Leaching of heavy metals and barium from forest roads reinforced with fly ash
Lindroos A.-J., Ryhti K., Kaakkurivaara T., Uusitalo J., Helmisaari H.-S. (2019). Leaching of heavy metals and barium from forest roads reinforced with fly ash. Silva Fennica vol. 53 no. 2 article id 10088. https://doi.org/10.14214/sf.10088
Highlights
- Heavy metal concentrations were generally low in percolation and ditch water samples of ash roads, but elevated concentrations were found in some parts of ash roads
- Risk for heavy metal leaching is negligible if road parts producing high concentrations are rare.
Abstract
The aim of this study was to determine the effect of leaching of heavy metals (Cr, As, Cd, Cu, Ni, Pb, Zn, Co, Mo) and earth-alkaline metal, barium (Ba), on the percolation and ditch water quality from the forest roads that contained ash in the road structures. Water quality was studied in the immediate vicinity below the ash layers as well as deeper in the road structure. Water quality was also determined in the drainage water in ditches that crossed the forest roads. A mixture of wood and peat based fly ash was used in the road structures. The treatments were: 1) no ash, 2) a 15 cm layer of ash/gravel mixture, 3) a 20 cm layer of ash/gravel mixture, 4) a 25 cm layer of ash, and 5) a 50 cm layer of ash. Large variation in the concentrations of Cr, As, Cu, Ni, Pb, Mo and Ba in the percolation water, even within the same treatment, caused difficulties to generalize the results. The concentrations of Cr, As, Ni, Pb, Mo and Ba in water samples were high in some treatment plot lysimeters containing ash compared to the control (no ash). On the other hand, many lysimeters had low and similar concentrations in water samples in the treatment plots containing ash compared to concentrations in the control plots. The ash in the roads did not affect the concentrations in the ditches. The leaching is uneven and seems to take place only from some parts of the ash layer. Risk for leaching is minimal if such parts are not widely spread.
Keywords
recycling;
lysimeter;
fly ash;
forest road rehabilitation;
environmental impact assessment;
low-volume road
-
Lindroos,
Natural Resources Institute Finland (Luke), Latokartanonkaari 9, FI-00790 Helsinki, Finland
E-mail
antti.lindroos@luke.fi

- Ryhti, University of Helsinki, Department of Forest Sciences, P.O. Box 27, FI-00014 University of Helsinki, Finland E-mail kira.ryhti@helsinki.fi
- Kaakkurivaara, Natural Resources Institute Finland (Luke), Latokartanonkaari 9, FI-00790 Helsinki, Finland E-mail tomi.kaakkurivaara@gmail.com
- Uusitalo, Natural Resources Institute Finland (Luke), Korkeakoulunkatu 7, FI-33720 Tampere, Finland E-mail jori.uusitalo@luke.fi
- Helmisaari, University of Helsinki, Department of Forest Sciences, P.O. Box 27, FI-00014 University of Helsinki, Finland E-mail helja-sisko.helmisaari@helsinki.fi
Received 29 November 2018 Accepted 12 April 2019 Published 25 April 2019
Views 71230
Available at https://doi.org/10.14214/sf.10088 | Download PDF

1 Introduction
Power and heating plants in Finland produce large amounts of ash due to the bioenergy production. The amount of fly ash (i.e. ash composed of fine particles) which originates from burning of wood and peat has grown since 2006 and was about 700 000 t in year 2017, of which the share of the forest industry was significant (Emilsson 2006; Biomassa Atlas 2018). In the future, it can be expected that the amount of wood based ash will increase further based on the ongoing investments of the Finnish forest industry. However, the government is planning to reduce the amount of waste going to landfills. Due to this aim to promote a circular economy, dumping charges have been raised recently to €70 per ton in Finland (Finlex 2015). Therefore, it is important to identify other ways to utilize ash rather than disposing of it in landfill. Wood ash has traditionally been utilized as a fertilizer for forest and agricultural soils provided that it fulfills the quality requirements set for fertilizers (Patterson et al. 2004; Moilanen et al. 2005). Other utilization possibilities are to use it as a binding material in cement (Wang et al. 2008; Pesonen et al. 2016) or road material (Lahtinen 2001; Edil and Benson 2007).
The utilization of ash as a fertilizer has been largely studied in forests (e.g. Saarsalmi et al. 2004, 2005, 2006, 2010; Hytönen 1998, 2003). In addition, the leaching of nutrients from ash has been studied (Saarsalmi et al. 2005) as well as leaching of heavy metals, e.g. cadmium (Perkiömäki et al. 2003). Ash may contain several heavy metals, e.g. Cu, Cd, Cr, Mn, Hg, Ni and Zn which in high concentrations can be toxic (Perkiömäki 2004). Cadmium (Cd) is one of the most problematic heavy metal in wood ash since it is a mobile (i.e. easily dissolved) heavy metal and toxic to living organisms (Perkiömäki 2004). Especially the fly ash fraction may contain increased amount of Cd as it concentrates in fine particle sized fly ash (Narodoslawsky and Obernberger 1996). Perkiömäki et al. (2003) did not, however, find elevated Cd concentrations in soil percolation water in boreal forest soils fertilized by wood based ash. Other heavy metals, e.g. copper (Cu), may also have negative effects on forest soil processes such as litter decomposition. Studies made near point sources, such as metal smelters, have shown that heavy metals may decrease the activity of forest microbes (Bååth 1989; Pennanen et al. 1996; Fritze et al. 2000).
