Bacterial fertilizer and filtered sludge enhance soil fertility and plant growth in a eucalyptus plantation
Ren H., Chen X., Qin X., Zhang S., Lv C., Zhou J., Chen H. (2024). Bacterial fertilizer and filtered sludge enhance soil fertility and plant growth in a eucalyptus plantation. Silva Fennica vol. 58 no. 5 article id 24042. https://doi.org/10.14214/sf.24042
Highlights
- Filtered sludge significantly increased eucalyptus growth, particularly after six months of application
- Bacterial fertilizer significantly improved soil enzyme activities, specifically urease and sucrase
- Bacterial fertilizer influenced overall soil fertility, promoting sustainable eucalyptus cultivation practices
- The study indicates the potential of biofertilizers as environmentally friendly alternatives to chemical fertilizers.
Abstract
Plant growth-promoting rhizobacteria (PGPR) and filtered sludge are widely used to improve soil fertility and plant yields. In this study, we evaluated the impact of sludge and/or PGPR application on the nutrient contents and enzyme activities of the soil as well as on plant growth. We planted bare-root eucalyptus seedlings in (1) soil amended with filtered sludge from Nanning sugar factory (FS), (2) soil amended with filtered sludge + PGPR (BF), and (3) non-amended soil (control). Soil fertility and eucalyptus growth were determined after 3, 6, 9, and 12 months. Results demonstrated that FS treatment significantly increased eucalyptus growth compared to the control, particularly after six months. Bacterial fertilizer (BF) also increased soil urease and sucrase activities, although differences diminished over the study period. Our findings suggest that the integration of bacterial fertilizers and filtered sludge can serve as an effective and environmentally friendly strategy to improve soil health and promote sustainable eucalyptus cultivation. This research contributes to the growing body of evidence supporting the use of bio-fertilizers in forestry practices, highlighting their potential to reduce or replace the use of chemical fertilizers while increasing plant productivity.
Keywords
nutrient content;
Bacillus megaterium;
enzyme activity;
PGPR;
plant yield
-
Ren,
College of Life and Environmental Sciences, Wenzhou University, Wenzhou, Zhejiang, 325035 China; Forestry College, Guangxi University, Daxue E Rd, Xixiangtang District, Nanning, Guangxi, 530004 China
 https://orcid.org/0000-0002-0156-0726
E-mail
renhan1225@163.com
https://orcid.org/0000-0002-0156-0726
E-mail
renhan1225@163.com

- Chen, College of Life and Environmental Sciences, Wenzhou University, Wenzhou, Zhejiang, 325035 China E-mail 21211270104@stu.wzu.edu.cn
- Qin, Forestry College, Guangxi University, Daxue E Rd, Xixiangtang District, Nanning, Guangxi, 530004 China E-mail qinxiaohong186@163.com
- Zhang, College of Life and Environmental Sciences, Wenzhou University, Wenzhou, Zhejiang, 325035 China E-mail 00811091@wzu.edu.cn
- Lv, Forestry College, Guangxi University, Daxue E Rd, Xixiangtang District, Nanning, Guangxi, 530004 China E-mail lvchengqun8@163.com
- Zhou, College of Life and Environmental Sciences, Wenzhou University, Wenzhou, Zhejiang, 325035 China E-mail rosechl@wzu.edu.cn
-
Chen,
College of Life and Environmental Sciences, Wenzhou University, Wenzhou, Zhejiang, 325035 China
 https://orcid.org/0000-0002-3043-6919
E-mail
hualin2100@wzu.edu.cn
https://orcid.org/0000-0002-3043-6919
E-mail
hualin2100@wzu.edu.cn

Received 2 July 2024 Accepted 16 October 2024 Published 22 October 2024
Views 39002
Available at https://doi.org/10.14214/sf.24042 | Download PDF

Supplementary Files
1 Introduction
Increasing awareness of the negative impact that artificial fertilizers have on the environment has prompted a global effort to develop more environmentally friendly biofertilizers from N-fixing bacteria and organic matter to replace or reduce the use of artificial fertilizer in forestry land (Ammar et al. 2023). Bio-fertilizers are defined as substances that contain living microorganisms, and can potentially promote plant growth and increase soil fertility (Arif et al. 2020). The main benefits associated with the application of biofertilizer include: an increase in soil nutrient availability due to the direct contribution of microbial exudation; an increase in soil nitrogen contributed by enhanced activity of N-fixing microorganisms (Tamreihao et al. 2018); increase in mineralization and solubilization of soil organic compounds into the forms that are more readily absorbed by the plants (Daniel et al. 2022); stimulation of synthesis of phytohormones related to plant growth (gibberellins, auxins, ethylene) (Orozco-Mosqueda et al. 2023) as well as the potential activation of disease resistant genes in plants promoted by the microorganisms in the bio-fertilizer (Zhu et al. 2022). However, compared to bio-fertilizers, inorganic fertilizers are more widely used in traditional forestry, especially for afforestation of fast-growing tree species. Currently, eucalyptus plantations rely on the massive application of inorganic fertilizer because soil nutrient availability regulated by microbial activity might decrease after continuous use of inorganic fertilizers (Wang et al. 2023b), although the removal of the logging residues might be another reason for decreased nutrient availability (Zhu and Wu 2023). Excessive use of inorganic fertilizers can cause soil compaction, acidification, and salinization (Wang et al. 2022). One approach to resolving this problem is to use biofertilizers instead of artificial or inorganic fertilizers.
Plant growth-promoting rhizobacteria (PGPR) has been widely used as bio-fertilizers and they have been shown to increase not only soil fertility and nutrient uptake by the plants (Ren et al. 2022) but also the diversity of the soil microbial community (Ren et al. 2020a). Some selected species of PGPR, such as Bacillus megaterium de Bary, 1884 (e.g., B. megaterium var. phosphatic), Bacillus subtilis (Ehrenberg, 1835) Cohn, 1872, and Bacillus licheniformis (Weigmann, 1898) Chester, 1901, indirectly protect the plant from diseases and pests by competing with plant pathogens for nutrients (Miljaković et al. 2020) and by directly carrying out biological N-fixation in the terrestrial ecosystem (Yousuf et al. 2017). Filter sludge (FS) from sugar factories is a byproduct of sugar production that is rich in organic matter and nutrients such as nitrogen (N), phosphorus (P), and potassium (K), which has the potential to improve soil fertility and plant growth (Wang et al. 2024). FS can be used to improve soil nutrient conditions after aerobic fermentation, thereby enhancing plant growth. Additionally, FS can also serve as an essential carrier for PGPR to produce bio-fertilizers due to its rich organic matter content (Zhang et al. 2014). A remarkable point in the use of bio-fertilizers is the potential synergistic beneficial effect of applying both PGPR and FS. Interest in the application of a mixture of PGPR and organic matter as a bacterial fertilizer is growing in the agriculture and forestry sectors.
