Created substrates do not fully mimic natural substrates in restoration: the occurrence of polypores on spruce logs
Komonen A., Halme P., Jäntti M., Koskela T., Kotiaho J. S., Toivanen T. (2014). Created substrates do not fully mimic natural substrates in restoration: the occurrence of polypores on spruce logs. Silva Fennica vol. 48 no. 1 article id 980. https://doi.org/10.14214/sf.980
Highlights
- Polypore communities were more homogeneous among created than among natural logs
- The old-growth forest indicator Phellinus ferrugineofuscus occurred frequently on natural logs, but occupied only a few created logs
- Results show that created logs do not fully mimic natural logs.
Abstract
Many protected areas have been under intensive forest management prior to protection and thus lack natural ecosystem structures and dynamics. Dead wood is a key structure in forests harboring hundreds of threatened species. We investigated the ecological success of dead wood creation as a boreal forest restoration measure. We analysed whether the polypore communities of chain-saw felled and girdled (subsequently fallen) Norway spruce (Picea abies (L.) H. Karst.) logs differ from naturally formed spruce logs of similar decay stage and size. The study was conducted in Leivonmäki National Park in central Finland 8 years after the restoration measures. The average number of polypore species was highest on the chain-saw felled logs and most of the common polypore species were most frequent on this substrate. However, among the natural logs, number of species increased more steeply with increasing number of logs, suggesting greater variation in community composition on this substrate. The old-growth forest indicator Phellinus ferrugineofuscus occurred frequently on natural logs, occupied a few girdled logs but was absent from chain-saw felled logs. Our results show that from the polypore perspective created logs do not fully mimic natural logs, suggesting that creating substrates for species may pose a challenge for restoration.
Keywords
Norway spruce;
boreal forest;
ecological restoration;
dead wood;
protected area management;
substrate quality;
wood-decaying fungi
-
Komonen,
Department of Biological and Environmental Science, P.O. Box 35, FI-40014, University of Jyväskylä, Finland
E-mail
atte.komonen@jyu.fi

- Halme, Department of Biological and Environmental Science, P.O. Box 35, FI-40014, University of Jyväskylä, Finland E-mail panu.halme@jyu.fi
- Jäntti, Department of Biological and Environmental Science, P.O. Box 35, FI-40014, University of Jyväskylä, Finland E-mail mari.j.jantti@student.jyu.fi
- Koskela, Department of Biological and Environmental Science, P.O. Box 35, FI-40014, University of Jyväskylä, Finland E-mail tuuli.e.koskela@student.jyu.fi
- Kotiaho, Department of Biological and Environmental Science, P.O. Box 35, FI-40014, University of Jyväskylä, Finland E-mail janne.kotiaho@jyu.fi
- Toivanen, Department of Biological and Environmental Science, P.O. Box 35, FI-40014, University of Jyväskylä, Finland; Current: Birdlife Finland, Annankatu 29 A 16, FI-00100 Helsinki, Finland E-mail tero.toivanen@birdlife.fi
Received 16 August 2013 Accepted 22 January 2014 Published 14 February 2014
Views 178305
Available at https://doi.org/10.14214/sf.980 | Download PDF

1 Introduction
Setting-aside pristine land for conservation purposes is not enough to halt the ongoing biodiversity loss. Due to anthropogenic land use, pristine areas are globally scarce and even completely absent in many places (Foley et al. 2005; Ellis 2011). Indeed, many protected areas have been intensively used by humans for decades or centuries before protection (Wallenius et al. 2010), and they lack many structures and processes, which are typical for natural ecosystems (Kuuluvainen 2002). Thus, to promote biodiversity conservation, there is clearly a need for more active measures, including ecological restoration (Convention on Biological Diversity 2010). Ecological restoration is an intentional human intervention in altered ecosystems, which aims at bringing back natural structures and processes (Society for Ecological Restoration… 2004; Halme et al. 2013)
In natural forest ecosystems, dead wood is a characteristic feature. It has become scarce in managed forests, largely due to the commercial forest management over the past centuries (Harmon et al. 1986; Stokland et al. 2012). For example, in Fennoscandian boreal forests, the volume of dead wood has declined over 90%. Because about 25% of forest species are dependent on dead wood (Siitonen 2001), many of these species have declined and are increasingly threatened by regional and national extinction (Rassi et al. 2010; Gärdenfors et al. 2010). Given the lack of dead wood in formerly managed, currently protected forests, dead wood creation as a restoration measure has become a pivotal practice in protected area management in Fennoscandia (Kuuluvainen et al. 2002; Toivanen and Kotiaho 2007; Vanha-Majamaa 2007; Olsson et al. 2011; Halme et al. 2013), but relatively little is still known about the ecological success of these restoration measures (but see Toivanen and Kotiaho 2010; Laarmann et al. 2013; Penttilä et al. 2013).