One possible way to utilize ash is as a road material, especially in forest roads, which are part of the low-volume road network. These are public and private roads which have low daily traffic, are paved with gravel or even surfacing with local soil materials and have less demanding design specifications (Kaakkurivaara 2018). The situation of ash utilization in forest road construction and rehabilitation has changed recently in Finland. At the beginning of 2018, the same environmental permission procedure covers both the forest roads and public roads. This act harmonizes and clarifies the practices of the environmental administration (Finlex 2017). Earlier, the public and forest roads had different regulations due to the structural differences between these road types. The public roads are commonly paved by asphalt, which ensures that the ash structure is not under the influence of water that drains through the road structure, which would possibly cause leaching of heavy metals. Utilization of wood or wood and peat based ash has not yet been widely studied on forest roads, although some studies exist; Kaakkurivaara et al. (2016) and Bohrn and Stampfer (2014) stated that ash improved the bearing capacity compared to the non-ash road structure. Kaakkurivaara and Korpunen (2017) developed a cost calculation model for the ash structure construction work, which showed that ash structures were suitable options for rehabilitation of forest road. So far, only a few studies on heavy metal leaching have been conducted; Oburger et al. (2016) studied wood based grate ash and fluidized bed ash and their contents of dissolved organic carbon (DOC), As, Ni, Al, Fe and Mn as well as pH during a two year survey, and concluded that the concentration levels were environmentally acceptable. Nordmark et al. (2014) found a declining trend of Al, Cl, K, Ca, pH and SO4 in a wood based fly ash structure during a three year survey, and they deduced that the ash can be used without threatening the surrounding environment. However, these leaching studies did not include peat based ash, which may have initially different concentrations of heavy metals than wood ash, e.g. higher concentrations of arsenic (As) (Huotari 2012). It should be taken into account that the contents of heavy metals in ash depend on several factors: the combustion temperature, burning technique, fuels used and combustion gas filtering technique (Korpijärvi et al. 2009). Consequently, it is difficult to make direct and general conclusions from the above mentioned studies. If the utilization of wood and peat based fly ash in forest roads is to be environmentally safe, consideration should be given to possible leaching of heavy metals.
The aim of this study was to determine the water quality related to the concentrations of dissolved heavy metals and barium (Ba) (Table 1) in percolation water inside the forest roads containing ash and in drainage water in ditches that crossed these forest roads. The concentrations in percolation water were studied in the immediate vicinity below the ash layers at different depths inside the forest road. We hypothesized that possible dissolution of heavy metals and Ba from ash layers would be evenly distributed phenomena and similar in all locations from the ash containing road since the homogenous ash layers were constructed inside the forest roads.
| Table 1. The upper limit concentrations (quality limit, QL) used in the study to indicate a good water quality. Measured heavy metals and earth alkaline metal, barium, with their chemical signs and density values (kg l–1) are indicated. | |||
| Measured parameter | Density (kg l–1) | Quality limit (QL) | Source |
| Heavy metals | |||
| Arsenic (As) | 5.72 | 10 µg l–1 | Finlex (2001) |
| Cadmium (Cd) | 8.65 | 5 µg l–1 | Finlex (2001) |
| Cobalt (Co) | 8.90 | 1 µg l–1 | Lahermo et al. (2002) |
| Copper (Cu) | 8.96 | 2000 µg l–1 | Finlex (2001) |
| Chromium (Cr) | 7.19 | 50 µg l–1 | Finlex (2001) |
| Lead (Pb) | 11.35 | 10 µg l–1 | Finlex (2001) |
| Molybdenum (Mo) | 10.22 | 70 µg l–1 | Lahermo et al. (2002) |
| Nickel (Ni) | 8.90 | 20 µg l–1 | Finlex (2001) |
| Zinc (Zn) | 7.13 | 3000 µg l–1 | Finlex (2001) |
| Earth alkaline metal | |||
| Barium (Ba) | 3.59 | 700 µg l–1 | WHO (2011) |
2 Material and methods
The experiment was established on forest roads located in Jämsä, central Finland. The surrounding landscape of the experimental roads consisted of forestland; mainly wetlands and partly upland mineral soils (Table 2). The composition of the surrounding forests varied from young stands to mature managed stands. The treatment plots were built on the top of the initial road as a part of the rehabilitation of the forest roads. Five different treatments were (i.e. four ash treatments and a control) similarly conducted on two forest road sections; 5 treatment plots on the forest road 1 (FR1) and 5 plots on forest road 2 (FR2) (Fig. 1).
