Growth and nutrient dynamics of transplanted Quercus variabilis seedlings as influenced by pre-hardening and fall fertilization
Wang J., Yu H., Li G., Zhang F. (2016). Growth and nutrient dynamics of transplanted Quercus variabilis seedlings as influenced by pre-hardening and fall fertilization. Silva Fennica vol. 50 no. 2 article id 1475. https://doi.org/10.14214/sf.1475
Highlights
- High pre-hardening fertilization favored seedling growth and nutrient storage at the rapid growth and hardening phases following transplantation. Overall, high fall fertilization was beneficial only at the hardening phase
- The combination of 100 mg N seedling–1 during pre-hardening with 36 mg N seedling–1 during hardening was recommended for satisfactory transplanting performance for Quercus variabilis.
Abstract
Stored nutrient reserves are closely correlated with survival and growth of transplanted seedlings. Previous studies have proven that combining pre-hardening fertilization (PF) with fall fertilization (FF) built seedling nutrient reserves more effectively; however, their effect on transplanting performance is poorly documented. We investigated the independent and interacting effects of 2 levels of PF and 4 levels of FF on seedling growth, nutrient acquisition and accumulation during different growth phases 1 year after transplanting of Quercus variabilis Blume in a nursery. High PF benefited nutrient reserves and subsequent transplanted seedling growth and tissue nutrient storage at the end of the rapid growth and hardening phases. Fall fertilization with 36 mg N increased stem dry mass and tissue nutrient content at the end of the hardening phase. At the conclusion of establishment, PF and FF showed a significant interaction for N and K uptake from soil. At the end of the rapid growth and hardening phases, high PF consistently increased nutrient uptake. Enhanced N and K uptake occurred following application of 36 mg N of FF at the end of the hardening phase. Distinct roles for PF and FF on 3 phases of transplanted seedlings demonstrated the necessity to evaluate fertilization in terms of nutrient reserves and subsequent transplanting performance in consecutive phases. Combining 100 mg N seedling–1 during pre-hardening with 36 mg N seedling–1 during fall yielded ideal transplanting performance for Quercus variabilis seedlings.
Keywords
nutrient loading;
nutrient storage;
nursery transplanting;
seedling quality
- Wang, Key Laboratory for Silviculture and Conservation, Ministry of Education; Beijing Laboratory of Urban and Rural Ecological Environment, Beijing Forestry University, 35 East Qinghua Road, Haidian District, Beijing 100083, China E-mail wjx198979@163.com
- Yu, Beijing Forestry Carbon Administration, room 201, No.1 Xiao Huang Zhuang Bei Jie, Dongcheng District, Beijing 100013, China E-mail yuhq@bfdic.com
-
Li,
Key Laboratory for Silviculture and Conservation, Ministry of Education; Beijing Laboratory of Urban and Rural Ecological Environment, Beijing Forestry University, 35 East Qinghua Road, Haidian District, Beijing 100083, China
E-mail
glli226@163.com

- Zhang, Beijing Forestry Carbon Administration, room 201, No.1 Xiao Huang Zhuang Bei Jie, Dongcheng District, Beijing 100013, China E-mail zhangf@bfdic.com
Received 6 September 2015 Accepted 29 January 2016 Published 12 February 2016
Views 160975
Available at https://doi.org/10.14214/sf.1475 | Download PDF

Supplementary Files
1 Introduction
Many forest ecosystems have developed in environments with marginal soils characterized by growth-limiting availability of macronutrients such as nitrogen (N) and phosphorus (P) (Lovett et al. 2004; Raven and Andrews 2010; Rennenberg and Schmidt 2010). Inadequate nutrition has been identified as one of the limiting factors constraining growth and establishment success of hardwood plantations (Kozlowski. 1987). Nutrient limitations in marginal lands can be ameliorated by fertilization at outplanting (Burdett et al. 1984). However, fertilization at outplanting may unintentionally stimulate growth of competing vegetation rather than target plants (Staples et al. 1999; Jobidon et al. 2003). Hence, nutrient reserves in seedlings through nursery fertilization may provide a better means to promote survival and growth of outplanted seedlings, especially on marginal land (Brockley 1988; Jacobs et al. 2005; Oliet et al. 2011).
Fertilization techniques, such as exponential fertilization during the pre-hardening period and fall fertilization during the hardening period, have been recommended to build rich nutrient reserves in seedlings, and increase the performance of outplanted seedlings in the field (Timmer 1996; Villar-Salvador et al. 2012) and transplanted seedlings in the nursery (Quroeshi and Timmer 2000; Salifu et al. 2008; Oliet et al. 2009). Exponential fertilization, relative to the practice to conventional fertilization which involves applying fertilizer in equal doses at regularly spaced intervals over the growing season, is achieved by applying fertilizer at exponential rates corresponding more closely to the desired relative growth rate of plants during their exponential phase of growth (Timmer 1996; Birge et al. 2006). This technique has been demonstrated with a variety of tree species, including Pinus spp. (Timmer and Armstrong 1987; Miller and Timmer 1994; Dumroese et al. 2005), Picea spp. (Quoreshi and Timmer 2000; Salifu and Timmer 2003a), Larix spp. (Qu et al. 2003), and Quercus spp. (Salifu and Jacobs 2006; Jacobs and Wilkinson 2009). Fall fertilization, another fertilization technique to facilitate nutrient loading, also improve field performance (Birchler et al. 2001; South and Donald 2002; Oliet et al. 2011) and transplanting performance (Van den Driessche 1985; Boivin et al. 2004) by alleviating nutrient dilution during the hardening period and building up nutrient reserves in seedlings (Van den Driessche 1985). This method has been used in most evergreen tree species (Birchler et al. 2001; South and Donald 2002; Boivin et al. 2004; Islam et al. 2009; Oliet et al. 2011; Andivia et al. 2012; Jonsdottir et al. 2013) and several deciduous conifer (Li et al. 2012, 2014; Zhu et al. 2013).
The above-mentioned studies have focused predominantly on the individual effects of either pre-hardening or fall fertilization on seedling nutrient reserves and transplanting performance in the nursery (or outplanting performance in the field). Because pre-hardening and hardening are 2 consecutive stages that seedlings experience, nursery practices that include fertilization regimes cannot be separated. In our previous study (Li et al. 2014), we examined combined effects of pre-hardening and fall fertilization on N translocation and storage in Quercus variabilis Blume seedlings. We found that adequate nutrient reserves could be effectively obtained with a combination of pre-hardening and fall fertilization and the effect of fall fertilization was dependent on pre-hardening fertilization. Because a transplanting trial was not include in the study, how stored nutrient reserves due to pre-hardening fertilization in combination with fall fertilization impact seedling growth and nutrient acquisition from soil remains unknown. The goal of seedling production is establishment success and transplanting (or outplanting) performance has been viewed as a tool to evaluate seedling culture practices (Mattsson 1996; Salifu and Timmer 2003b; Davis and Jacobs 2005). Therefore, it is critical to characterize transplanting performance to deepen our understanding of the combined role of pre-hardening and fall fertilization.
