The occurrence and pathogenicity of fungi associated with Orthotomicus erosus on Pinus brutia in the Southern Marmara, Türkiye
Acer S., Arslangündoğdu Z., Lehtijärvi A. (2023). The occurrence and pathogenicity of fungi associated with Orthotomicus erosus on Pinus brutia in the Southern Marmara, Türkiye. Silva Fennica vol. 57 no. 2 article id 10764. https://doi.org/10.14214/sf.10764
Highlights
- Three ophiostomatoid fungi species are recorded, associated with Orthotomicus erosus on Pinus brutia for the first time in Türkiye
- Ceratocystis ips has the highest frequency. The association between Leptographium wingfieldiiand Orthotomicus erosus occurred with high frequency
- While all three fungal species are severely pathogenic for pines in some regions, they are weak pathogens for Turkish pine in Türkiye.
Abstract
Fungal pathogens associated with bark beetles constitute one of the most significant problems to forest health. The Turkish pine (Pinus brutia Ten.) is a native species in the forests of Türkiye and occurs in the Mediterranean-type climate. The Southern Marmara is a natural occurrence area of Turkish pine in the Marmara Region. In the present study, trap logs were set up in pure Pinus brutia forests to investigate fungi associated with Orthotomicus erosus (Wollaston) (Mediterranean pine beetle) throughout Southern Marmara. Orthotomicus erosus adults, larvae, and their galleries were sampled and individually cultured on a 1% CSMA (cycloheximide–streptomycin malt agar) medium. Three ophiostomatoid fungi were identified using morphological characteristics and molecular genetic analyses: Ceratocystis (syn. Ophiostoma) ips (Rumbold) C. Moreau, Graphilbum sp. H.P. Upadhyay & W.B. Kendr., and Leptographium wingfieldii M. Morelet. All three species were new in records of the fungal flora of Türkiye. The most dominant of these species, Ceratocystis ips was isolated 69%. Unexpectedly, L. wingfieldii had a high-frequency association with O. erosus (27%). The pathogenicity tests showed that all three species could cause lesions on branches of Turkish pine but were non-pathogenic or weak pathogenic.
Keywords
Ophiostoma;
pathogenicity;
Graphilbum;
Leptographium;
Mediterranean pine beetle;
Turkish pine;
Southern Marmara
-
Acer,
Istanbul University-Cerrahpaşa, Department of Forest Entomology and Protection, Faculty of Forestry, 34473, Istanbul, Türkiye
E-mail
sacer@iuc.edu.tr

- Arslangündoğdu, Istanbul University-Cerrahpaşa, Department of Forest Entomology and Protection, Faculty of Forestry, 34473, Istanbul, Türkiye E-mail zeynel@iuc.edu.tr
- Lehtijärvi, Isparta University of Applied Sciences, Sütçüler Prof. Dr. Hasan Gürbüz Vocational School, 32950, Isparta, Türkiye E-mail askolehtijarvi@isparta.edu.tr
Received 30 June 2022 Accepted 12 June 2023 Published 22 June 2023
Views 104374
Available at https://doi.org/10.14214/sf.10764 | Download PDF

1 Introduction
Pinus brutia Ten. (Turkish or Calabrian pine) is an important representative species of the genus Pinus in Türkiye. Although its major distribution area is along the Mediterranean coast of Türkiye, it also grows in small stands in Palestine, Jordan, Syria, Iraq, Lebanon, the Greek Islands, Italy, and Cyprus (Sarıbaş and Ekici 2004; Kavgacı et al. 2017). The natural occurrence area of Turkish pine is in Southern Marmara for Marmara Region (Atalay et al. 1998). Turkish pine is a species with a high growth rate and desirable timber qualities for the wood and paper industries. Today, the proportion of Turkish pine in afforestation ranges from 40% to 50% in Türkiye (Ürgenç 1998).
Turkish pine is a fast-growing coniferous species native to the Mediterranean region that requires high rainfall but tolerates a wide temperature range (Fady et al. 2003). It has a high genetic diversity and adaptability (Korol et al. 2002). Pinus brutia requires temperatures of –5 to 12 ℃ and water-demanding humid/sub-humid climates with annual rainfall ranging from 400 to 1300 mm (Chambel et al. 2013). The elevation limits of this species are between 0–600 m in Marmara Region (Atalay et al. 1998), but in the southern part of its natural range, it can reach 1200–1400 m (Chambel et al. 2013). It shows a distinct preference for south-facing slopes with high insolation and avoids sites with fog or high rainfall. It prefers shallow soils, limestone, schist, and other bedrock types (Atalay et al. 1998; Şentürk et al. 2019).
Climate change alters the distributions and population structures of forest pests and pathogens, the way they interact with trees, and their evolutionary capacity, while also affecting the ability of forest systems to resist and tolerate attacks (Linnakoski et al. 2019). Reis et al. (2018) showed that in a Turkish pine forest characterized by semi-arid extreme summer drought, the annual diameter increments of trees in a local high-humidity area compared to a semi-arid field with the same elevation and aspect characteristics were significantly high. In addition, substantial increases or decreases in precipitation cause significant growth differences in diameter increment in Turkish pine stands where arid climatic conditions prevail. Furthermore, disturbances in water availability, particularly water shortage conditions, can have unfavourable effects on the tree host and benefit the infectivity of the pathogens in the host (Terhonen et al. 2019).
Pure natural stands of Turkish pine are mostly found in fire-prone areas in Türkiye (Turna and Bilgili 2006; Tavşanoǧlu and Gürkan 2009; Bilgili et al. 2019). Fire is a major disturbance in P. brutia forests, and several adaptations generally contribute to the post-fire regeneration of Turkish pine (Boydak 2004). However, fires cause wounds that disrupt living tissues, including the vascular cambium, resulting in a loss of function in surviving trees (Sutherland and Smith 2000). Post-fire stress causes injured trees to use available carbohydrate storage to replenish dead or injured fine roots, thereby depleting carbohydrate reserves. Then, these fire-weakened trees could succumb to second-order pests, diseases, or climatic stress (Otrosina et al. 1999; Menges and Deyrup 2001; Sword Sayer and Haywood 2006; Morgan Varner et al. 2009; Alexou and Dimitrakopoulos 2014).
Previous studies have shown that P. brutia contains a variety of biotic stressors. The pine processionary moth attacks (Thaumetopoea pityocampa Dennis & Schiff. and T. wilkinsoni Tams.) is a considerable problem faced by pure stands of P. brutia (Kanat et al. 2005; Sbay and Zas 2018). They are the primary insect defoliator of Mediterranean pines and can cause significant growth loss and overall decline (Jacquet et al. 2012). Defoliation by pine processionary moths removes photosynthetic material and affects several vital functions. Semiz et al. (2016) reported that the levels of GST (Glutathione S-transferase enzyme) transcripts were significantly high in moth feeding period samples of P. brutia. They also suggested that GST could be a valuable marker created by pine processionary moth herbivory stress on P. brutia. The combined effects of previous herbivory and drought on tree growth and carbohydrate pools show significant additive effects (Jacquet et al. 2014). Marchalina hellenica Genn. is known as the primary source of pine honey production, and pine honey producers spread the beetle by manually transporting it into different forest areas in Türkiye. It lives on pine trees, especially P. brutia and P. halepensis Mill. in Türkiye and Greece (Margaritopoulos et al. 2003; Akkuzu et al. 2006). Marchalina hellenica feeds by absorbing the sap, which is 20% protein and 80% carbohydrate, from the vascular bundles of trees and directly damages the photosynthetic tissue, weakening and stressing the pine trees (Bacandritsos et al. 2004). Yeşil et al. (2005) and Gallis (2007) showed that M. hellenica had a negative effect on the growth of infested P. brutia and P. halepensis trees in Türkiye and Greece.
Like other coniferous, Turkish pine is stressed and weakened by climate change, forest fires, and invasion of insect defoliators or scale insects and has trouble withstanding attacks by secondary biotic pests (Ciesla 2011; Linnakoski et al. 2019). Orthotomicus erosus (Wollaston) is the most abundant bark beetle species that attacks Turkish pine (Kalapanida-Kantartzi et al. 2010; Sarıkaya and Avcı 2011; İbiş and Sarıkaya 2012; Acer et al. 2021). It is named the Mediterranean pine beetle or the Mediterranean pine engraver beetle. Although O. erosus can attack Cedrus, Abies, and Picea spp., it primarily attacks pine species and reproduces only in Pinus spp. (Mendel and Halperin 1982). The beetle is a secondary pest that infests recently fallen trees and wounded and stressed living trees by forming galleries under the bark and reproducing in them. Stressed trees are more prone to attacks; therefore, attacks are more intense in successive years of drought and in trees ravaged by fire or storms. Weakened, infested trees often die, and where populations are high, massive attacks can lead to the death of healthy trees (Gil Sánchez and Pajares Alonso 1986). In Croatia, it was reported that O. erosus occurrence increased owing to cumulative stress on trees caused by drought intensity, frequency and aridification trends. Increased voltinism, high dispersal abilities by flight and easy transportation with the infested material of O. erosus altered the population level of this pest and became a crucial forest pest (Pernek et al. 2019). Due to attacks of bark beetles, especially O. erosus, from 2005 to 2015 in Balıkesir province alone, control measures were implemented in approximately 8700 ha of coniferous stands (Cebeci and Baydemir 2019).
Beetles carry a great diversity of wood-inhabiting fungi, and environmental factors, vector beetle communities, and, to some extent, fungal source communities are determinants of beetle-associated fungal communities (Seibold et al. 2019). The interactions between beetles, fungi, and the host plant have been well documented (Batra 1903; Beaver 1989; Schowalter and Filip 1993; Krokene and Solheim 1998; Harrington 2005; Six 2012; Hofstetter et al. 2015). Associations among pine species, bark beetles, and ophiostomatoid fungi are among the most significant host-beetle-mycobiota associations (Rane and Tattar 1987; Lieutier et al. 1989; Nevill et al. 1995; Dong Zhou et al. 2001; Jacobs and Wingfield 2001; Solheim et al. 2001; Sabbatini Peverieri et al. 2006; Romón et al. 2007). The ophiostomatoid fungi include nearly 400 species (including asexual stages) in 14 genera classified within the Ophiostomatales and Microascales, of which 134 species belong to the Ophiostoma sensu lato (De Beer et al. 2013, 2022). Few studies have investigated the association among Turkish pine-O. erosus-ophiostomatoid fungi (Ben Jamaa et al. 2007; Dori-Bachash et al. 2015). Furthermore, it has been reported that O. erosus transmits ophiostomatoid fungi to other pine species (Romón et al. 2007).