One group of organisms that has been heavily influenced by forest management is the polyporous wood-decaying fungi (Aphyllophorales: Polyporaceae) (Josefsson et al. 2010; Junninen and Komonen 2011). Polypores are an important functional component of boreal forests, since they recycle nutrients, especially the refractory digestible lignin and cellulose (Rayner and Boddy 1988; Boddy et al. 2008). Their fruit bodies and mycelia are also an important food source for invertebrates (Komonen 2003). Polypores are directly dependent on dead wood as a substrate, and in addition to dead wood quantity, also dead wood quality influences their occurrences and abundances. For example, physical features of dead wood, such as tree species, trunk size, decay stage and type (standing, snag or log) (Junninen et al. 2008; Junninen and Komonen 2011), as well as wood density and chemical features such as C : N ratio, moisture and lignin content (Rajala et al. 2012) are all important. Furthermore, the identity of the pioneer decayer species influences directly and/or indirectly the successional pathways and community turnover at later decay stages (Renvall 1995; Fukami et al. 2010; Lindner et al. 2011). Thus, it can be expected that for polypores it matters what restoration measures are used in dead wood creation.
In Fennoscandia, gap fellings, dead wood creation and prescribed burning are the main methods of forest restoration (Similä and Junninen 2012). Although they all result in increased dead wood amount, their objectives are slightly different. The effects of these restoration measures on fungal diversity have been studied quite little, and the existing studies have mostly focused on the effects of fire (Penttilä and Kotiranta 1996; Junninen et al. 2008; Olsson and Jonsson 2010; Berglund et al. 2011; Penttilä et al. 2013). However, a few studies have investigated the occurrence of polypores on different types of created dead wood. These studies have shown, for example, that the management history of the forest stand influences the polypore community on created logs (Olsson et al. 2011), that different types of created dead wood have different polypore communities (Berglund et al. 2011; see Selonen et al. 2005 and Toivanen et al. 2012 for similar results on clear cuts), and that on clear cuts the number of polypores is higher on cut pieces of dead wood than on stumps (Lindhe et al. 2004; Toivanen et al. 2012). None of the restoration studies, however, have compared polypore species on different types of created dead wood and respective natural logs in the same sites. It is a fundamental conservation issue whether the dead wood created in restoration resembles the natural dead wood from the species perspective.
In forest restoration, dead wood has been created primarily by chain-saw felling or girdling, or pushing trees down with machinery. Trees that are pushed down are likely to resemble wind-felled trees, and they may or may not retain root connection. However, many natural wind-fells have been weakened by wood-rotting fungi (Edman et al. 2007), and thus they are likely to be dissimilar to healthy trees that are pushed down. Girdled trees are likely to die slowly while standing, and thus resemble trees that have been weakened by insects, fungi, drought or paludification; all girdled trees do eventually fall down. Chain-saw felled trees are likely to resemble trees that have been broken off by wind or snow (Similä and Junninen 2012), although many wind- or snow-broken trees have in fact been weakened by fungal pathogens. Thus, healthy trees that are chain-saw felled in restoration may differ from natural wind- or snow-broken trees. It is not known whether chain-saw felled and girdled logs are suitable for the rare and threatened species for which they are expected to provide substrate. It is possible that different types of created and natural dead wood differ in both physio-chemical properties as well as competitive interactions (Groot 1972; Rajala et al. 2012).
Polypores are a suitable focus group for the study of the effects of forest management and restoration, since they are directly dependent on dead wood as substrate. In this study, we evaluate the suitability of dead wood created in restoration as a substrate for polypores over an eight-year period. We compare the species richness and community composition of polypores on chain-saw felled, girdled and subsequently fallen, and naturally fallen Norway spruce (Picea abies (L.) H. Karst) logs. We also analyse the occurrence of seven locally abundant polypore species (including the old-growth forest indicator Phellinus ferrugineofuscus) in different dead wood types.
2 Material and methods
2.1 Study area
This study was conducted in Leivonmäki National Park in Central Finland. The park was established in year 2003 and it is 30 km2 in area. All forests in the park have a recent management history, and consequently, the dead wood volume is generally low, only rarely exceeding 10 m3 ha–1 (for comparison, volumes of dead wood >10 cm in diameter are typically 50–130 m3 ha–1 in similar natural forests; Siitonen 2001). In the National park, ecological restoration was carried out during the winter 2003–2004. Restoration actions included creating dead wood by felling trees with chain-saw (cutting them from the base) and damaging trees by girdling. As a part of the actions, a restoration monitoring network was also established. The network consists of 50 m x 50 m plots randomly distributed in the park. On these plots, 5 or 10 m3 of dead wood was created by felling, whereas girdling was done only outside the plots.
