Reduced height of short day induced bud scale complex may partly explain early bud burst in Norway spruce seedlings
Luoranen J., Sutinen S. (2017). Reduced height of short day induced bud scale complex may partly explain early bud burst in Norway spruce seedlings. Silva Fennica vol. 51 no. 5 article id 7759. https://doi.org/10.14214/sf.7759
Highlights
- Short day treatment used in tree seedling nurseries affects the structure of apical buds
- Changes in bud structure may partly explain early bud burst and may be a reason for unburst buds of short day treated seedlings.
Abstract
Short day (SD) treatment is used as a dormancy induction in forest tree seedling nurseries in the boreal forest zone. However, SD treatment has caused early bud burst in the following spring, which may expose the seedlings to spring frosts. Because the mechanisms affecting earlier bud burst in SD treated seedlings are not fully understood yet, here we have studied the effect of SD treatment on the structure of buds in Norway spruce [Picea abies (L.) Karst.] seedlings. Seedlings were exposed to SD treatments or natural (CTRL) light and photoperiod in July in a nursery in Central Finland. The experiments included two lots of seedlings over two summers and the analyses were done under a stereo microscope. SD treatment advanced initiation of bud scales and formation of needle primordia, and thus the formation period was shorter in CTRL seedlings. In mature buds, no differences in primordial shoots were found between the treatments, whereas notable differences were found in bud scales. The SD buds had fewer and shorter bud scales than the CTRL buds. This led to significantly shorter bud scale complex and, consequently, to shorter buds in SD than in CTRL seedlings. Buds and needles matured earlier in SD treated seedlings. In the following spring, the primordial shoots started to elongate in both treatments around mid-May, when the SD buds started to break down, whereas CTRL buds started to break down in late May. The fewer number and shorter height of protective bud scales may expose buds to harsh winter temperatures and early loss of scales may predispose the SD buds to spring frosts.
Keywords
Picea abies;
elongation;
bud scale complex;
primordia;
shape
Received 26 June 2017 Accepted 15 September 2017 Published 28 September 2017
Views 83475
Available at https://doi.org/10.14214/sf.7759 | Download PDF

1 Introduction
In the boreal forest zone, well-hardened seedlings are wanted as they do not require frost protection during autumn in the nurseries and the risk of frost damage is decreased after autumn planting. Seedlings must also be well-hardened for freezer storage in late autumn. To produce these desired seedlings for forestry, the tree seedling nurseries in northern latitudes have used artificial shortening of photoperiod [short-day treatment (SD)] (Dormling et al. 1968; Heide 1974a; Colombo et al. 2001 and reference therein). SD treatment stops height growth and advances bud formation, which also hastens frost hardening during early autumn (Dormling et al. 1968; Colombo et al. 2001 and reference therein; Konttinen et al. 2003; Fløistad and Granhus 2010). The actual day and night temperatures during bud formation affect the size of the buds and the time required to form new buds: low temperature delays and high temperature increases the rate of bud formation (Pollard and Logan 1977; Colombo et al. 2003). In addition, higher temperature also increases the number of needle primordia and, thus, the size of the primordial shoot (Pollard and Logan 1977; Colombo et al. 2003). Day temperatures during SD affect the bud formation but the night temperatures modify this process (Olsen et al. 2014). If night temperatures are higher than day temperatures, the rate of bud formation slows down (Fløistad and Patil 2002).
SD treated seedlings tolerate early autumn frost better than untreated seedlings. On the other hand, earlier bud burst typical to SD treated seedlings leads to increased spring frost damage in many Picea species (Odlum and Colombo 1988; Bigras and D’Aoust 1993; Hawkins et al. 1996; Fløistad and Granhus 2010; Luoranen and Rikala 2015). SD treatment may also increase the number of buds that fail to burst (McClaren et al. 1994; Luoranen et al. 1994, 2006; Konttinen et al. 2003; Luoranen and Rikala 2015). Reduced survival and growth of conifer seedlings caused by spring frost has been associated with a decreasing frost tolerance of the previous year’s needles, together with visible bud development (Cannel and Smith 1984; Bigras et al. 2004; Luoranen et al. 2010) and recently also with the internal elongation of the primordial shoots in visible dormant buds (Luoranen et al. 2010).
Bud formation and development have been well studied in several tree species, especially on adult trees (Owens and Molder 1976; Owens et al. 1977; Sutinen et al. 2009, 2012). In conifer seedlings, the phenology of buds has also been studied for example in Douglas-fir (Pseudotsuga menziesii (Mirb.) Franco var. menziesii) (MacDonald and Owens 1993a,b, 2010) and various Picea species (Pollard and Logan 1977; El Kayal et al. 2011). The seedling studies have included also the effects of SD induced dormancy combined with other environmental conditions like temperature or drought. After induction treatments bud formation (bud set) as well as bud development preceding the bud burst in the following spring have been done without natural light controls (CTRL) and are mainly based on external features of the seedlings.
In our previous study, we found differences in bud structure between SD-treated and untreated Norway spruce (Picea abies (L.) Karst.) seedlings (Sutinen et al. 2015). Therefore, we aimed to clarify, how and when the SD treatment affects the formation of new buds compared to that in control buds, and can the treatment affect the bud burst in the following spring. To achieve the aims, we followed the buds of one-year-old Norway spruce container seedlings at the stereo microscopic level from their first bud burst, through the predetermined and neoformed growth, up to the completion of new bud after SD treatment and natural photoperiod, and further, during bud burst in the following spring during the years 2012–2015. Our hypothesis was that SD treatment will reduce the length of bud scales, which then affects the timing of bud burst.
2 Materials and methods
2.1 Seedlings and treatments
The growing of seedlings was carried out at the Suonenjoki Research Nursery of Natural Resources Institute Finland (Luke; 62°39´N, 27°03´E, altitude 142 m above sea level). Norway spruce seeds were source identified, stand collected, and of local origin (62°57´N–63°20´N, 26°57´E–27°57´E, Maaninka, Finland, critical night length of the origin is about 7 h). Seedlings were grown in hard plastic Plantek (BCC, Iso-Vimma, Finland) 81F trays (81 cells per tray, 546 cells m–2, cell volume 85 cm3) filled with base-fertilized (0.8 kg m–3 of 16N:8P:16K soluble fertilizer with micronutrients) and limed (2.0 kg m–3) light sphagnum peat (Kekkilä Co., Tuusula, Finland). Seeds for the first lot were sown on 20 June 2011 and for the second lot on 11 June 2013. Seedlings were raised according to standard nursery practice in Finland. They were irrigated 2–4 times per week depending on evapotranspiration and fertilized with 0.1% Forest-Superex solution (22-5-16 for N-P-K + micronutrients; Kekkilä Co., Tuusula, Finland) on average once per week. On 27 August 2013, seedlings were treated with Mogeton WP (quinoclamine) against liverwort (Marchantia sp.). In both years, seedling lots were grown in a greenhouse until the end of October, when they were transferred to an outdoor growing area. Before transfer, the seedling lots were divided into two parts in both years. One-half of the seedlings were for controls (CTRL), and the other half for SD treatments.