Following transplanting (outplanting), the development of most seedlings during the first growing season can be divided into 3 phases: establishment, rapid growth, and hardening (Jacobs and Wilkinson 2009). During the establishment phase, new growth of seedlings were more dependent on plant nutrient reserves than soil nutrient availability because of poor root–soil contact, root restriction, and slow root development that limited nutrient and water uptake (Burdett 1990; Nambiar and Sands 1993; Grossnickle 2005). As a result, a large body of research has focused on the relation of fertilization with internal nutrient reserves, nutrient remobilization and establishment success whether for transplanting trial (Van den Driessche 1985; Grelet et al. 2003; Salifu et al. 2008; Uscola et al. 2015) or outplanting trial (Salifu and Timmer 2001). Plants initiate roots when the soil temperature gradually becomes warmer (Grossnickle 2005) and N from soil uptake plays an increasingly important role for seedling growth (McAlister and Timmer 1998). This could explain why the role of nursery fertilization has received less attention during the rapid growth phase relative to the establishment phase. For example, Salifu and Timmer reported effect of pre-hardening fertilization on remobilization and growth after Picea mariana (Mill.) BSP seedlings were transplanted (2003b) and outplanted (2001) 120 days. At the end of the hardening phase, how nursery fertilization affects survival and growth performance is concentrated again. In addition, studies associated with outplanting performance (Villar-Salvador et al. 2012; references therein) outnumber transplanting performance (Quoreshi and Timmer 2000; Oliet et al. 2009). Although the 3 phases described above are consecutive, published papers precluded one or more phases when dealing with the relationship between fertilization and transplanting (or outplanting) performance (Grelet et al. 2003; Salifu and Timmer 2001, 2003b). Therefore, it is necessary to study nutrient accumulation and acquisition through the 3 distinct phases to explain conclusively the role of fertilization on transplanting (or outplanting) performance.
Q. variabilis, a source of industrial cork, is one of the most important afforestation tree species in China (Zhang and Lu 2002). As mentioned above, effects of pre-hardening fertilization alone and in combination with fall fertilization on N translocation and storage for this deciduous broadleaved tree have been studied (Li et al. 2014); although the relationship of built reserves in seedlings with their transplanting performance is still unknown. In the present study, our objective was to further elucidate whether their combined effects would promote growth, nutrient accumulation, and nutrient uptake at three growth phases after subsequent transplanting. To fulfill this objective, we investigated the independent and interacting effects of 2 levels of pre-hardening N fertilization, 4 levels of fertilization during the hardening period (fall fertilization), and 4 sampling dates during different growth phases of Q. variabilis after transplanting in a nursery.
2 Material and methods
2.1 Fertilization treatment
Acorns from the Q. variabilis center in Sizuolou Forest Farm (Beijing, China) were selected and stored prior to sowing, as detailed in Li and others (2014). On 18 March 2012, acorns were sown into containers filled with a 3:1 (v:v) peat (Pindstrup Seeding, pH 6.0, Screening 0–6 mm): vermiculite (5 mm diameter, Xinyang Jinhualan Mining Co., Henan, China) mixture. Each tray had 30 containers and each container was 8 cm × 20 cm (1050 cm3). Thirty-two filled trays were put onto a rolling bench under natural light in the greenhouse of Beijing Forestry University near Jiufeng Mountain, Beijing (39°54´N, 116°28´E).
Pre-hardening fertilization started from 1 April 2012, 2 weeks after germination, and was applied once a week for 20 weeks, terminating on 12 August (week 3 through week 22). Based on published N supplies for Quercus spp. (Salifu and Jacobs 2006; Li et al. 2014), 2 N fertilization levels (50, 100 mg N seedling-1) were chosen. N was applied exponentially according to Eq. 1 (Ingestad and Lund 1986 as modified by Timmer and Aidelbaum 1996):
NT was the desired amount (50, 100 mg N) to be added over the number of fertilizations (t). Ns is the initial N content in each seed. For our study, Ns was determined to be 27.85 mg N seed−1 and the total number of fertilizations (t) was 20. Therefore, r, the relative addition rate required to increase Ns to finial level NT + Ns of 77.85 and 127.85 mg N was 5.1 and 7.6 %, respectively. The calculation was described in detail by Li et al. (2014).
The quantity of N to apply for a specific week (Nt) was calculated using Eq. 2 (Ingestad and Lund 1986 as modified by Timmer and Aidelbaum 1996):
where Nt–1 is the cumulative amount of N applied during previous applications.
Each pre-hardening fertilization regime (hereafter, 50E, 100E) was applied to 16 trays (480 seedlings per regime; a total of 32 trays and 960 seedlings for the entire experiment). N was supplied as urea (Xilong Chemical Co., China), which is currently applied as an N source in Chinese nurseries. Elemental P and K were supplied as KH2PO4 (Damao Chemical Co., China). We applied corresponding amount of P and K seedling−1 per week for a cumulative total of 26.3 mg and 32.9 mg, respectively. Microelements were supplied by EDTA (Xilong Chemical Co., China) and DTPA (Jinke Fine Chemical Institute, Tianjin, China) to avert deficiency of other nutrients. And a cumulative total of 0.252 mg EDTA and 0.078 mg DTPA seedling−1 were supplemented. P, K, EDTA and DTPA were evenly split along with N. Each week the desired amounts of N in addition to P, K, EDTA, and DTPA were dissolved in water and 20 ml of solution was added with a hand-sprayer to each seedling. Seedlings were rinsed with distilled water after each application to avoid foliar fertilizer burn. The seedlings were irrigated as needed, about 2 times each week. From sowing (March 18) to the conclusion of pre-hardening fertilization (August 12), ambient temperature averaged 25:18 °C (day:night) as measured with a JL-18 Series thermometer (Huayan Instrument and Equipment Co., Shanghai, China) at 15-min intervals. The trays on raised benches were rotated weekly to minimize edge effect.
A 2-week short-day treatment from 13 August to 26 August (week 23 and week 24) commenced after pre-hardening fertilization was terminated by covering seedlings with blackout curtains from 17:00 to 08:00. When most seedlings had formed terminal buds, seedlings were taken outdoors on 27 August and again exposed to natural light conditions. Of the 16 trays per pre-hardening fertilization regime, groups of 4 trays were randomly selected and treated with one of 4 fall fertilization levels: control (no fall N); low N (12 mg N); intermediate N (24 mg N) and high N (48 mg N) (Li et al. 2014). Thus, the 4 fall N fertilization levels were applied within each of the two pre-hardening (50E, 100E) regimes as a factorial 2×4 design and 4 trays for each of 8 treatments served as 4 replications. Except for N, each seedling received the same amount of P, K, and chelated micronutrients. Cumulative supplementary P, K, EDTA, and DTPA were 10.52, 13.16, 1.52 and 0.46 mg, respectively. Each application of N, P, K, EDTA, and DTPA was evenly split for a total of 8 applications between 27 August and 15 October, 2012 (week 25 to week 32). All treatments were irrigated about every 5 days during and after fall fertilization to induce hardening. After 12 November (week 36), seedlings were stored outdoors under snow cover over winter prior to transplanting. From being taken outdoors (week 25) to being stored (week 36), ambient temperature averaged 15:10 °C (day:night). Temperature was monitored as described above.