2.2 Data collection
In this study, we compared the polypore communities of chain-saw felled and girdled Norway spruce logs to spruce logs of natural origin. The chain-saw felled logs were sampled inside 12 monitoring plots and the girdled logs outside the plots; logs of natural origin were from both inside and outside the monitoring plots. Outside the plots we selected only logs located in the immediate vicinity of the monitoring plots (i.e. on the same forest stands) to control for possible variation in local species pools. On the monitoring plots, data were collected in autumn 2011 and consisted of 173 chain-saw felled spruce logs and 187 natural spruce logs (only a subset of these logs could be included in the analyses; see below). Outside the plots data were collected in autumn 2012 and consisted of 27 girdled spruce logs and 23 natural spruce logs. 51% of the natural logs had been formed by uprooting and 49% by stem breakage (at the base of the tree, typically < 1.5 m height).
In the field, we determined the diameter of the logs (dbh) and decay stage at 1.3 m from the root base. Decay stage was measured with knife, using the scale of 1–5 (Renvall 1995): stage 1 represents fresh, bark covered and hard logs, and stage 5 almost completely decayed logs. The majority (77%) of the chain-saw felled logs were of decay stage 2 (range 1–3), while 59% of the girdled logs were of decay stage 1 (range 1–2). The difference in decay stage was most likely due to time lag between girdling and subsequent tree death and falling down, and thus slower decay of the girdled trees.
The presence or absence of polypore species on the logs was inventoried based on the presence of fruit bodies. If identification was uncertain in the field, specimens were collected for microscopic identification. The nomenclature of polypores follows Kotiranta et al. (2009) and the classification of old-growth forest indicators Niemelä (2005).
2.3 Data processing and analyses
To build meaningful (i.e., similar in terms of decay stage and diameter range) comparison groups for the analyses, we selected only natural logs representing decay stages 1 and 2 and only logs that had dbh > 15 cm. Natural logs of stage 1 were a comparison group for the girdled logs, and natural logs of stage 2 were a comparison group for the chain-saw felled logs. Ideally, one would have been able to compare logs that had died at the same time, however, it is practically impossible to determine the time-since-death of naturally downed wood. Thus, we had four log type groups in our analyses: girdled logs (n = 27, mean dbh = 23.3 cm, range = 17–33 cm), logs felled with chain-saw (n = 150, mean dbh = 25.3 cm, range = 16–44 cm), natural logs of decay stage 1 (n = 28, mean dbh = 25.8 cm, range = 16–45 cm), and natural logs of decay stage 2 (n = 35, mean dbh = 27.3 cm, range = 16–46 cm). The average diameter of the logs did not differ between the log type groups (ANOVA, F3,236 = 2.07, p = 0.104).
We used Kruskal-Wallis non-parametric test to analyze whether the species richness of polypores differed between the log types. We also calculated species accumulation curves for each log type to explore how the number of species increased with increasing number of logs. We further compared the species-specific frequencies of the seven most abundant species on different log types using χ2-test. The species occurrence data were x+1 -transformed to meet the test assumptions. Differences from the expected frequency were interpreted using adjusted standardized residuals, which indicate the importance of each cell to the ultimate chi-square value. The species accumulation curves were calculated with EstimateS version 9 (Colwell 2013) and the other statistical tests were performed with IBM SPSS Statistics 20.
3 Results
In total, we recorded 21 polypore species (Table 1), five of which are classified as indicators of old-growth forest. Seven species occurred on girdled logs, 13 species on chain-saw felled logs, six species on natural logs of decay stage 1 and 14 species on natural logs of decay stage 2. Six of the species occurred only on chain-saw felled logs, and seven species were recorded only on natural logs. On girdled logs there were no unique species. Four of the old-growth forest indicator species occurred on natural logs of decay stage 2, two species on natural logs of decay stage 1, one on girdled logs and one on chain-saw felled logs (Table 1). However, the total numbers of species per log type group are not directly comparable because of varying sample sizes among the groups; in particular, the sample of chain-saw felled logs was substantially larger than that of the other groups.
