Characteristics of boreal and hemiboreal herb-rich forests as habitats for polypore fungi
Hämäläinen K., Tahvanainen T., Junninen K. (2018). Characteristics of boreal and hemiboreal herb-rich forests as habitats for polypore fungi. Silva Fennica vol. 52 no. 5 article id 10001. https://doi.org/10.14214/sf.10001
Highlights
- Polypore species richness and diversity were affected positively by dead-wood diversity, and negatively by increasing latitude
- Red-listed species responded only to the abundance of large-diameter dead wood
- Main factor determining composition of polypore assemblages was host-tree species
- High proportion of deciduous dead-wood in herb-rich forests provides complementary effect on polypore assemblages in boreal forest landscapes.
Abstract
Herb-rich forests are often considered biodiversity hotspots in the boreal zone but their fungal assemblages, particularly those of wood-decaying fungi, remain poorly known. We studied herb-rich forests as habitats for polypores, a distinct group of wood-decaying fungi, and assessed the importance of tree- and stand-scale variables for polypore species richness, abundance, and diversity, including red-listed species. The data include 71 herb-rich forest stands in Finland and 4797 dead wood items, on which we made 2832 observations of 101 polypore species. Dead-wood diversity was the most important variable explaining polypore species richness and diversity, whereas increasing latitude had a negative effect. Red-listed species showed a positive response to the abundance of large-diameter dead wood, which, especially birch, supported also high general abundance of polypores. The composition of polypore assemblages reflected their host-tree species. The red-listed species did not show explicit patterns in the ordination space. Compared to old-growth spruce forests, herb-rich forests seem to host lower polypore species richness and less red-listed species. However, because of high proportion of deciduous trees in the dead wood profile, herb-rich forests have a clear complementary effect on polypore assemblages in boreal forest landscapes.
Keywords
biodiversity;
species richness;
coarse woody debris;
threatened species;
wood-decaying fungi
-
Hämäläinen,
School of Forest Sciences, University of Eastern Finland, P.O. Box 111, FI-80101 Joensuu, Finland
E-mail
karoham@uef.fi

- Tahvanainen, Department of Environmental and Biological Sciences, University of Eastern Finland, P.O. Box 111, FI-80101 Joensuu, Finland E-mail teemu.tahvanainen@uef.fi
- Junninen, Metsähallitus Parks & Wildlife Finland, c/o UEF/Borealis, P.O. Box 111, FI-80101 Joensuu, Finland E-mail kaisa.junninen@metsa.fi
Received 8 May 2018 Accepted 7 November 2018 Published 16 November 2018
Views 65259
Available at https://doi.org/10.14214/sf.10001 | Download PDF

Supplementary Files
1 Introduction
Boreal herb-rich forests are considered biodiversity hotspots providing primary habitat for 47% of all forest-dwelling red-listed species (Kotiranta et al. 2010) and they are top-priority habitats for conservation in the boreal zone. Intensive silvicultural actions, however, have profoundly altered characteristics of boreal forests during the past few decades, including herb-rich forests. One of the key structural elements affected by forestry is dead wood: at present, the volume of dead wood in Fennoscandian boreal forests is approximately 2–10 m3 ha–1 (Tonteri and Siitonen 2001), whereas the average volume in natural old-growth forests is generally 60–120 m3 ha–1 (Siitonen 2001). In addition to the sheer quantitative loss, forestry practices also decrease the diversity of dead wood. Consequently, the estimated 4000–5000 dead-wood associated species in Finland have been subject to great habitat deterioration, and it has been suggested that more than half of these species might eventually go extinct in managed forests (Siitonen 2001).
One group of organisms that is totally dependent on dead wood as their habitat is polypore fungi. Because of their fundamental role in the decomposition of dead wood, and their value as indicator species of valuable forest habitats (Niemelä 2016), polypores have become a focal species group for assessments of biodiversity (Ylisirniö et al. 2005), ecological impacts of forest management (Penttilä et al. 2004), and conservation value of forests (Lonsdale et al. 2008).
So far, the majority of polypore studies in the boreal zone have been conducted in coniferous forests, often focusing only on Picea abies (L.) H. Karst. -dominated forest types (Junninen and Komonen 2011). Few studies have focused on polypores of herb-rich forests. A more comprehensive understanding of herb-rich forests as polypore habitats and their polypore assemblages is crucial for identifying the most vital structures for saproxylic organisms, preserving biodiversity, applying appropriate ecological restoration practices, and allocating limited conservation resources.
In this study, we aim to answer to the following questions: (1) how do substrate- and stand-scale variables affect the abundance, species richness and diversity of polypore fungi in hemiboreal and boreal herb-rich forests; (2) do stand-scale variables affect differently common and red-listed species, and (3) what kind of polypore assemblages exist in herb-rich forests? In addition, we compare the results to those obtained from boreal coniferous heath forests in earlier studies.
2 Material and methods
2.1 Study area
The study comprises of 71 forest stands in 22 protected forest areas in hemiboreal, southern boreal and middle boreal vegetation zones in Finland (Fig. 1). The protected areas were chosen within 10×10 km2 squares, where the total area of protected herb-rich forests was at least 50 ha. Within each protected area, two to four forest stands (land-use and inventory units with apparently homogenous tree stand patterns) defined as herb-rich forest were randomly selected for polypore inventories. In the selection of stands, the only delimiting factor was that the stands should not be located next to each other. The study stands and forest areas are listed in Supplementary file S2.
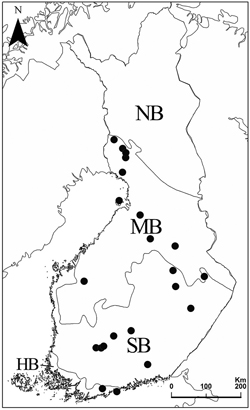
Fig. 1. Vegetation zones and the locations of the 22 forest areas. HB = hemiboreal, SB = southern boreal, MB = middle boreal, and NB = northern boreal zone.