2.2 Greenhouse transplanting trial
To analyze how fertilization influenced growth and nutrient content of the seedlings in the subsequent year, the transplanting trial was started on 21 March 2013 in the same greenhouse. Plants grew under natural day length with day:night temperatures averaging 22:15 °C, respectively. Prior to transplanting, we gently washed off the medium from the roots. The aim of bare rooting was to minimize the influence of the residual fertilizer in the medium from the previous year. Twenty seedlings from each tray were individually transplanted in a 6.8 L PVC tube (diameter = 12 cm and long = 60 cm) filled with washed sand, resulting in a total of 640 seedlings for the 8 treatments (20 seedlings per tray, 4 trays per treatment). All transplanted seedlings were then arranged as a completely randomized design and were irrigated every 4 to 5 days as necessary.
2.3 Sampling and measurements
To evaluate the responses of transplanted seedlings to fertilization, seedlings were sampled during the transplanting trial on week 0, 8, 21, 34 representing the end of initial phase (T0), establishment phase (T1), rapid growth phase (T3) and hardening phase (T4), respectively, according to Jacobs and Wilkinson (2009). For each sampling, 5 seedlings per treatment per replicate were collected (20 seedlings per pre-hardening fertilization × fall fertilization; 160 seedlings total each time). After growth medium was gently washed from roots, seedlings were measured for height (root collar to the tip of the terminal bud) and root collar diameter. We separated seedlings into foliage, stem, and root, dried them in an oven at 70 °C for 48 hours, and then weighed the material to determine dry mass. Within each replication, each tissue fraction (foliage, stem, and root) of the 5 seedlings was subsequently combined to a composite sample, ground, sieved through a 0.25-mm screen, and analyzed for N, P and K. Samples were wet digested in a heating block at 250 °C with a mixture of H2SO4 and H2O2 (as described above). We determined total N concentration by using semi-micro Kjeldahl distillation (UDK-152, Velp Scientifica, USA) (Bremner and Mulvaney 1982), P concentration by using Model 722 spectrophotometer (Agilent 8453, Germany), and K concentration by using atomic emit spectrometry (Spectra AA Varian 220 atomic absorption spectrometer, Varian Inc., USA). The total nutrient uptakes were each calculated by subtracting the nutrient content at transplanting from the total content in the current growing phase (Heiskanen et al. 2009).
2.4 Statistical data analysis
Morphological and nutritional data were evaluated by separate analysis of variance (ANOVA) using SPSS 16.0 (Chicago, Illinois, USA). For morphological attributes (n = 20), data were calculated from individual seedlings for each tray (pre-hardening fertilization; fall fertilization). For mineral nutrient attributes (n = 4), treatment means were computed from a composite sample for each tray (pre-hardening fertilization; fall fertilization). A two-way ANOVA was first used to analyze the effects of pre-hardening fertilization, fall fertilization, and their interactions on morphological and nutritional attributes. Separation of means for morphological and nutritional responses was ranked according to Duncan’s multiple range test at P = 0.05. The explore function of SPSS was used to examine data prior to the t test and ANOVA to ensure normality and variance homogeneity requirements and no transformations were necessary.
3 Results
3.1 Growth responses
At transplanting (T0), Q. variabilis seedlings from all fertilization treatments showed no statistically significant differences in any growth parameter (Table 1 in Supplementary file 1). Although most growth parameters still exhibited no significant differences at the end of establishment phase (T1), fall fertilization exhibited a significant effect on diameter. Fall fertilization slightly (low and intermediate rates) or significantly (high rate) increased diameter (Fig. 1).
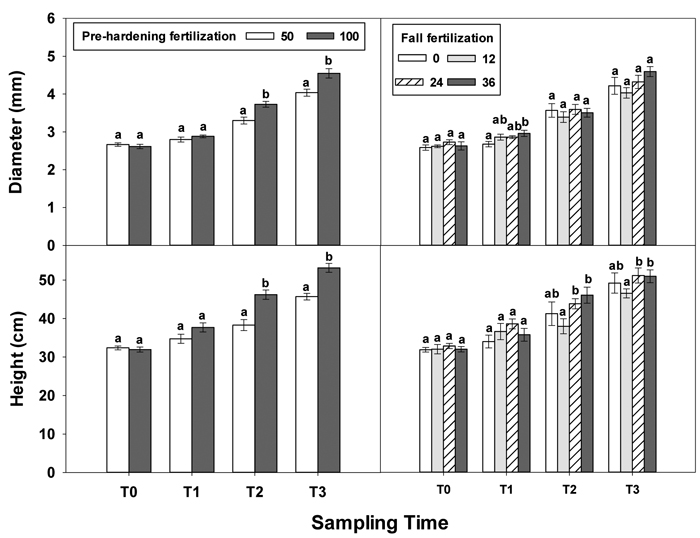
Fig. 1. Main effects of pre-hardening fertilization (50, 100 mg N seedling–1) and fall fertilization (0, 12, 24, 36 mg N seedling–1) on height and diameter (Means ± SE) of Quercus variabilis seedlings at transplanting (T0), at the end of establishment (T1), rapid growing (T2) and hardening (T3) stages after transplantation. Bars marked with different letters differ statistically for each stage according to Duncan`s test = 0.05.
Greater differences in all seedling growth parameters were observed at the end of the rapid growth phase (T2), with high pre-hardening fertilization facilitating height, diameter and tissue dry mass (Table 1 in Supplementary file 1, Figs. 1 and 2). Relative to low pre-hardening fertilization, high fertilization promoted 13% increase in height, 21% increase in diameter, 24% increase in foliage dry mass, 47% increase in stem dry mass, and 22% increase in root dry mass (Figs. 1 and 2). Similar growth responses to pre-hardening fertilization were observed after the end of the hardening phase (T3) except that significant differences occurred in foliage dry mass. The interaction of pre-hardening and fall fertilization affected the fallen foliage dry mass. Under low pre-hardening, intermediate and high fall fertilization could enhance foliage dry mass compared with the control (Fig. 3). Meanwhile, fall fertilization significantly affected stem dry mass (Table 1 in Supplementary file 1) and the high rate yielded the greatest stem dry mass (Fig. 2).
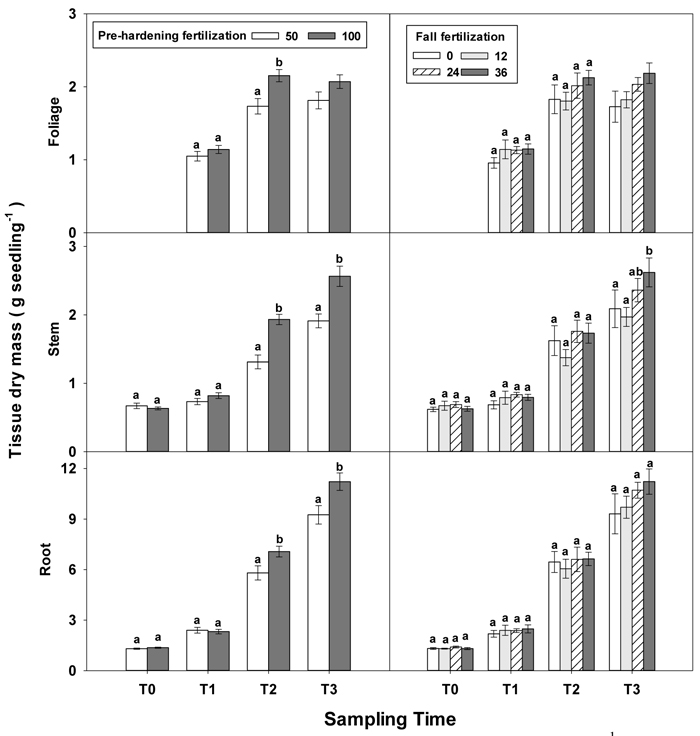
Fig. 2. Main effects of pre-hardening fertilization (50, 100 mg N seedling–1) and fall fertilization (0, 12, 24, 36 mg N seedling–1) on tissue dry mass (Means ± SE) of Quercus variabilis seedlings at transplanting (T0), at the end of establishment (T1), rapid growing (T2) and hardening (T3) stages after transplantation. Bars marked with different letters differ statistically for each tissue within the same stage according to Duncan`s test = 0.05. Because of the significant interaction effect between pre-hardening and fall fertilization, the mean foliage dry mass response at T3 was not presented. Foliage dry mass at T0 stage was not presented due to the absence of leaves for the deciduous tree species at transplanting.
