Germination differences between natural and afforested populations of Pinus brutia and Cupressus sempervirens
Al-Hawija B., Wagner V., Partzsch M., Hensen I. (2014). Germination differences between natural and afforested populations of Pinus brutia and Cupressus sempervirens. Silva Fennica vol. 48 no. 4 article id 1176. https://doi.org/10.14214/sf.1176
Highlights
- Silvicultural practices of raising and outplanting seedlings yielded contrasting outcomes in our species
- Afforested Pinus brutia populations acquired ability to tolerate drought stress at intermediate and hot temperatures compared to natural populations, which may indicate local adaptation
- Natural Cupressus sempervirens populations showed higher salt-tolerance than afforested populations
- Seed germination was optimal under intermediate temperatures and deionized water for both species.
Abstract
In afforestation, silvicultural processes of raising and planting seedlings under certain conditions can yield contrasting outcomes in tree stock performance. Moderate nursery conditions may select against stress tolerance whereas planting seedlings in stressful environments at afforestation sites may select for higher stress tolerance compared to natural populations. We compared germination performance between natural and afforested populations of Pinus brutia Ten. subsp. brutia and Cupressus sempervirens L. var. horizontalis (Mill.) under differing stress treatments. Seeds were collected from both natural stands and from afforested populations outside the natural distribution range, in Syria. Cold, intermediate and hot temperature regimes were simulated (8/4 °C, 20/10 °C and 32/20 °C) along with cold stratification, drought stress (–0.2 and –0.4 MPa), salt stress (50 and 100 mMol l–1), and deionized water (control) conditions. In addition, we tested the effects of seed weight and climatic conditions on seed germination. In general, intermediate temperatures were optimal for both population types. Afforested P. brutia populations outperformed natural ones under drought stress levels at hot and/or intermediate temperatures. Conversely, in C. sempervirens, cold stratification at all temperatures and higher salt stress at intermediate temperatures significantly decreased germination in afforested populations. Seed weight did not significantly affect germination percentages, which were however significantly negatively related to annual precipitation in P. brutia, and to annual temperature in C. sempervirens. We infer that silvicultural processes led to divergent outcomes in our species: local adaptation to drought stress and hot temperatures in afforested P. brutia populations and lower salt-stress tolerance in C. sempervirens.
Keywords
nursery;
silviculture;
drought stress;
cold stratification;
local adaptation;
salt stress;
Syria
-
Al-Hawija,
Martin-Luther-University Halle-Wittenberg, Institute of Biology/Geobotany and Botanical Garden, Am Kirchtor 1, D-06108 Halle/Saale, Germany
E-mail
batoulh@gmail.com

- Wagner, Department of Botany and Zoology, Masaryk University, Kotlářská 2, CZ-611 37 Brno, Czech Republic E-mail wagner@sci.muni.cz
- Partzsch, Martin-Luther-University Halle-Wittenberg, Institute of Biology/Geobotany and Botanical Garden, Am Kirchtor 1, D-06108 Halle/Saale, Germany E-mail monika.partzsch@botanik.uni-halle.de
- Hensen, Martin-Luther-University Halle-Wittenberg, Institute of Biology/Geobotany and Botanical Garden, Am Kirchtor 1, D-06108 Halle/Saale, Germany & German Centre for Integrative Biodiversity Research (iDiv) Halle-Jena-Leipzig, Deutscher Platz 5e, 04103 Leipzig, Germany E-mail isabell.hensen@botanik.uni-halle.de
Received 29 April 2014 Accepted 22 October 2014 Published 28 November 2014
Views 197519
Available at https://doi.org/10.14214/sf.1176 | Download PDF

Corrections
1 Introduction
Afforestation involves the cultivation of a forest at a site where forests do not grow naturally (FAO 2003). Planted forests (including afforestations) account for 6.6% of the world’s total forest area (FAO 2010), and they have important ecological and socio-economic benefits (Lal 2004; FAO 2005; Metzger and Hüttermann 2009).
Trees selected for afforestation have to be tolerant to considerable abiotic and biotic stress, particularly if they are planted outside their natural geographic distribution range. However, in tree domestication, the silvicultural practice of raising and planting seedlings may lead to contrasting selective outcomes for stress tolerance. First of all, trees are usually germinated in nurseries under relatively moderate and controlled conditions that differ strongly from natural field conditions, both in terms of abiotic and biotic factors (van den Driessche 1991). As such, selection processes are much reduced in nurseries compared to natural habitats (Muona and Harju 1989; Rajora 1999). This lack of exposure to stress may undermine species’ natural selection in respect of stress tolerance at the seed and seedling stages (White et al. 2007), resulting in a loss of stress tolerance among afforested populations. In addition, trees cultivated under artificial conditions are subject to a variety of treatments such as seed storage and seed sizing (Silen and Osterhaus 1979; Chaisurisri et al. 1993; El-Kassaby et al. 1992), both of which can lead to an erosion of genetic variation in plantation stock (El-Kassaby and Thomson 1996; Davidson et al. 1996). Furthermore, inbreeding can lead to declining seed viability and poor seed germination (Wilcox 1983; Muona and Harju 1989), which becomes ever more pronounced as populations become smaller (Ferriol et al. 2011). For example, in a study by Salzer and Gugerli (2012), small peripheral populations of Pinus cembra showed lower germination success than central core populations, indicating higher inbreeding depression among smaller, scattered populations. However, it is also possible that the practice of planting trees in stressful conditions beyond their natural range may select for higher stress tolerance among afforested populations. As a species becomes established in a stressful environment, it experiences divergent selection pressures (Espinoza et al. 2013). Selection pressures on phenotypes could lead to local adaptation (Pigliucci et al. 2006) and the development of local landraces (Espinoza et al. 2013). The fast formation of landraces in a species indicates a high potential to evolve and adjust its performance to changing climatic conditions (Bigras and Colombo 2000). Indeed, the development of land races has occurred in several conifer species under more stressful environments (Zobel and Talbert 1984; Ennos et al. 1998; Espinoza et al. 2013).
The performance of a progeny shows a high phenotypic plasticity in response to prevailing environmental conditions during seed maturation (maternal effect) (Skrøppa and Johnsen 2000; Donohue et al. 2009). For example, species may acquire resistance to cold only during embryo development under lower temperatures in the field, and they may lose this ability if temperatures rise during seed maturation (Finkeldey and Hattermer 2007). However, seeds from favorable maternal environments outperformed those from stressful maternal environments in terms of their germination response (Cendàn et al. 2013). In addition, genotypes developed in unfavorable maternal environment have aborted a higher proportion of their seeds than the genotypes from a favorable maternal environment (Kärkkäinen et al.1999).
