Recent unexpected decline of forest growth in North Finland: examining tree-ring, climatic and reproduction data
Mäkinen H., Nöjd P., Helama S. (2022). Recent unexpected decline of forest growth in North Finland: examining tree-ring, climatic and reproduction data. Silva Fennica vol. 56 no. 4 article id 10769. https://doi.org/10.14214/sf.10769
Highlights
- Tree-ring indices of Scots pine showed decadal variations and a prolonged reduction both on mineral soil sites and peatlands after the mid 2000s
- The indices of Norway spruce had less pronounced decadal variations and no trend-like reduction over the last 15 years
- Temperature and drought explain some part of the observed growth variability.
Abstract
After a decades-long increasing trend, the recent results of the National Forest Inventory (NFI) reported a decline of forest growth in North Finland. The aim of this study was to assess climatic and reproduction influences behind the growth decline. We used tree-ring data that had been collected by NFI using systematic sampling. The tree-ring width series were detrended using the regional curve standardisation (RCS) removing age-related trends. The resulting tree-ring indices of Scots pine (Pinus sylvestris L.) showed decadal variations with low increment in the 1990s, and high increment in the 1980s and the early years of the current century. Thereafter, a prolonged growth reduction for pine started both on the mineral soil sites and peatlands. The tree-ring indices of Norway spruce (Picea abies (L.) Karst.) had less pronounced decadal variations and no trend-like reduction over the last 15 years. High spring and summer temperatures were found to enhance radial growth, but high winter temperatures were related to low growth for pine and spruce in the following summer. Temperature variation, accompanied by variables indicating years of drought and intensive flowering, accounted for 34% annual growth variance of pine and 21–44% for spruce. Thus, the results imply that climatic factors may have to some extent contributed to the recent growth reduction of pine. Due to its ecological and economic consequences growth decline needs to be further monitored and investigated. Moreover, analyses of stand and age structure, potentially affecting the growth decline, were beyond the scope of this paper, but also warrant further investigation.
Keywords
Pinus sylvestris;
Picea abies;
precipitation;
temperature;
seed production;
tree-ring width;
growth variation
-
Mäkinen,
Natural Resources Institute Finland (Luke), Latokartanonkaari 9, 00790 Helsinki
 https://orcid.org/0000-0002-1820-6264
E-mail
harri.makinen@luke.fi
https://orcid.org/0000-0002-1820-6264
E-mail
harri.makinen@luke.fi

- Nöjd, Natural Resources Institute Finland (Luke), Latokartanonkaari 9, 00790 Helsinki E-mail pekka.nojd@luke.fi
-
Helama,
Natural Resources Institute Finland (Luke), Ounasjoentie 6, 96200 Rovaniemi, Finland
 https://orcid.org/0000-0002-9777-3354
E-mail
samuli.helama@luke.fi
https://orcid.org/0000-0002-9777-3354
E-mail
samuli.helama@luke.fi
Received 8 July 2022 Accepted 20 December 2022 Published 27 December 2022
Views 75298
Available at https://doi.org/10.14214/sf.10769 | Download PDF

Supplementary Files
1 Introduction
Arctic forests are strongly influenced by the ongoing changes in the global climate system. Clearly warmer temperatures have already been measured at the northern regions and the trend has been predicted to continue towards the end of the 21st century, but the projections on precipitation are more variable (Vihma et al. 2016; IPCC 2021). Large-scale greening detected in northern regions since the early 1980s based on remote sensing vegetation data is thought to be caused by climate warming (Myneni et al. 1997; Nemani et al. 2003). Fennoscandia belongs to the region where warming has been extensive since the 1970s with a strong Atlantic influence modifying the seasonal climatic patterns (Marshall et al. 2018) and affecting vegetation and tree growth (Henttonen et al. 2017; Helama et al. 2018).
Previous studies have indicated that summer temperature is the main climatic parameter influencing tree growth in northern Fennoscandia, (Mikola 1950; Helama et al. 2004; Korpela et al. 2011). In addition, high spring temperatures have enhanced snow and soil frost melting and consequently tree growth (Hordo et al. 2011; Helama et al. 2013). Apart from temperature, the Arctic moistening affects the Arctic forests of northern Fennoscandia through increased precipitation and cloud cover (Helama et al. 2018). However, growth response to precipitation has generally been weak in northern boreal forests (Salminen et al. 2009; Korpela et al. 2011; Fleischer et al. 2022), even though our previous results indicated that water availability and drought may influence tree growth also at the high northern latitudes (Henttonen et al. 2014). As climate changes, the factors limiting tree growth are in change and the established knowledge on climate-growth relations may not apply. Thus, the recent and future changes in climate may lead to increasing uncertainties in forest growth predictions.
Apart from the direct climate-growth relationship, tree growth is linked to fluctuations in climatic conditions via reproduction complicating climate-growth studies. Abundant seed production constitutes an important resource investment and relocated allocation from growth to reproduction (Pukkala 1987; Despland and Houlle 1997; Climent et al. 2008; Vilà-Cabrera et al. 2014).
Investigations on growth trends have indicated increasing forest productivity in central and northern Europe (Pretzsch et al. 2014; Henttonen et al. 2017; Socha et al. 2021). However, recent results of the Finnish National Forest Inventory (NFI) reported a decline of forest growth in North Finland, a deviation from a decades-long rising trend in volume growth (Korhonen et al. 2021a,b). The most recent annual volume increment estimate for North Finland was 3.3 M m3 a–1 (–11%) smaller than the previous one, most of the decrease being targeted to Scots pine (Pinus sylvestris L.). In addition, a negative trend in tree-ring data of Scots pine was found over the past ~15 years (Korhonen et al. 2021b). In contrast, the growth of Norway spruce (Picea abies (L.) Karst.) show only a slight reduction during the same period. Generally, the growth of Norway spruce in Fennoscandia has been expected to be less resilient to climate warming and associated increase in prolonged extreme weather events, rather than the growth of Scots pine (Kellomäki et al. 2018; Matkala et al. 2021). Due to its shallow root system and long transpiring crown, Norway spruce is sensitive to periods of limited water availability. Indeed, droughts have been shown to limit growth of Norway spruce trees and result in mortality in southern Finland (Mäkinen et al. 2001; Jyske et al. 2010).
The growth decline is widespread, as the NFI is based on systematic cluster sampling over the region and the confidence intervals for the growth estimates are narrow (Korhonen et al. 2021b). The extent of the decline is worrisome not only for forest owners but also for the society as a whole as wood consumption in the region is increasing due to a new bioproduct mill, the largest-ever investment by forest industries to Finland, and enlargements of sawmill industries, motivated by the recent high demand of pulp and saw timber. Moreover, additional mills are being planned. Lack of explanation for the substantial decline is a handicap for accurate assessment of annual sustainable cuts and for determining appropriate silvicultural practices enhancing forest growth.
The analyses of this study were motivated by the observed decline of the overall forest growth and the downturn of tree-ring indices of Scots pine in North Finland. Recent data show an increase in summer temperatures in the region since around 1980 (Irannezhad et al. 2015; Meier et al. 2022), linear trends corresponding to 2.1°C warming both of June–August and May–September seasons observed in Sodankylä meteorological station (central Finnish Lapland) since that date (calculated from open data available at fmi.fi). Contrary to expectations that warmer summers enhance northern conifer growth, the recent warming has not led to increased growth in North Finland (Korhonen et al. 2021b).