During the second growing seasons (2012 and 2014) the seedling lots were grown in an outdoor growing area until mid-July. Half of the seedlings were exposed to SD treatment between July 16 and August 2 in 2012, and July 21 and August 8 in 2014, with the use of a blackout curtain in the outdoor growing compound. During the blackout, the day length was 12 h. The control seedlings were grown under the natural photoperiod, which ranged from 18 h 58 min (16 July) to 17 h 54 min (8 August). After SD treatments, all seedlings were raised similarly in both treatments up to the completion of the experiments in 2013 (the first lot) and 2015 (the second lot).
Air temperature data was collected hourly at the height of 2 m from the snow melt up to the late growing season at the Suonenjoki Research Unit of Luke during each study year. The temperature sums with the threshold value of 0°C were calculated from daily mean temperatures. The temperature sums accumulated a day before each sampling day are given in Tables 1 and 2. At the seedling top level, in both treatments, the air temperature was also measured using two Hobo data loggers (type H08-032-08) from 16 July to 29 July in 2012. Due to technical problems, temperatures were not possible to monitor for a longer period. During this 13-day period, daily mean temperatures at a seedling level were 16.7 °C for SD and 15.4 °C for CTRL seedlings, and accumulated temperature sums were 234 and 216 d.d., respectively.
| Table 1. Sampling days in 2012 and 2014. Days from 0 indicate the number of days after beginning of short day (SD) treatment. The day number from the beginning of the year (DOY, day of the year) indicates the sampling day. It is calculated as the midpoint of the sampling dates of the two study years. Day 0 was the beginning date of SD treatment, which was July 16 in 2014 and July 21 in 2014. Samples were not taken on day 0 in 2014. The degree day (d.d.) temperature sums were calculated with a threshold value of 0 °C from the day of snow melt until the day preceding the sampling day. | |||||||
| DOY | Year 2012 | Year 2014 | |||||
| Date | d.d. | Days from 0 | Date | d.d. | Days from 0 | ||
| 198 | 16 July | 995 | 0 | 18 July | 1121 | –3 | |
| 202 | 19 July | 1037 | 3 | 23 July | 1227 | 2 | |
| 208 | 26 July | 1144 | 10 | 28 July | 1344 | 7 | |
| 211 | 30 July | 1224 | 14 | 30 July | 1383 | 9 | |
| 214 | 2 Aug | 1273 | 17 | 1 Aug | 1425 | 11 | |
| 218 | 6 Aug | 1342 | 21 | 6 Aug | 1534 | 16 | |
| 225 | 13 Aug | 1412 | 28 | 13 Aug | 1669 | 23 | |
| 228 | 16 Aug | 1480 | 31 | 15 Aug | 1700 | 25 | |
| 232 | 20 Aug | 1539 | 35 | 20 Aug | 1768 | 30 | |
| 235 | 23 Aug | 1575 | 38 | 22 Aug | 1794 | 32 | |
| 246 | 4 Sept | 1719 | 50 | 1 Sept | 1907 | 42 | |
| 252 | 10 Sept | 1771 | 56 | 8 Sept | 1999 | 49 | |
| 259 | 17 Sept | 1852 | 63 | 15 Sept | 2084 | 56 | |
| 266 | 24 Sept | 1912 | 70 | 22 Sept | 2154 | 63 | |
| 274 | 2 Oct | 1977 | 78 | 29 Sept | 2198 | 70 | |
| Table 2. Changes during bud development in the height (mm ± SD) of primordial shoots, whole bud and bud scale complex as well as the most common estimation for bud development phases and stages in springs before short day (SD) treatments in 2012 and 2014. The degree-day (d.d.) temperature sums with a threshold value of 0 °C were calculated from snow-melt each year until the day preceding the sampling day. The development stages are 0: no changes; 1: slightly swollen; 2: swollen, transparent bud scales; 3: bud scales loosened; and 4: bud burst and shoot elongated. Development phases are 0: all needle primordia have rounded tips, and the apex is wholly visible; 1: the tips of the needle primordia are pointed, beginning from the lowest part of the primordial shoot nearly up to the apex; 2: the needle primordia have elongated and are bending over the apex, and the elongation of the primordial shoot has started; 3: the apex can no longer be seen due to the elongation of the primordial shoot and needles; 4: needle primordia and shoot are elongating. Nm = not measured. | |||||||
| Year | Date | d.d. | Height of primordial shoot | Height of whole bud | Height of bud scale complex | Development phase of bud | Development stage of bud |
| 2012 | 24 April | 0 | 1.4 ± 0.1 | 2.5 ± 0.2 | 1.1 ± 0.2 | 1 | 0 |
| 26 April | 0 | 1.4 ± 0.2 | 2.3 ± 0.2 | 1.0 ± 0.2 | 1 | 0 | |
| 7 May | 60 | 2.0 ± 0.3 | 2.5 ± 0.2 | 0.5 ± 0.2 | 1 | 1 | |
| 14 May | 117 | 2.9 ± 0.4 | 3.1 ± 0.2 | 0.2 ± 0.3 | 4 | 2 | |
| 16 May | 142 | 3.6 ± 0.5 | 3.8 ± 0.4 | 0.2 ± 0.3 | 4 | 3 | |
| 18 May | 173 | 5.6 ± 0.8 | 5.6 ± 0.8 | 0.0 ± 0.0 | 4 | 3 | |
| 2014 | 24 March | nm | 1.5 ± 0.1 | 2.6 ± 0.1 | 1.1 ± 0.1 | 0 | 0 |
| 10 April | 18 | 1.6 ± 0.2 | 2.7 ± 0.2 | 1.1 ± 0.2 | 1 | 1 | |
| 23 April | 83 | 2.2 ± 0.3 | 3.0 ± 0.3 | 0.9 ± 0.2 | 2 | 1 | |
| 8 May | 146 | 3.3 ± 0.5 | 3.6 ± 0.3 | 0.3 ± 0.3 | 3 | 2 | |
2.2 Sampling
The study included two experiments during four years (2012–2013 and 2014–2015). Bud development was followed in the springs before SD treatments (2012 and 2014), and then in the following springs after SD treatments (2013 an 2015). The formation of new buds was followed from May 24 in 2012 and May 23 in 2014 in both SD and CTRL seedlings.