Seed germination is a crucial life stage (Fenner and Thompson 2005) that is strongly influenced by abiotic factors such as temperature, water stress and salinity, as well as their interaction (Simak and Kamra 1970; Rao and Singh 1985; Bewley and Black 1994; Baskin and Baskin 1998). However, very scarce studies have compared germination performance between natural and afforested populations. In a germination experiment, Zhu et al. (2006) found that afforested Pinus sylvestris var. mongolica populations lost their tolerance towards increased drought stress compared to that of natural populations. In general, increased germination performance under unfavorable germination conditions, such as low or high temperatures as well as drought or salinity stress, could be due to larger seed weight and/or the establishment and development of the parent populations under more stressful conditions (Skrøppa and Johnsen 2000; Croser et al. 2001; Zhu et al. 2006).
For the present study, we addressed the question as to whether afforested populations develop lower or higher stress tolerances at the seed stage compared to natural populations. We focused on P. brutia (Brutia pine) and C. sempervirens (Common cypress) stands in the Eastern Mediterranean. In the Mediterranean region, germination is often restricted to short periods in the wetter spring or autumn seasons, but it is unlikely to occur during the dry summer (García-Fayos et al. 2000; Quilichini and Debussche 2000). We chose these species because their general germination ecology is well known (e.g. Skordilis and Thanos 1995; Boydak et al. 2003; Piotto et al. 2003; Tilki and Dirik 2007; Giannini et al. 1999). In addition, our natural and afforested populations originated from locations with contrasting climatic regimes, which enabled us to explore the role of climatic conditions as co-variables.
For each study species, we compared the germination performance of seeds from natural and afforested populations and tested the following hypotheses: (1) Compared to natural Pinus brutia and Cupressus sempervirens populations, afforested populations have either lost or acquired their tolerance for cold and hot temperatures as well as for drought and salt stress; (2) The germination response of Pinus brutia and Cupressus sempervirens populations is influenced by seed weight, i.e. heavier seeds exhibit higher germination; and (3) Germination responses in natural and afforested populations of both species are influenced by climatic provenance, i.e. mean annual temperature and annual precipitation values of the seed collection sites. Specifically, we expected populations located in more arid regions to show enhanced germination.
2 Materials and methods
2.1 Study region and study species
Syria is an Eastern Mediterranean country with variable geographic, geological and climatic conditions that have influenced the abundance and structure of forest vegetation (Nahal and Zahoueh 2005). Semi-arid and arid habitats prevail in Syria (Ghazal 2008) with about 50% of soils being Aridisols, which occur particularly in arid areas with a mean annual precipitation of < 250 mm (Ilaiwi 2001). In addition, saline soils are widely distributed across coastal and inland areas in Syria (Al-Oudat and Qadir 2011). As such, both natural vegetation and man-made plantations grow in conditions of drought stress and salinity.
Pinus brutia and C. sempervirens are two important tree species in Syrian natural forests, whose natural ranges overlap along different zones in the coastal mountains (Nahal and Zahoueh 2005). Pinus brutia occurs naturally in a variety of bioclimatic zones (Thermo-Mediterranean, Eu-Mediterranean and Supra-Mediterranean) and altitudinal belts (from sea level to 1100 m) throughout the coastal mountains of Syria (Nahal and Zahoueh 2005; Ghazal 2008). It is dominant in the Eu-Mediterranean humid zone, where it forms pure stands at 100–450 m a.s.l in the Baer-Bassit Mountains of Latakia, and it occurs as natural forest in northwest Syria in the Aleppo (or “Kurds”) Mountain (Ghazal 2008).
Cupressus sempervirens natural forests grow in pure stands in the coastal mountains of Syria, or they are frequently associated with P. brutia (Zohary 1973). Beyond their natural distribution range, the two species are frequently planted to protect against soil and wind erosion in semi-arid and arid climate zones with mean annual precipitation levels of < 350 mm (Nahal and Zahoueh 2005). Previous studies have shown that an intermediate temperature of 20 °C is optimal for seed germination in both species, and that relatively lower temperatures along with decreasing water potentials significantly inhibit germination (e.g. Skordilis and Thanos 1995; Piotto et al. 2003; Tilki and Dirik 2007; Giannini et al. 1999). Cold stratification has been found to have a beneficial effect on the subsequent germination performance of P. brutia seeds (Skordilis and Thanos 1995; Tilki and Dirik 2007). For C. sempervirens, cold stratification over 3–4 weeks is recommended to improve germination (Piotto et al. 2003). So far, no research has been recorded on salt tolerance in both study species.
For the present study, we compared seed germination in natural and afforested populations originating from contrasting climates in Syria (Table 1). The afforested populations were established in the 1980s from seeds originating from older plantations that had previously been established in the 1950s in the vicinity of our studied sites (Fig. 1) [see Al-Hawija et al. (2014b) for more details on seed origins and geographic distances among sampled populations]. Thus, our afforested populations had gone through both a process of artificial selection in nurseries and they had been exposed to stressful environmental conditions at the afforestation sites (Table 1).
2.2 Seed collection
Cones from afforested and natural populations were collected in April–May in 2009 and 2010, respectively. We collected cones from six natural and five afforested P. brutia populations and three natural and six afforested C. sempervirens populations across different bioclimatic regions in Syria (Table 1). Pinus brutia cones were collected from natural populations in Latakia [coastal mountains; Eu-Mediterranean humid zone, electrical conductivity (henceforth: EC) generally less than 1 dS m–1; Jamal et al. (2005)] and from the Aleppo Mountain [Eu-Mediterranean, semi-arid zone; EC is 5 dS m–1 (Jamal et al. 2005)]. Seeds from afforested populations were collected in the Manbij district of Aleppo province (Eu-Mediterranean, semi-arid zone), where the EC ranged between 2 and 16 dS m–1 (Schweers et al. 2005). We also collected seeds from afforested populations in the Irano-Turanian arid zone in Deir Ezzor and Raqqa, where the EC ranged between 2 and 5 dS m–1 (Jamal et al. 2005). Salinity levels were included in relation to the respective study regions as we lacked specific data on soil salinity at our study populations.
| Table 1. An overview of Syrian Pinus brutia and Cupressus sempervirens study populations, incl. population type, geographic location, climatic conditions, population area, seed weight and percentage of seed viability, empty seeds and degraded embryos before the start of the experiment. View in new window/tab. |
Cupressus sempervirens cones were also collected from natural populations in Latakia and from afforested populations in the Manbij district and Deir Ezzor city. The original seed source for the Aleppo afforestations was the ‘Afrin’ afforestation in the Eu-Mediterranean semi-arid zone of northwest Syria, while the seed origin of Deir Ezzor afforestations was the ‘Al-Hasaka’ afforestation in the Irano-Turanian zone of northeast Syria (Fig. 1). These seed origins are geographically and climatically comparable to the studied afforested populations. Ultimately, the seed source for these afforestations can likely be traced back to natural forests of Syria, from which afforestation stock was collected when forestry began in the 1950s. Consequently, natural forests in Latakia and the Aleppo Mountain may represent the primary origin of afforested P. brutia populations, whereas Latakia could represent the primary natural origin of afforested C. sempervirens populations.
