Colchicine did not affect the viability of induced 2n pollen in Populus tomentosa
Liu Y., Zhang Y., Zhou Q., Wu J., Zhang P. (2019). Colchicine did not affect the viability of induced 2n pollen in Populus tomentosa. Silva Fennica vol. 53 no. 2 article id 10132. https://doi.org/10.14214/sf.10132
Highlights
- The number of colchicine injections and the meiotic stage at which they were administered both had a significant effect on the occurrence rate of induced 2n pollen in Populus tomentosa
- Treating male buds with 5000 ppm colchicine solution during meiosis led to a significant decrease in pollen production
- Colchicine injection could induce P. tomentosa to produce 2n pollen and did not lead to dysfunction of induced diploid pollen.
Abstract
Colchicine is widely used as a mutagen to induce production of diploid gametes in plants. However, whether colchicine affects induced pollen viability remains unclear. To clarify whether colchicine affected the viability of induced pollen, we induced production of diploid pollen by colchicine, followed by pollen germination in vitro and crossing induced pollen with normal gametes to produce triploid in Populus tomentosa Carrière. The results showed that the predominant meiotic stages and the number of colchicine injections had significant effects on the occurrence rates of induced 2n pollen. When the colchicine injection was given at diakinesis, a significant decrease in the pollen production per bud was observed (p < 0.001). The morphology of the colchicine-induced 2n pollen was similar to that of the natural 2n pollen in its ectexine structure. The pollen germination experiments revealed that there was also no significant difference in germination rates between the induced diploid pollen and natural 2n pollen grains, and 68 triploids were created by crossing colchicine-induced pollen. Our findings revealed that colchicine injection could induce P. tomentosa to produce 2n pollen and will not lead to dysfunction of induced diploid pollen.
Keywords
colchicine-induced 2n pollen;
pollen production;
pollen viability;
pollen germination rate;
triploid
- Liu, National Engineering Laboratory for Tree Breeding, Beijing Forestry University, Beijing 100083, China; College of Bioscience and Biotechnology, Beijing Forestry University, Beijing 100083, China E-mail 342767649@qq.com
- Zhang, National Engineering Laboratory for Tree Breeding, Beijing Forestry University, Beijing 100083, China; College of Bioscience and Biotechnology, Beijing Forestry University, Beijing 100083, China E-mail 409192881@qq.com
- Zhou, National Engineering Laboratory for Tree Breeding, Beijing Forestry University, Beijing 100083, China; College of Bioscience and Biotechnology, Beijing Forestry University, Beijing 100083, China E-mail 876034493@qq.com
- Wu, National Engineering Laboratory for Tree Breeding, Beijing Forestry University, Beijing 100083, China; College of Bioscience and Biotechnology, Beijing Forestry University, Beijing 100083, China E-mail 1269485709@qq.com
-
Zhang,
National Engineering Laboratory for Tree Breeding, Beijing Forestry University, Beijing 100083, China; College of Bioscience and Biotechnology, Beijing Forestry University, Beijing 100083, China
E-mail
zhangpd@bjfu.edu.cn

Received 5 January 2019 Accepted 11 June 2019 Published 17 June 2019
Views 73870
Available at https://doi.org/10.14214/sf.10132 | Download PDF

1 Introduction
Populus tomentosa Carrière (family Salicaceae) is an economically important tree species native to China. As a rapidly growing tree with high quality timber, it is widely used in China for landscape cultivation, ecological restoration and the production of lumber and pulp. Natural triploid P. tomentosa (2n = 3x = 57) was firstly described in 1992 by Zhu et al. (1995, 1998) and is characterized by extremely large leaves. The growth rate of the natural triploids is approximately twice that of diploid individuals, and the trees have longer fibers and improved pulp properties (Zhu et al. 1995, 1998; Yao and Pu 1998). Due to its high growth rate and timber quality, these natural triploid P. tomentosa are widely planted throughout Hebei, Henan, Shandong, and Shanxi provinces.
Crossing 2n pollen with a normal female gamete has been shown to be one of the most effective methods by which to produce triploid individuals (Nilsson-Ehle 1936; Müntzing 1936). Zhu (2006) reported that the average occurrence rate of natural 2n pollen in P. tomentosa is 3.9%. Subsequently, five triploids were created by crossing natural 2n pollen in P. tomentosa with normal female gametes in P. tomentosa × Populus bolleana Lauche (Zhu et al. 1998). However, owing to the low occurrence rate of natural 2n pollen, the production rate of triploids was less than 0.1%. To increase the occurrence rate of 2n pollen, novel methods for inducing polyploid production in plants via the use of spindle inhibitors, such as dinitroanilines or colchicine, have been successfully explored and used in some species (Vaughn and Lehnen1991; Hancock 1997; Zlesak et al. 2005; Dhooghe et al. 2009).
