The saprotrophic wood-degrading abilities of Rigidoporus microporus
Oghenekaro A. O., Daniel G., Asiegbu F. O. (2015). The saprotrophic wood-degrading abilities of Rigidoporus microporus. Silva Fennica vol. 49 no. 4 article id 1320. https://doi.org/10.14214/sf.1320
Highlights
- Rigidoporus microporus isolates displayed varying saprotrophic capabilities on wood blocks of Rubber tree (Hevea brasiliensis)
- Percentage mass loss of (Hevea brasiliensis) wood blocks caused by the pathogenic Rigidoporus microporus was significantly higher than that observed with the endophytic isolate
- The endophytic isolate has very poor saprotrophic ability on Hevea brasiliensis wood blocks.
Abstract
Saprotrophic wood-decaying abilities of Rigidoporus microporus (Polyporales, Basidiomycota) syn. Rigidoporus lignosus and the structural alterations induced in wood blocks of Hevea brasiliensis Muell. Arg were studied. Mass loss of wood blocks was analyzed after 3 and 6 months respectively and the patterns of decay by pathogenic and endophytic isolates of this fungus were investigated using light microscopy. Effects of temperature on growth of the isolates on malt extract agar were also investigated. The R. microporus isolated from a non-H. brasiliensis host caused the highest percentage mass loss (27.2% after 6 months), followed by isolates ED310 (21.1%) and M13 (15.7%), both collected from diseased H. brasiliensis plantations. The isolate initially identified as an endophyte showed very low saprotrophic wood decay capability (4.3% after 6 months). The optimal temperature for growth of the isolates was 30 °C; except for the endophytic isolate which showed highest growth at 25 °C. Wood samples degraded by the R. microporus isolates showed simultaneous attack of wood cell walls, typical of white rot fungi. Results of the study indicate variability in the wood degrading abilities of the isolates and the potential differences in their physiology are discussed. Our findings further support the need for a taxonomical revision of the Rigidoporus genus.
Keywords
simultaneous decay;
wood degradation;
white rot;
delignification
-
Oghenekaro,
Department of Forest Sciences, University of Helsinki, P.O. Box 27, FI-00014 University of Helsinki, Finland
E-mail
abbot.oghenekaro@helsinki.fi

- Daniel, Department of Forest Products/Wood Science, Swedish University of Agricultural Sciences, P.O. Box 7008, SE-75007 Uppsala, Sweden E-mail geoffrey.daniel@slu.se
- Asiegbu, Department of Forest Sciences, University of Helsinki, P.O. Box 27, FI-00014 University of Helsinki, Finland E-mail fred.asiegbu@helsinki.fi
Received 16 February 2015 Accepted 19 August 2015 Published 25 August 2015
Views 110862
Available at https://doi.org/10.14214/sf.1320 | Download PDF

1 Introduction
Rigidoporus microporus (Polyporales, Basidiomycota) syn. Rigidoporus lignosus is the most economically important pathogen of tropical rubber tree (Hevea brasiliensis Muell. Arg) plantations (Liyanage 1997) where it causes white rot disease (WRD). White rot disease was shown as a major problem for 43% of farmers in a smallholdings survey in Malaysia (Sail and Ahmad 2009). In Nigeria, WRD is responsible for 96 % of incidences of root diseases and results in killing of up to five trees per hectare per year in plantations (Omorusi, 2012). The taxonomy of the R. microporus complex is problematic. The source of the type specimen of R. microporus (Sw.) Overeem 1788 was from West Indies, but the source of the type specimen of the synonym R. lignosus (Klotzsch) Imazeki is not given in the literature and probably does not exist anymore (Ryvarden, 1976). In an earlier multigene phylogenetic study, we were able to separate R. microporus isolates causing WRD in Africa and Asia into two different groups (Oghenekaro et al. 2014). Non-pathogenic isolates from Peru and a herbarium specimen of a sample from Cuba identified as R. microporus were also separated into different groups (Oghenekaro et al. 2014). White rot disease attack of rubber wood plantations is well known throughout Southeast Asia, central, east and West Africa.
