Interactive effects of defoliation and climate change on compensatory growth of silver birch seedlings
Huttunen L., Ayres M. P., Niemelä P., Heiska S., Tegelberg R., Rousi M., Kellomäki S. (2013). Interactive effects of defoliation and climate change on compensatory growth of silver birch seedlings. Silva Fennica vol. 47 no. 3 article id 964. https://doi.org/10.14214/sf.964
Highlights
- The main components affecting growth compensation in silver birch seedlings are the timing and severity of foliage damage
- The ability to compensate growth is also dependent upon the limits of temperature and nutrient availability
- The responses of birches imply that folivory does not necessarily lead to reduced net productivity under changing climate
Abstract
Atmospheric warming increases the abundance of insect herbivores and intensifies the risk of defoliation, especially in high latitude forests. At the same time, the effects of increasing temperature and CO2 on plant responses to foliage damage are poorly understood. We examined if previous-year defoliation, varying between 0 and 75% of total leaf area, and different combinations of elevated temperature, CO2 and nutrient availability alter the growth of two-year old silver birch (Betula pendula Roth) seedlings. We measured the greatest height growth in seedlings that were fertilized and defoliated twice at the level of 50% of total leaf area, and subjected to elevated temperature with ambient CO2. The lowest growth was recorded in unfertilized seedlings that were defoliated twice at the level of 25% of total leaf area, and grew under ambient temperature with ambient CO2. The total biomass increased in all seedlings that were fertilized or grew under elevated temperature. The root: shoot ratios were low in defoliated seedlings, or seedlings subjected to fertilization or temperature elevation. Our conclusion is that ability of birches to compensate height growth is highly dependent upon the magnitude and frequency of defoliation on the limits of temperature and nutrient availability. These responses imply that folivory does not necessarily lead to reduced net productivity of trees under changing climate.
Keywords
root;
shoot ratio;
biomass;
Betula pendula;
elevated CO2;
elevated temperature;
folivory
-
Huttunen,
Section of Ecology, Department of Biology, University of Turku, FI-20014 Turku, Finland
E-mail
liisa.huttunen@utu.fi

- Ayres, Biological Sciences, Dartmouth College, Hanover, NH 03755, USA E-mail matt.ayres@dartmouth.edu
- Niemelä, Section of Biodiversity and Environmental Science, Department of Biology, University of Turku, FI-20014 Turku, Finland E-mail pekka.niemela@utu.fi
- Heiska, The Finnish Forest Research Institute, Punkaharju Unit, Finlandiantie 18, FI-58450 Punkaharju, Finland E-mail susanne.heiska@metla.fi
- Tegelberg, Digitarium - Digitization Centre of the Finnish Museum of Natural History and the University of Eastern Finland, Joensuu Science Park, P.O. Box 111, FI-80101 Joensuu, Finland E-mail riitta.tegelberg@helsinki.fi
- Rousi, The Finnish Forest Research Institute, Vantaa Unit, P.O. Box 18, FI-01301 Vantaa, Finland E-mail matti.rousi@metla.fi
- Kellomäki, Faculty of Science and Forestry, School of Forest Sciences, University of Eastern Finland, P.O. Box 111, FI-80101 Joensuu, Finland E-mail seppo.kellomaki@uef.fi
Received 22 March 2013 Accepted 27 June 2013 Published 10 July 2013
Views 188926
Available at https://doi.org/10.14214/sf.964 | Download PDF

1 Introduction
Climate warming increases the abundance of insect herbivores and expands their northern distributions, which will magnify the risks of foliar damage in mid- and high-latitude forests (Wolf et al. 2008; Jepsen et al. 2011). Anticipated changes in insect communities and intensified risk of defoliation would tend to reduce the annual net primary productivity, NPP (Pinkard et al. 2011; Jacquet et al. 2012) and cause a decrease in nutrient turnover rates of different forest types (Ritchie et al. 1998). For example a massive mountain pine beetle (Dendroctonus ponderosae Hopkins) outbreak dramatically decreased photosynthetic capacity and NPP in coniferous forests of northern America for a decade (Kurz et al. 2008). However, the consequences for biomass production depend on the extent to which plants are able to compensate for losses to herbivores. Several studies with trees have demonstrated partial compensation for insect feeding damages (Mattson et al. 2004; Osier and Lindroth 2004; Jacquet et al. 2012; Landhäusser and Lieffers 2012), whereas some perennial herbaceous plants can even overcompensate for lost foliage (Agrawal 2000; Wise and Abrahamson 2008). Here the term ‘overcompensation’ refers to greater re-growth and/or seed production following herbivory than without herbivory.
Plant responses to tissue loss are presumably influenced by evolutionary history and growth form, but also by plant physiological state, environmental conditions and resource availability, including water, CO2 and nutrients (Rosenthal and Kotanen 1994; Straus and Agrawal 1999; Eyles et al. 2009). Indeed, some prominent examples of over-compensative growth have come from experiments where plants were cultivated under conditions that favored their performance (Wise and Abrahamson 2008). It is possible that the ability of trees to recover from defoliation is improved by global patterns of increasing atmospheric CO2 and temperature, and modified by concurrent changes in mineral nitrogen availability (Anttonen et al. 2002; Niinemets et al. 2002; Zhang et al. 2006; Riikonen et al. 2010). For instance, long-term exposure to elevated CO2 has restored the photosynthetic capacity of defoliated trees to equivalent of that of intact counterparts growing under ambient CO2 (Handa et al. 2005). Furthermore, Huttunen et al. (2007) reported that a short-term exposure to elevated temperature and CO2 caused over-compensation of carbon uptake and biomass growth in defoliated silver birch (Betula pendula Roth) seedlings. However, the concurrent long-term effects of atmospheric warming and increased CO2 on productivity of trees that have suffered defoliation are still unknown (Pinkard et al. 2011).