Colchicine has frequently been applied to induce triploid production in 2n pollen in woody species during meiosis of pollen mother cells (PMCs). It can inhibit microtubule polymerization by binding to tubulin (Mashkina et al. 1989). For example, Kang et al. (1999) reported that the highest percentage of induced 2n pollen by colchicine was 88.0% and the suitable stage for induction of 2n pollen ranged from leptotene to diakinesis in P. tomentosa × Populus bolleana. In a subsequent study, 12 triploids were detected from 1280 offspring by crossing colchicine-induced 2n pollen (63.7%) with haploid female gametes in P. tomentosa × P. bolleana. The highest percentage of triploids was 12.9% (Kang et al. 2000). In another study, 62.1% of total 2n pollen was induced when four injections were given at 2 h intervals, producing two triploids in Populus × popularis Hsü. (Xi et al. 2011). In Eucalyptus (L’Hér) spp., 2n pollen was also induced by Yang et al (2016). They achieved 28.7% of 2n pollen induced by treating flower buds with 5000 ppm colchicine solution for 6 hours. However, little information exists on whether colchicine affects the viability of colchicine-indued-2n pollen in Populus spp.
The objective of this study was to determine the impact of colchicine on the viability of induced 2n pollen. We investigated morphology of 2n pollen induced by a 5000 ppm colchicine solution in P. tomentosa. 2n pollen germination in vitro followed by crossing induced 2n pollen with normal gametes to produce triploids. Our findings provide more specific information regarding triploid breeding in Populus spp.
2 Material and methods
2.1 Plant materials
Floral branches of a highly fertile male parent P. tomentosa clone 5088 and a female parent Populus alba L. × Populus glandulosa Uyeki were collected from the Guan Country Nursery, Shandong Province. The P. tomentosa clone 5088 was originally collected from Shanxi Province and P. alba × P. glandulosa was introduced from Korea and planted at the Guan Country Nursery in 1985 by Zhu (2006). The mean annual temperature of Guan Country is 13.3 ℃. Coldest temperatures on average occur in January and the warmest in July. Mean annual rainfall is 549.9 mm. All floral branch samples were wrapped in a plastic cloth and transported to Beijing Forestry University. All samples were detached and cultured in tap water under greenhouse conditions (10–20 ℃) to force floral development. No additional nutrition was supplied.
2.2 Treatment with a 5000 ppm colchicine solution
Meiosis of the PMCs was initiated in male samples after 52 h in the tap water culture (Zhao et al. 2017). We sampled 60–90 flower buds once every 4 h and treated them with a 5000 ppm colchicine solution. The samples were divided into three treatments receiving either three, five or seven injections of colchicine, with 2 h between each injection. Untreated male catkins were taken as the control group. The treated male samples were then hydroponically cultured until the catkins matured. Subsequently, pollen grains were sampled and stored in glass bottles with allochronic silica gel at 4 ℃ to dry them out.
We determined the occurrence rate of induced 2n pollen using methods described in Zhang and Kang (2013). Based on the assumption that one pollen mother cell has a certain volume and can divide into four or two parts, we predicted that diploids will display an increase in diameter by a factor of 1.28 compared with haploid pollen, provided that the volume has not changed during the cleavage process. Pollen grains with a diameter larger than 1.28 plus the average diameter of the tested group were considered to be diploid. The diameters of 500–700 pollen grains were analyzed for each sample.
2.3 Scanning electron microscopy
Fixed pollen grains were rinsed with ethanol and air-dried, before being sputter-coated with gold using a HITACHI E-1010 ion sputter coater (Japan). They were then observed under a HITACHIS-3400N microscope (Japan), using an accelerating voltage of 5 kV.
2.4 Pollen-germination in vitro
Pollen germination in vitro medium was prepared following the methods of Zhao et al. (2017). The medium was composed of 0.7% agar, 50 mg l–1 calcium chloride, and 120 mg l–1 boric acid. The pH was 6.0. Fresh induced 2n pollen was applied to slides carrying the germination medium. These slides were then placed onto 120 mm petri dishes with wet filter paper. Pollen germination was conducted in a climate chamber set to 26 ℃. After four hours of culture, we washed the sampled pollen grains with the liquid germination medium and collected them in a 1.5 ml centrifuge tube. After being centrifuged for five minutes at a speed of 1000 rpm, the grains were fixed in Carnoy’s solution (ethanol: acetic acid, 3:1) for 10 minutes. The germination rates of each sample were determined by an eyepiece micrometer. A total of 500–800 pollen grains were calculated per sample.