| Table 2. Background information of the experimental area near Jämsä, central Finland. | |
| Experimental area | |
| Location | 62°02´N, 24°54´E |
| Vegetation | Boreal |
| Forest land | wetlands, upland mineral soils |
| Mean precipitation (mm a–1) 1 | 636 |
| Mean effective temperature sum (degree days) 1 | 1333 |
| Mean growing season length (days) 1 | 170 |
| 1 Lindroos et al. (2008); mean for the period 1998–2004, data from the closest intensive monitoring plot of forest ecosystems in Finland for the experimental area (Juupajoki 61°52´N, 24°13´E, UN/ECE ICP Forests, Level II plot). | |
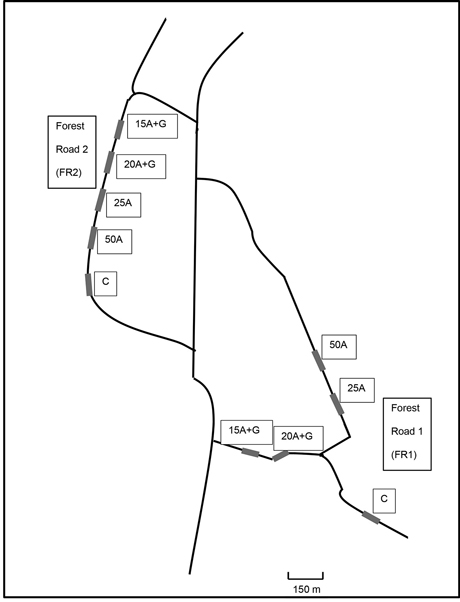
Fig. 1. Schematic picture of the location of test road sections containing ash structures near Jämsä, central Finland. Treatments: C = control (no ash), 15A + G = 15 cm layer of ash + gravel, 20A + G = 20 cm layer of ash + gravel, 25A = 25 cm layer of ash, 50A = 50 cm layer of ash.
A mixture of wood and peat based fly ash was used in the road structures, and the ash layer in forest roads consisted of pure ash or a mixture of ash and gravel. The treatments were: 1) control (no ash), 2) a 15 cm layer of ash and gravel mixture in ratio of 33/66, 3) a 20 cm layer of ash and gravel mixture in ratio of 50/50, 4) a 25 cm layer of ash, and 5) a 50 cm layer of ash.
Percolation water quality was studied during 2011–2014 in water samples with zero-tension lysimeters, which were located inside the roads in the immediate vicinity below ash layers and road surface as well as deeper in the road structure at the bottom level of the road (Fig. 2). In each experimental plot containing ash, three plate (two in control plots) lysimeters with a collection area of 0.1 m2 were installed at the top level of the road, below road surface, and three lysimeters at the bottom level of the road. The lysimeters were located vertically as a pair at the top and bottom levels close to each other, and horizontal distance of lysimeters was 20 m from each other within the treatment. The lysimeters were installed approximately under the wheel path so that the percolation water would be mainly collected from the center part of the road. The number of samples (Fig. 3–10) collected by the lysimeters was comparable between the treatment plots.
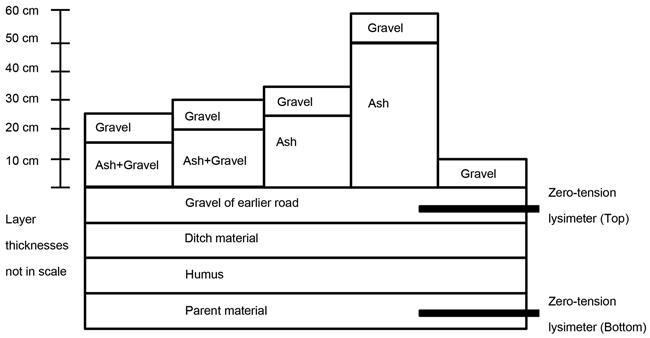
Fig. 2. The structure of the forest roads and location of zero-tension lysimeters.
Water samples were collected during 2011–2014 from the open ditches where drainage water from treatments was flowing along the forest roads and from the ditches that had no contact with experimental roads (background level). Water samples were collected from the ditches which had not yet crossed the ash roads (no ash effect) and from ditches that had drainage water from ash roads (possible ash effect). The open ditches went across FRs through an open tunnel. Water samples were collected from the middle of the ditch at a depth of about 10 cm below the water surface. Contamination from hands was avoided by using plastic gloves. The water flow in ditches varied from dry periods in summer to spring flooding due to snowmelt, but the samples were only collected during the time when the ditches were not flooding.
Water samples from the lysimeters and ditches were collected during the snow-free period (May–November) at about 4-week intervals. The samples in May also represented snowmelt water. The lysimeters were emptied one week before the sampling in order to prevent the over-flowing of containers connected to the lysimeters, and to collect water that was not chemically changed between the sampling occasions. Therefore, the samples collected from the lysimeters represented the percolation water that accumulated during the previous week before the sampling. The samples from the ditches were collected directly to sample bottles. Immediately after sampling, the water samples were sent to the laboratory and kept fridge-cold during the transportation and before analysis.
At the laboratory, the pH of the water samples was measured, and the samples filtered through a 0.45 µm membrane filter. Concentrations of Cr, As, Cd, Cu, Ni, Pb, Zn, Ba, Co and Mo were determined by an inductively coupled plasma atomic emission spectrophotometer (ICP-AES, iCAP 6500 Duo analyzer). Dissolved organic carbon (DOC) concentration was determined by a TOC analyzer (Shimadzu TOC-LCSH/CSN).
