The efficacy of Chondrostereum purpureum against sprouting of deciduous species after mechanized pre-commercial thinning
Laine T., Hamberg L., Saarinen V.-M., Saksa T. (2019). The efficacy of Chondrostereum purpureum against sprouting of deciduous species after mechanized pre-commercial thinning. Silva Fennica vol. 53 no. 3 article id 10195. https://doi.org/10.14214/sf.10195
Highlights
- Fungal treatments increased stump mortality compared to the control (cutting only)
- The fungal treatment did not decrease the number of sprouts per stump
- Application during mechanized pre-commercial thinning did not yield as high stump mortalities as in earlier treatments performed manually.
Abstract
The use of a white-rot fungus, Chondrostereum purpureum (Pers. Ex Fr.) Pouzar, as a biocontrol agent against sprouting has been studied with good results. The aim of the study was to investigate the efficacy of two pre-commercial thinning machines, Tehojätkä and Mense, to spread an inoculum of C. purpureum as a biocontrol agent on freshly cut birch (Betula pendula Roth and B. pubescens Ehrh.), European aspen (Populus tremula L.), rowan (Sorbus aucuparia L.), and goat willow (Salix caprea L.) stumps (the fungal treatment) and compare that to the control (cutting only, done by Tehojätkä). Efficacy was investigated in terms of stump mortality and the number of sprouts per stump. This study was conducted in one stand and sprouting was investigated for three years after treatment. The fungal treatment resulted in higher mortality of stumps (34.0% for Tehojätkä and 41.5% for Mense after three years), compared to the control (13.4%). However, the fungal treatment did not decrease the number of sprouts per stump compared to the control. The low occurrence of basidiomata indicates that the accuracy of the spreading mechanism was not satisfactory, causing low mortality figures for the fungal treatment compared to previous studies. In the future, this mechanized method may provide a promising alternative in sprout control if the spreading mechanisms, the accuracy of the treatment, and consequently the efficacy could be improved.
Keywords
vegetation management;
silviculture;
mechanization;
Chondrostereum purpureum;
fungal treatment;
stump sprouts
-
Laine,
Natural Resources Institute Finland (Luke), Natural resources, Juntintie 154, FI-77600 Suonenjoki, Finland
E-mail
tiina.laine@luke.fi

- Hamberg, Natural Resources Institute Finland (Luke), Bioeconomy and environment, Latokartanonkaari 9, FI-00790 Helsinki, Finland E-mail leena.hamberg@luke.fi
- Saarinen, Natural Resources Institute Finland (Luke), Natural resources, Juntintie 154, FI-77600 Suonenjoki, Finland E-mail mulinvuori@gmail.com
- Saksa, Natural Resources Institute Finland (Luke), Natural resources, Juntintie 154, FI-77600 Suonenjoki, Finland E-mail timo.saksa@luke.fi
Received 10 May 2019 Accepted 16 August 2019 Published 20 August 2019
Views 39592
Available at https://doi.org/10.14214/sf.10195 | Download PDF

1 Introduction
After artificial forest regeneration, young stand management, i.e., cutting of deciduous saplings, is needed to ensure the growth of conifers, mostly spruce (Picea abies (L.) H. Karst.) and Scots pine (Pinus sylvestris L.) (Gobakken and Næsset 2002; Huuskonen and Hynynen 2006; Äijälä et al. 2014; Uotila 2017). Vigorous regrowth of deciduous stump sprouts after early clearing (ca. five years after regeneration when the height of conifer saplings is 1–2 meter) causes a need for pre-commercial thinning (PCT) (when pines are 5–7 m and spruces 3–4 m in height) to ensure better growing conditions for more valuable coniferous trees (Kauppi et al. 1987; Johansson 2008; Äijälä et al. 2014; Uotila 2017). However, repeated cuttings increase the total cost of young stand management (Uotila 2017). Compared to the 1990-level, the costs of young stand management have more than doubled by the 2010s, accounting for the current highest costs, ca. EUR 58 million, of all silvicultural and forest improvement work types in Finland (Luke 2018).
The use of chemical herbicides to prevent sprouting would be efficient, but it is harmful to nature and is restricted by forest certification (PEFC 2017). Thus, an environmentally friendly alternative, a white-rot fungus Chondrostereum purpureum (Pers. Ex Fr.) Pouzar, has been studied (Wall 1990; Gosselin et al. 1999; Setliff 2002; Vartiamäki 2009; Hamberg et al. 2015). Results have been promising as manual application of C. purpureum inoculum on freshly cut birch stumps has resulted in mortality of more than 75% (Wall 1990; Roy et al. 2010; Vartiamäki 2009; Hamberg et al. 2015).
The aim of the study was to investigate the efficacy of two PCT-machines, Tehojätkä and Mense, together with the fungal treatment (C. purpureum applied on freshly cut stumps) for controlling competing deciduous trees in a juvenile conifer stand. We hypothesized that the fungal treatment increases stump mortality and decreases the number of sprouts per stump. In this study, the young stand management method is called “early PCT”, with the target that a young stand will be treated only once for cost-efficiency.
2 Material and methods
2.1 Study design
In 25 June 2015, early PCT was done on a Myrtillus site type stand, located in central Finland: Jyväskylä (62°15´N, 26°02´E). The stand was artificially regenerated to Norway spruce (Picea abies) in autumn 2010. Basal suspension of Chondrostereum purpureum (provided by Verdera Ltd.) included an efficient fungal strain R5 (same as in Hamberg et al. 2015) and diluted (1:100) with tap water just before the treatments.
The work was done by a lightweight mini-harvester Tehojätkä (Usewood Ltd., Finland) equipped with a boom-mounted UW40-cleaning head or Mense RP6L attached to the harvester boom (Fig. 1). Both machines were equipped with tanks for applying a suspension of C. purpureum mycelium that was connected through a hose along the boom to the cleaning heads. The suspension was pumped out when the clearing head was in use. In Tehojätkä, the C. purpureum inoculum was placed on the upper side of the cutting blade from which it flowed through holes, made in the blade, onto the surface of cut stumps. In Mense, C. purpureum inoculum was placed on both sides of the blade, fan-like, through a T-piece in the boom, about 50 cm above the blade. There was also a punctual nozzle that sprayed suspension to the tip of the blade.