Soil enzyme activity and nutrients serve as indicators of fertility status, primarily due to their important role in soil biogeochemical processes. Soil urease is an enzyme primarily produced by bacteria and fungi, and plays a critical role in the nitrogen cycle by converting urea into ammonia, water, and carbon dioxide (Fu et al. 2020). This conversion is essential for making nitrogen available to plants, which is vital for their growth and overall nutrition (Rechenmacher et al. 2017). Sucrase is not only an important participant in the soil carbon cycle but also one of the key factors in enhancing soil fertility and reflecting soil health status, and further promoting plant growth and development (Wang et al. 2020). The activity of catalase is closely related to the organic matter content, microbial population, and soil respiration intensity (Wang et al. 2021). Among the most valuable soil indicators used to assess soil fertility are soil nutrients (especially N, P, and K, etc.) and enzyme activities (including oxidoreductases, transferase, hydrolase, etc.), as they are essential for soil fertility, nutrient turnover, vegetation productivity, and soil microbial activity (Wang et al. 2023a).
Microorganism-based fertilizers have been applied to soils where agriculturally important crops are being planted, such as wheat (Khatri et al. 2020), paddy rice (AW et al. 2020), lettuce (Venancio et al. 2019), and sugarcane (Santos et al. 2018). However, few studies have examined the effects of the combined application of sludge and PGPR as a standalone fertilizer on the soil and forest ecosystem, and even less on the underlying mechanisms by which such bio-fertilizers affect the soil fertility in forest plantations. In this study, we determined the effects of filtered sludge and PGPR application on soil fertility and plant growth in a eucalyptus (Eucalyptus obliqua L’Hér.) plantation and identified the potential relationship between soil fertility and eucalyptus yield. The objective of our study was to evaluate the applications of bacterial fertilizer (BF) and FS on increasing soil fertility and plant growth of eucalyptus plantations, thereby providing an environmentally friendly alternative to conventional fertilizers in forestry practices. We hypothesized that (1) soil nutrient content, soil enzyme activity, and plant growth would increase with the application of bio-fertilizers and (2) soil N contents would significantly influence plant growth and yield.
2 Materials and methods
2.1 Experimental site
This study was conducted in the Liangfengjiang National Park in Nanning, Guangxi, China (107°45’–108°51’ E, 22°13’–23°32’ N). The site recorded an average annual precipitation of 1304 mm and a mean annual humidity of 79% from 2005–2015. The mean annual air temperature ranges from 21.1 ℃ to 22.5 ℃, with a maximum of 40.4 ℃ in the summer and a minimum of –2.4 ℃ in the winter. The soil is classified as Eutric Fluvisols (Shi et al. 2010), with an organic matter content of 2.5 g kg–1, a concentration of total N concentration of 0.79 g kg–1, total P concentration of 0.33 g kg–1, total K concentration of 0.81 g kg–1.
2.2 Filtered sludge and PGPR characterization
Filtered sludge (FS) was selected as the focal raw material for the fertilizer used in this study because Guangxi is a major producer of sugarcane, and FS (mainly sugar factory filter sludge) has been a major by-product from the production of sugarcane in the past 10 years. The nutrient content of FS is shown in Supplementary file S2. Eucalyptus DH32-29 (a clone of the hybrid Eucalyptus urophylla S.T. Blake × E. grandis W. Mill ex Maiden) was selected as the focal crop for our study because it has been the most prominent pulp and wood material in Guangxi since the 1970s (Zhang et al. 2023a).
Filtered sludge was supplied by Nanning Sugar Factory (Guangxi, China) and it consisted of the following components: 1.50 mg total N g–1, 0.27 mg total K g–1, 0.33 mg total P g–1, and pH 6.10 (Ren et al. 2020b). FS was transferred from the factory directly to the study site in 55 cm × 85 cm plastic bags.
Bacillus megaterium strain DU07 used in this study was first isolated from the eucalyptus rhizosphere in solid lysogeny broth (LB) in 2011 by the microbiological laboratory of the Forestry College at the Guangxi University (China). The culture was stored in an Ultra-low Temperature Freezer at –80 ℃. A frozen stock of B. megaterium strain DU07 was cultured in liquid LB at 28°C with shaking at 120 r min–1 for 6 days and then diluted to 9 × 109 CFU g–1 with sterile water.
2.3 Experimental setup and treatments
To determine the effects of the filtered sludge and PGPR on the soil fertility and plant growth we applied two different treatments on the same day the eucalyptus seedlings were planted: (1) 62.5 t ha–1 filtered sludge from sugarcane (FS); and (2) 62.5 t ha–1 FS + inoculation of 2 ml per seedling of 3 × 109 CFU ml–1 PGPR (BF). A control was also included in which the soil was not subjected to any treatment. The study site was divided into three 16 m × 16 m blocks: one block was kept as the control while the other two were each treated with FS or BF. We planted 81 seedlings within 16 m × 16 m blocks, spaced 2 m apart (Suppl. file S1).
E. urophylla × grandis DH32-29 seedlings were supplied as bare-root seedlings from Dongmen Tree Farm in Nanning, China. The original average height of the seedlings (N = 10) was approximately 25 cm, which was measured using a measuring tape before planting. The roots of the seedlings were trimmed, and 243 seedlings were then planted in June 2011.