Mean values and standard deviations (S.D.) for the period 2011–2014 were calculated for the determined parameters of each treatment plot separately and for the ditches that crossed the ash containing FRs. The numbers of percolation and ditch water samples are presented in the Fig. 3–10. Heavy metal and Ba concentrations in water samples were compared with the water quality limits (Table 1). The upper limit concentrations (quality limit, QL) indicate a good water quality. Finlex (2001) determines the upper permissible concentrations for household water in Finland in water from the dug groundwater based wells. Lahermo et al. (2002) reports high concentrations in ground water based wells (no Finlex value available), and for Mo the upper permissible concentration used in Finland before Finlex (2001) where no limit is presented. WHO (2011) determines the quality limit for drinking water.
3 Results
The mean chromium (Cr) concentrations in the percolation water were elevated in the two treatment plots that contained ash: at the top of the road in the treatment “ash(50 cm)” (FR1) and “ash(25 cm)” (FR2). The concentrations highly varied within these plots, and the Cr concentrations were not elevated for the “ash(50 cm)” in FR2 and “ash(25 cm)” in FR1. In the other treatment plots at both depths, the mean Cr concentrations were low compared to the quality limit value (Table 1). No signs of elevated Cr concentrations due to the ash in the road structures could be detected in the water of the ditches that crossed FR1 and FR2 (Fig. 3 and 4).
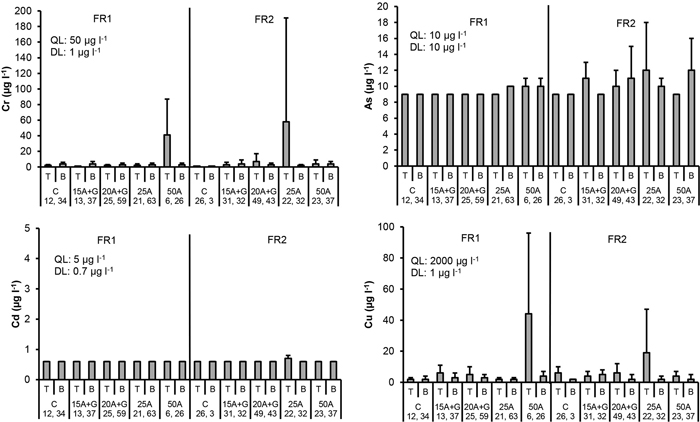
Fig. 3. Cr, As, Cd and Cu concentrations in percolation water collected from the top (T) and bottom (B) part of the two experimental forest roads (FR1, FR2). Mean and standard deviation for 2011–2014. Treatments: C = control (no ash), 15A + G = 15 cm layer of ash + gravel, 20A + G = 20 cm layer of ash + gravel, 25A = 25 cm layer of ash, 50A = 50 cm layer of ash. QL = quality limit (see Table 1). DL = detection limit of analytical equipment. Number of samples is indicated for each treatment plot.
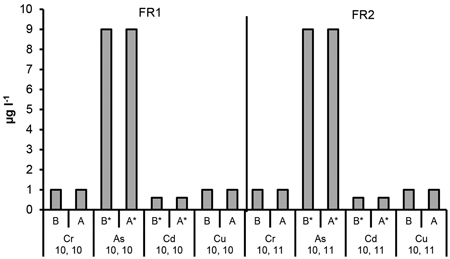
Fig. 4. The Cr, As, Cd and Cu concentrations in ditch water collected before (B, no ash effect) and after (A, incl. possible ash effect) the ditch crossed the experimental forest road containing ash layers (FR1, FR2). Mean and standard deviation for 2011–2014. * = below DL. Number of samples is indicated for each parameter.
The detection limit for arsenic (As) was 10 µg l–1 which is also the quality limit value used in our study (Table 1). A few concentrations were above this limit at some individual points within the plots containing ash. Generally, the As concentrations were below the detection limit, and the mean concentrations close or below 10 µg l–1. No signs of elevated As concentrations due to ash were detected in ditch water (Fig. 3 and 4).
No signs of elevated cadmium (Cd) concentrations were found in either the percolation or ditch water. The concentrations were generally below the detection limit (Fig. 3 and 4).
The mean copper (Cu) concentrations in the percolation water were higher compared to the other plots at the top of the roads in the plot “ash(50 cm)” (FR1) and “ash(25 cm)” (FR2). Still, these mean values were clearly below the quality limit value (Table 1). No signs of elevated Cu concentrations were found in the percolation water in the other plots or in ditch water (Fig. 3 and 4).
The highest mean nickel (Ni) concentrations were detected in the plots “ash(25 cm)” and “ash(50 cm)” at the top of the road in FR2, but the variation was high in these plots. The mean values were generally close or higher than 20 µg l–1 in many plots including the control plot that contained no ash. No signs of elevated Ni concentrations were found in the ditch water due to roads containing ash (Fig. 5 and 6).
