Longitudinal differences in Scots pine shoot elongation
Andersson Gull B., Persson T., Fedorkov A., Mullin T. J. (2018). Longitudinal differences in Scots pine shoot elongation. Silva Fennica vol. 52 no. 5 article id 10040. https://doi.org/10.14214/sf.10040
Highlights
- More northerly Scots pine origins exhibit earlier onset and cessation of shoot growth
- Continental origins show more northern phenological behaviour
- Heat accumulation requirements for onset are not fixed and may be lower when accumulating slower
- Scots pine may suffer from spring frost due to earlier growth onset in a warming climate
- Phenological traits show potential to adapt to new climate conditions by breeding.
Abstract
Phenology can have a profound effect on growth and climatic adaptability of long-lived, northern tree species such as Scots pine (Pinus sylvestris L.), where the onset of growth in the spring is triggered mainly by accumulated heat, while cessation of growth is related to the joint effect of photoperiod and temperature. In this study, the objectives were: (1) to compare shoot phenology of genetic material from Scandinavia (maritime climate origin) and northern Russia (continental climate origin) sources, under field conditions in both Scandinavia and Russia (maritime and continental growth conditions); and (2) to estimate the heritabilities of phenological parameters. The material used was part of a larger provenance test series involving Scots pine populations and open-pollinated plus-tree families from Russia, Sweden and Finland. Terminal shoot elongation was measured on multiple occasions during the seventh growing season from seed at a trial near Bäcksjön (Sweden) and Syktyvkar (northern Russia). We calculated the regression of relative shoot elongation over accumulated heat sum above +5 °C using an exponential expression. Seedlings of Swedish and Russian provenance had similar heat-sum requirements for growth onset and cessation in both trials. More northern provenances started onset and cessation at a lower temperature sum, but heat accumulation requirements for onset were not fixed. Scots pine may suffer from spring frost due to earlier growth onset in a warming climate. Variation and heritability of phenological traits show potential to adapt Scots pine to new climate conditions by breeding.
Keywords
Pinus sylvestris;
climate change;
adaptation;
shoot phenology;
heritability of phenological traits;
growth onset;
growth cessation
-
Andersson Gull,
The Swedish Forestry Research Institute (Skogforsk), Box 3, SE-918 21 Sävar, Sweden
 https://orcid.org/0000-0003-3556-3172
E-mail
bengt.anderssongull@skogforsk.se
https://orcid.org/0000-0003-3556-3172
E-mail
bengt.anderssongull@skogforsk.se
- Persson, The Swedish Forestry Research Institute (Skogforsk), Box 3, SE-918 21 Sävar, Sweden E-mail torgny.persson@skogforsk.se
-
Fedorkov,
The Institute of Biology of Komi Scientific Centre of the Ural Branch of the Russian Academy of Sciences (IB Komi SC UB RAS), Kommunisticheskaya St., 28, Syktyvkar, 167982, Russia
 https://orcid.org/0000-0001-7800-7534
E-mail
fedorkov@ib.komisc.ru
https://orcid.org/0000-0001-7800-7534
E-mail
fedorkov@ib.komisc.ru
-
Mullin,
The Swedish Forestry Research Institute (Skogforsk), Box 3, SE-918 21 Sävar, Sweden
 https://orcid.org/0000-0003-4924-1836
E-mail
tim.mullin@skogforsk.se
https://orcid.org/0000-0003-4924-1836
E-mail
tim.mullin@skogforsk.se

Received 22 August 2018 Accepted 10 November 2018 Published 16 November 2018
Views 54204
Available at https://doi.org/10.14214/sf.10040 | Download PDF

1 Introduction
Scots pine (Pinus sylvestris L.) is one of the most abundant and economically important forest tree species in northern parts of Europe (Mullin et al. 2011). Its natural range spans 33 degrees of latitude, or about 3700 km from the Sierra Nevada Mountains in Spain to northern Norway, and across over 10 000 km from Portugal and Scotland in the west to near the Pacific Ocean in eastern Russia (Boratyński 1991; Little and Critchfield 1969; Ruotsalainen and Persson 2013). Across such a huge range, phenology can have a profound effect on growth and climatic adaptability of a long-lived, northern tree species.
“Growth rhythm” refers to the timing and duration of annual developmental events (sensu Warwell and Shaw 2018 and references therein). Geographic variation in Scots pine traits describing growth rhythm in provenance field trials has been reported by numerous studies, mainly in Scandinavia, showing a clinal relationship between early phenology and latitude of origin (see reviews by Aitken and Hannerz 2001; Hannerz et al. 2002; Repo et al. 2001). The start of Scots pine growth in the spring is triggered mainly by accumulated heat, while cessation of growth is related to the joint effect of photoperiod and temperature (Hänninen 2016 and the many references therein). Additional environmental factors such as winter chilling may affect bud break, while soil moisture, nutrition and light quality may affect bud set (as reviewed by Howe et al. 2003).