Loss of foliage often changes resource partitioning between above- and belowground parts of plants favoring shoot growth at the expense of root growth; this causes decreases in root: shoot ratios (Markkola et al. 2004). Removal of foliage increases fine root mortality leading to decreased absorption of nutrients from the soil (Tuomi et al. 1990). Moreover, if defoliation stimulates photosynthesis in the remaining or re-sprouting leaves (Handa et al. 2005; Turnbull et al. 2007), the consequence may be a disproportionate change in carbon versus nutrient reserves in nutrient limited species as birches (Zhang et al. 2006). Therefore, an increase in nutrient availability has a potential to improve the ability of birches to compensate growth and recover after severe defoliation (Eyles et al. 2009). In fact, fertilization has enhanced the height and biomass growth of previously defoliated silver birch seedlings (Huttunen et al. 2007). This is especially relevant because the broad impacts of global change frequently include increased atmospheric deposition of nitrogen (Galloway et al. 2004) and accelerated mineralization rates of organic nitrogen in soil (Verburg 2005). On the other hand, defoliation may weaken the ability of roots to store resources as a result of increased carbon allocation to aboveground organs (Huttunen et al. 2012). Compromised store reserves may cause delays in leaf emergence and growth resumption in the season following insect outbreak (Tuomi et al. 1989).
The objective of this study was to examine if previous-year defoliation and different combinations of elevated temperature, CO2 and nutrient availability alter the growth of two-year old silver birch seedlings. Earlier, in a similar kind of system, Huttunen et al. (2007; 2008) examined the direct effects of defoliation on the birch seedlings. In the present study, the height and biomass growth of the same birches were quantified to test the following hypotheses:
- Interactive effects of defoliation, elevated temperature, CO2 and nutrient availability increase resource investment to the aboveground organs resulting in over-compensation of shoot height and biomass growth.
- Resource allocation to aboveground organs decreases belowground storage, which is evident as reduced root: shoot ratios in defoliated seedlings.
2 Material and methods
2.1 Plant material and treatments
Throughout summer 2002, silver birch seedlings that originated from south-eastern Finland (61°48´N, 29°18´E) were grown in 16 climate-controlled closed-top chambers at the Mekrijärvi Research Station (62°47´N, 30°58´E), University of Eastern Finland. The chambers had an internal volume of 19.3 m3 and a ground area of 5.2 m2. The seedlings were planted in trays containing cells with a volume of 1.2 dm3 filled with unfertilized commercial peat (VAPO,Vapo Oy, Jyväskylä, Finland). Each seedling tray constituted a fertilizer and defoliation treatment combination unit for 24 seedlings. These units were placed in chambers totaling six units per chamber (Fig. 1A).
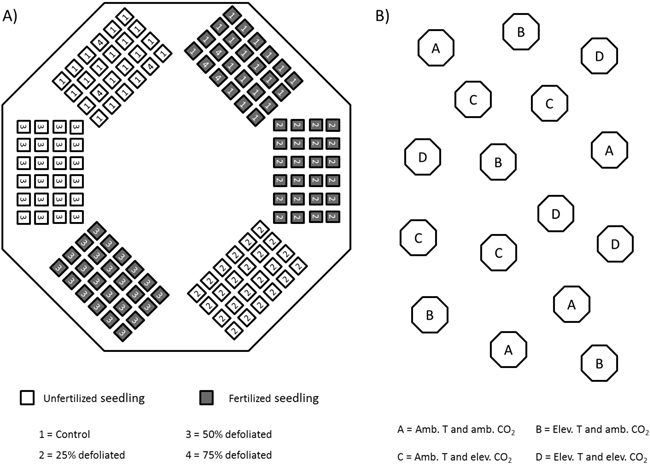
Fig. 1. Schematic figure of silver birch seedlings in climate-controlled closed-top chambers in 2002. A) randomized placement of seedling-trays in each chamber; B) location of chambers in the field.
The climate treatments in chambers included combinations of ambient temperature tracing the natural temperature outside with ambient CO2 (= 360 parts per million, ppm = µmol mol–1; climate 1), ambient temperature with elevated CO2 (= 720 µmol mol–1; climate 2), elevated temperature being 2 °C higher than the ambient temperature with ambient CO2 (climate 3) and elevated temperature with elevated CO2 (climate 4). The treatments were designed to correspond to the scenario of an increased temperature and a doubled atmospheric CO2 concentration in situ eastern Finland (Kellomäki and Väisänen 1997). Each treatment was replicated in four chambers (Fig. 1B). The temperature and CO2 were automatically adjusted at target levels by a computer-controlled heating and cooling system together with a set of magneto-electric valves controlling the supply of pure CO2 (CO2 transmitter; GMP 111, Vaisala, Finland, CO2 control module; ISM 112, Gantner Electronic, Austria). A heat exchanger linked to a refrigeration unit (CAJ-4511YHR, L’Unité Hermetique, La Verpilliére, France) was installed in each chamber. A fan drew unfiltered air into chambers through a duct with airflow rate varying from 0.2 to 0.4 m3 s–1. The flow rate was determined periodically depending on the season and weather conditions. Detailed description of the chamber system is provided by Kellomäki et al. (2000). To reduce the effect of within-chamber variation, the seedling-trays were twice re-positioned during the summer.
In each chamber, the seedling-trays were randomly assigned into two fertilizer treatments, which were 0and 130 kg nitrogen (N) ha–1 year–1, or 0and 63.7 mg N per seedling, respectively. This amount corresponded to the commonly used optimal amount of N applied for 1-year-old birch seedlings in Finnish forest nurseries (Juntunen and Rikala 2001). Fertilizer was dissolved in water and applied weekly for seven weeks starting in early June. The first three times the fertilizer was Kekkilä SuperX-9 (NPK: 19-4-20), and the following four times, it was Kekkilä SuperX-5 (NPK: 12-5-27). Same quantity of fertilizer, 60.7 mg per seedling containing of 11.5 or 7.3 mg N depending on the fertilizer was applied each time to maintain the prescribed levels of N. To prevent excessive dehydration, all the seedlings were irrigated daily throughout the summer. Detailed description of the different fertilizer treatments and seedling material is provided by Huttunen et al. (2007; 2008).