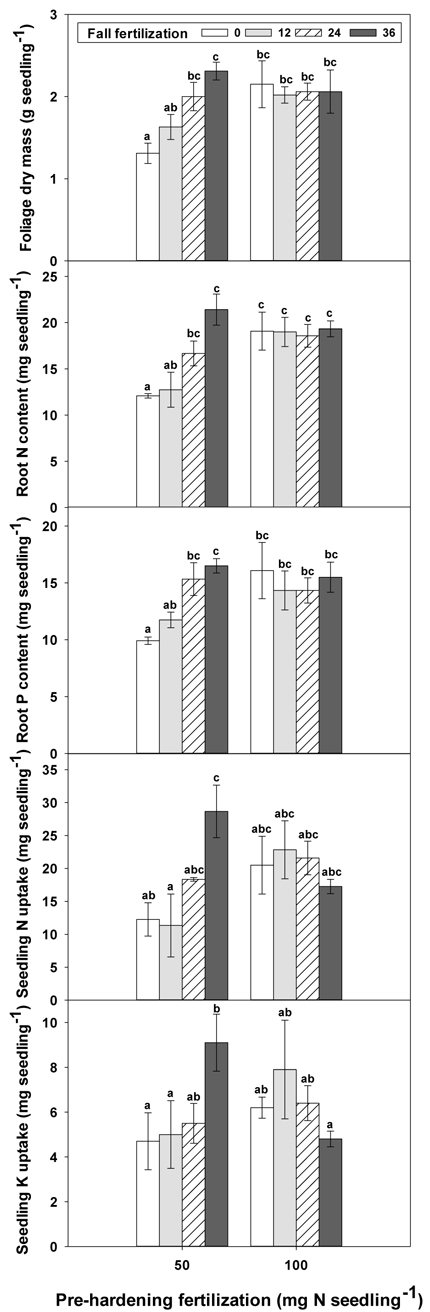
Fig. 3. The interaction of pre-hardening fertilization (50, 100 mg N seedling–1) and fall fertilization (0, 12, 24, 36 mg N seedling–1) on Quercus variabilis seedlings foliage dry mass at the end of hardening stage (T3), root N content at the end of establishment stage (T1), and root P content at the end of hardening stage (T3), and on seedling N and K uptake from soil during establishment stage (T1-T0) after transplantation. Bars marked with different letters differ statistically according to Duncan`s test = 0.05.
3.2 Nutrient accumulation
Only root N and stem N were affected by fertilization at T0 (Table 2 in Supplementary file 1). Seedlings reared at high pre-hardening fertilization had greater stem and root N content (Fig. 4). At T1, high pre-hardening fertilization resulted in accumulation of both stem N and foliage K (Figs. 4–6), whereas root N at this time was affected by the interaction of pre-hardening and fall fertilization (Fig. 3). Intermediate and high fall fertilization led to enhanced N only under low pre-hardening fertilization (Fig. 3). The combination of low pre-hardening and high fall fertilization consistently yielded the maximum values of root and stem N.
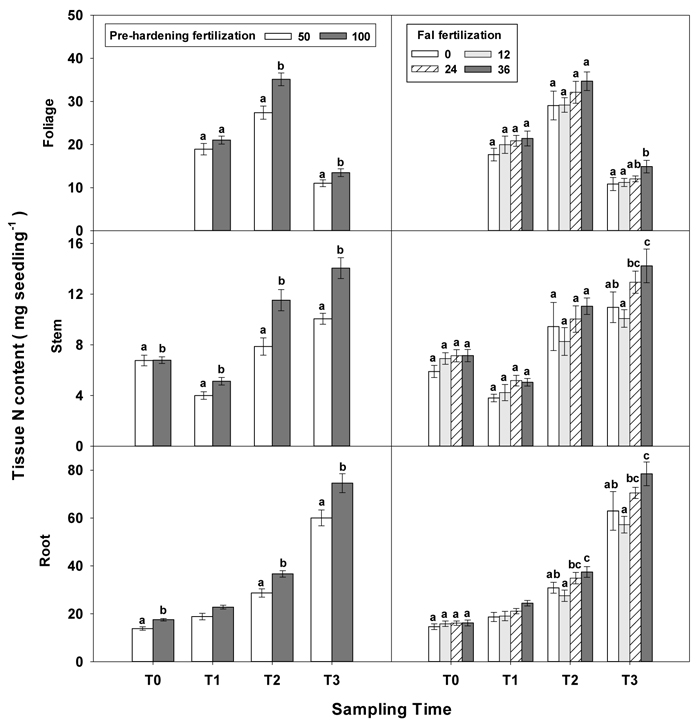
Fig. 4. Main effects of pre-hardening fertilization (50, 100 mg N seedling–1) and fall fertilization (0, 12, 24, 36 mg N seedling–1) on tissue N content (Means ± SE) of Quercus variabilis seedlings at transplanting (T0), at the end of establishment (T1), rapid growing (T2) and hardening (T3) stages after transplantation. Bars marked with different letters differ statistically for each tissue within the same stage according to Duncan`s test = 0.05. Foliage N content at T0 stage was not presented due to the absence of leaves for the deciduous tree species at transplanting.
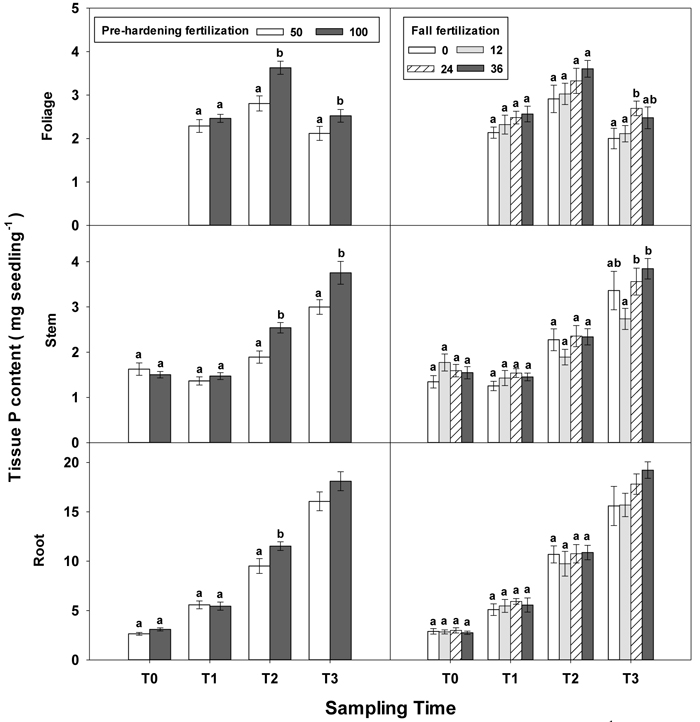
Fig. 5. Main effects of pre-hardening fertilization (50, 100 mg N seedling–1) and fall fertilization (0, 12, 24, 36 mg N seedling–1) on tissue P content (Means ± SE) of Quercus variabilis seedlings at transplanting (T0), at the end of establishment (T1), rapid growing (T2) and hardening (T3) stages after transplantation. Bars marked with different letters differ statistically for each tissue within the same stage according to Duncan`s test = 0.05. Because of the significant interaction effect between pre-hardening and fall fertilization, the mean root P content response at T3 was not presented. Foliage P content at T0 stage was not presented due to the absence of leaves for the deciduous tree species at transplanting.