White rot fungi are known as active wood degraders secreting a wide range of hydrolytic and oxidative enzymes involved in plant polymer biomineralization (Daniel 2014). Cellulose degradation is carried out by a range of cellobiohydrolases and endoglucanases (Hori et al. 2013). Lytic polysaccharide monooxygenases are also implicated in cellulose breakdown (Bey et al. 2013). White rot fungi degrade lignin using a plethora of ligninolytic peroxidases and laccases (Daniel 2014). Ligninolytic peroxidases include lignin, manganese and versatile peroxidases that may be produced in different amounts according to the white rot species involved (Floudas et al. 2012; Fernandez-Fueyo et al. 2012). Laccases may also be produced in high amounts by white rot basidiomycetes and are implicated in various biological processes in addition to lignin biodegradation in both bacteria and fungi (Furukawa et al. 2014). A typical example is Pycnoporus cinnabarius (Jacq.) Fr. which has been shown to secrete large amounts of laccase into culture media (Levasseur et al. 2014).
Apart from being a serious pathogen, R. microporus is a typical white rot basidiomycete with saprotrophic abilities degrading the major components of wood including lignin. Epidemiological studies of H. brasiliensis natural forest and plantations showed high fungal density in soil (Nandris et al. 1988) suggesting a capacity for high biodegradative ability of plant residues by R. microporus. Majority of previous studies on R. microporus has been directed at population biology and molecular phylogeny (Kaewchai et al. 2010; Oghenekaro et al. 2014), pathogenicity (Farid et al. 2009; Kaewchai et al. 2009; Madushani et al. 2013), host-parasite interactions (Nicole et al, 1985; Nicole et al. 1986a; 1986b), peroxidases (Geiger et al. 1989) and laccases, with the latter being isolated, purified and studied in detail (Nicole et al. 1992; Bonomo et al. 1998; 2001; Cambria et al, 2000; 2011; 2012). In vitro studies have focused on biological control of the pathogen (Kaewchai and Soytong, 2010; Ogbebor et al. 2015).
There are presently no studies on the effects of temperature on in vitro growth of isolates of the fungus causing WRD and almost nothing is known about the role of the fungus when the tree is eventually killed. The present study primarily focused on assessment of saprotrophic wood decay ability of a tropical rubber tree pathogen (Rigidoporus microporus) compared to isolates described as endophytes or saprotrophs on host and non-host trees. To achieve this, we conducted initial studies to assess wood decay capacity of a sub-set of representative R. microporus isolates including non-pathogenic South American isolate previously identified as an endophyte (Martin et al. 2015).
2 Materials and methods
2.1 Fungal isolates
The Rigidoporus microporus isolates used were: ED310 (Nigeria), M13 (Malaysia), MUCL45064 (Cuba) and MS564b (Peru). The isolates, ED310 and M13 were isolated from diseased H. brasiliensis plantations. MUCL45064 was isolated from an un-identified angiosperm in 2003 and identified as R. microporus according to records from BCC/MUCL [Belgian Co-ordinated collections of Micro-organisms (http://bccm.belspo.be/about-us/bccm-mucl)]. MS564b was isolated from sapwood of H. brasiliensis from the wild and identified as R. microporus (Martin et al. 2015). Isolates ED310, M13 and MS564b are deposited in culture collections of Forest Pathology Group at Department of Forest Sciences, University of Helsinki. The isolates used in this study were derived from isolates that were identified in our earlier work using multigene phylogeny and found to belong to 4 different clades. The isolates were previously grouped together as R. microporus. The African and Asian isolates were more closely related and were isolated from WRD diseased H. brasiliensis trees (Oghenekaro et al. 2014). The fungi were routinely maintained on 2% w/v malt extract agar plates (MEA).