2.5 Triploid production by crossing colchicine-induced 2n pollen
Female flower buds were pollinated with fresh colchicine-induced pollen from P. tomentosa clone 5088 when all stigmas were receptive. The female floral branches were then hydroponically cultured for a further four weeks. Seeds were collected after maturation and germinated in sterile soil. When the seedlings reached approximately 5 cm in height they were transferred into 8 × 10 cm containers with nutritious soil to promote growth. Surviving seedlings were transplanted into the field on reaching approximately 25 cm.
2.6 Ploidy analysis by flow cytometry and chromosome counting
Flow cytometry measurements were done using a flow cytometer (BD FACSCalibur, Franklin Lakes, NJ, USA). Young leaves were chopped with a sharp razor blade. 0.5 g of the chopped leaves were placed in a 55-mm plastic petri dish with nuclei extraction solution (Galbraith et al. 1983) (0.2 mMTris–HCl, 45 mM MgCl2, 30 mM sodium citrate, 20 mM 4-morpholinepropane sulfonate, 1% (v/v) Triton X-100, pH 7.0) and then filtered through a 40 µm nylon mesh. The filtrate was collected and centrifuged at 1000 rpm for five minutes. Subsequently, the suspension of released nuclei was stained with 50 µl of 4′, 6-diamidino-2-phenylindole (DAPI, 10 mg ml–1) for 5 min. At least 2000 nuclei of each sample were detected and three samples were collected per plant. The leaf sample from a known diploid plant of P. tomentosa (2n = 2x = 38) served as a control. The standard diploid peak was set to appear at about channel 50 of relative fluorescent intensity.
The ploidy level of each putative triploid was confirmed ultimately by chromosome counting. Stem tips from the seedlings were sampled and pretreated with para-dichlorobenzene solution at room temperature for 4 hours. Then, the tips were washed once in water and fixed in fresh Carnoy’s solution for at least 24 hours at 4 ℃. The fixed stem tips were hydrolyzed in 38% HCl: ethanol (1:1) for 15 minutes at room temperature and then rinsed three times with distilled water for 30 minutes. The hydrolyzed samples were stained with carbol fuchsin, crushed with a cover slip. Metaphase chromosomes were observed at 100X oil lens magnification using an Olympus BX61 microscope (Japan).
2.7 Statistical analysis
We used a Chi squared (χ2) test to estimate the difference between the expected and observed triploid production rates. We expected the colchicine-induced 2n pollen to have the same fertilization ability as haploid pollen. The observed triploid production rate was determined by the number of real triploid plants and the total number of seedlings in each cross combination.
To reveal the differences among the number of colchicine injections and dominant meiotic stages, we conducted analyses of variance using the GLM-univariate procedure in the SPSS software (SPSS for Windows, Version13, SPSS, Chicago, IL, USA). Because of the heterogeneity in variance, the values of occurrence rates of induced 2n pollen (p–1) were transformed prior to analysis. Partial correlation between the triploid production rate and the occurrence rate of induced 2n pollen was also calculated, and statistical significance was tested for using the SPSS PROC CORR software.
3 Results
3.1 2n pollen production induced by 0.5% colchicine solution
After the male buds were treated with colchicines in P. tomentosa, bud development progressed slowly and the anthers became brown and dry. Some male buds died after treatment, and so pollen could not be collected from them.
Colchicine-induced 2n pollen was collected from all surviving mature catkins (Fig. 1a). Relatively few natural 2n pollen grains were observed in the control group (Fig. 1b). Fig. 2 presents the occurrence rates of colchicine-induced 2n pollen in the different treatments. The average occurrence rates of colchicine-induced 2n pollen ranged from 8.6 to 56.5% (Fig. 2). The GLM-univariate analysis indicated that the predominant meiotic stages (F = 45.822, p = 0.000) and the number of colchicine injections (F = 10.150, p = 0.004) had significant effects on the occurrence rate of colchicine-induced 2n pollen. Additionally, LSD multiple-comparison tests showed that there were no significant differences in colchicine-induced 2n pollen occurrence between the zygotene, pachytene, diplotene and diakinesis stages. Nevertheless, they were, at the α = 0.05 level, all significantly higher than that of the leptotene stage and metaphase Ⅰ. This shows that 2n pollen should be induced with colchicine between zygotene to diakinesis. The occurrence rate of colchicine-induced 2n pollen for samples given seven injections was significantly higher than in samples given only three. However, there were no significant differences between the occurrence rates of colchicine-induced 2n pollen between samples with five injections and those with seven.