To follow the bud development preceding the bud burst, the sampling was started during early spring each year (between 24 March and 6 May in 2012–2015, Tables 2,3). The interval between sampling dates varied from a few days up to two weeks and was decided partly according to the forecasted temperature and partly to the knowledge about the relation between the temperature sum and primordial shoot growth in the developing buds (Sutinen et al. 2012) To follow the bud formation (bud set), the first samples were taken in early June 2012 and 2014. From the start of the SD treatment (day 0), the sampling was done at intervals of two to three days up to 10 September in 2012 and up to 25 August in 2014. The last sampling was done on 2 October in 2012 and on 29 September in 2014. Twenty samplings were done during 67 days in 2012 and 23 samplings during 70 days in 2014. The real sampling dates, the days from the beginning of the SD treatment (day 0) and the estimated common sampling days (day of the year, DOY) are given in Table 1. All sampling dates were not included in Table 1, but if some events were met during those days, the events are mentioned in the text.
| Table 3. Changes during bud development in the height (mm ± SD) of primordial shoots, whole bud and bud scale complex as well as the most common estimation for bud development phases and stages in the spring after previous summer short day (SD) treatments in 2013 and 2015. The degree-day (d.d.) temperature sums with a threshold value of 0 °C were calculated from snow-melt each year until the day preceding the sampling day. The bold text in the table indicates the statistically significant difference between SD and untreated control (CTRL) in a given day (p < 0.05, t-test). The development stages are 0: no changes; 1: slightly swollen; 2: swollen, transparent bud scales; 3: bud scales loosened; and 4: bud burst and shoot elongated. Development phases are 0: all needle primordia have rounded tips, and the apex is wholly visible; 1: the tips of the needle primordia are pointed, beginning from the lowest part of the primordial shoot nearly up to the apex; 2: the needle primordia have elongated and are bending over the apex, and the elongation of the primordial shoot has started; 3: the apex can no longer be seen due to the elongation of the primordial shoot and needles; 4: needle primordia and shoot are elongating. | ||||||||||||
| Height of primordial shoot | Height of whole bud | Height of bud scale complex | Development phase of bud | Development stage of bud | ||||||||
| Year | Date | d.d. | CTRL | SD | CTRL | SD | CTRL | SD | CTRL | SD | CTRL | SD |
| 2013 | 6 May | 85 | 1.8 ± 0.2 | 1.9 ± 0.2 | 2.8 ± 0.4 | 2.7 ± 0.3 | 1.1 ± 0.3 | 0.7 ± 0.3 | 2 | 2 | 0 | 0 |
| 13 May | 142 | 2.6 ± 0.5 | 2.7 ± 0.5 | 3.4 ± 0.5 | 3.1 ± 0.5 | 0.8 ± 0.2 | 0.4 ± 0.3 | 3 | 3 | 1 | 2 | |
| 15 May | 168 | 3.4 ± 0.3 | 3.4 ± 1.0 | 4.0 ± 0.5 | 3.8 ± 0.9 | 0.6 ± 0. 4 | 0.4 ± 0.3 | 3 | 3 | 1 | 2 | |
| 17 May | 195 | 4.5 ± 1.0 | 4.1 ± 1.0 | 4.8 ± 0.9 | 4.3 ± 1.0 | 0.3 ± 0.2 | 0.2 ± 0.2 | 4 | 4 | 2 | 3 | |
| 20 May | 245 | 7.1 ± 2.0 | 7.2 ± 1.7 | 7.3 ± 1.9 | 7.2 ± 1.6 | 0.1 ± 0.1 | 0.0 ± 0.1 | 4 | 4 | 2 | 4 | |
| 2015 | 21 April | 18 | 1.4 ± 0.6 | 1.4 ± 0.2 | 2.9 ± 0.4 | 2.3 ± 0.8 | 1.5 ± 0.3 | 0.9 ± 0.6 | 1 | 1 | 0 | 0 |
| 5 May | 83 | 1.7 ± 0.1 | 1.8 ± 0.4 | 2.9 ± 0.3 | 2.5 ± 0.4 | 1.2 ± 0.4 | 0.7 ± 0.2 | 1 | 1 | 0 | 0 | |
| 10 May | 122 | 1.9 ± 0.3 | 2.2 ± 0.2 | 3.1 ± 0.5 | 2.8 ± 0.5 | 1.2 ± 0.7 | 0.5 ± 0.3 | 2 | 2 | 1 | 1 | |
| 15 May | 162 | 2.0 ± 0.3 | 2.8 ± 0.3 | 3.0 ± 0.2 | 3.3 ± 0.6 | 1.0 ± 0.4 | 0.5 ± 0.2 | 3 | 3 | 1 | 2 | |
| 18 May | 180 | 3.0 ± 0.3 | 3.2 ± 0.3 | 4.0 ± 0.8 | 3.5 ± 0.8 | 1.0 ± 0.5 | 0.3 ± 0.3 | 3 | 3 | 1 | 2 | |
| 20 May | 191 | 3.2 ± 0.3 | 4.0 ± 0.3 | 4.0 ± 0.8 | 4.0 ± 1.0 | 0.8 ± 0.4 | 0.1 ± 0.1 | 3 | 3 | 1 | 2 | |
| 25 May | 242 | 5.2 ± 0.4 | 5.1 ± 0.3 | 5.1 ± 1.0 | 5.3 ± 1.2 | 0.1 ± 1.0 | 0.1 ± 0.3 | 3 | 4 | 2 | 3 | |
On each sampling date, ten SD and ten CTRL seedlings were randomly sampled. However, in spring 2012, before the start of the treatments, total of 10 seedlings were sampled in each time. In total 460 seedlings were sampled in spring and summer of 2012, and 100 seedlings in spring and early summer of 2013. Similarly, 460 samples were taken in 2014 and 120 samples in 2015. On each sampling a 2-cm piece was cut from the top of each seedling and placed in test tube containing the fixative solution (2% glutaraldehyde in a cacodylate buffer; pH 7.0). The sample tubes were transported, in a cool bag, to the laboratory of Joensuu Research Unit of Luke for morphological bud examination. The samples were prepared under a stereomicroscope (Wild, Heerbrugg, Switzerland) and the photographs for analyses were taken under this microscope with a digital camera (Leica Microsystems CD Camera, Heerbrugg, Switzerland). The observations and measurements were done from the photographs using Adobe Photoshop (version 6.0, Adobe Systems Nordic AB, Kista, Sweden). We will report first the bud formation in 2012 and 2014 in SD and CTRL seedlings and, then the bud burst before (2012 and 2014) and after (2013 and 2015) SD treatments.
2.3 Bud formation
For analysis of bud formation in CTRL and SD seedlings during the two exposure summers in 2012 and 2014, the top of the seedlings with neo-formed needles was photographed at 6× and 12× objective magnifications under a stereo microscope. After this, the neo-formed needles from the vicinity of the apex were carefully removed. To follow the elongation and formation of new terminal buds (later called only buds) the photographs were taken at 6×, 12×, 25× and 50× objective magnifications during both study years. The measurements were done as follows: first, from the top of apex to the lowermost row of needle primordia in the primordial shoot (shoot height, see Fig. 1a); and second, the outer surface of the bud from the outermost bud scales up to the top of the bud (height of the whole bud; see Fig. 2e and Figs. 1b,d). The number of needle primordia rows was counted during the photographing and was verified later from the photographs (Templeton et al. 1993).
