Effects of restoration fire on dead wood heterogeneity and availability in three Pinus sylvestris forests in Sweden
Eriksson A.-M., Olsson J., Jonsson B. G., Toivanen S., Edman M. (2013). Effects of restoration fire on dead wood heterogeneity and availability in three Pinus sylvestris forests in Sweden. Silva Fennica vol. 47 no. 2 article id 954. https://doi.org/10.14214/sf.954
Abstract
Restoration fires are increasingly used as a conservation tool in Sweden to recreate forests with characteristics of previous forests that were periodically disturbed by fires and promote fire-dependent species. Restoration fires can result in large inputs of fresh dead wood, but there are risks of losing some of the existing, pre-fire dead wood. To assess these counteracting effects we studied the heterogeneity and availability of dead wood before and after three restoration fires in boreal Scots pine forests. Specifically, we studied volumes of stumps, high stumps, snags and logs. The fires decreased the total volume of pre-fire dead wood (23-41%) and consumed logs in late decay stages (26-54%) to a higher extent than logs in earlier stages. The input of new fresh dead wood after the fires exceeded losses of pre-fire dead wood and resulted in a net increase of dead wood in all three sites. The added dead wood consisted of fresh snags killed by the fires. Fire also affected log characteristics: reducing their vegetation coverage (60-98%), decreasing their ground contact (4-50%) and increasing their surface area of charred wood (>50%). Such changes have important consequences for the micro environmental conditions inside logs, but have been rarely studied in relation to restoration fires. Our results show that restoration fire causes changes in dead wood availability and characteristics of logs. The results imply that ideally stands with low abundance of rare and heavily decayed wood substrates should be burned to optimize dead wood values. Alternatively, management practices should include protection of these substrates during restoration fires.
Keywords
Scots pine;
CWD;
prescribed burning;
decay stage;
charred wood
-
Eriksson,
Department of Natural Sciences, Mid Sweden University, SE-851 70, Sundsvall, Sweden
E-mail
anna-maria.eriksson@miun.se

- Olsson, Department of Ecology and Environmental Science, Umeå University, SE-901 87, Umeå, Sweden E-mail jorgen.m.olsson@slu.se
- Jonsson, Department of Natural Sciences, Mid Sweden University, SE-851 70, Sundsvall, Sweden E-mail bengt-gunnar.jonsson@miun.se
- Toivanen, Department of Natural Sciences, Mid Sweden University, SE-851 70, Sundsvall, Sweden E-mail sara.toivanen@lansstyrelsen.se
- Edman, Department of Natural Sciences, Mid Sweden University, SE-851 70, Sundsvall, Sweden E-mail mattias.edman@miun.se
Received 30 October 2012 Accepted 11 April 2013 Published 17 July 2013
Views 163783
Available at https://doi.org/10.14214/sf.954 | Download PDF

1 Introduction
In boreal forests, fire has long been the major disturbance factor, creating a diverse landscape with stands in different successional stages (Zackrisson 1977; Ryan 2002). Therefore, many species have specific adaptations to fire disturbance and the following successional stages are often species-rich, featuring specialized species of vascular plants, insects and fungi (Engelmark and Hytteborn 1999).
In boreal Fennoscandia the fire regime has varied depending on vegetation type, altitude, climate and human activities (Niklasson and Granström 2000; Carcaillet 2007). Human activities such as traditional livelihoods with high use of fire i.e slash and burn cultivation had a great impact on the annual burned areas and the fire regimes (Wallenius 2011). Efficient fire suppression programs started in the end of the 19th century to protect timber resources (Östlund et al. 1997; Lehtonen 1998; Groven and Niklasson 2005). Fire suppression together with changed human use of forest from traditional livelihoods to modern agriculture and forestry resulted in that the annual burned area dropped and fire regimes changed in the whole Fennoscandia (Wallenius 2011).
Before the end of the 19th century the fire return interval ranged between decades to centuries in dry to mesic Scots pine (Pinus sylvestris L.) forests (Zackrisson 1977; Engelmark 1984; Niklasson and Granström 2000). Today fire is almost absent as a disturbance factor in Fennoscandian boreal forests and the expected fire return interval is hundreds or thousands of years (Granström 2001).
Forest fires can generate large inputs of dead wood (Spies et al. 1988; Siitonen 2001; Sidoroff 2007), which is a key substrate for forest species richness and important for structural heterogeneity, nutrient cycling and carbon dynamics (Harmon et al. 1986; Berg et al. 1994). Unfortunately, intensive forest harvesting and efficient fire suppression have caused a decrease in dead wood (Fridman and Walheim 2000), adversely affecting many boreal forest species (Siitonen 2001). Coarse dead wood, particularly, is crucial for a large fraction of the red-listed forest species in Fennoscandia. However, the abundance of coarse dead wood has strongly decreased and there is an especially acute lack of coarse pine wood as a result of intensive selective cutting at the end of the 19th and beginning of the 20th centuries (Linder and Östlund 1998). Further, due to the short rotation times prevailing in current forestry the regeneration rate of coarse pine wood is very low, and the efficient fire suppression, together with forest management practices, has favored the more shade-tolerant Norway spruce (Picea abies (L.) Karst.) relative to Scots pine (Linder 1998). Coarse, dead pine wood is generally much more abundant in old-growth forest compared to managed stands (Siitonen 2001; Rouvinen et al. 2002), but protected pine and mixed coniferous forests account for only about 0.63% of the total area of productive forest below the mountainous region border in Sweden (Statistics Sweden 2010).