The aim of this study was to assess potential climatic influences behind the growth decline. Moreover, we compared the climate-growth relationships of Scots pine and Norway spruce to unveil potential differences between these two main tree species of the region. As site properties may have a profound effect on growth response, we analysed climate-growth relationship of both tree species separately on mineral soils and on peatlands where growth frequently is limited by excessive amount of water, not lack of it (Edvardsson et al. 2015; Blanchet et al. 2017; Nöjd et al. 2017). We used the extensive tree-ring data systematically sampled as a part of the NFI. Thus, the data represent the forests in North Finland (except for the three northernmost municipalities), in contrast to targeted sampling of old trees on ecologically marginal sites (cf., Klesse et al. 2018).
2 Material and methods
A systematically sampled set of increment cores were collected from North Finland by the NFI during the years 2004–2021. The study region of this paper consists of the NFI sampling regions of ‘Lapland and Kuusamo’ and ‘Ostrobothnia and Kainuu’ (Korhonen et al. 2021a), excluding ‘Upper Lapland’, i.e., the three northernmost municipalities (Enontekiö, Utsjoki and Inari). The sampling design in the NFI has been based on systematic cluster sampling (Tomppo et al. 2011; Korhonen et al. 2021a). Tree measurements were made on circular sample plots where the plot radius depend on stem diameter. Sub-samples of the trees were selected as sample trees by weighting the sampling probability with stem basal area. The NFI data included a high number of stands and sample trees (23 048 Scots pine and 8277 Norway spruce trees, hereafter pine and spruce) on forest land and poorly productive forest land. However, the sample size of the cored trees has decreased over the recent years because an increasing number of sample plot clusters have been established as permanent which means that the sample trees are not cored (Supplementary file S1). In the ongoing cycle (NFI13), the proportion of temporary clusters is 20%.
The data were stratified into four subgroups according to tree species (pine, spruce, but excluding deciduous trees which together represent 18% of the total volume of growing stock in the region) and main site type (mineral soils, peatlands). There are 3.8 M ha of peatlands on productive and poorly productive forest land, which make up 34% of the total area of productive and poorly productive forest land in North Finland (Korhonen et al. 2021a).
On every sample tree, an increment core was collected from the bark to the pith at breast height (1.3 m). The rings of the cores were measured to 0.01 mm using the WinDendroTM software (Regent Instruments Inc., Quebec, Canada) and visually cross-dated. The trees with less than six rings were excluded from this study. The tree-ring width series of years 1970–2021 were detrended by using the regional curve standardisation (RCS) removing age-related trends from tree-ring data (Briffa et al. 1992; Helama et al. 2017). The RCS curve was calculated by averaging the tree-ring widths according to their age from pith outward (adjusted by pith offset estimate) and the mean of all the series was modelled by a stiff spline (Suppl. file S2). The tree-ring indices (IRindt,i) were calculated as ratios between the measured values (IRt,i) and the RCS curve values (IRRCS,i) for the appropriate tree age i for tree t:

The tree-ring indices were then averaged by calendar year into a mean time series. The RCS standardisation was separately performed for the four subgroups, i.e., pine and spruce on mineral soil sites and peatlands. A plot was classified as peatland site if the thickness of the peat layer covering the mineral soil was over 30 cm.
Data on cone production were included in the analysis to account for growth reductions due to intensive flowering and cone production. The intensity of cone production was obtained from the Natural Resources Institute Finland (Pekka Helenius, unpublished). The data contained the average number of cones per sample tree in 34 and 18 stands for pine and spruce, respectively, in North Finland. The cone crop measurements were initiated in 1979, i.e., they did not cover the early part of the study period. To identify the years of intensive flowering and cone production (‘mast’ years), a threshold of 60 and 66 cones per tree for pine and spruce, respectively, was defined based on the 90th quantile of cone production and visual inspection of the cone data. If the cone production exceeded the threshold, the year in question in spruce and the previous year in pine was marked as an intensive flowering year (Flowering = 1) and the other years were marked as normal years (Flowering = 0). For pine, the years of abundant number of cones were also marked as years of intensive cone production (seed maturation) as cone development period of pine is one year longer than that of spruce (Cone = 1/0).
Weather data (daily mean temperature, precipitation sum, global radiation) were obtained from the Finnish Meteorological Institute. The data set covering the period 1970 to 2021 consist of daily values interpolated into a 10 × 10 km grid (Venäläinen and Heikinheimo 2002). The weather data for each plot having sample trees were extracted and averaged using the NFI plot-level latitude and longitude coordinates. Because the mean temperature and global radiation were significantly correlated, global radiation was omitted from the analyses. Because the impact of severe droughts on tree growth is not well known at the high northern latitudes, we identified the years with lowest July precipitation as drought years (Dry = 1) and the other years as normal years (Dry = 0), in the same way as for the flowering and cone production, based on the 90th quantile and visual inspection of July precipitation distribution. Accordingly, the years with lowest June precipitation were identified as drought years, but they were unrelated to the growth variation and the variable was not retained in the final analysis.
Pearson correlation coefficients over years 1970–2021 were calculated between the tree-ring indices and mean monthly temperatures and precipitation sums from the June of the previous year to the August of the current year. The correlations were calculated separately for pine and spruce and for mineral soils and peatlands. To quantify and simultaneously estimate the effects of the weather variables, drought, flowering and cone production, a linear model was fit to the mean chronologies for both tree species and main site types. Because the physiological processes and rates of reactions that lead to ring formation are often non-linear rather than linear (Cook and Pederson 2011; Wilmking et al. 2020), a natural log-transformation was applied to the increment indices and to the weather variables, except for the winter temperatures and the dummy variables for flowering, cone production and drought. The Durbin-Watson statistic was used to test for autocorrelation in the residuals of the model. The statistical analyses were performed in the SAS software (transreg, arima and reg procedures), version 9.4 (SAS Institute Inc. 2013).
3 Results
3.1 Growth variation
The tree-ring indices of pine showed highly similar annual variation on mineral soil sites and peatlands (Fig. 1A). In the 1970s, years with especially high increment were 1976 and 1979. In the 1980s, the tree-ring indices of pine were positive for several years, but the 1990s was a decade of low increment with especially low indices in 1992 and 1995. Thereafter, the growth recovered, and the indices were positive until 2006. A prolonged growth decline started after 2006 and the tree-ring indices of pine have been negative for the latter part of 2000s and the whole 2010s, except in 2011 and 2012. On the peatland sites, the recent decline has been even greater than on the mineral soil sites and the indices have fallen below the low values of the early 1970s.
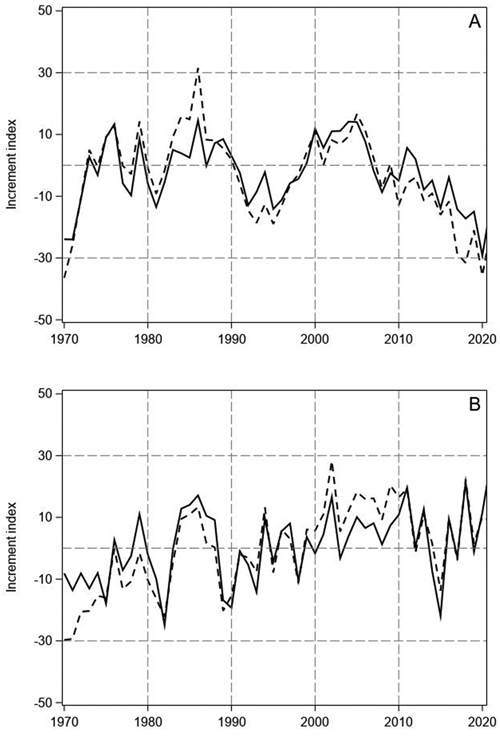
Fig. 1. Tree-ring indices of Scots pine (A) and Norway spruce (B) on mineral soil (continuous line) and on peatlands (dashed line) in North Finland.