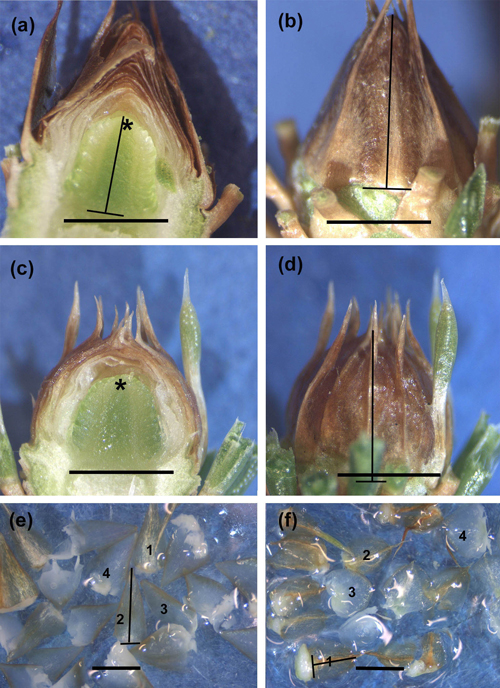
Fig. 1. Longitudinally cut untreated control (CTRL) (a,b) and short day (SD) treated (c,d) buds sampled on 21 April 2015, as well as bud scales removed from CTRL (e) and SD (f) buds on 22 September 2014. The development phase of the primordial shoots (a,c), as well as the external phenological stage of buds (b,d), is 0 (no changes/all needle primordia have rounded tips and the apex is wholly visible) in both treatments. Observe the higher scale complex (a) and, thus, longer outside measure (b) in CTRL buds compared to those in SD buds (c,d). Instead, the primordial shoots are equally long (a,c). Observe the typical conic shape of the CTRL bud and bud scales with combined needle-scale structure and thorns compared to more rounded shape of SD bud and scales (d,f). The lines on the buds show how the height of the primordial shoot (a) and that of the whole bud (b,d) was measured. Similarly, the lines in (e,f) show how the height was measured from four medium size scales (e,f). Asterisk = apex, bar = 2 mm in each picture.
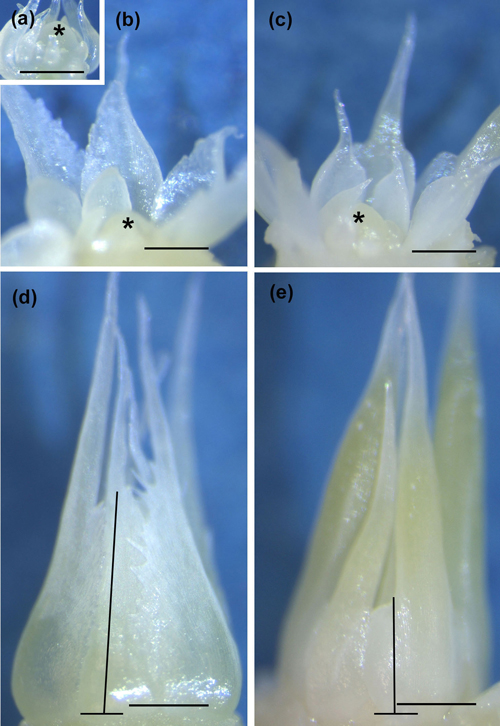
Fig. 2. Innermost neo-formed needles around the apex in a control seedling (CTRL) sampled on 23 May 2014 (a). Innermost bud scales around the apex (b,c) and uppermost bud scales (d,e) in early bud formation in CTRL (b,d) and short day (SD) treated (c,e) seedlings sampled on 30 July (SD) and on 4 August (CTRL) 2014. Observe the conic CTRL scales (b, d) and cup-shaped SD scales (c), as well as a combined scale-needle structure of the outermost scales in SD-treated buds (e). The lines in (d) and (e) show how the height of bud scales was measured. Asterisk = apex, bar = 0.2 mm in each picture.
In 2014, when the bud scales were rigid enough to be removed, their number was counted from each bud by removing the scales carefully with the very thin point tweezers. Furthermore, all bud scales from each bud of each seedling were photographed from the three last samplings in the liquid in a bell jar. From the photographs, four medium-sized scales were measured. As the shape of the bud scales appeared to be different between the treatments the upper point for scale measurements did not include the thorns that were sticking out from the scales (see Figs. 2d,e and Figs. 1e,f). The thorns were also excluded when measuring the height of the whole buds (see Figs. 1b,d). The height of bud scale complex (defined by MacDonald and Owens 1993a) was calculated by subtracting the height of the primordial shoot from the height of the whole bud.
Except for the bud scale measurements mentioned above and the maturation of the neo-formed needles (from watery pale green to dark green, followed in 2014), all other bud analyses were done during both study years. The day when the first bud scales emerged was noted. The formation and the outward maturation of the buds were followed from the phase when the bud scales started to form minute buds. Bud maturation was divided according to the color of the outermost bud scales into three classes as follows: 0= no bud, 1 = white color, and 2 = brown.
2.4 Bud development and bud burst in spring
For analysis of bud development during the four springs, the buds from every seedling were cut longitudinally into two halves. For the morphological studies, the outer surface and the inner parts of the longitudinally cut buds were photographed at 6× and 12× objective magnification under a stereo microscope. After that, the bud scales were removed, and the outer surface of the primordial shoots was photographed digitally from different angles at 12×, 25× and, in some cases, at 50× objective magnification.
The externally visible phenological stages of the buds were evaluated according to Luoranen et al. (2010): Stage 0: no changes; Stage 1: slightly swollen; Stage 2: swollen, transparent bud scales; Stage 3: bud scales loosened; and Stage 4: bud burst and shoot elongated. The seedlings where all the buds had reached stage 4 were not sampled (microscopic analysis) further. The phases of bud development towards bud burst inside the bud scales were determined from phase 0to phase 4 as in Luoranen et al. (2010): Phase 0: all needle primordia have rounded tips, and the apex is wholly visible; Phase 1: the tips of the needle primordia are pointed, beginning from the lowest part of the primordial shoot nearly up to the apex; Phase 2:, the needle primordia have elongated and are bending over the apex, and the elongation of the primordial shoot has started; Phase 3: the apex can no longer be seen due to the elongation of the primordial shoot and needles; Phase 4: needle primordia and shoot are elongating.