2.2 Wood samples and decay tests
Wood decay studies were carried out using wood blocks obtained from Rubber tree (H. brasiliensis). Wood blocks were generated from the tree clone NIG 801 (Umar et al. 2010) of H. brasiliensis at the Rubber Research Institute of Nigeria. Three wood blocks measuring 3 x 1 x 0.5 cm were dried at 65 °C to constant mass. Three wood blocks per treatment were then weighed and placed in 100 ml Erlenmeyer flasks containing vermiculite (fraction size – 1 mm) and nutrient solution (gl–1: NH4NO3 – 0.6, K2HPO4 – 0.4, KH2PO4 – 0.5, MgSO4.7H2O – 0.4 and glucose – 1.0) in the ratio 1:6 (1 g vermiculite to 6 ml nutrient solution). Flasks containing wood blocks were stoppered with cotton wool and aluminum foil and autoclaved for 20 mins. Three agar square plugs (5 mm2) from actively growing isolates of R. microporus were inoculated into each flask with wood blocks. Sterile agar square plugs of 2% MEA inoculated into separate flasks containing wood blocks served as controls. There were three replications. Inoculated flasks were placed in an improvised incubation chamber overlaid with wet paper towels to provide humidity and prevent water loss. The chamber was covered with a lid and incubated at 25 °C. Humidity in the chamber was maintained at 60–80% by wetting the paper towels periodically at intervals of 2 weeks. Two time points were chosen, with sets of blocks harvested after three months and six months. At the end of each incubation period, the wood blocks were removed from the flask and adhering mycelia scraped off using a scalpel. Wood blocks were then dried in an oven at 105 °C for 24 hrs. Percentage dry matter loss with respect to the original dry mass of the wood blocks was then calculated.
2.3 Growth rate and temperature studies
Growth rates of the isolates at different temperature (15, 20, 25, and 30 °C) were studied in vitro using MEA. Five mm2 agar plug of an actively growing culture was placed on the center of 9 cm Petri dish containing MEA. Three replicates were prepared for each isolate and temperature. Measurement of the diameter of colony growth was done daily for each replicate. Mean values were calculated.
2.4 Light microscopy observations of wood decay
To observe patterns of fungal colonization and decay by the R. microporus isolates, sections were cut from the wood blocks using a razor blade and examined using light microscopy. Transverse (T.S), radial and tangential longitudinal (L.S) sections were stained with either 1% w/v Safranin O or 1% w/v aniline blue (to stain and visualize hyphae) respectively. Sections were mounted in fresh glycerol on glass slides and observed with a Leica DMLB light microscope with images recorded using a Leica DC 300 camera.
2.5 Statistical analysis
The Levene test was used for testing homogeneity of variances (p > 0.05) and normality of the error variances. Normality of variances and uniformity of residuals was confirmed by the skewness and kurtosis values for the data distribution. To better satisfy conditions for normality and uniformity of variance, logarithmic values of the data distribution was used for the skewness and kurtosis test. Percentage mass loss was compared separately at 3 and 6 months, using one-way analyses of variance to determine significance at 5% level (p ≤ 0.05). Interaction of temperature and isolate on hypha length on media was tested using two-way analysis of variance at 5% level (p ≤ 0.05). Differences between the means (within groups) of the percentage mass losses caused by the different fungal isolates were tested using Tukey HSD (Honestly Significant Difference) test. Statistical analysis was performed with SPSS 21.0 Statistical package (IBM Corporation, New York, U.S.A).
3 Results
3.1 Mass loss
The R. microporus isolates studied showed large variations in their saprotrophic wood decay ability on rubber wood blocks. Wood blocks were densely colonized with characteristic mycelial strands of R. microporus by three of the isolates while isolate MS564b showed poor and faint colonization. Isolate MUCL45064 showed the greatest capacity for saprotrophic attack degrading 19.3% and 27% of the wood after 3 and 6 months respectively. This was followed by ED310 (13.7% and 21.2%) and M13 (11.3% and 15.7%) isolates (Fig. 1). The isolate MS564b showed only weak attack and 4.3% mass loss after 6 months. According to Tukey’s HSD test, there was no significant difference between isolates ED310 and M13 after 3 months. (p > 0.05). There was no significant difference in percentage mass loss between the non-pathogenic endophytic isolate (MS564b) and the control both at 3 and 6 months (p > 0.05) (Fig. 1).
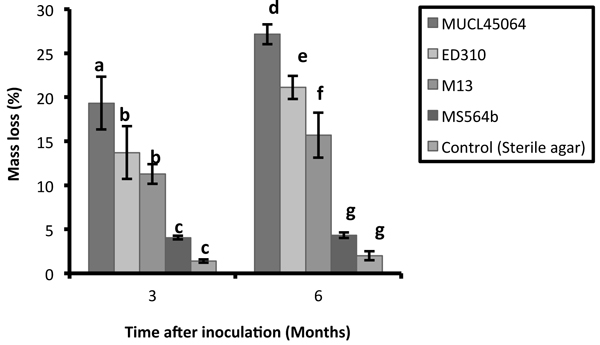
Fig. 1. Mass loss of Hevea brasiliensis wood blocks after 3 and 6 months caused by isolates of Rigidoporus microporus. Values with different letters differed significantly within groups according to Tukey HSD test (P < 0.05). Error bars indicate standard errors of the mean.