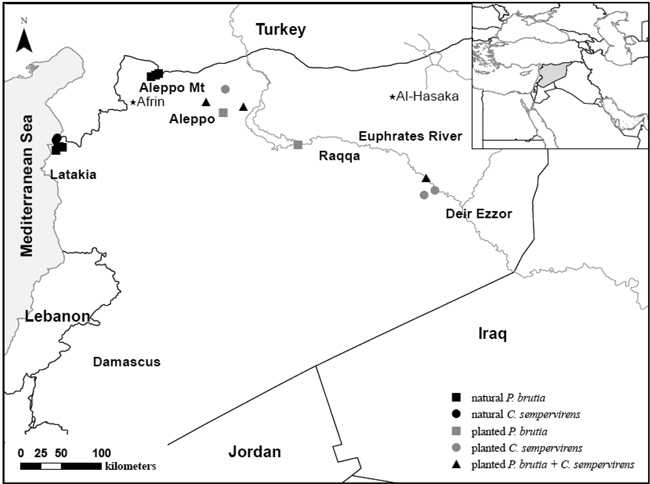
Fig. 1. Sample locations of natural and afforested Pinus brutia and Cupressus sempervirens populations in Syria. Seed origins of the afforestations are marked with a star.
A total of 75–100 cones were randomly collected from each population from at least 20 mother trees spread across the spatial distribution of each population, with distances between populations ranging from 3 to 426 km. Cones were then dried in the open air to stimulate opening, after which they were cracked mechanically. Seeds from each population were pooled and thoroughly mixed with 300 seeds from 30 bulk samples being weighed (Table 1). Seeds were then stored in paper bags under ambient laboratory conditions.
2.3 Germination experiment
The germination experiment was initiated in February 2010 and 2011, approximately one year after each collection phase. We used a full factorial design in which we crossed three different temperature regimes (8/4 °C, 20/10 °C and 32/20 °C; 12 h of warm, white light, 12 h of darkness) with six treatments (de-ionized water as a control, cold stratification, drought stress: –0.2 and –0.4 MPa, and salt stress: 50 and 100 mMol l–1). All six treatments were applied for each temperature regime and for both species. In the case of the cold stratification treatment, we incubated seeds at 5 °C for four weeks under moist condition. Drought stress and salt stress effects were investigated using different concentrations (–0.2 and –0.4 MPa) of polyethylene glycol (PEG-4000) and sodium chloride NaCl (50 and 100 mMol l–1) solutions. As no information was available on the soil water content or salinity of our study populations, the levels employed were in line with previous general findings for the East Mediterranean region (Thanos and Skordilis 1987; Boydak et al. 2003; Tilki and Dirik 2007).
We used four Petri dishes per treatment (72 Petri dishes per population). For the cold stratification treatment, we used 25 seeds of P. brutia and 30 seeds of C. sempervirens per Petri dish. For all other treatments, we chose 20 seeds of P. brutia and 25 seeds of C. sempervirens per Petri dish. Seeds were chosen randomly and placed on filter paper in standard glass Petri dishes (7 cm diameter). Dishes were placed in three programmed incubators, each with one of the aforementioned temperature regimes that simulated temperature fluctuations in winter, spring and autumn in the study region. Although it has been shown that the intermediate temperature is optimal for seed germination in both species (see above), the cold and hot temperature regimes were applied to explore the differences in responses between the two population types to relatively non-optimal conditions.
Seeds were regarded as having germinated when they showed a visible radicle, and they were subsequently scored and removed every second or third day. The location of the dishes in the chambers was rotated after each census, and dishes with PEG and NaCl solutions were covered with Parafilm to prevent evaporation. Filter papers and solutions were changed as needed in order to keep water potentials and salt concentrations steady throughout the germination experiment. The experiment lasted for 45 days.
Before the experiment, we tested 100 randomly chosen seeds per population for viability (with triphenyl tetrazolium chloride; Hendry and Grime 1993), degradation and emptiness (Table 1). Shortly after the end of the experiment, we checked non-germinated seeds under the microscope and recorded whether seeds were filled or had degraded embryos (data not shown).
2.4 Data analysis
We estimated the final germination percentage as the number of seeds germinated from the total seeds (i.e. 20 or 30) per Petri dish during the experiment. Germination rates were calculated using a modified Timson’s index (Timson 1965; Pérez-Fernández et al. 2006): the cumulative sum of all germinated seeds per Petri dish during the course of the experiment divided by the number of days (n = 45). However, as the germination percentage and rate were highly correlated (data not shown), we included only germination percentage in all subsequent analyses. It is noted that one afforested population (Deir Ezzor 3) did not show any germination, and it was consequently not include it in the statistical analysis. We extracted climatic variables (Table 1) per population from the WORLDCLIM database (resolution of 2.5 arc minutes, Hijmans et al. 2005).
We used linear mixed-effects models (LMMs) to analyze the effect of population type (afforested vs. natural populations), temperature regime and treatment combinations and their interactions, and we used seed weight (ln-transformed) as a covariate on germination percentage (arcsine square-root – transformed). The arcsine square-root transformation was performed on our data before statistical analysis to ensure homogeneity of variance. For each species, we performed three individual models, with each model testing different treatment combinations (cold stratification, drought stress and salt stress). The results for the water control were always included as an additional level in the treatment combinations.
We started with a full model using population type, temperature regimes and treatment combinations and their interactions as a fixed effect, and seed weight as a covariate. To account for spatial clustering, we used population as a random effect (justified following model comparisons). Estimates in our models were fit with restricted maximum likelihood (REML). All analyses were performed separately for the two species using the ‘nlme’ package (Pinheiro et al. 2012) in R (R Core Team 2013). We also implemented the ‘multcomp’ package (Hothorn et al. 2008) to perform a Tukey’s pairwise post hoc test (P < 0.05) for the significant interactions.
For both species, we used general linear models to test the influence of mean annual temperature and mean annual precipitation at the seed collection site (henceforth: annual precipitation) on mean germination percentage under the intermediate temperature regime (20/10 °C) and the water control. This resulted in a model comprising the effects of population types (natural vs. afforested) and climatic variables. In order to generate a minimal adequate model for each response variable, we generated a full model and simplified it by removing all non-significant terms (P > 0.05) in a stepwise-backward procedure. Model selection was based on the corrected Akaike Information Criterion (AIC; Akaike 1974) using the ‘sme’ package (Berk et al. 2013).
