Proximity to charred logs in burned forests likely affects decomposition processes in the soil
Čugunovs M., Tuittila E.-S., Kouki J. (2020). Proximity to charred logs in burned forests likely affects decomposition processes in the soil. Silva Fennica vol. 54 no. 1 article id 10084. https://doi.org/10.14214/sf.10084
Highlights
- Standardised organic substrate decomposition was tentatively observed to be faster adjacent to non-charred downed logs than away from the logs or adjacent to charred logs
- A spatial linkage was observed between non-charred logs and decomposition in the soil in burned boreal forests
- Proximity to a charred log may provide a micro-environment where decomposition rates differ from the surrounding forest soil.
Abstract
We studied the spatial decomposition rates of standardised organic substrates in soils (burned boreal pine-dominated sub-xeric forests in eastern Finland), with respect to charred and non-charred coarse woody debris (CWD). Decomposition rates of rooibos plant litter inside teabags (C:N = 42.870 ± 1.841) and pressed-sheet Nordic hardwood pulp (consisting of mainly alpha-cellulose) were measured at 0.2 m distance from 20 charred (LC0.2) and 40 non-charred logs (LNC0.2). We also measured decomposition at 60 plots located 3–10 m away from downed logs (L3,10). The rooibos decomposition rate constant ‘k’ was 8.4% greater at the LNC0.2 logs than at the L3,10 or LC0.2 logs. Cellulose decomposed more completely in 1 micron mesh bags at LNC0.2 (44% of buried bags had leftover material) than at LC0.2 (76%) or L3,10 (70%). Decomposition of cellulose material was rapid but varied greatly between sampling plots. Our results indicate that decomposition of the standardised organic matter was more rapid close to CWD pieces than further away. However, only the plots located near non-charred logs (LNC0.2) exhibited high decomposition rates, with no corresponding increase observed at the charred logs (LC0.2). This suggests a possible noteworthy indirect effect of forest burning on soil organic matter (SOM) decomposition rates close to charred CWD after forest fires. We urge for more studies on this tentative observation as it may affect the estimates on how fires affect carbon cycling in forests.
Keywords
coarse woody debris;
forest fire;
prescribed burning;
cellulose decomposition;
tea-bag method
-
Čugunovs,
University of Eastern Finland, School of Forest Sciences, Yliopistokatu 7, P.O. Box 111, FI-80101 Joensuu, Finland
E-mail
mihails.cugunovs@gmail.com

-
Tuittila,
University of Eastern Finland, School of Forest Sciences, Yliopistokatu 7, P.O. Box 111, FI-80101 Joensuu, Finland
 https://orcid.org/0000-0001-8861-3167
E-mail
eeva-stiina.tuittila@uef.fi
https://orcid.org/0000-0001-8861-3167
E-mail
eeva-stiina.tuittila@uef.fi
-
Kouki,
University of Eastern Finland, School of Forest Sciences, Yliopistokatu 7, P.O. Box 111, FI-80101 Joensuu, Finland
 https://orcid.org/0000-0003-2624-8592
E-mail
jari.kouki@uef.fi
https://orcid.org/0000-0003-2624-8592
E-mail
jari.kouki@uef.fi
Received 23 November 2018 Accepted 17 January 2020 Published 11 February 2020
Views 76345
Available at https://doi.org/10.14214/sf.10084 | Download PDF

1 Introduction
Fire is an important disturbance agent across forests in various landscapes (Pinchot 2011). In particular, large-scale and severe wildfires in boreal forests often dramatically change the landscape and impact the carbon cycle (Stocks et al. 2001). Nowadays, wildfires in Fennoscandian boreal forests are rare and well-controlled (Wallenius 2011), although silvicultural and restoration burnings are practiced annually (Similä and Junninen 2012). In protected areas, forests are burned to safeguard biodiversity and to re-create natural features, such as dead wood (Kouki 2016). It is currently recommended that prescribed forest burnings are also maintained in managed forests, to protect biodiversity and overall ecosystem integrity (Heikkala et al. 2016). Of the three types of forest burning, wildfire is generally considered the most severe, followed by silvicultural burning and restoration burning (Čugunovs et al. 2017).
Dead wood is an ecologically important part of natural boreal forests (Harmon et al. 1986; Sippola et al. 1998; Siitonen et al. 2000; Kunttu et al. 2015) and burning often creates charred and non-charred coarse woody debris (CWD) as trees are killed and fall over (Heikkala et al. 2014). Dead wood (CWD and slash) that is present on the ground before burning can also increase the severity of the burn by providing additional fuels (Knapp et al. 2005; Čugunovs et al. 2017). Coarse woody debris is known to host a multitude of forest species, such as polypore fungi (Suominen et al. 2018) and saproxylic beetles (Similä et al. 2003). Coarse woody debris also affects soil microenvironmental conditions in its vicinity, for example, by buffering moisture levels (Goldin and Hutchinson 2014) and changing the local temperature regime (Brown and Naeth 2014). Furthermore, CWD is the link where organic matter and nutrients are transferred from the tree biomass back to the soil (Laiho and Prescott 1999, 2004). Therefore, CWD can alter the decomposition of soil organic matter (SOM) and thus further drive changes in the soil-vegetation-atmosphere carbon cycle, site fertility and overall ecosystem functioning (Gonzalez-Polo et al. 2013; Stutz and Lang 2017).
Charred CWD that originates from burning may have different effects on the ecosystem and on SOM decomposition than non-charred CWD. The surface of charred CWD is more recalcitrant to decomposition (Preston and Schmidt 2006), and the albedo is lower due to the darker surface colour leading to increased local temperature sums (Gleason et al. 2013). Charred CWD also hosts different decomposer fungal species to non-charred CWD (Suominen et al. 2018), and hence the decomposer community and its functioning may also be different.
In this study, we focus on the role that charred wood may have on soil decomposition processes in the proximity of burned logs. We investigate the effects of location on SOM decomposition with respect to charred and non-charred CWD pieces created during silvicultural burnings. Based on previous knowledge (described above), we hypothesise that SOM decomposition in burned and cut forests will be altered closer to CWD pieces, likely due to an input of organic matter from logs to the nearby soil. We also hypothesise that decomposition rates will be different between positions located near charred and non-charred CWD, because of the differences in ecological properties and decomposer assemblages on the charred and non-charred wood.