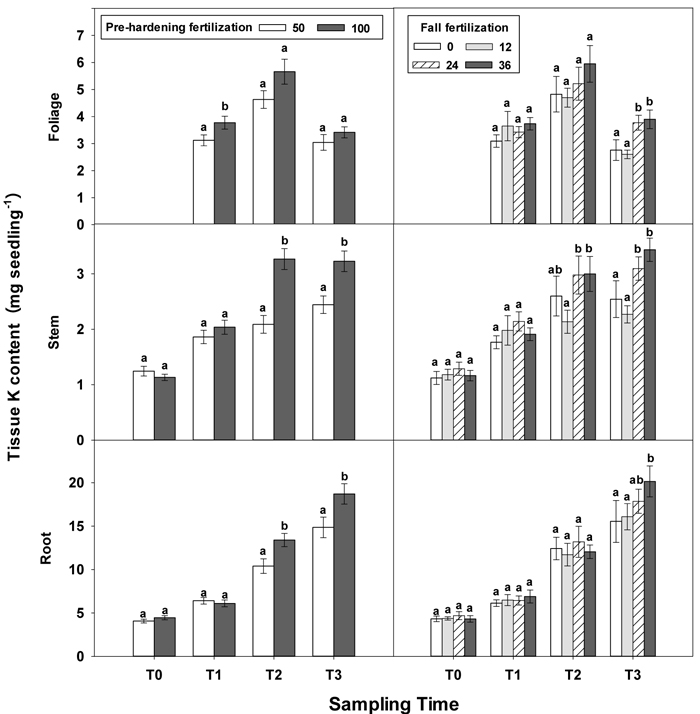
Fig. 6. Main effects of pre-hardening fertilization (50, 100 mg N seedling–1) and fall fertilization (0, 12, 24, 36 mg N seedling–1) on tissue K content (Means ± SE) of Quercus variabilis seedlings at transplanting (T0), at the end of establishment (T1), rapid growing (T2) and hardening (T3) stages after transplantation. Bars marked with different letters differ statistically for each tissue within the same stage according to Duncan`s test = 0.05. Foliage K content at T0 stage was not presented due to the absence of leaves for the deciduous tree species at transplanting.
Similar to growth responses, more tissue nutrient parameters yielded statistically significant differences at T2 and most of these parameters were enhanced by pre-hardening fertilization (Table 2 in Supplementary file 1). All tissue nutrients except foliage K were significantly increased by pre-hardening fertilization. Compared with low pre-hardening fertilization, high fertilization increased N content by 28%, 46% and 28% in root, stem and foliage, respectively; and increased P content by 21%, 32% and 29% in root, stem and foliage, respectively; and increased K content by 29% and 57% in root and stem, respectively (Figs. 4–6). Furthermore, root N was also influenced by fall fertilization, with seedlings receiving high fall fertilization showing greater root N than the control (Fig. 4).
Pre-hardening fertilization and fall fertilization affected most of the nutrient parameters at T3. Compared with low pre-hardening fertilization, high fertilization increased N content by 24%, 40% and 23% in root, stem and foliage, respectively; P content by 27% and 19% in stem and foliage, respectively; and K content by 26% and 33% in root and stem, respectively (Figs. 4–6). Additionally, most of these parameters and foliage K were also influenced by fall fertilization at T3. Relative to the control, high fall fertilization led to significant enhancement of tissue N content, whereas greater foliage P content resulted from intermediate fall fertilization (Figs. 4 and 5). Intermediate and high fall fertilization consistently had greater K content in foliage and stems (Fig. 6). Overall, intermediate and high fall fertilization induced to some extent tissue nutrient accumulation (Figs. 4–6). Root P was affected by the interaction of pre-hardening and fall fertilization and showed similar trends with root N at T1 (Fig. 3).
3.3 Nutrient uptake from soil
During the establishment phase (T1–T0), the interaction of pre-hardening and fall fertilization significantly affected N and P uptake (Table 3 in Supplementary file 1). Combination of low pre-hardening and high fall fertilization consistently yielded the maximum values of these attributes (Fig. 3). From transplanting to the rapid growth phase (T2–T0), N, P, and K uptake of transplanted seedlings were all increased by pre-hardening fertilization (Fig. 7). Seedlings receiving high pre-hardening fertilization exhibited increased N uptake by 36%, P uptake by 32%, and K uptake by 42%. Over the entire growing season (T3–T0), both pre-hardening and fall fertilization, but not their interaction, resulted in significantly increased N and K uptake. Compared with low pre-hardening fertilization, N and K uptake in high fertilization regimes were increased by 28% and 32%, respectively. However, significant increment by fall fertilization only occurred at the high rate and these two parameters were increased by 39% and 43% in high fall fertilization rate relative to the control.
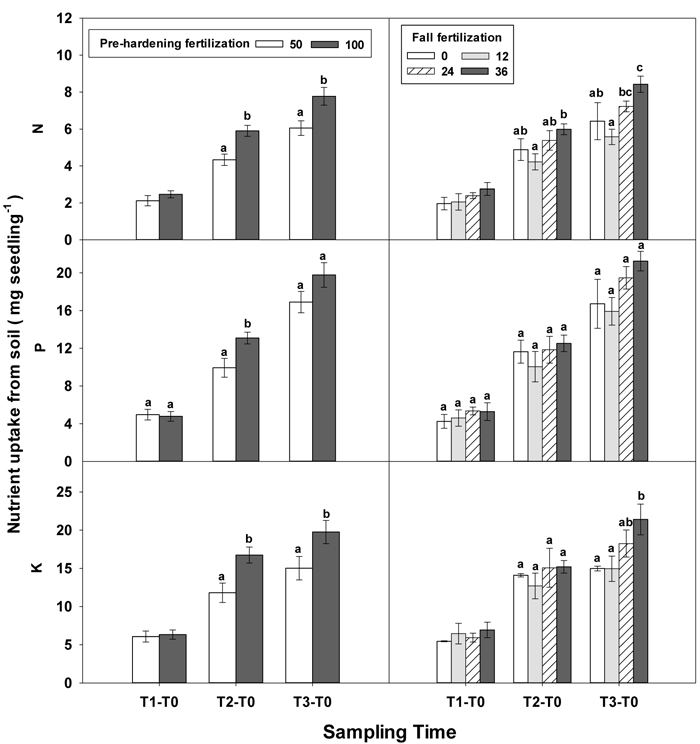
Fig. 7. Main effects of pre-hardening fertilization (50, 100 mg N seedling–1) and fall fertilization (0, 12, 24, 36 mg N seedling–1) on N, P and K uptake of Quercus variabilis seedlings from soil during establishment (T1-T0), from transplanting to the rapid growing (T2-T0) and hardening (T3-T2) stages and the whole growing season (T3-T0) after transplantation. Bars marked with different letters differ statistically for each stage according to Duncan`s test = 0.05. Because of the significant interaction effect between pre-hardening and fall fertilization, the mean N and K uptake from soil at T1-T0 stage was not presented.