Seedlings under each fertilizer and climate treatment combination were allocated to 0, 25, 50 or 75% defoliation. Defoliation at levels of 25 and 50% of total leaf area was carried out twice, on July 1st and 29th, by tearing the corresponding amount from the apical portion of each leaf. The second tissue removal was directed at those leaves that had developed along with height growth after the first tissue removal. Defoliation at the level of 75% of total leaf area was performed once, on July 29th; for the treatment three seedlings were randomly selected among intact seedlings in each fertilizer treatment per chamber. This was done to investigate carbon allocation and accumulation between vegetative and storage organs of the seedlings after late-season defoliation. Further details of defoliation treatments are provided in Huttunen et al. (2007).
In October 2002, the chamber system had to be disabled because of operating costs. A total of 384 seedlings comprising of 12 seedlings per treatment combination, or three seedlings per treatment combination per chamber were assigned for the second year experiment. All seedlings were dormant; their height growth was terminated, and bud formation and leaf abscission completed. This enabled their outdoor storage in an open shelter (10 m × 10 m, height 1.5 m), which was fenced with chicken wire and covered with a plastic tarpaulin to prevent damage by hares (Lepus timidus L.). Each seedling was positioned at random in the shelter.
Prior to the bud burst in spring 2003, the general condition and height of seedlings was determined. A total of 218 plants suffered from broken stems or had died during the winter, and they were excluded from analyses. The exclusion concerned the entire treatment group of seedlings that were unfertilized, exposed to ambient temperature with ambient CO2 and defoliated at the level of 75% of total leaf area. The overwintered 166 seedlings comprised of different treatment combinations including 3 to 12 seedlings per combination with mean heights (±standard errors) varying from 17.3 (±2.3) cm to 47.8 (±4.0) cm. These seedlings were transferred to Joensuu (62°40’N, 29°45’E), Finland, and individually transplanted into plastic pots with a volume of 2 dm3 (15.5 cm in diameter, 13.5 cm in depth). Pre-fertilized commercial peat (1.5 g N kg–1, VAPO, Vapo Oy, Jyväskylä, Finland) was used as a growth medium to maximize seedling survival after transplanting. The amount of N was considered to be minor compared to the actual N treatment represented later on. During transplanting, the roots were carefully checked. All the seedlings appeared not to suffer from a lack of space, and their roots looked generally normal.
About three weeks after bud burst in early June 2003, seedlings were allocated to environments that corresponded to those at the Mekrijärvi chamber system. Climate 1 (ambient, see above) was in a laboratory greenhouse, and climates 2, 3 and 4 in Conviron chambers (Conviron, Controlled Environments Ltd, Winnipeg, Canada). The separate climate treatments were constant during day and night. Relative humidity of the air was regulated at 70% corresponding to a long-term average of June-September period at Mekrijärvi in 1998–2002. The ambient temperature was adjusted at 20 °C and the elevated temperature at 25 °C. The CO2 concentration for the ambient treatment was 360 µmol mol –1, and for the elevated treatment 720 µmol mol –1. In Conviron chambers, humidity, temperature, and CO2 regulation was managed by CMP5090 control system keeping the conditions at target levels throughout the experiment. In greenhouse, the regulation was executed by temperature and CO2 sensor technology connected to thermostats and electric ventilators. The location of seedlings of each environment was changed weekly during the experiment to reduce the effect of within-chamber variation. Fig. 2 illustrates the experimental setup in 2003.
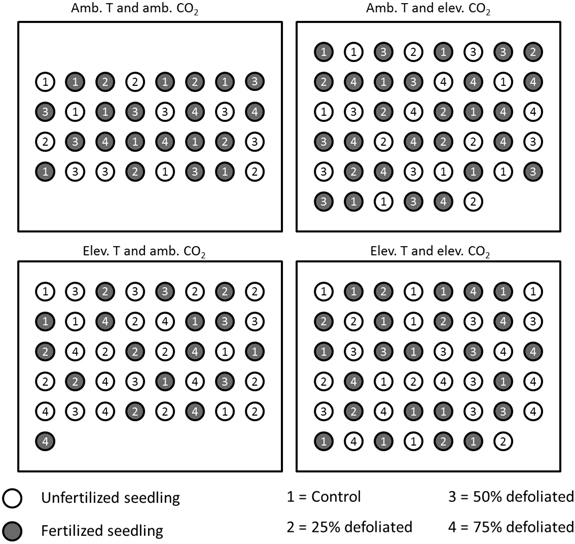
Fig. 2. Placement of differently treated silver birch seedlings in the built environments in 2003 (figure is not drawn to scale). Within each climate treatment, all the seedlings were located randomly, and their positions re-randomized each week. The distance between the seedlings was 20 cm.
Photon flux density (PFD) in the built environments was measured by LI-COR LI-185B quantum/radiometer/photometer (LI-COR, Lincoln, NE, USA). In Conviron chambers, the PFD was adjusted by data loggers installed inside the chambers. The PFD was automatically tuned at different levels according to the time of day by glow lamps (100 W, 230 V; 48 lamps per m2). The PFD between 06:00 h and 23:00 h was 600 μmol m–2 s–1. From 23:00 h to 01:00 h and from 04:00 h to 06:00 h, it was 400 μmol m–2 s–1. Between 01:00 h and 04:00 h, the PFD was 200 μmol m–2 s–1. From August to September, the PFD was adjusted at 500 μmol m–2 s–1, and the lamps were on from 07:00 h to 20:00 h. In the greenhouse, PFD followed natural periodicity and was assisted with 18 high pressure sodium lamps (400 W and 230 V; 1.5 lamps per m2) with a PFD of 600 μmol m–2 s–1. The lamps were switched off at nights and at bright day-light, when the PFD exceeded 600 μmol m–2 s–1. In the built environments, the PFD did not fully correspond to that prevailing at 62°N; therefore it may have affected the photoperiod of the seedlings. However, the PFD was in the range of saturating irradiance for leaf water conductance in silver birch, which varies between 100 and 800 µmol m–2 s–1 and is dependent on foliage position on the crown (Sellin and Kupper 2005). Since leaf water transport affects stomata and thus the diffusion of CO2, it has an effect on photosynthesis (Sellin and Kupper 2005). Moreover, saturation point for the photosynthetic PFD in silver birch is around 900 µmol m–2 s–1; exceeding this point causes no increase in photosynthesis (Wang et al. 1995).
