The availability of cavity trees along an age gradient in fresh pine forests
Zawadzka D., Drozdowski S., Zawadzki G., Zawadzki J. (2016). The availability of cavity trees along an age gradient in fresh pine forests. Silva Fennica vol. 50 no. 3 article id 1441. https://doi.org/10.14214/sf.1441
Highlights
- The density of cavity trees in pine-dominated, managed forests varied in relation to stand age and was highest in stands older than 130 years of age
- Cavities excavated by woodpeckers dominated among all cavities
- The number of trees with cavities appears insufficient to ensure the effective protection of bird diversity in managed stands of Augustów Forest.
Abstract
Given their importance as a resource for many forest organisms, tree cavities were inventoried in the managed pine forests of north-east Poland, in relation to the: 70–100, 101–130 and >130 year age-classes within the clear-cutting system. The densities at which cavities were present was found to depend on forest age, given that stands 70–100 years old were characterised by an average density of 0.62 trees ha–1, while forests older than 130 years reported 3.28 trees ha–1. Stands aged 70–100 years differed from those aged 130+ in having just 0.27 trees ha–1 of cavity trees, as compared with 2.91 trees ha–1. The total volume of cavity trees in stands up to 100 years old was 0.37 m3 ha–1 on average, as compared with 5.42 m3 ha–1 in stands over 130 years old. The cavities created by woodpeckers constituted 76% of all of those found, and included 53% excavated by great spotted woodpeckers (Dendrocopos major L.) and 23% by black woodpeckers (Dryocopus martius L.) The proportion of cavities excavated by D. major was highest in the youngest age class of stands. There, cavities made by D. martius constituted only 8% of the total, as compared with 31% in the oldest stands. The abundance of cavity trees thus differed along an age gradient, though in any event the availability of cavity trees appears to be too limited to provide for the needs of hole-nesting birds. Forest managers must thus take more account than hitherto of the need to protect cavity trees.
Keywords
Pinus sylvestris;
forest management;
Poland;
Augustów Forest;
cavity-nesting birds;
woodpeckers
- Zawadzka, Institute of Forest Science, University of Łódź, Branch in Tomaszów Mazowiecki, Konstytucji 3 Maja 65/67, 97-200 Tomaszów Mazowiecki, Poland E-mail dorota_zaw@wp.pl
-
Drozdowski,
Department of Silviculture, Warsaw University of Life Sciences - SGGW, Nowoursynowska 159, 02-776 Warsaw, Poland
E-mail
stanislaw_drozdowski@sggw.pl

- Zawadzki, Eagle Conservation Committee, Okółek 14, 16-506 Giby, Poland E-mail grzesiekgfz@op.pl
- Zawadzki, Eagle Conservation Committee, Okółek 14, 16-506 Giby, Poland E-mail jerzy_zaw@wp.pl
Received 20 August 2015 Accepted 14 April 2016 Published 16 May 2016
Views 115138
Available at https://doi.org/10.14214/sf.1441 | Download PDF

1 Introduction
Forests managed for timber mainly differ from their natural counterparts in lacking a full cycle of forest development. As a consequence, commercial forests are characterised by reduced biodiversity, especially where specialised forest species dependent on large, dead or hollow trees are concerned (Angelstam et al. 2004; Jonsson et al. 2005; Juutinen et al. 2005; Gutowski et al. 2006; Smith 2007; Stachura-Skierczyńska et al. 2009; Vatka et al. 2014). One of the important differences between managed and natural forests is the density of cavity trees, which is clearly lower in commercial, managed stands (Remm and Lõhmus 2011; Robles et al. 2011; Walankiewicz et al. 2014).
Tree cavities are significant, natural components of forest ecosystems that support biodiversity (Camprodon et al. 2008; Sławski 2014). Their presence is an indicator of a forest’s naturalness, and this determines opportunities for many specialized groups of organisms to occur (Zawadzka and Zawadzki 2006; Walankiewicz et al. 2014). Cavities form habitats for many species, from invertebrates through to mammals, fungi, and plants, including numerous rare and threatened species. In North America, cavity trees are shown to be used by at least 67 vertebrate species for nesting, feeding or wintering, with 30% among these exposed and at risk (Bunnell 2013). Numbers of cavities are shown to be correlated with the distribution, numbers and species diversity of secondary-hole-nesting birds (Camprodon et al. 2008).
In Polish forests, cavities provide breeding sites for over 30 bird species. Bats and some rodents also find breeding and daytime shelter in cavity trees (Lewis 1995; Ruczyński et al. 2010). It is believed that a reduction in cavity resource in forests may have globally limited 10–40% of the populations of birds and mammals using them as breeding sites and shelters (Cockle et al. 2011). Cavities are also the preferred habitat for many rare insect species, including saproxylic beetles, as well as other invertebrates (Nilsson and Baranowski 1997; Ranius 2002; Ranius et al. 2009). In the Polish part of the Białowieża Primeval Forest, 455 beetle species live in cavities and standing dead trees (Byk 2001). In turn, a deficiency of old, dying trees in forest ecosystems is shown to reduce the distribution of saproxylic beetles (Gutowski et al. 2006).
Cavity trees are of low economic value (value of furnace fuel), but high biological value. For example, cavity trees left standing after death become some of the most important sources of large-sized, standing dead wood in forest ecosystems. Such trees are critically important for many endangered forest species, and for the proper functioning and continuity of ecological processes (Czerepko 2008; Cockle et al. 2011).