| Table 1. Polypore species recorded, and the percentage of logs occupied by the species on each log type group. Species are ranked according to the total number of observations. | ||||
| Species | Girdled | Chain-saw felled | Natural decay 1 | Natural decay 2 |
| n = 27 | n = 150 | n = 28 | n = 35 | |
| Trichaptum abietinum | 48 | 99 | 36 | 60 |
| Fomitopsis pinicola | 70 | 91 | 43 | 63 |
| Antrodia serialis | 15 | 71 | 49 | |
| Skeletocutis carneogrisea | 79 | 4 | 9 | |
| Ischnoderma benzoinum | 16 | |||
| Phellinus ferrugineofuscus* | 7 | 11 | 37 | |
| Skeletocutis amorpha | 4 | 11 | 4 | |
| Postia tephroleuca | 7 | 6 | 14 | |
| Postia caesia | 7 | 3 | 9 | |
| Phellinus viticola* | 4 | 9 | ||
| Pycnoporellus fulgens* | 2 | |||
| Gloeophyllum sepiarium | 1 | |||
| Fomitopsis rosea* | 6 | |||
| Junghuhnia luteoalba* | 3 | |||
| Oligoporus stipticus | 1 | |||
| Antrodia sinuosa | 3 | |||
| Oligoporus fragilis | 3 | |||
| Gloeophyllum odoratum | 1 | |||
| Physisporinus vitreus | 3 | |||
| Skeletocutis papyracea | 3 | |||
| Spongiporus undosus | 1 | |||
| * Old-growth forest indicator species according to Niemelä (2005). | ||||
There was a significant difference in the average number of polypore species per log between the different log types (Kruskal-Wallis test: χ2 = 113.1, df = 3, P < 0.001; Fig. 1). According to the pairwise comparisons, chain-saw felled logs hosted on average more species than natural logs of decay stage 2 (χ2 = 50.0, p < 0.001), but there was no difference between girdled logs and natural logs of decay stage 1 (χ2 = 20.7, p = 0.26). Other pairwise comparisons were not meaningful in the context of the present study due to differences in decay stages.
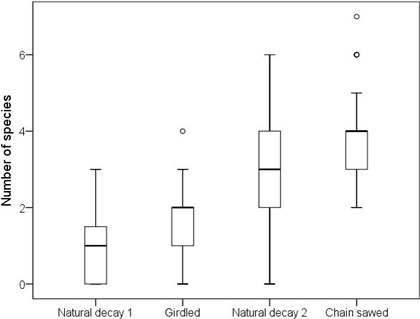
Fig. 1. The number of polypore species per log on natural logs of decay stage 1 (n = 28), girdled logs (n = 27), natural logs of decay stage 2 (n = 35) and chain-saw felled logs (n = 150). The horizontal lines represent medians, boxes 25% to 75% quartiles, whiskers extend to 1.5 times the height of the box or to the minimum or maximum values; circles represent extreme values that do not fall between the whiskers.
According to the species accumulation curves, a standardized sample of 27 chain-saw felled logs, girdled logs, natural logs of decay stage 1 and natural logs of decay stage 2 hosted on average 8.8, 7, 5.9 and 13 species, respectively. The differences between chain-saw felled logs and natural logs of decay stage 2, and between girdled logs and natural logs of decay stage 1 were not statistically significant, judged from the overlapping 95% confidence intervals. However, the accumulation curves illustrate that the number of species increased much steeper on natural logs of decay stage 2 than on chain-saw felled logs, suggesting larger variation in community composition among the natural logs (Fig. 2).
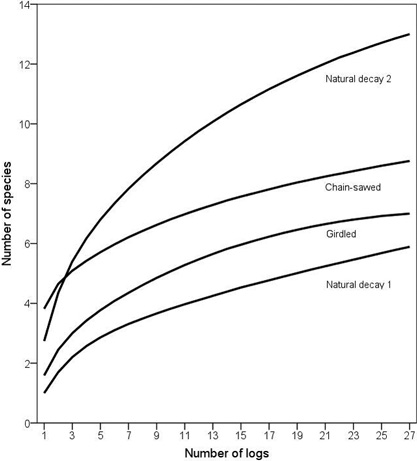
Fig. 2. Species accumulation curves of polypores on increasing number of logs for each of the log type groups. To increase clarity, 95% confidence intervals are not presented; see Results for statistical differences.
The occurrences of the six (out of seven) most common polypore species was dependent on the log type (for all, χ2 > 9.5, df = 3, P < 0.05), the only exception being Skeletocutis amorpha (χ2 = 4.3, df = 3, P = 0.24). The old-growth forest indicator Phellinus ferrugineofuscus was among the most common species in our material. On chain-saw felled logs, P. ferrugineofuscus occurred less frequently than expected, whereas the other six species were more frequent than expected. On natural logs of decay stage 2, P. ferrugineofuscus occurred more frequently than expected (Table 2).