Only a few previous studies on ophiostomatoid species have been conducted in Türkiye. Ophiostoma ulmi (Buisman) Nannf. and Ophiostoma novo-ulmi Braiser are responsible for Dutch elm disease in Europe, North America, and Asia. Although the disease agent was not detected, elm deaths were reported in Türkiye by Acatay (1940). Karahan and Maden (1979) isolated Graphium (Ceratocystis) ulmi M.B. Schwarz from elm trees and Graphium sp. from poplar trees in Central Anatolia. In 1980, Brasier (1991) reported O. novo-ulmi from Southern Türkiye. Sümer (1983) investigated the spread of the disease based on elm deaths in Türkiye. Lehtijärvi et al. (2018) isolated Ceratocystis platani (J.M. Walter) Engelbrecht & Harrington, which causes canker stain disease of Platanus trees on the European side of Istanbul. Many studies have discussed fungi associated with bark beetles on pines worldwide (e.g. Jacobs et al. 2004; Sabbatini Peverieri et al. 2006; Jankowiak and Kot 2011; Dori-Bachash et al. 2015). However, no study has been conducted on the fungal species carried by bark beetles associated with any coniferous species in Türkiye.
The objective of this study was to isolate and identify common fungi associated with O. erosus, which attacks Turkish pine in both natural and planted Turkish pine forests in the Southern Marmara Region of Türkiye. In addition, the pathogenicity of the isolated fungi was studied by inoculating Turkish pine branches in planted forest.
2 Materials and methods
2.1 Sample collection and morphological observations
Our research area was located in the Balıkesir and Çanakkale Provinces in the southern part of the Marmara Region of Türkiye (39°20´ to 40°45´N, 25°37´ to 28°30´E) (Fig. 1). The area includes coastal regions in the Marmara and Aegean seas of Türkiye and is the location of the Strait of Çanakkale (Doğukan et al. 2008; CSB 2013). The climate in the region is the characteristic transitional climate between the Mediterranean Sea and the Black Sea. The average annual temperature is 14.8 ℃, with a maximum summer temperature of 30.6 ℃ and a minimum winter temperature of 2.7 ℃. The average annual rainfall is approximately 615.4 mm (MGM 1998). The prevailing soil types in the FAO system are eutric cambisol and orthic luvisol (FAO 2022). Forty-three percent of the Southern Marmara Region is covered with forests, of which 66% Turkish pine (Karagöz and Demirci 2006). This region is the boundary of the natural distribution of Turkish pine in the north.
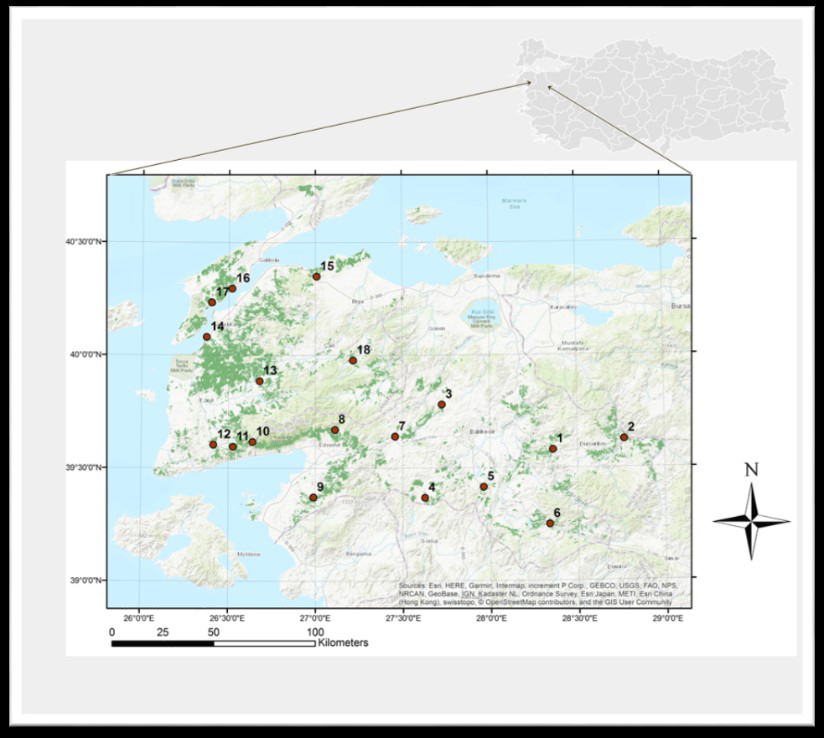
Fig. 1. Map of Southern Marmara, Türkiye, showing study sites denoted by 1–18 (with the distribution area of Turkish pine shown in dark green).
Eighteen representative study sites were selected, covering the distribution range of Turkish pine within the study area. The sites contained 20- to 25-year-old pure Turkish pine stands. In February 2014, 15 to 20 trap logs, approximately 1 m long and 0.2 m in diameter (bark thickness ~ 2 cm), were set in each study site, using the technique described by Tribe (1992). To examine the presence of fungi, O. erosus individuals and samples of blue-stained wood infested by this bark beetle were collected from trap logs in all 18 Turkish pine stands. The study sites, elevations, and geographical coordinates were also noted (Table 1) and indicated on a map using ArcGIS 10.2 (ESRI 2014) (Fig. 1). Trap logs were checked every 20 days, and three logs at each site were inspected for the presence of beetle entrance holes. All beetles brought to the laboratory were morphologically identified using a LEICA S8APO stereomicroscope (Grüne 1979; Selmi 1998).
| Table 1. Geolocation of study sites where wood traps were established in Southern Marmara, Türkiye. | |||||
| Study site | Geographical position | Elevation (m) | Study site | Geographical position | Elevation (m) |
| 1. Kireç | 39°34’46.6”N 28°21’42.1”E | 508 | 10. Küçükkuyu | 39°37’02.6”N 26°38’19.3”E | 557 |
| 2. Gökçedağ | 39°37’29.5”N 28°46’12.2”E | 467 | 11. Yeniçam | 39°35’43.2”N 26°31’35.2”E | 379 |
| 3. Balya | 39°46’52.1”N 27°43’33.5”E | 448 | 12. Ayvacık | 39°36’14.7”N 26°24’52.80”E | 269 |
| 4. Savaştepe | 39°22’07.0”N 27°37’34.9”E | 275 | 13. Bayramiç | 39°53’11.2”N 26°40’44.9”E | 204 |
| 5. Konakpınar | 39°24’57.7”N 27°57’44.8”E | 442 | 14. Çınarlı | 40°4’53.6”N 26°22’24.9”E | 43 |
| 6. Sınıdırgı | 39°14’58.3”N 28°20’17.4”E | 321 | 15. Dişbudak | 40°20’59.8”N 27°00’24.3”E | 133 |
| 7. İvrindi | 39°38’26.2”N 27°27’25.7”E | 310 | 16. Gelibolu | 40°17’45.3”N 26°31’11.8”E | 26 |
| 8. Edremit | 39°40’15.3”N 27°06’43.5”E | 372 | 17. Eceabat | 40°14’04.0”N 26°24’10.9”E | 107 |
| 9. Burhaniye | 39°22’19.5”N 26°59’19.8”E | 311 | 18. Yenice | 39°58’41.6”N 27°13’0.6”E | 380 |
2.2 Fungal isolation and identification
Trap logs were debarked and then the bark beetle adults (O. erosus), larvae, pupae, and galleries were collected. The larvae, pupae, and adults and pieces of the galleries measuring 1 cm2 were surface sterilized with 0.05% NaClO, washed three times with distilled water, and blotted on filter paper. Fungal isolations were performed in Petri dishes on autoclaved 1% CSMA (cycloheximide–streptomycin malt agar; 10 g malt extract, 15 g agar, 1000 ml distilled water, 5 ml cycloheximide solution, and 100 mg streptomycin). Each insect and gallery piece was placed separately in a Petri dish. The cultures were incubated at 20 ℃ in the dark until colonies were formed. The actively growing colonies were subcultured on 2% malt extract agar (MEA) and incubated at 20 ℃. The fungal isolates were classified into three morphological groups according to the culture morphology. Representatives of each of these groups were identified based on the morphological characteristics of the ascomata, ascospores, conidiophores, and conidia and DNA sequencing.
The fruiting structures were mounted in lactophenol on glass slides and characterized using LEICA DM 750 light microscope. Measurements were made of 50 of each morphological structure so that the ranges and average size values could be calculated. The growth ability was determined at different temperatures ranging from 5 °C to 35 °C at intervals of 5 °C; three replicates were used. Agar disks (5 mm in diameter) taken from the edge of a freshly grown colony were placed on 2% MEA medium (20 ml) in 90 mm petri dishes. Colony diameters (two perpendicular measurements) on each plate were determined at 3, 5, and 7 d after incubation, and growth rates were calculated in millimetres per day. Fungal structures were compared with the species descriptions given in the literature (e.g. Jacobs and Wingfield 2001; Wingfield et al. 1993). The cultures used in this study were stored in the culture collection of Istanbul University-Cerrahpaşa, Faculty of Forestry, Department of Forest Entomology and Protection in Istanbul.