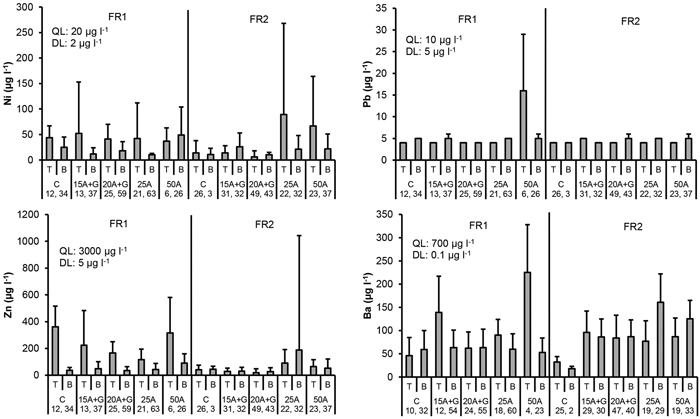
Fig. 5. Ni, Pb, Zn and Ba concentrations in percolation water collected from the top (T) and bottom (B) part of the two experimental forest roads (FR1, FR2). Mean and standard deviation for 2011–2014. Treatments: C = control (no ash), 15A + G = 15 cm layer of ash + gravel, 20A + G = 20 cm layer of ash + gravel, 25A = 25 cm layer of ash, 50A = 50 cm layer of ash. QL = quality limit (see Table 1). DL = detection limit of analytical equipment. Number of samples is indicated for each treatment plot.
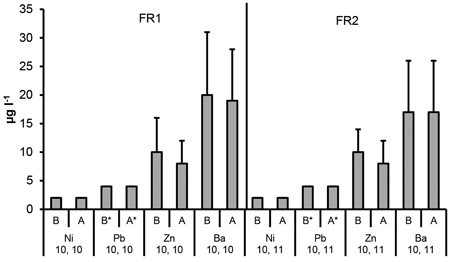
Fig. 6. The Ni, Pb, Zn and Ba concentrations in ditch water collected before (B, no ash effect) and after (A, incl. possible ash effect) the ditch crossed the experimental forest road containing ash layers (FR1, FR2). Mean and standard deviation for 2011–2014. * = below DL. Number of samples is indicated for each parameter.
Mean concentrations of lead (Pb) in the percolation water were generally below the detection limit of 5 µg l–1 both in the treatments with or without ash. The only exception was the plot “ash(50 cm)” in FR1, where the mean Pb concentration was the highest (16 µg l–1) at the top of the road. No signs of elevated Pb concentrations were found in the ditch water (Fig. 5 and 6).
No signs of elevated zinc (Zn) concentrations were detected due to ash, since the highest mean concentration was determined for the control plot with no ash at the top of FR1. No signs of elevated Zn concentrations were found in the ditch water from roads containing ash. Mean Zn concentrations in all the treatment plots in the percolation water as well as in the ditch water were below the quality limit (Table 1, Fig. 5 and 6).
Mean barium (Ba) concentrations were higher compared to the control plots in all the plots containing ash in FR2 at both depths as well as at the top of FR1 in the plots “ash(15 cm ash + gravel)”, “ash(25 cm)” and “ash(50 cm)”. Mean Ba concentration was, however, lower than the quality limit value used in our study (Table 1). No signs of elevated Ba concentrations were found in the ditch water from roads containing ash (Fig. 5 and 6).
The highest cobalt (Co) concentrations were measured at the bottom level of FRs and not immediately below the ash layers in the top level of FRs, and this was also the case in the control plot of FR1. Therefore, the possible effect of ash on the concentrations was difficult to detect. No signs of elevated Co concentrations were found in the ditch water due to roads containing ash (Fig. 7 and 8).
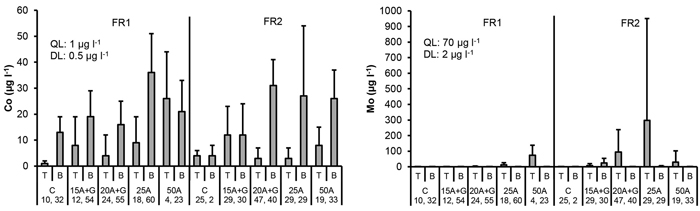
Fig. 7. Co and Mo concentrations in percolation water collected from the top (T) and bottom (B) part of the two experimental forest roads (FR1, FR2). Mean and standard deviation for 2011–2014. Treatments: C = control (no ash), 15A + G = 15 cm layer of ash + gravel, 20A + G = 20 cm layer of ash + gravel, 25A = 25 cm layer of ash, 50A = 50 cm layer of ash. QL = quality limit (see Table 1). DL = detection limit of analytical equipment. Number of samples is indicated for each treatment plot.
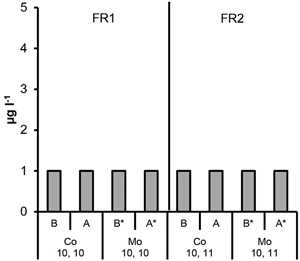
Fig. 8. The Co and Mo concentrations in ditch water collected before (B, no ash effect) and after (A, incl. possible ash effect) the ditch crossed the experimental forest road containing ash layers (FR1, FR2). Mean and standard deviation for 2011–2014. * = below DL. Number of samples is indicated for each parameter.
Elevated molybdenum (Mo) concentrations were detected in the plots that contained ash compared to the control plots without ash, and the highest mean values were measured at top level of FRs. In the treatment “ash(50 cm)” in FR1 as well as “ash(20 cm ash + gravel)” and “ash(25 cm)” in FR2, these mean values at top level of FRs were above the quality limit used in this study (Table 1). However, no signs of elevated Mo concentrations were found in the ditch water due to roads containing ash (Fig. 7 and 8).