The tree-ring indices of spruce also showed similar variation on the mineral soil sites and peatlands, but the indices had less pronounced decadal variations than for pine (Fig. 1B). Like for pine, a period of high increment occurred in the 1980s. Moreover, the indices of spruce were mainly positive in the early and middle 2000s, like the indices of pine. However, no continuous growth decline could be found for spruce during the last 15 years, only the sudden low peak of 2015. The low visual parallelism of the pine and spruce indices (Fig. 1) was confirmed by their low correlation on the mineral soil sites and peatlands (pine vs spruce on mineral soils r = 0.22, p = 0.12; pine vs spruce on peatlands r = 0.23, p = 0.11).
3.2 Correlations with weather variables
The tree-ring indices of pine were positively correlated with the spring temperature (April–May), especially on the mineral soil sites (Fig. 2A,B). High July temperatures seemed to promote pine growth on the mineral soil sites, but no correlation was found for peatlands. In addition, high winter temperatures (December of the previous year, January of the current year) were related to low tree-ring indices of pine in the following summer on both mineral soil sites and peatlands. The correlations with monthly precipitation were low on both main site types, and generally negative on peatlands.
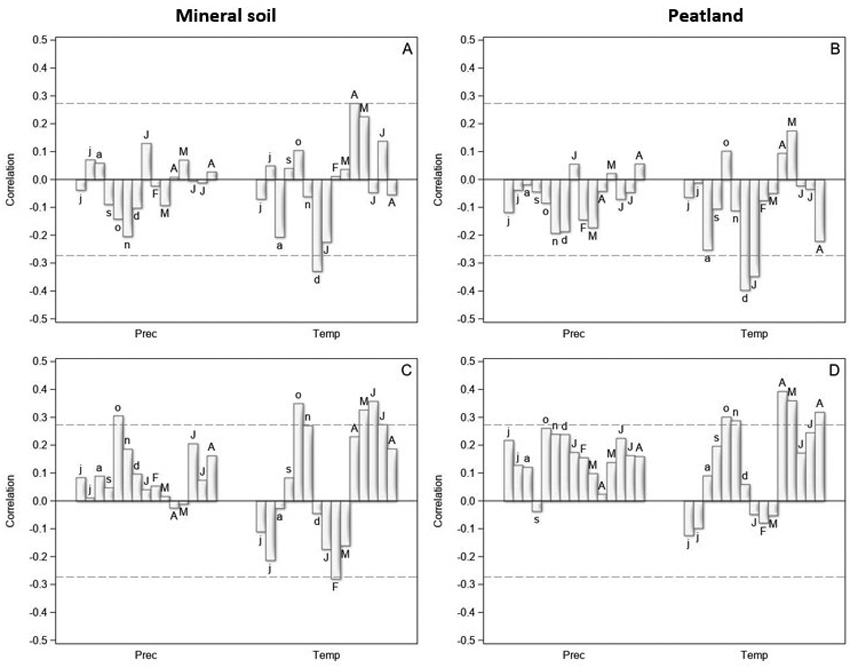
Fig. 2. The correlation coefficients between the tree-ring indices and monthly precipitation sums (Prec) and mean temperatures (Temp) from June of the previous year (small letters) to August of the current year (capital letters) in years 1970–2021 for Scots pine (upper row) and Norway spruce (lower row) on mineral soil (left column) and on peatland (right column) in North Finland. The dashed horizontal lines are 0.05 significance levels.
Warm spring and early summer were related to high tree-ring indices also for spruce (Fig. 2C,D). In addition, high June and July temperatures seemed to promote spruce growth on both main site types. High winter temperatures seemed to be detrimental for spruce growth on the mineral soil sites, but the highest negative correlations occurred a few months later (February, March) than for pine. On peatlands, the correlations between the winter temperatures and tree-ring indices were low. The correlations with precipitation were mainly positive and higher on the peatlands than on the mineral soil sites.
3.3 Models explaining the growth variation
Based on the correlation analyses, winter, early summer and midsummer temperatures were selected as independent variables to the linear model, in addition to the variables indicating the years of intensive flowering and cone production and midsummer droughts. As already indicated by the correlations, high early and midsummer temperatures were positively related to the tree-ring indices of pine, and high winter temperatures seemed to impair growth in the following summer (Table 1). Moreover, the years of intensive flowering (1981, 1995, 1996, 2003) and droughts (1980, 1994, 2018, 2019) were negatively related to the tree-ring indices of pine on both main site types. However, no relationship was found to the years of intensive cone production (variable Cone, see the Material and methods). The relationships between the weather variables and tree-ring indices were similar in spruce as in pine, except that the winter temperatures were not related to the tree-ring indices of peatland spruces (Table 1). In addition, no relationship was found between spruce growth and the years of intensive flowering (1986, 1989, 1994, 2018) and midsummer drought.
| Table 1. Parameter estimates and their standard errors (S.E.) of the tree-ring index models in years 1970–2021 for Scots pine and Norway spruce on mineral soil and peatlands in North Finland. | ||||
| Scots pine | Norway spruce | |||
| Parameter | S.E. | Parameter | S.E. | |
| Mineral soil | ||||
| Int. | –0.625 | 0.373 | –1.257 | 0.246 |
| T–12,1 | –0.012 | 0.004 | - | |
| T2,3 | - | –0.018 | 0.005 | |
| ln(T4,5) | 0.082 | 0.028 | 0.064 | 0.027 |
| ln(T6) | - | 0.420 | 0.096 | |
| ln(T7) | 0.152 | 0.137 | - | |
| Flower | –0.061 | 0.051 | - | |
| Dry | –0.127 | 0.052 | - | |
| R2 | 0.34 | 0.44 | ||
| Pr<DW | <0.01 | <0.01 | ||
| Peatland | ||||
| Int. | –0.289 | 0.066 | –0.921 | 0.503 |
| T–12,1 | –0.020 | 0005 | - | |
| ln(T4,5) | 0.081 | 0.039 | 0.111 | 0.038 |
| ln(T7) | - | 0.291 | 0.186 | |
| Flower | –0.083 | 0.071 | - | |
| Dry | –0.188 | 0.071 | - | |
| R2 | 0.33 | 0.21 | ||
| Pr<DW | <0.01 | <0.01 | ||
| T–12,1: mean temperature of previous year December and current year January T2,3: mean temperature of current year February and March T4,5: mean temperature of current year April and May T6: mean temperature of current year June T7: mean temperature of current year July Flower: intensity of flowering and cone production Dry: intensity of drought in current year July Pr<DW: p-value for testing positive autocorrelation | ||||
The predictions of the linear model had similarities with the annual and decadal growth variations of pine both on the mineral soil sites and peatlands (Fig. 3A,B). However, the magnitude of the variation in the observed tree-ring indices was clearly higher than in the predicted indices. Thus, the predicted tree-ring indices for pine were lower during the first years after the turn of the century, and the growth reduction thereafter delayed and less steep than the observed one. Accordingly, the linear model reproduced the growth variation of spruce on the mineral soil sites, excluding the early 1970s, but the peaks of the observed indices were higher and the valleys lower than those of the predicted indices (Fig. 3C). In general, the predictive power of the models was rather low R2 ranging from 0.21 for spruce growing on the peatlands to 0.44 for spruce on the mineral soil (Table 1). Moreover, the Durbin-Watson pointed to positive autocorrelation in the residuals of the models.