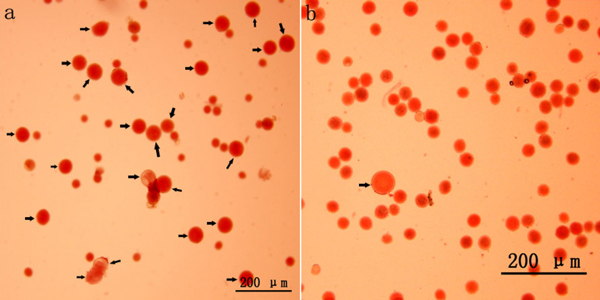
Fig. 1. 2n pollen production induced with colchicine in Populus tomentosa clone 5088 (Bar = 200 μm). (a) 2n pollen induced with colchicine in P. tomentosa clone 5088 (arrow). (b) Natural 2n pollen occurred in P. tomentosa clone 5088 (arrow).
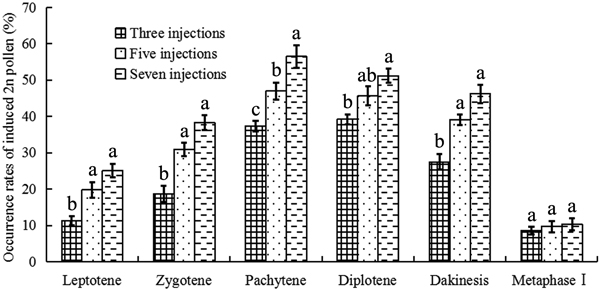
Fig. 2. 2n pollen production induced by colchicine in Populus tomentosa clone 5088. The values indicated by the different letters were significantly different within the phase at α = 0.05 according to the LSD test.
3.2 The effects of colchicine injection on pollen production and pollen morphology
All flower branches on which male flower buds were treated at diakinesis were hydroponically cultured until pollen was released from the anthers. Five flower buds per treatment were separately tested to evaluate the effects of colchicine injection on pollen production. Total pollen production per bud in different treatments is presented in Fig. 3. The average pollen production per flower bud in the control group was 39.4 ± 1.3 mg. When the colchicine injection was given during diakinesis, a significant decrease in the pollen production was observed (p < 0.001). In buds given seven injections, the average pollen production was 2.8 ± 0.4 mg, which was significantly lower than those of buds given three or five injections, suggesting that an increase in the number of colchicine injections would result in a significant reduction in pollen production.
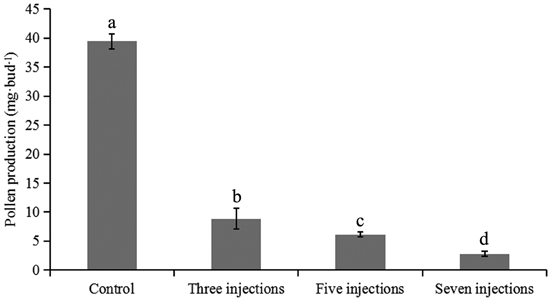
Fig. 3. Pollen production per bud after different numbers of colchicine injections were given in Populus tomentosa clone 5088. The values indicated by the different letters were significantly different at α = 0.05 in LSD test.
The morphology of the pollen was examined using scanning electron microscopy. The images revealed that the pollen collected in the control group was homogenous, spherical, with few corrugations and granulated marks on the surface (Figs. 4a, d, g). No aperture was observed. After three or seven injections were given, a number of 2n pollen grains were produced (Figs. 4b, c). The colchicine-induced 2n pollen (Figs. 4e, f, h, i) was similar to the natural 2n pollen in its ectexine structure (Fig. 4d). No large difference in ectexine structure was found between the haploid pollen grains in the treatment and control groups (Figs. 4h, i), as the haploid pollen was not the result of the colchicine injection.

Fig. 4. Scanning electron micrographs of pollen grains of male flower buds in Populus tomentosa clone 5088 after receiving three or seven colchicine injections at diakinesis and from male flower buds cultured in tap water at 25 ℃ as control. (a) Morphology of pollen grains derived from the control group. (b) Morphology of colchicine-induced pollen grains after three colchicine injections were given and 2n pollen grains. (c) Morphology of colchicine-induced pollen grains after seven colchicine injections were given and 2n pollen grains. (d) Details of ectexine structure of natural 2n pollen. (e) Details of ectexine structure of colchicine-induced 2n pollen after three colchicine injections. (f) Details of ectexine structure of colchicine-induced 2n pollen after seven colchicine injections. (g) Details of ectexine structure of haploid pollen from the control group. (h) Details of ectexine structure of haploid pollen after three injections. (i) Details of ectexine structure of haploid pollen after seven injections. Scale bar = 100 µm (a, b, and c) and 10 µm (d, e, f, g, h, and i).