Ideally, the study sites were natural or near-natural forests with limited anthropogenic impact. Unfortunately, information about the species composition and age structure of the tree layer, volume of dead wood, or forest management history were not available and are therefore not considered in the analyses.
2.2 Sampling
The inventories were organized by Metsähallitus Parks & Wildlife Finland and carried out by seven surveyors who all had expertise in polypore species identification. The data was collected during August to October 2009, the main season for the sporocarp formation of annual polypores in boreal zone (Niemelä 2016). In each forest stand, all dead wood items with a minimum diameter of 10 centimeters (later “CWD” or “coarse woody debris”) were inspected. Tree species, diameter, decay stage on a scale I-V (Renvall 1995) and dead wood type (log, branch, stump, snag, etc.) of all CWD were recorded regardless of presence of polypores. The diameter of standing dead wood items and fallen trunks was measured at breast height (130 cm from the base of the trunk) or, in the case of branches and other dead wood items, at their basal part.
All sporocarps of any single species growing on the same dead wood item were considered as one occurrence. Thus, the abundance of polypores equaled the number of occupied dead wood items for each species. The species were identified in the field, and in uncertain cases, specimens were collected for identification based on microscopic characters.
The aim was to survey completely the whole area of selected forest stands. However, to be able to investigate two stands within one day, inventory within one stand was restricted to four hours and the area covered was calculated afterwards using GPS-tracks. Therefore, within each stand, the exact area surveyed is known and all dead wood items within the surveyed area were investigated, even if the total area of the stand was not covered.
2.3 Statistical analyses
The data was analysed in R-program using packages car, Hmisc, MASS and vegan (Oksanen et al. 2013). The surveyed areas were calculated in ArcMap 10.3.1 using a 10-meter buffer around GPS track.
The response variables describing polypores were total species richness, number of occurrences, Shannon’s diversity index, species richness of red-listed species, and percentage of CWD items inhabited by polypores. Red-listed species included species classified as near-threatened, threatened, vulnerable or endangered by Kotiranta et al. (2010) (for the full species list, see Suppl. file S1). Since the data were strongly dominated by a few common species, and both rare and dominant species were of equal interest, Shannon’s diversity index (H’) was selected a priori to be used as a measurement of diversity. The environmental variables considered in the analyses were the total number of CWD items, number of CWD items per hectare, number of large (diameter > 30 cm) CWD items per hectare, CWD diversity, surveyed area and northern coordinate. The relationships between variables were visually inspected in a correlation matrix using the Spearman rank-order correlation analysis. The Spearman rank-order correlation test was used to analyse the co-variation between polypore and environmental variables.
To calculate the dead-wood diversity, frequencies of each unique combination of tree species, size class, dead-wood type, and decay class were calculated. The CWD items were classified to 10 cm diameter classes: 10–19 cm, 20–30 cm, and so on. The substrate types were classified into five categories: (1) whole standing dead trees and >1.3 m high snags; (2) downed intact and broken logs; (3) branches and roots; (4) natural stumps < 1.3 m high; (5) logging residue and man-made stumps, logs and bolts. To keep the data more robust, decay classes were reclassified into three categories: (1) decay stages I and II; (2) decay stage III; and (3) decay stages IV and V. This CWD classification system closely follows that of a commonly used index developed by Siitonen et al. (2000). As it can be assumed that not only the number of different niches available, but also the relative abundance of resource might be of importance for organisms utilizing them, the eventual CWD diversity was calculated as Shannon’s diversity index. Thus, the dead-wood diversity used in this study is the entropy of unique CWD type frequencies. This diversity index was selected because the relative importance of an individual CWD item for polypores decreases the more abundant that particular CWD type is. For example, the changes in less common substrates are presumed to be of greater importance.
To compare the importance of different CWD diameter fractions of each host-tree species for polypore species richness, species accumulation curves were calculated using “specaccum” function from R vegan package (Oksanen et al. 2013). In addition, Kruskal-Wallis test was used to test the significance of differences in the mean number of polypore occurrences in each size class. As the number of deciduous tree species was rather high, with only a few occurrences on some of them, most deciduous tree species were pooled together in order to keep the analyses more robust. This was supported by a prior cluster analysis using Sørensen dissimilarity index, which indicated similar polypore assemblages on CWD items of Acer platanoides L., Alnus glutinosa (L.) Gaertn., A. incana (L.) Moench, Prunus padus L., Salix caprea L., Salix L. spp., Sorbus aucuparia L., Tilia cordata Mill., and unidentified broadleaved deciduous tree species, treated as “other deciduous trees”. Picea abies, Pinus sylvestris L., Betula spp. (B. pendula Roth and B. pubescens Ehrh.) and Populus tremula L. were kept separate in the analyses. The CWD items of different tree species or a group of tree species were divided into 10-cm diameter classes. Occurrences of polypores were relativized to the number of CWD items of each tree species in every sizeclass, since both the surveyed area and abundance of different tree species varied greatly among forest stands.
The effects of CWD diversity, number of large (diameter > 30 cm) CWD units per hectare, and latitude on the polypore species richness in forest stands were analyzed with linear regression and multiple regression analysis. As the species richness of red-listed species was not normally distributed, generalized linear model (GLM) with a Poisson distribution and model log link function was used to study the relationship between the stand variables and the number of red-listed species. Because the four hours’ time limit to survey each area likely caused an artefact in the relationship between the size of the surveyed area and the polypore species richness, forest-stand area was not included as a covariate in the models. Rather, all results are constrained to the four-hour inventory time and not area. At first step, the independent variables were used as separate covariates on their own. Analysis of variance was performed for each model and the resulting sum of squares and significance levels were compared to evaluate whether the differences in model fits were significant. Secondly, CWD diversity, number of large logs per hectare and northern coordinate were included as covariates simultaneously in multiple regression analysis or, in case of red-listed species, in GLM.
