logging (in 2012) and the change in number of Usnea longissima thalli (diffLog) from 2005/06 to 2012. Black circles (and black fitted line) show trees not influenced by logging, whereas red crosses (and red line) show trees that were influenced by the experimental logging events.
| Table 2. Parameter estimates of the linear model (ANCOVA) between change in number of Usnea longissima thalli on individual trees (diffLog, dependent variable), and logging influence (categorical) and tree density (basal area) in 2012 (independents) (model R2 = 0.06, F = 11.5, p < 0.0001, n = 381). The interaction term between the two independent variables was not significant (t = 0.078, p = 0.94). | ||||
| Variable | Coefficient | SE | t-value | p-value |
| Intercept | 0.407 | 0.083 | 4.88 | <0.0001 |
| Logging influence (categorical) | 0.034 | 0.022 | 1.57 | 0.118 |
| Basal area in 2012 (m2ha–1) | −0.017 | 0.005 | −3.73 | 0.0002 |
Finally, we checked whether individual tree age or growth pattern up to 2005/06 influenced the change in number of U. longissima thalli. None of these variables had any explanatory power (all rs ≤ | 0.15 |, all p ≥ 0.20, n = 75) (Table 3).
| Table 3. Spearman rank correlations between change (diffLog) in number of Usnea longissima thalli (from 2005/06 to 2012) and size, age, and different growth variables of individual trees (n = 75). Note that the growth variables were measured in 2005/06. | ||
| Variable | Spearman rank | |
| rs | p-value | |
| DBH, cm | −0.13 | 0.29 |
| Total age, yrs | 0.001 | 0.99 |
| Mean tree-ring width last 5 yrs, mm | −0.06 | 0.63 |
| Mean tree-ring width last 10 yrs, mm | −0.04 | 0.75 |
| Mean tree-ring width last 20 yrs, mm | −0.01 | 0.96 |
| GI 5 yr / 5 yr * | −0.14 | 0.24 |
| GI 10 yr / 10 yr | −0.11 | 0.34 |
| GI 10 yr / 20 yr | −0.01 | 0.90 |
| GI 10 yr / 10 yr, max-value after 1950 | 0.06 | 0.59 |
| GI 10 yr / 10 yr, min-value after 1950 | −0.15 | 0.20 |
| GI 10 yr / 10 yr, max-value after 1975 | 0.04 | 0.75 |
| GI 10 yr / 10 yr, min-value after 1975 | −0.08 | 0.48 |
| * GI 5 yr / 5 yr = (mean tree ring width 2001–2005) / (mean tree ring width 1996–2000). (See Storaunet et al. 2008 for further details.) | ||
4 Discussion
We did a re-inventory of U. longissima thalli on Norway spruce trees in ten forest stands where experimental selective loggings had been conducted 5 to 8 years before. The total number of trees with lichen thalli present did not change much, principally because the lichen-bearing trees were avoided during the logging operation. However, total number of U. longissima thalli increased with 34%. Number of thalli increased more where the forest was open, whether or not the low tree density was caused by the logging events. At high tree densities, change in number of U. longissima thalli was negligible, but here the initial abundance of thalli also was rather low.
U. longissima is known to have high growth rates in open habitats, enabling the photosynthesizing green algae to be active for longer periods, provided that the microclimate is sufficiently humid (Gauslaa and Solhaug 1996; Keon and Muir 2002; Gauslaa et al. 2007). On the other hand, loss rates, in terms of wind, snow and ice break-off and mortality due to light-damage to the photobiont, also seem to be highest in open, exposed habitats (Keon and Muir 2002). Over time, fragmentation of thalli may lead to a downward shift of the lichen population in the canopy, presumably at higher rates in open forests due to higher wind speed and more snow in the canopy during winter season. However, Norway spruce trees in open habitats commonly have more low branches that could catch such fragments. We did not measure the height distribution of thalli within trees, neither the heights of the lowermost branches. The general impression was that the green canopy typically extended three fourths down the stems with dead branches below, although many of the trees inhabited by U. longissima even had lower green branches. Most commonly, the U. longissima thalli resided in the lower half of the canopy and on the dead branches. We did not take notice of a downward shift in the vertical distribution of the thalli between the two survey periods, but as said, this was not quantitatively assessed.