During the second year experiment, fertilizer treatments for seedlings were 0and 100 kg N ha–1 year–1, or 0and 49.0 mg N per plant, respectively. Compared with the first year experiment (Huttunen et al. 2007; 2008), the amount of fertilizer was reduced to minimize the risk of metabolic stress. The fertilizer (Kekkilä Taimi-Superx; NPK: 19-4-20) was dissolved in water, and the solution was given once a week for nine weeks, starting at June 18th. The same quantity of fertilizer, 28.7 mg per seedling containing 5.5 mg N, was applied each time. All the seedlings were irrigated daily with 0.1 dm3 of water to prevent excessive dehydration.
2.2 Measurements
Height of the seedlings was measured weekly until the termination of the experiment in early September. By this time, over 80% of the seedlings had formed apical buds. The height growth of seedlings had started to decelerate earlier, by the end of July.
After the final height measurement, the plants were cut from the root collar, and the shoots were divided into stems including branches and leaves. Five fully grown leaves of each harvested plant were randomly sampled for the leaf area measurement using a portable meter (LI-COR LI-3000, LI-COR, Lincoln, NE, USA). Thereafter all the leaves were dried at 60 °C for 48 h and weighed. The leaf mass per area (SLW, mg mm–2) of the individual leaves and the mass of all the leaves were used to estimate the total leaf area of a plant. The roots were carefully cleaned from adhering peat, and dried with stems at 105 °C for 48 h. Then the dry mass of each plant part was determined for calculations of the root: shoot ratios (dry mass of roots: dry mass of stems + leaves).
2.3 Statistical analyses
Linear mixed models (the MIXED procedure, SAS 9.3 software, SAS Institute Inc., Cary, NC, USA) were applied to analyze the effects of CO2, temperature, fertilization and defoliation and their interactions on the annual height increment (cm), the total biomass (g), the leaf area (cm2) and the root: shoot ratio. Defoliation was treated as a continuous variable. This was done to investigate if increase in defoliation level caused a trend in the response variables. The height of the seedlings at the beginning of the second year experiment (the initial height, IH) was included in the model as a covariate. Due to the split-plot design and a lack of uncorrelated replicates for climate treatments in 2003, the four-way interaction, i.e., CO2 × temperature × fertilization × defoliation treatment combination was treated as a random effect in all analyses; in this case, defoliation was as a categorical variable. The degrees of freedom for the error terms were computed using the Satterthwaite method. Data were log-transformed to achieve normal distributions and homogeneity of variances for the residuals; the normality and homogeneity were tested using scatter plots and Q-Q plots, where “Q” stands for quantile. The reported results in text and figures are the mean values that are obtained from untransformed data. In text, the standard errors are presented in brackets after the mean values. In figures, the trend lines are based on the least squares means provided by SAS/MIXED. The relationship between the treatment combinations and the amount of dead seedlings was tested using logistic regression analysis (The LOGISTIC procedure, SAS 9.3).
3 Results
3.1 Seedling exclusion
A total of 218 seedlings (56.8% of all plants) were excluded from the analyses due to broken stems or death during the winter 2002–2003. The exclusion was the highest under the combination of ambient temperature with ambient CO2 including 64 seedlings (Table 1). Considerable amount of unfertilized plants or plants that were defoliated at a level of 75% of total leaf area were excluded (Table 1). However, the differences in the proportion of excluded plants in the climate × fertilization × defoliation combinations were not statistically significant, P = 0.249.
| Table 1. Summary of cross tabulations for survived and dead seedlings. The term “survived” refers to overwintered seedlings and “dead” to seedlings that were winterkilled or suffered from broken stems. The percentage (%) of each group indicates the proportion of all the 384 seedlings in the 2003 experiment. | ||||||||||
| Treatment | ||||||||||
| Amb. T and amb. CO2 | Amb. T and elev. CO2 | Elev. T and amb. CO2 | Elev. T and elev. CO2 | Total | ||||||
| Number | % | Number | % | Number | % | Number | % | Number | % | |
| Survived | 32 | 8.3 | 46 | 12.0 | 41 | 10.7 | 47 | 12.2 | 166 | 43.2 |
| Dead | 64 | 16.7 | 50 | 13.0 | 55 | 14.3 | 49 | 12.8 | 218 | 56.8 |
| Total | 96 | 25.0 | 96 | 25.0 | 96 | 25.0 | 96 | 25.0 | 384 | 100.0 |
| Fertilized | Unfertilized | Total | ||||||||
| Number | % | Number | % | Number | % | |||||
| Survived | 90 | 23.4 | 76 | 19.8 | 166 | 43.2 | ||||
| Dead | 102 | 26.6 | 116 | 30.2 | 218 | 56.8 | ||||
| Total | 192 | 50.0 | 192 | 50.0 | 384 | 100.0 | ||||
| Control | 25% defoliated | 50% defoliated | 75% defoliated | Total | ||||||
| Number | % | Number | % | Number | % | Number | % | Number | % | |
| Survived | 49 | 12.8 | 41 | 10.7 | 40 | 10.4 | 36 | 9.4 | 166 | 43.2 |
| Dead | 47 | 12.2 | 55 | 14.3 | 56 | 14.6 | 60 | 15.6 | 218 | 56.8 |
| Total | 96 | 25.0 | 96 | 25.0 | 96 | 25.0 | 96 | 25.0 | 384 | 100.0 |
3.2 Absolute annual height increment
Irrespective of treatment, the annual height increment in all seedlings varied between 5 cm (absolute minimum) and 76 cm (absolute maximum). Defoliation, temperature and fertilization had significant main effects on the height gain (P = 0.028, P = 0.006 and P < 0.001, respectively); on average, the plant height increment responded increasingly to elevated levels of temperature, nutrients or defoliation (Fig. 3). Between treatment combinations, the greatest mean height increment, 55.2 (±5.5) cm, was recorded in seedlings that were subjected to elevated temperature with ambient CO2, fertilized and defoliated once at the level of 50% of total leaf area. As a point of comparison, the seedlings that were subjected to the same climate and fertilization treatments, but not defoliated gained an average annual height of 44.3 (±4.4) cm, and seedlings under the same climate treatment without defoliation and fertilization increased their height by 11.6 (±2.1) cm (Fig. 3). Under ambient temperature with ambient CO2, the average annual height growths were the lowest; for example, those seedlings that were intact and not fertilized gained 11.0 (±2.1) cm’s growth. Here the height gain was 2.3 cm lower than in their unfertilized counterparts, which were defoliated by 50% of total leaf area (Fig. 3). Under the same climate treatment, the estimated response in fertilized seedlings to increasing levels of defoliation was negative, although fertilizing typically increased the growth (Fig. 3).