2.4 Field sampling and lab measurements
Soil samples (3 replicates) were collected from each block in September 2011 (M3), December 2011 (M6), March 2012 (M9), and June 2012 (M12). We demarcated three 4 m × 4 m quadrats in each block, and the focal eucalyptus tree was located in the middle of each quadrat. We collected four soil subsamples (0–20 cm) tightly adhering to the roots of each focal eucalyptus tree using a soil corer and then mixed these soil subsamples to a composite sample following the quartering method for soil sampling. Then one composite rhizosphere soil was collected in each quadrat around the focal eucalyptus. All soil samples were then air-dried and sieved through a 100-mesh after grinding.
The levels of soil total nitrogen (TN) were analyzed via a flow injection auto-analyzer (Technicon, AA3, Hamburg, Germany). Soil available nitrogen (AN) was measured in a C/N analyzer (Vario, Max, CN). The levels of available potassium (AK) and available phosphorus (AP) were determined with a flow injection auto-analyzer (Technicon, AA3, Hamburg, Germany) following the method of (Qi et al. 2018). Soil catalase (CAT), urease, and sucrase activities were determined by a colorimetric method (Lei et al. 2023). The methods for measuring soil enzyme activities are shown in Suppl. file S4.
To determine the effects of the soil treatments on the eucalyptus plants, nine (including the focal eucalyptus and eight eucalyptus around) plants were selected from each quadrat from which the soil samples were taken. In total of 27 eucalyptus were selected for plant measurements in each treatment and the control. The plant heights and stem diameters were then measured with a measuring tape and Vernier caliper, respectively. The measurements were carried out 3 (M3), 6 (M6), 9 (M9), and 12 (M12) months after planting, and plant stem volumes were then calculated according to formula shown in Suppl. file S5 (Lie and Xue 2019).
2.5 Statistical analysis
The effects of treatments on soil fertility (soil nutrients and soil extracellular enzyme activities) and plant growth (stem diameter, height, and stem volume) were evaluated by the Kruskal-Wallis test performed in R 3.4.2 (R Core Team 2018). Where the main effects were significant (p < 0.05), the Mann-Whitney test was used to determine differences between pairs. Mean CAT, urease, and sucrase values by plot were calculated in logarithmic and back-transformed by using exponential.
The panel model is constructed using fixed effects regression, which aims to eliminate the influence of the time dimension and find out the main impact factor in the section dimension. The panel model examines the influence of independent variables (CAT, Urease, Sucrase, TN, AK, AP, AN) on dependent variables (Volume) using panel data. Based on the results of the F-test, Breusch-Pagan test, and Hausman test, it was recommended to select the fixed effects (FE) model for our analysis (Suppl. file S3). The fixed effect model was performed in R 3.4.2 (R Core Team, 2017) using the plm package (Zeileis and Croissant 2010).
3 Results
3.1 Plant growth
There was no significant effect of FS and BF on plant height and timber volume three months after planting (Table 1). Plant diameter was significantly decreased by FS application (0.73 cm) relative to the control (0.79 cm). Eucalyptus seedlings planted in FS- or BF-treated soil exhibited significant increases in stem diameter and volume compared with those planted in control soil after six months. The average stem diameters determined for the trees planted in FS-treated, BF-treated, and control soils were 2.57 cm, 2.78 cm, and 2.22 cm, respectively, while the average stem volumes of the trees 0.041, 0.045, and 0.036 m3 tree–1 (Table 1). Eucalyptus seedlings planted in BF-treated soil also displayed greater height and were also taller (2.10 m) than those planted in the control block (1.85 m) whereas no significant difference in height was observed between those planted in FS-treated soil and control soil.
| Table 1. Means and standard errors of eucalyptus growth measured at four different time points in Guangxi, China; Different lowercase letters show the statistically significant differences at ɑ = 0.05 level among the three different soils (BF-treated, FS-treated, and control soils). Significances are indicated in bold for p < 0.05. * = significant different. | |||
| Treatments | Diameter (cm) | Height (m) | Stem volume (m3 tree–1) |
| M3 | |||
| BF | 0.83 ± 0.021 a | 0.56 ± 0.035 | 0.010 ± 0.00045 |
| FS | 0.73 ± 0.015 b | 0.55 ± 0.025 | 0.0092 ± 0.00032 |
| Control | 0.79 ± 0.035 a | 0.60 ± 0.023 | 0.010 ± 0.00049 |
| p | 0.047* | 0.17 | 0.061 |
| Cohen’s f | 0.6 | 0.44 | 0.51 |
| M6 | |||
| BF | 2.78 ± 0.062 a | 2.10 ± 0.061 a | 0.045 ± 0.0013 a |
| FS | 2.57 ± 0.20 a | 1.97 ± 0.081 b | 0.041 ± 0.0015 b |
| Control | 2.22 ± 0.067 b | 1.85 ± 0.029 b | 0.036 ± 0.0010 c |
| p | 0.039* | 0.037* | 0.027* |
| Cohen’s f | 0.25 | 0.61 | 0.67 |
| M9 | |||
| BF | 4.99 ± 0.04 a | 6.70 ± 0.046 a | 0.12 ± 0.0011 a |
| FS | 4.31 ± 0.11 b | 6.33 ± 0.17 b | 0.11 ± 0.0033 b |
| Control | 3.52 ± 0.17 c | 5.23 ± 0.20 b | 0.083 ± 0.0038 b |
| p | 0.027* | 0.027* | 0.02* |
| Cohen’s f | 0.69 | 0.69 | 0.7 |
| M12 | |||
| BF | 7 ± 0.21 a | 10.32 ± 0.39 a | 0.19 ± 0.0073 a |
| FS | 6.09 ± 0.10 b | 9.27 ± 0.15 ab | 0.16 ± 0.0012 ab |
| Control | 5.04 ± 0.18 b | 6.68 ± 0.22 b | 0.12 ± 0.0046 b |
| p | 0.027* | 0.03* | 0.027* |
| Cohen’s f | 0.69 | 0.7 | 0.69 |
After nine and twelve months, all measured variables of the plants (plant diameter, height, and stem volume) showed significantly higher values for the seedlings planted in BF-treated soils than for the seedlings planted in the control soil (Table 1). It is important to note that the plant growth was significantly improved following BF treatment since M6 is relative to the control, indicating that bacterial fertilizer may make a great contribution to plant nutrient supply.