Longitudinal differences in autumn frost hardiness among Scandinavian and northern Russian Scots pine populations have been revealed by artificial freezing tests with one-year-old seedlings. The autumn frost hardiness of Russian (continental) populations was higher than that of Scandinavian (maritime) sources from corresponding latitudes (Andersson and Fedorkov 2004). While these differences can be explained by differences in phenology, young Scots pine exhibit sylleptic shoot growth, whereas that in older trees is predominantly proleptic (sensu Hallé et al. 1978). This implies that the most meaningful assessments of phenological differences are facilitated by older trees.
Field tests of seed provenances offer a good opportunity to assess and predict the effects of environmental (climate) change on forests (Lindgren and Persson 1997; Mátyás 1994; Mátyás 1997). The traits that describe the annual growth cycle are the most informative to predict the effects of climatic changes on growth and survival (Beuker 1994; Beuker and Koski 1997; Beuker et al. 1998; Hänninen 1991). Changes in the growth rhythm of trees are likely to occur in a changing temperature environment, even if photoperiod remains stable (Warwell and Shaw 2018). More detailed information on the genetic basis of growth rhythm would help us to build more reliable models of possible adaptation processes (Savolainen et al. 2007). Although Scots pine phenology has been widely discussed, little is actually known about the genotypic and spatial variation in phenological characteristics of this species across northern Europe, from the Urals to Scandinavia.
The objectives of this study were: (1) to compare shoot phenology of Scots pine populations and plus tree progenies from Scandinavia (maritime climate origin) and northern Russia (continental climate origin) sources, under field conditions in both Scandinavia and Russia (maritime and continental growth conditions); and (2) to estimate the heritabilities of phenological parameters.
2 Materials and methods
2.1 Genetic material and field trials
The material used for this study was part of a larger provenance test series involving Scots pine populations and open-pollinated plus-tree families from Russia, Sweden and Finland. These trials were originally established at four sites in Sweden and three in the Komi Republic (Russia). Two of these trials were selected for assessment of phenology: one was a clear-cut area on a sandy silty moraine, with deep, fresh soil and no moving ground water near Bäcksjön in northern Sweden; and the second was a clear-cut area on dry, sandy soil near Syktyvkar in northern Russia (Table 1).
| Table 1. Characteristics of the Scots pine field trials in Sweden and Russia. | |||||||||
| Country | Name | Latitude, longitude | Altitude (m a.s.l.) | Continentality index | Area (ha) | Plot size | Spacing (m) | No. of seedlings | |
| 2007a | Normalb | ||||||||
| Sweden | Bäcksjön | 63°56´N, 20°21´E | 75 | 25.5 | 18.6 | 1.3 | 1×1 | 2.2×2 | 2785 |
| Russia | Syktyvkar | 61°40´N, 51°03´E | 132 | 38.5 | 31.9 | 1.1 | 1×1 | 2×2 | 2128 |
| a Continentality index calculated per Lockwood (1985). b Average over 10-year period 1998–2007 from SMHI (2018) at Bäcksjön and Novakovskiy and Elsakov (2014) at Syktyvkar. | |||||||||
According to Lockwood (1985), “continentality index” is the difference in monthly mean temperatures between the coldest (February) and warmest (July) month. As summarized in Table 1, the difference in continentality index between Bäcksjön and Syktyvkar in the year of assessment (2007) is 13 °C; this is consistent with the 10-year average difference of 13.3 °C (Novakovskiy and Elsakov 2014; SMHI 2018), so that Syktyvkar has a much more continental climate compared to Bäcksjön.
Seedling materials were raised separately for each field test site. Seeds were sown in the spring of 2001 and cultivated using similar greenhouse protocols in 90 cm3 and 108 cm3 plastic containers in Sweden and Russia, respectively. Test materials were out-planted at each site in a completely randomized single-tree plot design with 5 to 12 seedlings per entry. Test materials were planted in the spring of the second growing season from seed at Bäcksjön, while those at Syktyvkar were planted in the fall of the second season.