The height of the primordial shoot and that of the whole bud were measured from the photographs taken at 6× and 12× objective magnification. The measurements for primordial shoot height were: 1) the distances from the lowest row of needle primordia up to the top of the shoot apex and; 2) for the height of the whole bud, the distances from the lowest primordial row up to the top of the outermost bud scales. The top of the whole bud was determined similarly to as described above; the thorns that stick out from the buds were not included in the measurements (see Figs. 1b,d). Similarly, as above, the height of the bud scale layers (the bud scale complex) was calculated by subtracting the height of the primordial shoot from the height of the whole bud.
2.5 Statistical methods
The effect of the treatments on different bud characteristics measured at the stereomicroscopic level was analyzed separately for each sampling time using a t-test. The correlation between the primordial shoot elongation and accumulated temperature sum (d.d.) were analyzed with Pearson’s correlation test.
3 Results
3.1 Bud formation
After bud bursting in both years up to the formation of first bud scales, the primordia at the base of the apex (Fig. 2a) were grown to neo-formed needles, which maturated later during the bud formation. Bud scale formation started on days 7 and 5 in SD seedlings (2012 and 2014, respectively) and day 10 in CTRL seedlings in both years. The number of scales was calculated only in 2014 and could be started on 6 August when the scales were rigid enough to be removed (DOY 218, Fig. 3a). After the start of counting, the scale number increased for 23 days in SD buds and for 49 days in CTRL buds (Fig. 3a). SD buds had significantly more scales than the CTRL buds (p = 0.021) on day 16 (DOY 218). During the next two weeks, the number of the scales increased in neither of the treatments. Later, during the four last samplings, the CTRL buds had significantly more scales than SD buds (p = 0.001 every time, Fig. 3a). Furthermore, the height of the bud scales in CTRL buds on DOY 244, 265 and 272 in 2014 were 2 ± 0.1, 2.3 ± 0.1 and 2.1 ± 0.1 mm, respectively, whereas during the same times the height of the scales in SD buds was always 1.8 ± 0.1 mm. The difference between the treatments was significant during the two last measurements (p = 0.338, 0.005 and 0.017 for the three measure occasions). The bud scales also differed qualitatively between the treatments in both 2012 and 2014. In CTRL buds the form of scales was always conical, whereas that in SD buds was always spoon-like.
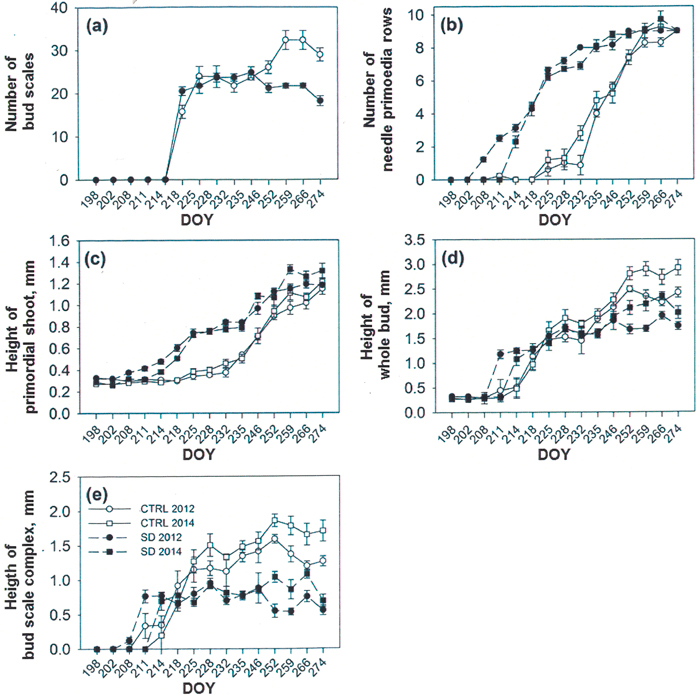
Fig. 3. Formation of new buds in untreated control (CTRL) and short day (SD) exposed seedlings in summers 2012 and 2014. The number of bud scales (a) was counted only in 2014, whereas number of rows of needle primordia (b), height (mm) of primordial shoot (c) and that of the whole bud (d) were followed in both 2012 and 2014. The calculated height of the bud scale complex is shown in (e). The graphs were started on the day of year (DOY) 198, i.e. some days before the appearance of first forming structures. Observe in (a) that the formation of bud scales was started before the DOY 214, but the scales were not rigid enough to remove until the day 214.
The appearance of first tiny buds followed the formation of scales, even before the scales could be removed (i.e. the height of the buds was first higher in SD than in CTRL seedlings up to the DOY 246, after which the CTRL buds were higher) (compare Fig. 3a and Fig. 3d). The white color of the scales (class 1) in the buds started in late July (DOY 211–214) and lasted up to 22/23 August (DOY 235) in both treatments (Table 1). After this, the outermost scales in SD buds turned to brown (class 2) within 11 days in 2012 and within 17 days in 2014 (i.e. during the first weeks of September). In the CTRL seedlings, the outermost bud scales turned to brown within 24 days in 2012 and within 39 days in 2014 (i.e. from mid-August to early September). The change in color of current needles from pale watery green to dark green, typical to the mature spruce needles, was seen in SD seedlings on 13 August (DOY 225) and in CTRL seedlings on 1 September (DOY 246) in 2014.
3.2 Needle primordia, primordial shoots, and bud scale complex
The first row of needle primordia was seen in SD buds on day 10 in 2012 (26 July; DOY 208) and on day 11 in 2014 (1 August; DOY 214). In control buds, the first row of needle primordia was seen about two weeks later (12 August, DOY 225) (Fig. 3b). The completion of primordial rows lasted 66 days in 2012 and 60 days in 2014 in SD buds, compared to 49 days in CTRL buds in both years. Most times the differences between the treatments were statistically significant in both years (p = 0.001–0.047), except for the last three samplings, when no difference was found (p = 0.432–0.814) (Fig. 3b). In both treatments, the number of rows of needle primordia was 9 in short and 13 in long rows, giving a total of 117 needle primordia in mature primordial shoots. Elongation of the primordial shoot followed the same pattern as the formation of primordial rows (Fig. 3c).
Elongation of the whole bud started earlier, and the buds were significantly longer in SD seedlings (around 1 mm) compared to CTRLs (less than 0.5 mm) in both years in late July (DOY 211 in 2012 and 214 in 2014, p = 0.006–0.043) (Fig. 3d). From 22/23 August (DOY 235) the CTRL buds were close to 2 mm long and were significantly (p = 0.001–0.034) longer than the SD buds, which were at that time around 1.5 mm long. The significant difference was found up to the completion of samplings during both study years (p = 0.001–0.026) (Fig. 3d).