3.2 Effects of temperature on growth rate
On MEA, isolates MUCL45064 and MS564b grew at all the temperatures tested while ED310 and M13 did not grow at 15 °C after 6 days (Fig. 2). Highest hyphae growth length after 6 days (78.3 mm) was observed for M13 at 30 °C (Fig. 2). A two way ANOVA at the 5% level (p ≤ 0.05) to test interaction of temperature and isolate on hyphae length on media showed that there was significant effect (F = 103.26, p = 0.001). Consequently, we did not consider the effects of temperature and isolate type individually. However, there was generally an increase in growth as temperature increased except with the endophytic isolate, MS564b. For the endophytic isolate, mean growth at 25 °C (52.1 mm) was higher than at 30 °C (41 mm) after 6 days (Fig 2).
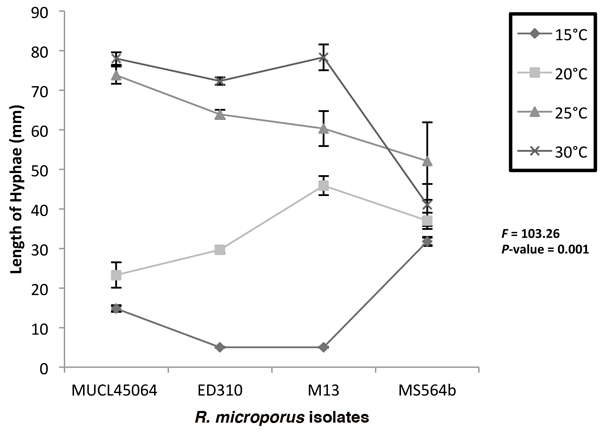
Fig. 2. Growth of Rigidoporus microporus isolates at different temperatures on malt extract agar after 6 days. Two way ANOVA shows interactive effect of isolates and temperature is significant (P < 0.05) and only this interaction was significant. The F and P values for the time x isolate combinations are indicated. Error bars indicate standard deviations of the mean.
3.3 Light microscopy observations of wood decay
Light microscopy was carried out to confirm the mass loss results and to visualize the attack on the wood material. All four isolates produced simultaneous attack and characteristic cell wall thinning typical of white rot fungi. The ED310, MUCL45064 and M13 isolates showed typical hyphal colonization and spreading into the rays and vessels as well as to other cellular elements. First signs of delignification occurred in the ray cells where the red staining of lignified cells changed to pinkish indicating a gradual selective lignin loss from the ray cells towards the fibers. Corresponding cell wall thinning occurred as delignification progressed (Fig. 3A). As decay continued, ultimately, the middle lamella holding the fibers together was delignified resulting in fiber separation (Fig. 3B). Examples of fungal delignification spreading from the rays to the secondary wall (S2) of fibers are shown in Figure 3A, B, C. Hyphae of most of the isolates after staining with aniline blue were observed in the vessels and ray cells (Fig. 3D, E, F). While hyphae colonization of vessels was evident, the vessel walls showed strong resistance to decay by the isolates.