The material selected for assessment included 12 Swedish and 10 Russian Scots pine populations (Table 2, Fig. 1), spanning latitudinal gradients from 60–68°N and 60–64°N, respectively, as well as a total of 19 single-tree open-pollinated (OP) families collected from three stands in Sweden, and 20 OP families from two stands in Russia (Table 3, Fig. 1). At time of assessment, each entry in the population and family studies was represented by 3 to 12 seedlings and 2 to 10 seedlings, in the Swedish and Russian field trials, respectively.
| Table 2. Geographic origin of the populations studied. | ||
| Provenance designation | Latitude, longitude | Altitude (m a.s.l.) |
| Populations from Sweden | ||
| Almajärvi | 68°02´N, 23°08´E | 320 |
| Lainio | 67°40´N, 22°22´E | 350 |
| Tärendö | 67°05´N, 22°40´E | 250 |
| Harads | 66°10´N, 18°54´E | 200 |
| Arvidsjaur | 65°35´N, 19°40´E | 400 |
| Skellefteå | 65°00´N, 20°30´E | 150 |
| Ramsele | 63°30´N, 16°25´E | 400 |
| Sollefteå | 63°00´N, 17°00´E | 200 |
| Stöde | 62°25´N, 16°37´E | 200 |
| Ånge | 62°15´N, 15°55´E | 300 |
| Edsbyn | 61°30´N, 15°30´E | 400 |
| Malung | 60°30´N, 13°45´E | 450 |
| Populations from Russia | ||
| Ertom | 63°33´N, 47°50´E | 130 |
| Ertom | 63°31´N, 47°45´E | 130 |
| Mitrofanovo | 63°12´N, 56°03´E | 140 |
| Kyltovo | 62°12´N, 51°00´E | 160 |
| Komsomolsk | 62°07´N, 56°36´E | 170 |
| Ust-Kulom | 61°41´N, 53°40´E | 190 |
| Vilgort | 61°39´N, 50°46´E | 120 |
| Sherjg | 61°36´N, 54°19´E | 110 |
| Kuratovo | 60°55´N, 49°30´E | 180 |
| Koygorodok | 60°27´N, 51°00´E | 150 |
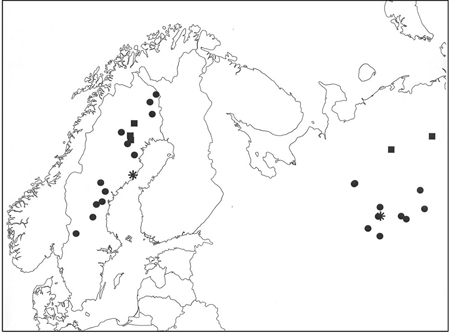
Fig. 1. Location of the Scots pine populations (●), stands with selected plus trees (■) and field trials (*).
| Table 3. Geographic origin of the open-pollinated families studied. | |||
| Stand designation | Number of OP families | Latitude, longitude | Altitude (m a.s.l.) |
| Half-sib families from Sweden | |||
| Ranesvare | 6 | 66°37´N, 20°27´E | 200 |
| Moskosel | 7 | 66°00´N, 19°59´E | 290 |
| Arvidsjaur | 6 | 65°46´N, 20°03´E | 375 |
| Half-sib families from Russia | |||
| Ust-Chilma | 11 | 65°23´N, 52°21´E | 50 |
| Usinsk | 9 | 66°05´N, 57°30´E | 75 |
2.2 Field measurements
Terminal shoot elongation was measured through the seventh growing season from seed in 2007, on nine occasions at Bäcksjön and eleven at Syktyvkar. The first measurement was performed in early May, before bud flush, by marking a reference datum 100 mm below the tip of the terminal bud. Subsequent measurements determined shoot elongation with respect to the datum. Consecutive measurements were carried out to July 7 and July 18 in Syktyvkar and Bäcksjön, respectively, and a final measurement performed in mid-September when elongation was complete.
The analysis of growth rhythm was based on proportions of shoot elongation achieved at various assessment dates relative to the total shoot elongation measured at the last occasion of the year. Relative shoot elongation at onset and at cessation were defined as that occurring on those assessment dates when the test-wide average shoot elongation was closest to 10% and 90%, respectively (Nilsson 2001).
Temperature data were obtained from weather stations located about 15 kilometres from each field trial, considered to be representative of the trial site conditions over a wide and relatively uniform topographic area. The vegetative growth period was considered to start on the fourth successive day with daily mean temperature above +5 °C. Temperature sums were accumulated from this point in degree-days (d.d.) above a +5 °C threshold. The end of the vegetative growth period was defined as the day before four successive days with daily mean temperatures below +5 °C.
2.3 Statistical analysis
The gradually increasing shoot elongation during the growing period was visualized (expressed) by fitting relative shoot elongation (y) to the cumulative heat sum (x), by the exponential expression (Nilsson 2001). Coefficients b, r and c were estimated by regression analysis (SAS 2001), to obtain growth curves from: (i) the Bäcksjön and Syktyvkar trials (all populations merged within each trial); and (ii) different population region categories (Swedish and Russian) at different trials. For these analyses, we used only data for all individuals measured from the bulk-collected populations (half-sib families excluded) that were selected for the study (Table 1).