Identification based on morphology was confirmed by DNA sequencing of representative isolates (Tab. 1). DNA was extracted from pure fungal cultures after incubation for 8–10 d at 20 ℃ in 2% MEA. For DNA preparation, agar plugs taken from MEA cultures were planted onto sterile cellophane sheets overlaid on 2% MEA plates. After 3–7 d of growth at 20 ℃ in the dark, the mycelium was harvested from the cellophane sheet by scraping the surface with a scalpel (Kim et al. 2003; Roe et al. 2010). DNA extraction was performed using the EURx GeneMATRIX Plant & Fungi DNA Purification Kit, following the manufacturer’s instructions. The internal transcribed spacer (ITS) and 5.8S regions of the nuclear rRNA operon were amplified using the primers ITS1 (5’-CCGTAGGTGAACCTGCGG-3’) and ITS4 (5’-CCTCCGCTTATTGATATGC-3’) (White et al. 1990; Kim et al. 2003). Amplification of the D1/D2 region of the nuclear large subunit of the rRNA (LSU) gene was conducted using the primers NL1 (5´-GCATATCAATAAGCGGAGGAAAAG-3´) and NL4 (5´-GGTCCGTGTTTCAAGACGG-3´) (Guadet et al. 1989; Kolařík et al. 2006). Each PCR amplification was performed in a total volume of 35 µl consisting of 0.3 µM of each primer, 1x PCR buffer, 0.2 µM dNTPs, and 2 U Taq DNA Polymerase (Solis Biodyne FIREPol, Tartu, Estonia). The conditions used for the thermal cycling were as follows: an initial denaturation of the DNA at 95 °C for 5 min, followed by 40 cycles consisting of denaturation at 95 °C for 45 s, annealing at 57 °C for 45 s, extension at 72 °C for 60 s, and a final extension at 72 °C for 5 min. The PCR products were separated on a 1.5% agarose gel and visualized under UV light. Amplification products were purified with MAGBIO (HighPrep PCR Clean-up System; MagBio Genomics, Inc., Gaithersburg, Maryland, USA) and sequenced at Macrogen Europe B. V. (Amsterdam, The Netherlands). ITS and LSU sequences were compared by a BLAST search against DNA sequences deposited in NCBI GenBank (1988) to identify the most similar available sequences. The ITS sequences of C. ips and L. wingfieldii and, LSU sequences of Graphilbum sp. are deposited in GenBank with accession numbers OM86751–OM867520, OM885001–O M885004, and OM883868 and OM883867, respectively (Table 2).
| Table 2. Representative fungal isolates of Ophiostomatoid fungi associated with Orthotomicus erosus on Pinus brutia during the current study. | |||||||
| Taxon | Isolate name | Study site | GenBank accession no. | Close match in BLAST | Accessison of match | Identity % | |
| LSU | ITS | ||||||
| Ceratocystis ips | CZ27.52 | 18 | - | OM867517 | Ophiostoma ips* | OM468593 | 99.7 |
| CZ51.1 | 1 | - | OM867518 | O. ips | OM468597 | 99.7 | |
| CZ24.21 | 15 | - | OM867519 | O. ips | OM468593 | 99.7 | |
| CZ30.21 | 4 | - | OM867520 | O. ips | OM468597 | 100 | |
| Graphilbum sp. | CZ20.19 | 11 | OM883868 | - | Graphilbum rectanglosporium | OM514754 | 99.1 |
| CZ30.6 | 4 | OM883867 | - | G. rectanglosporium | OM514754 | 99.1 | |
| CZ30.24 | 4 | - | - | - | - | - | |
| CZ44.9 | 17 | - | - | - | - | - | |
| Leptographium wingfieldii | CZ33.40 | 7 | - | OM885001 | Leptographium wingfieldii | KP691916 | 99.7 |
| CZ41.7 | 14 | - | OM885002 | L. wing.fieldii | KP691916 | 100 | |
| CZ40.25 | 13 | - | OM885003 | L. wing.fieldii | KP691916 | 100 | |
| CZ42.32 | 15 | - | OM885004 | L. wing.fieldii | KP691916 | 100 | |
| * Ophiostoma ips (syn. for Ceratocystis ips). | |||||||
Information about the fungal strains used in this study is summarized in Tables 2 and 3. ITS and LSU sequence in this study and 47 isolates of ophiostomatoid fungi were aligned with ClustalW using Bioedit v.7.2.5 (Thompson et al. 1994). ML phylogenetic analysis was performed using MEGA v.11.0.13. For the phylogenetic relationship of C. ips estimated T92+G evolutionary model determined by Model Test based on Akaike Information Criteria (AIC) was applied. The estimated proportion of the shape parameter for the gamma distribution (G) was set to 0.39. For the phylogenetic relationship of Graphilbum sp., the TN93+G evolutionary model and gamma distribution (G) were set to 0.1. The phylogenetic tree of Graphilbum sp. was rooted in Ceratocystiopsis manitobensis (J. Reid & Georg Hausner) Zipfel, Z.W. de Beer & M.J. Wingf. while L. wingfieldii was determined by the T92 evolution model, the tree was rooted in Ophistoma quercus (Georgev.) Nannf., in Melin & Nannfeldt. (Tamura et al. 2021). The best-fit model of nucleotide substitution was calculated in MEGA 11 under default parameters using the Bayesian information criterion (Schwarz 1978). Support for the nodes was estimated from 1000 replications (Jankowiak 2012). Final adjustments were made in iTOL (https://itol.embl.de) (Letunic and Bork 2021).
| Table 3. List of reference sequences used for the phylogenetic tree in this study and their GenBank accesion. | |||||||
| Species name | Isolate number | Type1 | Isolated from | Country2 | Collector | GenBank accession numbers3 | |
| LSU | ITS | ||||||
| Ceratocystiopsis manitobensis | UM237 | T | P. resinosa/Manitoba beetle gallery | CAN | J. Reid | EU913674 | |
| Graphilbum anningense | CXY1939 | P. yunnanensis/T. yunnanensis | CHN | H.M. Wang | MH325162 | ||
| G. cf. rectangulosporium | VPRI43763 | P. radiata | AUS | A.J. Carnegie | MW046118 | ||
| G. cf. rectangulosporium | VPRI43843 | P. taeda | AUS | C. Trollip | MW046119 | ||
| G. crescericum | CMW22828 | T | P. radiata/H. palliatus | ESP | P. Romón | OM514749 | |
| G. fragrans | CBS 279.54 | T | SWE | A. Mathiesen-Kaeaerik | MH868872 | ||
| G. fragrans | CBS 279.54 | P. sylvestris | SWE | T.C. Harrington | AF198248 | ||
| G. ipis-grandicollis | VPRI43762 | T | Pinus radiata/I. grandicollis | AUS | A.J. Carnegie | MW046117 | |
| G. rectangulosporium | CMW26258 | M. Procter | OM514754 | ||||
| Grosmannia aurea | CMW667 | A | Pinus contorta var. latifolia | CAN | R.W. Davidson | OM501387 | |
| Leptographium longiclavatum | SL-Kw1436 | CAN | AY816686 | ||||
| L. lundbergii | CMW2190 | T | P. sylvestris | NOR | H. Roll-Hansen | OM501432 | |
| L. pyrinum | CMW509 | T | D. adjunctus | USA | K.R.W. Davidson J. | OM501445 | |
| L. terebrantis | CMW29841 | T | D. terebrantis | USA | S.J. Baras | JF798477 | |
| L. wingfieldii | CBS 645.89 | E | T. piniperda | FRA | M. Morelet | AY935603 | |
| L. wingfieldii | MB192 | P. halepensis/T. destruens gallery | ISR | M. Dori-Bachash | KP691916 | ||
| L. wingfieldii | CMW4741 | P. densiflora | JPN | H. Masuya | OM501461 | ||
| L. wingfieldii | CBS 648.89 | E | P. brutia | GRC | Mich.-Ska. | AY935611 | |
| L. wingfieldii | MCC 125 | P. densiflora | JPN | M. Masuya | AY935608 | ||
| L. wingfieldii | CMW2096 | P. strobus /T. piniperda | EUR | M. Morelet | AY553398 | ||
| L. wingfieldii | CMW2096 | T | FRA | FIN | AY553398 | ||
| Ophiostoma bicolor | CBS492.77 | T | Picea glauca | CAN | S.M. Alamouti | DQ268604 | |
| O. floccosum | CMW34182 | T | Wood | SWE | A. Mathiesen-Käärik | KU184431 | |
| O. fuscum | CMW23196 | T | Picea abies/P. chalcographus | FIN | Linnakoski | HM031504 | |
| O. ips | AK188 | Ips acuminatus | UKR | K.V. Davydenko | KU663983 | ||
| O. ips | CMW7075 | T | I. i̇nteger | USA | CT Rumbold | AY546704 | |
| O. ips | MB176 | P. halepensis/O. erosus | ISR | Dori-Bachash | KP691908 | ||
| O. ips | CMW6418 | P. elliottii/O. erosus | ZAF | XD Zhou | AY546702 | ||
| O. ips | S36.9 | Pine wood | PRT | C.S. Vicente | OM468593 | ||
| O. ips | S40.1a | P. pinaster | PRT | C.S. Vicente | OM468597 | ||
| O. ips | MCC 023 | Tp beetle | JPN | H. Masuya | AY194935 | ||
| O. japonicum | CMW2202 | T | I. typographus japonicus/Picea jezoensis | JPN | Y. Yamaoka | OM501492 | |
| O. montium | CMW15419 | P. contorta | USA | B. Bentz | OM501498 | ||
| O. piceae | C1087 | T | DEU | Münch | AF198226 | ||
| O. pseudobicolor | CFCC52683 | T | I. subelongatus/Larix gmelinii | CHN | Q. Lu | MK748188 | |
| O. quercus | CMW2467 | T | Quercus spp. | FRA | M. Morelet | AY466626 | |
| O. rectangulosporium | MAFF 238951 | JPN | N. Ohtaka | AB235158 | |||
| 1 T = ex-type, E = ex-epitype, A = authentic isolate. 2 AUS: Australia, CAN: Canada, CHN: China, DEU: Germany, ESP: Spain, EUR: Europe, FIN: Finland, FRA: France, GRC: Greece, ISR: Israel, JPN: Japan, NOR: Norway, PRT: Portugal, SWE: Sweden, UKR: Ukraine, USA: United States of America, ZAF: South Africa. 3 ITS: internal transcribed spacer, LSU: ribosomal large subunit. | |||||||
2.3 Pathogenicity tests
Pathogenicity tests were conducted using four representative isolates of each fungal species (Table 2) from managed Turkish pine stands in the Istanbul Regional Directorate of Forestry in June 2019. The mean diameter at the breast height of the trees was 20 cm. The inoculum was prepared by growing the fungal isolates on 2% MEA at 20 ℃ in the dark. Sterile 2% MEA plugs were used as negative controls. Depending on the appropriate trees in the study area, each of the 12 isolates was tested in two trees, and five branches was inoculated in each tree; 24 trees were inoculated in total. Inoculations were made by removing the outer bark from the branches with a 5 mm cork borer, and 5 mm diameter agar plugs cut from the tested isolates were then placed with the mycelium facing downward into the wounds. Controls (a total of 40 branches in 8 trees) were inoculated with sterile 2% MEA. A total of 160 branches in 32 trees, including control, were assigned to pathogenicity treatments. All inoculation points were sealed with removed bark and masking tape to reduce desiccation. Inoculations were harvested after 20 weeks and measured. Re-isolations of the fungi were attempted from the inoculation points. Small pieces of tissue were cut from the edges of necrotic areas with a sterile scalpel and plated onto 1% CSMA. The plates were incubated in the dark at 21 ℃ for 3 weeks. Re-isolation plates were examined to confirm that the inoculated fungi caused the lesions by assessing colony morphology and microscopic characteristics. Analysis was performed with ANOVA using the GLM procedure in SAS software (SAS 2022). The lesion length data did not meet the normality assumption (Kolmogorov–Smirnov test, p = 0.13) and were log-transferred before analysis to correct for heteroscedasticity. The data were compared using Tukey’s multiple comparison test. The re-isolation results were tested using the logit model in SPSS in IBM SPSS version 21 for Windows (IBM 2021).