The situation has changed during the last decade, since use of controlled forest fires in Sweden has increased. To meet the FSC forest certification criteria, forest owners are required to burn a certain area each year (FSC 2010). In addition, regional county administrations are applying restoration fires in nature reserves to promote threatened fire-dependent species and recreate forests with characteristics of previous forests that were periodically disturbed by fires. However, given the low amounts of protected old-growth pine forest, there is a potential conflict in burning reserves to benefit fire-dependent species and create ecologically valuable dead wood, since there is also a high risk of rare wood substrates being consumed by the fires. This may potentially cause skewed distributions in the decay stages of dead wood in forest stands, with potential negative effects for species that depend on wood substrates other than fresh, fire-affected wood created by the fires.
Several studies have evaluated the value of restoration fires as a conservation tool in Fennoscandian boreal forest (Vanha-Majamaa et al. 2007; Junninen et al. 2008; Olsson and Jonsson 2010). However, to our knowledge no previous studies have addressed the effects of restoration fire on the abundance and heterogeneity of dead wood (but see Covington and Sackett 1992; Stephens and Finney 2002; Knapp et al. 2005; Stephens and Moghaddas 2005 for examples of studies on prescribed fires and their effects, among other things on fuel reduction, in boreal North America). There is a growing body of information about the habitat requirements of species depending on dead wood. Important dead wood properties for many wood-dependent species include, tree species (Jonsell et al. 1998), wood size (Økland et al. 1996) and decay stage (Renvall 1995). Three further factors affecting the micro environmental conditions (and assemblages of wood-inhabiting species) inside the log, that have been largely neglected, are the logs’ surface coverage by vegetation (non-vascular plants; mainly bryophytes and lichens) (Vanha-Majamaa et al. 2007), their ground contact (Dynesius et al. 2010) and the degree of charred wood from previous fires (Renvall 1995).
In this study we compared the heterogeneity and availability of dead wood before and after three restoration fires. The overall aim of the study is to compare the effect of restoration fire on the heterogeneity and abundance of dead wood. The study include three restoration fires planned and preformed by different operators. Hence the study should rather be viewed as a documentation of three typical restoration fires than a controlled experiment. In addition to the overall effects of the fire at stand scale we also investigated how
- fire consumption of logs is influenced by log decay stage and log base diameter
- log characteristics such as ground contact, charred surface area and vegetation cover change after fire
- tree mortality and addition of new dead wood are influenced by tree base diameter and fire intensity.
2 Methods
2.1 Studied forests
We studied forests subjected to restoration fires in three areas: Trollmosseskogen (68°03ʹN, 14°71ʹE), Långsidberget (68°87ʹN, 14°87ʹE) and Berga (71°30ʹN, 17°31ʹE), all located in the middle boreal zone of Sweden (Ahti et al. 1968). In all three areas, the tree layer is strongly dominated by Scots pine (Pinus sylvestris L.) with Norway spruce (Picea abies (L.) Karst.) only occurring at mesic locations. There are also scattered occurrences of birch (Betula pubescens Ehrh., Betula pendula Roth.) and aspen (Populus tremula L.). Trollmosseskogen and Långsidberget are both nature reserves with total areas of 548 ha and 296 ha, respectively. Within these reserves the study was carried out in a 6.3 ha stand and three smaller stands with a combined area of 5.3 ha, respectively. Berga is a private 40 ha pine forest, owned by the forest company Holmen skog, 12 ha of which was included in our study (Table 1).
| Table 1. Total area of restoration fires, surveyed area and area of sampled plots in the three studied forests, Trollmosseskogen, Långsidberget and Berga. Number of dead wood objects (logs, stumps, high stumps and snags) and measured variables are presented. *Only logs measured. | ||||
| Trollmosseskogen | Långsidberget | Berga | Measured variables | |
| Restoration fire area | 70 ha | 52 ha | 32 ha | |
| Surveyed area | 6.3 ha | 5.3 ha | 12 ha | Pre-fire dead wood Consumption of pre-fire dead wood Fire severity |
| Number of dead wood objects | 328 | 193 | 153* | |
| Total area of sampled plots | 0.4 ha | 0.5 ha | 1 ha | Pre- and post fire basal areas Tree mortality and dead wood addition Fire intensity |
Generally, about 20% of the tree layer (mostly spruce) is removed prior to a restoration fire. However, we only included uncut stands in our study, where the basal area ranged from low (8.2 m2ha–1) in Berga, due to low productivity conditions in this site, to closer to average levels for the region at Trollmosseskogen and Långsidberget (16.3 and 23 m2ha–1, respectively).The total volumes of pre-fire dead wood (logs, stumps, high stumps and snags) were 11.8, 15.0 and 1.5 m3 ha–1 at Trollmosseskogen, Långsidberget and Berga, respectively. Fire histories of the three forests, derived from fire scars in living and dead Scots pine wood, were obtained from the local County administration and Holmen skog. The fire history of Trollmosseskogen is approximate because it was derived from data obtained from a nearby nature reserve. Six to eighteen fires have been dated in the three forests, with an average apparent fire interval of 35–52 years. The latest fire years were 1888, 1920 and 1828 in Trollmosseskogen, Långsidberget and Berga, respectively.