Relative shoot elongation was further analysed according to the linear model:
![]()
where yijk is the relative shoot elongation for individual seedlings at onset (closest to 10% total elongation) or cessation (closest to 90% total elongation); si is the fixed effect of ith test site (Bäcksjön or Syktyvkar); rj is the fixed effect of the jth region of origin (Swedish or Russian); d is a regression coefficient; L is latitudinal origin of the stand (°N); and eijk is the random residual. The model was fitted by the GLM procedure in the SAS statistical package (SAS 2001). Interactions were tested but found to be not significant.
Analyses of narrow-sense heritability for relative shoot elongation were made using the half-sib material (Table 2), according to the model:
![]()
where yijklm is relative shoot elongation for individual seedlings at onset (closest to 10% total shoot elongation) or cessation (closest to 90% total shoot elongation), si is the fixed effect of the ith test site (Bäcksjön or Syktyvkar), rj is the fixed effect of the jth region of origin (Swedish or Russian); bjk is the random effect of kth stand within the ith region; fjkl is the random effect of the lth family within the jkth stand; sbijk is the interaction between the ith site and jkth stand; sfijkl is the interaction between the ith site and jklth family; and ejklm(i) is the residual error within the ith site under an assumption of different residuals. Single-site analyses were also carried out using the Model 2, but the terms for site and its interaction with stand and family were dropped (referred to as Model 3).
Estimates of variance components were obtained iteratively by the computer program ASReml (Gilmour et al. 2009). Standard errors (SEs) of the parameter estimates were calculated by first-order Taylor series approximations using the post-processing module of ASReml. Assuming that open-pollinated families account for ¼ of the total additive genetic variation, individual narrow-sense heritability (h2) was calculated from the variance components as:
![]()
![]()
where ![]() and
and ![]() are the stand, family, site-by-stand interaction, site-by-family interaction, and residual variances, respectively.
are the stand, family, site-by-stand interaction, site-by-family interaction, and residual variances, respectively.
3 Results
Five years after planting, the average survival at the Bäcksjön and Syktyvkar trials was 83% and 54%, respectively. At Bäcksjön, the low mortality was essentially random and not associated with any common cause. At Syktyvkar, trees succumbed to root damage by larvae of Melolontha hippocastani and needle damage by Phacidium infestans, and in some cases, were also damaged by hare grazing and snow breakage. Average within-trial tree height (standard deviation) was 91.6 (23.2) cm in Bäcksjön after the sixth growing season from seed, and 49.2 (20.9) cm after the seventh season in Syktyvkar.
The sigmoid growth curve model fits our measured data well (Figs. 2a and 2b), with pseudo R2 values from 0.9425 to 0.9829 for all six curves (data not shown). The required heat sum for the curve-predicted shoot elongation onset, i.e., 10% of total average elongation, was lower (about 38 d.d.) at Bäcksjön than at Syktyvkar (about 75 d.d.), Fig. 2a. Onset of growth was approximately the same for Russian and Swedish origins (Fig. 2b), at both sites. In contrast to onset, the heat sum required for growth cessation, i.e., 90% of total average elongation, was similar for both trials, i.e., 313 d.d. and 330 d.d. for Bäcksjön and Syktyvkar, respectively. Similar to onset, the difference between origins was small also for growth cessation. It should be noted that latitude of origin is not controlled in the comparisons of origin (Fig. 2b).
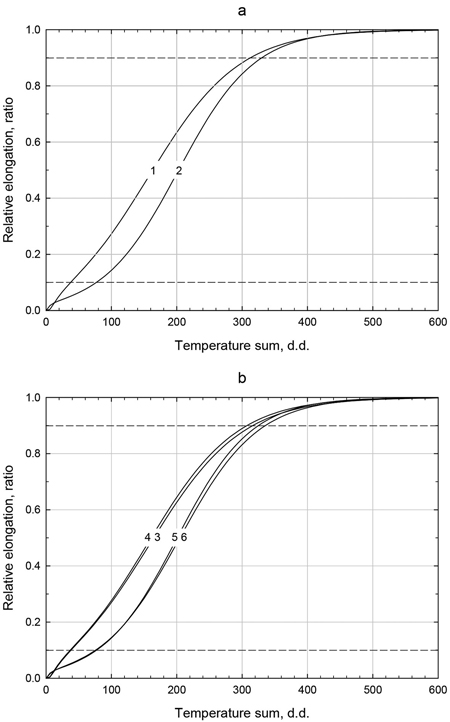
Fig. 2. Relationships between heat sum and relative shoot elongation estimated from the sigmoid function y = 1/(1 + be(rx+c/x)) based on: (a) populations at (1) Bäcksjön and (2) Syktyvkar test sites; and (b) Swedish (3, 5) and Russian (4, 6) material in Bäcksjön (3, 4) and Syktyvkar (5, 6) field trials, respectively.
The relationships between heat sum and Julian date over the growth periods at each site are shown in Fig. 3. The start of the growth period occurred at an earlier Julian day in Syktyvkar compared to Bäcksjön and the accumulation of d.d. was faster, resulting at an earlier Julian day for onset in Syktyvkar and at a greater heat sum. Growth cessation occurred at approximately the same Julian day and accumulated heat sum at both localities.