3 Results
3.1 Pinus brutia
Seed viability was relatively high in natural and afforested population types of P. brutia (mean = 87.5 ± 7.80%). Most non-viable seeds in both population types contained degraded embryos (Table 1). In general, there was no difference in seed germination between the two types of P. brutia populations (Table 2; A-C: Population type). The temperature regimes significantly influenced germination percentage (Table 2; A-C: Temperature). The intermediate temperatures produced the highest germination while the cold temperatures and, to a lesser extent, the hot temperatures produced lower germination percentages (Fig. 2). In all treatments, afforested and natural populations differed in their germination percentage under different temperature regimes (Table 2; A-C: Population type × Temperature, Fig. 2).
| Table 2. Results of linear mixed-effects models performed for Pinus brutia. Models tested whether Population type (natural vs. afforested), Temperature (8/4 °C, 20/10 °C and 32/20 °C), Treatment (including, cold stratification, drought stress: –0.2 MPa and –0.4 MPa, salt stress: 50 mMol l–1 and 100 mMol l–1 and the “deionized water” as a control), and their interactions had a significant effect on seed germination. Population was used as a random effect and seed weight as ln-transformed. | |||||||||
| Model/Treatment | A: Cold stratification | B: Drought stress | C: Salt stress | ||||||
| Fixed effect | d.f. | F-value | p-value | d.f. | F-value | p-value | d.f. | F-value | p-value |
| Population type | 1 | 0.66 | 0.440 | 1 | 4.11 | 0.077 | 1 | 0.63 | 0.450 |
| Temperature | 2 | 255.23 | <0.0001 | 2 | 729.47 | <0.0001 | 2 | 926.59 | <0.0001 |
| Treatment | 1 | 111.05 | <0.0001 | 2 | 158.34 | <0.0001 | 2 | 37.25 | <0.0001 |
| Seed weight | 1 | 0.24 | 0.637 | 1 | 0.24 | 0.634 | 1 | 0.04 | 0.864 |
| Population type × Temperature | 2 | 9.97 | 0.0001 | 2 | 33.56 | <0.0001 | 2 | 5.91 | 0.003 |
| Population type × Treatment | 1 | 0.89 | 0.346 | 2 | 15.21 | <0.0001 | 2 | 2.75 | 0.065 |
| Temperature × Treatment | 2 | 12.84 | <0.0001 | 4 | 43.15 | <0.0001 | 4 | 15.09 | 0.0001 |
| Population type × Temperature × Treatment | 2 | 0.06 | 0.935 | 4 | 2.90 | 0.021 | 4 | 0.938 | 0.441 |
| Bold numbers indicate significant effects (P < 0.05). | |||||||||
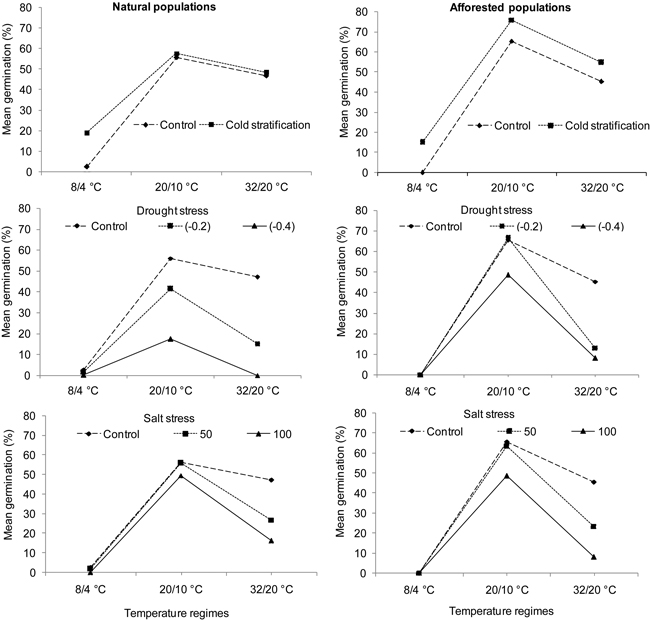
Fig. 2. Interaction plots showing germination percentages of natural (left) and afforested (right) Pinus brutia populations across three different temperature regimes and treatments. Note that germination percentage is shown as not arcsine – transformed. Control = deionized water. Drought stress was tested at levels of –0.2 MPa and –0.4 MPa, and salt stress at concentrations of 50 mMol l–1 and 100 mMol l–1.
Cold stratification significantly increased germination percentage in P. brutia (Table 2; A: Treatment). This effect was revealed in the natural population type only at the cold temperatures (Table 2; A: Temperature × Treatment, Fig. 2). By comparison, both drought and salt stress significantly decreased germination percentage in P. brutia (Table 2; B and C: Treatment, Fig. 2). Under the drought stress treatment, differing responses were revealed in relation to the population types (Table 2; B: Population type × Treatment). A Pairwise-Tukey test showed that afforested populations outperformed natural ones under drought stress level (–0.2) at the intermediate temperatures and under the drought stress level (–0.4 MPa) at both intermediate and hot temperatures (Table 2; B: Population type × Temperature × Treatment; Fig. 2). Moreover, salt stress (50 and 100 mMol l–1) inhibited seed germination in P. brutia, which occurred similarly in both population types under the hot temperature regime (Table 2; C: Temperature × Treatment, Fig. 2).
Seed weight had no significant influence on the germination percentage of P. brutia (Table 2; A-C: Seed weight). According to the minimum adequate model, annual precipitation (AIC: 10.37) was the only climatic predictor that significantly influenced mean germination percentage (F (9, 1) = 6.91, p = 0.034), and had a negative effect (Fig. 4). Adding population type (AIC: 15.84) or annual temperature (AIC: 16.69) did not improve the fit of the minimum adequate model showing that these variables do not affect germination percentage.
3.2 Cupressus sempervirens
Seed viability tested at the beginning of the experiment was relatively low in C. sempervirens (mean = 48.67 ± 17.57%). Moreover, most of the non-viable seeds were empty or had degraded embryos; an observation that was more pronounced in the afforested populations from the arid region (Table 1). We detected no effect of population type on seed germination of C. sempervirens (Table 3; A-C: Population type). Temperature regimes significantly influenced seed germination (Table 3; A-C: Temperature), with the highest germination percentages being revealed at the intermediate temperatures (Fig. 3). In all treatments except for cold stratification, population types reacted differently to temperature regimes (Table 3; A-C: Population type × Temperature). Germination percentage in C. sempervirens was significantly affected by cold stratification, with population types reacting differently to the treatment (Table 3; A: Population type × Temperature × Treatment). A Pairwise-Tukey test showed a decreased germination percentage in the afforested populations in response to this treatment at all temperature regimes but an enhanced germination of the natural populations under the cold temperature regime (Table 3; A: Population type × Temperature × Treatment, Fig. 3).