Based on the tree density analyses before the logging events took place (Storaunet et al. 2008), number of thalli peaked at medium tree densities, i.e. less U. longissima thalli was found both at high and low tree densities. The present results corroborate these findings, although it seems that number of thalli may increase also at lower tree densities. If the weather and soil conditions ensure that the trees and branches where the lichens grow do not desiccate, the lichen may thrive also if the forest is very sparsely stocked. During 2009–2012 both precipitation and relative humidity in the region were higher than the previous years (Fig. 6). This may have contributed to the marked increase in the number of lichen thalli that we recorded, particularly in the trees not influenced by the selective logging.
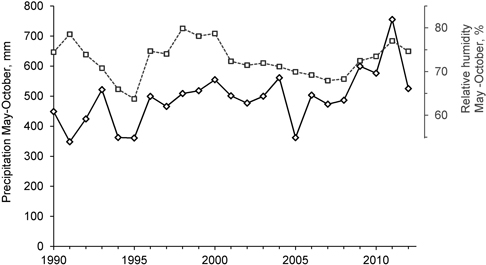
Fig. 6. Precipitation (solid line, left axis) and relative humidity (dotted line, right axis) in the region, from 1st of May to 31st of October, from 1990 to 2012. Data from The Norwegian Meteorological Institute (www.eklima.no), Biri (precipitation) and Lillehammer (relative humidity) meteorological stations.
In favorable conditions, U. longissima has a great potential for growth (Keon and Muir 2002; Gauslaa et al. 2007). In the Pacific Northwest it is shown that the lichen can increase its length twofold in a year (Rolstad and Rolstad 2008). If many long lichen threads are tangled, it can be difficult to separate different threads, resulting in a possible underestimation of the number of lichen thalli. A short thallus doubling its length will, with the present survey method, count the same and thereby also contribute to underestimation. On the other hand, if one long strand of thallus breaks up into several smaller ones, this would result in overestimation. In our study, the same persons followed the same methodology when counting thalli and we assume the errors to be moderate, and presumably on the conservative side.
The results show that selective loggings in productive spruce forests with relatively high timber volumes gave positive effects on number of U. longissima thalli. Previously, we suggested that leaving 100 to 150 m3ha–1 (approximately 400 medium-sized trees per ha) after logging would be a conservative approach (Storaunet et al. 2008). The present results suggest that even lower timber volume can be left after logging, provided that the humidity remains relatively high. In the mature spruce forests of the region, this implies that approximately 50% of the growing stock can be harvested. This might, however, reduce lichen abundance in occasional dry summers. Another important prerequisite is that no lichen-bearing trees are cut. This implies that the lichen flora must be thoroughly surveyed, and that trees with U. longissima must be marked in the field prior to logging. It is also important to evaluate how suitable the forest stands are for selective logging (e.g. following Lexerød and Eid 2006), since certain stand conditions may result in increased vulnerability to wind throws and tree desiccation (as happened in stand E8). Finally, similar to regular thinning operations, selective logging may reduce the speed of lower branch dieback, possibly prolonging the suitability of the canopy for U. longissima.
It is known that many epiphytic lichens grow more vigorously on slow-growing trees, possibly due to such trees offering more stable growing conditions or providing more favorable chemical micro-habitat (Gauslaa and Holien 1998; Gauslaa et al. 1998). In 2005/06 we found that trees with U. longissima showed lower absolute growth and more strongly declining growth compared to trees without the lichen. However, the lichen abundance (number of thalli per tree) was not related to the same growth patterns (Storaunet et al. 2008). In the present study, we found no effects of the same growth variables on the change in number of thalli from 2005/06 to 2012. It should be noted though, that we did not core the trees in 2012, implying that the growth patterns during the last years were not included in the present analyses.