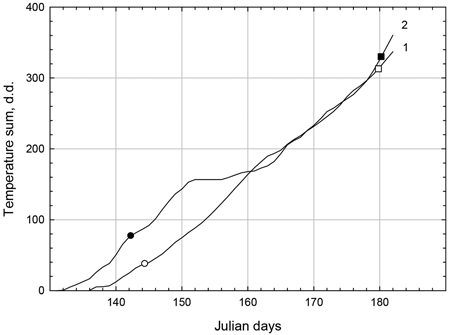
Fig. 3. Temperature sum accumulation over Julian days for populations at Bäcksjön (1) and Syktyvkar (2) test sites. The temperature sum at 10% (○, ●) and 90% (□, ■) estimated shoot elongation is indicated by symbols in Bäcksjön (○, □) and Syktyvkar (●, ■) field trials, respectively.
To include both east-west region of origin (Russian/Swedish) and latitude of origin, analyses of variance were performed according to Model 1 for populations. The actual measuring days closest to onset (10% of shoot elongation) and cessation (90% of shoot elongation) were chosen for the analyses (Table 4). According to the sigmoidal functions, the Julian days for 10 and 90% shoot elongation in Bäcksjön are 24 May and 29 June, respectively, and those for Syktyvkar are 22 May and 29 June, respectively (data not shown). For the onset of growth, the regression coefficient describes a latitude effect of 0.00728 units, showing that northern populations started earlier than those from the south (Table 5). When controlling for latitude of origin, Russian populations had about 2 percent units longer relative shoot elongation than those from Sweden at the time of onset (Table 5), as the Russian populations started elongation earlier than those from Sweden.
| Table 4. Selected date and corresponding actual mean shoot elongation representing growth onset and cessation. | ||||||
| Onset | Cessation | |||||
| Field trial | Date | Degree days | (%) | Date | Degree days | (%) |
| Syktyvkar | May 20 | 50.4 | 8.7 | July 1 | 360.4 | 93.1 |
| Bäcksjön | May 24 | 36.6 | 11.1 | June 27 | 296.3 | 88.2 |
| Table 5. Onset of growth, parameter estimates from Model 1 with p-values. | ||||
| Source | Parameter | Estimate | Standard error | p-value |
| Site | s | –0.02363a | 0.00559 | <0.0001 |
| Region of origin | r | 0.02013 | 0.00613 | 0.0012 |
| Regression coefficient for latitude | d | 0.00728 | 0.00131 | <0.0001 |
| a differences between Russian and Swedish test sites include dates of measurement and other site-specific environmental effects. | ||||
A latitudinal effect was also found for growth cessation, where northern populations terminated growth earlier (Table 6). In accordance to onset, Russian populations had longer relative shoot elongation (2.7 percent units) than those from Sweden at the time of growth cessation (93% and 88% at Syktyvkar and Bäcksjön, respectively), i.e., Russian populations terminated shoot elongation earlier than did Swedish populations (Table 6). Note that sites are also confounded with calendar date of measurement in these analyses (Tables 5 and 6).
| Table 6. Cessation of growth, parameter estimates from Model 1 with p-values. | ||||
| Source | Parameter | Estimate | Standard error | p-value |
| Site | s | 0.04869a | 0.008523 | <0.0001 |
| Region of origin | r | 0.02685 | 0.00937 | 0.0045 |
| Regression coefficient for latitude | d | 0.00932 | 0.00199 | <0.0001 |
| a differences between Russian and Swedish test sites include dates of measurement and other site-specific environmental effects. | ||||
For the family analyses, Model 2 failed to reject the null hypothesis, i.e., the SE of heritabilities for both onset and cessation were larger than the heritability estimates themselves (Table 7). Single-site analyses with Model 3 showed differences between sites. At Bäcksjön, heritability estimates for both onset and cessation were large and significant in relation to their SEs, while those at Syktyvkar were lacking significance (Table 7).
| Table 7. Narrow-sense heritabilities (h2 ± S.E.) of Scots pine phenological traits. | ||||
| Model 2 | Model 3 | |||
| Field trial | Onset | Cessation | Onset | Cessation |
| Syktyvkar (Russia) | Not estimable | 0.021 ± 0.052 | 0.084 ± 0.251 | Not estimable |
| Bäcksjön (Sweden) | Not estimable | 0.061 ± 0.151 | 0.482 ± 0.181 | 0.501 ± 0.165 |
4 Discussion
In general, traits related to phenology of Scots pine provenances from Scandinavia and northern Russia are correlated to latitude of origin. Bud burst and onset of shoot growth are triggered mainly by the accumulation of heat, while shoot growth cessation is mainly driven by the joint effect of heat accumulation and photoperiod, and shoot and needle frost- hardening is mainly governed by photoperiod (Hänninen 2016 and the many references therein). In the regions of study, northern latitudes are associated with differences in photoperiod and lower mean temperatures. Differences in altitude of origin are minor and less related to autumn frost hardiness than to latitude of origin (Sundblad and Andersson 1995).