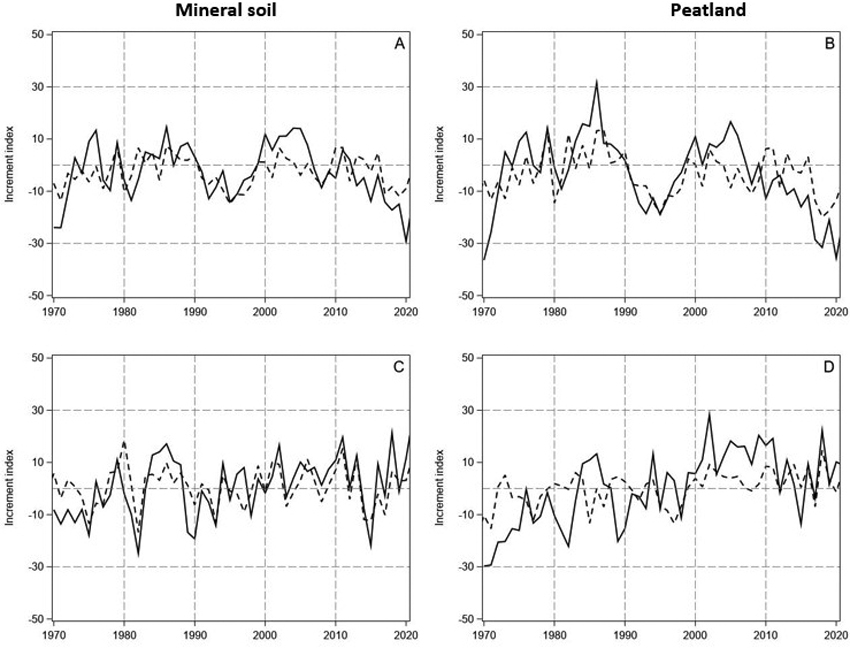
Fig. 3. The measured and predicted tree-ring indices (continuous and dashed lines, respectively) for Scots pine (A and B, upper row) and Norway spruce (C and D, lower row) in years 1970–2021 on mineral soil (left column) and on peatland (right column) in North Finland. The indices were predicted by using the models in Table 1.
4 Discussion
Forests trees have been shown grow faster under current climate than trees in otherwise similar conditions a few decades ago in Central Europe and Fennoscandia (Henttonen et al. 2017; Socha et al. 2021). As climate is expected to become warmer, the increasing trend in growth has been projected to continue (Kellomäki et al. 2018; Härkönen et al. 2019). The observed lower growth of pine, both in terms of the dimensionless tree-ring indices (i.e., mean growth of individual trees) and annual volume increment measured as m3 ha–1 a–1, contradicts the predictions. The results of this study demonstrated that a parallel decline has occurred both on the mineral soil and peatland sites, with a greater drop on the peatlands. Despite the considerable decline of pine, no trend-like reduction was found for spruce growth. The variation in weather and flowering accounted for one-third of the pine growth variance and the variables describing weather were also able to partly reproduce the recent decline.
The results of this study corroborate the results of several previous studies (Mäkinen et al. 2000; Helama et al. 2004; Korpela et al. 2011) in that high summer temperatures promote tree growth at the northern latitudes. However, the correlations with summer temperature were rather low. Based on a global network of tree-ring data, Babst et al. (2019) found a positive correlation with summer temperature, but the temperature response of boreal trees decreased towards the late 20th century. At cold sites metabolic rates are slow and warming promotes growth, but as temperature continues to increase, growth-temperature relationship tends to weaken or even disappear (Mäkinen et al. 2003; Andreu-Hayles et al. 2011; Williams et al. 2011). Accordingly, several studies have reported divergent growth trends and a loss of climate sensitivity of trees (D’Arrigo et al. 2008; Wilmking et al. 2020). Moreover, there are species-specific differences in growth response to divergence-related underlying factors (Büntgen et al. 2006).
High spring temperatures have also enhanced radial increment according to some northern studies (cf., Hordo et al. 2011; Henttonen et al. 2014; Babst et al. 2019) and the correlations of the trees we studied with April/May temperatures were mostly higher than for those with June and July temperatures. In northern Finland, the permanent snow cover typically melts in late April/May, thus, warm periods in spring promote snow and soil frost melting and consequently prolong growing season (Helama et al. 2013).
The growth of pines on both mineral soil sites and peatlands, as well as the growth of spruces on the mineral soil sites, responded negatively to mild winters, as also found by Suvanto et al. (2016) for the northern spruce provenances growing in North Finland. Accordingly, in cold-dry regions, the response to winter temperature became negative towards the late 20th (Babst et al. 2019). Andreassen et al. (2006) found that February temperatures were negatively correlated with radial growth in northern Norway and at high altitudes of south-eastern Norway, as also reported for northern Finland by Helama and Sutinen (2016). Andreassen et al. (2006) suggested that mild winter temperatures result in an earlier break of the dormant phase and, thus, increase frost damage risk. Moreover, repeated freeze-thaw cycles under elevated winter temperatures with shallow snowpack may have damaged fine roots in moist boreal forests (Cleavitt et al. 2008; Sutinen et al. 2014). High temperatures also affect respiration during winter as trees maintain their respiration during warm and dark periods (Vesala et al. 2010). Ögren et al. (1997) showed that spruce down-regulated respiration faster than pine, which improved the conservation of sugar storage. Moreover, the results by Linkosalo et al. (2014) showed that spruce regain the potential for photosynthetic activity more rapidly than pine when temperatures increase during the cold months and, therefore, spruce is more prone to frost damage. As the predicted effects of climate change in northern Europe are the strongest during winter (IPCC 2021), the results presented in this study indicate that high winter temperatures could, at least party, counterbalance growth-promoting effects of increasing summer temperatures.
For pine, the correlations between the tree-ring indices and monthly precipitation were lower than those with temperature on both main site types (cf., Salminen et al. 2009; Korpela et al. 2011). However, the results of our previous study implied that low water availability may influence pine growth also at the high northern latitudes (Henttonen et al. 2014). Even though the correlation analysis of this study did not confirm an overall connection between the variations of precipitation and tree growth, the dummy variable for exceptionally dry summers, i.e., years of low July precipitation, improved the fit of the linear model indicating that exceptionally low water availability may reduce pine growth. In contrast, the variable for dry summers was not significant for spruce, which typically grows on less drought prone sites (Sutinen et al. 2002). Our observations are consistent with the global tree-ring data set (Babst et al. 2019), in which a moderate increase in water limitation of growth was found across the boreal zone during 1960–1990.