3.3 In vitro Colchicine-induced 2n pollen germination
The colchicine-induced pollen contained a mixture of haploid pollen and 2n pollen grains, since meiosis of PMCs in P. tomentosa clone 5088 was asynchronous. To evaluate the viability of colchicine-induced 2n pollen, fresh colchicine-induced pollen grains were used for the germination test. We used the medium supplemented with 50 mg l–1 calcium chloride and 120 mg l–1 boric acid (Fig. 5a). The mean pollen germination rate was approximately 26.4% after 4 h in culture. The germinated natural 2n pollen and induced 2n pollen grains are shown in Figs. 5b, c, respectively. The germination rates of the 2n pollen are shown in Fig. 6. The average germination rate of natural 2n pollen in the control group after 4 h in culture was 26.3%. This was slightly higher than the germination rates of colchicine-induced 2n pollen derived from the groups treated with three injections (25.1%), five injections (23.4%) and seven injections (23.0%). Analysis using the GLM-univariate method showed that no significant difference existed between the germination rates of 2n pollen derived from the groups treated with different number of injections, suggesting that colchicine injection did not significantly affect the viability of 2n pollen.
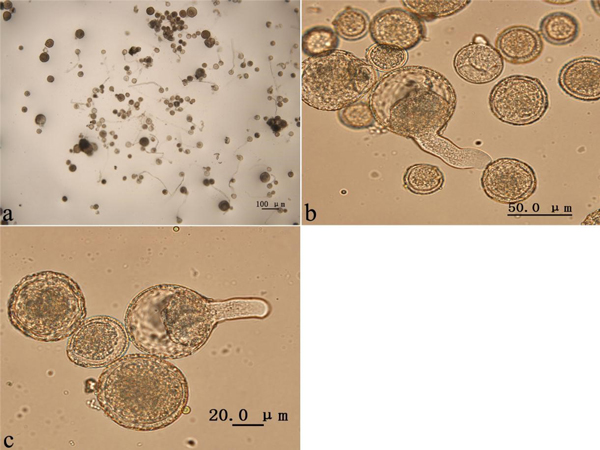
Fig. 5. Colchicine-induced 2n pollen from Populus tomentosa clone 5088 after the germination test in pollen germination medium. (a) Germination of pollen grains treated with colchicine. (b) Germination of natural 2n pollen grains. (c) Germination of colchicine-induced 2n pollen grains.
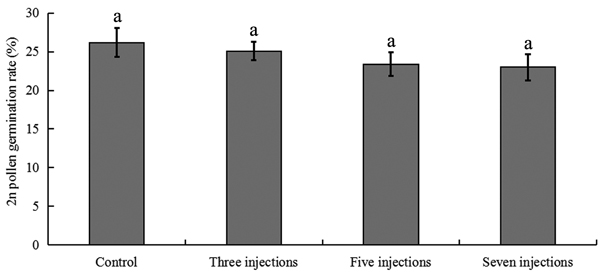
Fig. 6. 2n pollen viability in flower buds treated with 5000 ppm colchicine solution in Populus tomentosa clone 5088. Control corresponds to 25 ℃, i.e. standard culture conditions. The values indicated by the same letters were not significantly different at α = 0.05 in LSD test.
3.4 Triploid production by crossing colchicine-induced 2n pollen
After pollinated female flower buds were cultured for 4 weeks, a total of 3346 seeds were produced from all treated cross combinations and the untreated group. These seeds produced 1135 seedlings to be scored later in the experiment (Table 1). All young seedlings were detected by flow cytometric analysis and 68 putative triploids were achieved (Fig. 7a). Each putative triploid was a real triploid (2n = 3x = 57, Fig. 7b), as confirmed by somatic chromosome counting. All real triploids were derived from the cross combinations pollinated with different rates of induced 2n pollen. There were no triploids obtained in the control group, suggesting that P. alba. × P. glandulosa did not generate 2n eggs and that natural 2n pollen in P. tomentosa did not take part in fertilization.
| Table 1. Seed number, seedling number and triploid production by crossing different occurrence rates of induced 2n pollen via 5000 ppm colchicine solution in Populus tomentosa clone 5088 with P. alba × P. glandulosa. | |||||
| Occurrence rate of 2n pollen (%) | Seed number | Seedling number | Triploid number | Triploid production rate (%) | χ2 |
| 19.8 | 527 | 169 | 8 | 4.7 | |
| 30.9 | 392 | 134 | 9 | 6.7 | |
| 47.0 | 368 | 121 | 16 | 13.2 | |
| 45.7 | 522 | 187 | 18 | 9.6 | 117.7a |
| 39.1 | 608 | 204 | 15 | 7.4 | |
| 9.7 | 483 | 149 | 2 | 1.4 | |
| 1.6 (Control ) | 446 | 171 | 0 | 0 | |
| Total | 3346 | 1135 | 68 | ||
| a indicating extremely significant (p < 0.01) | |||||
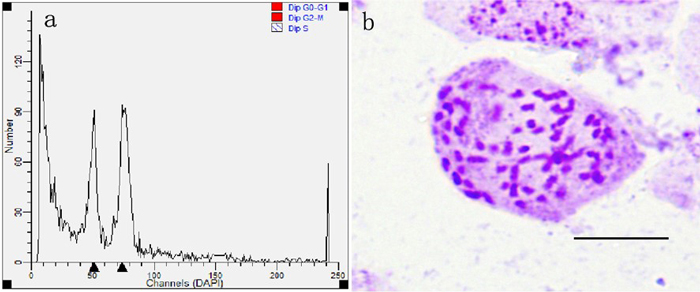
Fig. 7. Ploidy level determination by flow cytometric analysis and chromosome counting in progeny came from the cross combinations with colchicine-induced 2n pollen (Bar = 10 μm). (a) Histogram of flow cytometric analysis of young leaves derived from diploid and triploid plants. (b) Chromosome with 2n = 3x = 57 from triploid seedlings.