2 Material and methods
2.1 Study sites
This study was performed in three sites (replicates) located within 10 km of each other in Lieksa municipality, eastern Finland. The sites are part of the larger “FIRE” experiment (Fire and retention trees in facilitating biodiversity in boreal forests) that includes a total of 24 experimental forests (see Hyvärinen et al. (2005), Heikkala et al. (2014), and Kouki (2016), http://forest.uef.fi/jarikouki/project_fire.htm). Initially, the three sites sampled for the current study were randomly allocated to specific harvest and burning treatments, and all three sites were subjected to clearcutting with 50 m3 ha–1 green tree retention, followed by prescribed burning. This treatment is considered equivalent to silvicultural prescribed burning (Čugunovs et al. 2017) with a notable level of green tree retention. The three sampled forest stands, each 3–5 ha in size (stand background data given in Table 1), are located at the southern edge of the middle boreal zone (Ahti et al. 1968). Annual average temperature in the study area is 2 °C, with a January mean of –12 °C and a July mean of 15.8 °C. Temperature sum in the study area is 1000–1100 degree-days (Ilmatieteen laitos 2016). Annual precipitation is on average 650–700 mm (Ilmatieteen laitos 2020).
| Table 1. Characteristics of the study sites – tree stand and soil parameters. | |||||||
| Parameter | Site | N obs. | Mean | Min | Max | Stdev | CV, % |
| Tree stand volume, m3 ha–1 in 2000, pre-harvest | 1 | 1 | 331.2 | ||||
| 2 | 1 | 233.7 | |||||
| 3 | 1 | 179.0 | |||||
| Tree stand basal area, m3 ha–1 in 2000, pre-harvest | 1 | 1 | 32.2 | ||||
| 2 | 1 | 26.6 | |||||
| 3 | 1 | 19.0 | |||||
| Soil organic layer thickness at sampling time in 2016, cm data by S. Tatsumi, unpublished | 1 | 3 | 3.5 | 3.0 | 4.5 | 0.85 | 24 |
| 2 | 6 | 2.5 | 1.5 | 4.0 | 1.05 | 42 | |
| 3 | 6 | 4.2 | 2.5 | 8.0 | 2.18 | 53 | |
| Soil organic layer thickness after burning and harvest in 2001, cm (data published in Laamanen 2002) | 1 | 191 | 2.4 | 0 | 7.5 | 1.25 | 52 |
| 2 | 152 | 2.9 | 0.3 | 7.6 | 1.35 | 46 | |
| 3 | 198 | 3.3 | 0.4 | 8.5 | 1.55 | 47 | |
| Soil organic layer pHH2O at sampling time in 2016, data by S. Tatsumi, unpublished | 1 | 3 | 4.6 | 4.4 | 4.8 | 0.20 | 4 |
| 2 | 6 | 4.5 | 4.0 | 5.0 | 0.38 | 8 | |
| 3 | 6 | 4.6 | 4.3 | 5.0 | 0.32 | 7 | |
| Soil organic layer water content when burying the tea bags in 2016, percent of dry soil weight (Tatsumi, unpublished) | 1 | 3 | 92 | 62 | 140 | 42 | 45 |
| 2 | 6 | 130 | 29 | 212 | 72 | 56 | |
| 3 | 6 | 157 | 65 | 335 | 116 | 74 | |
| N obs. – total number of observations for parameter per site, Mean – sample arithmetic mean, Min – sample minimum value, Max – sample maximum value, Stdev – sample standard deviation, CV, % – coefficient of variance. All the study sites (1,2,3) were burned clearcut sites with 50 m3 ha–1 green tree retention, treatments executed in 2001. Approximate coordinates (with spatial accuracy of ca. 10 km) of the study sites´ locations (based on EUREF-FIN coordinate reference system): Site 1 – 63°18´N, 30°30´E, Site 2 – 63°20´N, 30°39´E, Site 3 – 63°16´N, 30°48´E. Soil organic layer thickness after burning and harvest in 2001 based on Laamanen J. (2002). Tulen voimakkuuden vaikutus metsikön pienalaiseen vaihteluun. [The effect of fire severity on small-scale spatial heterogeneity in forest stands]. MSc thesis. University of Joensuu, Faculty of Forest Sciences, Finland. 44 p. + 12 app. | |||||||
Prior to the experimental stand-level harvest and burning treatments, the experimental sites were 150-year-old coniferous sub-xeric heath forest and belonged to the Empetrum-Vaccinium forest site type in the Finnish forest type classification system (sensu Hotanen et al. 2008). The stands were dominated by Scots pine (Pinus sylvestris L.) and Norway spruce (Picea abies (L.) H. Karst.). Birch trees (Betula pendula Roth and Betula pubescens Ehrh.) were also present in small numbers. The International Plant Names Index (International Plant Names Index 2004) was used for taxonomic reference. Some minor, selective, single-tree harvesting was done in the sites during the late 1800s and early 1900s, but otherwise no forest management had taken place before the experimental burnings, and the sites had never been clearcut (Hyvärinen et al. 2006). The harvests were carried out during the winter of 2000/2001, and the prescribed burnings were conducted over two consecutive days (under similar weather conditions) at the end of June 2001 (Hyvärinen et al. 2005). The dynamics of trees 11 years after the burnings is reported in detail by Heikkala et al. (2014) and Hämäläinen et al. (2016).
The three forest sites used in this study featured ecological conditions highly typical of the forest type studied and were deliberately selected to serve as replicates for the treatments (locations with respect to logs). The purpose of replication was not to control or select exactly similar forest characteristics for treatments and sampling but rather to allow the ecological variation that typically occurs in similar forest types (Table 1). This sampling coverage was achieved mainly by random allocation of treatments to the study forests, although for logistic reasons, the number of stand-level replications in a large-scale experiment is bound to remain rather low. All three sites were located in the same region within 10 km of each other, but with a between-replicate distance of >3 km to ensure landscape-level independence between replicates.