U. longissima is presently categorized as Endangered in the Norwegian Red List (Kålås et al. 2010). Environmental certification standards in Norway do not allow forest stands with redlisted species to be clearcut. Given the relatively high number of U. longissima locations in certain areas, which is the case in our study area, carefully planned selective loggings may be a better alternative than a long-term hands-off policy.
Acknowledgements
Funding was provided by Utviklingsfondet for skogbruket (Norwegian Agricultural Authority), Skogtiltaksfondet (The Norwegian Forest Owners’ Federation), and Mjøsen Skog SA. Thanks to the forest owners for offering their properties at our disposal. Permit to conduct selective loggings in woodland key habitats were given by Mjøsen Skog SA under the forest certification system (PEFC), and approved by local authorities. Comments from two anonymous reviewers helped improving the clarity of the paper.
References
Dettki H., Esseen P.-A. (1998). Epiphytic macrolichens in managed and natural forest landscapes: a comparison at two spatial scales. Ecography 21: 613–624. http://dx.doi.org/10.1111/j.1600-0587.1998.tb00554.x.
Esseen P.-A., Ehnström B., Ericson L., Sjöberg K. (1997). Boreal forests. Ecological Bulletins 46: 16–47.
Esseen P.-A., Ericson L., Lindström H., Zackrisson O. (1981). Occurrence and ecology of Usnea longissima in central Sweden. Lichenologist 13: 177–190. http://dx.doi.org/10.1017/S0024282981000224.
Gaarder G. (1997). Huldrestry og andre kryptogamer i fuktige granskoger i sørlige deler av Oppland. NOA-Rapport, Report 1997-1. Siste Sjanse – Naturvernforbundet i Oslo og Akershus, Oslo. 85 p. ISBN 82-90895-09-7. [In Norwegian].
Gärdenfors U. (ed.). (2010). The 2010 Red List of Swedish species. Swedish Species Information Centre, Swedish University of Agricultural Sciences, Uppsala, Sweden. 590 p. ISBN 978-91-88506-35-1.
Gauslaa Y., Holien H. (1998). Acidity of boreal Picea abies – canopy lichens and their substratum, modified by local soils and airborne acidic depositions. Flora 193: 249–257.
Gauslaa Y., Solhaug K.A. (1996). Differences in the susceptibility to light stress between epiphytic lichens of ancient and young boreal forest stands. Functional Ecology 10: 344–354.
Gauslaa Y., Ohlson M., Rolstad J. (1998). Fine-scale distribution of the epiphytic lichen Usnea longissima on two even-aged neighbouring Picea abies trees. Journal of Vegetation Science 9: 95–102. http://dx.doi.org/10.2307/3237227.
Gauslaa Y., Palmqvist K., Solhaug K.A., Holien H., Hilmo O., Nybakken L., Myhre L.C., Ohlson M. (2007). Growth of epiphytic old forest lichens across climatic and successional gradients. Canadian Journal of Forest Research 37: 1832–1845. http://dx.doi.org/10.1139/X07-048.
Gjerde I., Sætersdal M., Blom H.H. (2007). Complementary hotspot inventory – a method for identification of important areas for biodiversity at the forest stand level. Biological Conservation 137: 549–557. http://dx.doi.org/10.1016/j.biocon.2007.03.007.
Gjerde I., Brandrud T.E., Ohlson M., Ødegaard F. (2010). Woodland. In: Kålås J.A., Henriksen S., Skjelseth S., Viken Å. (eds.). Environmental conditions and impacts for Red List species. Norwegian Biodiversity Information Centre, Norway. p. 67–78. ISBN-13: 978-82-92838-28-0.
Josefsson T., Hellberg E., Östlund L. (2005). Influence of habitat history on the distribution of Usnea longissima in boreal Scandinavia: a methodological case study. Lichenologist 37: 555–567. http://dx.doi.org/10.1017/S0024282905015355.