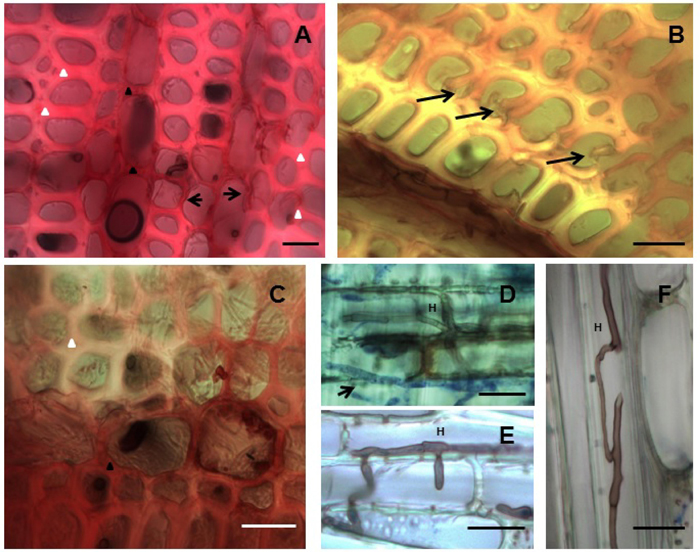
Fig. 3. Simultaneous white rot decay of Hevea brasiliensis wood by Rigidoporus microporus. (A) MUCL45064, Decay progressing from ray towards (arrows) the fibers and cell wall thinning through simultaneous attack. (B) ED310, Early stage of fibre separation due to attack of middle lamellae. (C) MS564b, Delignification spreading from across fibers as seen by the loss of staining (upper fibre regions). (D) ED310, Hypha (H) stained with aniline-blue (arrow) in the rays. (E) ED310, Hypha (H) penetrating vessel. (F) MS564b, hyphae (H) spreading through a fibre. Fig 3A-C –Transverse sections stained with safranin. Fig. 3D-F-Longitudinal sections stained with aniline blue. Bars: 20 µm.
4 Discussion
In this study, the results indicated that the R. microporus isolates previously isolated from trees displayed varying abilities to cause mass loss of rubber wood suggesting differences in their saprotrophic abilities. Interestingly, the values for percentage mass loss recorded were comparable to other studies using strains of the sapstain fungus Lasiodiploidia theobromae (Pat.) Griffon & Maubl. cultivated on H. brasiliensis and inoculated under similar conditions (Encinas and Daniel 1996; 1997). The isolate MUCL45064 collected from a non-H. brasiliensis host showed higher wood decay ability than the isolates which are proven pathogens on rubber trees. This isolate also had the highest growth on MEA at 25 °C, the same temperature used for the wood decay experiment. It is difficult to speculate on whether the higher saprotrophic ability of this isolate also suggests a potential to be pathogenic on H. brasiliensis plantations especially if it undergoes a host-shift. Possibility for a host-shift of R. microporus from other trees to H. brasiliensis or vice-versa has been proven through molecular studies (Oghenekaro et al. 2014). The fungus has recently been reported for the first time as pathogenic on Artocarpus nobilis Thw. (Moraceae family) in Sri Lanka (Madushani et al. 2013). An interesting feature concerned the endophytic isolate which showed very poor saprotrophic ability; perhaps this is associated with its behavior as a sapwood endophyte of H. brasiliensis in the natural forest (Martin et al. 2015).
Of the four isolates studied, the MUCL45064, ED310 and M13 isolates showed characteristic mycelial strand development in culture. R. microporus produces rhizomorphs in the soil and also mycelial strands under culture conditions (Richard and Button 1996). The absence of mycelial strands in the non-pathogenic isolate further points to an endophytic life-style. This isolate was previously isolated from the sapwood of healthy H. brasilieneis trees showing no symptoms of WRD (Martin et al. 2015). The effect of different temperature on the growth rates of the fungi also varied among the isolates. The varying saprotrophic abilities of the different isolates suggests that the isolates collectively considered as R. microporus (Mycobank, GenBank) and used for this study are most likely different species as suggested by Oghenekaro et al. (2014) through molecular phylogenetic studies. This supports the consensus that the R. microporus/lignosus species complex should be revised.
Light microscopy observations of H. brasiliensis degraded wood confirmed typical white rot decay. The differential pink and red Safranin staining of cell walls showed evidence of delignification as reported in previous studies (Srebotnik and Messner 1994; Schwarze and Engels 1998). The isolates colonized the wood blocks through the rays, vessels and then fibers. Direct hyphal penetration through wood cell walls was observed for all isolates except for the endophytic isolate whose hyphae only colonized and spread in the rays. There was widespread distribution of hyphae throughout vessels, fibres and tracheids in the wood blocks especially with isolate ED310 (Schwarz et al., 2004; Schwarz 2007). Simultaneous cell wall thinning and preferential degradation of wood cell walls was observed for the MUCL45064, ED310 and M13 isolates. Degradation of cell wall materials particularly around the middle lamella regions was also evident. The saprotrophic decay patterns observed are similar to those reported for other basidiomycete white rot fungi like Heterobasidion annosum (Daniel et al. 1998); Lenzites stereoides (Fr.) Ryvarden and Ganoderma lucidum (Curtis) P. Karst (Nagadesi et al. 2013); Phanerochaete chrysosporium Burdsall (Koyani and Rajput 2014); Schizophyllum commune Fries and Flavodon flavus (Klotzsch) Ryvarden (Padhiar and Albert 2012).