| Table 2. The results of the χ2-test exploring the frequency of the seven most frequent polypore species on the four log type groups. The adjusted standardized residuals (ASR) indicate whether the species was more (positive values) or less (negative values) frequent than expected on the log type. Significant differences from the expected frequency are underlined. | ||||||||
| Girdled n = 27 | Chain-saw felled n = 150 | Natural decay 1 n = 28 | Natural decay 2 n = 35 | |||||
| % logs | ASR | % logs | ASR | % logs | ASR | % logs | ASR | |
| Trichaptum abietinum | 48 | –4.5 | 99 | 9.5 | 36 | –6.3 | 60 | –3.3 |
| Fomitopsis pinicola | 70 | –1.2 | 91 | 6.0 | 43 | –5.0 | 63 | –2.6 |
| Antrodia serialis | 15 | –4.2 | 71 | 7.0 | 0 | –5.7 | 49 | –0.6 |
| Skeletocutis carneogrisea | 0 | –5.4 | 79 | 11.1 | 4 | –5.4 | 9 | –5.5 |
| Ischnoderma benzoinum | 0 | –1.3 | 16 | 3.1 | 0 | –1.4 | 0 | –1.7 |
| Phellinus ferrugineofuscus | 7 | –0.1 | 0 | –5.4 | 11 | 0.6 | 37 | 6.9 |
| Skeletocutis amorpha | 4 | –0.9 | 11 | 2.1 | 4 | –0.9 | 0 | –1.2 |
4 Discussion
Our study shows that the restoration measures that are applied to create dead wood matters for polypores. There were clear differences in species richness and community composition between the chain-saw felled, girdled and natural logs. Although the ability of polypores to utilize created dead wood has been documented (Lindhe et al. 2004; Berglund et al. 2011; Olsson et al. 2011), it was still somewhat unexpected that the chain-saw felled logs hosted on average more polypore species than the natural logs of the same decay stage. The chain-saw felled logs hosted also species which were rare or absent from the other types of substrates; however, all these species are known to occur also on naturally formed dead wood in the study area. The higher mean species richness on the chain-saw felled logs was largely caused by a few polypore species that were particularly frequent on these logs, and there was much more variation in the community composition among the natural logs. This variation was well illustrated by the species accumulation curves, i.e. the cumulative number of species on the natural logs increased much steeper with increasing number of logs.
The species thriving in the current managed forest landscape are likely to be well capable to utilize man-made substrates (e.g. cut stumps, logging residues; see Toivanen et al. 2012). Indeed, the most common species of our study, Trichaptum abietinum and Fomitopsis pinicola, were most frequent on the chain-saw felled logs. Also Antrodia serialis, Skeletocutis carneogrisea, S. amorpha and Ischnoderma benzoinum were clearly more frequent on the chain-saw felled logs than on any other log type. Skeletocutis carneogrisea is a successor species growing on the dead fruit bodies of T. abietinum. It was interesting that it colonized 80% of the chain-saw felled logs on which T. abietinum occurred, while the corresponding figure for natural logs of decay stage 2 was only 15%. The old-growth forest indicator Phellinus ferrugineofuscus was the only locally common species that was more frequent than expected on natural logs and simultaneously occurred less frequently than expected on the chain-saw felled logs.
The primary goal of the restoration actions is not to increase species richness as such (Hobbs and Kramer 2008), but to recreate lost substrates for the original and threatened species which are still present or possibly able to colonize the area from the surrounding landscape (Ennallistamistyöryhmä 2003; Society for Ecological Restoration…2004). Thus, the differences in community composition between the log types may be a more important finding than the differences in the species richness. Indeed, the old-growth forest indicator species were well represented on natural logs, i.e. there were four indicator species on natural logs, whereas only one indicator species was observed on chain-saw felled logs despite the much larger sample size. In particular, the old-growth forest indicator P. ferrugineofuscus did not occur at all on chain-saw felled logs, but was among the most frequent species on natural logs. Interestingly, this species occurred on girdled logs, which suggests that species succession may also differ between the different types of created substrates and provides further support that for the polypores the chosen restoration measure does matter. From the practical point of view this implies that different restoration measures should be used if increased substrate heterogeneity is aimed for.
All the species that inhabit a given log do not produce fruit bodies, or the timing of fruit body production may be different from the inventory period, and thus some species remain undetected in the inventories (Halme and Kotiaho 2012). For example, DNA of Phellinus viticola and P. nigrolimitatus have been found from the early decay stages (Rajala et al. 2012), although these species typically produce fruit bodies only in mid- to late decay stages (Renvall 1995; Jönsson et al. 2008). Of course, it is impossible to say what proportion of the species present as mycelia in early decay stages ever produces fruit bodies, i.e. reproduces successfully. However, the fact that surveys based on fruit bodies are likely to underestimate the species richness of a log should influence our conclusions only if the rate or timing of fruit body production differs between the studied dead wood types (see Halme and Kotiaho 2012).
A diverse fungal fauna is likely to be present already in living trees (Parfitt et al. 2010), and fungi may actually cause the death of a tree or predispose trees to windfall or stem breakage. It is likely that many of the natural logs of this study had already been weakened by fungi at the time of tree death, while a larger proportion of the chain-saw felled logs were probably healthy prior to felling. Thus, chain-saw felled logs may be, on average, a more competition free substrate, which could favor the establishment of the common pioneer species (Berglund et al. 2011). Naturally formed dead wood may also be more resistant to decaying agents, because of remaining root connection.