2.2 Restoration fires
The Trollmosseskogen and Långsidberget nature reserves were burned by the local county administrations in 2004 and 2006, respectively. The private forest in Berga was burned by the forest company Holmen Skog in 2001. In all three forests the fires burned for one to three days in either July or August. The total area burned was 70, 52 and 30 ha in Trollmosseskogen, Långsidberget and Berga, respectively. The weather was dry prior to the fires, and during the fires the temperature varied between 14 and 27 ºC, the air humidity between 30–60% and the wind velocity between 1–4 m/s. The lowest temperature (14 °C) and the highest humidity (60%) occurred at the end of the fire (01:00 am) at Långsidberget.
2.3 Measurements of dead wood
The study design involved a before-and-after fire design. To quantify losses of dead wood in each stand, all pieces of coarse dead wood from pine trees, such as logs, stumps (height 0.5–1.5 m), high stumps (1.5–4.0 m) and snags (> 4.0 m) larger than 10 cm in base diameter were measured (Table 1). At Berga only logs were sampled (the material from Berga is a part from another study were stumps, high stumps and snags were not measured, see Olsson and Jonsson 2010). For all wood substrates the length or height were recorded. The diameter of intact logs was recorded at one location (base, top was set to 1 cm) and that of logs deformed by decay or fire at five locations (base, 2/5 from the base, middle, 4/5 from the base and top). Only base and top diameters were measured for stumps, high stumps and snags. Size or decay stage related effects of the fires on stumps, high stumps and snags were not analyzed since there were too few samples.
The degree of ground contact, the amount of charred surface area and vegetation (moss) cover of logs at each site (except for Berga were vegetation cover was not measured) were assessed visually to the nearest 10%. Finally, the wood decay stage was recorded for all wood substrates using the first six decay stages of the classification system presented by McCullough (1948), as modified by Söderström (1988).
To quantify the additions of dead pine wood after the fires, we established 0.1 ha plots randomly within the forest stands (Table 1). The number of plots in each stand was chosen in proportion to the burned stand area (n = 4, 5 and 10 in Trollmosseskogen, Långsidberget and Berga respectively, corresponding to 6 to 9% of the total burned uncut area; Table 1).Within each sample plot we measured the base diameter of all living pines with a base diameter ≥ 10cm before the fires. After the fires we also measured the height of all pines killed by the fires. Trees killed in the fire were measured 2005 and 2006, one (Långsidberget), two (Trollmosseskogen) and five years (Berga) after the fires. The trees killed in the fire were then used to assess additions of dead wood and tree mortality by size. The basal area of living and dead pine, spruce and birch trees before and after the fires, were also measured with a relascope from the center of each sample plot.
2.4 Measurements of humus consumption and flame height
Fire severity and fire intensity are the two main determinants of the effects of a fire on living trees and dead wood. Fire severity is often used to describe the ecological impact of a fire (Schimmel and Granström 1996; DeBano et al. 1998), but there are no consistent criteria to classify it (Ryan 2002). However, a good field indicator is the degree of consumption of the organic soil layer (i.e humus layer; Van Wagner 1983). Fire intensity is proportional to the flame length in a spreading fire, and determines the survival of plant parts above ground (Van Wagner 1973; Alexander 1982). To obtain information on the severity of the fires at stand level we measured the humus depth before and after fire at randomly selected points around the base in a subset of the logs, at two sites; Trollmosseskogen (n = 56) and Långsidberget (n = 21). Fire intensity was assessed within the 0.1 ha plots by measuring the height of continuously blackened and charred bark on each of the living and dead pine trees after the fires, at the sides of the trees where the flames had reached the highest points.
2.5 Statistical analysis
All volumes of dead wood were calculated according to the Conic-paraboloid formula (Fraver et al. 2006):

where L = length, Ab = the cross-sectional area at the base and Au = the cross-sectional area at the top. For deformed logs, the volume was first calculated separately for each wood section then the total volume was summed.
To test how decay stage and base diameter influence the consumption of logs following restoration fire we constructed a GLM model in R version 2.15.2 ( R Development Core team 2011). The consumption of logs was analyzed with a binomial error distribution and a logit link.
A second GLM model was constructed to test how tree base diameter and fire intensity influence the tree mortality. The tree mortality was analyzed with a binomial error distribution and a logit link.
For both GLM models, fire site was included as a factor. Initially the models included all factors and their interactions. By model simplification (Crawley 2007) we stepwise removed non-significant terms (p > 0.05). The steps were repeated until the model contained nothing but significant terms.
3 Results
3.1 General effects of the restoration fires
The basal area in all forests was dominated by Pinus sylvestris. In Långsidberget, the basal areas of Picea abies and Betula sp. were higher than in the other forests and represented about 1/2 and 1/3, respectively of the mean basal area of Pinus sylvestris trees. The combined pre-fire basal area of all living pine, spruce and birch trees was 16.3, 23.0 and 8.2 m2 ha–1 at Trollmosseskogen, Långsidberget and Berga, respectively. After the fire the basal area of living trees decreased and the basal area of standing dead trees increased (Fig. 1).