2.2 Decomposition data collection
We measured decomposition rates of standard substrates using the tea-bag method (Keuskamp et at. 2013), which is a cost-effective and widely applied way to collect uniform decomposition data across ecosystems by using standardised store-bought tea bags. The method entails burying two tea bags at each sampling point: one rooibos and one green tea bag, which differ in their decomposability, for approximately 90 days. The tea bags used in this method and in our study were manufactured by Lipton, Unilever corporation. The green tea consisted of 89% green tea, and the rooibos material consisted of 93% rooibos: both were supplemented with natural flavouring (Keuskamp et al. 2013). A mesh size of 0.25 mm allowed microorganisms and mesofauna to enter the bags but excluded macrofauna (Keuskamp et al. 2013). The series number for Lipton green tea bags was EAN: 87 22700 05552 5, and for Lipton rooibos bags – EAN: 87 22700 18843 8. Based on the measurements by the tea bag method authors of a set of the standardised tea bags, the C:N ratio (mean and standard deviation) of green tea is 12.229 ± 0.129, and of rooibos material –42.870 ± 1.841 (Keuskamp et al. 2013). Further physical and chemical parameters of the standardised teabag substrates can be found in Keuskamp et al. (2013).
By using two litter types with different decomposition rates (faster-decomposing green tea and more recalcitrant rooibos), it is possible to estimate both the decomposable fraction of the litter and the decomposition rate constant without building a time series. Instead, we use a snapshot-type approach by measuring litter decomposition (green tea and rooibos) after ca. 90 days. This method also allows an examination of the environmental conditions that lead to partial stabilisation of the labile litter fraction into the more recalcitrant pool (stabilisation factor).
For the tea bag method, one Lipton green tea and one Lipton rooibos bag per sample plot is buried in the soil at the desired depth (usually 8 cm or at the organic-mineral interface). Then, the tea bags are recovered after ca. 90 days, oven-dried, and measured for substrate weight loss. From the difference in tea bag substrate weights (after vs. before), the incubation time and the chemical composition of the green tea and rooibos litters, the decomposition rate constant ‘k’ and stabilisation factor ‘S’ can be calculated using the equations provided below. A detailed explanation of the method is provided in Keuskamp et al. (2013).
To quantify the impact of spatial position on decomposition rates in relation to downed logs, we buried tea bag pairs at 40 sampling plots in each of the three sites. In each site, we chose 20 sampling plots (LC0.2, LNC0.2) adjacent to charred and non-charred downed logs (20 logs per site). The logs were at least 15 cm in diameter and had >30 cm uninterrupted log-ground contact. In each site, we also placed 20 sampling plots at a minimum distance of 10 m from the nearest log (L10). In one of the three sites with abundant downed logs, we had to reduce the minimum distance to 3 m (L3). In each site, there was a total of 40 sampling plots with 80 tea bags buried.
The 20 logs selected per site (60 in total) were sampled for length, diameter, surface charring (Yes/No), proportion of surface charred, and decomposition stage based on knife penetration depth and physical integrity (on an ordinal scale from 1 to 5; see Renvall 1995). We also attempted to identify the species of the logs whenever possible. The logs used in the sampling were mostly of natural origin, but no distinction was made between cut and natural logs in data collection. Some logs were probably on the ground before the experimental burnings were carried out in 2000/2001, while many logs were retention trees that fell over after the burning was carried out.
Of the 60 logs sampled, 16 were identified as Scots pine, 9 as Norway spruce, and one as birch. The remaining 34 logs could not be identified due to the advanced phase of their decomposition (as the bark needed for identification was absent). However, it is likely that the majority of the unidentified logs were Scots pine or possibly the occasional spruce but not birch as they tend to decompose more rapidly. The forest stand that produced the CWD was highly dominated by Scots pine. The mean diameter of the sampled logs was 24.2 cm (S.D. 6.2 cm). Of the 60 sampled logs, 20 were charred and 18 belonged to decay class 1, 14 logs belonged to decay class 2, 15 logs belonged to decay class 3, 6 logs belonged to decay class 4, and 7 logs belonged to decay class 5. The log decay stage classification is based on Renvall (1995). The log decay classes were determined by the depth to which a knife blade penetrated into the decaying log. In decay class 1, the wood is hard, bark almost intact, and the pushed knife penetrates only a few mm into the wood; in decay class 2, the wood is fairly hard, and the knife penetrates ca. 1–2 cm into the wood, with Scots pine already losing most of its bark, and Spruce bark starting to break up; in decay class 3 the wood is fairly soft, knife penetrates fairly easily ca. 3–5 cm into the wood; in decay class 4 the wood is soft, the whole blade of the knife easily penetrates into the wood, trunks extensively decayed and covered by bryophytes and lichens; in decay class 5 the wood is very soft, almost completely decomposed and disintegrates easily down between fingers, the trunk considerably shrunken and almost totally covered with bryophytes, lichens and/or dwarf shrubs (summarized from Renvall 1995). The method is widely applied in forest inventories and, e.g., in a slightly modified version, also in the National Forest Inventory in Finland.
We buried the tea bag pairs at the interface of the organic and mineral soil layers at each of the 40 sample plots per site, leading to variable depth of burial depending on the thickness of the moss and humus layer. The residence time of tea bags in the soil was 79–80 days. Tea bags were incubated in the field in summer 2016, i.e. 15 years after experimental burning. After tea bag retrieval, we followed the original laboratory protocol for the analysis (Keuskamp et al. 2013), with the exception that we dried the tea bags at 60 °C instead of 70 °C.
In addition to the tea bag index method, we used decomposition bags containing cellulose material with three different mesh sizes (1 mm, 50 μm, 1 μm), to further study the impact of spatial position in relation to downed logs. The cellulose pulp used in this experiment was a Nordic hardwood dissolving pulp obtained from the Stora Enso Uimaharju pulp mill. The feedstock for pulp production was 90% silver birch and 10% European aspen with ca. 88% alpha-cellulose content, the remainder being fragments of hemicelluloses and trace amounts of lignin, wood extractives and inorganic compounds. The cellulose pulp was pressed into sheets of ca. 3 mm thickness and these sheets were cut into ca. 7 × 7 cm squares and put into the mesh bags.