During the twentieth century, cavity trees were removed from forests in Poland in line with recommendations on maintaining “forest health”. The consequence was a significant reduction in the occurrence of hole-dependent species, due to the fragmentation or disappearance of their habitat (Gutowski et al. 2006). Only towards the end of the 20th century did the approach of foresters to cavity trees and dead wood change. Today, cavity trees in Poland’s managed forests are defined as “biocenotic” trees, and it is recommended that they be left in stands to die a natural death and then decay (Forest Protection Instruction 2004, 2012). However, there is no general rule concerning minimal or optimal numbers of such trees per unit of area. Moreover, with a view to the ecological processes of cavity creation being protected, it is recommended that susceptible tree species (especially aspen and birch) and individual trees of impaired health (Referowska-Chodak 2010) should be left in place. According to the Programme for the Endorsement of Forest Certification (PEFC), the number and distribution of cavity trees should be such as to ensure a high level of forest biodiversity (Referowska-Chodak 2010). However, in the case of Poland’s forests (other than the Białowieża Primeval Forest), there is a lack of data concerning the frequency of occurrence or density of cavity trees.
The aim of the work detailed here was thus to assess numbers of cavities in the trees forming stands of fresh pine forest stands that are managed in line with the clear-cutting system. A further aim was to relate the abundance to ages of stands and origins of cavities (i.e. created by woodpeckers or developed naturally). The occurrence of cavities was primarily of interest given the importance of the availability of nesting sites for the so-called secondary cavity-nesting birds present in the forest under study, most especially those of species listed in Annex 1 to the “Birds Directive” (Council Directive 2009/147/EC on the conservation of wild birds). The volume of dead, standing trees with cavities was also estimated to assess the importance of cavity trees in increasing the resource of dead wood present in a forest.
It was anticipated that results obtained would allow for the generation of recommendations concerning cavity trees to be left in managed stands (in the course of thinning and final cutting), most importantly in the interests of secondary cavity-nesting birds, as well as other forest-dwelling organisms of specialized habitat requirements.
2 Materials and methods
The study was carried out in the Polish part of Puszcza Augustowska, i.e. Augustów Forest (NE Poland, 53°54´N, 23°15´E, Fig. 1), which covers 1140 km2. The area is fairly flat, at altitudes between 135 and 190 m above sea level. The mean annual temperature is 6.5 °C, while the growing season is of (only) 135 days’ duration, on average, and snow cover lasts for about 100 days. Forests cover 93% of the area in question, lakes for 6%. Tree stands here are composed of Scots pine (Pinus sylvestris L.) (78%), Norway spruce (Picea abies (L.) Karst.) (8%), black alder (Alnus glutinosa Gaertn.) (9%), birch (Betula verrucosa Ehrh.) (5%), and pedunculate oak (Quercus robur L.) (1%). The undergrowth is formed mainly from spruce trees, while there is also a shrub layer consisting of young spruces as well as specimens of common juniper (Juniperus communis L.) (Sokołowski 2010).
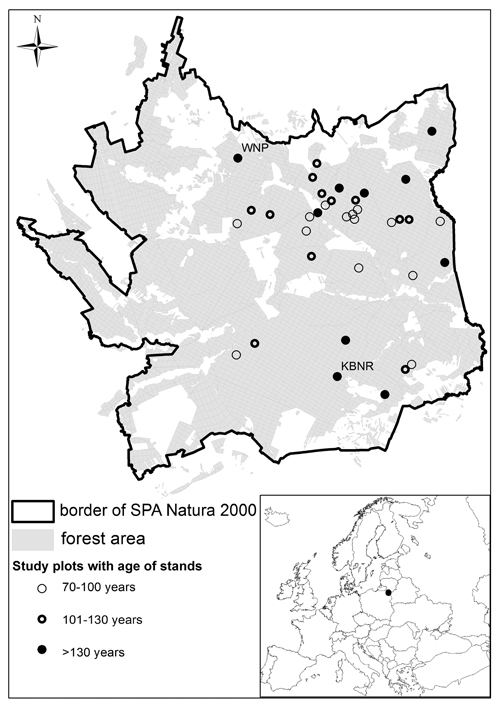
Fig. 1. Locations of study plots (WNP - Wigry National Park, KBNR - Kuriańskie Bagno Nature Reserve).
The mean age of tree stands in the forest is just 60 years, with stands more than 100 years old accounting for just 5% of the total forest area. Where site types are concerned, it is the so-called fresh pine forest – with a high share of bilberry (Vaccinium myrtillus L.) that covers almost 40% of the area, while fresh mixed/coniferous forest with bilberry and bush-grass (Calamagrostis epigeios (L.) Roth) covers 27%. Boggy coniferous forest sites with labrador tea (Rhododendron tomentosum, Harmaja) in turn cover about 7% of the forest area (Sokołowski 2010).
Augustów Forest is included within the Natura 2000 network as the PLB200002 Special Protection Area for Birds (SPA). Thirty-nine bird species, including eleven cavity-nesters, enjoy protection in the “Augustów Forest” SPA. Equally, most of Augustów Forest is managed commercially by six Forest Districts (FDs) of the State Forests National Forest Holding Lasy Państwowe. All of the Forest Districts are FSC (Forest Stewardship Council)- and PEFC-certified, and thus take full account of biodiversity protection. The Lake Wigry (Wigierski) National Park (WNP), established in 1989 and encompassing 151 km2, is located in the western part of this Forest (Sokołowski 2010).