Analysis of variance with type III sum of squares was used to test the significance of the differences in model fits. In this analysis, each variable was tested on the condition of all the other variables being included simultaneously in the model.
Finally, polypore assemblages were graphically represented and visually assessed by using nonmetric multidimensional scaling (NMDS) from R vegan package (Oksanen et al. 2013). In NMDS, the data matrix was transformed first by Wisconsin double standardization and then by square root. The latter divides species abundances by their maxima and stands by stand totals, effectively downweighting the effect of very abundant species. A distance matrix applying Bray-Curtis index was thereafter calculated to circumvent problems, such as the strong influence of null values, associated with Euclidean-based distances. The maximum number of starts was set as “trymax = 100” in order to avoid getting stuck into a local minimum. An ordination of polypore assemblages by study sites was conducted, and the relative similarity or dissimilarity between polypore species were presented in the ordination. In addition, to visually assess the dissimilarity of polypore assemblages between host-tree species, an ordination of polypore assemblages by tree species was performed.
The relationship between polypore assemblage structures and environmental variables was interpreted by fitting environmental vectors onto the species ordination. The environmental vectors used were the northern coordinate, size of the surveyed area, and the number of CWD of different tree species per hectare. The envfit function in vegan package performs linear regression of environmental variables against the ordination configuration, and finds the maximal correlation between the variable and the species scores. The significance of fitted environmental variables was based on 999 random permutations. Secondly, polypore assemblages found on different tree species in each forest stand were ordinated. The function ordihull was applied, with the tree species as class centroids.
3 Results
The polypore data included 2832 occurrences of 101 polypore species (Table 1). Altogether 30 occurrences of 12 red-listed species were recorded. No species occurred in all of the 71 study sites, and only four species (Fomes fomentarius (L.: Fr.) J. Kickx f, Fomitopsis pinicola (Sw.:Fr.) P. Karst., Trichaptum abietinum (Dicks.: Fr.) Ryvarden, and Phellinus igniarius coll. (L. : Fr.) Quél.) were recorded on more than 50% of the study sites. These four species accounted for over half of all polypore observations. On the other hand, 15 species were found only once, and over 50% of species were recorded five times or less. At most, five occurrences of red-listed species were recorded from one study site.
| Table 1. Dead-wood (CWD) tree species, total numbers of CWD units, the mean numbers and standard deviations of each tree species CWD in study areas, and polypore numbers found on them. | ||||||
| Tree species | Total no. of CWD units | Mean (SD) no. of CWD units/ha | Polypore occurrences | Polypore species | Unique species | Red-listed species |
| Acer platanoides | 4 | 0.08 (0.4) | 2 | 2 | 0 | 0 |
| Alnus glutinosa | 219 | 3.7 (16.3) | 152 | 20 | 1 | 1 |
| Alnus incana | 944 | 25.7 (61.7) | 264 | 30 | 1 | 1 |
| Betula spp. | 1054 | 19.4 (23.5) | 932 | 41 | 8 | 2 |
| Corylus avellana | 1 | 0.03 (0.2) | 1 | 1 | 0 | 0 |
| Juniper communis | 3 | 0.07 (0.3) | 0 | 0 | 0 | 0 |
| Picea abies | 1399 | 24.2 (25.8) | 993 | 44 | 16 | 6 |
| Pinus sylvestris | 274 | 5.1 (10.2) | 103 | 24 | 4 | 1 |
| Populus tremula | 242 | 4.5 (10.0) | 163 | 20 | 6 | 2 |
| Prunus padus | 54 | 0.9 (2.7) | 28 | 10 | 0 | 0 |
| Salix caprea | 298 | 5.8 (13.1) | 147 | 24 | 2 | 1 |
| Salix spp. | 12 | 0.7 (4.9) | 4 | 2 | 0 | 0 |
| Sorbus aucuparia | 131 | 2.4 (6.8) | 31 | 15 | 1 | 1 |
| Tilia cordata | 11 | 0.2 (0.8) | 3 | 2 | 0 | 0 |
| Unidentified | 56 | 1.2 (5.0) | 2 | 3 | 0 | 0 |
| Unidentified conifer | 43 | 1.1 (4.1) | 3 | 3 | 0 | 0 |
| Unidentified deciduous | 56 | 1.1 (2.8) | 4 | 4 | 0 | 0 |
| Total | 4797 | 96.2 (77.2) | 2832 | 101 | 39 | 12 |
The maximum number of polypore species observed in one site was 30, while the two most species-poor sites had three species only (mean 12.7 species; SD ± 5.5 species). The surveyed area varied between 0.16 and 3.1 hectares (mean 0.91 ha; SD ± 0.55 ha). The species and study sites are presented in more detail in Suppl. files S1 and S2, respectively.
3.1 Composition of dead wood
The dead wood data included 4797 dead wood items of which 2069 were inhabited by polypores. Tree species composition of dead wood varied greatly among study sites, from pure deciduous stands to those dominated by P. abies. In the majority of the study sites, deciduous trees constituted at least 58% of dead wood items (mean 59%; SD ± 31%), but the single most common tree species, by average, was P. abies (Fig. 2). The amount of dead wood was also highly variable, from 7.2 to 399.2 CWD items per hectare (mean 96.2; SD ± 77.2).