The calculated height of the bud scale complex initially followed the pattern of visible height growth of the buds. In early August (DOY 214), the bud scale complex in SD buds was significantly higher compared to CTRL buds (p = 0.017 in 2012 and 0.05 in 2014). From 13 August (DOY 225) the scale complex was always higher in CTRL buds. The difference was significant to the completion of samplings from 23 August (DOY 235) in 2012 (p = 0.001–0.002) and from 13 August (DOY 225) in 2014 (p = 0.001–0.008) (Fig. 3e). The difference in bud scale complex between the treatments was also seen in a longitudinal section of the buds (Figs. 1a,c). Also, the appearance of mature buds and that of the scales in CTRL seedlings was conic, and the outermost conic bud scales with their thorns were interwoven in the top of the bud (Figs. 1a,b,e). In SD seedlings, the bud scales were spoon-like, and the shape of the bud was globular. The outermost bud scales in SD buds had thorns, which stuck out separately from the top of the buds (Figs. 1c,d,f).
The growing season in 2014 was warmer than in 2012 and, thus, the accumulated temperature sum at the end of samplings was 1977 d.d. in 2012 compared to 2198 d.d. in 2014. Consequently, during the last four sampling times, the heights of the whole buds and the height of the bud scale complexes were greater in 2014 than in 2012, both in CTRL and SD buds (Figs. 3d,e).
3.2 Structure and phenology of buds in spring
The height of primordial shoot during the first samplings was between 1.4–1.9 mm in both treatments and each spring (Table 2). The inner phases and external stages of the buds were between 0and 1 during the first samplings in 2012, 2014, and 2015 (Tables 2,3, Figs. 1a–d). Elongation of primordial shoots was started at 60–85 d.d. when the shoot development phase was around 2 (Tables 2,3). The elongation of the primordial shoots and that of the whole buds correlated positively with the temperature sums (TS, d.d.) and were 0.787 (p = 0.01) in CTRL and 0.829 (p = 0.01) in SD seedlings. Contrary to that, the height of bud scale complex showed negative correlations with the TS both in CTRL (–0.567, p = 0.01) and SD seedlings (–0.70, p = 0.01).
In 2013 and 2015, after the previous summer SD treatments, the height of the whole SD buds remained shorter than that of CTRL buds also during the first samplings in the following spring (Table 3, Figs. 1a–d). Due to the simultaneous elongation of the primordial shoot in both treatments, the calculated height of the bud scale complex was significantly less in SD than in CTRL buds during the two first samplings in 2013, and up to the latest sampling in 2015 (Table 3). The internal development phase 3 was achieved around mid-May in both treatments in 2013 and 2015 (Figs. 4a,c). Contrary to that, the external phenological stage at that time was 1 in CTRL and 2 in SD buds (Table 3, Figs. 4b,d). After mid-May, the external stage of SD buds continued to be more advanced compared to CTRL buds up to the last samplings before bud burst in late May in 2013 and 2015 (Table 3).

Fig. 4. Longitudinally cut untreated control (CTRL) bud sampled on 20 May (a,b) and short day (SD) treated (c,d) bud sampled on 18 May, in 2015. The inner development phase in both CTRL and SD buds is the phase 3 (the apex can no longer be seen due to the elongation of the primordial shoot and needles) (a,c), whereas the external phenological stage of the buds is the stage 1 (slightly swollen) in CTRL (b) and the stage 2 (swollen, transparent bud scale) in SD (d) buds. Asterisk = apex, bar = 1 mm in each picture.
4 Discussion
This study shows that bud formation shares similarities between control and SD-treated seedlings. Our hypothesis on the reduced length of bud scales due to SD treatment and the effect of this change on the timing of bud burst between SD and CTRL seedlings in the following spring was confirmed. In addition, we found differences in initiation time, duration of organ completion, and especially in the number, structure, and shape of the organs.
SD-induced rapid start of bud scale initiation was seen here and has also been reported in Douglas fir by MacDonald and Owens (1993b), in black spruce (Picea mariana [Mill.]) by Colombo et al. 2003 and in white spruce (Picea glauca [Moench] Voss) by El Kayal et al. (2011). Compared to CTRL-seedlings, the formation of needle primordia started about ten days earlier in SD-treated seedlings, and their formation lasted about 65 days. This agrees with 70 days in SD-treated white spruce (El Kayal et al. 2011) and with 70–90 days in SD-treated coastal Douglas-fir (MacDonald and Owens 1993a, 2010) seedlings. In CTRL seedlings, the formation of needle primordia lasted only 49 days. Thus, the rate of needle primordia formation in CTRL buds differed from that in SD buds, as well as from that described in SD buds in coastal Douglas-fir (MacDonald and Owens 2010). Although the rate of needle primordia formation differed between the treatments, the number of primordia rows was the same after completion of bud formation, which has also been reported by Colombo et al. (2003).
The elongation rate of the primordial shoot also differed between the treatments but the final height was the same. Outward maturation of new buds (brown color), as well as that of current needles (dark green), was advanced in SD compared to CTRL. The bud maturation is known to correlate to better frost tolerance of SD-treated spruce seedlings in late autumn compared to CTRL seedlings (Colombo et al. 1989, 2003; Konttinen et al. 2003). The increase in frost hardiness of seedlings, however, is not dependent on the structural maturation of buds and needles per se, as noted by Colombo et al. (2001), but includes many biochemical and biophysical changes in the buds, needles, and stems (Ide et al. 1989; Zwiazek et al. 2001).
The most notable difference between the treatments was found in the bud scales. The scales in SD buds showed always cup-shape compared to conical shape in CTRL buds. Similar cup-shaped scales in the buds have been described earlier in Norway spruce (Templeton et al. 1993 see Figure 3a; Luoranen et al. 2010 see Figure 1a) and in coastal Douglas-fir exposed to SD treatments or moisture stress in long-day conditions (MacDonald and Owens 1993a,b). In our case, SD buds also had fewer scales, and they were shorter compared to scales in CTRL buds. Differences in the number of bud scales have also been reported in coastal Douglas-fir seedlings, where gradual exposure to SD induced more scales than abrupt exposure, and where moisture stress produced more scales in the long day compared to short day conditions (MacDonald and Owens 1993a,b). The conical shape of the scales seen in CTRL seedlings here have not been reported earlier in Norway spruce seedlings, but the shape can be seen in the figures of the buds sampled from mature Norway spruce grown in the field (Sutinen et al. 2009, 2012). The shape and the reduced height of the bud scales also affected the shape and height of the new buds. To our knowledge, the differences in shapes, heights, and numbers of scales in Norway spruce buds between SD and CTRL seedlings has not been previously reported, mainly because of the lack of natural CTRL and omitting the study of bud scales.
The increase in the number of the buds that fail to burst has been reported in conifer seedlings exposed to SD treatment during the preceding summer (McClaren et al. 1994; Konttinen et al. 2003; Luoranen et al. 1994, 2006; Luoranen and Rikala 2015). This phenomenon might be related to the bud scales, which have several functions in protecting the primordial shoot (Ide et al. 1998). From the structural point of view, the changes in bud scale complex (fewer and shorter bud scales) due to SD treatment may expose the buds to frost damage during harsh winter conditions and consequently cause the death of the primordial shoot. The death of the buds can be seen as a tanned primordial shoot under the unburst bud scales (Konttinen et al. 2003).