Research was conducted in the 2005–2012 period, in a study area consisting of typical commercial forest (34 plots, each plot was equal one forest sub-compartment), as well as stands within Wigry National Park (1 plot) and the Kuriańskie Bagno Nature Reserve (KBNR) (1 plot) (Fig. 1). Cavity trees were thus counted in a total of 36 plots (corresponding with forest sub-compartments). This in most cases denoted plot sizes of ca. 10 ha, though the 2 supporting the oldest forest stands were of 6 and 12 ha, respectively. Each plot covered area of homogeneous age of stand. The borders of study plots were determined in field using a GPS receiver equipped with digital maps of the study area. The age structure of the stands under investigation was in the 70–210-year range. Specifically stands aged 70–100 years were present on 13 plots, as were those 101–130 years old. The remaining 10 plots supported stands >130 years old. Stands were those characterizing the site type of “fresh coniferous forest”, or else fresh mixed/coniferous forest. In each case, stands were dominated by Scots pine with an admixture of Norway spruce.
Cavity trees and number of cavities present in each were inventoried on the study plots by groups of 4–10 people walking slowly and inspecting all trees carefully. All measurements were supported by a GPS receiver equipped with digital maps of the study area. Trees with cavities were chalk-marked to avoid double counting. The information recorded for each identified cavity tree was as follows: tree species, living or dead tree, number of cavities and origin (excavated by woodpeckers or formed naturally). Cavities were identified from the ground using binoculars. Only cavities potentially useful to birds, with a minimum diameter of about 2 cm, were included. Apart from cavities regarded as having formed naturally, those excavated by woodpeckers were divided into the categories of created by great spotted woodpeckers (Dendrocopos major, L.), or by black woodpeckers (Dryocopus martius, L.). Cavities excavated by the latter species are recognized as holes with a large oval entrance, ca. 7–12 cm wide and 7–15 cm long, located at least 12 m above the ground (Fig. 2a). In turn, cavities created by the great spotted woodpecker have a round entrance of diameter 4–6 cm, and are located at a range of different heights (from 2–15 m above the ground) (Fig. 2b). Natural cavities in trees were larger than those excavated by woodpeckers, and also included cracks and slots in trunks. Natural cavities unsuitable for the nesting of secondary hole-nesters were excluded.

Fig. 2. Comparison of cavities excavated by (a) Black Woodpecker (a), and (b) Great Spotted Woodpecker (b). A Tengmalm’s Owl uses a cavity excavated by a Black Woodpecker (c), and a Black Woodpecker is photographed in the process of excavating a cavity in a live pine tree (d). (Photography by G. Zawadzki).
As the diameter at breast high (dbh) of each cavity tree was not measured, we used the following procedure. Estimates of the total volume (V) of cavity trees (living and dead) on a given study plot made use of available digital stand descriptions (http://www.bdl.lasy.gov.pl), and specifically of quadratic mean diameters (Dg), heights (Hg) and stand age, i.e. typical parameters for volume estimation in monospecific and even-aged stands. Collected information on Dg, Hg and stand age, as well as yield tables elaborated by Czuraj (1991) for single standing trees, were then used to extract specific mean volumes of trees ![]() for each plot. Finally, each plot’s total volume of cavity trees (V) was obtained by multiplying the inventoried numbers of cavity trees (N) by the value for the site-specific mean volume of trees
for each plot. Finally, each plot’s total volume of cavity trees (V) was obtained by multiplying the inventoried numbers of cavity trees (N) by the value for the site-specific mean volume of trees![]()
Prior to statistical analysis, checks were made for normality of distribution of data on the densities and volumes of cavity trees (using the Shapiro-Wilk test), as well as the homogeneity of their variance (Levene’s test). This was done separately for 1) live, dead and all cavity trees in the different age classes, 2) natural cavities, or holes excavated by either black or great spotted woodpeckers in different age classes. No differences in variance or deviations from a normal distribution were noted within the samples. One-way analysis of variance (ANOVA) and Tukey’s HSD test (α = 0.05) were then used, with all calculations carried out using Statistica 10.0 software (StatSoft, Inc.).
3 Results
Taken together, the study plots were found to contain 277 cavity trees (of 8 species), with a total of 919 cavities. Specifically, the cavity trees were 204 pines (74%), 60 birches (22%), 5 spruces (2%), 4 oaks (1%), and one specimen each of aspen, small-leaved lime (Tilia cordata Mill.), crack willow (Salix fragilix L.) and hazel (Corylus avellana L.). The mean number of cavities per tree was 3.3 (range 1–6). In the 70–100 year-old stands, cavities were found mostly in birches (61%) (Fig. 3). The proportion of cavities in pines was markedly higher among trees in stands over 100 years old: 75% in 101–130 year-old stands and 90% in stands older than 130 years (Fig. 3).