We placed sets of three cellulose pulp sheets, each within a bag with different mesh size at every sampling plot next to the tea bags, at the interface of the organic and mineral soil layers (40 sampling plots and 120 cellulose bags per site). The varying mesh size is expected to separate decomposing agents entering the bags. A mesh size of 1 mm would let in bacteria, fungi and some soil fauna, as well as fine roots of grasses and other plants. The 50 μm mesh size would exclude soil fauna and all but the smallest fine roots, while letting fungal mycelia and bacteria enter. The smallest mesh size (1 μm) is expected to shield out fungal mycelia, with mainly bacteria decomposing the cellulose inside the bags. The bags were ca. 10 × 10 cm in size and each included ca. 3–4 grams of cellulose material (a roughly square piece of pressed-sheet cellulose). They were buried on 1–2.8.2016 and retrieved on 28–29.9.2017, for a total field residence time of approximately 14 months (ca. 15–16 years after the experimental burnings).
2.3 Data analysis
To obtain the decomposition rate constant ‘k’ for each sampling point we fitted function

where:
![]()
where Ar is the decomposable fraction of rooibos litter, Hr is hydrolysable fraction of rooibos litter, and S is the stabilisation factor, where Wr is the difference between the initial and final rooibos litter weight:
![]()
and where ‘days’ is the incubation time in days of each tea bag pair.
To obtain the litter stabilisation factor (S parameter), which describes the inhibiting effect of environmental conditions on the decomposition of the labile fraction of litter (Keuskamp et al. 2013), we fitted function:

where ag is the decomposable fraction and Hg is the hydrolysable fraction of green tea, where:

where Mgreen_End is the mass of remaining green tea after incubation, and Mgreen_Init is the initial mass of green tea before incubation. The values for Hr and Hg were taken from Keuskamp et al. (2013).
Thus, the decomposable fraction and stabilisation factor ‘S’ is first taken from the decomposition dynamics of green tea litter (Eqs. 4–5) and then linked to the decomposition dynamics of rooibos litter (Eqs. 1–3) to obtain the decomposition rate constant ‘k’ from simultaneous decomposition of rooibos litter and green tea over ca. 90 days, as per standard protocol (the incubation time can be lower depending on e.g. field conditions, Eq. 1).
The ‘k’ and ‘S’ parameters obtained from the tea bags were analysed with a linear mixed-effects model using the nlme package (Pinheiro et al. 2017) in the R software (R Core Team 2013) for differences between positions next-to-charred-logs, next-to-non-charred-logs and away-from-logs (LC0.2, LNC0.2, L3,10). The three forest stands sampled were designated as random groups in the mixed-effects model. The pairwise comparisons for the treatment factor levels were based on Tukey post-hoc t-tests, executed in multcomp package (Hothorn et al. 2008) in R software (R Core Team 2013).
For the cellulose decomposition bags, percent remaining mass was similarly compared between locations with respect to log proximity (LC0.2, LNC02., L3,10), using a linear mixed-effects model. The model was employed separately for the three decomposition bag mesh sizes. For the 1 mm mesh size, the data were log-transformed to correct for the non-normality of residuals. In addition, the probability of cellulose material “surviving” (i.e. some left after the decomposition period) in the bags was tested by a random-effect generalised binomial model with a logit link function (“lme4” package in R; Bates et al. 2015). Tukey Honest Significant Difference tests were used within R software for pairwise comparisons of remaining cellulose mass and cellulose survival in bags by mesh size and treatment.
3 Results
3.1 Log effects on substrate decomposition
We found no difference in tea bag rooibos litter decomposition rate ‘k’ [F(3,52) = 0.4273, p = 0.7342], or cellulose mass loss [F(2,30) = 1.951, p = 0.1598] at LC0.2 and LNC0.2 (sample plots nearby logs) between logs of different species (i.e. Scots pine, Norway spruce, birch). We also found no difference in tea bag rooibos litter decomposition rate ‘k’ [F(4,51) = 0.5858, p = 0.6744], or cellulose mass loss [F(4,28) = 0.6163, p = 0.6545] at LC0.2 and LNC0.2 between logs of different decay classes.
3.2 Log effects on tea bag litter decomposition
We found a clear indication towards the effect of sample plot position with respect to downed logs on the tea bag litter decomposition rate constant ‘k’, [F(2,102) = 2.3505, p = 0.1005]. The decomposition of rooibos litter over the 79–80 day field incubation period was nearly statistically significantly faster at LNC0.2 than at L3,10, the statistical significance level or the probability to commit a Type-I error being close to the conventional α-value of 0.05 (Fig. 1a, pairwise Tukey t-test p-value at 0.0787, z-value at –2.15); decomposition rate parameter ‘k’ of rooibos litter was 8.4% greater at LNC0.2 than at L3,10. Rooibos decomposition rates were not at all significantly different at LC0.2 and L3,10 ([z = –0.976, p = 0.5893], Fig. 1a), indicating that a positive “log-effect” on decomposition was not evident close to the charred logs.
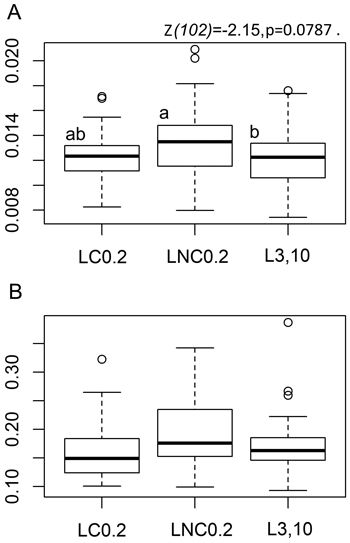
Fig. 1. Tea bag parameter boxplots by position with respect to logs. A: Boxplots for rooibos teabag litter decomposition rate constant - ‘k’ parameter, B: Boxplots for the stabilisation factor showing the inhibiting effect of environmental conditions on the decomposition of the labile fraction of green tea litter - ‘S’ parameter. LC0.2 = position next to charred logs, 0.2 m from log-ground contact zone, LNC0.2 = position next to non-charred logs, 0.2 m from log-ground contact zone, L3,10 = position away from logs, 3 or 10 m to the nearest downed log. Lower case letters denote pairwise differences between the positions based on post-hoc Tukey multiple comparison t-tests. The boxplots show aggregated data from three replicate forest sites used in the study, 40 sample plots in each of the three sites (20 near logs and 20 further away from logs) with one green tea bag and one rooibos bag per sample plot. Tea-bag incubation time in the field was 79–80 days.