The results of the present study reveal differences in the wood decay capability of host and non-host pathogenic and endophytic isolates as well as in their physiology. The study further sheds light on the potential contribution of the isolates to carbon cycling through their saprotrophic decay activities on dead trees already killed by this fungus. This imperatively should have important implications on the management of trees in rubber plantations infected by the pathogens.
Acknowledgements
We thank the Delta State Government of Nigeria for a postgraduate scholarship to A.O.O. We are also grateful to Academy of Finland for project funding.
References
Bey M., Zhou S., Poidevin L., Henrissat B., Coutinho PM., Berrina J., Sigoillot J. (2013). Cello-Oligosaccharide oxidation reveals differences between two lytic polysaccharide monooxygenases (family GH61) from Podospora anserina. Applied and Environmental Microbiology 79: 488–496. http://dx.doi.org/10.1128/AEM.02942-12.
Bonomo R.P., Boudet A.M., Cozzolino R., Rizzarelli E., Santoro A.M., Sterjiades R., Zappalà R. (1998). A comparative study of two isoforms of laccase secreted by the ‘white- rot’ fungus Rigidoporus lignosus, exhibiting significant structural and functional differences. Journal of Inorganic Biochemistry 71(3–4): 205–211. http://dx.doi.org/10.1016/S0162-0134(98)10057-0.
Bonomo R.P., Cennamo G., Purrello R., Santoro A.M., Zappalà R. (2001). Comparison of three fungal laccases from Rigidoporus lignosus and Pleurotus ostreatus: correlation between conformation changes and catalytic activity. Journal of Inorganic Biochemistry 83(1): 67–75. http://dx.doi.org/10.1016/S0162-0134(00)00130-6.
Cambria M., Cambria A., Ragusa S., Rizzarelli E. (2000). Production, purification and properties of an extracellular laccase from Rigidoporus lignosus. Protein Expression and Purification 18(2): 141–147. http://dx.doi.org/10.1006/prep.1999.1126.
Cambria M.T., Ragusa S., Calabrese V., Cambria A. (2011). Enhanced laccase production in white-rot fungus Rigidoporus lignosus by the addition of selected phenolic and aromatic compounds. Applied Biochemistry and Biotechnology 163(3): 415–422. http://dx.doi.org/10.1007/s12010-010-9049-2.
Cambria M.T., Gullotto D., Garavaglia S., Cambria A. (2012). In silico study of structural determinants modulating the redox potential of Rigidoporus lignosus and other fungal laccases. Journal of Biomolecular Structure and Dynamics 30(1): 89–101. http://dx.doi.org/10.1080/07391102.2012.674275.
Daniel G. (2014). Fungal and bacterial biodegradation: white rots, brown rots, soft rots, and bacteria. In: Schultz T. et al. (eds.). Deterioration and protection of sustainable biomaterials. ACS Symposium Series, American Chemical Society, Washington, DC. p. 23–54. http://dx.doi.org/10.1021/bk-2014-1158.ch002.
Daniel G., Asiegbu F., Johansson M. (1998). The saprotrophic wood-degrading abilities of Heterobasidium annosum intersterility groups P and S. Mycological Research 102(8): 991–997. http://dx.doi.org/10.1017/S0953756297005935.
Encinas O., Daniel G. (1996). Decay capacity of different strains of the blue stain fungus Lasiodiplodia theobromae on various wood species. Material Und Organismen 30(4): 239–258.
Encinas O., Daniel G. (1997). Degradation of the gelatinous layer in aspen and rubberwood by the blue stain fungus Lasiodiplodia theobromae. International Association of Wood Anatomists Journal 18(2): 107–115. http://dx.doi.org/10.1163/22941932-90001471.
Farid A.M., Lee S.S., Maziah Z., Patahayah M. (2009). Pathogenicity of Rigidoporus microporus and Phellinus noxius against four major plantation tree species in peninsular Malaysia. Journal of Tropical Forest Science 21: 289–298.