As illustrated in Fig. 2, the onset of growth started at a lower temperature sum in the Swedish trial, indicating that a more maritime climate will promote shoot elongation to start earlier. The more maritime conditions at the Bäcksjön trial in comparison to the Syktyvkar trial, mimic global warming where winter temperatures are expected to increase more than summer temperatures (Kjellström et al. 2018), resulting in more maritime conditions.
In a warming climate, Scots pine may break bud and begin shoot elongation at an earlier Julian date due to two factors: (i) temperature sum above +5 °C will start to accumulate earlier (Kjellström et al. 2018); and (ii) the temperature sum required for growth onset may be lower (as illustrated in Figs. 2 and 3). Thus, Scots pine may suffer from spring and early summer frost under climate warming conditions, especially if these conditions also comprise a more maritime climate. Still, the faster temperature accumulation in Syktyvkar may affect the morphological stages to lag behind the temperature accumulation, such that onset may start later at a higher temperature sum (Sarvas 1972).
Hänninen et al. (1993) analysed models for growth onset with temperature as the driving parameter. The model predicted hastened onset in a warmer environment. Empirical data confirmed earlier onset, but of lower magnitude than did the model, indicating that additional driving parameters and/or more detailed calculation of accumulated temperature sum is needed. Usually, as in our investigation, heat sum calculations are imprecise. Daily mean temperatures above +5 °C are calculated here as the mean between daily maximum and minimum temperatures (irrespective of day length and difference between day and night temperatures). In addition, temperatures below +5 °C may also contribute to heat accumulation impacting phenology (e.g., Chuine 2000).
Our data showed a lower temperature-sum requirement for onset at the more maritime Bäcksjön trial, where heat accumulation was slower. The Bäcksjön trial was located more than 2 degrees of latitude further north, and it is unsure whether the slower accumulation was due to a more northern or a more maritime climate. For both sites, the mean temperature during the growing season of 2007 was slightly warmer (0.5 °C) compared to the 10-year average during 1998 to 2007 (Novakovskiy and Elsakov 2014; SMHI 2018). January, February and November were cooler, while the remaining months were warmer at both sites.
Continentality indices were higher than the 10-year average in both Bäcksjön and in Syktyvkar by a similar amount (6.9, 6.6 °C higher, respectively, Table 1). The fall of 2006 was also somewhat warmer than the 10-year average, but followed by winter temperatures well below zero. Based on the above data, we consider that the climate in 2007 did not deviate substantially from the climate normal at either site. Our data show that exactly the same material had different temperature-sum requirements in different climatic conditions.
Seedlings of Swedish and Russian origin had similar temperature-sum requirements for growth onset and cessation in both trials. More northern origins started onset and cessation at a lower temperature sum (see regression coefficients for latitude in Tables 5 and 6). The lack of difference between Swedish (on average 2 degrees more northern latitude) and Russian origins may be due to a more continental origin compensating for a southern latitude. This has also been shown for autumn frost hardiness where continental origins from Russia behaved as did Swedish origins from 4 degrees further north (Andersson and Fedorkov 2004).
Nilsson (2001) found that early onset and early cessation of shoot elongation were related to populations with a northern latitude of origin for both Scots pine and lodgepole pine (Pinus contorta Dougl. var. latifolia Engelm.), although to a smaller extent for lodgepole pine. In contrast, the maximum growth in June was not related to latitude of origin for either species. Nilsson (2001) also showed that the temperature sum for onset and cessation varied between years, indicating that the difference between Syktyvkar and Bäcksjön in temperature sum at onset may be explained partly by a year effect. Still, the earlier onset and cessation in our study for continental origins (Russia) compared to maritime (Sweden), controlling for latitude of origin, were stable at both sites.
Chuine et al. (2006) found few, or no differences, in response of shoot elongation to temperature among widely distributed provenances of lodgepole pine and western white pine (Pinus monticola L.), respectively. Phenology differences were instead related to differences in the number of internodes set the preceding summer. However, the cell growth rate response started at lower temperatures for lodgepole pine than for western white pine, showing species-specific responses to temperature.
In our study, more northern origins started onset and cessation at a lower temperature sum, i.e. at an earlier date. Notivol et al. (2007) also found latitudinal differences in number of days to growth initiation (from sowing) and growth cessation when studying first-year growth of Scots pine under greenhouse conditions. A latitudinal cline, with northern populations being earlier, was observed for cessation, while for growth initiation such a cline was lacking.