3 Results
3.1 Fungal identification
Based on culture morphology, isolates were divided into three distinct groups. The first group produced perithecia, the second formed only mycelia, and the third group contained the Leptographium anamorph. The morphological and molecular evidence showed that three ophiostomatoid fungi associated with O. erosus were identified from Turkish pine in this study. They were Ceratocystis ips (syn. Ophiostoma ips), Graphilbum sp., and Leptographium wingfieldii.
Ceratocystis ips (Rumbold) C. Moreau (syn. Ophiostoma ips (Rumbold) Nannf.), Revue Mycol., Paris 17 (Suppl. Colon. no. 1): 22 (Moreau 1952). The fungus grew optimally at 25 ℃ to 68 mm in diameter on 2% MEA in 10 days. No growth was found either below 5 ℃ or above 35 ℃ on MEA; the colony was pale brown to hyaline (i.e., glossy and translucent); and the hyphae of the fungus were immersed (Fig. 2a). The perithecia were globose and dark brown to black, (203.5–) 305.4 – 473.5 (–635.1) µm in diameter, ornamented with light brown aseptate hyphae and the necks were nearly cylindrical and dark brown, becoming brown at the apex, (137.6–) 1182.2 (–1393.1)µm in length with absent ostiolar hyphae. The ascospores were hyaline and one-celled and had a hyaline gelatinous sheath appearing pillow-shaped, (3.2–) 4.04 – 5.56 (–6.1) × (1.43–) 2.1 – 3.1 (–3.4) µm (Fig. 2b). Regarding the size of the measurements, in this study they were variable, compared to Rumbold (1931), Wingfield and Marasas (1980), Hutchison and Reid (1988), Pérez-Vera et al. (2009), and Kim et al. (2011).

Fig. 2. (a) Colony characteristics of Ceratocystis ips on 2% MEA, 25 ℃ for 10 d: (b) light microscopic micrographs; peritechia, ostium and ascospores of C. ips, (c) phylogenetic estimate, based on the ITS and 5.8S regions of the nuclear rRNA operon sequence analysis, showing potential phylogenetic relationships. The tree is rooted in Graphilbum fragrans. The tree was constructed with the MEGA program and evaluated using the bootstrap procedure (1000 replicates). Only bootstrap values >50% were provided. The analysis involved 20 nucleotide sequences. There were 1354 positions in the final dataset. The isolates obtained in this study are shown in bold.
Molecular identification of the CZ24.21, CZ27.52, CZ51.1 and CZ30.21 isolates was made using the amplified sequence of the ITS gene region of the genomic DNA. A BLAST search of the GenBank database using the determined sequence revealed the highest similarity (99.7% to 100%) to that of Ophiostoma ips. Three distinct groups are apparent from the phylogenetic analysis. All isolates of C. ips were placed in one clade and separated from another containing the sapstain species O. fuscum Linnak., Z.W. de Beer & M.J. Wingf., O. bicolor R.W. Davidson & D.E. Wells, O. montium (Rumbold) Arx. O. japonicum Yamaoka & M.J. Wingf., and Ophiostoma pseudobicolor Z. Wang & Q. Lu, in Wang, Liu, Wang, Meng, Liu, Decock, Zhang & Lu. The remaing were placed in the third clade (Fig. 2c).
Graphilbum sp. H.P. Upadhyay & W.B. Kendr., Mycologia 67: 800 (1975). Hyaline colonies on 2% MEA showed optimal growth at 20 ℃ and were 19–21 mm in diameter after 10 days (Fig. 3a). No growth was observed at either 5 ℃ or 35 ℃. There were only hyaline hyphae observed, with no conidia or perithecium.
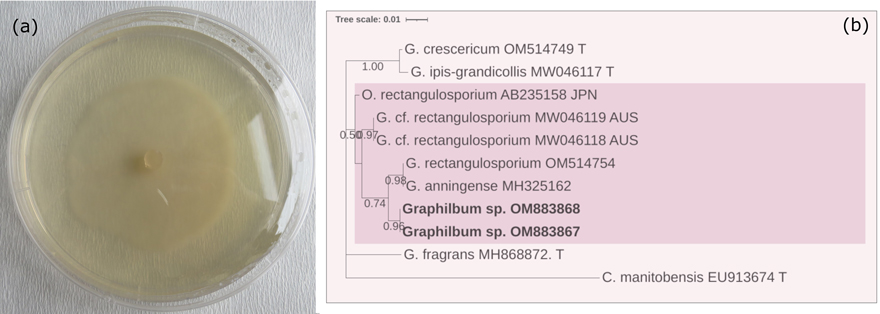
Fig. 3. (a) Colony characteristics of Graphilbum sp. grown on 2% MEA, 25 ℃ for 10 d. (b) Based on the LSU sequence analysis, a phylogenetic estimate showed potential phylogenetic relationships. The tree was constructed with the MEGA program and evaluated using the bootstrap procedure (1000 replicates). Only bootstrap values >50% were provided. This analysis included 11 nucleotide sequences. There were 896 positions in the final dataset. The isolates obtained from this study were printed in bold.
A BLAST search of the GenBank database using the determined sequence revealed that the highest similarity (99.1%) was with that of Graphilbum rectangulosporium (Ohtaka, Masuya & Yamaoka) Z.W. de Beer & M.J. Wingf. In the phylogenetic tree, our Graphilbum sp. isolates were placed in one clade together with Graphilbum anningense H.M. Wang, Q. Lu & Zhen Zhang and G. rectangulosporium (Fig. 3b).
Leptographium wingfieldii M. Morelet, Ann. Soc. Sci. Nat. Arch. Toulon et du Var 40(1): 43 (Morelet 1988). It grew optimally at 25 ℃ and covered all 90 mm of the petri dish on 2% MEA medium in 10 d (Fig. 4a). No growth was observed at either –5 ℃ or 35 ℃. The dark brown colonies were immersed and covered with aerial mycelia. Conidiogenous apparatus observed that singly or in groups is light in colour and (63.6–) 66.3 – 93.5 (–117,3) µm long. The conidia were highly variable in length and width and occasionally slightly clavate, transparent, non-segmented, (4.9–) 5.7 – 7.5 (–8.7) × (1.8–) 2.5 – 3.1 (–4.2) µm. The ageing hyphae were dark brown, while the younger hyphae were yellowish. The conidiophores of these isolates also had a slightly yellowish colour, similar to those of the conidiophores of L. wingfieldii (Fig. 4b). These findings were like those reported by Jacobs and Wingfield (2001) for this species.

Fig. 4. (a) Colony characteristics of Leptographium wingfieldii grown on 2% MEA, 25 ℃ for 10 d. (b) Light microscopic micrographs: conidiophores and conidia of L. wingfieldii, (c) Phylogenetic estimate based on the ITS and 5.8S regions of the nuclear rRNA operon sequence analysis, showing the potential phylogenetic relationships. The tree is rooted in O. quercus. The tree was constructed with the MEGA program and evaluated using the bootstrap procedure (1000 replicates). Only bootstrap values >50% were provided. This analysis involved 18 nucleotide sequences. There were 842 positions in the final dataset. The isolates obtained from this study were printed in bold.
A BLAST search of the GenBank database using the determined sequence revealed that the highest similarity (99.7% to 100%) was with that of L. wingfieldii. In the phylogenetic tree, four L. wingfieldii isolates obtained from P. brutia attacked by O. erosus grouped together with other reference isolates of L. wingfieldii. Also, Grosmannia aurea (Rob.-Jeffr. & R.W. Davidson) Zipfel, Z.W. de Beer & M.J. Wingf. and Leptographium lundbergii Lagerb. & Melin, in Lagerberg, Lundberg & Melin, and Leptographium pyrinum R.W. Davidson strains were grouped in this section (Fig. 4c).
In total, 694 fungal isolates were obtained from 664 adults, 17 larvae or pupae, and 23 gallery systems of O. erosus. Associated with O. erosus, 481 C. ips isolates were obtained from all but two of the study sites while 24 Graphilbum sp. and 187 L. wingfieldii isolates were taken from 10 and 11 study sites, respectively. The most dominant species was C. ips, with an average isolation frequency of 69%, followed by frequency of 27% Leptographium wingfieldii and 4% Graphilbum sp. (Table 4).
| Table 4. Fungal species detected from Orthotomicus erosus adults, larvae, pupa and gallery (number of fungal isolates frequency) collected at all study sites. | ||||||
| Fungal Species | Study site/s | Gallery | Larvae/Pupae | Beetle | Total | % |
| Ceratocystis ips | 1,2,3,4,5,6,8,10,11,12,13,14,15,16,17,18 | 13 | 15 | 463 | 481 | 69 |
| Graphilbum sp. | 1,2,3,4,6,11,12,13,15,17 | 2 | - | 24 | 26 | 4 |
| Leptographium wingfieldii | 1,2,3,4,5,6,7,10,13,14,15 | 8 | 2 | 177 | 187 | 27 |
3.2 Pathogenicity tests
The lesion lengths (F = 5.52, p < 0.0001) were significantly affected by inoculation treatment in the linear model. In each case, no evidence was found to suggest the inoculation was responsible for wilting, drying, or mortality of branches of Turkish pine trees. All inoculations probably resulted in colonization, achieved by formed phloem necrosis and resin exudation around inoculation points. Phloem necrosis was seen on controls, but only several had resin exudations. The lesion lengths on the phloem caused by C. ips (with a lesion length average of 51 mm, CZ51.1) and L. wingfieldii (45 mm, CZ41.7) were more significant statistically than the ones caused by the controls (F = 19.32, p < 0.0001). However, there was a statistically significant difference in the lengths of lesion caused by C. ips and L. wingfieldii. Graphilbum sp. isolates induced significantly shorter lesions compared to C. ips and L. wingfieldii and did not differ from the control excluding one isolate (CZ20.19) (F = 4.664, p < 0.0001). There is a significant difference among the inoculated trees, by lesion length (F = 3.57, p < 0.0001). The third and sixth P. brutia individuals inoculated with respectively CZ27.52 and CZ51.1 isolates of C. ips, and the twelfth individual inoculated with the CZ41.7 isolate of L. wingfieldii caused significantly larger lesions than the others. All tested fungi were re-isolated from tissue surrounding lesions. The colony morphologies and microscopic characteristics of re-isolation colonies were confirmed with original pure cultures. However, infection from inoculated trees was confirmed in 91% of C. ips, 64% of L. wingfieldii and 52% of Graphilbum sp. (Table 5).