The spring frosts typical to boreal areas have been risky, especially to SD treated seedlings (Odlum and Colombo 1988; Bigras and D’Aoust 1993; Fløistad and Granhus 2010; Luoranen and Rikala 2015). During bud development, the vascular tissue is differentiating and allows water to move to the elongating primordial shoots (the development phases from 1 to 3–4) (de Faÿ et al. 2000; Luoranen et al. 2010; Sutinen et al. 2012). The development phases from 2 onwards are frost sensitive (Luoranen et al. 2010). Thus, the SD seedlings with earlier bud burst are especially at risk to freezing. Similarly, earlier bud burst in juvenile trees compared to adult trees seems to explain the greater risk of young seedlings and saplings for springtime bud damage, rather than the freezing resistance of the buds as such (Vitasse et al. 2014).
Temperatures during bud formation seemed to affect the height of the bud scale complex: in warmer summer it became higher than in cooler summer. The structure of bud scale complex might be considered as a “memory” effect. This could explain, at least on part, why the bud burst is delayed in the following spring in cases, where the SD seedlings were exposed also to higher temperature during bud formation (Dormling et al. 1968; Heide 1974b; Søgaard et al. 2008; Olsen et al. 2014; Wallin et al. 2017). SD induced changes in the activity of dormancy-related genes has also been reported to determine the timing of dormancy induction, dormancy release and forthcoming bud burst in Norway spruce seedlings (Wallin et al. 2017).
The earlier bud burst in SD-treated seedlings might be a reason to increased growth of treated seedlings in some studies (Odlum and Colombo 1988; Hawkins et al. 1996; Konttinen et al. 2007: Luoranen and Rikala 2015). We did not observe any differences in the number of needle primordia, which may explain why no differences in first-year growth of SD-treated and untreated seedlings are not always found (Konttinen et al. 2003).
In conclusion, we describe the positive and negative aspects of SD treatments to buds of Norway spruce seedlings in natural field conditions in the boreal forest zone. The earlier maturation of SD buds together with earlier hardening of current needles help to protect the seedlings from autumn frost. However, the fact that SD treatment reduced the height of bud scales, as well as their number compared to CTRL, may predispose the primordial shoots to low temperatures during winter and spring. The equal elongation and development of the primordial shoot in relation to temperature accumulation in both CTRL and SD buds lead to earlier loosening of scanty bud scales from SD buds compared to CTRL buds having taller and more scales. The difference in loosening of bud scales was seen as advanced bud burst in SD-treated seedlings already during late May, which is the most probable period when the primordial shoots with inadequate scale protection may be exposed to spring frost in boreal areas.
Acknowledgements
We thank Auli Lehtinen, Mervi Ahonpää, Marja-Leena Jalkanen and Hanna Ruhanen for their assistance in the field, as well as Seija Repo for her assistance in the laboratory, and Leena Karvinen for graphic design. We also thank Dr. Johanna Riikonen and four anonymous referees for their valuable comments on the manuscript, as well as Ms. Ella Pesonen for correcting the language. The paper has been professionally proofread by Proof-Reading Service.com. The study was financed by the Finnish Forest Research Institute (project 3554) and Natural Resources Institute Finland (project 41007-00001300).
References
Bigras F.J., D’Aoust A.L. (1993). Influence of photoperiod on shoot and root frost tolerance and bud phenology of white spruce seedlings (Piceaglauca). Canadian Journal of Forest Research 23(2): 219–228. https://doi.org/10.1139/x93-029.
Bigras F.J., Coursolle C., Margolis H.A. (2004). Survival and growth of Picea glauca seedlings as a function of freezing temperatures and exposure times during budbreak and shoot elongation. Scandinavian Journal of Forest Research 19(3): 206–216. https://doi.org/10.1080/02827580410024115a.
Cannel M.G.R., Smith R.I. (1984). Spring frost damage on young Picea sitcensis. 2. Predicted dates of budburst and probability of frost damage. Forestry 57(2): 177–197. https://doi.org/10.1093/forestry/57.2.177.
Colombo S.J., Glerum C., Webb D.P. (1989). Winter hardening in first-year black spruce (Picea mariana) seedlings. Physiologia Plantarum 76: 1–9. https://doi.org/10.1111/j.1399-3054.1989.tb05444.x.
Colombo S.J., Menzies M.I., O’Reilly C. (2001). Influence of nursery cultural practices on cold hardiness of coniferous forest tree seedlings. In: Bigras F.J., Colombo S.J. (eds.). Conifer cold hardiness. Kluwer Academic Publishers, Dordrecht, The Netherlands. p. 223–252. https://doi.org/10.1007/978-94-015-9650-3_9.
Colombo S.J., Glerum C., Webb D.P. (2003). Daylength, temperature and fertilization effects on desiccation resistance, cold hardiness and root growth potential of Picea mariana seedlings. Annals of Forest Science 60(4): 307–317. https://doi.org/10.1051/forest:2003022.
de Faÿ E., Vacher V., Humbert F. (2000). Water-related phenomena in winter buds and twigs of Picea abies L. (Karst.) until bud-burst: a biological, histological and NMR study. Annals of Botany 86(6): 1097–1107. https://doi.org/10.1006/anbo.2000.1276.
Dormling I., Gustafsson Å., von Wettstein D. (1968). The experimental control of the life cycle in Picea abies (L.) Karst. I. Some basic experiments on the vegetative cycle. Silvae Genetica 17: 44–64.
El Kayal W., Allen C.C.G., Ju C.J-T., Adams E., King-Jones S., Zaharia L.I., Abrams S.R., Cooke J.E.K. (2011). Molecular events of apical bud formation in white spruce, Picea glauca. Plant, Cell and Environment 34: 480–500. https://doi.org/10.1111/j.1365-3040.2010.02257.x.
Fløistad I., Granhus A. (2010). Bud break and spring frost hardiness in Picea abies seedlings in response to photoperiod and temperature treatments. Canadian Journal of Forest Research 40(5): 968–976. https://doi.org/10.1139/X10-050.
Fløistad I., Patil G.G. (2002). Growth and terminal bud formation in Picea abies seedlings grown with alternating diurnal temperature and different light qualities. Scandinavian Journal of Forest Research 17(1): 15–27. https://doi.org/10.1080/028275802317221046.
Hawkins C.D.B., Eastham A.M., Story T.L., Eng R.Y.N., Draper D.A. (1996). The effect of nursery blackout application on Sitka spruce seedlings. Canadian Journal of Forest Research 26(12): 2201–2213. https://doi.org/10.1139/x26-249.
Heide O.M. (1974a). Growth and dormancy in Norway spruce ecotypes (Picea abies) I. Interaction of photoperiod and temperature. Physiology Plantarum 30: 1–12. https://doi.org/10.1111/j.1399-3054.1974.tb04983.x.