The across-site analyses in Model 2 showed non-significant or non-estimable heritability estimates for onset and cessation, while single-site analyses (Model 3) showed that heritability estimates from Bäcksjön were significant and of similar magnitude as other phenology traits in Scots pine (Persson et al. 2010). Notivol et al. (2007) also found high heritabilities for both growth initiation (h2 = 0.64) and cessation (h2 = 0.71) in their study of first year growth of Scots pine, which is in line with our results from the Bäcksjön trial. In Syktyvkar, the additive variance was either not significant or non-estimable for onset and cessation, respectively. While heritability in Bäcksjön shows potential to improve adaptation to new climatic conditions by selection and breeding, the lack of significance in Syktyvkar may be explained by fewer degrees of freedom available from fewer seedlings per family.
Acknowledgements
This work was supported by TC4F (Trees and Crops for Future, Sweden); the European Community’s Seventh Framework Programme (FP7/2007–2013) under grant agreement n° 211868 (Project Noveltree); and Föreningen Skogsträdsförädling (The Swedish Tree Breeding Association). The authors are grateful for the helpful reviews provided by two referees that greatly improved the manuscript.
References
Aitken S.N., Hannerz M. (2001). Genecology and gene resource management strategies for conifer cold hardiness. In: Bigras F.J., Colombo S.J. (eds.). Conifer cold hardiness. Kluwer Academic. p. 23–53. https://doi.org/10.1007/978-94-015-9650-3_2.
Andersson B., Fedorkov A. (2004). Longitudinal differences in Scots pine frost hardiness. Silvae Genetica 53(1–6): 76–79. https://doi.org/10.1515/sg-2004-0014.
Beuker E. (1994). Adaptation to climatic changes of the timing of bud burst in populations of Pinus sylvestris L. and Picea abies (L.) Karst. Tree Physiology 14(7–8–9): 961–970. https://doi.org/10.1093/treephys/14.7-8-9.961.
Beuker E., Koski V. (1997). Adaptation of tree populations to climate as reflected by aged provenance tests. World Series (IUFRO) 6: 103–108.
Beuker E., Valtonen E., Repo T. (1998). Seasonal variation in the frost hardiness of Scots pine and Norway spruce in old provenance experiments in Finland. Forest Ecology and Management 107(1–3): 87–98. https://doi.org/10.1016/S0378-1127(97)00344-7.
Boratyński A. (1991). Range of natural disturbution. In: Giertych M., Mátyás C. (eds.). Genetics of Scots pine. Elsevier Science Publishers. p. 19–30.
Chuine I. (2000). A unified model for budburst of trees. Journal of Theoretical Biology 207(3): 337–347. https://doi.org/10.1006/jtbi.2000.2178.
Chuine I., Rehfeldt G.E., Aitken S.N. (2006). Height growth determinants and adaptation to temperature in pines: a case study of Pinus contorta and Pinus monticola. Canadian Journal of Forest Research 36(5): 1059–1066. https://doi.org/10.1139/x06-005.
Gilmour A.R., Gogel B.J., Cullis B.R., Thompson R. (2009). ASReml User Guide, Release 3.0. VSN International Ltd., Hemel Hempstead, UK.
Hallé F., Oldeman R.A.A., Tomlinson P.B. (1978). Tropical trees and forests: an architectural analysis. Springer-Verlag, Berlin. https://doi.org/10.1007/978-3-642-81190-6.
Hannerz M., Thorsen A., Mattsson S., Weslien J. (2002). Pine weevil (Hylobius abietis) damage to cuttings and seedlings of Norway spruce. Forest Ecology and Management 160(1–3): 11–17. https://doi.org/10.1016/S0378-1127(01)00467-4.
Hänninen H. (1991). Does climatic warming increase the risk of frost damage in northern trees? Plant, Cell & Environment 14(5): 449–454. https://doi.org/10.1111/j.1365-3040.1991.tb01514.x.
Hänninen H. (2016). Boreal and temperate trees in a changing climate: modelling the ecophysiology of seasonality. Biometeorology. Springer Science Media, Dordrect, the Netherlands. https://doi.org/10.1007/978-94-017-7549-6.
Hänninen H., Kellomäki S., Laitinen K., Pajari B., Repo T. (1993). Effect of increased winter temperature on the onset of height growth of Scots pine: a field test of a phenological model. Silva Fennica 27(4): 251–257. https://doi.org/10.14214/sf.a15679.
Howe G.T., Aitken S.N., Neale D.B., Jermstad K.D., Wheeler N.C., Chen T.H.H. (2003). From genotype to phenotype: unraveling the complexities of cold adaptation in forest trees. Canadian Journal of Botany 81(12): 1247–1266. https://doi.org/10.1139/b03-141.