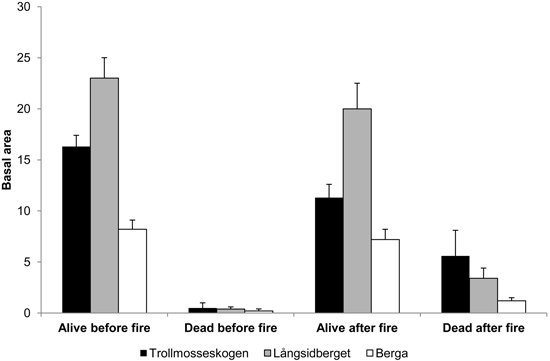
Fig. 1. Mean basal area (m2 ha–1) ± SE of all living and standing dead trees (Pinus sylvestris, Picea abies and Betula spp. ) before and after fire in the three studied forests: Trollmosseskogen, Långsidberget and Berga.
The reductions in average thickness of the humus layers, used as a measure of fire severity, were 30% at both Trollmosseskogen and Långsidberget after the fires. However, in both of these forests the fire severity was patchy and the humus consumption ranged between 0and 70% among the sample points. In 17 and 24% of the sample points more than 50% of the humus was consumed at Trollmosseskogen and Långsidberget, respectively. The highest levels of charred bark on tree trunks showed that flame heights, used as an indicator of fire intensity, were on average 3.7, 2.2 and 1.1 m at Trollmosseskogen, Långsidberget and Berga, respectively.
3.2 Dead wood losses
Fire decreased the total volume of pre-fire dead wood (logs, stumps, high stumps and snags) per hectare in the forests at Trollmosseskogen, Långsidberget and Berga (only logs) from 11.8 to 7.0, 15.0 to 11.6 and 1.5 to 1.1 m3 ha–1, respectively, corresponding to 23–41% losses of dead wood (Table 2). Further, reductions in mean volume were recorded for all types of dead wood (logs, stumps, high stumps and snags; Table 3), the reduction was greatest (70%) for high stumps at Trollmosseskogen.
| Table 2. Dead coarse pine wood (m3 ha–1) before and after the fires in Trollmosseskogen, Långsidberget and Berga (only logs and snags). | |||
| Dead wood (m3 ha–1) | |||
| Trollmosseskogen | Långsidberget | Berga | |
| Before fire a) | 11.8 | 15.0 | 1.5 |
| Loss a) | 4.8 | 3.4 | 0.4 |
| Addition b) | 35.0 | 3.8 | 2.0 |
| After fire c) | 42.0 | 15.4 | 3.1 |
| a) Based on studied stands within the burned area. b) Based on 0.1 ha plots within the studied stands. c) Calculated. | |||
| Table 3. Mean volume (m3, ± SE) of individual dead pine wood before and after the restoration fires in Trollmosseskogen, Långsidberget and Berga. *Only logs measured. | ||||||||||||
| Mean volume per wood type (m3) | ||||||||||||
| All wood types | Trollmosseskogen | Långsidberget | Berga | |||||||||
| Before fire | After fire | % loss | n | Before fire | After fire | % loss | n | Before fire | After fire | % loss | n | |
| Logs | 0.3 ± 0.03 | 0.2 ± 0.02 | 33 | 188 | 0.6 ± 0.07 | 0.5 ± 0.06 | 17 | 101 | 0.13 ± 0.01 | 0.09 ± 0.01 | 31 | 153 |
| Stumps | 0.1 ± 0.01 | 0.05 ± 0.01 | 50 | 82 | 0.2 ± 0.02 | 0.1 ± 0.01 | 50 | 56 | * | * | * | * |
| High stumps | 0.2 ± 0.03 | 0.06 ± 0.02 | 70 | 16 | 0.2 ± 0.03 | 0.1 ± 0.02 | 50 | 13 | * | * | * | * |
| Snags | 0.3 ± 0.02 | 0.2 ± 0.03 | 33 | 42 | 0.5 ± 0.07 | 0.4 ± 0.06 | 20 | 23 | * | * | * | * |
| Mean | 0.2 ± 0.02 | 0.1 ± 0.01 | 50 | 328 | 0.4 ± 0.04 | 0.3 ± 0.03 | 25 | 193 | 0.13 ± 0.01 | 0.09 ± 0.01 | 31 | 153 |
The results of the GLM model showed that all three factors, decay stage, base diameter and fire area, influenced the consumption of logs (p < 0.05). Model simplification resulted in no significant interactions between the factors (Table 4).
| Table 4. Results from the GLM model of the effect of base diameter, decay stage and fire area on the consumption of logs. No interaction term was significant and included in the model. | ||||
| Estimate | SD | Z-value | P-value | |
| Base diameter | –0.02380 | 0.01099 | –2.165 | 0.03 |
| Decay stage | 0.65499 | 0.09969 | 6.570 | <0.001 |
| Fire area | –0.41851 | 0.14822 | –2.823 | 0.005 |
Losses were greatest for logs in late decay stages (5 and 6), ranging from 26 to 54%. Losses of logs in middle decay stages (3 and 4) and early decay stages (1 and 2) were 13–35%, and just 1–13%, respectively (Fig. 2). Although the consumption of wood in late decay stages was high, few logs were completely consumed and the total number of logs decreased only marginally after the fires (Table 5). Although large logs where statistically more consumed than small, the effect was relatively small and exhibiting large variation among logs (Fig. 3).