Table 1 presents the occurrence rates of colchicine-induced 2n pollen and the triploid production rates. Partial correlation analysis between the percentage of triploids and the frequency of induced 2n pollen indicated that the triploid production rates had a significant positive correlation with 2n pollen occurrence rates (r = 0.960, p = 0.000). This suggested that a higher frequency of colchicine-induced 2n pollen helps increase the percentage of triploids. The observed triploid production rate was not consistent with the expected triploid production rate according to the χ2 test. However, the expected triploid production rates were significantly higher than the observed triploid production rate, suggesting that colchicine-induced 2n pollen was weakly competitive during fertilization compared to haploid pollen.
4 Discussion
4.1 Colchicine and induced 2n pollen production
Crossing 2n pollen with haploid female gametes is commonly used to produce triploids in Populus spp. Zhu (2006) documented that ten hybrid triploids in P. tomentosa were produced by crossing natural 2n pollen with haploid gametes in P. tomentosa × P. bolleana. However, less than 0.1% triploidy was achieved because of the lower percentage of natural 2n pollen. 63.7% colchicine-induced 2n pollen was used to crossed with haploid female gametes by Kang et al. (2000) and 16 triploids were obtained. The highest frequency of triploids was 12.9%, suggesting that a higher percentage of 2n pollen can improve the rate of triploid production.
In the present study, colchicine was successfully applied to induce chromosome doubling during microsporegenesis and 68 triploids were produced. Among all the treatments, the highest rate triploid production was 13.2%. This is slightly higher than that achieved in cross combinations with induced 2n pollen as documented by Kang et al. (2000). The expected triploid production rate was significantly higher than the observed triploid production rate. This suggested that the induced 2n pollen did, compared with haploid pollen, have weak competition during fertilization. According to Zhao et al. (2017), slow-growing pollen-tubes of 2n pollen were responsible for the lower triploid production.
It is crucial that the mutagenic agent be applied to cells at a suitable stage. To induce 2n pollen in P. tomentosa × P. bolleana, Kang et al. (1999) documented that the most effective stage is the pachytene. Li et al. (2014) reported that the most suitable stage for induction of 2n pollen by colchicine in P. alba was varied from leptotene to pachytene. Additionally, Lu et al. (2013) recorded that the most effective stage for inducing 2n female gametes via exposure to high temperature in Populus adenopoda Maxim. is from pachytene to diakinesis. In this study, the suitable stage for induction of 2n pollen with colchicine varied from zygotene to diakinesis during the development of PMCs. This is slightly earlier than the suitable stages for inducing chromosome doubling using high temperature. The disparity could be partially explained by the slower diffusion of colchicine inside the anther, whereas heat treatment is more direct.
The number of colchicine injections is also an important factor to consider when inducing chromosome doubling. In previous studies, the number of colchicine injections significantly affected the occurrence rate of colchicine-induced 2n pollen in P. tomentosa × P. bolleana (Kang et al. 1999), P. × popularis (Xi et al. 2011) and P. alba (Li et al. 2014). The percentage of induced 2n pollen changed with the number of colchicine injections. More injections resulted in higher occurrence rates of colchicine-induced 2n pollen. However, 2n pollen production in each treated catkin significantly decreased when injections were excessive (Kang et al. 1999; Zhao et al. 2017). A suitable number of colchicine injections must be determined to produce the most 2n pollen.