4 Discussion
4.1 Necessity of fall fertilization for pre-hardening nutrient-loaded seedlings
As described in the conceptual model by Timmer (1996), the fact that seedling N content increased by exponential fertilization rates without significantly changing total dry mass at transplanting (T0) reflected that both rates induced luxury consumption. It follows that additional fertilization during fall led to slight, but not significant, enhancement in seedling N. However, the minor effect by fall fertilization was exaggerated at the end of the growing season (T3). Relative to the control, high fall fertilization (36 mg N seedling–1) contributed to the maximum values of stem dry mass, stem and root N content, and stem and root K content. This effect was linked with the benefit of fall fertilization on N and K uptake from soil (T3–T0). Boivin et al. (2004) also demonstrated that nursery fall fertilization stimulated nutrient uptake from soil when Picea mariana seedlings were transplanted in a nursery. Our results indicated that transplanting performance should be fully considered to determine whether seedlings are further added by fertilization during fall.
4.2 Distinct transplanting performance at three growth phases and in relation to pre-hardening and fall fertilization
During the plantation establishment phase, fast root egress into the soil and quick nutrient absorption from soil are crucial prerequisites for transplanted seedlings (Heiskanen et al. 2009). In their review, Millard and Grelet (2010) quantified a range of species and concluded that N uptake can occur for typically 3 to 5 weeks after transplanting. In our study, Q. variabilis transplanted seedlings absorbed more than 20 mg N, 4 mg P, and 5 mg K from soil at the end of establishment (week 8). Moreover, the interaction of pre-hardening and fall fertilization promoted their N and K uptake. Fall fertilization under low pre-hardening fertilization could slightly or significantly benefit Q. variabilis transplanted seedlings’ N or K uptake. Enhancement in N and K absorption due to fertilization promoted Q. variabilis transplanted seedling N (root and stem) and K (foliage) storage, whereas fertilization promoted Q. rubra L. transplanted seedlings to store N and P, but not K, in foliage at the end of the establishment phase (60 days after transplanting) (Salifu et al. 2008). Additionally, although not quantified directly, the both N and P declined in stems from T0 to T1 and this strongly indicates that stems serve to translocate nutrients to support new growth of newly planted seedlings. This finding has been confirmed in other studies (Nambiar 1987; Hawkins et al. 1998; Salifu et al. 2008).
In contrast to the establishment phase, the role of pre-hardening fertilization was fully realized at the end of the rapid growth phase (week 21). High pre-hardening fertilization consistently favored transplanted seedling growth (height, diameter, tissue dry mass), nutrient storage (N, P, and K in any tissue) and nutrient uptake from soil (N, P, and K). Our results are in agreement with earlier both transplanted (Salifu and Timmer 2003b) and outplanted (Salifu and Timmer 2001) studies on Picea mariana seedlings. The evidence that superior transplanting performance of fertilization occurred in the rapid growth phase was presumably attributed to sink strength and nutrient uptake. Compared with the establishment phase, Q. variabilis transplanted seedlings had greater growth and requirements for nutrients during the rapid growth phase (Yang et al. 2012). In addition, the expanded root system with the high fertilization increased nutrient uptake and further stimulated photosynthesis and transplanted seedling growth (Salifu and Timmer 2003b). Enhanced sink strength led to a feedback cycle that promoted root growth and nutrient uptake (Burdett 1990).
Similar to the end of the rapid growth phase, high pre-hardening fertilization promoted transplanted seedling growth and nutrient storage as well as soil nutrient uptake at the end of the hardening phase. Greater dry mass and nutrient uptake was also observed in high-fertilized Picea mariana seedlings after one transplanting season (Quoreshi and Timmer 2000). Contrary to the end of the rapid growth phase, transplanted seedlings with fall fertilization (especially the high rate) generally exhibited superior stem dry mass, tissue nutrient storage, and enhanced soil nutrient uptake at the end of the growing season. After one transplanting season, fall-fertilized Picea abies (L.) Karst seedlings also had better growth performance and larger nutrient reserves (Rikala et al. 2004). Due to deficiencies in sampling during the establishment and rapid growth phases, it was unknown whether the beneficial effect of fall fertilization existed in these phases for Picea abies seedlings.
4.3 Role of leaf decomposition for evaluating nursery fertilization
Leaves of most deciduous tree species abscise in the fall and nutrients move from the foliar tissues to stems and roots prior to abscission (Aerts 1996). We observed that P (2.3 mg), and K (3.2 mg), and especially N (12.3 mg) content at the end of the hardening phase consistently declined in comparison to those values at the end of the rapid growth phase (3.2, 5.1, and 31.3 mg, respectively). Furthermore, we found that high pre-hardening and fall fertilization resulted in more nutrients stored in the fallen leaves of transplanted (two-year old) seedlings. Desirable foliage nutrient accumulation by high fertilization also occurred in our earlier studies on one-year old Q. variabilis (Li et al. 2014) and Larix olgensis Henry (Zhu et al. 2013) seedlings in the nursery. It should be noted that the fate of fallen leaves between nursery and field conditions determines their different roles in nutrient cycling. In the nursery, fallen leaves are removed as litter, whereas in the field these leaves decompose on the soil surface and gradually return nutrients to soil. If Q. variabilis seedlings were shipped and outplanted in the field rather than transplanted in the nursery, greater nutrient content in the fallen leaves owing to high nursery fertilization would return more nutrients to support subsequent seedling growth. Several studies have focused on how nursery fertilization built nutrient reserves and subsequently impacted seedling nutrient accumulation (Salifu and Timmer 2001; Boivin et al. 2004; Thiffault et al. 2012). Therefore more attention should be paid to nutrient cycling from fallen leaves to fully understand the role of nursery fertilization on field performance, especially for deciduous tree species.
5 Conclusions
The effect of fall fertilization on dry mass and nutrient accumulation at transplanting (T0) was minor; however, by the end of growing season (T3) following transplanting its effect was pronounced. These results indicated that even for pre-hardening nutrient-loaded seedlings, fall fertilization was necessary and transplanting performance should be fully considered to determine whether seedlings are further aided by fall fertilization. High pre-hardening fertilization promoted seedling growth and nutrient accumulation at the end of the rapid growth and hardening phases while the rate of 36 mg N fall fertilization yielded similar effects only at the end of the hardening phase, demonstrating that pre-hardening fertilization and fall fertilization played disparate roles at these distinct transplanting phases. Moreover, at the end of establishment, the effect of fall fertilization on N and K uptake from soil was dependent on pre-hardening regimes. At the conclusion of both rapid growth and hardening phases, high pre-hardening fertilization consistently increased nutrient uptake. Enhancement in N and K uptake occurred at the rate of 36 mg N of fall fertilization at the end of the hardening phase. Thus, it is necessary to evaluate fertilization by assessing seedling nutrient reserves in combination with dynamic transplanting performance. Using this approach, the combination of 100 mg N during pre-hardening with 36 mg N during hardening was recommended for satisfactory transplanting performance.