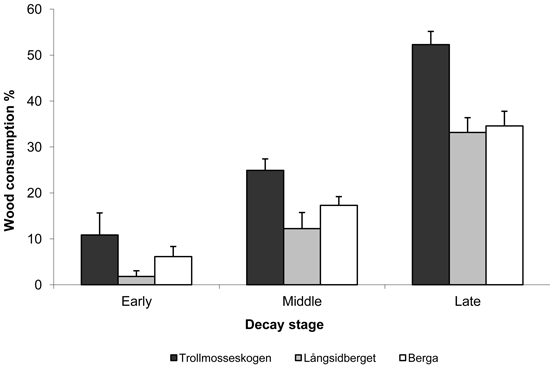
Fig. 2. Mean proportions (%) of wood in logs in indicated decay stages (± SE) consumed by fire in the three study areas. Early, middle and late decay stages refer to decay stages 1 & 2, 3 & 4 and 5 & 6, respectively (Söderström 1988 classification).
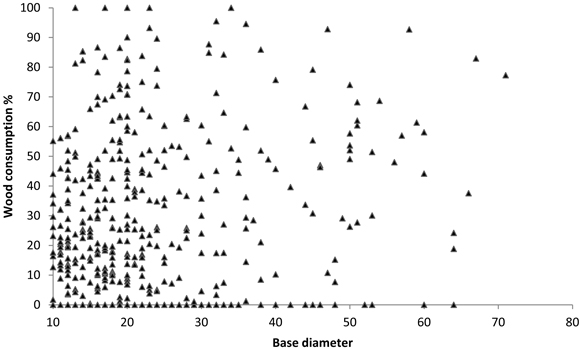
Fig. 3. Wood consumption of logs in relation to base diameter in all study areas. Each triangle representing one log, n = 188, 101, 153 in Trollmosseskogen, Långsidberget and Berga, respectively.
3.3 Changes in log characteristics
Fire increased the mean proportion of charred surface area on logs in all forests from very low values up to almost 60% and more (Table 5). In Trollmosseskogen and Långsidberget the mean areas of vegetation cover on logs decreased by 60 and 98%, respectively. The mean ground contact decreased slightly, by 11% and 4% after the fires in Trollmosseskogen and Långsidberget, while at Berga the ground contact decreased by 50%. Further, the fires also changed the morphology of the logs, as both their length and base diameter decreased (Table 5).
| Table 5. Numbers of logs and their characters before and after the fires. All values are mean values ± SE. *Not measured at Berga. | ||||||
| Logs | Trollmosseskogen | Långsidberget | Berga | |||
| Before fire | After fire | Before fire | After fire | Before fire | After fire | |
| Number | 188 | 182 | 101 | 101 | 153 | 153 |
| Base diameter (cm) | 23.5 ± 0.9 | 18.9 ± 1.0 | 29.7 ± 1.5 | 26.6 ± 1.5 | 18.7 ± 0.5 | 16.9 ± 0.5 |
| Length (m) | 9.0 ± 0.5 | 7.6 ± 0.4 | 10.3 ± 0.7 | 9.6 ± 0.7 | 7.5 ± 0.3 | 6.9 ± 0.3 |
| Charred wood (%) | 3 ± 2 | 57 ± 2 | 9 ± 2 | 68 ± 2 | 0 ± 0 | 63 ± 2 |
| Moss cover (%) | 15 ± 2 | 6 ± 1 | 42 ± 4 | 1 ± 1 | * | * |
| Ground contact (%) | 44 ± 2 | 39 ± 2 | 46 ± 3 | 44 ± 3 | 59 ± 3 | 30 ± 2 |
3.4 Dead wood additions
Additions of dead wood in terms of volumes of fire-killed trees amounted to 35.0, 3.8 and 2.0 m3 ha–1 in Trollmosseskogen, Långsidberget and Berga, respectively (Table 2). The results from the GLM model showed that all three factors, base diameter, fire intensity and fire area, influenced tree mortality and hence the addition of dead wood (p < 0.05). Model simplification resulted in no significant interactions between the factors (Table 6). Within each fire area the mean base diameter of trees killed by fire was smaller than the base diameter of trees surviving fire. Further, the fire intensity (mean flame height) was greater for tress killed by fire compared to trees surviving fire (Fig. 4).
| Table 6. Results from the GLM model of the effect of tree base diameter, fire intensity and fire area on the tree mortality. No interaction term was significant and included in the model. | ||||
| Estimate | SD | Z-value | P-value | |
| Base diameter | 0.1793 | 0.0261 | 6.868 | <0.001 |
| Fire intensity | –0.7348 | 0.1260 | –5.830 | <0.001 |
| Fire area | 0.5994 | 0.2258 | 2.654 | 0.008 |
The fires increased the volume of dead wood in all forests, since more dead wood was added than lost, but there was large variation between the forests in this respect, the increase ranged from 2% at Långsidberget to 255% at Trollmosseskogen where the fire intensity was highest. The additions largely resulted from the creation of new fresh snags.