Also for spruce growing on the mineral soil sites, the correlations between the tree-ring indices and precipitation were generally lower than those with temperature, excluding the correlation with the precipitation of previous year October. It is hard to find a plausible physiological explanation for a high correlation between growth and precipitation in late autumn, especially when the correlation with the precipitation of previous year September was low. Moreover, the correlations between growth and precipitation of several months were relatively high for spruce growing on peatlands. That is surprising as -unlike on mineral soils- soil water availability is abundant on peatlands (Smiljanić et al. 2014; González de Andrés et al. 2022). Moist soils on peatlands have been associated with reduced growth due to low soil temperature and anaerobic conditions (Hökkä et al. 2012; Edvardsson et al. 2015). A potential explanation could be that most of tree roots are in the top peat layer due to high water table levels. When the surface peat layer dries out shallow-rooted trees may be prone to drought. In Finland, one quarter of the productive forests grow on drained peatlands (Hökkä et al. 2012). The large-scale drainage of peatlands in the 1960s and 1970s aimed to increase forest growth. Despite the ditching, soil water conditions are different on drained peatlands and mineral soil sites. However, the increasing growth trend due the ditching may have concurred with an age-dependent growth pattern of young forests and thus was probably partly removed in the RCS standardisation. Yet, an increasing trend over the study period is evident in the tree-ring indices of spruce on the peatlands, coincidently with the increasing trend in precipitation over the study period. Therefore, we decided not to include precipitation in the model describing growth variation of spruce, except the dummy variable for exceptionally low July precipitation, even though it was not statistically significant for spruce. These inconsistent results emphasise the need for future studies on the effects of water availability on spruce growth, especially because climate change is expected to make growing conditions less favourable for Norway spruce (Kellomäki et al. 2018; Matkala et al. 2021).
Estimates of resource allocation from growth to reproduction in Pinus spp. vary between 10% and 18% of annual biomass/volume increment of tree stems (Ovington 1961; Linder and Troeng 1981; Cannell 1985; Pukkala 1987). Seed production also has a pronounced negative effect (up to 20%) on stem volume growth of Norway spruce (Pukkala 1987) and the reduction of ring width in masting years of high seed production has been up to 50% (Selås et al. 2002). In northern Finland, seed production is infrequent due to the harsh climate (Koski and Tallqvist 1978) and, thus, the number of masting years remains low. Accordingly, we found no relationship between the annual variation of flowering and tree-ring indices, but the years of intensive flowering were associated with reduced pine growth. No relationship between the flowering and radial growth was found for spruce. Cone development of Scots pine takes place over a period of four calendar years, i.e., three growing seasons are involved in cone initiation, flowering and seed maturation, but seed production of Norway spruce requires only three calendar years, i.e., seeds fall during the spring following the flowering year (Sarvas 1962). As the process is shorter for spruce, one would expect the relationship between flowering and radial growth to be stronger. As warm summers promote both flowering and tree growth (Despland and Houlle 1997), the confounding effect appears to be more prominent for spruce. Moreover, it must be taken into account that the data sets used in the analyses do not describe flowering/cone production and growth in the same trees. However, a synchronous negative relation between ring widths and seed production has been found at sites up to 250 km apart for spruces and 500 km apart for pines (Koenig and Knops 1998).
The RCS chronology has potential to preserve long-term growth changes due to climate, but it is sensitive to inhomogeneity in the data set (Cook et al. 1995; Helama et al. 2017). The RCS curve may be influenced by changes in non-climatic conditions over time. Even the large sample size in the NFI data does not remove effects of systematic changes in forest management over time. Changing stand structure and between-tree competition, as well as age structure, may affect the RCS chronology more than chronologies standardized using a flexible curve fitted individually to each series. Using a flexible curve to detrend the series would, in any case, remove any long-term growth trend from the resulting chronology, and such method cannot be used when a potential decline in growth is studied.
It is noteworthy that there is a strong reduction in sample size towards present, especially after the first decade of the 21st century (Suppl. file S1). As a caveat, this reduction overlaps with the growth decline and could impair the estimation of the recent growth variations. However, the tree-ring indices calculated based only on the trees cored during 2019–2021 are very similar to the indices based on the whole data set (Suppl. file S3).
It is noteworthy that although the model (Table 1) was linear, the variables (except the winter temperatures and dummy variables) were logarithmically transformed prior to the calculations, which affects the interpretation of the regression coefficients. Another indication of non-linear relationships between climate and growth is the acceptance of dummy variables (e.g., drought) in the regression, i.e., the growth variation was not related to the annual variation of precipitation and flowering, but growth was reduced after a certain threshold. Regressions used to model the growth variability accounted for 34% of annual growth variance of pine and 21–44% (depending on the soil type) of spruce. This means that a large portion of growth variation remained unexplained. Moreover, the models were fitted over the period of growth decline and were not validated on data withheld from the calibration, both increasing the risk of statistical overfitting. In addition, the Durbin-Watson statistics indicated that the residuals from the models were not randomly distributed, which could mean that a yet to be defined factor or factors contributing to growth variation may exist. As a high number of climatic variables were examined, non-climatic factors (e.g., changes in stand and age structure) are strong candidates. An implication of the Durbin-Watson statistics could be that the climate-growth relationship may not have remained stable over the study period.
Alternatively, the fact that the indices were averaged over an extensive area (11.298 M ha) could have led to a situation where factors related to climate and/or reproduction but affecting tree growth over more limited areas may have been partially masked when calculating the mean time-series (see below). Combined, the coefficients we obtained may not represent climatic relationships over the full study region and period. Such generalisations in modelling could also have lowered the R2 of the models.
In interpreting the results, several changes over time in forest management need to be considered. At the start of our monitoring period, a large part of forests in North Finland had not been managed intensively. The mean productivity was low, not only because of the cold climate, but also because high stand age and former selective dimensional cuttings (highgrading), which often resulted in stands with low density (Ilvessalo 1957). Management has become much more intensive during our monitoring period.
The most obvious change is large-scale regeneration of old pine stands and, thus, pine forests in North Finland are on average much younger today than in early 1970s (Tomppo et al. 2011; Korhonen et al. 2021a). In addition, favouring pine in regeneration and converting former spruce dominated forests on mineral soils into pine stands has been extensive. This means that the average site fertility of the current pine and spruce forests may be significantly different than it used to be 40–50 years ago. This needs to be considered in interpreting the results for spruce.
The estimated annual volume increment of the forests of North Finland has increased from 12.0 M m3 a–1 of the early 1970s to the most recent one of 27.4 M m3 a–1 (Korhonen et al. 2021b), a result of not only a more favourable age-structure, but also a much higher growing stock. However, the most recent reported age-structure suggests that a large share of regenerated pine stands are past their peak growth (Korhonen et al. 2021a,b), which probably partly explains the fact that the most recent growth estimate for the region was somewhat lower than the previous one.
Our approach, based on a statistically representative sample from the forests of the region, differs from those dendrochronological studies focusing on site chronologies. In the latter, data collection is targeted to test a certain, pre-selected hypothesis. A researcher operating on a sample-based data does not have this advantage. On the other hand, generalizing findings based on subjectively selected material is bound to be problematic (Cherubini et al. 1998; Nehrbass-Ahles et al. 2014; Klesse et al. 2018). In this study, the growth correlations and the variance explained by weather variables were lower than in several previous dendroclimatic studies from the region. This may be at least partly explainable by tree age and size-related differences in climate sensitivity, reported in several previous studies (Sceicz and Macdonald 1994), that may dilute the signal in sample-based data. Moreover, there is large heterogeneity in the NFI data in respect to latitude, longitude, altitude, site properties, management practices etc., resulting in less variability in tree-ring time series. The climatic signal in tree-ring chronologies could be masked and thus more difficult to relate to coarse (large-scale) weather variables.