| Table 3. Results of linear mixed-effects models performed for Cupressus sempervirens. Models tested whether Population type (natural vs. afforested), Temperature (8/4 °C, 20/10 °C and 32/20 °C), Treatment (including, cold stratification, drought stress: –0.2 MPa and –0.4 MPa, salt stress: 50 mMol l–1 and 100 mMol l–1 and the “deionized water” as a control), and their interactions had a significant effect on seed germination. Population was used as a random effect and seed weight as ln-transformed. | |||||||||
| Model/Treatment | A: Cold stratification | B: Drought stress | C: Salt stress | ||||||
| Fixed effect | d.f. | F-value | p-value | d.f. | F-value | p-value | d.f. | F-value | p-value |
| Population type | 1 | 1.81 | 0.226 | 1 | 0.67 | 0.448 | 1 | 0.10 | 0.758 |
| Temperature | 2 | 85.43 | <0.0001 | 2 | 344.37 | <0.0001 | 2 | 230.52 | <0.0001 |
| Treatment | 1 | 3.79 | 0.048 | 2 | 78.69 | <0.0001 | 2 | 56.08 | <0.0001 |
| Seed weight | 1 | 1.96 | 0.219 | 1 | 1.34 | 0.298 | 1 | 0.39 | 0.557 |
| Population type × Temperature | 2 | 0.01 | 0.986 | 2 | 5.38 | <0.001 | 2 | 7.34 | <0.001 |
| Population type × Treatment | 1 | 28.91 | <0.0001 | 2 | 0.23 | 0.790 | 2 | 4.29 | 0.014 |
| Temperature × Treatment | 2 | 4.37 | 0.014 | 4 | 7.21 | <0.0001 | 4 | 6.21 | 0.0001 |
| Population type × Temperature × Treatment | 2 | 8.93 | <0.001 | 4 | 3.42 | 0.009 | 4 | 0.34 | 0.846 |
| Bold numbers indicate significant effects (P < 0.05). | |||||||||
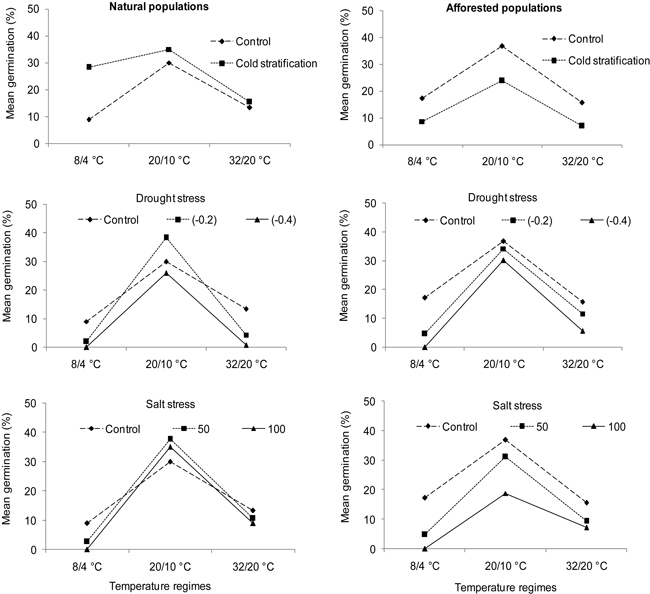
Fig. 3. Interaction plots showing germination percentages of natural (left) and afforested (right) Cupressus sempervirens populations across three different temperature regimes and treatments. Note that germination percentage is shown as not arcsine – transformed. Control = deionized water. Drought stress was tested at levels of –0.2 MPa and –0.4 MPa, and salt stress at concentrations of 50 mMol l–1 and 100 mMol l–1.
Drought and salt stress revealed significant effects on seed germination of C. sempervirens (Table 3; B and C: Treatment). Natural and afforested population types reacted similarly and differently to drought stress and salt stress treatments, respectively (Table 3; B and C: Population type × Treatment). A Pairwise-Tukey test showed that both natural and afforested population types exhibited decreased germination percentages compared to the control under a drought stress of –0.4 MPa at the cold and hot temperatures. Moreover, a drought stress level of –0.2 MPa affected population types differently at the varying temperature regimes (Table 3; B: Population type × Temperature × Treatment). In comparison to the control, a reduction in germination percentage of the natural type occurred under the cold and hot temperature regimes but only under the cold temperature regime in the afforested population type (Fig. 3). Interestingly, under the intermediate temperature regime, natural populations of C. sempervirens showed enhanced germination under drought stress (–0.2 MPa) compared to the control (Fig. 3), albeit to a non-significant degree. Natural C. sempervirens populations showed higher salt tolerance than afforested populations. In this regard, a Pairwise-Tukey test demonstrated a significant decline in seed germination from afforested populations compared to natural ones under the higher salt stress (100 mMol l–1) at the intermediate temperatures (Fig. 3). In afforested populations, both salt stress levels significantly decreased seed germination relative to the control under all temperature regimes (Fig. 3).
We did not find a significant relationship between germination percentage and seed weight under all treatments (Table 3; B-C: Seed weight). In addition, the minimum adequate model (AIC: 9.93) showed that annual temperature significantly negatively influenced the mean germination percentage (F (7, 1) = 5.77, p = 0.049) (Fig. 4). Population type (AIC: 14.50) and annual precipitation (AIC: 14.25) did not have a significant effect on seed germination of C. sempervirens.
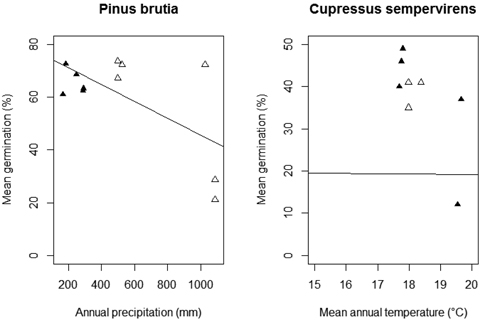
Fig. 4. Relationship between mean germination percentage and annual precipitation and mean annual temperature per population of Pinus brutia and Cupressus sempervirens, respectively (natural populations: empty triangles; afforested populations: solid triangles).
4 Discussion
Trees planted for afforestation need to tolerate considerable abiotic and biotic stress when they are planted outside their native range. Our study demonstrated that the silvicultural practice of raising and planting seedlings led to two different selective outcomes in our study species. In P. brutia, afforested populations acquired an ability to tolerate drought stress at intermediate and hot temperatures. The result may indicate a first sign of local adaptation to more stressful conditions and the development of well adapted land races. However, in C. sempervirens, afforested populations lost their ability to tolerate higher salt stress.
4.1 Local adaptation among afforested Pinus brutia populations
In the case of P. brutia, afforested populations showed significantly higher germination than natural populations under both drought stress levels (–0.2 and –0.4 MPa) and under both intermediate and hot temperature regimes. Afforested populations and their planted parent populations grow in areas with a more arid climate than natural populations. Our results indicate that within two generations, the more arid climate has selected for increased drought stress and higher temperature tolerance among afforested populations. Interestingly, Zhu et al. (2006) found contrasting results for Pinus sylvestris var. mongolica populations, where natural populations had higher germination percentages under increased simulated drought stress compared to afforested populations. The authors attributed their results to the fact that seeds from natural forests developed under lower precipitation, suggesting adaption to more drought conditions.