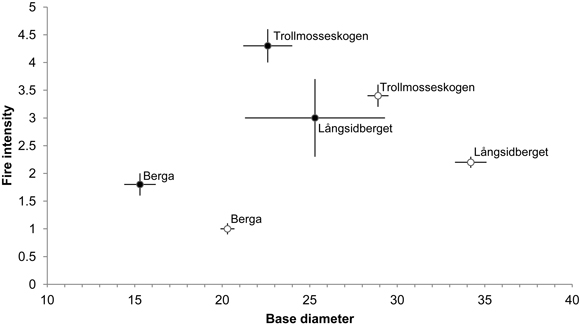
Fig. 4. Mean base diameter (cm) (± SE) in relation to mean fire intensity (i.e flame heigth (m)) (± SE) of dead (●) and alive (○) Pinus sylvestris trees following restoration fire. Numbers of dead and alive trees are: 42, 4 and 26, and 95, 126 and 301 at Trollmosseskogen, Långsidberget and Berga respectively.
4 Discussion
4.1 Influence of the fires on dead wood
The restoration fires consumed a large proportion of the existing dead wood and strongly altered the dead wood composition in all studied forests.
The proportion consumed varied between different wood types; higher volumes (up to 70%) of stumps and high stumps were consumed than of logs. This was probably because the stumps and high stumps were mostly in late decay stages. It is likely that stumps in late decay stages behave as logs in late decay stages, which were most heavily consumed. Highly decomposed wood has a lower density and thus greater flammability in dry weather conditions (DeBano et al. 1998). Prescribed fires have also reportedly consumed very high proportions (76 to 99%) of highly decayed logs in North American boreal forests (Covington and Sackett 1992; Stephens and Finney 2002), and higher proportions of logs in late decay stages than logs in early decay stages (Stephens and Moghaddas 2005).
The restoration fires also created large inputs of fresh dead wood. In all forests, the inputs of dead wood exceeded the total losses of existing dead wood, although it was most pronounced in Trollmosseskogen. This is in accordance with findings that the amount of dead wood in boreal forest is usually highest in early successional stages after fires and other large-scale disturbances (Spies et al. 1988; Siitonen 2001). The fires primarily killed smaller trees compared to larger trees in all areas and the mortality was related to the fire intensity.
Kolström and Kellomäki (1993) found that fire tolerance increased with tree size. Trees that were not directly killed by the fire may die indirectly from insect and fungi attacks later on. Thus we expect that additional mortality may occur at Långsidberget and Trollmosskogen that were sampled one and two years after the fire. The results from Berga 5 years after fire suggests that the indirect mortatlity may be rather low, since the total amount of dead wood after 5 years was still small. However, the fire intensity was also lowest at Berga, indicating that fire behavior is the most important factor influencing tree mortality
The inputs of fresh dead wood, in combination with losses of logs, stumps, high stumps and snags in late decay stages reduced the variability of dead wood after the fires. Thus, restoration fires such as these provide more homogenous sets of dead wood with respect to decay stages in individual stands. The fires also caused changes in factors that affect the microclimate within and around the logs, e.g. increasing their charred surface areas and reducing their vegetation coverage. An increase in black, charred surface area may also raise the temperature of logs, as more light is absorbed, thereby increasing evaporation rates and reducing their moisture content. Fire-associated factors that influence the microclimate of logs also included reductions in moss cover (observed on all examined logs) and ground contact. The latter leads to logs receiving less moisture and drying more rapidly, since greater proportions of their surface area are exposed to wind and sun. These observed changes all lead to a drier and warmer microclimate within and around affected logs. In Trollmosseskogen and Långsidberget the mean ground contact of logs decreased slightly after the fires, while in Berga the mean ground contact decreased more substantially. These differences may be related to the greater consumption of logs in Trollmosseskogen and Långsidberget causing logs to become deformed and collapse after the fires.
4.2 Management implications
Careful planning and clear goals are important in any application of restoration fire, particularly since its use in conservation programs is increasing. For example, one important goal is to create substrates, such as fire-killed and fire-affected dead wood, for fire-dependent species. Therefore, knowledge about fires’ effects on the consumption of existing dead wood is essential. Our study clearly shows that much of the dead pine wood in late decay stages is consumed by fire in boreal forests. Such wood is a key substrate for many red-listed species (Renvall 1995; Gärdenfors 2010), but due to intensive forestry it has become rare in the Fennoscandian boreal forest landscape (Linder and Östlund 1998). Thus, there is a trade-off between maintaining crucial dead wood substrates and restoring values associated with burned forests. Thus, when selecting sites for restoration fires it might be advisable to exclude sites with high abundance of rare and sensitive wood substrates (i.e. large and highly decayed logs).
Depending on its intensity, fire can generate a high density of dead wood, e.g. Sidoroff et al. (2007) found that burning young, managed pine stands increased the amount of dead wood by 10 m3 ha–1. Thus, fires can provide important additions of dead wood to increase biodiversity in managed forest stands, but forest managers must be aware that fire intensity and the following inputs of dead wood can be strongly affected by diverse factors, for example, ignition patterns, wind speeds and the moistness of humus (Ryan 2002).
Because fire decreases dead wood heterogeneity (i.e dead wood types and decay stages) at the stand level it is also important to plan and apply fires in a larger landscape context.
For example, restoration fires could be applied to different forest stands within a landscape at different time intervals, thereby maintaining the heterogeneity of wood types required for the full spectrum of associated species at landscape-level in both spatial and temporal dimensions.