| Table 5. Length (and standard error) of the lesion in phloem in branches of Turkish pine 4 months after inoculation with Ophiostomatoid spp. | ||||||
| Species | Isolate no. | Tree no. | Mean lesion length (mm) | Re-isolation % | ||
| 1th tree | 2nd tree | Total | ||||
| Ceratocystis ips | CZ24.21 | 1b/2a | 52 ± 27.1 | 15.8 ± 4.9 | 33.9a ± 26.5 | 95.5 |
| CZ27.52 | 3c/4b | 67 ± 11.5 | 43.4 ± 6.8 | 55.2bc ± 15.3 | 86.4 | |
| CZ51.1 | 5a/6c | 55.8 ± 8.6 | 75.2 ± 30.5 | 65.5c ± 30.5 | 86.4 | |
| CZ30.21 | 7b/8b | 50.4 ± 9.9 | 51.8 ± 10.7 | 51.1bc ± 9.7 | 95.5 | |
| Total | 51.4 ± 24.2 | 91 | ||||
| Leptographium wingfieldii | CZ33.40 | 9b/10a | 63.8 ± 69.9 | 25 ± 11.1 | 44.4b ± 51.4 | 86.4 |
| CZ41.7 | 11b/12c | 48.2 ± 6.7 | 83.6 ± 22.9 | 65.9c ± 24.5 | 68.2 | |
| CZ40. 25 | 13a/14a | 33.6 ± 20.2 | 35.2 ± 5.8 | 34.4b ± 14.0 | 31.8 | |
| CZ42.32 | 15a/16b | 24 ± 15.1 | 44.6 ± 19.6 | 34.3b ± 19.8 | 68.2 | |
| Total | 44.8 ± 32.3 | 64 | ||||
| Graphilbum sp. | CZ20.19 | 17b/18a | 43.6 ± 9.5 | 28.6 ± 12.2 | 36.1b ± 13.0 | 77.3 |
| CZ30.6 | 19a/20a | 40.6 ± 14.2 | 21.4 ± 5.3 | 31.0a ± 14.3 | 40.9 | |
| CZ30.24 | 21a/22a | 30.6 ± 13.9 | 28.4 ± 13.8 | 29.5a ± 13.1 | 4.5 | |
| CZ44.9 | 23a/24a | 31.8 ± 3.4 | 26.6 ± 9.3 | 29.2a ± 7.2 | 86.4 | |
| Total | 31.5 ± 12.1 | 52 | ||||
| Control | 25a – 32a | 25.3a ± 7.7 | ||||
| Lesion length data were transformed (log10) before analysis though non-transformed values were reported. The same letters within the columns are not significantly different from one another at p < 0.0001 | ||||||
4 Discussion
To date, no study of ophiostomatoid species was conducted on conifers in Türkiye. In the present study, based on morphological characteristics, DNA sequence comparisons and phylogenetic tree analysis, three ophiostomatoid fungi species, including C. ips, Graphilbum sp., and L. wingfieldii, were identified associated with O. ersosus on P. brutia from Türkiye. All were new records for Türkiye’s fungal flora but known commonly worldwide.
Ceratocystis ips is well-known both as a sapstainer and also as a tree pathogen (Mathiesen-Käärik 1960; Wingfield and Marasas 1980; Hutchison and Reid 1988; Lieutier et al. 1991; Jacobs and Wingfield 2001; Kirisits 2007; Min et al. 2009; Pérez-Vera et al. 2009; Jankowiak 2012; Davydenko et al. 2017). Zhou et al. (2007) found a higher genetic diversity in the North American than in the European population and suggested that North America could be the possible source region of C. ips. The microscopy measurements of C. ips were variable (Rumbold 1931; Hutchison and Reid 1988; Pérez-Vera et al. 2009; Kim et al. 2011). One of the three groups formed from phylogenetic analyses includes C. ips strains isolated from different geographies accompanying the C. ips isolates in the present study. C. ips was the most frequent (69%) fungal species isolated from O. erosus. Our results were in agreement with previous reports by Zhou et al. (2007) in which C. ips constituted 60% of the fungi isolated from O. erosus in South Africa. In addition, Dori-Bachash et al. (2015) found that C. ips was the most frequently encountered (52.4%) fungal species isolated from O. erosus in Israel. Ceratocystis ips was first described from Ips calligraphus (Germar), which was inhabiting Pinus echinata Miller, Pinus sylvestris L., and Pinus rigida Miller (Rumbold 1941). On the other hand, C. ips was isolated from O. erosus inhabiting P. sylvestris, Pinus nigra J.F. Arnold, Pinus pinaster Aiton, P. halepensis, P. pinea L., Pinus patula Schiede ex Schltdl. & Cham., Pinus elliottii Engelm, and Pinus radiata D. Don in preceding studies in other countries (Dong Zhou et al. 2001; Ben Jamaa et al. 2007; Ghaioule et al. 2007; Romón et al. 2007; Dori-Bachash et al. 2015; Musvuugwa et al. 2016).
In the present study, Graphilbum sp. produced neither a conidial nor a sexual stage; we identified this species via the results of rDNA sequencing and phylogeny analysis. They had LSU sequences that were similar to the sequence of G. rectangulosporium. Ophiostoma species without teleomorph or anamorph in culture are unusual (Kim et al. 2011). However, Ohtaka et al. (2006) reported a new species from Japan, Ophiostoma rectangulosporium, Ohtaka, Masuya & Yamaoka, with only teleomorph stage in culture via molecular analysis and phylogenetic tree. Besides, G. rectangulosporium was identified from O. erosus, on Turkish pine in Israel (Dori-Bachash et al. 2015). On the other hand, Graphilbum sp. isolates were phylogenetically closely related to G. anningense in this study. However, G. anningense forms the conidial stage in the 2% MEA medium and differs in colony characteristics and growth rate (Wang et al. 2019).
Microscopic measurements, colony characteristics and growth rate of L. wingfieldii isolates in this study were similar to the records in Jacobs and Wingfield (2001). In addition, four L. wingfieldii isolates from P. brutia attacked by O. erosus grouped with referance isolates of L. wingfieldii on the ML phylogenetic tree. Additionally, G. aurea, L. lundbergii, and L. pyrinum strains were placed close to this clade. L. wingfieldii has been reported to be the most common fungal species associated with Tomicus spp., especially Tomicus piniperda (L.) and Tomicus destruens (Wollaston) (Solheim and Långström 1991; Långström et al. 1993; Jankowiak and Kurek 2003; Jacobs et al. 2004; Sabbatini Peverieri et al. 2006; Ben Jamaa et al. 2007; Dori-Bachash et al. 2015). It was also reported that L. wingfieldii was isolated from Hylastes opacus Erichson in England (Wingfield and Gibbs 1991). In North America, the fungus was associated with T. piniperda as well as Ips pini (Say, T.) and Dendroctonus valens LeConte (Jacobs et al. 2004). Romón et al. (2007) obtained only one isolate of L. wingfieldii out of 219 isolates from O. erosus in Spain. Dori-Bachash et al. (2015) isolated only one specimen from the gallery created by O. erosus in their research. In our study, L. wingfieldii was isolated with the second-highest frequency of 27% from 187 O. erosus individuals and gallery walls in 11 of 18 study sites. In this aspect, our study was the first to show that there was a significant association between O. erosus and L. wingfieldii.
We found only a few studies of fungal species associated with bark beetles of Turkish pine. Ben Jamaa et al. (2007) performed pathogenicity tests of fungal species isolated from O. erosus and T. piniperda on P. halepensis, P. pinaster, and P. brutia in Tunisia. Even though they did not confirm those fungal species (O. ips and Ophiostoma minus (Hedgc.) Syd. & P. Syd. and L. wingfieldii) on Turkish pine, they performed pathogenicity tests with those fungal species from other trees. In addition, they showed that the most susceptible species was Turkish pine. In Israel, three ophiostomatoid species, C. ips, G. rectangulosporium, and L. wingfieldii, were isolated from O. erosus, T. destruens, and Pityogenes calcaratus (Eichhoff) occurring on P. brutia and P. halepensis in forests (Dori-Bachash et al. 2015). In agreement with these reports, we obtained the same species from O. erosus on P. brutia but also L. wingfieldii in surprisingly high frequency. In the present study, the most abundant isolated fungus associated with O. erosus was C. ips, consistent with findings reported by Ben Jamaa et al. (2007) and Dori-Bachash et al. (2015).
Graphilbum sp. isolates did not cause significantly different lesions from the control. Hence, those isolates appeared to be non-pathogenic to Turkish pine. In agreement with our results, Dori-Bachash et al. (2015) showed with inoculation experiments that G. rectangulosporium isolates did not cause lesions on or deaths of P. halepensis or P. brutia seedlings. On the other hand, Jankowiak (2012) and Davydenko et al. (2017) showed that the inoculation of G. rectangulosporium isolates to Scots pine seedlings created significantly larger necrotic lesions than C. ips, as well as a loss of needles, an overall decline in health, and even death.
The lesion lengths on the phloem caused by C. ips and L. wingfieldii were significantly different from the controls. However, C. ips induced significantly larger lesions compared to L. wingfieldii. We contradict by Dori-Bachash et al. (2015), who reported that C. ips did not cause any lesions or wilting on P. brutia and P. halepensis in Israel. We agree with Nevill et al. (1995), who showed that single and combination inoculations of C. ips caused vertical lesions on 15- to 18-year-old loblolly pine trees, in Alabama.
Ben Jamaa et al. (2007) reported that C. ips caused shorter lesions than L. wingfieldii on P. halepensis in Tunisia. They suggested that L. wingfieldii was the most virulent agent and C. ips an intermediately virulent agent. In addition, they performed mass inoculations with two isolates of L. wingfieldii on P. brutia and P. halepensis trunks and revealed that P. brutia was more susceptible. Lieutier et al. (1989) isolated five ophiostomatoid fungal species, including C. ips and L. wingfieldii, from bark beetles of P. sylvestris in France. They proposed that L. wingfieldii was the most aggressive species, while C. ips was intermediately aggressive in pathogenicity experiments. Solheim et al. (2001) isolated three ophiostomatoid species, including L. wingfieldii, from Tomicus spp. on P. sylvestris, and then inoculated them to pine trees and showed that L. wingfieldii was more virulent. Dori-Bachash et al. (2015) showed that L. wingfieldii was responsible for the death of 2-year-old P. halepensis and P. brutia seedlings. Our results showed that C. ips was more aggressive than L. wingfieldii and were not in the agreement above studies.