The stressful environmental conditions at afforestation sites can exert severe selection pressure on species, which is indicated by the differing mortality rates recorded among provenances (Schiller et al. 2004). Although there is no data on the mortality rate of outplanted P. brutia trees after afforestation establishment, investigations on different afforestation projects performed in semiarid areas suggest a mortality average of ca. 80% or 60% for Pinus halepensis (Maestre and Cortina 2004; Schiller et al. 2004). If the same rate applies for P. brutia, it may be assumed that the surviving individuals could cope with stressful and variable environmental conditions at the afforestation sites. A parallel study (Al-Hawija et al. 2014b) showed that afforested populations are also genetically distinct, compared to natural populations, which may indicate that afforested populations are in the process of developing into different land races (White et al. 2007).
4.2 Loss in stress tolerance among afforested Cupressus sempervirens populations
In C. sempervirens, seeds from afforested populations showed significantly lower germination under cold stratification compared to the control. In addition, at the end of the cold-stratification experiment, most of the non-germinated seeds were non-viable and highly infested with fungi, suggesting that the stratification conditions may not have been optimal for afforested C. sempervirens populations. Likewise, in Abies amabilis, stratified seeds displayed more fungal growth than unstratified seeds and exhibited lower germination (Davidson et al. 1996). Edwards (1980) reviewed several studies and revealed that immature seeds tend to show reduced germination as a result of prechilling, and that they are more susceptible to disease. Maturity differences can be found among cones within the same tree and even among seeds within any one cone (Edwards 1982). Cones of C. sempervirens can remain on the tree as long as twenty years (Piotto et al. 2003); it may therefore be assumed that our afforested seeds maintain different levels of maturity and some of them may remain immature.
Our results showed that afforested populations lost their tolerance towards higher salt stress at the intermediate temperatures. The loss in salt tolerance among afforested populations could be attributed to different effects: First, high levels of inbreeding among the small and scattered afforested populations: In this study, the vast majority of non-viable seeds were empty or had degraded embryos. Conifer species are characterized by a high number of lethal equivalents (Savolainen et al.1992). Consequently, most selfed-embryos die during seed development (Charlesworth and Charlesworth 1987; Kärkkäinen et al. 1999). Further, Salzer and Gugerli (2012) found that 76% of seeds collected in small peripheral stands were empty; by comparison, seeds from large core populations showed an average of 30% embryo abortion. The authors considered the high embryo abortion rate is an indication of inbreeding depression at the earliest life stage. Second, the maternal environment could have affected salt tolerance: In conifer species, the maternal environment effect is more pronounced compared to other species because the seed tissues (i.e. the seed coat, megagametophyte and the embryo) that control germination are mainly maternal (El-Kassaby et al. 1992; Linkeis et al. 2010). Moreover, all hormones and provisioning transcripts that regulate germination processes are transmitted from the mother trees to seeds (Nakabayashi et al. 2005). It is possible that the more favorable maternal environment in natural habitats allows progeny seeds to resist higher salt concentrations. Similar results were revealed by Cendàn et al. (2013) for Pinus pinaster, where seeds from the favorable maternal environment germinated earlier and to a greater extent than seeds from the stressful maternal environment.
Last but not least, the result could be due to higher water content within seeds of natural populations: These populations grew under remarkably higher annual precipitation levels compared to afforested populations. Their seeds could have contained more water to withstand salt stress. Croser et al. (2001) similarly explained higher germination success in seeds of Pinus banksiana under salt stress by virtue of their larger seed size and more solutes.
4.3 The effect of seed weight and climatic provenance
Seed germination in P. brutia and C. sempervirens was not affected by seed weight. Likewise, no relationship between seed mass and germination parameters was observed in Pseudotsuga menziesii (El-Kassaby et al. 1992) or Pinus strobus (Parker et al. 2006).
In addition, our results demonstrated for P. brutia that afforested populations from localities with higher annual precipitation showed decreased germination. This is in line with other studies that showed that seeds from humid provenances were more susceptible to high water stress than seeds from arid provenances (Boydak et al. 2003; Tilki and Dirik 2007). Conversely, in C. sempervirens, natural population seeds from localities with lower annual temperatures exhibited higher germination percentages. This can be interpreted again as a more favorable maternal environment in the natural area of the species (Cendàn et al. 2013).
In conclusion, the results of our study show that raising seedlings in nurseries and outplanting them into relatively more stressful environments at afforestation sites can be two-fold: Afforested P. brutia populations showed signs of local adaptation to increased drought and hot temperatures, while afforested C. sempervirens populations appeared to lose their tolerance to salt-stress. Our finding indicates that for P. brutia, the stressful environmental conditions at afforestation sites compared to those prevailing in natural forests induces selection pressures, which in turn result in local adaptation and a development of land races within the species.
The different trend in germination performance between natural and afforested C. sempervirens populations may have mainly resulted from inbreeding depression in the afforested populations, particularly arid regions, which was evidenced by the higher numbers of empty seeds and degraded embryos. In a previous study by Al-Hawija et al. (2014a), seedlings from the respective natural C. sempervirens populations outperformed the afforested ones under different temperature and moisture regimes. This result, together with the current study results, calls for an urgent protection of the natural forest resources of this species in Syria.
As the intermediate temperatures were demonstrated to be optimal for seed germination of population types in both species, it is practically beneficial when such conditions prevail during the germination phase in nurseries.
The positive effect of cold stratification on the activation of germination was more pronounced in afforested populations of P. brutia. This result substantiates the beneficial effect of cold stratification in terms of breaking dormancy and enhancing the germination percentage of P. brutia seeds. Such an approach can be used to facilitate nurseries for large scale afforestation programs. With regard to C. sempervirens, cold stratification improved seed germination in natural population seeds at cold temperatures, which is of relevance where plant sowing can be performed under cold conditions. The negative influence of cold stratification on the afforested populations suggests that this treatment is not recommended for the afforested C. sempervirens populations.
Acknowledgements
We are thankful to Dr. Abdul Munem AlHafez for his invaluable help in collecting materials, Christine Voigt for her assistance in the lab and Dr. Susanne Lachmuth for her support with the statistical analyses. We also thank Daniel McCluskey, who kindly proofread our English, and the two anonymous reviewers for their valuable comments which helped us to significantly improve this manuscript.
References
Akaike H. (1974). A new look at statistical model identification. IEEE Transactions on Automatic Control AU-19: 716–723. http://dx.doi.org/10.1109/TAC.1974.1100705.
Al-Hawija B.N., Lachmuth S., Welk E., Hensen I. (2014a). Performance of seedlings from natural and afforested populations of Cupressus sempervirens under different temperature and moisture regimes. Plant Species Biology. http://dx.doi.org/10.1111/1442-1984.12060.
Al-Hawija BN., Wagner V., Hensen I. (2014b). Genetic comparison between natural and planted populations of Pinus brutia and Cupressus sempervirens in Syria. Turkish Journal of Agriculture and Forestry 38: 267–280. http://dx.doi.org/10.3906/tar-1211-24 .
Al-Oudat M., Qadir M. (2011). The halophytic flora of Syria. International Center for Agricultural Research in the Dry Areas (ICARDA), Aleppo, Syria. 186 p. http://www.icarda.cgiar.org/content/halophytic-flora-syria.