Acknowledgements
We thank the County Administrations of Jämtland and Dalarna counties and David Rönnblom at Holmen Skog AB for providing access to the restoration fire areas. We also thank Daniel Lussetti for help during the field work. The study was performed with financial support from The Centre for Environmental Research (CMF) to Jörgen Olsson and The Swedish Research Council for Environment, Agricultural Sciences and Spatial Planning (Formas) to Mattias Edman and Bengt-Gunnar Jonsson.
References
Ahti T., Hämet-Ahti L., Jalas J. (1968). Vegetation zones and their sections in north-western Europe. Annales Botanici Fennici 5: 167–211.
Alexander M.E. (1982). Calculating and interpreting forest fire intensities. Canadian Journal of Botany 60: 349–357.
Berg A., Ehnström B., Gustafsson L., Hallingbäck T., Jonsell M., Weslien J. (1994). Threatened plant, animal, and fungus species in Swedish forests: distribution and habitat associations. Conservation Biology. 8: 718–731.
Berglund H., O’Hara R.B., Jonsson B.G. (2009). Quantifying habitat requirements of tree-living species in fragmented boreal forests with Bayesian methods. Conservation Biology 23: 1127–1137.
Berglund H., Jönsson M.T., Penttilä R., Vanha-Majamaa I. (2011). The effects of burning and dead-wood creation on the diversity of pioneer wood-inhabiting fungi in managed boreal spruce forests. Forest Ecology and Management 261: 1293–1305.
Carcaillet C., Bergman I., Delorme S., Hörnberg G., Zackrisson O. (2007). Long-term fire frequency not linked to prehistoric occupations in northern Swedish boreal forest. Ecology 88: 465–477.
Covington W.W., Sackett S.S. (1992). Soil mineral nitrogen changes following prescribed burning in ponderosa pine. Forest Ecology and Management 54: 175–191.
Crawley M.J. (2007). The R book. John Wiley and Sons Ltd, Chichester, England.
Dahlberg A., Stokland J. (2004). Vedlevande arters krav på substrat – en sammanställning och analys av 3600 arter. Skogsstyrelsen, Jönköping. [In Swedish].
DeBano L.F., Neary D.G., Ffolliott P.F. (1998). Fire’s effects on ecosystems. John Wiley and Sons, New York.
Dynesius M., Gibb H., Hjälten J. (2010). Surface covering of downed logs: drivers of a neglected process in dead wood ecology. Plos One 5: e13237.
Engelmark O. (1984). Forest fires in the Muddus national park (Northern Sweden) during the past 600-years. Canadian Journal of Botany 62: 893–898.
Engelmark O., Hytteborn H. (1999). Coniferous forests. Acta Phytogeographica Suecia 84: 55–74.
Fraver S., Ringvall A., Jonsson B.G. (2007). Refining volume estimates of down woody debris. Canadian Journal of Forest Research 37: 627–633.
Fridman J., Walheim M. (2000). Amount, structure and dynamics of dead wood on managed forestland in Sweden. Forest Ecology and Management 131: 23–36.
FSC. 2010. Svensk FSC-standard för certifiering av skogsbruk. Forest Stewardship Council A.C., Uppsala. [In Swedish].
Gärdenfors U. (2010). The 2010 red list of Swedish species. ArtDatabanken. SLU, Uppsala.
Granström A. (2001). Fire management for biodiversity in the European boreal forest. Scandinavian Journal of Forest Research 3: 62–69.
Groven R., Niklasson M. (2005). Anthropogenic impact on past and present fire regimes in a boreal forest landscape of southestern Norway. Canadian Journal of Forest Research 35: 2719–2726.
Harmon M.E., Franklin J.F., Swanson F.J., Sollins P., Gregory S.V., Lattin J.D., Anderson N.H., Cline S.P., Aumen N.G., Sedell J.R., Lienkaemper G.W., Cromack K., Cummins K.W. (1986). Ecology of coarse woody debris in temperate ecosystems. Advances in Ecological Research 15: 133–302.
Jonsell M., Weslien J., Ehnström B. (1998). Substrate requirements of red-listed saproxylic invertebrates in Sweden. Biodiversity and Conservation 7: 749–764.
Junninen K., Kouki J., Renvall P. (2008). Restoration of natural legacies of fire in European boreal forests: an experimental approach to the effects on wood-decaying fungi. Canadian Journal of Forest Research 38: 202–215.
Kaila L., Martikainen P., Punttila P. (1997). Dead trees left in clear cuts benefit saproxylic Coleoptera adapted to natural disturbance in boreal forest. Biodiversity and Conservation 6: 1–18.
Knapp E.E., Keeley J.E., Ballenger E.A., Brennan T.J. (2005). Fuel reduction and coarse woody debris dynamics with early season and late season prescribed fire in a Sierra Nevada mixed conifer forest. Forest Ecology and Management 208: 383–397.
Kolström T., Kellomäki S. (1993). Tree survival in wildfires. Silva Fennica 4: 277–281.
Lehtonen H. (1998). Fire history recorded on dead pine trunks and stumps: influence of land use and fires on forest structure in North Karelia. Scandinavian Journal of Forest Research 13: 462–468.
Linder P. (1998). Structural changes in two virgin boreal forest stands in central Sweden over 72 years. Scandinavian Journal of Forest Research 13: 451–461.