It should be considered that there was a significant difference in lesion length among the inoculated trees in this study. The third and sixth P. brutia individuals inoculated with respectively CZ27.52 and CZ51.1 isolates of C. ips, and the twelfth individual inoculated with the CZ41.7 isolate of L. wingfieldii were more susceptible. These results suggest that susceptibility to each isolate obtained from the current study varies in different Turkish pine individuals.
5 Conclusion
The study included isolations of pure cultures of fungi from Mediterranean pine engraver beetles and their galleries, DNA sequencing, phylogenetic tree, and pathogenicity tests. We identified three species of ophiostomatoids for the first time in Türkiye. The study demonstrated the occurrence of fungi on O. erosus, discoloration of infested wood, and pathogenicity to the Turkish pine. Our findings showed that C. ips was the most frequent species associated with O. erosus on Turkish pine. In addition, our research recorded an important association between O. erosus and L. wingfieldii for the first time. In Türkiye, the fungi associated with bark beetles, especially those causing damage to a certain tree species, should be studied in detail. In addition, the results of the pathogenicity tests of our pioneering study showed that all three species could cause lesions on Turkish pine branches but that they were weak pathogens.
Acknowledgements
We thank the editors and two anonymous reviewers for valuable comments to improve the manuscript. We would like to thank the Balıkesir and Çanakkale Regional Directorates of Forestry for their support. We are also grateful to Mustafa Baydemir from the Balıkesir Regional Directorate of Forestry for the assistance with fieldwork. In addition, we are thankful to the Istanbul Regional Directorate of Forestry for letting us apply pathogenicity tests.
Funding
This work was supported by Istanbul University‒Cerrahpaşa, Scientific Research Projects [number 30163]. In addition, the corresponding author acknowledges Istanbul University–Cerrahpaşa, Institute of Graduate Studies, for this research. The data of this study was obtained from the PhD thesis (Acer 2020) entitled “Scolytinae Species (Coleoptera–Curculionidae) Damaging on Pinus brutia Ten. and their Associated Fungi in Balikesir Regional Directorate Forests, Turkey”.
Authors’ contributions
Sabiha Acer: Served as the primary author of the manuscript and conducted the analysis.
Zeynel Arslangündoğdu: Contributed to field studies and also to manuscript design.
Asko Lehtijärvi: Contributed to the editing and revisions of the manuscript.
References
Acatay A (1940) Türkiye’de Karaağaç Ölümü. [Dutch elm disease in Türkiye]. Orman ve Av. 9: 269–273.
Acer S (2020) Scolytinae species (Coleoptera-Curculionidae) damaging on Pinus brutia Ten. and their associated fungi in Balıkesir Regional Directorate forests, Turkey. Istanbul University-Cerrahpaşa, Doctoral thesis, İstanbul, Türkiye.
Acer S, Arslangündoğdu Z, Hızal E, Kumbaşlı M (2021) Relationships between bark beetle diversity and habitat characteristics in pine forests of South Marmara, Turkey. Appl Ecol Environ Res 19: 263–277. https://doi.org/10.15666/aeer/1901_263277.
Akkuzu E, Arslangündoǧdu Z, Selmi E (2006) Contribution to the knowledge of scale insects (Homoptera: Coccoidea) of coniferous trees from Turkey. J Biol Sci 6: 591–595. https://doi.org/10.3923/jbs.2006.591.595.
Alexou M, Dimitrakopoulos AP (2014) Early physiological consequences of fire as an abiotic stressor in metabolic source and sink of young Brutian pine (Pinus brutia Ten.). Tree Physiol 34: 1388–1398. https://doi.org/10.1093/treephys/tpu098.
Atalay İ, Sezer Lİ, Çukur H (1998) Kızılçam (Pinus brutia Ten.) Ormanlarının Ekolojik Özellikleri ve Tohum Nakli Açısından Bölgelere Ayrılması. [The eclologic porperties of red pine (Pinus brutia Ten.) forests and their regioning in terms of seed tranfer]. T.C. Orman Bakanlığı, Orman Ağaçları ve Tohumları Islah Araştırma Müdürlüğü.
Bacandritsos N, Saitanis C, Papanastasiou I (2004) Morphology and life cycle of Marchalina hellenica (Gennadius) (Hemiptera: Margarodidae) on pine (Parnis Mt.) and fir (Helmos Mt.) forests of Greece. Int J Entomol 40: 169–176. https://doi.org/10.1080/00379271.2004.10697413.
Batra LR (1903) Ecology of ambrosia fungi and their dissemination. Trans Kansas Acad Sci 66: 213–236. https://doi.org/10.2307/3626562.
Beaver RA (1989) Insect–fungus relationships in the bark and ambrosia beetles. In: Wilding N, Collins P, Hammond PM, Webber JF (eds) Insect-fungus Interactions, vol. 14 in Symposium of the Royal Entomological Society. Academic Press, London, England, pp 121–143. https://doi.org/10.1016/b978-0-12-751800-8.50011-2.
Ben Jamaa ML, Lieutier F, Yart A, Jerraya A, Khouja ML (2007) The virulence of phytopathogenic fungi associated with the bark beetles Tomicus piniperda and Orthotomicus erosus in Tunisia. For Pathol 37: 51–63. https://doi.org/10.1111/j.1439-0329.2007.00478.x.
Bilgili E, Coskuner KA, Usta Y, Saglam B, Kucuk O, Berber T, Goltas M (2019) Diurnal surface fuel moisture prediction model for Calabrian pine stands in Turkey. iForest 12: 262–271. https://doi.org/10.3832/ifor2870-012.
Boydak M (2004) Silvicultural characteristics and natural regeneration of Pinus brutia Ten.– a review. Plant Ecol 171: 153–163. https://doi.org/10.1023/B:VEGE.0000029373.54545.d2.
Brasier CM (1991) Ophiostoma novo-ulmi sp. nov., causative agent of current Dutch elm disease pandemics. Mycopathologia 115: 151–161. https://doi.org/10.1007/BF00462219.
Cebeci HH, Baydemir M (2019) Bark beetles of Balıkesir Region in Turkey. Entomol News 128: 109–119. https://doi.org/10.3157/021.128.0210.
Chambel MR, Climent J, Pichot C, Ducci F (2013) Mediterranean pines (Pinus halepensis Mill. and brutia Ten.). In: Luc E. Pâques (ed) Forest tree breeding in Europe. Springer Dordrecht, pp 229–265. https://doi.org/10.1007/978-94-007-6146-9_5.
Ciesla W (2011) Forest entomology: a global perspective. Wiley-Blackwell. https://doi.org/10.1002/9781444397895.
CSB (2013) İl Çevre Durum Raporu- Balıkesir. [Environmental Situation Report of Balıkesir]. T.C. Çevre ve Şehircilik Bakanlığı, Balıkesir Çevre ve Şehircilik İl Müdürlüğü, Balıkesir.
Davydenko K, Vasaitis R, Menkis A (2017) Fungi associated with Ips acuminatus (Coleoptera: Curculionidae) in Ukraine with a special emphasis on pathogenicity of ophiostomatoid species. Eur J Entomol 114: 77–85. https://doi.org/10.14411/eje.2017.011.
De Beer ZW, Seifert KA, Wingfield MJ (2013) A nomenclator for ophiostomatoid genera and species in the Ophiostomatales and Microascales. In: Seifert KA, de Beer ZW, Wing MJ (eds) The ophiostomatoid fungi: expanding frontiers. Biodiversity Series, pp 245–322.
De Beer ZW, Procter M, Wingfield MJ, Marincowitz S, Duong TA (2022) Generic boundaries in the Ophiostomatales reconsidered and revised. Stud Mycol 101: 57–120. https://doi.org/10.3114/sim.2022.101.02.
Doğukan H, Baran Ş, Yorulmaz H, Yenici E (2008) Çanakkale İli Çevre Durum Raporu. [Çanakkale Province environmental status report]. T.C Çevre ve Orman Bakanlığı, Çanakkale Valiliği İl Çevre ve Orman Müdürlüğü, Çanakkale.
Dong Zhou X, Wilhelm de Beer Z, Wingfield BD, Wingfield MJ, Wing MJ (2001) Ophiostomatoid fungi associated with three pine-infesting bark beetles in South Africa. Sydowia 53: 290–300.
Dori-Bachash M, Avrahami-Moyal L, Protasov A, Mendel Z, Freeman S (2015) The occurrence and pathogenicity of Geosmithia spp. and common blue-stain fungi associated with pine bark beetles in planted forests in Israel. Eur J Plant Pathol 143: 627–639. https://doi.org/10.1007/s10658-015-0713-9.
ESRI (2014) Environmental Systems Research Institute. https://www.esri.com/en-us/home.
Fady B, Semerci H, Vendramin GG (2003) EUFORGEN technical guidelines for genetic conservation and use for Aleppo pine (Pinus halepensis) and brutia pine (Pinus brutia). International Plant Genetic Resources Institute, Rome, Italy. ISBN 978-92-9043-571-6, ISBN 92-9043-571-2.
FAO (2022) The soil of Europe. FAO Soils Portal. https://storage.googleapis.com/fao-maps-catalog-data/geonetwork/fao_unesco_soil_map/Europe_V_sheet2.pdf. Accessed 20 November 2022.
Gallis AT (2007) Evaluation of the damage by insect Marchalina hellenica (Genn.) in eastern Attica, Greece. Conclusions for sustainable management of forests ecosystems. Proceedings of the 10th International Conference on Environmental Science and Technology Kos island, Greece, 5–7 September 2007.
Ghaioule D, Ibn Al Khattab O, El Omari H, Rahouti M (2007) Ophiostoma ips colonization of phloem and sapwood in maritime pine logs. Tunis J Plant Prot: 85–97.
Gil Sánchez LA, Pajares Alonso JA (1986) Los escolítidos de las coníferas en la Península Ibérica. [Scolitids of conifers in the Iberian Peninsula]. INIA Monographs. ISBN 9788474982503.
Grüne S (1979) Handbuch zur bestimmung der Europäischen Borkenkäfer. [Brief illustrated key to European bark beetles]. Verlag M & H Schapfer. ISBN 3-7944-0103-4.