The environmental stabilisation of the labile litter fraction, as measured by decomposition of green tea material, tended to be greater at LNC0.2 than at LC0.2 or at L3,10 (Fig. 1b), [F(2,111) = 2.1328, p = 0.1233]. The related differences in the ‘S’ parameter (12% and 10% respectively) would have been, however, statistically significant only at risk levels of p = 0.225 (z-value at 1.643) and p = 0.149 (z-value at –1.857) respectively.
Overall, the patterns in rooibos and green tea material decomposition (‘k’ and ‘S’ parameters) were similar over the three different treatments (Fig. 1a–b).
3.3 Log effects on cellulose decomposition
After approximately 14 months of residence time in the field, ca. 40% of the buried decomposition bags still contained some material. There was no difference between treatments in the amount of cellulose material left in bags within the same mesh size (Fig. 2, for 1 mm bags [F(2,14) = 1.939, p = 0.181], for 50 micron bags [F(2,45) = 0.372, p = 0.691] and for 1 micron bags [F(2,72) = 0.613, p = 0.544]). There was also no difference in the amount of cellulose left between the three mesh sizes either for all the treatments pooled ([F(2,357) = 0.215, p = 0.807]), or in the analysis by treatment type (for LC0.2 [F(2,60) = 0.134, p = 0.875], for LNC0.2 [F(2,93) = 0.031, p = 0.97], and for L3,10 [F(2,117) = 1.081, p = 0.343]), based on general linear models (data not shown).
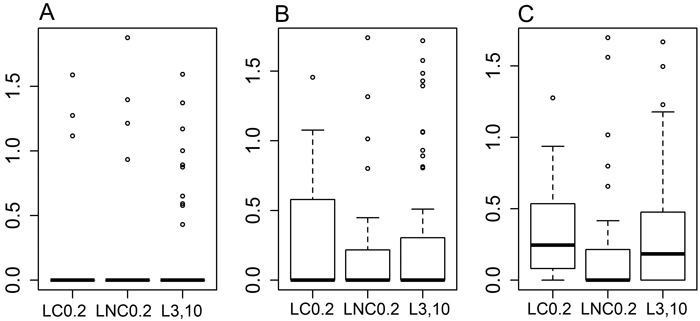
Fig. 2. Boxplots for the proportion of cellulose mass (%) remaining in the decomposition bags, data transformed into decimal logarithms. A: bags with mesh size of 1 mm, B: bags with mesh size of 50 μm, C: bags with mesh size of 1 μm. LC0.2 = position next to charred logs, 0.2 m from log-ground contact zone, LNC0.2 = position next to non-charred logs, 0.2 m from log-ground contact zone, L3,10 = position away from logs, 3 or 10 m to the nearest downed log. The boxplots show aggregated data from three replicate forest sites used in the study, where cellulose bags were buried in each forest site at 40 sample plots (20 near logs and 20 further away from logs), three bags per plot – one of each mesh size. Cellulose incubation time in the field was ca. 14 months.
However, based on the binomial model and pairwise comparisons, there were evident and nearly statistically significant differences between the treatments as to how much cellulose material would survive the decomposition period in the 1-micron mesh size bags. A smaller proportion of bags at LNC0.2 (44%) had material left in them compared to bags at LC0.2 (76%), [z = –2.144, p = 0.0793], or at L3,10 (70%), [z = –2.334, p = 0.0499].
4 Discussion
Our results indicate that organic matter decomposition in burned and harvested boreal forest soils could be influenced by the proximity of downed logs. Decomposition rates for rooibos litter were tentatively observed to be more rapid at LNC0.2 than at 3–10-m distance (L3,10) from the nearest coarse dead wood pieces (Fig. 1a). The absolute difference in the rooibos decomposition rate constant ‘k’ between treatments is relatively small (ca. 0.002) compared to the range of ‘k’ values between different ecosystems reported in Keuskamp et al. 2013 (ca. 0.02), although the wider range in the latter is based on data that spans different countries and ecosystems. In our case, the narrow between-treatment difference in rooibos decomposition rates is still noteworthy because it was observed in very similar boreal background conditions. Our three replicate study sites were chosen to represent the same forest site type, while still allowing ecological variation that is typical of natural ecosystems. Furthermore, we found that decomposition appeared to be slower at LC0.2 than at LNC0.2, and that there was no difference between the treatments at LC0.2 and L3,10.
The reasons for these differences could be due to soil moisture and temperature differences between the microsites, or differences in decomposer communities and decomposer activity. For instance, Finér et al. (2016) showed that a higher soil temperature sum enhanced decomposition in clear-cut boreal forests and that it was hindered by reduced moisture rates. Soil microbial activity is lowered for a considerable time after burning (Köster et al. 2015), and Suominen et al. (2018) found that burned stumps harbour different fungal species than unburned stumps, which could also affect decomposition rates. Such mechanisms could potentially be affected by small-scale differences in burning severity and its impacts on the soil microsites with respect to CWD pieces. In addition, leaching (Abiven et al. 2011) and fragmentation and particulate transport of recalcitrant pyrogenic compounds (Preston and Schmidt 2006) from the charred CWD pieces to the nearby soil could have caused slower decomposition of the standardised substrate by altering the microenvironmental conditions that control SOM decomposition. Our study, however, was solely focused on exploring the spatial associations between the decomposition rates and microsite type (log/no log, charred/non-charred). As such, tentatively identifying the probable effect is the first step in the process to determine the complex mechanisms that drive the heterogeneity.