Generally the mean pH values were higher in the ash treatments than in the control plots at the top level of FRs. The mean pH values were also elevated due to ash containing FRs in the ditch water (Fig. 9 and 10). There were no signs of the influence of the ash neither for the percolation nor ditch water DOC values (Fig. 9 and 10).
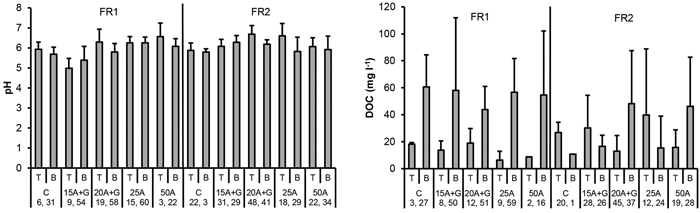
Fig. 9. pH and DOC concentration in percolation water collected from the top (T) and bottom (B) part of the two experimental forest roads (FR1, FR2). Mean and standard deviation for 2011–2014. Treatments: C = control (no ash), 15A + G = 15 cm layer of ash + gravel, 20A + G = 20 cm layer of ash + gravel, 25A = 25 cm layer of ash, 50A = 50 cm layer of ash. Number of samples is indicated for each treatment plot.
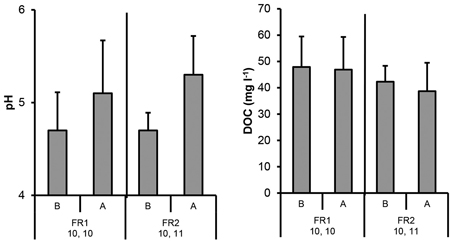
Fig. 10. The pH and DOC concentrations in ditch water collected before (B, no ash effect) and after (A, incl. possible ash effect) the ditch crossed the experimental forest road containing ash layers (FR1, FR2). Mean and standard deviation for 2011–2014. Number of samples is indicated.
4 Discussion
We studied the effect of dissolution of heavy metals and barium from fly ash on the quality of water percolating through forest roads as well as in ditches that crossed the experimental forest roads. Water quality is one of the important aspects to be studied when exploring the possible impacts of recycled ash on environment. However, water quality does not cover all the possible environmental impacts related to ash use. For example, we did not study nutrient and heavy metals fluxes in this study, as it would have required detailed and reliable information about the water fluxes. Ash roads should not negatively affect the water quality. Therefore, it is important to quantify the possible effects of ash use in forest roads on the element concentrations of percolation and drainage waters.
The concentrations of Cr, As, Cu, Ni, Pb, Mo and Ba highly varied in the water percolating through the forest roads, and this fact made it difficult to generalize the results. There were higher concentrations of Cr, As, Ni, Pb, Mo and Ba in some lysimeters within the treatment plots that contained ash as compared with the control (no ash). The highest individual concentrations were determined in plots containing the highest amount of ash (25 or 50 cm pure ash). On the other hand, concentrations were low in many treatments with or without ash.
A risk for the leaching of heavy metals (Cr, As, Ni, Pb, Mo) and Ba occurs if ash layers producing high concentrations (individual points) are common in the road. If such parts are exceptions, a risk for high leaching seems to be negligible. Future studies should concentrate on the reasons for such a large variation in the percolation water.
It is important that ash to be used in forest roads is of good and even quality. In our study, the chemical composition of the ash varied slightly since it was produced on several different occasions in the power plant, and stored in different lengths of time before the ash use. Also, some variation was probably caused by the compaction of the ash layers resulting in loose ash layers with a large surface area for chemical reactions. Ash layers were not compacted during construction by a separate operation, and the road structures were assumed to compact over time in use. The role of compacted ash layers in the leaching processes is undoubtedly an important aspect to be studied in the future.
The roads containing ash did not affect the water quality in the ditches that crossed the FRs. This is a positive finding, but it should be kept in mind that the hydrological conditions strongly affect the concentrations and may in some cases mask the possible effect. For example, if the water volume in the ditches after crossing the ash containing roads had increased, this may have a diluting effect on the concentrations of leached elements. Hydrological conditions varied in our ditches from low to high water volume, and these changes undoubtedly affect the concentrations. Our results however reflect the current situation in water chemical quality, which can be compared to the current threshold values for the quality of household water of the dug groundwater wells (Finlex 2001; Lahermo et al. 2002; WHO 2011). The concentrations of many parameters in the ditch water fulfilled these quality criterions set for household water of the dug groundwater wells, and no signs of increased concentrations were detected in ditches crossing the ash containing roads.
Vanhanen et al. (2014) reported that the concentrations of heavy metals were low in the ground water, which was under the influence of drainage waters from the forest roads constructed using wood based ash in Finland. Their study setup was very similar in many ways compared to our study, although they focused on ground water quality, and not percolation and ditch water quality as this study. They stated that a three year study was, however, a quite short monitoring period. Nordmark et al. (2014) found a declining trend in the Al, Cl, K, Ca, pH and SO4 concentrations in wood based fly ash structure during a three year survey. Our results are in agreement with these studies in respect that concentrations were low in many parts in forest road structures containing ash, and leaching did not negatively affect the ditch water quality. On the other hand, Oburger et al. (2016) found leaching of e.g. Ni and As from wood ash used in forest roads, and elevated concentrations of these metals were higher than the chemical quality limits set for household water in Austria. In our study, concentrations of Cr, As, Ni, Pb and Mo were occasionally high in the ash containing road structures in some points of the roads. The quality of the ash can vary due to many factors, and therefore only good and even quality ash should be used in road construction and as a fertilizer, and the leaching properties of different substances from ash should be known. Kaakkurivaara (2018) reported that a good bearing capacity was reached already by using a mixture of 15 or 20 cm ash and gravel. This structure seemed to be a good option from the point of view of both the bearing capacity and element leaching in our study area.