Heide O.M. (1974b). Growth and dormancy in Norway spruce ecotypes. II. After-effects of photoperiod and temperature on growth and development in subsequent years. Physiology Plantarum 31: 131–139. https://doi.org/10.1111/j.1399-3054.1974.tb03117.x.
Ide H., Price W.S., Arata Y., Ishikawa M. (1998). Freezing behaviors in leaf buds of cold-hardy conifers visualized by NMR microscopy. Tree Physiology 18(7): 451–458. https://doi.org/10.1093/treephys/18.7.451.
Konttinen K., Rikala R., Luoranen J. (2003). Timing and duration of short-day treatment of Picea abies seedlings. Baltic Forestry 9: 2–8.
Konttinen K., Luoranen J., Rikala R. (2007). Growth and hardening of Picea abies seedlings after various night lenght treatments. Baltic Forestry 13: 140–148.
Luoranen J., Rikala R. (2015). Post-planting effects of early-season short-day treatment and summer planting on Norway spruce seedlings. Silva Fennica 49(1) article 1300. https://doi.org/10.14214/sf.1300.
Luoranen J., Puttonen P., Rikala R. (1994). Lyhytpäiväkäsittely kuusen paakkutaimien kasvatuksessa. [Short day treatment as part of growing of Norway spruce seedlings]. Metsätieteen aikakauskirja 1994(1): 51–67. [In Finnish]. https://doi.org/10.14214/ma.6046.
Luoranen J., Rikala R., Konttinen K., Smolander H. (2006). Summer planting of Picea abies container-grown seedlings: effects of planting date on survival, height growth and root egress. Forest Ecology and Management 237(1–3): 534–544. https://doi.org/10.1016/j.foreco.2006.09.073.
Luoranen J., Sutinen S., Rikala R. (2010). Predicting spring frost sensitivity by bud development and temperature sum in Norway spruce seedlings. Trees 24(5): 809–817. https://doi.org/10.1007/s00468-010-0451-8.
MacDonald J.E., Owens J.N. (1993a). Bud development in coastal Douglas-fir under controlled-environment conditions. Canadian Journal of Forest Research 23(6): 1203–1212. https://doi.org/10.1139/x93-152.
MacDonald J.E., Owens J.N. (1993b). Bud development in coastal Douglas-fir seedlings in response to different dormancy-induction treatments. Canadian Journal of Botany 71(10): 1280–1290. https://doi.org/10.1139/b93-153.
MacDonald J.E., Owens J.N. (2010). Physiology and growth of containerized coastal Douglas fir seedlings given different durations of short days to induce dormancy. HortScience 45(3): 342–346. http://hortsci.ashspublications.org/content/45/3/342.short.
McClaren E.L., Krasowski M.J., Hawkins D.B. (1994). Summer plant culling criteria of interior spruce: keeping the bad and throwing the good? General Technical Repport RM-257: 116–129.
Odlum K.D., Colombo S.J. (1988). Short day exposure to induce budset prolongs shoot growth in the following year. In: Landis T.D. (ed.). Proceedings, Combined Meeting of the Western Forestry Nursery Association. USDA Forest Service General Technical Report RM-167: 57–59. https://npn.rngr.net/npn/npn/publications/proceedings/1988/odlum.pdf.
Olsen J.E., Lee Y., Junttila O. (2014). Effect of alternating day and night temperature on short day-induced bud set and subsequent bud burst in long days in Norway spruce. Frontiers in Plant Science volume 5 article 691. 11 p. https://doi.org/10.3389/fpls.2014.00691.
Owens J.N., Molder M. (1976). Bud development in Sitka spruce. I. Annual growth cycle of vegetative buds and shoots. Canadian Journal of Botany 54(3–4): 313–325. https://doi.org/10.1139/b76-029.
Owens J.N., Molder M., Langer H. (1977). Bud development in Picea glauca. I. Annual growth cycle of vegetative buds and shoot elongation as they relate to date and temperature sums. Canadian Journal of Botany 55(21): 2728–2745. https://doi.org/10.1139/b77-312.
Pollard D.F.W., Logan K.T. (1977). The effects of light intensity, photoperiod, soil moisture potential, and temperature on bud morphogenesis in Picea species. Canadian Journal of Forest Research 7(2): 415–421. https://doi.org/10.1139/x77-052.
Søgaard G., Johnsen Ø., Nilsen J., Junttila O. (2008). Climatic control of bud burst in young seedlings of nine provenances of Norway spruce. Tree Physiology 28(2): 311–320. https://doi.org/10.1093/treephys/28.2.311.
Sutinen S., Partanen J., Viherä-Aarnio A., Häkkinen R. (2009). Anatomy and morphology in developing vegetative buds on detached Norway spruce branches in controlled conditions before bud burst. Tree Physiology 29(11): 1457–1465. https://doi.org/10.1093/treephys/tpp078.
Sutinen S., Partanen J., Viherä-Aarnio A., Häkkinen R. (2012). Development and growth of primordial shoots in Norway spruce buds before visible bud burst in relation to time and temperature in the field. Tree Physiology 32(8): 987–997. https://doi.org/10.1093/treephys/tps063.
Sutinen S., Luoranen J., Haataja L., Rikala R. (2015). Lyhytpäiväkäsittely ohentaa silmusuomukerrosta: miten käy taimien pakkaskestävyyden? [Short day treatment reduces the thickness of bud scale complex: how it affects the frost hardiness?]. Taimiuutiset 2/2015: 10–13. [In Finnish]. http://urn.fi/URN:NBN:fi-fe2016051212317.
Templeton C.W.G., Odlum K.D., Colombo S.J. (1993). How to identify bud initiation and count needle primordia in first-year spruce seedlings. The Forestry Chronology 69(4): 431–437. https://doi.org/10.5558/tfc69431-4.
Vitasse Y., Lenz A., Hich G., Körner C. (2014). Earlier leaf-out rather than difference in freezing resistance puts juvenile trees at greater risk of damage than adult trees. Journal of Ecology 102: 981–988. https://doi.org/10.1111/1365-2745.12251.
Wallin E., Gräns D., Jacobs D.F., Lindström A., Verhoef N. (2017). Short-day photoperiods affect expression of genes related to dormancy and freezing tolerance in Norway spruce seedlings. Annals of Forest Science 74(59): 1–14. https://doi.org/10.1007/s13595-017-0655-9.
Zwiazek J.J., Renault S., Croser C., Hansen J., Beck E. (2001). Biochemical and biophysical changes in relation to cold hardiness. In: Bigras F.J., Colombo S.J. (eds.). Conifer cold hardiness. Kluwer Academic Publishers, Dordrecht, The Netherlands. p. 165–186. https://doi.org/10.1007/978-94-015-9650-3_7.
Total of 38 references.
Send to email