Fernandez-Fueyo E., Ruiz-Duenas F.J., Ferreira P., Floudas D., Hibbett D.S et al. (2012). Comparative genomics of Ceriporiopsis subvermispora and Phanerochaete chrysosporium provide insight into selective ligninolysis. Proceedings of the National Academy of Sciences of the United States of America 109: 5458–5463. http://dx.doi.org/10.1073/pnas.1119912109.
Floudas D., Binder M., Riley R., Barry K., Blanchette R.A. et al. (2012). The Paleozoic origin of enzymatic lignin decomposition reconstructed from 31 fungal genomes. Science 336: 1715–1719. http://dx.doi.org/10.1126/science.1221748.
Furukawa T., Bello F.O., Horsfall L. (2014). Microbial enzyme systems for lignin degradation and their transcriptional regulation. Frontiers in Biology 9(6): 448–471. http://dx.doi.org/10.1007/s11515-014-1336-9.
Geiger J.P., Rio B., Nicole M., Nandris D. (1989). Peroxidase production in tissues of the rubber tree following infection by root rot fungi. Physiological and Molecular Plant Pathology 34: 241–256. http://dx.doi.org/10.1016/0885-5765(89)90047-7.
Hori C., Gaskell J., Igarashi K., Samejima M., Hibbett D et al. (2013). Genome wide analysis of polysaccharide degrading enzymes in eleven white and brown rot polyporales provides insight into mechanisms of wood decay. Mycologia 105: 1412–1427. http://dx.doi.org/10.3852/13-072.
Kaewchai S., Soytong K. (2010). Application of biofungicides against Rigidoporus microporus causing white root disease of rubber trees. Journal of Agricultural Technology 6: 349–363.
Kaewchai S., Wang H.K., Lin F., Hyde K.D., Soytong K. (2009). Genetic variation among isolates of Rigidoporus microporus causing white root disease of rubber trees in southern Thailand revealed by ISSR markers and pathogenicity. African Journal of Microbiology Research 3: 641–648.
Kaewchai S., Lin F., Wang H.K., Soytong K. (2010). Characterization of Rigidoporus microporus isolated from rubber trees based on morphology and ITS sequencing. Journal of Agricultural Technology 6: 289–298.
Koyani R.D., Rajput K.S. (2014). Light microscopic analysis of Tectona grandis L.f. wood inoculated with Irpex lacteus and Phanerochaete chrysosporium. European Journal of Wood and Wood Products 72(2): 157–164. http://dx.doi.org/10.1007/s00107-013-0763-7.
Levasseur A., Lomascolo A., Chabrol O., Ruiz-Dueñas F.J., Boukhris-Uzan E et al. (2014). The genome of the white-rot fungus Pycnoporus cinnabarinus: a basidiomycete model with a versatile arsenal for lignocellulosic biomass breakdown. BMC Genomics 15(1): 486. http://dx.doi.org/10.1186/1471-2164-15-486.
Liyanage A.S. (1997). Rubber. In: Hillocks R.J., Waller J.M. (eds.). Soilborne diseases of tropical crops. CAB International, Wallingford, UK. p. 331–347.
Madushani H.K. I., Fernando T.H. P.S., Wijesundara R.L. C., Siriwardane D. (2013). First report of white root disease of Artocarpus nobilis in Sri lanka caused by Rigidoporus microporus. Journal of the National Science Foundation of Sri Lanka 42(2): 197–198.
Martin R., Gazis R., Skaltsas D., Chaverri P., Hibbett D.S (2015). Unexpected diversity of basidiomycetous endophytes in sapwood and leaves of Hevea. Mycologia. http://dx.doi.org/10.3852/14-206.
Nagadesi P.K., Arya A., Albert S. (2013). Delignification pattern of wood decay by white rot fungi in teak (Tectona grandis L. f.). Journal of the Indian Academy of Wood Science 10(1): 1–8. http://dx.doi.org/10.1007/s13196-013-0085-8.
Nandris D., Nicole M., Geiger J.P. (1988). Root rot diseases of rubber tree. I. Severity, dynamics and characterization of epidemics. Canadian Journal of Forest Research 18: 1248–1254. http://dx.doi.org/10.1139/x88-192.