5 Conclusions
The mean concentrations of all the measured parameters were low in many treatment plots that contained ash, and the mean concentrations decreased in the road structure from the top to the bottom level of the road. The ash containing forest roads did not negatively affect the concentrations of water in ditches that crossed the forest roads. Some lysimeters close to the ash layer had elevated concentrations of heavy metals (e.g. As, Ni, Mo) and Ba in percolation water, when others necessarily did not. Therefore, the leaching from the forest roads seems to take place from some points from the ash layer and not evenly from the entire ash layer. A risk for leaching of heavy metals and Ba seems to be low if the roads only rarely contain such high leaching parts. On the other hand, a risk for leaching should be considered if the points with high concentrations are widely spread in the road, and water from these parts is flowing directly to side-ditches and groundwater without percolation through the soil material of the road structure.
Acknowledgements
The work described in this paper is part of research coordinated by Luke (Natural Resources Institute Finland). The bulk of funding for the research was provided by EU’s European Agricultural Fund for Rural Development: Europe investing in rural areas.
References
Bååth E. (1989). Effects of heavy metals in soil on microbial processes and populations. Water, Air and Soil Pollution 47(3–4): 335–379. https://doi.org/10.1007/BF00279331.
Biomassa Atlas. (2018). https://www.luke.fi/biomassa-atlas/. [Cited 12 June 2018]
Bohrn G., Stampfer K. (2014). Untreated wood ash as a structural stabilizing material in forest roads. Croatian Journal of Forest Engineering 35(1): 81–88.
Edil T., Benson H. (2007). Demonstration of ash utilization in low volume roads. Research report 2007–12. Minnesota Department of Transportation. 233 p.
Emilsson S. (2006). International handbook: from extraction of forest fuels to ash recycling. Skogstyrelsen, Swedish Forest Agency. 42 p.
Finlex (2001). Sosiaali- ja terveysministeriön asetus pienten yksiköiden talousveden laatuvaatimuksista ja valvontatutkimuksista. 401/2001. https://www.finlex.fi/fi/laki/alkup/2001/20010401. [Cited 12 June 2018]. [In Finnish].
Finlex (2015). Laki jäteverolain 5 ja 6 §:n muuttamisesta. 1401/2015. [Waste tax Act]. https://www.finlex.fi/fi/laki/alkup/2015/20151401. [Cited 12 June 2018]. [In Finnish].
Finlex (2017). Valtioneuvoston asetus eräiden jätteiden hyödyntämisestä maarakentamisessa. [Waste utilization Act]. 843/2017. https://www.finlex.fi/fi/laki/alkup/2017/20170843. [Cited 13 June 2018]. [In Finnish].
Fritze H., Pennanen T., Haimi J., Siira-Pietikäinen A., Vanhala P. (2000). Effects of heavy metals on soil microflora. In: Mälkönen E. (ed.). Forest condition in a changing environment – the Finnish case. Kluwer Academic Publishers, Netherlands. p. 260–265. https://doi.org/10.1007/978-94-015-9373-1_30.
Huotari N. (2012). Tuhkan käyttö metsälannoitteena. Metsäntutkimuslaitos. 47 p. http://urn.fi/URN:ISBN:978-951-40-2371-2. [In Finnish].
Hytönen J. (1998). Effect of peat ash fertilization on the nutrient status and biomass production of short-rotation willow on cut-away peatland area. Biomass and Bioebergy 15(1): 83–92. https://doi.org/10.1016/S0961-9534(97)10050-2.
Hytönen J. (2003). Effects of wood, peat and coal ash fertilization on Scots pine foliar nutrient concentrations and growth on afforested former agricultural peat soils. Silva Fennica 37(2): 219–234. https://doi.org/10.14214/sf.503.
Kaakkurivaara T. (2018). Innovative methods for measuring and improving the bearing capacity of forest roads. Dissertationes Forestales 251. 57 p. https://doi.org/10.14214/df.251.
Kaakkurivaara T., Korpunen H. (2017). Increased fly ash utilization – value addition through forest road reconstruction. Canadian Journal of Civil Engineering 44(3): 223–231. https://doi.org/10.1139/cjce-2016-0193.
Kaakkurivaara T., Kolisoja P., Uusitalo J., Vuorimies N. (2016). Fly ash in forest road rehabilitation. Croatian Journal of Forest Engineering 37(1): 119–130.
Korpijärvi K., Mroueh U.M., Merta E., Laine-Ylijoki J., Kivikoski H., Järvelä E., Wahlström M., Mäkelä E. (2009). Energiantuotannon tuhkien jalostaminen maarakennuskäyttöön. [Processing of fly ash for earth construction]. VTT Research notes 2499. VTT Technical Research Centre of Finland. 104 p. [In Finnish].