3.2 Soil enzyme activities
No significant effects of FS and BF were detected on soil enzyme activity (catalase, sucrase, and urease) at nine, and 12 months after treatment (Fig. 1). However, three months after treatment, soil sucrase activity in the BF-treated soil was significantly increased (10.96 mg g–1 (24h)–1) relative to FS-treated (7.54 mg g–1 (24h)–1) and control (8.29 mg g–1 (24h)–1) soils, but neither FS nor BF had a significant effect on soil CAT and urease activities. After six months, soil urease activity was significantly (p < 0.01) increased in the BF-treated soil (11.71 mg g–1 (24h)–1) when compared with FS-treated (8.11 mg g–1 (24h)–1) and the control soil (7.16 mg g–1 (24h)–1). However, there was no significant difference in soil CAT and sucrase activities among the three soils.
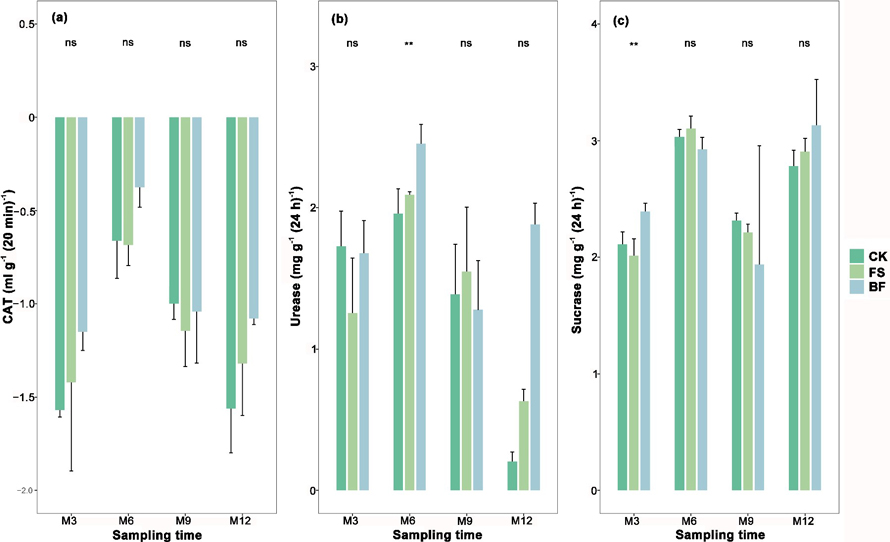
Fig. 1. Means and standard errors of soil enzyme activity variables (a, Catalase; b, Urease; c, Sucrase) three, six, nine, and twelve months after planting in soil treated with filtered sludge (FS) and bacterial fertilizer composed of filtered sludge + plant growth-promoting rhizobacteria (BF). ** indicate statistically significant differences among treatments and CK at α = 0.01, “ns” indicates “no significance”. View larger in new window/tab.
3.3 Soil nutrient contents
There was no significant effect of either BF or FS application on the levels of available P (AP) and available N (AN) in the soil, whereas the effects on the levels of total N (TN) and available K (AK) varied over time, and were more obvious 12 months after treatment (Fig. 2).
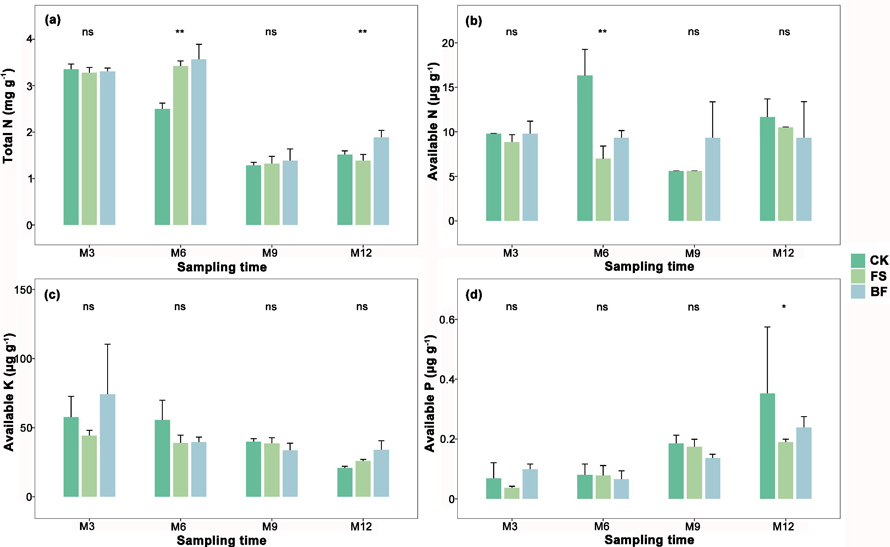
Fig. 2. Means and standard errors of soil nutrients (a, total N (TN); b, available K (AK); c, available N (AN); d, available P (AP)) three, six, nine, and twelve months after treatments with filtered sludge (FS) and bacterial fertilizer composed of filtered sludge + plant growth-promoting rhizobacteria (BF). *, ** indicate statistically significant differences among treatments and CK at α = 0.05, α = 0.01, and “ns” indicates “no significance”. View larger in new window/tab.
In terms of soil TN and AK, no significant effect was observed for either the M3 or M9 soil samples following the application of FS and FB when compared with the control soil samples taken at the same time (Fig. 2). After six months, TN was significantly higher in the FS-treated soil (3.43 mg g–1) and BF-treated soil (3.57 mg g–1) than in the control soil (2.50 mg g–1), whereas soil AN was significantly decreased in both BF- (9.33 μg g–1) and FS-treated soils (7.0 μg g–1). FS and BF had no significant effect on soil nutrients nine months after application.
Twelve months after application, a significant increase in soil TN (1.89 mg g–1) was detected in the BF soil compared with that in the control soil (1.52 mg g–1), but the TN level in the FS soil (1.39 mg g–1) was not significantly different from that in the control soil (Fig. 2). Moreover, the AK level in the BF soil also increased compared to that in the control soil (34 μg g–1 versus 21 μg g–1), whereas the AK level in the FS soil (26 μg g–1) was slightly higher than that in the control soil, although the difference was not statistically significant. This indicated that the co-application of FS and PGPR probably contributed to better soil fertility over a longer period, resulting in a higher level of nutrients in the soil than the application of FS alone.