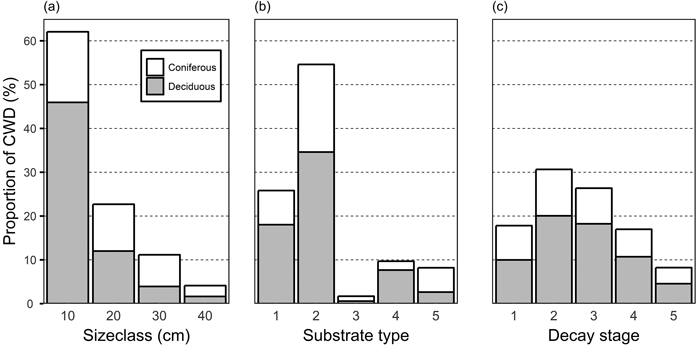
Fig. 2. Proportion of coniferous and deciduous coarse woody debris (CWD) among size classes (a), substrate types (b), and decay stages (c). Substrate types: 1, standing dead trees and >1.3 m high snags; 2: downed intact and broken logs; 3: branches and roots; 5: natural stumps, <1.3 m high; 6: logging residual and man-made stumps, logs, and bolts. CWD items not identified as either coniferous or deciduous are not displayed.
The diameter distribution of the CWD was skewed towards small diameter (Fig. 2a). More than 60% of CWD belonged to the smallest size class, and only 15% belonged to size class 30 cm and larger. Among different substrate types, whole fallen trees and broken logs formed more than half of all CWD (Fig. 2b). The different tree species produce CWD of different qualities and, consequently, the relative proportion of coniferous and deciduous trees varied by substrate types. Deciduous trees were dominant across all stages of decay, but the relative proportion of coniferous trees was highest in decay classes I and V (Fig. 2c).
3.2 Relationships between stand characteristics and polypores
Several environmental variables correlated significantly (p < 0.05) with variables describing polypore assemblages in the study sites, but some environmental variables were also inter-correlated (Table 2). Polypore diversity (r = –0.43), species richness (r = –0.42), and the percentage of dead wood units inhabited by polypores (r = –0.54) significantly (p < 0.001) decreased towards higher latitudes. Polypore species richness and diversity had by far the strongest positive correlation with CWD diversity (r = 0.64, p < 0.001 and r = 0.69, p < 0.001, respectively). Similar results were provided by the linear model, where the diversity of dead wood (F1,69 = 57.73, p < 0.001) explained 45.5% of the variation in the number of all species (Table 3). Furthermore, in the multiple linear regression model with CWD diversity, latitude and number of large CWD units per hectare as explanatory variables, both the CWD diversity (F1,67 = 44.84, p < 0.001) and latitude (F1,67 = 9.49, p = 0.003) retained their explanatory power on the variation of species richness, whereas the number of large CWD units (F1,67 = 0.01, p = 0.931) did not. In overall, 52% of the variation in polypore species richness (F3,67 = 24.93, p < 0.001) was explained by the multiple regression model.
| Table 2. Spearman rank-order correlation coefficients between stand-scale variables, percentage of coarse woody debris (CWD) items inhabited by polypores, polypore species richness, number of occurrences, red-listed species richness and polypore diversity. | ||||||||||
| N coordinate | Area | CWD total | CWD/ha | CWD30/ha | CWD diversity | Inhabited | Species richness | Occurrences | Red-listed species | |
| Area | –0.12 | |||||||||
| CWD total | 0.27* | 0.23 | ||||||||
| CWD/ha | 0.33** | –0.47*** | 0.72*** | |||||||
| CWD30/ha | –0.34** | –0.03 | 0.16 | 0.14 | ||||||
| CWD diversity | –0.16 | 0.26* | 0.53*** | 0.31** | 0.32** | |||||
| Inhabited | –0.54*** | 0.01 | –0.42*** | –0.39*** | 0.27* | –0.18 | ||||
| Species richness | –0.42*** | 0.34** | 0.39*** | 0.13 | 0.38** | 0.64*** | 0.35** | |||
| Occurrences | –0.19 | 0.35** | 0.55*** | 0.27* | 0.30* | 0.46*** | 0.40*** | 0.78*** | ||
| Red-listed species | –0.07 | 0.18 | 0.07 | –0.10 | 0.28* | 0.09 | 0.23* | 0.26* | 0.19 | |
| Polypore diversity | –0.43*** | 0.20 | 0.25* | 0.07 | 0.39*** | 0.69*** | 0.12 | 0.85*** | 0.44*** | 0.17 |
| CWD total, total number of CWD items; CWD/ha, coarse woody debris items per hectare; CWD30/ha, number of CWD items with a diameter of >30 cm per hectare; Inhabited, percentage of CWD items inhabited; Occurrences, total number of polypore observations; Red-listed species, number of R-L species. * = p < 0.05; ** = p < 0.01; *** = p < 0.001. | ||||||||||
| Table 3. Summary of linear regression models for total polypore species richness. Variables used in the models were the diversity of coarse woody debris (CWD) items, northern coordinate, and number of large (diameter > 30 cm) CWD items per hectare. | ||||
| Variable | Coefficient | p | F1,69 | R2 |
| Diversity of CWD | 6.939 | <0.001 | 57.73 | 0.455 |
| N-coordinate | –1.039×10–5 | <0.001 | 13.76 | 0.166 |
| Large CWD per hectare | 0.187 | 0.006 | 8.05 | 0.105 |
Surprisingly, the number of CWD units per hectare did not have significant correlations with either polypore diversity or polypore species richness, regardless of it having a significant positive correlation with CWD diversity.
The only stand-scale variable that correlated significantly with the red-listed species richness was the number of large CWD per hectare (r = 0.28, p < 0.01). This was also the only significant explanatory variable for red-listed species in the generalized linear model (χ2 = 4.414, p = 0.036), but it explained only 6.8% of the variation in red-listed species richness. However, in a model where all the variables were included simultaneously, none of the explanatory variables could significantly explain the variation in species richness after accounting for the effect of the other variables (p > 0.05).
3.3 The effect of substrate quality on polypore species richness and occurrences
The highest number of species and occurrences were recorded on P. abies, which also had by far the highest number of both unique and red-listed species (Table 1). Altogether, 70 species were recorded on deciduous trees and 50 species on conifers, and 39 polypore species were met on one host-tree species only.