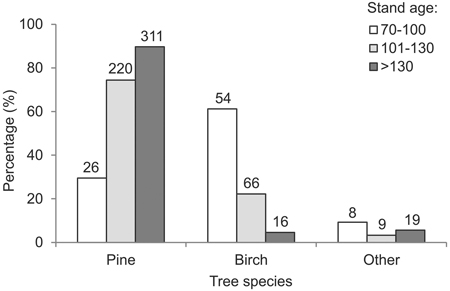
Fig. 3. Relative frequency of occurrence of cavity trees of different species, by age intervals (numbers above bars denote absolute numbers of cavity trees of the given species in the given age category).
Mean cavity-tree densities varied in relation to the age of the stand (df = 2, F = 13.5, p < 0.001): from 0.62 trees ha–1 in stands up to 100 years old to 3.28 trees ha–1 in stands older than 130 years (Fig. 4). Live trees increasingly prevailed among cavity trees in forest of successively greater age; a density of 0.27 trees ha–1 in the 70–100-year age interval compared with 2.91 trees ha–1 in the >130 years age interval (df = 2, F = 14.6, p < 0.001) (Fig. 4). The abundance of dead trees with cavities was limited (up to 1.4 trees ha–1) and was not found to vary with stand age (df = 2, F = 0.9, p = 0.426). It was nevertheless greatest in 101–130 year-old stands (Fig. 4).
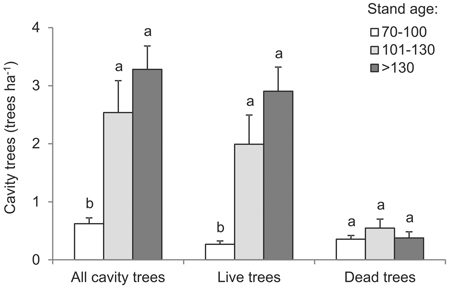
Fig. 4. Mean density of cavity trees, live and dead, by age intervals (vertical lines denote standard error of mean value; the same letters located by mean values indicate homogeneous groups, Tukey’s HSD test, p < 0.05).
The total volume of all cavity trees was greater in older forest. In 70–100 year-old stands, it was 0.37 m3 ha–1 on average, while in >130 year-old stands the corresponding figure was 5.42 m3 ha–1, albeit with a range between 0.1 and 9.5 m3 ha–1 (df = 2, F = 19.9, p < 0.001) (Fig. 5). The structure characterizing the volume of living trees was similar. In younger stands, the total volume of living trees with cavities was on average below 0.2 m3 ha–1, as compared with ca. 4.8 m3 ha–1 in the oldest stands (Fig. 5) (range 0–9.3 m3 ha–1) (df = 2, F = 17.4, p < 0.001). The volume of dead cavity trees was greater in the oldest stands – at 0.66 m3 ha–1, as compared with 0.2 m3 ha–1 in stands less than 100 years old (df = 2, F = 3.5, p = 0.043) (Fig. 5). In each case (including all cavity trees, living trees only or dead trees only), the total volume of trees per unit area was significantly greater in progressively older stands.
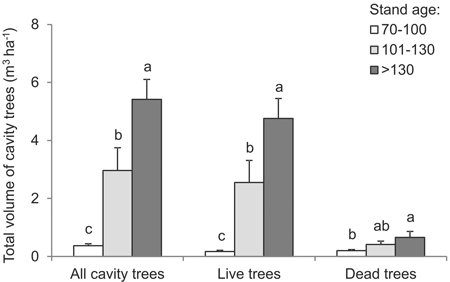
Fig. 5. Total volume of cavity trees, live or dead, by age intervals (vertical lines denote standard error of mean value; the same letters located near the median values indicate homogeneous groups, Tukey-test HSD, p < 0.05).
Most (76% of) cavities were made by woodpeckers, including 53% made by great spotted woodpeckers and 23% made by black woodpeckers. The cavities excavated by the former species were most abundant in the trees of the youngest stand-age class, being significantly less abundant in older stands (df = 2, F = 3.8, p = 0.033) (Fig. 6); the proportion was at 46% as opposed to 66%. In turn, black woodpecker cavities account for just 8% of the total where younger stands are concerned, as compared with 31% among the oldest trees (df = 2, F = 8.1, p = 0.001) (Fig. 6). In all circumstances, the share of naturally-formed cavities is apparently limited, if highest in the 101–130 year-old stands (df = 2, F = 0.6, p = 0.556) (Fig. 6). In forests under 100 years old, naturally-formed cavities were almost exclusively present in birch trees (75%, n = 9). In turn, in the oldest age classes, they were mainly found in pines (86%, n = 69).
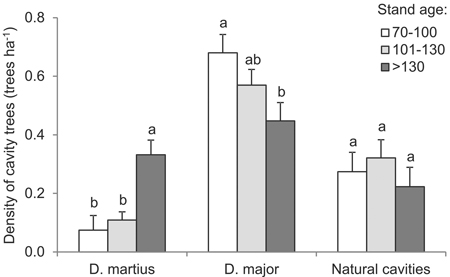
Fig. 6. Mean density of trees with cavities excavated by the Black Woodpecker or Great Spotted Woodpecker, as well as natural cavities, by age intervals (vertical lines denote standard errors of mean values, while the presence of the same letters by mean values indicates homogeneous groups, Tukey’s HSD test, p < 0.05.