It is known that the successional pathways of polypore species on logs varies depending on the pioneer polypore species (Renvall 1995; Lindner et al. 2011; Rajala et al. 2012) and dead wood type (Berglund et al. 2011). Future research should focus on whether and how these early differences in community composition and species richness influence the fungal communities of later successional stages. This is important, since the decay of a spruce log typically takes 60–80 years in boreal forests (Mäkinen et al. 2006), and depending on the fungal group the highest species richness, especially that of red-listed species, is found during mid- to advanced decay stages (Junninen and Komonen 2011; Rajala et al. 2012).
Our study shows that the way dead wood is formed does matter for polypore fungi, and that dead wood created with restoration measures may not fully mimic the natural processes of dead wood formation. However, using different restoration measures to create dead wood may improve the ecological success of restoration, because different methods may initiate different succession trajectories resulting in different polypore communities. Although we could not directly compare the girdled trees to the felled trees due to the difference in decay stage, the results show that a specialized indicator species of old-growth forest favored girdled logs as a substrate compared to the chain-saw felled logs. Although the time scale of our study is relatively long (8 years following restoration), the polypore species succession of the logs is still at its early stages. Therefore, the species succession should be followed in the future and particular attention should be paid to the girdled trees, which were still at the first stage of wood decay at the time of this study.
Acknowledgements
We are grateful to Metsähallitus for collaboration in planning and conducting the restoration actions. We thank Noora Vartija for assisting in the polypore surveys and Kaisa Junninen for commenting the manuscript. This study was funded by Maj and Tor Nessling Foundation (grant to TT) and Post Doc Pool (grant to PH).
References
Berglund H., Jönsson M., Penttilä R., Vanha-Majamaa I. (2011). The effects of burning and dead-wood creation on the diversity of pioneer wood-inhabiting fungi in managed boreal spruce forests. Forest Ecology and Management 261: 1293–1305. http://dx.doi.org/10.1016/j.foreco.2011.01.008.
Boddy L., Frankland J.C., van West P. (2008). Ecology of saprotrophic basidiomycetes. Academic Press, Elsevier, Amsterdam. 386 p.
Colwell R.K. (2013). EstimateS: statistical estimation of species richness and shared species from samples. Version 9. User guide and application. http://viceroy.eeb.uconn.edu/estimates/index.html.
Convention on Biological Diversity (2010). COP 10 decision X/2: strategic plan for biodiversity 2011–2020. http://www.cbd.int/decision/cop/?id=12268. [Cited 10 Feb 2013].
Edman M., Jönsson M., Jonsson B.G. (2007). Fungi and wind strongly influence the temporal availability of logs in an old-growth spruce forest. Ecological Applications 17: 482–490. http://dx.doi.org/10.1890/06-0852.
Ellis E.C. (2011). Anthropogenic transformation of the terrestrial biosphere. Philosophical Transactions of the Royal Society A – Mathematical, Physical and Engineering Sciences 369: 1010–1035. http://dx.doi.org/10.1098/rsta.2010.0331.
Ennallistamistyöryhmä (2003). Ennallistaminen suojelualueilla. Ennallistamistyöryhmän mietintö. Suomen ympäristö 618. 220 p.
Foley J.A., DeFries R., Asner G.P., Barford C., Bonan G., Carpenter S.R., Chapin F.S., Coe M.T., Daily G.C, Gibbs H.K., Helkowski J.H., Holloway T., Howard E.A., Kucharik C.J., Monfreda C., Patz J.A., Prentice I.C., Ramankutty N., Snyder P.K. (2005). Global consequences of land use. Science 309: 570−574. http://dx.doi.org/10.1126/science.1111772.
Fukami T., Dickie I.A., Wilkie J.P., Paulus B.C., Park D., Roberts A., Buchanan P.K., Allen R.B. (2010). Assembly history dictates ecosystem functioning: evidence from wood decomposer communities. Ecology Letters 13: 675–684. http://dx.doi.org/10.1111/j.1461-0248.2010.01465.x.
Gärdenfors U. (ed.) (2010). The 2010 red list of Swedish species. Artdatabanken, Swedish Agricultural University, Uppsala, Sweden. 589 p.
Groot R.C.D. (1972). Growth of wood-inhabiting fungi in saturated atmospheres of monoterpenoids. Mycologia 64: 863–870. http://dx.doi.org/10.2307/3757941.
Halme P., Kotiaho J.S. (2012). The importance of timing and number of surveys in fungal biodiversity studies. Biodiversity and Conservation 21: 205–219. http://dx.doi.org/10.1007/s10531-011-0176-z.