The degree to which the environmental conditions stabilised the decomposition of the labile litter fraction (‘S’ parameter that reflects the decomposition of green tea bags), was slightly greater at LNC0.2 than at LC0.2 (Fig. 1b). This can mean that the tentative differences seen in decomposition rates (‘k’ parameter of tea bag rooibos material), i.e. better conditions for SOM decomposition at non-charred logs, originate from changes in decomposition rates of the more stable and recalcitrant litter fraction, and not the labile fraction that is more prone to leaching.
The pattern found for the number of 1-micron mesh size cellulose bags with some undecomposed material was similar to the pattern observed for tea bag litter decomposition. Less bags with undecomposed cellulose were found at LNC0.2 than at L3,10 or at LC0.2, thereby indicating a more rapid rate of decomposition near non-charred logs. This pattern observed with the 1-micron bags could be reflective of the different bacterial communities that exist in a range of microsites with respect to CWD. Alternatively, it could mean that moisture conditions and leaching of cellulose was greater near non-charred logs. Greater moisture and cellulose leaching could be expected at non-charred logs because of slower evaporation rates due to lower temperature sums in the absence of dark-coloured char that would intensively absorb solar radiation (e.g. Gleason et al. 2013). The percentage of bags where the cellulose was not completely decomposed was rather small, especially for the 1-mm mesh bags (approximately 14%). This result shows that decomposition of a simple compound, such as cellulose, proceeds rather rapidly in burnt sub-xeric boreal forests. Obviously, it would be expected that the cellulose pulp in the 1-mm mesh bags would decompose fastest, as more decomposing agents could access the substrate, including the roots of grasses and their associated microorganism communities. Therefore, in our site conditions, a shorter incubation time for cellulose bags would probably have been more appropriate to better capture the variation in decomposition rates between treatments and to avoid total cellulose loss from the majority of the bags (86%). The forest sites in this study also have a thin and highly variable humus layer (often only 1–2 cm, and usually not more than 10 cm). Our observation of rapid decomposition rates differs from those reported for the wetter forests of Pacific Alaska that exhibit a much thicker humus layer, which probably further reinforces the slower decomposition of cellulose there over the course of a year (McClellan et al. 1990). Moreover, drainage conditions have been shown to control mineralisation in boreal forests, especially for the well-drained class of forest soils (Wickland and Neff 2008) – our sub-xeric forest sites are well-drained and drier than the ones sampled in McClellan et al. (1990). We also observed a rather substantial variation among the sample plots in percent mass cellulose left in the bags (Fig. 2). This is probably explained by local small-scale variations in the soil (Liski 1995; Čugunovs et al. 2017), which would include the humus layer thickness under which the bags were buried.
Our study from the boreal zone supports previous findings that described the importance of downed logs in forest soil functioning. For example, Gonzalez-Polo et al. (2013) found increased soil enzymatic activity and litter decomposition rates close to CWD pieces in Argentinian temperate forests. Similarly, Stutz and Lang (2017) found increased C:N ratio and base saturation in the soil under CWD pieces in German temperate forests.
However, the role that log charring may have on decomposition rates in the proximity of logs is not widely documented. Although the spatial pattern of soil processes with respect to charred and non-charred CWD seems preliminarily identifiable, the mechanisms and processes that might connect CWD to soil functioning are currently unclear, which hinders our understanding of the role of charring. For example, fungal nutrient translocation or priming (Rousk et al. 2014; Wild et al. 2014) in the log-ground contact zone are possible contributing mechanisms. Although fungal translocation of nutrients to the CWD in boreal forests is an important process (Rinne et al. 2017), we are not aware of any published field data explaining the most likely distances that this fungal translocation of nutrients might take place. Furthermore, it is probable that the interaction of fire with CWD and/or spatial burning patterns leading to CWD charring may alter many properties of the soil microenvironment near CWD, such as humus layer thickness, microclimate and decomposer community, thereby leading to differences in decomposition rates. To explore the various potential mechanisms responsible for CWD impacts on forest soils, future studies that combine gradients of several distances from the downed logs, and molecular carbon and nitrogen tracing methods are critically needed. The tea bag index method can then be used as an additional robust and cost-effective means to estimate the environmental controls on litter decomposition rates.
Another major challenge is in upscaling current and subsequent, individual log-level findings to the ecosystem level. It seems intuitive that the effects of proximity to CWD pieces on decomposition rates may be evident at the forest ecosystem level, particularly if there is a large abundance of dead wood. However, these processes can be altered with charred wood in extensively burned areas. If our log-level findings are directly transferable to the ecosystem level (as they ought to be), the observed patterns indicate a new mechanism on how forest fires may influence carbon dynamics related to CWD. Future studies would need intensive sampling of forest stands with varying amounts of dead-wood and different disturbance types and intensities of fire disturbances to conclude whether the functioning of the whole ecosystem is changed due to CWD and its interaction with the disturbance.
In conclusion, our study suggests noteworthy spatial differences in SOM decomposition rates in boreal forests after fire, in respect to the position of downed logs. Decomposition of SOM was tentatively observed to be more rapid near non-charred CWD pieces than near charred CWD or further away from CWD. These findings provide novel ideas on how fire disturbance in boreal forests may alter soil functioning in connection with downed logs. This being the case, CWD together with fire may have effects on forest ecosystem functioning that are poorly known yet may influence post-disturbance succession considerably by affecting temporal dynamics of humus formation and nutrient release from dead wood and maintaining the small-scale heterogeneity in forest ecosystem properties. However, despite the general importance of our finding, we stress the tentative nature of our observation. We urge for studies that take into account more thoroughly the local within-site variation in decomposition patterns near downed logs and log characteristics, to establish the overall validity of our finding.
Acknowledgements
We thank Jarmo Pennala and Risto Ikonen for their help in the fieldwork. Mihails Čugunovs’ work was supported by University of Eastern Finland, Doctoral Programme in Forests and Bioresources. Dr. Shinichi Tatsumi provided the background soil data on the study sites. We are grateful to prof. Lauri Mehtätalo for his help with the sampling design and statistical analyses. Metsähallitus offered the forest land for this research and conducted the practical harvest and burning treatments according to the study design. We also thank two anonymous reviewers and the journal editor for their constructive comments on the manuscript.