Heterozygosity within an unreduced gamete relies on the mechanism of 2n gamete formation. Veilleux (1985) and Bretagnolle and Thompson (1995) have reviewed the mechanism of 2n gamete formation. Genetic composition was different in 2n gametes produced between first division restitution (FDR) and second division restitution (SDR). 2n gametes formed by FDR theoretically transmit approximately 80% parental heterozygosity to the offspring, whereas 2n gametes formed by SDR transmit around 40% (Mendiburu and Peloquin 1977). Therefore, FDR-type 2n gametes are more valuable than SDR-type 2n gametes in sexual polyploidization in plants (Bretagnolle and Thompson 1995). Wang et al. (2010) induced 2n eggs with colchicine which appeared to be totally homozygous because they originated from mitotic inhibition of functional microspores. Not only FDR 2n female gametes but also SDR 2n female gametes were created by high temperature stress in P. pseudo-simonii Carr. × P. nigra L.‘Zheyin#3’ (Wang et al. 2012), P. adenopoda (Lu et al. 2013) and P. tomentosa (Kang et al. 2015). In the present study, all PMCs treated with colchicine took place from leptotene to metaphaseⅠ, leading to FDR-type 2n pollen production.
4.2 Colchicine, male bud development and induced 2n pollen viability
Colchicine is considered one of the strongest toxic mutagenic agents for 2n gamete induction in plants. Accordingly, after male buds were treated with colchicine in P. tomentosa clone 5088, buds developed slowly and the anthers dried out and became brown. Some eventually died.
Male buds can also respond to colchicine injection by producing less pollen. Kang and Wang (2010) reported that pollen production decreased as the number of colchicine injections increased and significant differences in pollen production per catkin were observed after a different number of colchicine injections were given in P. tomentosa × P. bolleana. In the present study, our findings are consistent with those of Kang and Wang (2010). Also, when colchicine injection was given at the diakinesis stage of PMCs, a significant decrease in the pollen production was observed (p < 0.001). In buds that were given seven injections, the pollen production was 2.8 ± 0.4 mg per bud, which was significantly lower than that of buds given three or five injections.
High temperatures are also used to generate 2n gametes in plants (Li et al. 2017). Pécrix et al. (2011) reported that incubation at 36 ℃ in early meiosis could lead to not only the appearance of numerous 2n pollen grains but also a decrease in viability and pollen ectexine defects in Rosa spp. Compared to natural 2n pollen, the morphological analysis of the colchicine-induced 2n pollen using scanning electron microscopy demonstrated that no significant defects in ectexine structure existed between colchicine-induced 2n pollen. Subsequently, the colchicine-induced pollen germination experiments revealed that no significant difference in germination rate existed between colchicine-induced 2n pollen and natural 2n pollen, and 68 triploids derived from induced 2n pollen were obtained, suggesting that colchicine injection will not lead to dysfunction of the induced 2n pollen.
Acknowledgements
We thank Guan Country nursery of Shandong province for providing the plant material. We also thank Dr. Joe Lambert for his linguistic editing. This study was financially supported by the National Natural Science Foundation of China (31570646).
References
Bretagnolle F., Thompson J.D. (1995). Gametes with the somatic chromosome number: mechanisms of their formation and role in the evolution of autopolyploid plants. New Phytologist 129(1): 1–22. https://doi.org/10.1111/j.1469-8137.1995.tb03005.x.
Dhooghe E., Grunewald W., Leus L., Van Labeke M.C. (2009). In vitro polyploidization of Helleborus species. Euphytica 165(1): 89–95. https://doi.org/10.1007/s10681-008-9763-9.
Galbraith D.W., Harkins K.R., Maddox J.M., Ayres N.M., Sharma D.P., Firoozabady E. (1983). Rapid flow cytomtric analysis of the cell cycle in intact plant tissue. Science 220(4601): 1049–1051. https://doi.org/10.1126/science.220.4601.1049.
Hancock J. (1997). The colchicine story. HortScience 32(6): 1011–1012. https://doi.org/10.21273/HORTSCI.32.6.1011.
Kang N., Bai F.Y., Zhang P.D., Kang X.Y. (2015). Inducing chromosome doubling of embryo sac in Populus tomentosa with high temperature exposure for hybrid triploids. Journal of Beijing Forestry University 37: 79–86. [In Chinese].
Kang X.Y., Wang J. (2010). Studies on technique of polyploid inducing in Populus. Science Press, Beijing. p. 44–65. [In Chinese].
Kang X.Y., Zhu Z.T., Lin H.B. (1999). Study on the effective treating period for pollen chromosome doubling of Populus tomentosa × P. bolleana. Scientia Silvae Sinicae 35: 21–24. [In Chinese].
Kang X.Y., Zhu Z.T., Zhang Z.Y. (2000). Breeding of triploids by the reciprocal crossing of Populus alba × P . glandulosa and P. tomentosa × P. bolleana. Journal of Beijing Forestry University 22(6): 8–11. [In Chinese].
Li Y., Guo Q., Wang J., Kang X.Y. (2014). Colchicine-induced pollen chromosome doubling and its cytological effects in Populus alba L.. Journal of Nuclear Agricultural Sciences 28: 749–756. [In Chinese].