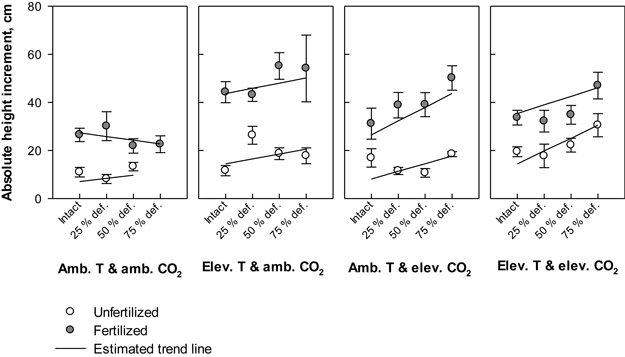
Fig. 3. Absolute height increment (cm) of silver birch seedlings during the growing season 2003. The plot results are based on untransformed data, where mean values of different treatment combinations with the standard errors are presented. The trend line is based on the least squares means (LS means) provided by SAS/MIXED. Abbreviations: Amb. T = ambient temperature; Elev. T = elevated temperature; Amb. CO2 = ambient CO2; Elev. CO2 = elevated CO2.
3.3 Biomass, leaf area and root: shoot ratio
Total biomass of all seedlings ranged between 1.7 and 44.3 g dry mass (DW), and was significantly affected by fertilizing or temperature (P < 0.001 and P = 0.008, respectively). In fertilized seedlings, the biomass was approximately 18.3 (±0.7) g DW, while in unfertilized seedlings the mass was less than half, 8.5 (±0.5) g DW. The seedlings that were subjected to elevated temperature treatment, gained a biomass of 14.9 (±0.8) g DW. Exposure to ambient temperature treatment resulted in a lower biomass gain, 12.6 (±0.8) g DW.
Total leaf area of all seedlings varied between 73 (absolute minimum) and 2569 cm2 (absolute maximum), and was significantly affected by fertilizing (P < 0.001). The leaf area was more than double in fertilized compared with unfertilized seedlings; 989.1 (±45.4) cm2 vs. 429.5 (±24.6) cm2, respectively. Also CO2 × temperature interaction had a significant effect on the leaf area (P = 0.029); under ambient temperature with elevated CO2, the leaf area was the lowest, 493.3 (±43.1) cm2, while under elevated temperature with elevated CO2, the leaf area was the highest, 979.8 (±77.7) cm2. Under ambient temperature with ambient CO2 and elevated temperature with ambient CO2, the leaf areas were 621.8 (±61.0) cm2 and 805.5 (±61.2) cm2, respectively. In general, elevation in temperature significantly increased the leaf area (P < 0.001).
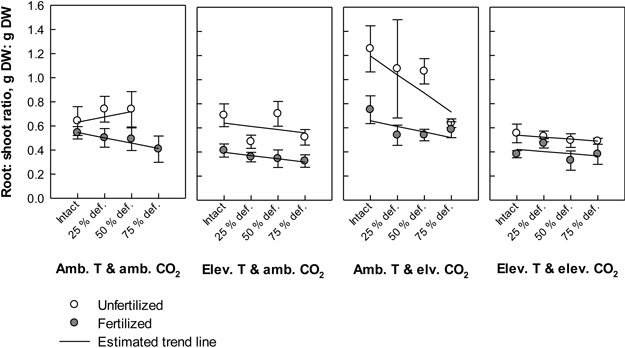
Fig. 4. Root: shoot ratios (biomass of roots in proportion to biomass of stems + leaves) of silver birch seedlings in different treatment combinations at the end of the experiment. The plot results are based on untransformed data, where mean values of different treatment combinations with the standard errors are presented. The trend line is based on the least squares means (LS means) provided by SAS/MIXED. Abbreviations: Amb. T = ambient temperature; Elev. T = elevated temperature; Amb. CO2 = ambient CO2; Elev. CO2 = elevated CO2.
Defoliation induced a near significant trend in the root: shoot ratio (P = 0.064). The higher the defoliation level was the lower the root: shoot ratios tended to be (Fig. 4). However, the estimated trend was positive in unfertilized seedlings under ambient temperature with ambient CO2. Typically, fertilizing lowered the root: shoot ratios, and the effect was depended on the climate treatment (CO2 × temperature × fertilization; P = 0.032, Fig. 4). The highest ratios were recorded in unfertilized seedlings under ambient temperature with elevated CO2, and the lowest ratios in fertilized seedlings under elevated temperature with ambient CO2. In general, elevation in temperature decreased the ratios (P < 0.001, Fig. 4), while elevation in CO2 increased them (P = 0.008, Fig. 4).
4 Discussion
Less than half of the silver birch seedlings overwintered without death or damage, and nearly 60% died during the winter or suffered from broken stems. It seems that none of the explanatory variables clearly accounted for the cause leading to stem breakage or winterkill. However, it is typical that overwinter mortality rates are high in the early seedling years of birches due to compromised energy reserves, or incomplete preparation against frost including poor shoot lignification during autumn (Moura et al. 2010; Renou-Wilson et al. 2010).