Guadet J, Julien J, Lafay JF, Brygoo Y (1989) Phylgeney of some Fusarium species, as determined by large-subunit RNA sequence comparison. Mol Biol Evol 6: 227–242.
Harrington TC (2005) Ecology and evolution of mycophagous bark beetles and their fungal partners. In: Vega FE, Blackwell M (eds) Ecological and evolutionary advances in insect-fungal associations. Oxford University Press, pp 257–291.
Hofstetter RW, Dinkins-Bookwalter J, Davis TS, Klepzig KD (2015) Symbiotic associations of bark beetles. In: Vega FE, Hofstetter RW (eds) Bark beetles: biology and ecology of native and invasive species. Elsevier Inc., pp 209–245. https://doi.org/10.1016/B978-0-12-417156-5.00006-X.
Hutchison LJ, Reid J (1988) Taxonomy of some potential wood-staining fungi from New Zealand 1. Ophiostomataceae. New Zeal J Bot 26: 63–81. https://doi.org/10.1080/0028825X.1988.10410099.
İbiş HM, Sarıkaya O (2012) Bark beetle species diversity in brutian pine (Pinus brutia Ten.) Forests of İzmir Province in Turkey. Proceedings of the Forestry Science and Practice for The Purpose of Sustainable Development of Forestry, 20 Years of The Faculty of Forestry in Banja Luka, pp 533–543.
IBM C (2021) Released 2012. IBM SPSS statistics for Windows, version 21.0. IBM Corp, Armonk, NY.
Jacobs K, Wingfield MJ (2001) Leptographium species – tree pathogens, insect associates and agents of blue stain. The American Phytopathological Society. ISBN 0-89054-278-3.
Jacobs K, Bergdahl DR, Wingfield MJ, Halik S, Seifert KA, Bright DE, Wingfield BD (2004) Leptographium wingfieldii introduced into North America and found associated with exotic Tomicus piniperda and native bark beetles. Mycol Res 108: 411–418. https://doi.org/10.1017/S0953756204009748.
Jacquet JS, Orazio C, Jactel H (2012) Defoliation by processionary moth significantly reduces tree growth: a quantitative review. Ann For Sci 69: 857–866. https://doi.org/10.1007/s13595-012-0209-0.
Jacquet JS, Bosc A, O’Grady A, Jactel H (2014) Combined effects of defoliation and water stress on pine growth and non-structural carbohydrates. Tree Physiol 34: 367–376. https://doi.org/10.1093/treephys/tpu018.
Jankowiak R (2012) Ophiostomatoid fungi associated with Ips sexdentatus on Pinus sylvestris in Poland. Dendrobiology 68: 43–54.
Jankowiak R, Kot M (2011) Ophiostomatoid fungi associated with bark beetles (Coleoptera: Scolytidae) colonizing branches of Pinus sylvestris in southern Poland. Polish Bot J 56: 287–293.
Jankowiak R, Kurek M (2003) The early stages of fungal succession in Pinus sylvestris phloem and sapwood infested by Tomicus piniperda. Dendrobiology 56: 27–36.
Kalapanida-Kantartzi M, Milonas DN, Buchelos CT, Avtsiz DN (2010) How does pollution affect insect diversity. A study on bark beetle entomofauna of two pine forests in Greece. J Biol Res 13: 67–74.
Kanat M, Alma MH, Sivrikaya F (2005) Effect of defoliation by Thaumetopoea pityocampa (Den. & Schiff.) (Lepidoptera: Thaumetopoeidae) on annual diameter increment of Pinus brutia Ten. in Turkey. Ann For Sci 62: 91–95. https://doi.org/10.1051/forest:2004095.
Karagöz G, Demirci M (2006) Orman Varlığımız. [Our forest wealth]. T.C. Çevre ve Orman Bakanlığı, Orman Genel Müdürlüğü.
Karahan O, Maden S (1979) Diseases of elms (Ulmus spp.) and poplars (Populus spp.) in Central Anatolia and the causal agent. Plant Prot Bull 41: 175–180.
Kavgacı A, Šilc U, Başaran S, Marinšek A, Başaran MA, Košir P, Balpınar N, Arslan M, Denli Ö, Čarni A (2017) Classification of plant communities along postfire succession in Pinus brutia (Turkish red pine) stands in Antalya (Turkey). Turk J Botany 41: 299–307. https://doi.org/10.3906/bot-1609-34.
Kim JJ, Kim SH, Lee S, Breuil C (2003) Distinguishing Ophiostoma ips and Ophiostoma montium, two bark beetle-associated sapstain fungi. FEMS Microbiol Lett 222: 187–192. https://doi.org/10.1016/S0378-1097(03)00304-5.
Kim JJ, Hyun MW, Suh DY, Kim SH, Shin SC (2011) Ophiostoma ips isolated from reddish brown stained Japanese red pine wood. Plant Pathol J 27: 397. https://doi.org/10.5423/PPJ.2011.27.4.397.
Kirisits T (2007) Fungal associates of European bark beetles with special emphasis on the ophiostomatoid fungi. In: Lieutier F, Day KR, Battisti A, Grégoire JC, Evans HF (eds) Bark wood boring insects living trees eur a synth. Springer Dordrecht, pp 181–235.
Kolařík M, Sláviková E, Pažoutová S (2006) The taxonomic and ecological characterization of the clinically important heterobasiodiomycete Fugomyces cyanescens and its association with bark beetles. Czech Mycol 58: 81–98. https://doi.org/10.33585/cmy.58106.
Korol L, Shklar G, Schiller G (2002) Diversity among circum-Mediterranean populations of Aleppo pine and differentiation from brutia pine in their isoenzymes: additional results. Silvae Genet 51: 35–41.
Krokene P, Solheim H (1998) Ecology and population biology pathogenicity of four blue-stain fungi associated with aggressive and nonaggressive bark beetles. Phytopathology 88: 39–44. https://doi.org/10.1094/PHYTO.1998.88.1.39.
Långström B, Solheim H, Hellqvist C, Gref R (1993) Effects of pruning young Scots pines on host vigour and susceptibility to Leptographium wingfieldii and Ophiostoma minus, two blue-stain fungi associated with Tomicus piniperda. Eur J For Path 23: 400–415. https://doi.org/10.1111/j.1439-0329.1993.tb00820.x.
Lehtijärvi A, Oskay F, Doğmuş Lehtijärvi HT, Aday Kaya AG, Pecori F, Santini A, Woodward S (2018) Ceratocystis platani is killing plane trees in Istanbul (Turkey). For Pathol 48, artile id e12375. https://doi.org/10.1111/efp.12375.
Letunick I, Bork P (2021) Interactive tree of life (iTOL) v5: an online tool for phylogenetic tree display and annotation. Nucleic Acids Res 49: 293–296. https://doi.org/10.1093/nar/gkab301.
Lieutier F, Yart A, Garcia J, Ham MC, Morelet M, Levieux J, Levie J (1989) Champignons phytopathogènes associés à deux coléoptères scolytidae du pin sylvestre (Pinus sylvestris L.) et étude préliminaire de leur agressivité envers l’hôte. [Phytopathogenic fungi associated with two bark beetles of scots pine (Pinus sylvestris L.), and preliminary study of their aggressiveness for the host]. Ann des Sci For 46: 201–216. https://hal.archives-ouvertes.fr/hal-00882474.
Lieutier F, Yart A, Jay-Allemand CH, Delorme L (1991) Preliminary investigations on phenolics as a response of Scots pine phloem to attacks by bark beetles and associated fungi. Eur J For Path 21: 354–364. https://doi.org/10.1111/j.1439-0329.1991.tb00773.x.
Linnakoski R, Kasanen R, Dounavi A, Forbes KM (2019) Editorial: forest health under climate change: effects on tree resilience, and pest and pathogen dynamics. Front Plant Sci 10, article id 1157. https://doi.org/10.3389/fpls.2019.01157.
Margaritopoulos JT, Bacandritsos N, Pekas AN, Stamatis C, Mamuris Z, Tsitsipis JA (2003) Genetic variation of Marchalina hellenica (Hemiptera: Margarodidae) sampled from different hosts and localities in Greece. Bull Entomol Res 93: 447–453. https://doi.org/10.1079/ber2003260.
Mathiesen-Käärik A (1960) Studies on the ecology, taxonomy and physiology of Swedish insect-associated blue stain fungi, especially the genus Ceratocystis. OIKOS 11: 1–25. https://doi.org/10.2307/3564881.
Mendel Z, Halperin J (1982) The biology and behavior of Orthotomicus erosus in Israel. Phytoparasitica 10: 169–181. https://doi.org/10.1007/BF02994526.
Menges ES, Deyrup MA (2001) Postfire survival in South Florida slash pine: interacting effects of fire intensity, fire season, vegetation, burn size, and bark beetles. Int J Wildland Fire 10: 53–63. https://doi.org/10.1071/WF01009.
MGM (1998) Meteoroloji Genel Müdürlüğü. [TSMS, Turkish State Meteorological Service]. https://www.mgm.gov.tr. Accessed 20 October 2022.
Min L, Zhou XD, de Beer ZW, Wingfield NJ, Sun JH (2009) Ophiostomatoid fungi associated with the invasive pine-infesting bark beetle, Dendroctonus valens, in China. Fungal Divers 338: 133–145.
Moreau C (1952) Coexistence des formes Thielaviopsis et Graphium chez une souche de Ceratocystis major (van Beyma) nov. comb. [Coexistence of Thielaviopsis and Graphium forms in a strain of Ceratocystis major (van Beyma) nov. comb.]. Rev Mycol 17: 17–25.
Morelet M (1988) Observations sur trois deutéromycètes inféodés aux pins. [Observations on three deuteromycetes dependent on pines]. Ann la Société des Sci Nat d’Archéologie Toulon du Var. 40: 41–45.
Morgan Varner J, Putz FE, O’Brien JJ, Kevin Hiers J, Mitchell RJ, Gordon DR (2009) Post-fire tree stress and growth following smoldering duff fires. For Ecol Manage 258: 2467–2474. https://doi.org/10.1016/j.foreco.2009.08.028.
Musvuugwa T, Dreyer LL, Roetes F (2016) Future danger posed by fungi in the Ophiostomatales when encountering new hosts. Fungal Ecol 22: 83–89. https://doi.org/10.1016/j.funeco.2016.01.004.
NCBI (1988) National Center for Biotechnology Information, National Library of Medicine (US). https://www.ncbi.nlm.nih.gov/. Accessed 10 November 2022.