3.4 Correlation between plant yield and soil nutrient contents
The specific factors affecting eucalyptus yield were determined using a fixed effects model on plant yield and soil fertility during the trial period (Table 2). According to the fixed effects model for panel data (F = 35.26, p < 0.001, R2overall = 0.81), sucrase (estimate = 0.003, p < 0.001) had a positive and significant effect on eucalyptus yield, while CAT (estimate = −0.1, p = 0.02), AK (estimate = −0.001, p = 0.025), TN (estimate = −0.044, p < 0.001) had a negative effect on eucalyptus yield (Table 2).
| Table 2. Coefficients of fixed effects model for panel data to determine the relationship between eucalyptus stem volume and soil fertility in our study. Significances were bold when p < 0.05. * = significant different; *** = extreme significant different. | |||
| Soil quality parameter | Estimate | Std. error | Pr (> |t|) |
| (Intercept) | 0.18 | 0.024 | |
| CAT | –0.1 | 0.041 | 0.02* |
| Urease | 0.002 | 0.002 | 0.29 |
| Sucrase | 0.003 | 0.001 | 0.000*** |
| Total N | –0.044 | 0.006 | 0.000*** |
| Avail K | –0.001 | 0 | 0.025* |
| Avail N | –0.001 | 0.001 | 0.53 |
| Avail P | 0.032 | 0.051 | 0.53 |
| F = 35.26, p = 0.000***, R2within = 0.91, R2between = 0.77, R2overall = 0.81 | |||
In general, the activity of the sucrase enzyme could significantly and positively influence the stem volume of eucalyptus in our study.
4 Discussion
4.1 Effect of FS and BF on plant growth
This study investigated the effects of BF and FS on soil nutrient contents and enzyme activities important for soil fertility in a eucalyptus plantation. In our study, plant diameter was significantly decreased by FS three months after planting, possibly because the main ingredient in FS is calcium carbonate, which could immediately lead to changes in the plant habitat (including soil water retention capacity and soil pH), resulting in growth suppression of the eucalyptus seedlings. BF significantly increased plant growth, including plant height and stem diameter, from six months onward, which is in line with the finding that PGPR can increase the growth of maize plants after one month of planting in PGPR-applied soil (González-Díaz et al. 2019). BF exerted a stronger effect on plant growth than FS in the long term (> 6 months) because the co-application of rhizobacteria and organic matter may supply sufficient organic matter to increase the level of bacterial activity (Amelung et al. 2001; Xu et al. 2021). This would benefit both plant growth and yield through soil organic matter mineralization. PGPR application is known to influence soil TN via its effects on biological N fixation in the soil, further influencing plant-soil interactions (Timofeeva et al. 2023).
4.2 Effect of FS and BF on soil nutrient content
When the filtered sludge was abundantly applied to the field at the test site, the TN in the soil significantly increased as a result of the improvement in the soil environment, which was beneficial for the metabolism and growth of N-fixation-related soil microbes (Chen et al. 2013). However, the significant decrease in the available N in the soil in the short term may be a result of the competition between the plants and microbes for N because of the unbalanced C:N ratio following the application of filtered sludge (Mao et al. 2024).
Soil nitrobacteria take part in the fixation of N in the soil, and the optimum pH for this bacterial growth has been shown to be 7.5–8.2 (slight alkalinity) (Norton and Ouyang 2019). Remarkably, the filtered sludge used in our experiment has previously been found to have a pH of about 6.10 (Ren et al. 2020b), and other studies have verified that the accumulation of PGPR may also contribute to soil acidification (Qin et al. 2024). Taken together, our results suggest that the applied filtered sludge interacted with the soil environment and decreased the available N in the soil due to increases in denitrification because of the changes in pH (Pan et al. 2023). However, the mechanism of this influence has not been identified and is, therefore, a subject for further study.
The bacterial strain DU07 used in this study belonged to the phylum Firmicutes (Ren et al. 2020b). A positive correlation between soil TN and the abundance of soil Firmicutes has been demonstrated in the Loess Plateau soil of China (Liu et al. 2020), which seems to support our result that increasing the amount of applied PGPR would contribute to improved soil TN. In addition, PGPR may stimulate the synthesis of secondary metabolites, which could increase the accumulation of potassium compounds in the roots of eucalyptus plants (Salla et al. 2014). Our previous study also demonstrated that the application of PGPR to the soil can cause an increase in soil K content compared with the control soil (Ren et al. 2020b), which is consistent with the phenomenon observed in this study.
4.3 Effect of FS and BF on soil enzyme activity
The trends toward increased sucrase and urease activities suggest that increased hydrolase activity may also contribute to the changes in soil N following PGPR treatment, although the effect of BF on soil enzyme activity was only statistically significant after three and six months (M3 and M6 soil samples). Soil sucrase is the main hydrolase that participates in the decomposition of soil organic matter (including cellulose, hemicellulose, and lignin), converting it into mineralized nitrogen through mineralization, a process that may help explain the changes in sucrase activity that we observed (Fu et al. 2018). The positive effect of PGPR on the C conversion status, respiration intensity, and bacterial abundance in the soil has been demonstrated by the application of PGPR to artificial soil (Vuolo et al. 2022), which is in line with our finding that soil sucrase activity was increased upon the application of the bacterial fertilizer.
Soil urease activity can potentially reflect the supply of organic nitrogen and the transformation of nutrients into the soil (Han et al. 2017). The activity of PGPR in the soil also promotes the proliferation and metabolism of soil microbial communities, thereby increasing the sources of soil enzymes (Pérez-Montaño et al. 2014). Taken together, our results suggest that PGPR interacts with the soil environment to increase the levels of enzyme activity in the soil, although the mechanism was not identified. Additional measurements on soil pH, bulk density, and additional micronutrients in a future study would help elucidate the long-term influence of PGPR on soil enzyme activity.
4.4 Effect of FS and BF on soil fertility
A positive effect of BF treatment on soil fertility was observed, demonstrating that the application of bacterial fertilizer was beneficial to soil fertility. Remarkably, BF mainly improved soil fertility, as a result of nutrient accumulation and increased enzyme activities in the soil. Other authors have reported the significant effects of soil microbial activity on the cycling of soil nutrients during plant–soil transformation (Przybylska et al. 2024).