Among those silver birch seedlings that survived through the winter, the annual height increment was the greatest in the plants that were subjected to elevated temperature with ambient CO2, fertilized and defoliated twice by 50% of total leaf area. The second greatest height gain was recorded in seedlings that grew under the same climate and fertilizer treatments, but were defoliated once by 75%. The height gain was also high in plants that grew under elevated CO2 with elevated or ambient temperature, and were both fertilized and defoliated by 75% of total leaf area (Fig. 3). These responses are in accordance with previous findings; for example Handa et al. (2005) reported that defoliated conifers under free air CO2 enrichment prioritized the production of needles and consequently over-compensated their height growth. Typically, elevated temperature or CO2 enhances growth by accelerating carbon assimilation (Riikonen et al. 2005; Mäenpää et al. 2011) and stimulating both cell production and expansion (Riikonen et al. 2010; Mäenpää et al. 2011). Defoliation and fertilizing have similar effects on trees; they both enhance photosynthesis, which increases carbohydrate availability for the growing organs (Huttunen et al. 2007; Turnbull et al. 2007; Maier et al. 2008). On the other hand, the effect of defoliation was dependent on the frequency and level of damage (Tuomi et al. 1989; Saravesi et al. 2008). Trees that repeatedly lose foliage but re-foliate during the same season are forced to compromise their nutrient and energy reserves; this affects their growth in the following season (Kaitaniemi et al. 1999; Landhäusser and Liefferes 2012), and explains lower height increment in seedlings that were defoliated twice by 25% compared with seedlings that were defoliated once by 75%. Also the timing of defoliation affects the growth; it seems that single late-season defoliation inflicts over-compensated height growth in most silver birch seedlings in the following year. In late season, plant growth terminates naturally by declining day length, and demand for assimilates in aboveground plant parts decreases. Since foliage damage intensifies photosynthesis in remaining leaves (Huttunen et al. 2007; Turnbull et al. 2007), the surplus assimilates are possibly directed to storage organs having a positive influence on growth in the season following defoliation (Huttunen et al. 2013).
Irrespective of defoliation treatment, total biomass was the highest in fertilized birch seedlings, or seedlings that were subjected to elevated temperature. Here, potential indicators for the increased biomass are increased leaf production, branching and stem diameter growth, which are typically enhanced by ample nutrient availability or elevated temperature due to stimulated metabolism in aboveground organs (Niinemets et al. 2002; Huttunen et al. 2007). Namely, it has been found that leaf dry mass strongly correlates with sapwood area indicating that increased vaporization and water requirements in shoots increase conducting tissue and thus diameter and mass in stem (Kaufmann and Troendle 1981). Furthermore, as total biomass, the photosynthetically active leaf area of birch seedlings increased by fertilization, or by temperature elevation; increase in leaf area is a typical response to increased nutrients and warmth for many tree species (Maier et al. 2008; Mäenpää et al. 2011; Kleczewski et al. 2012).
Ample nutrients and elevated temperature clearly decreased the root: shoot ratios of seedlings (Fig. 4; Ericsson 1995; Kuokkanen et al. 2004; Kleczewski et al. 2012). Also defoliation singly tended to cause a decrease in the ratio. In both defoliated seedlings and seedlings that grew under elevated temperature with high nutrients, increased shoot growth versus reduced root growth is in accordance with the optimal partitioning theory that predicts increased allocation of resources into those plant organs that provide the most growth limiting resources (Kobe et al. 2010). Resource allocation towards shoots in defoliated seedlings, or in seedlings under high nutrients and temperature supports the assumption that under these conditions assimilated carbon and light have become growth limiting factors (Tilman 1990). If global warming increases abundance of insect herbivores (Jepsen et al. 2011), or nutrient availability by accelerating mineralization rates or enhancing atmospheric nitrogen deposition (Galloway et al. 2004; Verburg 2005), the net effect in silver birch seedlings is to shift resource allocation toward shoot growth and away from root growth (Fig. 4; Kleczewski et al. 2012). It would not be surprising if there were some general adverse consequences for plants with low root: shoot ratios. For example, roots function as carbohydrate storage organs (Scott 2008), so there could be limits on energetic reserves. Shallow root systems may also diminish the ability to tolerate dry periods, which can increase the risk of plant mortality. However, elevated CO2 increased the root: shoot ratios of the birch seedlings. This goes along with previous findings in paper birch (Betula papyrifera Marsh.) and trembling aspen (Populus tremuloides Michx.); under elevated CO2, these tree species increased significantly their root mass (King et al. 2001), which is explained by accelerated carbon assimilation and allocation into roots (Riikonen et al. 2005). This tendency has possibly affected the root: shoot ratios of silver birch seedlings; the increased amounts of photosynthates, such as sucrose, glucose and fructose, provide substrates for insoluble cell wall components cellulose, hemicellulose, pectin and lignin increasing potential for root mass growth (Gibson 2012).
Silver birch seedlings that survived through the winter displayed an impressive general capability to recover from defoliation and compensate the height growth at nutrient rich soil under elevated temperature with both ambient and elevated CO2. These responses imply that increased folivory does not necessarily lead to decreased NPP under changing climate. However, the lowest root: shoot ratios were recorded in seedlings that grew with increased levels of nutrients and elevated temperature, or in seedlings that were defoliated, while the ratios were the highest in seedlings that were subjected to elevated CO2 with low nutrients; these responses give support for the optimal partitioning theory.
Although earlier reports point out that artificial defoliation may not include all the effects on plant responses compared with real insect defoliation (Straus and Agrawal 1999), we used this form of damage to standardize the tissue loss with greater precision than could be achieved using real herbivores. Also the conditions during the winter months may not represent the conditions that seedlings would have experienced if both temperature and CO2 concentration increased as anticipated. Nevertheless, survived birch seedlings were dormant and therefore the winter may not greatly have confounded the results. To conclude, it seems that the main components affecting recovery of silver birch seedlings are the timing and severity of foliage damage, which determine the magnitude of growth compensation within the limits of resource availability and climate conditions.