4 Discussion
4.1 The abundance of cavity trees
Data on the abundance of tree cavities in temperate forests are limited, though the numbers of cavities present in natural forest are known to be significantly higher than in commercial stands. In the Białowieża Primeval Forest, there are 12.5 trees ha–1 in the national park, as compared with just 3 trees ha–1 in the managed part of the forest (Walankiewicz et al. 2014). Correspondingly, the abundance of cavity-nesters was significantly higher in unmanaged than managed stands (Czeszczewik et al. 2015). Age and size gradients of stands are found to be associated with progressively greater numbers of cavities (Walters et al. 2002). Likewise, Sławski (2014), in analyzing the forest structure of pine stands in a clear cut system, showed that the probability of cavity trees being present in stands under 100 years old is only 10%, as compared with ca. 60% in forests over 200 years old. In managed forests, the main source of cavities is woodpecker activity (Lõhmus et al. 2005; Cockle et al. 2011; Remm and Lõhmus 2011). A prevalence of cavities made by woodpeckers is also characteristic of coniferous stands (review in: Remm et al. 2006). However, the upper limits for cavities excavated by woodpeckers do not usually exceed 10–20 cavities ha–1 (Remm and Lõhmus 2011), while the maximum number of cavities can reach 72 ha–1 in mixed forests of the European hemiboreal zone (review in: Remm et al. 2006).
Carlson (1994) showed the importance for secondary cavity-nesting bird species of cavity trees being left in clear-cutting areas. It is in turn the scarcity of cavity trees that limits populations of secondary hole-nesting birds in managed forests, especially in younger stands (Camprodon et al. 2008; Bunnell 2013; Walankiewicz et al. 2014). The availability of cavities created by woodpeckers (i.e. the main source of cavities in managed forest) is related to their numbers. For this reason, woodpeckers are regarded as keystone taxa (Gorman 2004; Kosiński et al. 2010). In our opinion, it is possible to use the amount of dead wood (a consequences of cavity trees being left to die naturally) as an index indirectly evaluative of the diversity of forest birds. Study by Lõhmus (2016) indicates that further good indicators may be the abundance of the fruit-bodies of pine-decaying fungus Phellinus pini (Brot.). Numbers of this latter species were found to correlate well with densities of populations of cavity-nesters.
4.2 Recommendations for cavity protection
Thus far, there have been few recommendations regarding the effective densities of cavity trees in managed forests of Central Europe, unlike in North America (DeGraaf and Shigo 1985; ONMR 2001; Bunnell 2013). The different aims characterizing commercial and protected forests ensure that the densities of cavities will always be higher in natural forests. This makes it important to estimate the numbers of cavities that secondary-nesters might need in managed forests, and to determine how this total could be arrived at, given the way commercial forests remain the dominant form of forest use and management. A determination of an optimal range for numbers of cavity trees per unit area can be made use of in practical guidelines for the implementation of sustainable forest management, e.g. within the FSC and PEFC forest certification systems (Referowska-Chodak 2010).
In managed forests, the main cause of limited availability of cavities is the elimination of cavity trees, as well as trees in poor health (e.g., with fungus as potential places of hole creation (Lõhmus 2016), dead trees) during sanitary cutting and harvesting. The black and great spotted woodpeckers prefer live pine trees in which to create cavities (Gorman 2004). Weakened trees, attacked by insects and fungi or of reduced immunity for other reasons, are often selected by woodpeckers as they excavate cavities. Thus, if the supply of cavities is to be increased, such weakened trees will need to be left in stands regularly, as thinning is carried out.
Most cavities in coniferous trees are made by woodpeckers, and these cavities are more resistant to decomposition by fungi than those in deciduous trees (Walankiewicz et al. 2014). 76% of all the cavities found in study plots in the Augustów Forest had been created by woodpeckers. The results obtained in our study document the state of the forest as shaped by the economic activities of foresters. Densities of <1 cavity tree per hectare found in stands younger than 100 years old are not sufficient to meet requirements as regards nest sites of such common passerine or near-passerine birds in the study area as pied flycatcher (Ficedula hypoleuca Pall.), six species of tit (Paridae), redstart (Phoenicurus phoenicurus L.), nuthatch (Sitta europaea L.) and wryneck (Jynx torquilla L.). Since such species can potentially achieve population densities ranging between one and a few pairs per 10 ha of pine forest (Sikora et al. 2007) it should be concluded that the cavity-excavating activity of great spotted woodpeckers in the Augustów Forest is such as to limit densities of populations of such birds. Additionally, not all holes that are present are suitable for nesting, on account of their quality, size or location (Remm et al. 2008). The use of cavities located close to one another can also be limited by the territoriality of birds (Lõhmus and Remm 2005; Remm et al. 2008). Similarly, certain holes are left unavailable on account of their being occupied by other organisms, e.g. mammals or invertebrates (Ruczyński et al. 2010). According to Remm et al. (2006), only 483 out of 731 cavities in study plots of riverine forests in Estonia (i.e. 68%) were actually suitable for hole-nesting birds.