Nicole M., Geiger J.P., Nandris D. (1985). Defense reactions of Hevea brasiliensis to root rot diseases. European Journal of Forest pathology 15: 320–322. http://dx.doi.org/10.1111/j.1439-0329.1985.tb01106.x.
Nicole M., Geiger J.P., Nandris D. (1986a). Root rot diseases of Hevea brasiliensis. II. Some host reactions. European Journal of Forest pathology 16: 37–55. http://dx.doi.org/10.1111/j.1439-0329.1986.tb01050.x.
Nicole M., Geiger J.P., Nandris D. (1986b). Penetration and degradation of suberized cells of Hevea brasiliensis infected with root rot fungi. Physiological and Molecular Plant Pathology 28: 181–185. http://dx.doi.org/10.1016/S0048-4059(86)80062-5.
Nicole M., Chamberland H., Geiger J.P., Lecours N., Valero J., Rio B., Ouellette G.B. (1992). Immunocytochemical localization of laccase L1 in wood decayed by Rigidoporus lignosus. Applied and Environmental Microbiology 58(5): 1727–1739.
Ogbebor N.O., Adekunle A.T., Eghafona O.N., Ogboghodo A.I. (2015). Biological control of Rigidoporus lignosus in Hevea brasiliensis in Nigeria. Fungal Biology 119: 1–6. http://dx.doi.org/10.1016/j.funbio.2014.10.002.
Oghenekaro A.O., Miettinen O., Omorusi V.I., Evueh G.A., Farid M.A., Gazis R., Asiegbu F.O. (2014). Molecular phylogeny of Rigidoporus microporus isolates associated with white rot disease of rubber trees (Hevea brasiliensis). Fungal Biology 118: 495–506. http://dx.doi.org/10.1016/j.funbio.2014.04.001.
Omorusi V.I. (2012). Effects of white root rot disease on Hevea brasiliensis (Muell. Arg.) - challenges and control approach. In: Dhal N.K., Sahu S.C. (eds.). Plant Science. http://www.intechopen.com/books/plant-science/effects-of-white-root-rot-disease-on-Hevea-brasiliensis-muell-arg-challenges-and-control-approach.
Padhiar A., Albert S. (2012). Anatomical studies on decaying wood of Mangifera indica by two white rot fungi Schizophyllum commune and Flavadon flavus. Journal of the Indian Academy of Wood Science 9(2): 143–153. http://dx.doi.org/10.1007/s13196-012-0079-y.
Richard T., Botton B. (1996). Growth and mycelial strand production of Rigidoporus lignosus with various nitrogen and carbon sources. Mycopathologia 134: 83–89. http://dx.doi.org/10.1007/BF00436869.
Ryvarden L. (1976). Type studies in the Polyporaceae 4. Species described by J.F Klotzsch. Memoirs of the New York Botanical Garden 28: 199–207.
Sail R.M., Ahmad M. (2009). Enhancing socio-economy of rubber smallholders through effective transfer of technology. National Rubber Economic Conference 2009 (NREC), Nikko Hotel, Kuala Lumpur, 23–24 June, 2009. p. 134–142.
Schwarze F.W.M.R., Engels J. (1998). Cavity formation and the exposure of peculiar structures in the secondary wall (S2) of tracheids and fibres by wood degrading basidiomycetes. Holzforschung 52: 117–123. http://dx.doi.org/10.1515/hfsg.1998.52.2.117.
Schwarz F.W.R.M., Engels J., Mattheck C. (2004). Fungal strategies of wood decay in trees. Springer, Heidelberg. 185 p.
Schwarze F.W. M.R. (2007). Wood decay under the microscope. Fungal Biology Reviews 21(4): 133–170. http://dx.doi.org/10.1016/j.fbr.2007.09.001.
Srebotnik E., Messner K. (1994). A simple method that uses differential staining and light microscopy to assess the selectivity of wood delignification by white rot fungi. Applied and Environmental Microbiology 60: 1383–1386.
Umar H.Y., Esekhade T.U., Idoko S.O., Ugwa I.K.(2010). Production analysis of budded rubber stumps in Rubber Research Institute of Nigeria (RRIN). Journal of Agricultural Science 1: 109–113.
Total of 42 references