5 Conclusions
The extensive tree-ring data, systematically sampled as a part of the NFI, revealed that pine growth has declined over the last 15 years in North Finland both on mineral soils and peatland sites. Thereagainst, spruce growth has not declined over the same period. Although temperature variation, accompanied by variables indicating years of drought, did not account for an especially large part of the growth variation, one can deduct that these factors have played a role behind the recent growth decline of pine, to an extent that remains to be detailed and validated. The possible mechanistic causes for tree growth sensitivity to weather extremes rather than monthly or seasonal means are still poorly understood and could provide an additional explanation. As no trend-like growth reduction was found for spruce indicates that growth decline has been species-dependent. Due to its detrimental ecological and economic consequences the phenomenon needs to be further monitored and investigated.
Authors’ contributions
Conceptualization (SH, PN, HM), data curation and analysis (HM), original draft preparation (HM), writing (PN, SH, HM), project administration (SH). All authors have read and agreed to the published version of the manuscript.
Declaration of openness of research materials, data, and code
Data available on request from the corresponding author.
Funding
Natural Resources Institute Finland (Luke) (project 41007-00212500), Academy of Finland (decision No. 315495).
Acknowledgements
The study was conducted at the Natural Resources Institute Finland under the GRAF project. We thank Helena Henttonen for making the NFI tree-ring data available, Pekka Helenius for the data on the flowering and cone production, Kari T. Korhonen and Hannu Salminen for constructive comments on the manuscript.
References
Andreassen K, Solberg S, Tveito OE, Lystad SL (2006) Regional differences inclimatic responses of Norway spruce (Picea abies L. Karst) growth in Norway. Forest Ecol Manag 222: 211–221. https://doi.org/10.1016/j.foreco.2005.10.029.
Andreu-Hayles L, D’Arrigo R, Anchukaitis KJ, Beck PSA, Frank D, Goetz S (2011) Varying boreal forest response to Arctic environmental change at the Firth River, Alaska. Environ Res Lett 6, article id 045503. https://doi.org/10.1088/1748-9326/6/4/045503.
Babst F, Bouriaud O, Poulter B, Trouet V, Girardin MP, Frank DC (2019) Twentieth century redistribution in climatic drivers of global tree growth. Sci Adv 5, article id eaat4313. https://doi.org/10.1126/sciadv.aat4313.
Blanchet G, Guillet S, Calliari B, Corona C, Edvardsson J, Stoffel M, Bragazza L (2017) Impacts of regional climatic fluctuations on radial growth of Siberian and Scots pine at Mukhrino mire (central-western Siberia). Sci Total Environ 574: 1209–1216. https://doi.org/10.1016/j.scitotenv.2016.06.225.
Briffa KR, Jones PD, Bartholin TS, Eckstein D, Schweingruber FH, Karlén W, Zetterberg P, Eronen M (1992) Fennoscandian summers from AD 500: temperature changes on short and long timescales. Clim Dynam 7: 111–119. https://doi.org/10.1007/BF00211153.
Büntgen U, Frank D, Schmidhalter M, Neuwirth B, Seifert M, Esper J (2006) Growth/climate response shift in a long subalpine spruce chronology. Trees 20: 99–110. https://doi.org/10.1007/s00468-005-0017-3.
Cannell MGR (1985) Dry matter partitioning in tree crops. In: Cannell MGR, Jackson JE (eds) Attributes of trees as crop plants, Institute of Terrestrial Ecology, Huntingdon, UK, pp 160–194.
Cherubini P, Dobbertin M, Innes JL (1998) Potential sampling bias in long-term forest growth trends reconstructed from tree rings: a case study from the Italian Alps. Forest Ecol Manag 109: 103–118. https://doi.org/10.1016/S0378-1127(98)00242-4.
Cleavitt NL, Fahey TJ, Groffman PM, Hardy JP, Henry KS, Driscoll CT (2008) Effects of soil freezing on fine roots in a northern hardwood forest. Can J Forest Res 38: 82–91. https://doi.org/10.1139/X07-133.
Climent J, Prada MA, Calama R, Chambel MR, de Ron DS, Alia R (2008) To grow or to seed: ecotypic variation in reproductive allocation and cone production by young female Aleppo pine (Pinus haplensis, Pinacea). Am J Bot 95: 833–842. https://doi.org/10.3732/ajb.2007354.
Cook ER, Pederson N (2011) Uncertainty, emergence, and statistics in dendrochronology. In: Hughes MK, Swetnam TW, Diaz HF (eds) Dendroclimatology: progress and prospects. Springer, Dordrecht, Heidelberg, London, New York, pp 77–112. https://doi.org/10.1007/978-1-4020-5725-0_4.
Cook ER, Briffa KR, Meko DM, Graybill DA, Funkhouser G (1995) The ‘segment length curse’ in long tree-ring chronology development for palaeoclimatic studies. Holocene 5: 229–237. https://doi.org/10.1177/095968369500500211.
D’Arrigo R, Wilson R, Liepert B, Cherubini P (2008) On the ‘divergence problem’ in northern forests: a review of the tree-ring evidence and possible causes. Global Planet Change 60: 289–305. https://doi.org/10.1016/j.gloplacha.2007.03.004.
Despland E, Houlle G (1997) Climate influences on growth and reproduction of Pinus banksiana (Pinacea) at the limit of the species distribution in eastern North America. Am J Bot 84: 928–937. https://doi.org/10.2307/2446283.
Edvardsson J, Rimkus E, Corona C, Šimanauskienė R, Kažys, J, Stoffel M (2015) Exploring the impact of regional climate and local hydrology on Pinus sylvestris L. growth variability – a comparison between pine populations growing on peat soils and mineral soils in Lithuania. Plant Soil 392: 345–356. https://doi.org/10.1007/ s11104-015-2466-9.
Fleischer P, Pichler V, Merganič J, Gömöryová E, Homolák M, Fleischer P. Jr. (2022) Declining growth response of Siberian spruce to climate variability on the taiga–tundra border in the Putorana mountains (Northwest Siberia). Forests 13, article id 131. https://doi.org/10.3390/f13010131.
González de Andrés E, Shestakova TA, Scholten RC, Delcourt CJF, Gorina NV, Camarero JJ (2022) Changes in tree growth synchrony and resilience in Siberian Pinus sylvestris forests are modulated by fire dynamics and ecohydrological conditions. Agr Forest Meteorol 312, article id 108712. https://doi.org/10.1016/j.agrformet.2021.108712.
Härkönen S, Neumann M, Mues V, Berninger F, Bronisz K, Cardellini G, Chirici G, Hasenauer H, Koehl M, Lang M, Merganicova K, Mohren F, Moiseyev A, Moreno A, Mura M, Muys B, Olschofsky K, Del Perugia B, Rørstad PK, Solberg B, Thivolle-Cazat A, Trotsiuk V, Mäkelä A (2019) A climate-sensitive forest model for assessing impacts of forest management in Europe. Environ Model Softw 115: 128–143. https://doi.org/10.1016/j.envsoft.2019.02.009.
Helama S, Sutinen R (2016) Inter- and intra-seasonal effects of temperature variation on radial growth of alpine treeline Norway spruce. J Mt Sci 13: 1–12. https://doi.org/10.1007/s11629-015-3665-9.
Helama S, Holopainen J, Timonen M, Ogurtsov MG, Lindholm M, Meriläinen J, Eronen M (2004) Comparison of living-tree and subfossil ringwidths with summer temperatures from 18th, 19th and20th centuries in northern Finland. Dendrochronologia 21: 147–154. https://doi.org/10.1078/1125.7865.00049.