Acknowledgements
This work was funded through the Finnish Centre of Excellence Programme (2000–2005), under the Centre of Excellence for Forest Ecology and Management (Project No. 64308). The Academy of Finland, the National Technology Agency (Tekes) and the Nordic Centre of Excellence Tundra within the ADAPT programme are acknowledged from financial support. We thank the technical staff at the Mekrijärvi Research Station of the University of Eastern Finland, Joensuu campus and Ms. Maini Mononen for running the climate chamber system during the summers 2002 and 2003 and for assistance in the laboratory. We also wish to thank Doc. Heli Peltola, Dr. Jaakko Heinonen and Dr. Tiina Ylioja for valuable advice concerning the manuscript.
References
Agrawal A.A. (2000). Overcompensation of plants in response to herbivory and the by-product benefits of mutualism. Trends in Plant Science 5: 309–313.
Anttonen S., Piispanen R., Ovaska J., Mutikainen P., Saranpää P., Vapaavuori E. (2002). Effects of defoliation on growth, biomass allocation, and wood properties of Betula pendula clones grown at different nutrient levels. Canadian Journal of Forest Research 32: 498–508.
Ericsson T. (1995). Growth and shoot: root ratio of seedlings in relation to nutrient availability. Plant and Soil 168–169: 205–214.
Eyles A., Pinkard E.A., Mohammed C. (2009). Shifts in biomass and resource allocation patterns following defoliation in Eucalyptus globulus growing with varying water and nutrient supplies. Tree Physiology 29: 753–764.
Galloway J.N., Dentener F.J., Capone D.G., Boyer E.W., Howarth R.W., Seitzinger S.P., Asner G.P., Cleveland C.C., Green P.A., Holland E.A., Karl D.M., Michaels A.F., Porter J.H., Townsend A.R., Vöosmarty C.J. (2004). Nitrogen cycles: past, present, and future. Biogeochemistry 70: 153–226.
Gibson L.J. (2012). The hierarchical structure and mechanics of plant materials. Journal of the Royal Society Interface 9: 2749–2766.
Handa T.I., Körner C., Hättenschwiler S. (2005). A test of the treeline carbon limitation hypothesis by in situ CO2 enrichment and defoliation. Ecology 86: 1288–1300.
Huttunen L., Niemelä P., Peltola H., Heiska S., Rousi M., Kellomäki S. (2007). Is a defoliated silver birch seedling able to overcompensate the growth under changing climate? Environmental and Experimental Botany 60: 227–238.
Huttunen L., Niemelä P., Julkunen-Tiitto R., Heiska S., Tegelberg R., Rousi M., Kellomäki S. (2008). Does defoliation induce chemical and morphological defenses in the leaves of silver birch seedlings under changing climate? Chemoecology 18: 85–98.
Huttunen L., Niemelä P., Ossipov V., Rousi M., Klemola T. (2012). Do warmer growing seasons ameliorate the recovery of mountain birches after winter moth outbreak? Trees – Structure and Function 26: 809–819.
Huttunen L., Blande J., Li T., Rousi M., Klemola T. (2013). Effects of warming climate on early-season carbon allocation and height growth of defoliated mountain birches. Plant Ecology 214: 373–383.
Jacquet J.-S., Orazio C., Jactel H. (2012). Defoliation by processionary moth significantly reduces tree growth: a quantitative review. Annals of Forest Science 69: 857–866.
Jepsen J.U., Kapari L., Hagen S.B., Schott T., Vindstad O.P.L., Nilssen A.C., Ims R.A. (2011). Rapid northwards expansion of a forest insect pest attributed to spring phenology matching with sub-Arctic birch. Global Change Biology 17: 2071–2083.
Juntunen M.-L., Rikala R. (2001). Fertilization practice in Finnish forest nurseries from the standpoint of environmental impact. New Forests 21: 141–158.
Kaitaniemi P., Neuvonen S., Nyyssönen T. (1999). Effects of cumulative defoliations on growth, reproduction, and insect resistance in mountain birch. Ecology 80: 524–532.
Karlsson P.S., Weih M. (2003). Long-term patterns of leaf, shoot and wood production after insect herbivory in the mountain birch. Functional Ecology 17: 841–850.
Kaufmann M.R., Troendle C.A. (1981). The relationship of leaf area and foliage biomass to sapwood conducting area in four subalpine forest tree species. Forest Science 27: 477–482.
Kellomäki S., Väisänen H. (1997). Modelling the dynamics of the forest ecosystem for climate change studies in the boreal conditions. Ecological Modelling 97: 121–140.
Kellomäki S., Wang K.-Y., Lemettinen M. (2000). Controlled environment chambers for investigating tree response to elevated CO2 and temperature under boreal conditions. Photosynthetica 38: 69–81.
King J.S., Pregitzer K.S., Zak D.R., Sober J., Isebrands J.G., Dickson R.E., Hendrey G.R., Karnosky D.F. (2001). Fine-root biomass and fluxes of soil carbon in young stands of paper birch and trembling aspen as affected by elevated atmospheric CO2 and tropospheric O3. Oecologia 128: 237–250.
Kleczewski N.M., Herms D.A., Bonello P. (2012). Nutrient and water availability alter belowground patterns of biomass allocation, carbon partitioning, and ectomycorrhizal abundance in Betula nigra. Trees – Structure and Function 26: 525–533.
Kobe R.K., Iyer M., Walters M.B. (2010). Optimal partitioning theory revisited: nonstructural carbohydrates dominate root mass responses to nitrogen. Ecology 91: 166–179.
Kuokkanen K., Niemelä P., Matala J., Julkunen-Tiitto R., Heinonen J., Rousi M., Henttonen H., Tahvanainen J., Kellomäki S. (2004). The effects of elevated CO2 and temperature on the resistance of winter-dormant birch seedlings (Betula pendula) to hares and voles. Global Change Biology 10: 1504–1512.