In Augustów Forest, the number of black woodpecker holes is very low in stands younger than 100 years of age, making nesting impossible for larger hole-nesting birds, such as Tengmalm’s owl (Aegolius funereus L.) (Fig. 2c), stock dove (Columba oenas L.), goldeneye (Bucephala clangula L.), and goosander (Mergus merganser L.). However, in stands more than 130 years old (Fig. 2d), the proportion of holes that are made by black woodpeckers is more favourable for this group of birds. Unfortunately, such stands will become progressively less well-represented, given an obligatory cutting age for pine in the Forest Districts in Augustów Forest equal to 120 years. Our study indicates that the black woodpecker prefers to excavate cavities in stands more than 130 years old, ensuring that an established age of cutting of 120 years may impinge upon the population of black woodpeckers as such, while also reducing the number of secondary cavity-nesters using the holes they make. In Poland, there are many other forest complexes in which the age at felling is below 100 years, with this presumably ensuring densities of cavity trees even lower than in Augustów Forest. For this reason, Poland’s State Forests recommend that islands of old growth be left in all clear-cutting areas, with these being of area 10 ares or more, and accounting for no less than 5% of the regeneration area (Silvicultural Guidelines 2012). Such practices do not fully protect old trees, which are needed if large cavity-nesters are to find a place. The consistent retention of islands of old growth is also important if other old forest-dependent groups of organisms and habitats are to gain real protection. Fortunately, the practice also proves advantageous when it comes to the maintenance of forest ecosystems of higher resilience and stability (Bernadzki 1993; Lachat et al. 2010). It is also recommended as an important tool in semi-natural silviculture (Pommerening and Murphy 2004). If the number of old cavity trees in Augustów Forest is not to be reduced, given the current ages of final cutting established at 120 years, it would be advisable to leave some of the oldest stands to ensure natural regeneration.
A common practice in managed forests is to provide nest boxes for birds, in an effort to compensate for any shortfall in numbers of the cavities made by great spotted woodpeckers primarily suitable for small Passerine secondary-cavity nesters, especially in stands less than 100 years old. Equally, the availability of nesting places for large-sized secondary cavity nesters depends almost exclusively on a location high (at least 10 m) above the ground, and hence excavated by black woodpeckers. It is not practicable for nest boxes to be put out at such a height.
Cavity pine trees left in a stand after they die may become a valuable source of the dead wood still present in a commercial forest. This is particularly important given the severe strong deficiency of dead wood where this kind of forest in Poland is concerned (Czerepko 2008). The results of an inventory performed for a BioSoil project further reinforce the idea that there is too small a share of dead wood in Polish forests, all the more so in regard to large standing or lying dead trees. This situation could be improved if some trees are left standing until the time of their natural death, in old growth islands left in clear cuts or as cavity trees (Bernadzki 1993; Czerepko 2008). Living cavity-trees dominate the inventory in the Augustów Forest and may constitute a potentially valuable source of large standing trees. This may represent an additional ecological effect of the protection of cavity trees.
5 Conclusions
Our study showed that the managed stands of NE Poland’s Augustów Forest have too small a number of trees with cavities to ensure the effective protection of forest bird diversity. The shortage of cavity trees in forests less than 100 years old is especially severe. As most cavities present in trees of this age are present in birches, the protection of trees of this species as thinning operations are being conducted is as important as the policies whereby some pines that have been damaged or attacked by insects or fungi are left as potential places for cavities to be created, as well as dead trees are left standing. The number of holes made by black woodpeckers is markedly greater where stands exceed 130 years of age. It is the abundance of this species which therefore determines nesting possibilities for the large secondary hole-nesters subject to protection in the context of the Natura 2000 PLB200002 Augustów Forest Special Protection Area. However, as the optimum density of black woodpecker cavities is present in stands older than the current final felling age, a greater availability of cavities excavated by black woodpeckers will only be assured if islands of old growth are left in all cutting areas until the time of the natural death of trees, and their subsequent decomposition. Some of the still-extant stands more than 120 years old should also be excluded from harvesting, or left for natural regeneration. In general, extant cavity trees should be inventoried and assured of effective protection in the course of thinning operations.
Acknowledgements
We thank Dr James R.A. Richards for improving the English text as well as Dr Asko Lõhmus for valuable comments and suggestions.
References
Angelstam P., Roberge J.-M., Lõhmus A., Bergmanis M., Brazaitis G., Dönz-Breuss M., Edenius L., Kosiński Z., Kurlavicius P., Lārmanis V., Lukins M., Mikusiński G., Racinskis E., Strazds M., Tryjanowski P. (2004). Habitat modelling as a tool for landscape-scale conservation - a review of parameters for focal forest birds. Ecological Bulletins 51: 427–453.
Bernadzki E. (1993). Zwiększenie różnorodności biologicznej przez zabiegi hodowlano-leśne [Increasing Biodiversity by Means of Silvicultural Intervention]. Sylwan 3: 29–36.
Bunnell F.L. (2013). Sustaining cavity-using species: patterns of cavity use and implications to forest management. ISRN Forestry, article id 457698. 33 p. http://dx.doi.org/10.1155/2013/457698.
Byk A. (2001). Próba waloryzacji drzewostanów starszych klas wieku w Puszczy Białowieskiej na podstawie struktury zgrupowań chrząszczy (Coleoptera) związanych z rozkładającym się drewnem pni martwych drzew stojących i dziupli. In: Szujecki A. (eds.) Próba szacunkowej waloryzacji lasów Puszczy Białowieskiej metoda zooindykacyjną. [An attempt at the valorization of older tree-stands in Białowieża Primeval Forest based on the structure of communities of beetles (Coleoptera) connected to decaying wood of standing dead tree trunks and hollows]. In: Szujecki A. (eds.) Attempt at the Tentative Valorization of the Forests of Białowieża Primeval Forest by the Zooindication Method]. Wydawnictwo SGGW, Warszawa. 333–367 p. ISBN 83-7244-256-8. [In Polish].