Li Y.J., Tian M.D., Zhang P.D. (2017). Embryo sac chromosome doubling in Populus alba × P. glandulosa induced by high temperature exposure to produce triploids. Breeding Science 67(3): 233–238. https://doi.org/10.1270/jsbbs.16193.
Lu M., Zhang P.D., Kang X.Y. (2013). Induction of 2n female gametes in Populus adenopoda Maxim by high temperature exposure during female gametophyte development. Breeding Science 63(1): 96–103. https://doi.org/10.1270/jsbbs.63.96.
Mashkina O.S., Burdaeva L.B., Belozerova M.M., Vyunova L.N. (1989). Method of obtaining diploid pollen of woody species. Lesoved 1: 19–25.
Mendiburu A.O., Peloquin S.J. (1977). The significance of 2n gametes in potato breeding. Theoretical and Applied Genetics 49(2): 53–61. https://doi.org/10.1007/BF00275164.
Müntzing A. (1936). The chromosomes of a grant Populus tremula. Hereditas 21(2–3): 383–393. https://doi.org/10.1111/j.1601-5223.1936.tb03206.x.
Nilsson-Ehle H. (1936). Note regarding the gigas form of Populus tremula found in nature. Hereditas 21(2–3): 72–382.
Pécrix Y., Géraldine R., Hélène F., Mireille C., Serge G., Manuel L.B. (2011). Polyploidization mechanisms: temperature environment can induce diploid gamete formation in Rosa sp.. Journal of Experimental Botany 62(10): 3587–3597. https://doi.org/10.1093/jxb/err052.
Vaughn K., Lehnen L. (1991). Mitotic disrupter herbicides. Weed Science 39(3): 450–457. https://doi.org/10.1017/S0043174500073215.
Veilleux R.E. (1985). Diploid and polyploid gametes in crop plants: mechanisms of formation and utilization in plant breeding. Plant Breeding Reviews 3: 253–288. https://doi.org/10.1002/9781118061008.ch6.
Wang J., Kang X.Y., Li D.L., Chen H.W., Zhang P.D. (2010). Induction of diploid eggs with colchicine during embryo sac development in Populus. Silvae Genetica 59(1–6): 40–48. https://doi.org/10.1515/sg-2010-0005.
Wang J., Li D.L., Kang X.Y. (2012) Induction of unreduced megaspores with high temperature during megasporogenesis in Populus. Annals of Forest Science 69(1): 59–67. https://doi.org/10.1007/s13595-011-0152-5.
Xi X.J., Jiang X.B., Li D., Guo L.Q., Zhang J.F., Li B.L. (2011). Induction of 2n pollen by colchicine in Populus × popularis and its triploids breeding. Silvae Genetica 60(1–6): 155–160. https://doi.org/10.1515/sg-2011-0021.
Yang J., Yao P.Q., Li Y., Mo J.Y., Wang J.Z., Kang X.Y. (2016). Induction of 2n pollen with colchicine during microsporogenesis in Eucalyptus. Euphytica 210(1): 69–78. https://doi.org/10.1007/s10681-016-1699-x.
Yao C.L., Pu J.W. (1998). Timber characteristics and pulp properties of the triploid of Populus tomentosa. Journal of Beijing Forestry University 20: 18–21. [In Chinese].
Zhang P.D., Kang X.Y. (2013). Occurrence and cytological mechanism of numerically unreduced pollen in diploid Populus euphratica. Silvae Genetica 62(1–6): 285–291. https://doi.org/10.1515/sg-2013-0034.
Zhao C.G., Tian M.D., Li Y.J., Zhang P.D. (2017). Slow-growing pollen-tube of colchicine-induced 2n pollen responsible for low triploid production rate in Populus. Euphytica 213: 94. https://doi.org/10.1007/s10681-017-1881-9.
Zhu Z.T. (2006). Genetic improvement of Populus tomentosa. China Forestry Press, Beijing. p. 155–213. [In Chinese].
Zhu Z.T., Lin H.B., Kang X.Y. (1995). Studies on allotriploid breeding of Populus tomentosa B301 clones. Scientia Silvae Sinicae 31: 499–505. [In Chinese].
Zhu Z.T., Kang X.Y., Zhang Z.Y. (1998). Studies on selection of natural triploid of Populus tomentosa. Scientia Silvae Sinicae 34: 22–31. [In Chinese].
Zlesak D.C., Thill C.A., Anderson N.O. (2005). Trifluralin-mediated polyploidization of Rosa chinensis minima (Sims) Voss seedlings. Euphytica 141(3): 281–290. https://doi.org/10.1007/s10681-005-7512-x.
Total of 29 references.