In general, a change in plant yield in response to soil urease and sucrase activities demonstrates that the selected soil parameter might serve as a sensitive indicator of the plant’s response to PGPR (Zhang et al. 2023b). PGPR has been shown in other studies to promote eucalyptus growth mainly via the accumulation of N in the roots, stem, and foliage (Lan et al. 2023). Soil TN was found to be negatively correlated with plant yield, probably because of the decomposition and mineralization of filtered sludge following the application of FS and BF to the soil, especially with the accumulation of N in the case of the BF-treated soil. Furthermore, soil TN negatively and significantly influenced plant yield in the experiment. In most cases, soil TN has been found to positively correlate with plant volume in most specified species (Matkala et al. 2020), however, the cycling and accumulation of N in the plant and soil systems may not be the key factor affecting plant growth when low phosphorus is the restriction factor (Herbert et al. 2003).
5 Conclusions
This study provides compelling evidence that the application of bacterial fertilizer, in conjunction with filtered sludge, significantly enhances soil fertility and promotes the growth of eucalyptus in a one-year eucalyptus plantation. The findings demonstrate that the combined use of Plant growth-promoting rhizobacteria (PGPR) and filtered sludge not only improves soil nutrient availability but also stimulates critical enzyme activities that are essential for maintaining soil fertility and promoting plant health. Our results also indicate that eucalyptus seedlings exhibited significant improvements in growth parameters in BF treatment. Also, the beneficial effects of PGPR are most significant after six months of application, suggesting a time-dependent response in plant growth linked to enhanced soil conditions. The observed increase in soil urease and sucrase activities further supports the hypothesis that PGPR application can facilitate nutrient cycling and improve the overall biological health of the soil. The implications of this research extend beyond the immediate benefits observed in eucalyptus plantations. The results advocate for the integration of environmentally friendly bio-fertilizers into forestry management practices, particularly in regions where the reliance on chemical fertilizers has led to soil degradation and reduced fertility. This study not only reinforces the potential of bacterial fertilizers as a viable alternative to conventional fertilizers but also paves the way for future research aimed at optimizing bio-fertilizer formulations and application strategies. Continued exploration of the synergistic effects of microbial and organic amendments will be essential for advancing sustainable forestry practices and ensuring the resilience of forest ecosystems in the face of environmental challenges.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Funding
This research was conducted with financial support from the National Natural Science Foundation of China (32201326).
Acknowledgments
We thank Hui Zhang and Zhiwei Peng for their assistance with data collection and manuscript preparation. We thank Caili Zhu and Yanhong Huang for their assistance with sample collection. We thank Dr. Baoling Huang and Dr. Jessica R. Miesel for their guidance and mentoring of Han Ren. Finally, we highly appreciate Dr Alan K Chang (Wenzhou University) for his dedicated work in editing the entire manuscript.
Data availability statement
The data presented in this study are available at the Zenodo repository: https://doi.org/10.5281/zenodo.13352574.
References
Amelung W, Miltner A, Zhang X, Zech W (2001) Fate of microbial residues during litter decomposition as affected by minerals. Soil Sci 166: 598–606. https://doi.org/10.1097/00010694-200109000-00003.
Ammar EE, Rady HA, Khattab AM, Amer MH, Mohamed SA, Elodamy NI, AL-Farga A, Aioub AAA (2023) A comprehensive overview of eco-friendly bio-fertilizers extracted from living organisms. Environ Sci Pollut Res 30: 113119–113137. https://doi.org/10.1007/s11356-023-30260-x.
Arif I, Batool M, Schenk PM (2020) Plant microbiome engineering: expected benefits for improved crop growth and resilience. Trends Biotechnol 38: 1385–1396. https://doi.org/10.1016/j.tibtech.2020.04.015.
AW X, Z L, WC L, ZH Y (2020) The effect of plant growth-promoting rhizobacteria (PGPR) on arsenic accumulation and the growth of rice plants (Oryza sativa L.). Chemosphere 242, article id 125136. https://doi.org/10.1016/j.chemosphere.2019.125136.
Cela F, Avio L, Giordani T, Vangelisti A, Cavallini A, Turrini A, Sbrana C, Pardossi A, Incrocci L (2022) Arbuscular mycorrhizal fungi increase nutritional quality of soilless grown lettuce while overcoming low phosphorus supply. Foods 11, article id 3612. https://doi.org/10.3390/foods11223612.
Chen F, Zheng H, Zhang K, Ouyang Z, Wu Y, Shi Q, Li H (2013) Non-linear impacts of Eucalyptus plantation stand age on soil microbial metabolic diversity. J Soils Sediments 13: 887–894. https://doi.org/10.1007/s11368-013-0669-3.
Daniel AI, Fadaka AO, Gokul A, Bakare OO, Aina O, Fisher S, Burt AF, Mavumengwana V, Keyster M, Klein A (2022) Biofertilizer: the future of food security and food safety. Microorganisms 10, article id 1220. https://doi.org/10.3390/microorganisms10061220.
Fu D, Yang H, Wang L, Yang S, Li R, Zhang W, Ai X, Ai Y (2018) Vegetation and soil nutrient restoration of cut slopes using outside soil spray seeding in the plateau region of southwestern China. J Environ Manage 228: 47–54. https://doi.org/10.1016/j.jenvman.2018.08.108.
Gómez-Godínez LJ, Fernandez-Valverde SL, Martinez Romero JC, Martínez-Romero E (2019) Metatranscriptomics and nitrogen fixation from the rhizoplane of maize plantlets inoculated with a group of PGPRs. Syst Appl Microbiol 42: 517–525. https://doi.org/10.1016/j.syapm.2019.05.003.
Gouda S, Kerry RG, Das G, Paramithiotis S, Shin HS, Patra JK (2018) Revitalization of plant growth promoting rhizobacteria for sustainable development in agriculture. Microbiol Res 206: 131–140. https://doi.org/10.1016/j.micres.2017.08.016.