Nevill R, Kelley W, Field Office A, Perry T (1995) Pathogenicity to loblolly pines of fungi recovered from trees attacked by southern pine beetles. South J Appl For 19: 78–83. https://doi.org/10.1093/sjaf/19.2.78.
Ohtaka N, Masuya H, Yamaoka Y, Kaneko S (2006) Two new Ophiostoma species lacking conidial states isolated from bark beetles and bark beetle-infested Abies species in Japan. Can J Bot 84: 282–293. https://doi.org/10.1139/B05-164.
Otrosina WJ, Bannwart D, Roncadoiri RW (1999) Root-infecting fungi associated with a decline of longleaf pine in the southeastern United States. Plant Soil 217: 145–150. https://doi.org/10.1023/a:1004645115446.
Pérez-Vera OA, Alvarado-Rosales D, Cárdenas-Soriano E, Equihua-Martínez A, Cibrián-Tovar D, Álvarez-Moctezuma JG, Mejía-Sánchez D, Harrington TC (2009) Ophiostoma ips associated with the bark beetle (Dendroctonus adjunctus) in hartweg pine. Rev Mex Micol 30: 9–18.
Pernek M, Lacković N, Lukić I, Zorić N, Matošević D (2019) Outbreak of Orthotomicus erosus (Coleoptera, Curculionidae) on Aleppo pine in the Mediterranean Region in Croatia. South-East Eur For 10: 19–27. https://doi.org/10.15177/seefor.19-05.
Rane KK, Tattar TA (1987) Pathogenicity of blue-stain fungi associated with Dendroctonus terebrans. Plant Dis 71: 879–883. https://doi.org/10.1094/PD-71-0879.
Reis M, Dutal H, Abız B, Tat S (2018) Impacts of climate change on annual diameter increment of natural Calabrian pine (Pinus brutia Ten.) forests in Kahramanmaras. Turkish J For 19: 219–225. https://doi.org/10.18182/tjf.407487.
Roe AD, Rice A V., Bromilow SE, Cooke JEK, Sperling FAH (2010) Multilocus species identification and fungal DNA barcoding: insights from blue stain fungal symbionts of the mountain pine beetle. Mol Ecol Resour 10: 946–959. https://doi.org/10.1111/j.1755-0998.2010.02844.x.
Romón P, Zhou XD, Iturrondobeitia JC, Wingfield MJ, Goldarazena A (2007) Ophiostoma species (Ascomycetes: Ophiostomatales) associated with bark beetles (Coleoptera: Scolytinae) colonizing Pinus radiata in northern Spain. Can J Microbiol 53: 756–767. https://doi.org/10.1139/W07-001.
Rumbold CT (1931) Two blue-staining fungi associated with bark-beetle infestation of Pines. J Agric Res 43: 847–873.
Rumbold CT (1941) A blue stain fungus, Ceratostomella montium n. sp., and some yeasts associated with two species of Dendroctonus. J Agric Res 62: 589–601.
Sabbatini Peverieri G, Capretti P, Tiberi R (2006) Associations between Tomicus destruens and Leptographium spp. in Pinus pinea and P. pinaster stands in Tuscany, central Italy. For Pathol 36: 14–20. https://doi.org/10.1111/j.1439-0329.2006.00427.x.
Sarıbaş M, Ekici B (2004) Contribution to Pinus brutia Ten.’s natural spreading in Western Black Sea Region. ZKü J Bartin Fac For 6: 127–135.
Sarıkaya O, Avcı M (2011) Bark beetle fauna (Coleoptera: Scolytinae) of the coniferous forests in the Mediterranean Region of Western Turkey, with a new record for Turkish fauna. Turkish J Zool 35: 33–47. https://doi.org/10.3906/zoo-0901-8.
SAS (2022) SAS Institute Inc, Cary, NC, USA.
Sbay H, Zas R (2018) Geographic variation in growth, survival, and susceptibility to the processionary moth (Thaumetopoea pityocampa Dennis & Schiff.) of Pinus halepensis Mill. and P. brutia Ten.: results from common gardens in Morocco. Ann For Sci 75, article id 69. https://doi.org/10.1007/s13595-018-0746-2.
Schowalter TD, Filip GM (1993) Beetle-pathogen interactions in conifer forests. Academic Press. ISBN 0126289700.
Schwarz G (1978) Estimating the dimension of a model. Ann Stat 6: 461–464. https://doi.org/http://dx.doi.org/10.1214/aos/1176344136.
Seibold S, Müller J, Baldrian P, Cadotte MW, Štursová M, Biedermann PHW, Krah FS, Bässler C (2019) Fungi associated with beetles dispersing from dead wood – let’s take the beetle bus! Fungal Ecol 39: 100–108. https://doi.org/10.1016/j.funeco.2018.11.016.
Selmi E (1998) Türkiye Kabuk Böcekleri ve Savaşı. [Bark beetles of Türkiye and controls]. İstanbul Üniversitesi Yayınları. ISBN 975-404-466-X.
Semiz A, Celik-Turgut G, Semiz G, Özgün O, Sen A (2016) Association between herbivore stress and glutathione S-transferase expression in Pinus brutia Ten.. Cell Mol Biol 62: 89–94. https://doi.org/10.14715/cmb/2016.62.3.15.
Şentürk Ö, Gülsoy S, Tümer İ (2019) Potential distribution modeling and mapping of brutian pine stands in the inner parts of the middle Black Sea Region in Turkey. Polish J Environ Stud 28: 321–327. https://doi.org/10.15244/pjoes/81682.
Six DL (2012) Ecological and evolutionary determinants of bark beetle – fungus symbioses. Insects 3: 339–366. https://doi.org/10.3390/insects3010339.
Solheim H, Långström B (1991) Blue-stain fungi associated with Tomicus piniperda in Sweden and preliminary observations on their pathogenicity. Ann Sci For 48: 149–156. https://doi.org/10.1051/forest:19910203.
Solheim H, Krokene P, Långström B (2001) Effects of growth and virulence of associated blue-stain fungi on host colonization behaviour of the pine shoot beetles Tomicus minor and T. piniperda. Plant Pathol 50: 111–116. https://doi.org/10.1046/j.1365-3059.2001.00541.x.
Sümer S (1983) Karağaç Ölümü Hastalığının Türkiye Karaağaçlarının Yayılış Yörelerindeki Durumu. [Status of the Dutch elm disease and its distribution patterns within the elm populations in Türkiye]. İstanbul Üniversitesi Orman Fakültesi Derg 33: 141–166.
Sutherland EK, Smith KT (2000) Resistance is not futile: the response of hardwoods to fire-caused wounding. In: Yausy DA (ed) Proceedings of workshop on Fire, People, and the Central Hardwoods Landscape. U.S. Department of Agriculture, Forest Service, Northeastern Forest Experiment Station, pp 111–115.
Sword Sayer MA, Haywood JD (2006) Fine root production and carbohydrate concentrations of mature longleaf pine (Pinus palustris P. Mill.) as affected by season of prescribed fire and drought. Trees 20: 165–175. https://doi.org/10.1007/s00468-005-0022-6.
Tamura K, Stecher G, Kumar S (2021) MEGA11: Molecular Evolutionary Genetics Analysis version 11. Mol Biol Evol 38: 3022–3027. https://doi.org/10.1093/molbev/msab120.
Tavşanoǧlu Ç, Gürkan B (2009) Post-fire regeneration of a Pinus brutia (Pinaceae) forest in Marmaris National Park, Turkey. Int J Bot 5: 107–111. https://doi.org/10.3923/ijb.2009.107.111.
Terhonen E, Langer GJ, Bußkamp J, Răscuţoi DR, Blumenstein K (2019) Low water availability increases necrosis in Picea abies after artificial inoculation with fungal root rot pathogens Heterobasidion parviporum and Heterobasidion annosum. Forests 10, article id 55. https://doi.org/10.3390/f10010055.
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22: 4673–4680. https://doi.org/10.1093/nar/22.22.4673.
Tribe GD (1992) Colonisation sites on Pinus radiata logs of the bark beetles, Orthotomicus erosus, Hylastes angustatus and Hylurgus ligniperda (Coleoptera: Scolytidae). J Entomol Soc South Afr 55: 77–84. https://hdl.handle.net/10520/AJA00128789_3199.
Turna I, Bilgili E (2006) Effect of heat on seed germination of Pinus sylvestris and Pinus nigra ssp. pallasiana. Int J Wildl Fire 15: 283–286. https://doi.org/10.1071/WF05069.
Ürgenç S (1998) Ağaçlandırma Tekniği. [Afforestation technique]. İstanbul Üniversitesi Yayınları ISBN 975-404-446-5.
Wang HM, Wang Z, Liu F, Wu CX, Zhang SF, Kong XB, Decock C, Lu Q, Zhang Z (2019) Differential patterns of ophiostomatoid fungal communities associated with three sympatric Tomicus species infesting pines in south-western China, with a description of four new species. MycoKeys 50: 93–133. https://doi.org/10.3897/mycokeys.50.32653.
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and applications. Academic Press Inc., pp 315–322. https://doi.org/10.1016/B978-0-12-372180-8.50042-1.
Wingfield MJ, Gibbs JN (1991) Leptographium and Graphium species associated with pine-infesting bark beetles in England. Mycol Res 95: 1257–1260. https://doi.org/10.1016/S0953-7562(09)80570-4.
Wingfield MJ, Marasas W (1980) Ceratocystis ips associated with Orthotomicus erosus (Coleoptera: Scolytidae) on Pinus spp. in the Cape Province of South Africa. Phytophylactica 12: 65–69.
Wingfield MJ, Seifert KA, Webber JF (1993) Ceratocystis and Ophiostoma – taxonomy, ecology and pathogenicity. The American Phytopathological Society. ISBN 0-89054-156-6.
Yeşil A, Gürkan B, Saraçoǧlu Ö, Zengin H (2005) Effect of the pest Marchalina hellenica Gennadius (Homoptera, Margarodidae) on the growth parameters of Pinus brutia Ten. in Mugla Region (Turkey). Polish J Ecol 53: 451–458.
Zhou X, Burgess TI, De Beer ZW, Lieutier F, Yart A, Klepzig K, Carnegie A, Portales JM, Wingfield BD, Wingfield MJ (2007) High intercontinental migration rates and population admixture in the sapstain fungus Ophiostoma ips. Mol Ecol 16: 89–99. https://doi.org/10.1111/j.1365-294X.2006.03127.x.
Total of 113 references.