References
Abiven S., Hengartner P., Schneider M.P.W., Singh N., Schmidt M.W.I. (2011). Pyrogenic carbon soluble fraction is larger and more aromatic in aged charcoal than in fresh charcoal. Soil Biology and Biochemistry 43(7): 1615–1617. https://doi.org/10.1016/j.soilbio.2011.03.027.
Ahti T., Hämet-Ahti L., Jalas J. (1968). Vegetation zones and their sections in northwestern Europe. Annales Botanici Fennici 5: 169–211.
Bates D., Maechler M., Bolker B., Walker S. (2015). Fitting linear mixed-effects models using lme4. Journal of Statistical Software 67(1): 1–48. https://doi.org/10.18637/jss.v067.i01.
Brown R.L., Naeth M.A. (2014). Woody debris amendment enhances reclamation after oil sands mining in Alberta, Canada. Restoration Ecology 22: 40–48. https://doi.org/10.1111/rec.12029.
Čugunovs M., Tuittila E.-S., Mehtätalo L., Pekkola L., Sara-Aho I., Kouki J. (2017). Variability and patterns in forest soil and vegetation characteristics after prescribed burning in clear-cuts and restoration burnings. Silva Fennica vol. 51 no. 1 article 1718. https://doi.org/10.14214/sf.1718.
Finér L., Jurgensen M.F., Palviainen M., Piirainen S., Page-Dumroese D. (2016). Does clear-cut harvesting accelerate initial wood decomposition? A five year study with standard wood material. Forest Ecology and Management 372: 10–18. https://doi.org/10.1016/j.foreco.2016.03.060.
Gleason K.E., Nolin A.W., Roth T.R. (2013). Charred forests increase snowmelt: Effects of burned woody debris and incoming solar radiation on snow ablation. Geophysical Research Letters 40: 4654–4661. https://doi.org/10.1002/grl.50896.
Goldin S.R., Hutchinson M.F. (2014). Coarse woody debris reduces the rate of moisture loss from surface soils of cleared temperate Australian woodlands. Soil Research 52(7): 637–644. https://doi.org/10.1071/SR13337.
Gonzalez-Polo M., Fernandez-Souto A., Austin A.T. (2013). Coarse woody debris stimulates soil enzymatic activity and litter decomposition in an old-growth temperate forest of Patagonia, Argentina. Ecosystems 16(6): 1025–1038. https://doi.org/10.1007/s10021-013-9665-0.
Hämäläinen A., Hujo M., Heikkala O., Junninen K., Kouki J. (2016). Retention tree characteristics have major influence on the post-harvest tree mortality and availability of coarse woody debris in clear-cut areas. Forest Ecology and Management 369: 66–73. https://doi.org/10.1016/j.foreco.2016.03.037.
Harmon M.E., Franklin J.F., Swanson F.J., Sollins P., Gregory S.V., Lattin J.D., Anderson N.H., Cline S.P., Aumen N.G., Sedell J.R., Lienkaemper G.W., Cromack K. Jr., Cummins K.W. (1986). Ecology of coarse woody debris in temperate ecosystems. Advances in Ecological Research 15: 133–302. https://doi.org/10.1016/S0065-2504(08)60121-X.
Heikkala O., Suominen M., Junninen K., Hämäläinen A., Kouki J. (2014). Effects of retention level and fire on retention tree dynamics in boreal forests. Forest Ecology and Management 328: 193–201. https://doi.org/10.1016/j.foreco.2014.05.022.
Heikkala O., Martikainen P., Kouki J. (2016). Decadal effects of emulating natural disturbances in forest management on saproxylic beetle assemblages. Biological Conservation 194: 39–47. https://doi.org/10.1016/j.biocon.2015.12.002.
Hotanen J.-P., Nousiainen H., Mäkipää R., Reinikainen A., Tonteri T. (2008). Metsätyypit – opas kasvupaikkojen luokitteluun. [Forest types – a guide for classification of site-types]. Metsäntutkimuslaitos. 182 p. ISBN-13: 978-952-5694-22-2.
Hothorn T., Bretz F., Westfall P. (2008). Simultaneous inference in general parametric models. Biometrical Journal 50(3): 346–363. https://doi.org/10.1002/bimj.200810425.
Hyvärinen E., Kouki J., Martikainen P., Lappalainen H. (2005). Short-term effects of controlled burning and green-tree retention on beetle (Coleoptera) assemblages in managed boreal forests. Forest Ecology and Management 212(1–3): 315–335. https://doi.org/10.1016/j.foreco.2005.03.029.
Hyvärinen E., Kouki J., Martikainen P. (2006). Fire and green-tree retention in conservation of red-listed and rare deadwood-dependent beetles in Finnish boreal forests. Conservation Biology 20: 1711–1719. https://doi.org/10.1111/j.1523-1739.2006.00511.x.
Ilmatieteen laitos (2016). http://ilmatieteenlaitos.fi/terminen-kasvukausi. [Cited 28 November 2016].
Ilmatieteen laitos (2020). https://ilmatieteenlaitos.fi/ilmastollinen-vertailukausi. [Cited 12 January 2020].
International Plant Names Index (2004). http://www.ipni.org/. [Cited 24 May 2018].
Keuskamp J.A., Dingemans B.J.J., Lehtinen T., Sarneel J.M., Hefting M.M. (2013). Tea Bag Index: a novel approach to collect uniform decomposition data across ecosystems. Methods in Ecology and Evolution 4: 1070–1075. https://doi.org/10.1111/2041-210X.12097.
Knapp E.E., Keeley J.E., Ballenger E.A., Brennan T.J. (2005). Fuel reduction and coarse woody debris dynamics with early season and late season prescribed fire in a Sierra Nevada mixed conifer forest. Forest Ecology and Management 208(1–3): 383–397. https://doi.org/10.1016/j.foreco.2005.01.016.
Köster K., Berninger F., Heinonsalo J., Lindén A., Köster E., Ilvesniemi H., Pumpanen J. (2015). The long-term impact of low-intensity surface fires on litter decomposition and enzyme activities in boreal coniferous forests. International Journal of Wildland Fire 25(2): 213–223. https://doi.org/10.1071/WF14217.