Han L, Su D, Lv S, Luo Y, Li X, Jiao J, Diao Z, Bu H (2017) Responses of biogeochemical characteristics and enzyme activities in sediment to climatewarming under a simulation experiment in geographically isolated wetlands of the Hulunbuir Grassland, China. Int J Environ Res Public Health 14: 1–17. https://doi.org/10.3390/ijerph14090968.
Herbert DA, Williams M, Rastetter EB (2003) A model analysis of N and P limitation on carbon accumulation in Amazonian secondary forest after alternate land-use abandonment. Biogeochemistry 65: 121–150. https://doi.org/10.1023/A:1026020210887.
Huo C, Mao J, Zhang J, Yang X, Gao S, Li J, He Q, Tang G, Xie X, Chen Z (2024) Fertilization- and irrigation-modified bacterial community composition and stimulated enzyme activity of Eucalyptus plantations soil. Int J Mol Sci 25, article id 1385. https://doi.org/10.3390/ijms25031385.
Jan MT, Roberts P, Tonheim SK, Jones DL (2009) Protein breakdown represents a major bottleneck in nitrogen cycling in grassland soils. Soil Biol Biochem 41: 2272–2282. https://doi.org/10.1016/j.soilbio.2009.08.013.
Khatri S, Sharma RK, Shridhar V (2020) Influence of cadmium-tolerant and plant growth-promoting rhizobacteria on cadmium accumulation and growth response of wheat seedlings under mountain ecosystem. Agric Res 9: 56–65. https://doi.org/10.1007/s40003-019-00407-9.
Lan Y, Liao L, Yao X, Ye S (2023) Synergistic effects of nitrogen and plant growth-promoting rhizobacteria inoculation on the growth, physiological traits and nutrient absorption of intercropped Eucalyptus urophylla × Eucalyptus grandis and Dalbergia odorifera. Trees – Struct Funct 37: 319–330. https://doi.org/10.1007/s00468-022-02350-9.
Lei J, Cao Y, Wang J, Chen Y, Peng Y, Shao Q, Dan Q, Xu Y, Chen X, Dang P, Yan W (2023) Soil nutrients, enzyme activities, and microbial communities along a chronosequence of Chinese fir plantations in subtropical China. Plants 12, article id 1931. https://doi.org/10.3390/plants12101931.
Li S, Liu Y, Wang J, Yang L, Zhang S, Xu C, Ding W (2017) Soil acidification aggravates the occurrence of bacterial wilt in south China. Front Microbiol 8, article id 703. https://doi.org/10.3389/fmicb.2017.00703.
Lie Z, Xue L (2019) Density effect and self-thinning in Eucalyptus urophylla stands. J For Res 30: 529–535. https://doi.org/10.1007/s11676-018-0685-7.
Liu Y, Zhu G, Hai X, Li J, Shangguan Z, Peng C, Deng L (2020) Long-term forest succession improves plant diversity and soil quality but not significantly increase soil microbial diversity: evidence from the Loess Plateau. Ecol Eng 142, article id 105631. https://doi.org/10.1016/j.ecoleng.2019.105631.
Matkala L, Salemaa M, Bäck J (2020) Soil total phosphorus and nitrogen explain vegetation community composition in a northern forest ecosystem near a phosphate massif. Biogeosciences 17: 1535–1556. https://doi.org/10.5194/bg-17-1535-2020.
Miljaković D, Marinković J, Balešević-Tubić S (2020) The significance of Bacillus spp. in disease suppression and growth promotion of field and vegetable crops. Microorganisms 8, article id 1037. https://doi.org/10.3390/microorganisms8071037.
Mukherjee A, Gaurav AK, Singh S, Yadav S, Bhowmick S, Abeysinghe S, Verma JP (2022) The bioactive potential of phytohormones: a review. Biotechnol Rep 35, article id e00748. https://doi.org/10.1016/j.btre.2022.e00748.
Nguyen TTN, Xu CY, Tahmasbian I, Che R, Xu Z, Zhou X, Wallace HM, Bai SH (2017) Effects of biochar on soil available inorganic nitrogen: a review and meta-analysis. Geoderma 288: 79–96. https://doi.org/10.1016/j.geoderma.2016.11.004.
Pan Y, She D, Shi Z, Cao T, Xia Y, Shan J (2023) Salinity and high pH reduce denitrification rates by inhibiting denitrifying gene abundance in a saline-alkali soil. Sci Rep 13, article id 2155. https://doi.org/10.1038/s41598-023-29311-7.
Pérez-Montaño F, Alías-Villegas C, Bellogín RA, Del Cerro P, Espuny MR, Jiménez-Guerrero I, López-Baena FJ, Ollero FJ, Cubo T (2014) Plant growth promotion in cereal and leguminous agricultural important plants: from microorganism capacities to crop production. Microbiol Res 169: 325–336. https://doi.org/10.1016/j.micres.2013.09.011.
Qi Y, Chen T, Pu J, Yang F, Shukla MK, Chang Q (2018) Response of soil physical, chemical and microbial biomass properties to land use changes in fixed desertified land. Catena 160: 339–344. https://doi.org/10.1016/j.catena.2017.10.007.
R Core Team (2018) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria.
Rahman MA, Lee SH, Ji HC, Kabir AH, Jones CS, Lee KW (2018) Importance of mineral nutrition for mitigating aluminum toxicity in plants on acidic soils: current status and opportunities. Int J Mol Sci 19, article id 3073. https://doi.org/10.3390/ijms19103073.
Rechenmacher C, Wiebke-Strohm B, De Oliveira-Busatto LA, Polacco JC, Carlini CR, Bodanese-Zanettini MH (2017) Effect of soybean ureases on seed germination and plant development. Genet Mol Biol 40: 209–216. https://doi.org/10.1590/1678-4685-gmb-2016-0107.
Ren H, Huang B, Fernández-garcía V, Miesel J, Yan L (2020a) Biochar and rhizobacteria amendments improve several soil properties. Microorganisms 8, article id 502. https://doi.org/10.3390/microorganisms8040502.
Ren H, Qin X, Huang B, Fernández-García V, Lv C (2020b) Responses of soil enzyme activities and plant growth in a eucalyptus seedling plantation amended with bacterial fertilizers. Arch Microbiol 202: 1381–1396. https://doi.org/10.1007/s00203-020-01849-4.