Camprodon J., Salvanya J., Soler-Zurita J. (2008). The abundance and suitability of tree cavities and their impact on hole-nesting bird populations in beech forest of NE Iberian Peninsula. Acta Ornithologica 43(1): 17–31. http://dx.doi.org/10.3161/000164508X345293.
Carlson A. (1994). Cavity breeding birds and clearcuts. Ornis Fennica 71: 120–122.
Cockle K.L., Martin K., Wesołowski T. (2011). Woodpeckers, decay, and the future of cavity-nesting vertebrate communities worldwide. Frontiers in Ecology and the Environment 9(7): 377–382. http://dx.doi.org/10.1890/110013.
Czerepko J. (eds.). (2008). Stan różnorodności biologicznej lasów w Polsce na podstawie powierzchni obserwacyjnych monitoringu. [The biodiversity condition of Polish forests on the basis of monitoring plots]. Instytut Badawczy Leśnictwa, Sękocin Stary. 135 p. ISBN 978-83-87647-75-9. [In Polish].
Czeszczewik D., Zub K., Stanski T., Sahel M., Kapusta A., Walankiewicz W. (2015). Effect of forest management on bird assemblages in the Białowieża Forest, Poland. iForest 8: 377–385.
Czuraj M. (1991). Tablice miąższości kłód odziomkowych i drzew stojących. [Tables of volume for logs and standing trees]. PWRiL, Warszawa. 362 p. ISBN 83-09-01262-4. [In Polish].
DeGraaf R.M., Shigo A.L. (1985). Managing Cavity Trees for Wildlife in the Northeast. USDA Forest Service, Northeastern Forest Experiment Station. http://www.treesearch.fs.fed.us/pubs/4138.
Gorman G. (2004). Woodpeckers of Europe. D&N Publishing Lambourn Woodlands, Hungerford, Berkshire. ISBN 1-872842-05-4.
Gutowski J.M., Buchholz L., Kubisz D., Ossowska M., Sućko K. (2006). Chrząszcze saproksyliczne jako wskaźnik odkształceń ekosystemów leśnych borów sosnowych. [Saproxylic beetles as indicators of the deformation of pine forest ecosystems]. Leśne Prace Badawcze 67(4): 101–144. [In Polish].
Instruction of Forest Protection (2004). General Directorate of The State Forest National Forest Holding, Warszawa. [In Polish].
Instruction of Forest Protection (2012). General Directorate of The State Forest National Forest Holding, Warszawa. [In Polish].
Jonsson B.G., Kruys N., Ranius T. (2005). Ecology of species living on dead wood – lessons for dead wood management. Silva Fennica 39(2): 289–309. http://dx.doi.org/10.14214/sf.390.
Juutinen A., Mönkkönen M., Sippola A.L. (2005). Cost-efficiency of decaying wood as a surrogate for overall species richness in boreal forest. Conservation Biology 20(1:) 74–84. http://dx.doi.org/10.1111/j.1523-1739.2005.00306.x.
Kosiński Z., Bilińska E., Dereziński J., Jeleń J., Kempa M. (2010). Dzięcioł czarny Dryocopus martius i buk Fagus sylvatica gatunkami zwornikowymi dla siniaka Columba oenas w Zachodniej Polsce. [The Black Woodpecker Dryocopus martius and the European Beech Fagus sylvatica as keystone species for the Stock Dove Columba oenas in Western Poland]. Ornis Polonica 51: 1–13. [In Polish].
Lachat T., Müller M., Bütller R. (2010). Empfehlungen für das Ausscheiden und die Beurteilung von Altholzinslen. Forschungsanstal WSL, Birmensdorf.
Lewis S.E. (1995). Roost fidelity for bats: a review. Journal of Mammalogy 76(2): 481–496. http://dx.doi.org/10.2307/1382357.
Lõhmus A. (2016). Habitat indicators for cavity-nesters: the polypore Phellinus pini in pine forests. Ecological Indicators 66: 275−280. http://dx.doi.org/10.1016/j.ecolind.2016.02.003.
Lõhmus A., Remm J. (2005). Nest quality limits the number of hole-nesting passerines in their natural cavity-rich habitat. Acta Oecologica 27(2): 125–128. http://dx.doi.org/10.1016/j.actao.2004.11.001.
Lõhmus A., Lõhmus P., Remm J., Vellak K. (2005). Old-growth structural elements in a strict reserve and commercial forest landscape in Estonia. Forest Ecology and Management 216(1–3): 201–215. http://dx.doi.org/10.1016/j.foreco.2005.05.031.
Nilsson S.G., Baranowski R. (1997). Habitat predictability and the occurrence of wood beetles in old-grown beech forests. Ecography 20(5): 491–498. http://dx.doi.org/10.1111/j.1600-0587.1997.tb00417.x.
OMNR (2001). Forest management guide for natural disturbance pattern emulation. Version 3.1. Ontario Ministry of Natural Resources, Queen’s Printer for Ontario, Toronto. ISBN 0-7794-2670-3.
Pommerening A., Murphy S.T. (2004). A review of the history, definitions and methods of continuous cover forestry with special attention to afforestation and restocking. Forestry 77(1): 27–44. http://dx.doi.org/10.1093/forestry/77.1.27.