Acknowledgements
The study was jointly funded by the Fundamental Research Funds for the Central Universities (Contract No. TD2011-8 and BLYJ201607), Special Funds for Beijing Municipal Common Construction Project with Central Universities, and Research and Demonstration of Key Technology of the Variety Breeding, Cultivation and Liquid Biofuels of High-starch Energy Plants (2015BAD15B01).
References
Aerts R. (1996). Nutrient resorption from senescing leaves of perennials: are there general patterns? Journal of Ecology 84: 597–608. http://dx.doi.org/10.2307/2261481.
Andivia E., Fernández M., Vázquez-Piqué J., Alejano R. (2012). Two provenances of Quercus ilex ssp. ballota (Desf.) Samp. nursery seedlings have different response to frost tolerance and autumn fertilization. European Journal of Forest Research 131: 1091–1101. http://dx.doi.org/10.1007/s10342-011-0578-1.
Birchler T.M., Rose R., Haase D.L. (2001). Fall fertilization with N and K: effects on Douglas-fir quality and performance. Western Journal of Applied Forestry 16: 71–79.
Birge Z.K.D., Salifu K.F., Jacobs D.F. (2006). Modified exponential nitrogen loading to promote morphological quality and nutrient storage of bareroot-cultured Quercus rubra and Quercus alba seedlings. Scandinavian Journal of Forest Research 21: 306–316. http://dx.doi.org/10.1080/02827580600761611.
Boivin J.R., Salifu K.F., Timmer V.R. (2004). Late-season fertilization of Picea mariana seedlings: intensive loading and outplanting response on greenhouse bioassays. Annals of Forest Science 61: 737–745. http://dx.doi.org/10.1051/forest:2004073.
Bremner J.M., Mulvaney C.S. (1982). Nitrogen-total. In: Page A.L. (ed.). Methods of soil analysis. American Society of Agronomy, Madison, WI. p. 595–624.
Brockley R.P. (1988). The effects of fertilization on early growth of planted seedlings - a problem analysis. FRDA Report 11. Forestry Canada and British Columbia Ministry of Forests, Victoria, British Columbia. 16 p.
Burdett A.N. (1990). Physiological processes in plantation establishment and the development of specifications for forest planting stock. Canadian Journal of Forest Research 20: 415–427. http://dx.doi.org/10.1139/x90-059.
Burdett A.N., Herring L.J., Thompson C.F. (1984). Early growth of planted spruce. Canadian Journal of Forest Research 14: 644–651. http://dx.doi.org/10.1139/x84-116.
Davis A.S., Jacobs D.F. (2005). Quantifying root system quality of nursery seedlings and relationship to outplanting performance. New Forests 30: 295–311. http://dx.doi.org/10.1007/s11056-005-7480-y.
Dumroese R.K., Page-Dumroese D.S., Salifu K.F., Jacobs D.F. (2005). Exponential fertilization of Pinus monticola seedlings: nutrient uptake efficiency, leaching fractions, and early outplanting performance. Canadian Journal of Forest Research 35: 2961–2967. http://dx.doi.org/10.1139/x05-226.
Grelet G.A., Alexander I.J., Millard P., Proe M.F. (2003). Does morphology or the size of the internal nitrogen store determine how Vaccinium spp. respond to spring nitrogen supply? Functional Ecology 17: 690–699. http://dx.doi.org/10.1046/j.1365-2435.2003.00776.x.
Grossnickle S.C. (2005). Importance of root growth in overcoming planting stress. New Forests 30: 273–294. http://dx.doi.org/10.1007/s11056-004-8303-2.
Hawkins B.J., Henry G., Kiiskila S.B.R. (1998). Biomass and nutrient allocation in Douglas-fir and amabalis fir seedlings: influence of growth rate and nutrition. Tree Phytologist 18: 803–810. http://dx.doi.org/10.1093/treephys/18.12.803.
Heiskanen J., Lahti M., Luoranen J., Rikala R. (2009). Nutrient loading has a transitory effect on the nitrogen status and growth of outplanted Norway spruce seedlings. Silva Fennica 43(2): 249–260. http://dx.doi.org/10.14214/sf.210.
Ingestad T., Lund A.B. (1986). Theory and techniques for steady state mineral nutrition and growth of plants. Scandinavian Journal of Forest Research 1: 439–453. http://dx.doi.org/10.1080/02827588609382436.
Islam M.A., Apostol K.G., Jacobs D.F., Dumroese R.K. (2009). Fall fertilization of Pinus resinosa seedlings: nutrient uptake, cold hardiness, and morphological development. Annals of Forest Science 66(7). 704 p. http://dx.doi.org/10.1051/forest/2009061.
Jacobs D.F., Salifu K.F., Seifert J.R. (2005). Growth and nutritional response of hardwood seedlings to controlled-release fertilization at outplanting. Forest Ecology and Management 214: 28–39. http://dx.doi.org/10.1016/j.foreco.2005.03.053.
Jacobs D.F., Wilkinson K.M. (2009). Planning crops and developing propagation protocols. In: Dumroese R.K., Luna T., Landis T.D. (ed.). Nursery manual for native plants: a guide for tribal nurseries - Volume 1: nursery management. Agriculture Handbook 730. U.S. Department of Agriculture, Forest Service, Washington, D.C., USA. p. 33–53.
Jobidon R., Roy V., Cyr G. (2003). Net effect of competing vegetation on selected environmental conditions and performance of four spruce seedling stock sizes after eight years in Quebec (Canada). Annals of Forest Science 60: 691–699. http://dx.doi.org/10.1051/forest:2003063.
Jonsdottir RJ, Sigurdsson BD, Lindström A (2013) Effects of nutrient loading and fertilization at planting on growth and nutrient status of Lutz spruce (Picea × lutzii) seedlings during the first growing season in Iceland. Scandinavian Journal of Forest 28: 631–641. http://dx.doi.org/10.1080/02827581.2013.824503.
Kozlowski T.T. (1987). Soil moisture and absorption of water by tree roots. Arboricultural Journal 13: 39–46.
Li G.L., Liu Y., Zhu Y., Li Q.M., Dumroese R.K. (2012). Effect of fall-applied nitrogen on growth, nitrogen storage, and frost hardiness of bareroot Larix olgensis seedlings. Silva Fennica 46: 345–354. http://dx.doi.org/10.14214/sf.45.
Li G.L., Zhu Y., Liu Y., Wang J.X., Liu J.J., Dumroese R.K. (2014). Combined effects of pre-hardening and fall fertilization on nitrogen translocation and storage in Quercus variabilis seedlings. European Journal of Forest Research 133: 983–992. http://dx.doi.org/10.1007/s10342-014-0816-4.
Lovett G.M., Weathers K.C., Arthur M.A. (2004). Nitrogen cycling in a northern hardwood forest: do species matter? Biogeochemistry 67: 289–308. http://dx.doi.org/10.1023/B:BIOG.0000015786.65466.f5.
Mattsson A. (1996). Predicting field performance using seedling quality assessment. New Forests 13: 223–248. http://dx.doi.org/10.1023/A:1006590409595.