Linder P., Östlund L. (1998). Structural changes in three mid-boreal Swedish forest landscapes, 1885- 1996. Biological Conservation 85: 9–19.
McAlister S. (1995). Species interactions and substrate specificity among log- inhabiting bryophyte species. Ecology 76: 2184–2195.
McCullough H.A. (1948). Plant succession on fallen logs in a virgin spruce-fir forest. Ecology 29: 508–513.
Muona J., Rutanen I. (1994). The short-term impact of fire on the beetle fauna in boreal coniferous forest. Annales Zoologici Fennici 31: 109–121.
Niklasson M., Granström A. (2000). Numbers and size of fires: long-term spatial explicit fire history in a Swedish boreal landscape. Ecology 81: 1484–1499.
Økland B., Bakke A., Hagvar S., Kvamme T. (1996). What factors influence the diversity of saproxylic beetles? A multiscaled study from a spruce forest in southern Norway. Biodiversity and Conservation 5: 75–100.
Olsson J., Jonsson B.G. (2010). Restoration fire and wood-inhabiting fungi in a Swedish Pinus sylvestris forest. Forest Ecolology and Management 259: 1971–1980.
Östlund L., Zackrisson O., Axelsson A.L. (1997). The history and transformation of a Scandinavian boreal landscape since the 19th century. Canadian Journal of Forest Research 27: 1198–1206.
Penttilä P., Kotiranta H. (1996). Short-term effects of prescribed burning on wood-rotting fungi. Silva Fennica 30: 399–419.
Penttilä R. (2004). The impacts of forestry on polyporous fungi in boreal forests. PhD Thesis. University of Helsinki, Helsinki, Finland.
Pugh G.J.F., Boddy L. (1988). A view of disturbance and life strategies in fungi. In: Boddy L., Waitling R., Lyon A.J.E. (eds.). Fungi and ecological disturbance. Proceedings of the Royal Society of Edinburgh. p. 3–11.
R Development Core Team. (2011). R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0. http://www.R-project.org/.
Renvall P. (1995). Community structure and dynamics of wood-rotting Basidiomycetes on decomposing conifer trunks in northern Finland. Karstenia 35: 1–51.
Rouvinen S., Kuuluvainen T., Karjalainen L. (2002). Coarse woody debris in old Pinus sylvestris dominated forests along a geographic and human impact gradient in boreal Fennoscandia. Canadian Journal of Forest Research 32: 2184–2200.
Ryan K.C. (2002). Dynamic interactions between forest structure and fire behavior in boreal forest ecosystems. Silva Fennica 36: 13–19.
Schimmel J., Granström A. (1996). Fire severity and vegetation response in the boreal Swedish forest. Ecology 77: 1436–1450.
Sidoroff K., Kuuluvainen T., Tanskanen H., Vanha-Majamaa I. (2007). Tree mortality after low-intensity prescribed fires in managed Pinus sylvestris stands in southern Finland. Scandinavian Journal of Forest Research 22: 2–12.
Siitonen J. (2001). Forest management, coarse woody debris and saproxylic organisms. Fennoscandian boreal forests as an example. Ecological Bulletins 49: 11–41.
Söderström L. (1988). Sequence of bryophytes and lichens in relation to substrate variables of decaying coniferous wood in northern Sweden. Nordic Journal of Botany 8: 89–97.
Spies T.A., Franklin J.F., Thomas T.B. (1988). Coarse woody debris in douglas-fir forests of western Oregon and Washington. Ecology 69: 1689–1702.
Statistics Sweden. 2010. Protected nature 2010. Statistics Sweden, Serie MI Miljövård SM 1101. [In Swedish].
Stephens S.L., Finney M.A. (2002). Prescribed fire mortality of Sierra Nevada mixed conifer tree species: effects of crown damage and forest floor combustion. Forest Ecology and Management 162: 261–271.
Stephens S.L., Moghaddas J.J. (2005). Fuel treatment effects on snags and coarse woody debris in a Sierra Nevada mixed conifer forest. Forest Ecology and Management 214: 53–64.
Tikkanen O.-P., Martikainen P., Hyvärinen E., Junninen K., Kouki J. (2006). Redlisted boreal forest species of Finland: associations with forest structure, tree species, and decaying wood. Annales Zoologici Fennici 43: 373–383.
Toivanen T., Kotiaho J.S. (2007). Mimicking natural disturbances of boreal forest: the effects of controlled burning and creating dead wood on beetle diversity. Biodiversity and Conservation 16: 3193–3211.
Vanha-Majamaa I., Lilja S., Ryömä R., Kotiaho J.S., Laaka-Lindberg S., Lindberg H., Puttonen P., Tamminen P., Toivanen T., Kuuluvainen T. (2007). Rehabilitating boreal forest structure and species composition in Finland through logging, dead wood creation and fire: The EVO experiment. Forest Ecology and Management 250: 77–88.
Van Wagner C.E. (1973). Height of crown scorch in forest fires. Canadian Journal of Forest Research 3: 373–378.
Van Wagner C.E. (1983). Fire behaviour in northern conifer forests. In: Wein R.W., Mac Lean D.A. (eds.). The role of fire in northern circumpolar ecosystems. John Wiley and Sons. New York. p. 65–80.
Zackrisson O. (1977). Influence and size of forest fires on the North Swedish boreal forest. Oikos 29: 22–32.
Total of 56 references