Among species accumulation curves, the smallest size class (10–19 cm) showed considerably lower species accumulation with sampling effort than the larger size classes (Fig. 3). The difference between the smallest and the larger size classes was clear also when the diameter specific species accumulation curves were assessed by each host tree species (not shown). The mean number of polypore occurrences per CWD item was highest in the largest size class in all deciduous tree species, most notably so in P. tremula and Betula spp. (Fig. 4). However, the difference in the mean number of polypore occurrences between the smallest and largest size classes was statistically significant only for Betula spp. (Kruskal-Wallis p < 0.05).
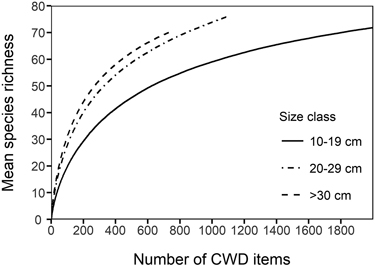
Fig. 3. Species accumulation curves for different size classes of coarse woody debris (CWD) items. The lines are mean (expected) number of species for a given number of CWD items sampled.
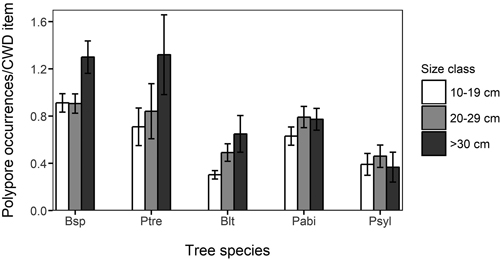
Fig. 4. Mean number of occurrences of polypores in different coarse woody debris (CWD) size classes and host tree species. The vertical lines indicate standard deviation. Bsp: Betula spp.;, Ptre: Populus tremula; Blt: Other deciduous trees, Pabi: Picea abies; Psyl: Pinus sylvestris.
3.4 Ordinations of polypore assemblages
In the two-dimensional NMDS solution locating tree species groups based on their polypore assemblages, conifers were located to the left in the ordination space, whereas deciduous trees were located to the right (Fig. 5). However, the convex hulls of conifers and deciduous tree species considerably overlapped due to few common polypore species, such as Fomitopsis pinicola, which occurred on every tree species group. When environmental vectors were fitted on NMDS solution locating polypore species, the amount of P. abies CWD per hectare (r = 0.39), amount of deciduous CWD per hectare (r = 0.35) and northern coordinate (r = 0.34) had the strongest significant (p < 0.05) correlations with species scores (Fig. 6) Although the red-listed species dependent on P. abies dead-wood were generally located right in the ordination space, associated with the direction of P. abies CWD environmental vector, the red-listed species did not show explicit patterns in the ordination space.
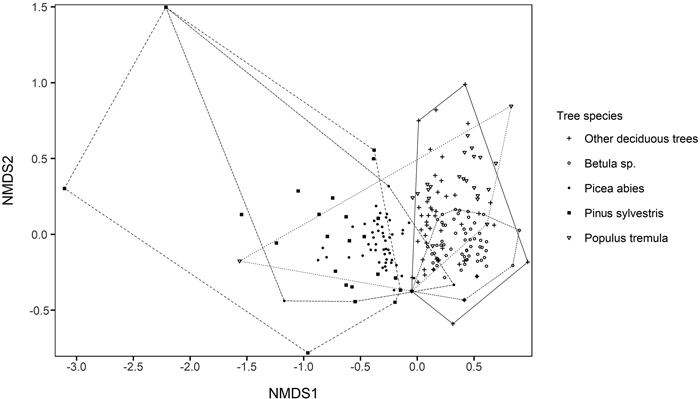
Fig. 5. Two-dimensional solution of non-metric multidimensional scaling (NMDS) ordination based on Bray-Curtis dissimilarities between polypore assemblages on different tree species in each forest stand. Stress value = 0.12. The polypore species scores of the NMDS solution are given in Supplementary file S3.
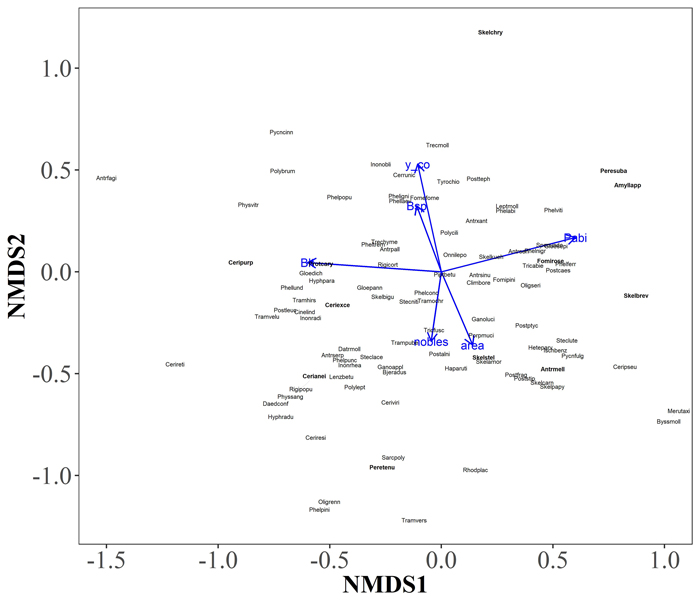
Fig. 6. A two-dimensional NMDS solution locating polypore species, and fitted environmental factors. Only environmental factors with p-value < 0.05 are displayed: Pabi, Picea abies (r = 0.39); Blt, other deciduous trees (r = 0.35); y_co, northern coordinate (r = 0.34); area, size of the surveyed area (r = 0.16); Bsp, Betula spp. (r = 0.13); nobles, so-called noble tree species (Tilia cordata, Acer platanoides and Corylus avellana) (r = 0.13). The tree species vectors are given as the number of coarse woody debris (CWD) of each tree species per hectare. Red-listed species are shown in bold. The polypore species scores of the NMDS solution are given in Supplementary file S4. View larger in new window/tab
4 Discussion
4.1 Dead wood profile in boreal and hemiboreal herb-rich forests
The tree species composition of dead wood in the study sites reflected tree layer rather typical of boreal herb-rich forests, in which the tree layer is usually spruce-dominated with admixture of both deciduous and coniferous trees (Hotanen et al. 2013). In the majority of the study sites, deciduous trees constituted more than half of all coarse woody debris, but there were also stands dominated by spruce and those comprising only of deciduous trees. The proportion of deciduous trees in CWD was, by average, almost two-fold in the herb-rich stands of this study compared to that in spruce-dominated old-growth forests (Siitonen et al. 2000; Juutilainen et al. 2014). Many of the deciduous tree species, such as S. aucuparia and A. incana, remain smaller in diameter than coniferous trees. This is reflected in the relative proportion of coniferous and deciduous trees in size classes: while deciduous trees constituted the majority in the smaller size classes, coniferous trees comprised more than half of the dead wood units with diameter greater than 30 cm.