McAlister J.A., Timmer V.R. (1998). Nutrient enrichment of white spruce seedlings during nursery culture and initial plantation establishment. Tree Physiology 18: 195–202. http://dx.doi.org/10.1093/treephys/18.3.195.
Millard P., Grelet G.A. (2010). Nitrogen storage and remobilization by trees: ecophysiological relevance in a changing world. Tree Physiology 30: 1083–1095. http://dx.doi.org/10.1093/treephys/tpq042.
Miller B.D., Timmer V.R. (1994). Steady-state nutrition of Pinus resinosa seedlings: response to nutrient loading, irrigation and hardening regimes. Tree Physiology 14:1327–38. http://dx.doi.org/10.1093/treephys/14.12.1327.
Nambiar E.K.S. (1987). Do nutrients retranslocate from fine roots? Canadian Journal of Forest Research 17: 913–918. http://dx.doi.org/10.1139/x87-143.
Nambiar E.K.S., Sands R. (1993). Competition for water and nutrients in forests. Canadian Journal of Forest Research 23: 1955–1968. http://dx.doi.org/10.1139/x93-247.
Oliet J.A., Tejada M., Salifu K.F., Collazos A., Jacobs D.F. (2009). Performance and nutrient dynamics of holm oak (Quercus ilex L.) seedlings in relation to nursery nutrient loading and post-transplant fertility. European Journal of Forest Research 128: 253–263. http://dx.doi.org/10.1007/s10342-009-0261-y.
Oliet J.A., Salazar J.M., Villar R., Robredo E., Valladares F. (2011). Fall fertilization of Holm oak affects N and P dynamics, root growth potential, and post-planting phenology and growth. Annals of Forest Science 68: 647–656. http://dx.doi.org/10.1007/s13595-011-0060-8.
Qu L.Y., Ali M., Quoreshi A.M., Koike T. (2003). Root growth characteristics, biomass and nutrient dynamics of seedlings of two larch species raised under different fertilization regimes. Plant and Soil 255: 293–302. http://dx.doi.org/10.1023/A:1026159709246.
Quoreshi A.M., Timmer V.R. (2000). Early outplanting performance of nutrient-loaded containerized black spruce seedlings inoculated with Laccaria bicolor: a bioassay study. Canadian Journal of Forest Research 30: 744–752. http://dx.doi.org/10.1139/x00-003.
Raven J.A., Andrews M. (2010). Evolution of tree nutrition. Tree Physiology 30: 1050–1071. http://dx.doi.org/10.1093/treephys/tpq056.
Rennenberg H., Schmidt S. (2010). Perennial lifestyle-an adaptation to nutrient limitation? Tree Physiology 30: 1047–1049. http://dx.doi.org/10.1093/treephys/tpq076.
Rikala R., Heiskanen J., Lahti M. (2004). Autumn fertilization in the nursery affects growth of Picea abies container seedlings after transplanting. Scandinavian Journal of Forest Research 19: 409–414. http://dx.doi.org/10.1080/02827580410030190.
Salifu K.F., Jacobs D.F. (2006). Characterizing fertility targets and multi-element interactions in nursery culture of Quercus rubra seedlings. Annals of Forest Science 63: 231–237. http://dx.doi.org/10.1051/forest:2006001.
Salifu K.F., Timmer V.R. (2001). Nutrient retranslocation response of Picea mariana seedlings to nitrogen supply. Soil Science Society of America Journal 65: 905–913. http://dx.doi.org/10.2136/sssaj2001.653905x.
Salifu K.F., Timmer V.R. (2003a). Optimizing nitrogen loading of Picea mariana seedlings during nursery culture. Canadian Journal of Forest Research 33: 1287–1294. http://dx.doi.org/10.1139/x03-057.
Salifu K.F., Timmer V.R. (2003b). Nitrogen retranslocation response of young Picea mariana to nitrogen-15 supply. Soil Science Society of America Journal 67: 309–317. http://dx.doi.org/10.2136/sssaj2003.3090.
Salifu K.F., Apostol1 K.G., Jacobs D.F., Islam M.A. (2008). Growth, physiology, and nutrient retranslocation in nitrogen-15 fertilized Quercus rubra seedlings. Annals of Forest Science 65(1). 101 p. http://dx.doi.org/10.1051/forest:2007073.
South D.B., Donald D.G.M. (2002). Effect of nursery conditioning treatments and fall fertilization on survival and early growth of Pinus taeda seedlings in Alabama, U.S.A. Canadian Journal of Forest Research 32: 1171–1179. http://dx.doi.org/10.1139/x02-039.
Staples T.E., Van Rees K.C.J., Van Kessel C. (1999). Nitrogen competition using 15N between early successional plants and planted white spruce seedlings. Canadian Journal of Forest Research 29: 1282–1289. http://dx.doi.org/10.1139/cjfr-29-8-1282.
Thiffault N., Hébert F., Jobidon R. (2012). Planted Picea mariana growth and nutrition as influenced by silviculture x nursery interactions on an ericaceous-dominated site. Silva Fennica 46(5): 667–682. http://dx.doi.org/10.14214/sf.918.
Timmer V.R. (1996). Exponential nutrient loading: a new fertilization technique to improve seedling performance on competitive sites. New Forests 13: 275–295. http://dx.doi.org/10.1023/A:1006502830067.
Timmer V.R., Aidelbaum A.S. (1996). Manual for exponential nutrient loading of seedlings to improve outplanting performance on competitive forest sites. NODA/NFP Technical Report TR-25. 21 p. + appendix. ISBN 0-662-24208-4.
Timmer V.R., Armstrong G. (1987). Growth and nutrition of containerized Pinus resinosa at exponentially increasing nutrient additions. Canadian Journal of Forest Research 17: 644–647. http://dx.doi.org/10.1139/x87-105.
Uscola M., Villar-Salvador P., Gross P., Maillard P. (2015). Fast growth involves high dependence on stored resources in seedlings of Mediterranean evergreen trees. Annals of Botany 115: 1001–1013. http://dx.doi.org/10.1093/aob/mcv019.
Van den Driessche R. (1985). Late season fertilization, mineral nutrient reserves, and translocation in planted Douglas-fir (Pseudotsuga menziesii (Mirb.) Franco) seedlings. Forest Science 31: 485–496.
Villar-Salvador P., Puértolas J., Cuesta B., Peñuelas J.L., Uscola M., Heredia-Guerrero N., Rey Benayas J.M. (2012). Increase in size and nitrogen concentration enhances seedling survival in Mediterranean plantations. Insights from an ecophysiological conceptual model of plant survival. New Forests 43: 755–770. http://dx.doi.org/10.1007/s11056-012-9328-6.
Yang Z.L., Ma L.Y., Jia Z.K., Wang Z., Gong N.N. (2012). Annual growth dynamics of one-year-old Quercus variabilis seedlings. Journal of Northeast Forestry University 40(5): 9–12. [In Chinese].
Zhang W.H., Lu Z.J. (2002). A study on the biological and ecological property and geographical distribution of Quercus variabilis population. Acta Botanica Boreali Occidentalia Sinica 22(5): 1093–1101. [in Chinese].
Zhu Y., Dumroese R.K., Pinto J.R., Li G.L., Liu Y. (2013). Fall fertilization enhanced nitrogen storage and translocation in Larix olgensis seedlings. New Forests 44: 849–861. http://dx.doi.org/10.1007/s11056-013-9370-z.
Total of 55 references.