Lahermo P., Tarvainen T., Hatakka T., Backman B., Juntunen R., Kortelainen N., Lakomaa T., Nikkarinen M., Vesterbacka P., Väisänen U., Suomela P. (2002). Tuhat kaivoa – Suomen kaivovesien fysikaalis-kemiallinen laatu vuonna 1999. Geologian tutkimuskeskus, Espoo. Tutkimusraportti 155. [In Finnish].
Lahtinen P. (2001). Fly ash mixtures as flexible structural materials for low-volume roads. Finnra Reports 70/2001. Finnish Road Administration. 95 p.
Lindroos A.-J., Derome J., Mustajärvi K., Nöjd P., Beuker E., Helmisaari H.-S. (2008) Fluxes of dissolved organic carbon in stand throughfall and percolation water in 12 boreal coniferous stands on mineral soils in Finland. Boreal Environment Research 13 (suppl. B): 22–34.
Moilanen M., Silverberg K., Hökkä H., Issakainen J. (2005). Wood ash as a fertilizer on drained mires – growth and foliar nutrients of Scots pine. Canadian Journal of Forest Research 35(11): 2734–2742. https://doi.org/10.1139/x05-179.
Narodoslawsky M., Obernberger I. (1996). From waste to raw material – the route from biomass to wood ash for cadmium and other heavy metals. Journal of Hazardous Materials 50: 157–168.
Nordmark D., Vestin J., Lagerkvist A., Lind B.B., Arm M., Hallgren P. (2014). Geochemical behavior of a gravel road upgraded with wood fly ash. Journal of Environmental Engineering 140(10): 05014002. https://doi.org/10.1061/(ASCE)EE.1943-7870.0000821.
Oburger E., Jäger A., Pasch A., Dellantonio A., Stampfer K., Wenzel W. (2016). Environmental impact assessment of wood ash utilization in forest road construction and maintenance – a field study. Science of the Total Environment 544: 711–721. https://doi.org/10.1016/j.scitotenv.2015.11.123.
Patterson S., Acharya S., Thomas J., Bertschi A., Rothwell R. (2004). Integrated soil and crop management, Barley biomass and grain yield and canola seed yield response to land application of wood ash. Agronomy Journal 96(4): 971–977. https://doi.org/10.2134/agronj2004.0971.
Pennanen T., Frostegård Å., Fritze H., Bååth E. (1996). Phospholipid fatty acid composition and heavy metal tolerance of soil microbial communities along two heavy metal-polluted gradients in coniferous forests. Applied Environmental Microbiology 62: 420–428.
Perkiömäki J. (2004). Wood ash use in coniferous forests. Finnish Forest Research Institute, Research Papers 917. 54 p. http://urn.fi/URN:ISBN:951-40-1912-1.
Perkiömäki J., Kiikkilä O., Moilanen M., Issakainen J., Tervahauta A., Fritze H. (2003). Cadmium-containing wood ash in a pine forest: effects on humus microflora and cadmium concentrations in mushrooms, berries, and needles. Canadian Journal of Forest Research 33: 2443–2451. https://doi.org/10.1139/x03-169.
Pesonen J., Yliniemi J., Kuokkanen T., Ohenoja K., Illikainen M. (2016). Improving the hardening of fly ash from fluidized-bed combustion of peat and wood with the addition of alkaline activator and Portland cement. Romanian Journal of Materials 46(1): 82–88.
Saarsalmi A., Mälkönen E., Kukkola M. (2004). Effect of wood ash fertilization on soil chemical properties and stand nutrient status and growth of some coniferous stands in Finland. Scandinavian Journal of Forest Research 19(3): 217–233. https://doi.org/10.1080/02827580410024124.
Saarsalmi A., Derome J., Levula T. (2005). Effect of wood ash fertilization on stand growth, soil, water and needle chemistry, and berry yields of lingonberry (Vaccinium vitis-idaea L.) in a Scots pine stand in Finland. In: Mandre M. (ed.). Utilisation of industrial wastes in forestry. Forestry Studies 42: 13–33.
Saarsalmi A., Kukkola M., Moilanen M., Arola M. (2006). Long-term effects of ash and N fertilization on stand growth, tree nutrient status and soil chemistry in a Scots pine stand. Forest Ecology and Management 235(1–3): 116–128. https://doi.org/10.1016/j.foreco.2006.08.004.
Saarsalmi A., Smolander A., Kukkola M., Arola M. (2010). Effect of wood ash and nitrogen fertilization on soil chemical properties, soil microbial processes, and stand growth in two coniferous stands in Finland. Plant and Soil 331: 329–340. https://doi.org/10.1007/s11104-009-0256-y.
Vanhanen H., Dahl O., Joensuu S. (2014). Utilization of wood ash as a construction material – sustainable use of wood ash. Sustainable Environment Research 24(6): 457–465.
Wang S., Miller A., Llamazos E., Fonseca F., Baxter L. (2008). Biomass fly ash in concrete: mixture proportioning and mechanical properties. Fuel 87(3): 365–371. https://doi.org/10.1016/j.fuel.2007.05.026.
WHO (2011). Guidelines for drinking-water quality. 4th edition.
Total of 35 references.