Helama S, Mielikäinen K, Timonen M, Herva H, Tuomenvirta H, Venäläinen A (2013) Regional climatic signals in Scots pine growth with insights into snow and soil associations. Dendrobiology 70: 27–34. https://doi.org/10.12657/denbio.070.003.
Helama S, Melvin TM, Briffa KR (2017) Regional curve standardization: State of the art. Holocene 27: 172–177. https://doi.org/10.1177/0959683616652709.
Helama S, Arppe L, Mielikäinen K, Oinonen M (2018) Arctic moistening provides negative feedbacks to riparial plants. Glob Change Biol 24: 2691–2707. https://doi.org/10.1111/gcb.14058.
Henttonen HM, Mäkinen H, Heiskanen J, Peltoniemi M, Laurén, A, Hordo M (2014) Response of radial increment variation of Scots pine to temperature, precipitation and soil water content along a latitudinal gradient across Finland and Estonia. Agr Forest Meteorol 198–199: 294–308. https://doi.org/10.1016/j.agrformet.2014.09.004.
Henttonen HM, Nöjd P, Mäkinen H (2017) Environment-induced growth changes in the Finnish forests during 1971 – 2010 – an analysis based on National Forest Inventory. Forest Ecol Manag 386: 22–36. https://doi.org/10.1016/j.foreco.2016.11.044.
Hökkä H, Salminen H, Ahti E (2012) Effect of temperature and precipitation on the annual diameter growth of Scots pine on drained peatlands and adjacent mineral soil sites in Finland. Dendrochronologia 30: 157–165. https://doi.org/10.1016/j.dendro.2011.02.004.
Hordo M, Henttonen HM, Mäkinen H, Helama S, Kiviste A (2011) Annual growth variation of Scots pine in Estonia and Finland. Balt For 17: 35–49.
Ilvessalo Y (1957) Suomen metsät metsänhoitolautakuntien toiminta-alueittain. Valtakunnan metsien inventoinnin tuloksia. [The forests of Finland by forestry board districts. Results of the national forest inventory]. Commun Inst For Fenn 47: 1–128. http://urn.fi/URN:NBN:fi-metla-201207171079.
IPCC (2021) Climate change 2021: the physical science basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change [Masson-Delmotte V, Zhai P., Pirani A, Connors SL, Péan C, Berger S, Caud N, Chen Y, Goldfarb L, Gomis MI, Huang M, Leitzell K, Lonnoy E, Matthews JBR, Maycock TK, Waterfield T, Yelekçi O, Yu R, Zhou B (eds)]. Cambridge University Press, In press.
Irannezhad M, Chen D, Kløve (2015) Interannual variations and trends in surface air temperature in Finland in relation to atmospheric circulation patterns, 1961–2011. Int J Climatol 35: 3078–3092. https://doi.org/10.1002/joc.4193.
Kellomäki S, Strandman H, Heinonen T, Asikainen A, Venäläinen A, Peltola H (2018) Temporal and spatial change in diameter growth of Boreal Scots pine, Norway spruce, and birch under recent-generation (CMPI5) global climate change projections for the 21th century. Forests 9, article id 118. https://doi.org/10.3390/f9030118.
Klesse S, DeRose RJ, Guiterman CH, Lynch AM, O’Connor CD, Shaw JD, Evans MEK (2018) Sampling bias overestimates climate change impacts on forest growth in the southwestern United States. Nat Commun 9, article id 5336. https://doi.org/10.1038/s41467-018-07800-y.
Koenig WD, Knops JMH (1998) Scale of mast-seeding and tree-ring growth. Nature 396: 225–226. https://doi.org/10.1038/24293.
Korhonen KT, Ahola A, Heikkinen J, Henttonen HM, Hotanen J-P, Ihalainen A, Melin M, Pitkänen J, Räty M, Sirviö M, Strandström M (2021a). Forests of Finland 2014–2018 and their development 1921–2018. Silva Fenn 55, article id 10662. https://doi.org/10.14214/sf.10662.
Korhonen KT, Henttonen HM, Räty M (2021b) Tuloksia valtakunnan metsien 13. inventoinnista 2019–2020. [Results of the National Forest Inventory 2019–2020]. Natural Resources Institute Finland. https://valtioneuvosto.fi/documents/1410837/102130909/Liite+4+VMI13_metsaneuvosto_korhonen_2021.pdf.
Korpela M, Nöjd P, Hollmén J, Mäkinen H, Sulkava M, Hari P (2011) Photo-synthesis, temperature and radial growth of Scots pine in northern Finland -identifying the influential time intervals. Trees 25: 323–332. https://doi.org/10.1007/s00468-010-0508-8.
Koski V, Tallqvist A (1978) Tuloksia monivuotisista kukinnan ja siemensadonmäärän mittauksista metsäpuilla. [Results of long-time measurements ofquantity of flowering and seed crop of forest trees]. Folia For 364: 1–60 p. http://urn.fi/URN:ISBN:951-40-0355-1.
Linder S, Troeng E (1981) The seasonal course of respiration and photosynthesis in strobili of Scots pine. Forest Sci 27: 267–276. https://doi.org/10.1093/forestscience/27.2.267.
Linkosalo T, Heikkinen J, Pulkkinen P, Mäkipää R (2014) Fluorescence measurements show stronger cold inhibition of photosynthetic light reactions in Scots pine compared to Norway spruce as well as during spring compared to autumn. Front Plant Sci 5, article id 264. https://doi.org/10.3389/fpls.2014.00264.
Mäkinen H, Nöjd P, Mielikäinen K (2000) Climatic signal in annual growth variation of Norway spruce (Picea abies (L.) Karst.) along a transect from central Finland to Arctic timberline. Can J For Res 30: 769–777. https://doi.org/10.1139/x00-005.
Mäkinen H, Nöjd P, Mielikäinen K (2001) Climatic signal in annual growth variation in damaged and healthy stands of Norway spruce (Picea abies (L.) Karst.) in southern Finland. Trees 15: 177–185. https://doi.org/10.1007/s004680100089.
Mäkinen H, Nöjd P, Kahle H-P, Neumann U, Tveite B, Mielikäinen K, Röhle H, Spiecker H (2003) Large-scale climatic variability and radial increment variation of Picea abies (L.) Karst. in central and northern Europe. Trees 17: 173–184. https://doi.org/10.1007/s00468-002-0220-4.
Marshall GJ, Kivinen S, Jylhä K, Vignols RM, Rees WG (2018) The accuracy of climate variability and trends across Arctic Fennoscandia in four reanalyses. Int J Climatol 38: 3878–3895. https://doi.org/10.1002/joc.5541.
Matkala L, Kulmala L, Kolari P, Aurela M, Bäck J (2021) Resilience of subarctic Scots pine and Norway spruce forests to extreme weather events. Agr Forest Meteorol 296, article id 108239. https://doi.org/10.1016/j.agrformet.2020.108239.
Meier HEM, Kniebusch M, Dieterich C, Gröger M, Zorita E, Elmgren R, Myrberg K, Ahola MP, Bartosova A, Bonsdorff E, Börgel F, Capell R, Carlén I, Carlund T, Carstensen J, Christensen OB, Dierschke V, Frauen C, Frederiksen M, Gaget E, Galatius A, Haapala JJ, Halkka A, Hugelius G, Hünicke B, Jaagus J, Jüssi M, Käyhkö J, Kirchner N, Kjellström E, Kulinski K, Lehmann A, Lindström G, May W, Miller PA, Mohrholz V, Müller-Karulis B, Pavón-Jordán D, Quante M, Reckermann M, Rutgersson A, Savchuk OP, Stendel M, Tuomi L, Viitasalo M, Weisse R, Zhang W (2022) Climate change in the Baltic Sea region: a summary. Earth Syst Dynam 13: 457–593. https://doi.org/10.5194/esd-13-457-2022.