Halme P., Allen K.A., Auniņš A., Bradshaw R.H.W., Brūmelis G., Čada V., Clear J.L, Eriksson A.-M., Hannon G., Hyvärinen E., Ikauniece S., Iršėnaitė R., Jonsson B.G., Junninen K., Kareksela S., Komonen A., Kotiaho J.S., Kouki J., Kuuluvainen T., Mazziotta A., Mönkkönen M., Nyholm K., Oldén A., Shorohova E., Strange N., Toivanen T., Vanha-Majamaa I., Wallenius T., Ylisirniö A.-L., Zin E. (2013). Challenges of ecological restoration: lessons from forests in northern Europe. Biological Conservation 167: 248–256. http://dx.doi.org/10.1016/j.biocon.2013.08.029.
Harmon M.E., Franklin J.F., Swanson F.J., Sollins P., Gregory S.V., Lattin J.D., Anderson N.H., Cline S.P., Aumen N.G., Sedell J.R., Lienkaemper G.W., Cromack Jr. K., Cummins K.W. (1986). Ecology of coarse woody debris on temperate ecosystems. Advances of Ecological Research 15: 133–302. http://dx.doi.org/10.1016/S0065-2504(08)60121-X.
Hobbs R.J., Kramer V.A. (2008). Restoration ecology: interventionist approaches for restoring and maintaining ecosystem function in the face of rapid environmental change. Annual Review of Environment and Resources 33: 39–61. http://dx.doi.org/10.1146/annurev.environ.33.020107.113631.
Jönsson M., Edman M., Jonsson B.G. (2008). Colonization and extinction patterns of wood-decaying fungi in a boreal old-growth Picea abies forest. Journal of Ecology 96: 1065–1075. http://dx.doi.org/10.1111/j.1365-2745.2008.01411.x.
Josefsson T., Olsson J., Östlund L. (2010). Linking forest history and conservation efforts: long-term impact of low-intensity timber harvest on forest structure and wood-inhabiting fungi in northern Sweden. Biological Conservation 143: 1803–1811. http://dx.doi.org/10.1016/j.biocon.2010.04.035.
Junninen K., Komonen A. (2011). Conservation ecology of boreal polypores: a review. Biological Conservation 144: 11–20. http://dx.doi.org/10.1016/j.biocon.2010.07.010.
Junninen K., Kouki J., Renvall P. (2008). Restoration of natural legacies of fire in European boreal forests: an experimental approach to the effects on wood-decaying fungi. Canadian Journal of Forest Research 38: 202–215. http://dx.doi.org/10.1139/X07-145.
Komonen A. (2003). Hotspots of insect diversity in boreal forests. Conservation Biology 17: 976–981. http://dx.doi.org/10.1046/j.1523-1739.2003.02076.x.
Kotiranta H., Saarenoksa R., Kytövuori I. (2009). Aphyllophoroid fungi of Finland. A check-list with ecology, distribution and threat categories. Norrlinia 19: 1–223.
Kuuluvainen T. (2002). Natural variability of forests as a reference for restoring and managing biological diversity in boreal Fennoscandia. Silva Fennica 36: 97–125.
Laarmann D., Korjus H., Sims A., Kangur A., Stanturf S.A. (2013). Initial effects of restoring natural forest structures in Estonia. Forest Ecology and Management 304: 303–311. http://dx.doi.org/10.1016/j.foreco.2013.05.022.
Lindhe A., Asenblad N., Toresson H. (2004). Cut logs and high stumps of spruce, birch, aspen and oak – nine years of saproxylic fungi succession. Biological Conservation 119: 443–454. http://dx.doi.org/10.1016/j.biocon.2004.01.005.
Lindner D.L., Vasaitis R., Kubartová A., Allmér J., Johannesson H., Banik M.T., Stenlid J. (2011). Initial fungal colonizer affects mass loss and fungal community development in Picea abies logs 6 yr after inoculation. Fungal Ecology 4: 449–460. http://dx.doi.org/10.1016/j.funeco.2011.07.001.
Mäkinen H., Hynynen J., Siitonen J., Sievänen R. (2006). Predicting the decomposition of Scots pine, Norway spruce, and birch stems in Finland. Ecological Applications 16: 1865–1879. http://dx.doi.org/10.1890/1051-0761(2006)016[1865:PTDOSP]2.0.CO;2.
Niemelä T. (2005). Käävät, puiden sienet. Botanical Museum, Finnish Museum of Natural History, Helsinki. 319 p.
Olsson J., Jonsson B.G. (2010). Restoration fire and wood-inhabiting fungi in a Swedish Pinus sylvestris forest. Forest Ecology and Management 259: 1971–1980. http://dx.doi.org/10.1016/j.foreco.2010.02.008.