Kålås J.A., Viken Å., Henriksen S., Skjelseth S. (eds.). (2010). The 2010 Norwegian red list for species. Norwegian Biodiversity Information Centre, Trondheim, Norway. 480 p. ISBN-13: 978-82-92838-26-6.
Keon D.B., Muir P.S. (2002). Growth of Usnea longissima across a variety of habitats in the Oregon Coast Range. Bryologist 105: 233–242. http://dx.doi.org/10.1639/0007-2745(2002)105[0233:GOULAA]2.0.CO;2.
Kohm K.A., Franklin J.F. (eds.). (1997). Creating a forestry for the 21st century. The science of ecosystem management. Island Press, Washington DC. 475 p. ISBN 1-55963-398.
Lexerød N.L., Eid T. (2006). Assessing suitability for selective cutting using a stand level index. Forest Ecology and Management 237: 503–512. http://dx.doi.org/10.1016/j.foreco.2006.09.071.
Mjøsen Skogeierforening. (2005). Biologisk viktige områder i Lillehammer – sluttrapport. Miljørapport 3/2005. Mjøsen Skogeierforening, Lillehammer. 20 p. http://www.mjosen.no/getfile.php/928975.1260.tadyxybvvb/Lillehammer_BVO.pdf. [Cited 31 May 2013]. [In Norwegian].
Moen A. (1999). National atlas of Norway: vegetation. Norwegian Mapping Authority, Hønefoss. 200 p. ISBN 82-7945-000-9.
Olsen S.R., Gauslaa Y. (1991). Usnea longissima, a lichen of ancient forest, threatened in Nordmarka, SE Norway. Svensk Botanisk Tidskrift 85: 342–346. ISSN 0039-646X. [In Swedish with English summary].
Rametsteiner E., Simula M. (2003). Forest certification – an instrument to promote sustainable forest management? Journal of Environmental Management 67: 87–98. http://dx.doi.org/10.1016/S0301-4797(02)00191-3.
Rassi P., Hyvärinen E., Juslén A., Mannerkoski I. (eds.). (2010). The 2010 Red List of Finnish species. Ympäristöministeriö & Suomen ympäristökeskus, Helsinki, Finland. 685 p. ISBN 978-952-11-3806-5.
Rolstad E., Rolstad J. (1996). The distribution of Usnea longissima in Nordmarka, southeastern Norway. Blyttia 54: 145–150. ISSN 0006-6269. [In Norwegian with English summary].
Rolstad J., Rolstad E. (1999). Does tree age predict the occurrence and abundance of Usnea longissima in multi-aged submontane Picea abies stands? Lichenologist 31: 613–625. http://dx.doi.org/10.1017/S0024282999000808.
Rolstad J., Rolstad E. (2008). Intercalary growth causes geometric length expansion in Methuselah’s beard lichen (Usnea longissima). Botany 86: 1224–1232. http://dx.doi.org/10.1139/B08-081.
Siitonen J. (2001). Forest management, coarse woody debris and saproxylic organisms: Fennoscandian boreal forests as an example. Ecological Bulletins 49: 11–41.
Sillett S.C., Goslin M.N. (1999). Distribution of epiphytic macrolichens in relation to remnant trees in a multiple-age Douglas-fir forest. Canadian Journal of Forest Research 29: 1204–1215. http://dx.doi.org/10.1139/x99-081.
Storaunet K.O., Rolstad J., Toeneiet M., Rolstad E. (2008). Effect of logging on the threatened epiphytic lichen Usnea longissima: a comparative and retrospective approach. Silva Fennica 42: 685–703.
Tønsberg T., Gauslaa Y., Haugan R., Holien H., Timdal E. (1996). The threatened macrolichens of Norway – 1995. Sommerfeltia 23: 1–258. ISBN 82-7420-029-2.
Vestad O. (ed.). (2008). Forvaltning av huldrestryforekomster. Prosjektrapport. Mjøsen Skog BA, Lillehammer. 38 p. ISBN 978-82-997078-1-7. [In Norwegian with English abstract].
Total of 29 references