Mikola P (1950) Puiden kasvun vaihteluista ja niiden merkityksestä kasvututkimuksissa. [On variations in tree growth and their significance to growth studies]. Commun Inst For Fenn 38: 1–131. http://urn.fi/URN:NBN:fi-metla-201207171070.
Myneni RB, Keeling CD, Tucker CJ, Asrar G, Nemani RR (1997) Increased plant growth in the northern high latitudes from 1981 to 1991. Nature 386: 689–702. https://doi.org/10.1038/386698A0.
Nehrbass-Ahles C, Babst F, Klesse S, Nötzli M, Bouriaud O, Neukom R, Dobbertin M, Frank D (2014) The influence of sampling design on tree-ring-based quantification of forest growth. Glob Change Biol 20: 2867–2885. https://doi.org/10.1111/gcb.12599.
Nemani RR, Keeling CD, Hashimoto H, Jolly WM, Piper SC, Tucker CJ, Myneni RB, Running SW (2003). Climate-driven increases in global terrestrial net primary production from 1982 to 1999. Science 300: 1560–1563. https://doi.org/10.1126/science.1082750.
Nöjd P, Korpela M, Hari P, Rannik Ü, Sulkava M, Hollmén J, Mäkinen H (2017) Effects of precipitation and temperature on the growth variation of Scots pine – a case study at two extreme sites in Finland. Dendrochronologia 46: 35–45. https://doi.org/10.1016/j.dendro.2017.09.003.
Ögren E, Nilsson T, Sundblad LG (1997) Relationship between respi- ratory depletion of sugars and loss of cold hardiness in coniferous seedlings over-wintering at raised temperatures: indications of different sensitivities of spruce and pine. Plant Cell Environ 20: 247–253. https://doi.org/10.1046/j.1365-3040.1997.d01-56.x.
Ovington JD (1961) Some aspect of energy flow in plantations of Pinus sylvestris. Ann Bot 25: 12–20. https://doi.org/10.1093/oxfordjournals.aob.a083728.
Pretzsch H, Biber P, Schütze G, Uhl E, Rötzer T (2014) Forest stand growth dynamics in Central Europe have accelerated since 1870. Nat Commun 5, article id 4967. https://doi.org/10.1038/ncomms5967.
Pukkala T (1987) Siementuotannon vaikutus kuusen ja männyn vuotuiseen kasvuun. [Effect of seed production on the annual growth of Picea abies and Pinus sylvestris. Silva Fenn 21: 145–158. https://doi.org/10.14214/sf.a15469.
Salminen H, Jalkanen R, Lindholm M (2009) Summer temperature affects the ratio of radial and height growth of Scots pine in northern Finland. Ann Forest Sci 66, article id 810. https://doi.org/10.1051/forest/2009074.
Sarvas R (1962) Investigations on the flowering and seed crop of Pinus silvestris. Commun Inst For Fenn 53: 1–198. http://urn.fi/URN:NBN:fi-metla-201207171085.
SAS (2013) Statistical analysis software. Users’ guide statistics, version 9.4. SAS Institute Inc., Cary.
Sceicz JM, Macdonald GM (1994) Age-dependent tree-ring growth responses of subarctic white spruce to climate. Can J Forest Res 24: 120–132. https://doi.org/10.1139/x94-017.
Selås V, Piovesan G, Adams JM, Bernabei M (2002) Climatic factors controlling reproduction and growth of Norway spruce in southern Norway. Can J Forest Res 32: 217–225. https://doi.org/10.1139/x01-192.
Smiljanić M, Seo J-W, Läänelaid A, van der Maaten-Theunissen M, Stajić B, Wilmking M (2014) Peatland pines as a proxy for water table fluctuations: Disentangling tree growth, hydrology and possible human influence. Sci Total Environ 500–501: 52–65. https://doi.org/10.1016/j.scitotenv.2014.08.056.
Socha J, Solberg S, Tymińska-Czabańska L, Tompalski P, Vallet P (2021) Height growth rate of Scots pine in Central Europe increased by 29% between 1900 and 2000 due to changes in site productivity. Forest Ecol Manag 490: article id 119102. https://doi.org/10.1016/j.foreco.2021.119102.
Sutinen R, Teirilä A, Pänttäjä M, Sutinen M-L (2002) Distribution and diversity of tree species with respect to soil electrical characteristics in Finnish Lapland. Can J For Res 32: 1158–1170. https://doi.org/10.1139/x02-076.
Sutinen S, Roitto M, Lehto T, Repo T (2014) Simulated snowmelt and infiltration into frozen soil affected root growth, needle structure and physiology of Scots pine saplings. Boreal Environ Res 19: 281–294. http://hdl.handle.net/10138/228600.
Suvanto S, Nöjd P, Henttonen HM, Beuker E, Mäkinen H (2016) Geographical patterns in the radial growth response of Norway spruce provenances to climatic variation. Agr Forest Meteorol 222: 10–20. https://doi.org/10.1016/j.agrformet.2016.03.003.
Tomppo E, Heikkinen J, Henttonen HM, Ihalainen A, Katila M, Mäkelä H, Tuomainen T, Vainikainen N (2011) Designing and conducting a forest inventory – case: 9th National Forest Inventory of Finland. Managing Forest Ecosystems 21. Springer, Heidelberg, Dordrecht, London, New York.
Venäläinen A, Heikinheimo M (2002) Meteorological data for agricultural applications. Phys Chem Earth 27: 1045–1050. https://doi.org/10.1016/S1474-7065(02)00140-7.
Vesala T, Launiainen S, Kolari P, Pumpanen J, Sevanto S, Hari P, Nikinmaa E, Kaski P, Mannila H, Ukkonen E, Piao SL, Ciais P (2010) Autumn temperature and carbon balance of a boreal Scots pine forest in southern Finland. Biogeosciences 7: 163–176. https://doi.org/10.5194/bg-7-163-2010.
Vihma T, Screen J, Tjernström M, Newton D, Zhang X, Popova V, Deser C, Holland M, Prowse T (2016) The atmospheric role in the Arctic water cycle: a review on processes, past and future changes, and their impacts. J Geophys Res 121: 586–620. https://doi.org/10.1002/2015JG003132.
Vilà-Cabrera A, Martinez-Vilalta J, Retana J (2014) Variation in reproduction and growth in declining Scots pine populations. Perspect Plant Ecol 16: 111–120. https://doi.org/10.1016/j.ppees.2014.02.005.
Williams AP, Xu C, McDowell NG (2011) Who is the new sheriff in town regulating boreal forest growth? Environ Res Let. 6, article id 041004. https://doi.org/10.1088/1748-%209326/6/4/041004.
Wilmking M, van der Maaten-Theunissen M, van der Maaten E, Scharnweber T, Buras A, Biermann C, Gurskaya M, Hallinger M, Lange J, Shetti R, Smiljanic M, Trouillier M (2020) Global assessment of relationships between climate and tree growth. Global Change Biol 26: 3212–3220. https://doi.org/10.1111/gcb.15057.
Total of 71 references.