Kjellström E., Nikulin G., Strandberg G., Christensen O.B., Jacob D., Keuler K., Lenderink G., van Meijgaard E., Schär C., Somot S., Sørland S.L., Teichmann C., Vautard R. (2018). European climate change at global mean temperature increases of 1.5 and 2 degrees C above pre-industrial conditions as simulated by the EURO-CORDEX regional climate models. Earth System Dynamics 9: 459–478. https://doi.org/10.5194/esd-9-459-2018.
Lindgren D., Persson A. (1997). Vitalization of results from provenance tests. In: Mátyás C. (ed.). Perspectives of forest genetics and tree breeding in a changing world. IUFRO World Series. IUFRO. p. 73–85.
Little E.L., Jr., Critchfield W.B. (1969). Subdivisions of the genus Pinus (Pines). USDA Forest Service, Washington, D.C.
Lockwood J.G. (1985). World climatic systems. Hodder Arnold, London, UK.
Mátyás C. (1994). Modeling climate change effects with provenance test data. Tree Physiology 14(7–8–9): 797–804. https://doi.org/10.1093/treephys/14.7-8-9.797.
Mátyás C. (1997). Effects of environmental change on the productivity of tree populations. World Series (IUFRO) 6: 109–121.
Mullin T.J., Andersson B., Bastien J.-C., Beaulieu J., Burdon R.D., Dvorak W.S., King J.N., Kondo T., Krakowski J., Lee S.J., McKeand S.E., Pâques L., Raffin A., Russell J., Skrøppa T., Stoehr M., Yanchuk A. (2011). Economic importance, breeding objectives and achievements. In: Plomion C., Bousquet J., Kole C. (eds.). Genetics, genomics and breeding of conifers. Genetics, Genomics and Breeding of Crop Plants. Science Publishers, Enfield, NH. p. 40–127.
Nilsson J.-E. (2001). Seasonal changes in phenological traits and cold hardiness of F1-populations from plus-trees of Pinus sylvestris and Pinus contorta of various geographical origins. Scandinavian Journal of Forest Research 16(1): 7–20. https://doi.org/10.1080/028275801300004361.
Notivol E., García-Gil M., Alía R., Savolainen O. (2007). Genetic variation of growth rhythm traits in the limits of a latitudinal cline in Scots pine. Canadian Journal of Forest Research 37(3): 540–551. https://doi.org/10.1139/X06-243.
Novakovskiy A., Elsakov V. (2014). Hydrometeorological database (HMDB) for practical research in ecology. Data Science Journal 13: 57–63. https://doi.org/10.2481/dsj.IFPDA-10.
Persson T., Andersson B., Ericsson T. (2010). Relationship between autumn cold hardiness and field performance in northern Pinus sylvestris. Silva Fennica 44(2): 255–266. https://doi.org/10.14214/sf.152.
Repo T., Nilsson J.-E., Rikala R., Ryyppö A., Sutinen M.-L. (2001). Cold hardiness of Scots pine (Pinus sylvestris L.). In: Bigras F., Colombo S. (eds.). Conifer cold hardiness, vol 1. Tree Physiology. Springer Netherlands. p. 463–493. https://doi.org/10.1007/978-94-015-9650-3_17.
Ruotsalainen S., Persson T. (2013). Scots pine – Pinus sylvestris. In: Mullin T.J., Lee S.J. (eds.). Best practices for tree breeding in Europe. Skogforsk (The Forestry Research Institute of Sweden), Uppsala. p. 49–63.
Sarvas R. (1972). Investigations on the annual cycle of development of forest trees. Active period. Communicationes Instituti Forestalis Fenniae 76: 1–110.
SAS (2001). The SAS system for Windows, version 8.0. SAS Institute Inc., Cary, NC.
Savolainen O., Bokma F., Knürr T., Kärkkäinen K., Pyhäjärvi T., Wachowiak W. (2007). Adaptation of forest trees to climate change. In: Koskela J., Buck A., Teissier du Cros E. (eds.). EUFORGEN Climate Change and Forest Genetic Diversity: implications for sustainable forest management in Europe, Paris, France, 15–16 March 2006. Bioversity International. p. 19–30.
SMHI (2018). Öppna data – meteorolgiska observationer. Swedish Meteorlogical and Hydrolgical Institute. https://opendata-download-metobs.smhi.se/explore/. [Cited October 2018].
Sundblad L.G., Andersson B. (1995). No difference in frost hardiness between high and low altitude Pinus sylvestris (L.) offspring. Scandinavian Journal of Forest Research 10(1–4): 22–26. https://doi.org/10.1080/02827589509382862.
Warwell M., Shaw R. (2018). Phenotypic selection on growth rhythm in whitebark pine under climatic conditions warmer than seed origins. Journal of Evolutionary Biology 31(9): 1284–1299. https://doi.org/10.1111/jeb.13301.
Total of 34 references.