4.2 Dead wood variables and polypores
Polypore species richness, diversity, and the number of occurrences correlated positively with both the quality and quantity of coarse woody debris. The diversity of CWD had the strongest positive correlations with polypore species richness and diversity, which is in accordance with earlier studies (Hottola et al. 2009). Among the generalized linear models with one covariate, the CWD diversity was also by far the best explanatory variable for species richness. These results suggest that the diversity of coarse woody debris efficiently reflects the range of niches available for different polypore species.
In contrast to the polypore species richness, the number of red-listed species was explained only by the number of large-diameter (>30 cm) dead wood items per hectare. The lack of response to most of the stand-scale variables is consistent with earlier studies, which have shown that red-listed species are most sensitive to the volume of dead wood (Hottola et al. 2009; Ylisirniö et al. 2016) and the availability of large logs (Sippola et al. 2004). Many of the red-listed species are confined to particular kinds of substrates, notably large diameter spruce and pine logs ( Nordén et al. 2013; Niemelä 2016). Thus, the availability of these certain substrate types is more important than a vast array of different substrates. However, our data included only 30 observations of red-listed species, and the small sample size results in low statistical power and increases the likelihood of type II error.
The importance of large-diameter dead wood units for polypore species richness and the number of occurrences is well acknowledged (Junninen and Komonen 2011). In the present study, however, it was found that there can be considerable differences between tree species. While the increase in the mean number of polypore occurrences from the smallest to the largest size class was notable in all deciduous tree species, the difference between size classes was significant only in Betula spp. In contrast, coniferous trees showed no increase in polypore occurrences with increasing substrate diameter. This might be due to the scarcity of old-growth spruce specialists in our data, compared to studies of old-growth spruce forests (Siitonen et al. 2005). When the species accumulation curves of dead wood of different diameter fractions were compared, CWD items in the larger size classes sustained clearly higher species richness than the same number of CWD items in the smallest size class. With the given sample size of our data, the species accumulation curves did not show signs of reaching an asymptote, suggesting that a greater number of polypore species are able to utilize large-diameter dead wood than that of smaller diameter.
The association of high species richness and number of occurrences with large diameter dead-wood likely results from several properties tied to the size of woody substrate. For instance, large CWD units offer plenty of resources due to their large volume and, because of their small surface-to-volume ratio, they maintain more stable microclimatic conditions than the smaller pieces of CWD (Bässler et al. 2010). Furthermore, the lower decay rate of large CWD units (Edman et al. 2007) allows for a longer colonization period, which may be crucial for many red-listed species (Renvall 1995) , and provides long-lasting habitats for wood-inhabiting fungi.
4.3 The effect of latitude
Few datasets allow consideration of latitudinal gradient as a variable explaining polypore diversity. Our results indicate that climatic factors are likely to have an influence on the polypore assemblages observed in herb-rich forests. The species richness, polypore diversity, and the percentage of trees inhabited by polypores all correlated negatively with latitude, regardless of the fact that CWD diversity was not affected. The northwards decreasing trend of the species richness and diversity, coupled with the lack of trend in the number of occurrences of polypores, reflects the pattern that a few common species tend to dominate northern herb-rich forests. In addition, regarding the multiple regression model with all the covariates included simultaneously, latitude had some explanatory power on polypore species richness independent to the other covariates.
To certain extent, the negative relationship between latitude and both polypore species richness and diversity can be explained by differences in the diameter distribution of dead wood across the gradient. The average tree size decreases towards higher latitudes due to shorter growing season, resulting in negative correlation with the northern coordinate and the amount of large-diameter dead wood units per hectare in the data. Thus, compared to their southern counterparts, the northern herb-rich forests sustain fewer large logs which, as discussed earlier, is a dead wood type favored by many polypore species (Renvall 1995; Sippola et al. 2004). In addition, climate is likely to have also direct influence on polypore assemblages. Bässler et al. (2010) found that temperature and radiation had significant impacts on species diversity and composition of wood-inhabiting fungi. The effect was stronger on species inhabiting fine woody debris than on those utilizing coarse woody debris, which might mean that in herb-rich forests where fine woody debris is proportionally more abundant than in old-growth conifer forests, the direct effects of climatic variables are stronger than in the old-growth.