Baskin C.C., Baskin J.M. (1998). Seeds: ecology, biogeography, and evolution of dormancy and germination. Academic Press, San Diego, California, USA.
Berk M. (2013). Sme: Smoothing-splines mixed-effects models. R package version 0.8. http://CRAN.R-project.org/package=sme.
Bewley J.D., Black M. (1994). Seeds, physiology of development and germination. Plenum Press, New York. 2 ed. 445 p.
Bigras F.J., Colombo S.J. (2001). Conifer cold hardiness. Kluwer Academic Publisher.
Boydak M., Duruk H., Tulku F., Alikoulu M. (2003). Effects of water stress on germination in six provenances of Pinus brutia seeds from different bioclimatic zones in Turkey. Turkish Journal of Agriculture and Forestry 27(2): 91–97.
Cendán C., Sampedro L., Zas R. (2013). The maternal environment determines the timing of germination in Pinus pinaster. Environmental and Experimental Botany 94: 66–72. http://dx.doi.org/10.1016/j.envexpbot.2011.11.022.
Charlesworth D., Charlesworth B. (1987). Inbreeding depression and its evolutionary consequences. Annual Review of Ecology and Systematics 18: 237–268. http://dx.doi.org/10.1146/annurev.es.18.110187.001321.
Croser C., Renault S., Franklin J., Zwiatek J. (2001). The effect of salinity on the emergence and seedling growth of Picea mariana, Picea glaucaand, and Pinus banksiana. Environmental Pollution115: 9–16. http://dx.doi.org/10.1016/S0269-7491(01)00097-5.
Davidson R.H., Edwards D.G.W., Sziklai O., El-Kassaby Y.A. (1996). Genetic variation in germination parameters among populations of Pacific silver fir. Silvae Genetica 45: 165–171.
Donohue K. (2009). Completing the cycle: maternal effects as the missing link in plant life histories. Philosophical Transactions of Royal Society B, Biological Sciences 364: 1059–1074. http://dx.doi.org/10.1098/rstb.2008.0291.
Edwards D.G.W. (1980). Maturity and quality of tree seeds – a state of the art review. Seed Science Technology 8: 625–657.
Edwards D.G.W. (1982). Collection, processing, testing, and storage of true fir seeds – a review. In: Oliver C.D., Kenady R.M. (eds.). Proceedings of the Biology and Management of True Fir in the Pacific Northwest Symposium. USDA Forest Service, Pacific North West Range and Experimental Station and University of Washington, Seattle, WA. p. 113–137.
El-Kassaby Y.A., Thomson A.J. (1996). Parental rank changes associated with seed biology and nursery practices in Douglas-fir. Forest Science 42(2): 228–235.
El-Kassaby Y.A., Edwards D.G.W., Taylor D.W. (1992). Genetic control of germination parameters in Douglas-fir and its importance for domestication. Silvae Genetica 41: 48–54.
Ennos R., Worrell R., Malcolm D.C. (1998). The genetic management of native species in Scotland. Forestry 71: 1–23. http://dx.doi.org/10.1093/forestry/71.1.1.
Espinoza S.E., Magni C.R., Martinez V.A., Ivkovic M. (2013). The effect of water availability on plastic responses and biomass allocation in early growth traits of Pinus radiata D. Don. Forest Systems 22(1): 3–14. http://dx.doi.org/10.5424/fs/2013221-02084.
FAO (2003). Definitions related to planted forests. http://www.fao.org/docrep/007/ae347e/ae347e02.htm.
FAO (2010). Global forest resources assessment 2010. http://www.fao.org/docrep/013/i1757e/i1757e.pdf.
FAO (2005). State of the world’s forests 2005. http://www.fao.org/docrep/007/y5574e/y5574e00.htm.
Fenner M., Thompson K. (2005). The ecology of seeds. Cambridge University Press, Cambridge, UK.
García-Fayos P., García-Ventoso B., Cerdà A. (2000). Limitations to plant establishment on eroded slopes in southeastern Spain. Journal of Vegetation Science 11: 77–86. http://dx.doi.org/10.2307/3236778.
Ghazal A. (2008). Landscape ecological phytosociological and geobotanical study of Eu-Mediterranean in West of Syria. Ph.D. theses. Faculty of Agricultural Sciences, University of Hohenheim, Germany. 260 p.
Giannini R., Capuana M., Giovannelli A. (1999). Raising plant material. In: Tessier du Cros E (ed.). Cypress. A practical handbook. Studio Leonardo, Firenze. p. 45–54.
Hendry G.A., Grime J.P. (1993). Methods in comparative plant ecology. Springer. 252 p.
Hijmans R.J., Cameron S.E., Parra J.L., Jones P.G., Jarvis A. (2005). Very high resolution interpolated climate surfaces for global land areas. International Journal of Climatology 25: 1965–1978. http://dx.doi.org/10.1002/joc.1276.
Hothorn T., Bretz F., Westfall P. (2008). Simultaneous inference in general parametric models. Biometrical Journal 50(3): 346–363. http://cran.r-project.org/web/packages/multcomp/vignettes/generalsiminf.pdf.
Ilaiwi M. (2001). Soils of the Syrian Arab Republic. In: Zdruli P., Steduto P., Lacirignola C., Montanarella L. (eds.). Soil resources of Southern and Eastern Mediterranean countries. CIHEAM, Bari. p. 227–242. (Options Méditerranéennes : Série B. Etudes et Recherches 34).
Jamal M., Arslan A., Dayoub K. (2005). Harnessing salty water to enhance sustainable livelihoods of the rural poor in four countries in West Asia and North Africa: Syria. General Commission for Scientific Agricultural Research–Ministry of Agriculture and Agrarian Reform. http://www.iwmi.cgiar.org/assessment/Research_Projects/harnessing_salty_water.htm.
Kärkkäinen K., Savolainen O., Koski V. (1999). Why do plants abort so many developing seeds: bad offspring or bad maternal genotypes? Evolutionary Ecology 13:305–317. http://dx.doi.org/10.1023/A:1006746900736.
Lal R. (2004). Food security soil carbon sequestration impacts on global climate change and food security. Science 304: 1623. http://dx.doi.org/10.1126/science.1097396.
Linkies A., Graeber K., Knight C., Leubner-Metzger G. (2010). The evolution of seeds. http://dx.doi.org/10.1111/j.1469-8137.2010.03249.x.
Maestre F.T., Cortina J. (2004). Are Pinus halepensis plantations useful as a restoration tool in semiarid Mediterranean areas? Forest Ecology and Management 198(1): 303–317. http://dx.doi.org/10.1016/j.foreco.2004.05.040.
Metzger J.O., Hüttermann A. (2009). Sustainable global energy supply based on lignocellulosic biomass from afforestation of degraded areas. Naturwissenschaften 96(2): 279–288. http://dx.doi.org/10.1007/s00114-008-0479-4.