Kurz W.A., Dymond C.C., Stinson G., Rampley G.J., Neilson E.T., Carroll A.L., Ebata T., Safranyik L. (2008). Mountain pine beetle and forest carbon feedback to climate change. Nature 452: 987–990.
Landhäusser S.M., Lieffers V.J. (2012). Defoliation increases risk of carbon starvation in root systems of mature aspen. Trees – Structure and Function 26: 653–661.
Mäenpää M., Riikonen J., Kontunen-Soppela S., Rousi M., Oksanen E. (2011). Vertical profiles reveal impact of ozone and temperature on carbon assimilation of Betula pendula and Populus tremula. Tree Physiology 31: 808–818.
Maier C.A., Palmroth S., Ward E. (2008). Short-term effects of fertilization on photosynthesis and leaf morphology of field-grown loblolly pine following long-term exposure to elevated CO2 concentration. Tree Physiology 28: 597–606.
Markkola A., Kuikka K., Rautio P., Härmä E., Roitto M., Tuomi J. (2004). Defoliation increases carbon limitation in ectomycorrhizal symbiosis of Betula pubescens. Oecologia 140: 234–240.
Mattson W.J., Kuokkanen K., Niemelä P., Julkunen-Tiitto R., Kellomäki S., Tahvanainen J. (2004). Elevated CO2 alters birch resistance to Lagomorpha herbivores. Global Change Biology 10: 1402–1413.
Moura J.C.M.S., Bonine C.A.V., Viana J.O.F., Dornelas M.C., Mazzafera P. (2010). Abiotic and biotic stresses and changes in the lignin content and composition in plants. Journal of Integrative Plant Biology 52: 360–376.
Niinemets Ü., Kull O. (1998). Stoichiometry of foliar carbon constituents varies along light gradients in temperate woody canopies: implications for foliage morphological plasticity. Tree Physiology 18: 467–479.
Niinemets Ü., Portsmuth A., Truus L. (2002). Leaf structural and photosynthetic characteristics, and biomass allocation to foliage in relation to foliar nitrogen content and tree size in three Betula species. Annals of Botany 89: 191–204.
Osier T.L., Lindroth R.L. (2004). Long-term effects of defoliation on quaking aspen in relation to genotype and nutrient availability: plant growth, phytochemistry and insect performance. Oecologia 139: 55–65.
Pinkard E.A., Battaglia M., Roxburgh S., O’Grady A.P. (2011). Estimating forest net primary production under changing climate: adding pests into the equation. Tree Physiology 31: 686–699.
Renou-Wilson F., Pöllänen M., Byrne K., Wilson D., Farrell E.P. (2010). The potential of birch afforestation as an after-use option for industrial cutaway peat lands. Suo – Mires and Peat 61: 59–76.
Riikonen J., Holopainen T., Oksanen E., Vapaavuori E. (2005). Leaf photosynthetic characteristics of silver birch during three years of exposure to elevated concentrations of CO2 and O3 in the field. Tree Physiology 25: 621–632.
Riikonen J., Percy K.E., Kivimäenpää M., Kubiske M.E., Nelson N.D., Vapaavuori E., Karnosky D.F. (2010). Leaf size and surface characteristics of Betula papyrifera exposed to elevated CO2 and O3. Environmental Pollution 158: 1029–1035.
Ritchie M.E., Tilman D., Knops J.M.H. (1998). Herbivore effects on plant and nitrogen dynamics in oak savanna. Ecology 79: 165–177.
Rosenthal J.P., Kotanen P.M. (1994). Terrestrial plant tolerance to herbivory. Trends in Ecology and Evolution 9: 145–148.
Saravesi K., Markkola A., Rautio P., Roitto M., Tuomi J. (2008). Defoliation causes parallel temporal responses in a host tree and its fungal symbionts. Oecologia 156: 117–123.
Scott P. (2008). Physiology and behaviour of plants. John Wiley & Sons, Chichester, UK. 305 p.
Sellin A., Kupper P. (2005). Effects of light availability versus hydraulic constraints on stomatal responses within a crown of silver birch. Oecologia 142: 388–397.
Strauss S.Y., Agrawal A.A. (1999). The ecology and evolution of plant tolerance to herbivory. Trends in Ecology & Evolution 14: 179–185.
Tilman D. (1990). Constraints and tradeoffs: toward a predictive theory of competition and succession. Oikos 8: 3–15.
Tuomi J., Niemelä P., Jussila I., Vuorisalo T., Jormalainen V. (1989). Delayed bud break: a defensive response of mountain birch to early-season defoliation? Oikos 54: 87–91.
Tuomi J., Niemelä P., Siren S. (1990). The panglossian paradigm and delayed inducible accumulation of foliar phenolics in mountain birch. Oikos 59: 399–410.
Turnbull T.L., Adams M.A., Warren C.R. (2007). Increased photosynthesis following partial defoliation of field-grown Eucalyptus globulus seedlings is not caused by increased leaf nitrogen. Tree Physiology 27: 1481–1492.
Verburg P.S.J. (2005). Soil solution and extractable soil nitrogen response to climate change in two boreal forest ecosystems. Biology and Fertility of Soils 41: 257–261.
Wang T., Tigerstedt P.M.A., Viherä-Aarnio A. (1995). Photosynthesis and canopy characteristics in genetically defined families of silver birch (Betula pendula). Tree Physiology 15: 665–671.
Wise M.J., Abrahamson W.G. (2008). Applying the limiting resource model to plant tolerance of apical meristem damage. The American Naturalist 172: 635–647.
Wolf A., Kozlov M.V., Callaghan T.V. (2008). Impact of non-outbreak insect damage on vegetation in northern Europe will be greater than expected during a changing climate. Climate Change 87: 91–106.
Zhang S., Dang Q.-L., Yü X. (2006). Nutrient and CO2 elevation had synergistic effects on biomass production but not on biomass allocation of white birch seedlings. Forest Ecology and Management 234: 238–244.
Total of 52 references