4.4 Polypore assemblages in herb-rich forests
The 101 polypore species found in herb-rich forests represent 40% of all polypore species in Finland. Comparison with species counts in earlier findings in other boreal forest types is problematic, as sampling methods and sample sizes are inconsistent. Nevertheless, our results indicate that the herb-rich forests may on the average host a higher number of species than broadleaved boreal forests (Markkanen and Halme 2012) or managed boreal forests (Ylisirniö et al. 2012), but a considerably lower number than old-growth spruce forests (Penttilä et al. 2004). In the few studies, which have compared polypore assemblages between habitat types, the highest number of polypore species were found in herb-rich forests (Sippola et al. 2004; Junninen and Kouki 2006; Juutilainen et al. 2016). It must be noted, however, that polypores are not necessarily affected by the fertility or forest site type per se (Junninen et al. 2006), but rather by the volume of dead wood that is promoted by the high volume of living trees (Sippola et al. 1998) which, in turn, is promoted by high fertility of the herb-rich forest types (Sippola et al. 2004). This is a subtle but crucial distinction when assessing the relative importance of various habitats for polypore assemblages ‒ herb-rich forests have the potential to host species-rich polypore assemblages if they have a sufficient amount of dead wood, not because of their fertility.
The polypore assemblages were strikingly different between tree species, with the clearest division between coniferous and deciduous trees. This supports the results of Baber et al. (2016), who noted that host-tree species is the major factor defining fungal species richness and community composition. Betula spp. and P. abies hosted both the highest numbers of polypore species and the highest number of polypore occurrences per CWD item among all tree species. The highest number of red-listed species was found on spruce, and 16 species were recorded exclusively on it. The high polypore species richness on both P. abies and Betula spp. is facilitated by the fact that these two were the most abundant tree species in CWD, resulting in plenty of resources for polypore species utilizing them.
In comparison to old-growth forests, relatively few red-listed species were found from our data set of herb-rich forests, as only 30 occurrences of 12 red-listed species were recorded on the total area of 63 hectares. This supports the earlier studies, in which it has been suggested that deciduous forests (Markkanen and Halme 2012) and woodland key-habitats, including herb-rich forests (Junninen and Kouki 2006), do not support as high densities of red-listed species as old-growth spruce forests. One possible factor to explain the scarcity of red-listed species may be that, at present, herb-rich forests mainly exist as small-sized, fragmented patches (Raunio et al. 2008). The red-listed polypores are often highly specialised in substrates with certain qualities (Juutilainen et al. 2017; Nordén et al. 2013) and are sensitive not only to the amount of local resources at the forest stand scale, but also to the amount of suitable resources at the landscape level (Nordén et al. 2013). Thus, their populations gradually decrease in isolated forest patches (Berglund and Jonsson 2008). A minimum area of 20 hectares has been suggested as a threshold value for the maintenance of species-rich polypore assemblages in boreal spruce forests (Junninen and Komonen 2011), which is considerably larger than the areas of our study sites. On the other hand, many of the species that favor herb-rich forest as their habitat are not considered threatened in red-list assessments, because they grow on relatively abundant substrates, such as deciduous fine woody debris. Therefore, the proportion of red-listed species of all polypore species is likely smaller in herb-rich forests than in old-growth forests. It is, however, necessary to get a more comprehensive understanding of the importance of herb-rich forests as red-listed species’ habitats for the conservation of these species.
5 Conclusions
In boreal forest landscapes, herb-rich forests are often considered biodiversity hotspots because of their rich flora and the associated biota. The fertility of the sites can also support rich mycobiota of soil fungi (Öpik et al. 2008), but the effect is less clear for wood-decaying fungi. We found that herb-rich forests can support rich polypore assemblages, including red-listed species, if sufficient amount of large-diameter dead wood is available. This applies particularly in southern parts of boreal zone, but also elsewhere herb-rich forests with their deciduous trees provide clear complementary effect on boreal polypore assemblages in forest landscapes.
Acknowledgements
We thank Panu Halme, Juha Kinnunen, Kimmo Kolehmainen, Mariko Lindgren, Jorma Pennanen and Keijo Savola for their invaluable field work and data collection. We extend our gratitude to Metsähallitus Parks & Wildlife Finland for providing the dataset, to two anonymous referees whose comments greatly improved the manuscript, and Jenny and Antti Wihuri foundation for financial support (author KH).
References
Baber K., Otto P., Kahl T., Gossner M.M., Wirth C., Gminder A., Bässler C. (2016). Disentangling the effects of forest-stand type and dead-wood origin of the early successional stage on the diversity of wood-inhabiting fungi. Forest Ecology and Management 377: 161–169. https://doi.org/10.1016/j.foreco.2016.07.011.
Bässler C., Müller J., Dziock F., Brandl R. (2010). Effects of resource availability and climate on the diversity of wood-decaying fungi. Journal of Ecology 98(4): 822–832. https://doi.org/10.1111/j.1365-2745.2010.01669.x.
Berglund H., Jonsson B.G. (2008). Assessing the extinction vulnerability of wood-inhabiting fungal species in fragmented northern Swedish boreal forests. Biological Conservation 141(12): 3029–3039. https://doi.org/10.1016/j.biocon.2008.09.007.
Edman M., Jönsson M., Jonsson B.G. (2007). Fungi and wind strongly influence the temporal availability of logs in an old-growth spruce forest. Ecological Applications 17(2): 482–490. https://doi.org/10.1890/06-0852.
Hotanen J.-P., Nousiainen H., Mäkipää R., Reinikainen A., Tonteri T. (2013). Metsätyypit – opas kasvupaikkojen luokitteluun. [Forest types – a guide to the identification of forest types]. Metsäkustannus Oy, Metsäntutkimuslaitos. 192 p.
Hottola J., Ovaskainen O., Hanski I. (2009). A unified measure of the number, volume and diversity of dead trees and the response of fungal communities. Journal of Ecology 97(6): 1320–1328. https://doi.org/10.1111/j.1365-2745.2009.01583.x.
Junninen K., Komonen A. (2011). Conservation ecology of boreal polypores: a review. Biological Conservation 144(1): 11–20. https://doi.org/10.1016/j.biocon.2010.07.010.
Junninen K., Kouki J. (2006). Are woodland key habitats in Finland hotspots for polypores (Basidiomycota)? Scandinavian Journal of Forest Research 21(1): 32–40. https://doi.org/10.1080/02827580500530009.