Kouki J. (2016). Ecology and biodiversity of boreal forests. http://forest.uef.fi/jarikouki/project_fire.htm. [Cited 28 Nov 2016].
Kunttu P., Junninen K., Kouki J. (2015). Dead wood as an indicator of forest naturalness: a comparison of methods. Forest Ecology and Management 353: 30–40. https://doi.org/10.1016/j.foreco.2015.05.017.
Laamanen J. (2002). Tulen voimakkuuden vaikutus metsikön pienialaiseen vaihteluun. [The effect of fire severity on small-scale spatial heterogeneity in forest stands]. MSc thesis. University of Joensuu, Faculty of Forest Sciences, Joensuu, Finland. 44 p. + 12 app.
Laiho R., Prescott C. (1999). The contribution of coarse woody debris to carbon, nitrogen and phosphorus cycles in three Rocky Mountain coniferous forests. Canadian Journal of Forest Research 29(10): 1592–1603. https://doi.org/10.1139/x99-132.
Laiho R., Prescott C. (2004). Decay and nutrient dynamics of coarse woody debris in northern coniferous forests: a synthesis. Canadian Journal of Forest Research 34(4): 763–777. https://doi.org/10.1139/x03-241.
Liski J. (1995). Variation in soil organic carbon and thickness of soil horizons within a boreal forest stand – effect of trees and implications for sampling. Silva Fennica 29(4): 255–266. https://doi.org/10.14214/sf.a9212.
McClellan M.H., Bormann B.T., Cromack K., JR. (1990). Cellulose decomposition in southeast Alaskan forests: effects of pit and mound microrelief and burial depth. Canadian Journal of Forest Research 20(8): 1242–1246. https://doi.org/10.1139/x90-163.
Pinchot G. (2011). The relation of forests and forest fires. Fire Ecology 7(3): 2–11. (original work published in National Geographic 1899). https://doi.org/10.4996/fireecology.0703002.
Pinheiro J., Bates D., DebRoy S., Sarkar D., R Core Team (2017). nlme: linear and nonlinear mixed effects models. R package version 3.1-131. https://CRAN.R-project.org/package=nlme.
Preston C.M., Schmidt M.W.I. (2006). Black (pyrogenic) carbon: a synthesis of current knowledge and uncertainties with special consideration of boreal regions. Biogeosciences 3(4): 397–420. https://doi.org/10.5194/bg-3-397-2006.
R Development Core Team (2013). R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0. http://www.R-project.org.
Renvall P. (1995). Community structure and dynamics of wood-rotting Basidiomycetes on decomposing conifer trunks in northern Finland. Karstenia 35(1): 1–51. https://doi.org/10.29203/ka.1995.309.
Rinne K.T., Rajala T., Peltoniemi K., Chen J., Smolander A., Mäkipää R. (2017). Accumulation rates and sources of external nitrogen in decaying wood in a Norway spruce dominated forest. Functional Ecology 31(2): 530–541. https://doi.org/10.1111/1365-2435.12734.
Rousk J., Hill P.W., Jones D.L. (2014). Priming of the decomposition of ageing soil organic matter: concentration dependence and microbial control. Functional Ecology 29(2): 285–296. https://doi.org/10.1111/1365-2435.12377.
Siitonen J., Martikainen P., Punttila P., Rauh J. (2000). Coarse woody debris and stand characteristics in mature managed and old-growth boreal mesic forests in southern Finland. Forest Ecology and Management 128: 211–225. https://doi.org/10.1016/S0378-1127(99)00148-6.
Similä M., Junninen K. (2012). Ecological restoration and management in boreal forests – best practices from Finland. Metsähallitus, Natural Heritage Services, Vantaa. 54 p.
Similä M., Kouki J., Martikainen P. (2003). Saproxylic beetles in managed and seminatural Scots pine forests: quality of dead wood matters. Forest Ecology and Management 174: 365–381. https://doi.org/10.1016/S0378-1127(02)00061-0.
Sippola A.-L., Siitonen J., Kallio R. (1998). Amount and quality of coarse woody debris in natural and managed coniferous forests near the timberline in Finnish Lapland. Scandinavian Journal of Forest Research 13(1): 204–214. https://doi.org/10.1080/02827589809382978.
Stocks B.J., Wotton B.M., Flannigan M.D., Fosbert M.A., Cahoon D.R., Goldammer J.G. (2001). Boreal forest fire regimes and climate change. In: Beniston M., Verstraete M.M. (eds.). Remote sensing and climate modeling: synergies and limitations. Kluwer Academic, Dordrecht, The Netherlands. p. 233–246.
Stutz K.P., Lang F. (2017). Potentials and unknowns in managing coarse woody debris for soil functioning. Forests 8(2): 37. https://doi.org/10.3390/f8020037.
Suominen M., Junninen K., Heikkala O., Kouki J. (2018). Burning harvested sites enhances polypore diversity on stumps and slash. Forest Ecology and Management 414: 47–53. https://doi.org/10.1016/j.foreco.2018.02.007.
Wallenius T. (2011). Major decline in fires in coniferous forests – reconstructing the phenomenon and seeking for the cause. Silva Fennica 45(1): 139–155. https://doi.org/10.14214/sf.36.
Wickland K.P., Neff J.C. (2008). Decomposition of soil organic matter from boreal black spruce forest: environmental and chemical controls. Biogeochemistry 87(1): 29–47. https://doi.org/10.1007/s10533-007-9166-3.
Wild B., Schnecker J., Eloy Alves R.J., Barsukov P., Bárta J., Čapek P., Gentsch N., Gittel A., Guggenberger G., Lashchinskiy N., Mikutta R., Rusalimova O., Šantručková H., Shibistova O., Urich T., Watzka M., Zrazhevskaya G., Richter A. (2014). Input of easily available organic C and N stimulates microbial decomposition of soil organic matter in arctic permafrost soil. Soil Biology and Biochemistry 75: 143–151. https://doi.org/10.1016/j.soilbio.2014.04.014.
Total of 47 references.