Ren H, Li Z, Chen H, Zhou J, Lv C (2022) Effects of biochar and plant growth-promoting rhizobacteria on plant performance and soil environmental stability. Sustainability 14, article id 10922. https://doi.org/10.3390/su141710922.
Salla TD, Ramos da Silva T, Astarita LV, Santarém ER (2014) Streptomyces rhizobacteria modulate the secondary metabolism of Eucalyptus plants. Plant Physiol Biochem 85: 14–20. https://doi.org/10.1016/j.plaphy.2014.10.008.
Samain E, Duclercq J, Ait Barka E, Eickermann M, Ernenwein C, Mazoyon C, Sarazin V, Dubois F, Aussenac T, Selim S (2023) PGPR-soil microbial communities’ interactions and their influence on wheat growth promotion and resistance induction against Mycosphaerella graminicola. Biology 12, article id 1416. https://doi.org/10.3390/biology12111416.
Santos RM, Kandasamy S, Rigobelo EC (2018) Sugarcane growth and nutrition levels are differentially affected by the application of PGPR and cane waste. Microbiologyopen 7, article id e00617. https://doi.org/10.1002/mbo3.617.
Schoenholtz S, Miegroet HV, Burger JA (2000) A review of chemical and physical properties as indicators of forest soil quality: challenges and opportunities. For Ecol Manage 138: 335–356. https://doi.org/10.1016/S0378-1127(00)00423-0.
Shi XZ, Yu DS, Xu SX, Warner ED, Wang HJ, Sun WX, Zhao YC, Gong ZT (2010) Cross-reference for relating Genetic Soil Classification of China with WRB at different scales. Geoderma 155: 344–350. https://doi.org/10.1016/j.geoderma.2009.12.017.
Tamreihao K, Mukherjee S, Khunjamayum R, Devi LJ, Asem RS, Ningthoujam DS (2018) Feather degradation by keratinolytic bacteria and biofertilizing potential for sustainable agricultural production. J Basic Microbiol 59: 4–13. https://doi.org/10.1002/jobm.201800434.
Timofeeva AM, Galyamova MR, Sedykh SE (2023) Plant growth-promoting soil bacteria: nitrogen fixation, phosphate solubilization, siderophore production, and other biological activities. Plants 12, article id 4074. https://doi.org/10.3390/plants12244074.
Venancio WS, Gomes JM, Nakatani AS, Hungria M, Araujo RS (2019) Lettuce production under reduced levels of N-fertilizer in the presence of plant growth-promoting Bacillus spp. bacteria. J Pure Appl Microbiol 13: 1941–1952. https://doi.org/10.22207/JPAM.13.4.06.
Vuolo F, Novello G, Bona E, Gorrasi S, Gamalero E (2022) Impact of plant-beneficial bacterial inocula on the resident bacteriome: current knowledge and future perspectives. Microorganisms 10, article id 2462. https://doi.org/10.3390/microorganisms10122462.
Wang L, Kaur M, Zhang P, Li J, Xu M (2021) Effect of different agricultural farming practices on microbial biomass and enzyme activities of celery growing field soil. Int J Environ Res Public Health 18, article id 12862. https://doi.org/10.3390/ijerph182312862.
Wang L, Hamel C, Lu P, Wang J, Sun D, Wang Y, Lee SJ, Gan GY (2023a) Using enzyme activities as an indicator of soil fertility in grassland – an academic dilemma. Front Plant Sci 14, article id 1175946. https://doi.org/10.3389/fpls.2023.1175946.
Wang Q, Hu XH, Ma YH, Li YL (2024) Enhancing sugar beet yield and quality in Northeast China: investigating the synergistic impact of sugar mill filter mud and biochar on black soil. Sci Hortic 326, article id 112680. https://doi.org/10.1016/j.scienta.2023.112680.
Wang X, Wang X, Sheng H, Wang X, Zhao H, Feng K (2022) Excessive nitrogen fertilizer application causes rapid degradation of greenhouse soil in China. Polish J Environ Stud 31: 1527–1534. https://doi.org/10.15244/pjoes/143293.
Wang Z, Zhu L, Gielen G, Wu Q, Huang K, Wen J, Wang X, Wang H, Lu S, Chen L, Wu L (2023b) Potential effects of soil chemical and biological properties on wood volume in Eucalyptus urophylla × Eucalyptus grandis hybrid plantations and their responses to different intensity applications of inorganic fertilizer. Environ Sci Pollut Res 30: 773–787. https://doi.org/10.1007/s11356-022-22238-y.
Xu Y, Ren S, Liang Y, Du A, Li C, Wang Z, Zhu W, Wu L (2021) Soil nutrient supply and tree species drive changes in soil microbial communities during the transformation of a multi-generation Eucalyptus plantation. Appl Soil Ecol 166, article id 103991. https://doi.org/10.1016/j.apsoil.2021.103991.
Yousuf J, Thajudeen J, Rahiman M, Krishnankutty S, P. Alikunj A, Mohamed MH (2017) Nitrogen fixing potential of various heterotrophic Bacillus strains from a tropical estuary and adjacent coastal regions. J Basic Microbiol 57: 922–932. https://doi.org/10.1002/jobm.201700072.
Zeileis A, Croissant Y (2010) Extended model formulas in R: multiple parts and multiple responses. J Stat Softw 34. https://doi.org/10.18637/jss.v034.i01.
Zhang C, Xiao X, Zhao L, Qin Y, Doughty R, Wang X, Dong J, Yang X (2023) Mapping Eucalyptus plantation in Guangxi, China by using knowledge-based algorithms and PALSAR-2, Sentinel-2, and Landsat images in 2020. Int J Appl Earth Obs Geoinf 120, article id 103348. https://doi.org/10.1016/j.jag.2023.103348.
Zhu L, Huang J, Lu X, Zhou C (2022) Development of plant systemic resistance by beneficial rhizobacteria: recognition, initiation, elicitation and regulation. Front Plant Sci 13, article id 952397. https://doi.org/10.3389/fpls.2022.952397.
Zhu Z, Wu L (2023) Fertilization and residue management improved soil quality of Eucalyptus plantations. Forests 14, article id 1570. https://doi.org/10.3390/f14081570.
Total of 53 references.