Junninen K., Similä M., Kouki J., Kotiranta H. (2006). Assemblages of wood-inhabiting fungi along the gradients of succession and naturalness in boreal pine-dominated forests in Fennoscandia. Ecography 29(1): 75–83. https://doi.org/10.1111/j.2005.0906-7590.04358.x.
Juutilainen K., Mönkkönen M., Kotiranta H., Halme P. (2014). The effects of forest management on wood-inhabiting fungi occupying dead wood of different diameter fractions. Forest Ecology and Management 313: 283–291. https://doi.org/10.1016/j.foreco.2013.11.019.
Juutilainen K., Mönkkönen M., Kotiranta H., Halme P. (2016). The role of novel forest ecosystems in the conservation of wood-inhabiting fungi in boreal broadleaved forests. Ecology and Evolution 6(19): 6943–6954. https://doi.org/10.1002/ece3.2384.
Juutilainen K., Mönkkönen M., Kotiranta H., Halme P. (2017). Resource use of wood-inhabiting fungi in different boreal forest types. Fungal Ecology 27(Part A): 96–106. https://doi.org/10.1016/j.funeco.2017.03.003.
Kotiranta H., Junninen K., Saarenoksa R., Kinnunen J., Kytövuori I. (2010). Aphyllophorales & Heterobasidiomycetes. In: Rassi P., Hyvärinen E., Juslén A., Mannerkoski I. (eds.). The 2010 Red List of Finnish species. Ministry of Environment & Finnish Environment Institute, Helsinki. p. 249–263.
Lonsdale D., Pautasso M., Holdenrieder O. (2008). Wood-decaying fungi in the forest: conservation needs and management options. European Journal of Forest Research 127(1): 1–22. https://doi.org/10.1007/s10342-007-0182-6.
Markkanen A., Halme P. (2012). Polypore communities in broadleaved boreal forests. Silva Fennica 46(3): 317–331. https://doi.org/10.14214/sf.43.
Niemelä T. (2016). Suomen käävät. [The polypores of Finland]. Finnish Museum of Natural History LUOMUS, Helsinki. 432 p.
Nordén J., Penttilä R., Siitonen J., Tomppo E., Ovaskainen O. (2013). Specialist species of wood-inhabiting fungi struggle while generalists thrive in fragmented boreal forests. Journal of Ecology 101(3): 701–712. https://doi.org/10.1111/1365-2745.12085.
Oksanen J., Blanchet F.G., Kindt R., Legendre P., Minchin P.R., O’Hara R.B., Simpson G.L., Solymos P., Stevens M.H.H., Wagner H. (2013). Package “vegan.” Community Ecology Package, Version 2. https://cran.r-project.org/web/packages/vegan/vegan.pdf. [Cited 8 May 2018].
Öpik M., Moora M., Zobel M., Saks Ü., Wheatley R., Wright F., Daniell T. (2008). High diversity of arbuscular mycorrhizal fungi in a boreal herb-rich coniferous forest. New Phytologist 179(3): 867–876. https://doi.org/10.1111/j.1469-8137.2008.02515.x.
Penttilä R., Siitonen J., Kuusinen M. (2004). Polypore diversity in managed and old-growth boreal Picea abies forests in southern Finland. Biological Conservation 117(3): 271–283. https://doi.org/10.1016/j.biocon.2003.12.007.
Renvall P. (1995). Community structure and dynamics of wood-rotting Basidiomycetes on decomposing conifer trunks in northern Finland. Karstenia 35(1): 1–51. https://doi.org/10.29203/ka.1995.309.
Siitonen J. (2001). Forest management, coarse woody debris and saproxylic organisms: Fennoscandian boreal forests as an example. Ecological Bulletins 49: 11–41.
Siitonen J., Martikainen P., Punttila P., Rauh J. (2000). Coarse woody debris and stand characteristics in mature managed and old-growth boreal mesic forests in southern Finland. Forest Ecology and Management 128(3): 211–225. https://doi.org/10.1016/S0378-1127(99)00148-6.
Siitonen P., Lehtinen A., Siitonen M. (2005). Effects of forest edges on the distribution, abundance, and regional persistence of wood‐rotting fungi. Conservation Biology 19(1): 250–260. https://doi.org/10.1111/j.1523-1739.2005.00232.x.
Sippola A.-L., Siitonen J., Kallio R. (1998). Amount and quality of coarse woody debris in natural and managed coniferous forests near the timberline in Finnish Lapland. Scandinavian Journal of Forest Research 13(1–4): 204–214. https://doi.org/10.1080/02827589809382978.
Sippola A.-L., Simila M., Mönkkönen M., Jokimäki J. (2004). Diversity of polyporous fungi (Polyporaceae) in northern boreal forests: effects of forest site type and logging intensity. Scandinavian Journal of Forest Research 19(2): 152–163. https://doi.org/10.1080/02827580410026294.
Tonteri T., Siitonen J. (2001). Lahopuu talousmetsissä valtakunnan metsien 9. inventoinnin tulosten mukaan – vertailu luonnonmetsiin. [Dead wood in managed forests according to the 9th National Forest Inventory – comparison to natural forests]. In: Siitonen J. (ed.). Monimuotoinen metsä. Metsäluonnon monimuotoisuuden tutkimusohjelman loppuraportti. Metsäntutkimuslaitoksen tiedonantoja 812: 57–72. http://urn.fi/URN:ISBN:951-40-1787-0.
Ylisirniö A.L., Mönkkönen M., Hallikainen V., Ranta-Maunus T., Kouki J. (2016). Woodland key habitats in preserving polypore diversity in boreal forests: effects of patch size, stand structure and microclimate. Forest Ecology and Management 373: 138–148. https://doi.org/10.1016/j.foreco.2016.04.042.
Total of 28 references.