Olsson J., Jonsson B.G., Hjältén J., Ericson L. (2011). Addition of coarse woody debris – the early fungal succession on Picea abies logs in managed forests and reserves. Biological Conservation 144: 1100–1110. http://dx.doi.org/10.1016/j.biocon.2010.12.029.
Parfitt D., Hunt J., Dockrell D., Rogers H.J., Boddy L. (2010). Do all trees carry the seeds of their own destruction? PCR reveals numerous wood decay fungi latently present in sapwood of a wide range of angiosperm trees. Fungal Ecology 3: 338–346. http://dx.doi.org/10.1016/j.funeco.2010.02.001.
Penttilä R., Kotiranta H. (1996). Short-term effects of prescribed burning on wood-rotting fungi. Silva Fennica 30: 399–419. http://dx.doi.org/10.14214/sf.a8501.
Penttilä R., Junninen K., Punttila P., Siitonen J. (2013). Effects of forest restoration by fire on polypores depend strongly on time since disturbance – a case study from Finland based on a 23-year monitoring period. Forest Ecology and Management 310: 508–516. http://dx.doi.org/10.1016/j.foreco.2013.08.061.
Rajala T., Peltoniemi M., Pennanen T., Mäkipää R. (2012). Fungal community dynamics in relation to substrate quality of decaying Norway spruce (Picea abies [L.] Karst.) logs in boreal forests. FEMS Microbiology Ecology 81: 494–505. http://dx.doi.org/10.1111/j.1574-6941.2012.01376.x.
Rassi P., Hyvärinen E., Juslèn A., Mannerkoski I. (eds.). (2010). Suomen lajien uhanalaisuus. Punainen kirja 2010. Ympäristöministeriö ja Suomen ympäristökeskus, Helsinki. 685 p.
Rayner A.D.M., Boddy L. (1988). Fungal decomposition of wood: its biology and ecology. John Wiley & Sons, Bath. 602 p.
Renvall P. (1995). Community structure and dynamics of wood-rotting Basidiomycetes on decomposing conifer trunks in northern Finland. Karstenia 35: 1–51.
Selonen V.A.O., Ahlroth P., Kotiaho J.S. (2005). Anthropogenic disturbance and diversity of species: polypores and polypore associated beetles in forest, forest edge and clear cut. Scandinavian Journal of Forest Research 20(Suppl 6): 49–58. http://dx.doi.org/10.1080/14004080510041002.
Society for Ecological Restoration International Science & Policy Working Group (2004). The SER international primer on ecological restoration. Society for Ecological Restoration International. https://www.ser.org/resources/resources-detail-view/ser-international-primer-on-ecological-restoration. [Cited 12 August 2013].
Siitonen J. (2001). Forest management, coarse woody debris and saproxylic organisms: Fennoscandian boreal forests as an example. Ecological Bulletins 49: 11–41.
Similä M., Junninen K. (eds.). (2012). Ecological restoration and management in boreal forests – best practices from Finland. Metsähallitus Natural Heritage Services, Vantaa. 50 p.
Stokland J.N., Siitonen J., Jonsson B.G. (2012). Biodiversity in dead wood. Ecology, Biodiversity and Conservation Series, Cambridge University Press. 509 p. http://dx.doi.org/10.1017/CBO9781139025843.
Toivanen T., Kotiaho J.S. (2007). Mimicking natural disturbances of boreal forests: the effects of controlled burning and creating dead wood on beetle diversity. Biodiversity and Conservation 16: 3193–3211. http://dx.doi.org/10.1007/s10531-007-9172-8.
Toivanen T., Kotiaho J.S. (2010). The preferences of saproxylic beetle species for different dead wood types created in forest restoration treatments. Canadian Journal of Forest Research 40: 445–464. http://dx.doi.org/10.1139/X09-205.
Toivanen T., Markkanen A., Kotiaho J.S., Halme P. (2012). The effect of forest fuel harvesting on the fungal diversity of clear-cuts. Biomass and Bioenergy 39: 84–93. http://dx.doi.org/10.1016/j.biombioe.2011.11.016.
Vanha-Majamaa I., Lilja S., Ryömä R., Kotiaho J.S., Laaka-Lindberg S., Lindberg H., Puttonen P., Tamminen P., Toivanen T., Kuuluvainen T. (2007). Rehabilitating boreal forest structure and species composition in Finland through logging, dead wood creation and fire: the EVO experiment. Forest Ecology and Management 250: 77–88. http://dx.doi.org/10.1016/j.foreco.2007.03.012.
Wallenius T., Niskanen L., Virtanen T., Hottola J., Brumelis G., Angervuori A., Julkunen J., Pihlström M. (2010). Loss of habitats, naturalness and species diversity in Eurasian forest landscapes. Ecological Indicators 10: 1093–1101. http://dx.doi.org/10.1016/j.ecolind.2010.03.006.
Total of 46 references