Muona O., Harju A. (1989). Effective population sizes, genetic variability, and mating systems in natural stands and seed orchards of Pinus sylvestris. Silvae Genetica 38: 221–228.
Nahal I., Zahoueh S. (2005). Syria. In: Merlo M., Croitoru L. (eds.). Valuing Mediterranean forests: towards total economic value. CABI International, Wallingford UK, Cambridge MA. p. 177–194.
Nakabayashi K., Okamoto M., Koshiba T., Kamiya Y., Nambara E. (2005). Genome-wide profiling of stored mRNA in Arabidopsis thaliana seed germination: epigenetic and genetic regulation of transcription in seed. Plant Journal 41: 697–709. http://dx.doi.org/10.1111/j.1365-313X.2005.02337.x.
Parker W.C., Noland T.L., Morneault A.E. (2006). The effects of seed mass on germination, seedling emergence, and early seedling growth of eastern White pine (Pinus strobus L.). New Forests 32: 33–49. http://dx.doi.org/10.1007/s11056-005-3391-1.
Pérez-Fernández M.A., Calvo-Magro E., Ferrer-Castán D. (2006). Simulation of germination of pioneer species along an experimental drought gradient. Journal of Environmental Biology 27: 679–685.
Pigliucci M., Murren C.J., Schlichting C.D. (2006). Phenotypic plasticity and evolution by genetic assimilation. The Journal of Experimental Biology 209: 2362–2367. http://dx.doi.org/10.1242/jeb.02070.
Pinheiro J., Bates D., Deb Roy S., Sarkar D., the R Development Core Team. (2012). nlme: Linear and nonlinear mixed effects models. R package version 3.1–103. http://cran.r-project.org/web/packages/nlme/index.html.
Piotto B., Bartolini G., Bussotti F., Asensio A.A.C., Garcia C., Chessa I., Ciccarese C., Ciccarese L., Crosti R., Cullum F.J., Noi A.D., GarciaFayos P., Lambardi M., Lisci M., Lucci S., Melini S., Reinoso J.C.M., Murranca S., Nieddu G., Pacini E., Pagni G., Patumi M., Garcia F.P., Piccini C., Rossetto M., Tranne G., Tylkowski T. (2003). Fact sheets on the propagation of Mediterranean trees and shrubs from seed. In: Piotto B., Di Noi A. (eds.). Seed propagation of Mediterranean trees and shrubs. APAT, Rome. p. 11–51.
Quilichini A., Debussche M. (2000). Seed dispersal and germination patterns in a rare Mediterranean island endemic (Anchusa crispa Viv., Boraginaceae). Acta Oecologica 21: 303–313. http://dx.doi.org/10.1016/S1146-609X(00)01089-4.
Rajora O.P. (1999). Genetic biodiversity impacts of silvicultural practices and phenotypic selection in white spruce. Theoretical and Applied Genetics 99: 954–961. http://dx.doi.org/10.1007/s001220051402.
Rao P.B., Singh S.P. (1985). Response breadths on environmental gradients of germination and seedling growth in two dominant tree species of central Himalayas. Annals of Botany 56: 783–794.
Salzer K., Gugerli F. (2012). Reduced fitness at early life stages in peripheral versus core populations of Swiss stone pine (Pinus cembra) is not reflected by levels of inbreeding in seed families. Alpine Botany 122: 75–85. http://dx.doi.org/10.1007/s00035-012-0106-z.
Savolainen O., Kärkkäinen K., Kuittinen H. (1992). Estimating numbers of embryonic lethals in conifers. Heredity 69:308–314. http://dx.doi.org/1038/hdy.1992.130.
Schiller G., Korol L., Shklar G. (2004). Habitat effects on adaptive genetic variation in Pinus halepensis Mill provenances. Forest Genetics 11: 325–335.
Schweers W., Rieser A., Zöbisch M., Thomson T. (2005). Development of soil salinity under irrigated land-use in a dry region of Syria. Conference on International Agricultural Research for Development. Stuttgart-Hohenheim, Germany. http://www.tropentag.de/2005/abstracts/full/563.pdf.
Silen R., Osterhaus C. (1979). Reduction of genetic base by sizing of bulked Douglas-fir seed lots. Tree Planter’s Notes 30: 24–30.
Simak M., Kamra S.K. (1970). Germination studies on Norway spruce (Picea abies) seed of different provenances under alternating and constant temperatures. International Seed Testing Association 35: 383–391.
Skordilis A., Thanos C.A. (1995). Seed stratification and germination strategy in the Mediterranean pines Pinus brutia and P. halepensis. Seed Science Research 5: 151–160. http://dx.doi.org/10.1017/S0960258500002774 .
Skrøppa T., Johnsen Ø. (2000). Patterns of adaptive genetic variation in forest tree species; the reproductive environment as an evolutionary force in Picea abies. In: Mátyás C. (ed.). Forest genetics and sustainability. Kluwer Academic Publications, Dordrecht, The Netherlands. p. 49–58.
Skrøppa T., Nikkanen T., Ruotsalainen S., Johnsen Ø. (1994). Effects of sexual reproduction at different latitudes on performance of the progeny of Picea abies. Silvae Genetica 43: 298–304.
Thanos C.A., Skordilis A. (1987). The effect of light, temperature and osmotic stress on the germination of Pinus halepensis and P. brutia seeds. Seed Science Technology 15: 163–174.
The R Development Core Team (2013) R: a language and environment for statistical computing. R Foundation for Statistical Computing. http://www.R-project.org.
Tilki F., Dirik H. (2007). Seed germination of three provenances of Pinus brutia (Ten.) as influenced by stratification, temperature and water stress. Journal of Environmental Biology 28(1): 133–136.
Timson J. (1965). New method of recording germination data. Letters of Nature 207: 216–217. http://dx.doi.org/10.1038/207216a0.
van den Driessche R. (1991). Influence of container nursery regimes on drought resistance of seedlings following planting. I. Survival and growth. Canadian Journal of Forest Research 21: 555–565. http://dx.doi.org/10.1139/x91-077.
White T.L., Adams W.T., Neale D.B. (2007). Forest genetics. Cambridge University Press Cambridge UK. 682 p.
Wilcox M.D. (1983). Inbreeding depression and genetic variances estimated from self- and cross- pollinated families of Pinus radiata. Silvae Genetica 32: 89–96.
Zhu J., Kang H., Tan H., Xu M. (2006). Effects of drought stresses induced by polyethylene glycol on germination of Pinus sylvestris var. Mongolia seeds from natural and plantation forests on sandy land. Journal of Forest Research 11(5): 319–328. http://dx.doi.org/10.1007/s10310-006-0214-y.
Zobel B., Talbert J. (1984). Applied forest tree improvement. John Wiley and Sons, New York, NY, USA. 505 p.
Zohary D. (1973). Geobotanical foundations of the Middle East. Vols. 1 and 2. Gustav Fischer Verlag and Swets & Zeitlinger, Stuttgart and Amsterdam.
Total of 67 references