Ranius T. (2002). Influence of stand size and quality of tree hollows on saproxylic beetles in Sweden. Biological Conservation 103(1): 85–91. http://dx.doi.org/10.1016/S0006-3207(01)00124-0.
Ranius T., Niklasson M., Berg N. (2009). Development of tree hollows in pedunculate oak (Quercus robur). Forest Ecology and Management 257(1): 303–310. http://dx.doi.org/10.1016/j.foreco.2008.09.007.
Referowska-Chodak E. (2010). Ochrona różnorodności biologicznej w systemach certyfikacji FSC i PEFC a gospodarka leśna w Polsce. [The effectiveness of biodiversity protection given by FSC and PEFC certification systems in the context of forest management in Poland]. Leśne Prace Badawcze 71(4): 429–439. [In Polish].
Remm J., Lõhmus A. (2011). Tree cavities in forests – the broad distribution pattern of a keystone structure for biodiversity. Forest Ecology and Management 262(4): 579–585. http://dx.doi.org/10.1016/j.foreco.2011.04.028.
Remm J., Lõhmus A., Remm K. (2006). Tree cavities in riverine forests: what determines their occurrence and use by hole-nesting passerines? Forest Ecology and Management 221(1–3): 267–277. http://dx.doi.org/10.1016/j.foreco.2005.10.015.
Remm J., Lõhmus A., Rosenvald R. (2008). Density and diversity of hole-nesting passerines: dependence on the characteristics of cavities. Acta Ornithologica 43(1): 83–91. http://dx.doi.org/10.3161/000164508X345365.
Robles H., Ciudad C., Matthysen E. (2011). Tree-cavity occurrence, cavity occupation and reproductive performance of secondary cavity-nesting birds in oak forests: the role of traditional management practices. Forest Ecology and Management 261(8): 1428–1435. http://dx.doi.org/10.1016/j.foreco.2011.01.029.
Rosenvald R., Lõhmus A., Kraut A., Remm L. (2011). Bird communities in hemiboreal old-growth forests: the roles of food supply, stand structure, and site type. Forest Ecology and Management 262(8): 1541–1550. http://dx.doi.org/10.1016/j.foreco.2011.07.002.
Ruczyński I., Nicholls B., MacLeod C.D., Racey P.A. (2010). Selection of roosting habitats by Nyctalus noctula and Nyctalus leisleri in Białowieża Forest – an adaptive response to forest management? Forest Ecology and Management 259(8): 1633–1641. http://dx.doi.org/10.1016/j.foreco.2010.01.041.
Sikora A., Rohde Z., Gromadzki M., Neubauer G., Chylarecki P. (eds.) (2007). Atlas rozmieszczenia ptaków lęgowych Polski 1985–2004. [The atlas of breeding birds in Poland 1985–2004]. Bogucki Wydawnictwo Naukowe, Poznań. 639 p. [In Polish].
Silvicultural Guidelines (2012). Zasady Hodowli Lasu. DGLP, Ośrodek Rozwojowo-Wdrożeniowy Lasów Państwowych, Bedoń. 72p. [In Polish].
Sławski M. (2014). Zmiany struktury lasu w szeregu rozwojowym drzewostanów sosnowych zagospodarowanych sposobem zrębowym. [Changes of forest structure in a chronosequence of secondary pine forest in a clear-cut system]. SGGW, Warszawa. 123 p. [In Polish].
Smith K.W. (2007). The utilization of dead wood resources by woodpeckers in Britain. Ibis 149 (s2): 183–192. http://dx.doi.org/10.1111/j.1474-919X.2007.00738.x.
Sokołowski A.W. (2010). Puszcza Augustowska. [Augustów Forest]. Centrum Informacyjne Lasów Państwowych, Warszawa. 291 p. ISBN 978-83-61633-03-7. [In Polish].
Stachura-Skierczyńska K., Tumiel T., Skierczyński M. (2009). Habitat prediction model for three-toed woodpecker and its implications for the conservation of biologically valuable forests. Forest Ecology and Management 258: 697–703. http://dx.doi.org/10.1016/j.foreco.2009.05.007.
Vatka E., Kangas K., Orell M., Lampila S., Nikula A., Nivala V. (2014). Nest site selection of a primary hole-nesting passerine reveals means to developing sustainable forestry. Journal of Avian Biology 45(2): 187–196. http://dx.doi.org/10.1111/j.1600-048X.2013.00250.x.
Walankiewicz W., Czeszczewik D., Stański T., Sahel M., Ruczyński I. (2014). Tree cavity resources in spruce-pine managed and protected stands of the Białowieża Forest, Poland. Natural Areas Journal 34(4): 423–428. http://dx.doi.org/10.3375/043.034.0404.
Walters R.J., Crowder B.L., Priddy A.J. (2002). Population viability analysis for red-cockaded woodpeckers using an individual-based model. Ecological Applications 12(1): 249–260. http://dx.doi.org/10.1890/1051-0761(2002)012[0249:PVAFRC]2.0.CO;2.
Zawadzka D., Zawadzki J. (2006). Ptaki jako gatunki wskaźnikowe różnorodności biologicznej i stopnia naturalności lasów. [Birds as indicators of biodiversity and level of forest naturalness]. Studia i Materiały Centrum Edukacji Przyrodniczo-Leśnej 14(4): 249–262. [In Polish].
Total of 45 references.