Fig. 1. Machines used in the study: Usewood Tehojätkä small-scale forest machine equipped with UW40-cleaning head (left) and Mense RP6L equipped on the boom of the harvester (right) Photos: Veli-Matti Saarinen and UW40-cleaning head: www.usewood.fi.
The study design included three blocks for each treatment: the fungal treatments by Tehojätkä or Mense (cutting and applying fungal inoculum on freshly cut stumps), and the control (cutting only) done by Tehojätkä. The distance between the blocks and their size was determined by terrain conditions. There was a minimum buffer zone of 10 m between the treatments and from trees of neighboring stands to avoid their immediate effects on sprouting (Hamberg et al. 2015). The inventories were done in autumn of 2016, 2017 and 2018, i.e., one to three years after treatment. Inventories were carried out by measuring a systematic regularly shaped grid of 15 circular sample plots (r = 1.0 m) in every block. The distance between the plots was 6 m. The data consisted of 135 sample plots, 45 per treatment. However, due to land use changes in the area, two out of three Tehojätkä blocks were destroyed after the 2017 inventory. Thus, the final inventory in 2018 included only 15 sample plots for Tehojätkä, i.e., altogether 105 plots.
All stumps (diameter > 0.5 cm) and stump sprouts were inventoried from the sample plots annually. The following characteristics were recorded or measured: tree species, the diameter (mm) of an investigated stump, and the number of living stump sprouts.. Tree species investigated were birch (Betula pendula Roth and B. pubescens Ehrh.), European aspen (Populus tremula L.), rowan (Sorbus aucuparia L.), and goat willow (Salix caprea L.). Since the effect of the fungal treatment on the sprouting of different tree species, i.e., their ability to produce both stump sprouts and root suckers is known (Hamberg et al. 2014, 2015; Hamberg and Hantula 2016), we concentrated on stump sprouts only to investigate possible differences in the efficacy of the PCT-machines. A stump was considered dead if it had no living sprouts. In 2016 and 2017, the occurrence of basidiomata of C. purpureum per stump (0 = basidiomata not found, and 1 = basidiomata found) was recorded.
2.2 Statistical analyses
The statistical program R was used in the analyses (R Core Team 2018). The mortality of stumps (0 = stump is alive, 1 = stumps is dead) and the number of sprouts on a living stump were investigated with generalized linear mixed models (GLMMs) using the function glmer in library lme4 (Bates et al. 2015). In the mortality model, a binomial distribution with logit link function was used, whereas in the stump sprout number model, a Poisson distribution with log link function was used. All stumps were included in the mortality models, but only living stumps were included in the stump sprout models (number). Models were estimated separately for each year.
Models included the following explanatory variables: (1) treatment (a factor with three levels: control, Tehojätkä, and Mense), (2) tree species (a factor with four levels: birch, aspen, rowan, and willow), (3) stand density (cut stumps and saplings), and (4) the diameter of an investigated stump (mm). Block and sample plot were included as nested random factors as conditions within the same block and sample plot may be more similar than on a randomly selected block or sample plot.
3 Results
The early PCT lowered the density of saplings by ca. 62% to the density of 5321 ± 3899 ha–1 (Table 1). Of all stumps investigated (n = 361), 34.1% were birch, 26.3% aspen, 21.3% rowan and, 18.3% willow. The mean diameter and height of cut stumps was 12.6 ± 5.4 and 31.6 ± 12.8 cm, respectively.
| Table 1. Number of stumps, their diameter and height, and stand density before and after the early pre-commercial thinning. | ||||
| Mense | Tehojätkä* | Control | Pooled* | |
| Number of stumps | 111 | 150 (38) | 100 | 361 (249) |
| Birch | 38 | 63 (11) | 22 | 123 (71) |
| Aspen | 21 | 24 (3) | 50 | 95 (74) |
| Rowan | 22 | 42 (15) | 13 | 77 (50) |
| Willow | 30 | 21 (9) | 15 | 66 (54) |
| Stump diameter (mm) | 13.3 ± 4.7 | 11.4 ± 4.5 | 13.7 ± 6.9 | 12.6 ± 5.4 |
| Birch | 13.2 ± 5.3 | 11.4 ± 5.1 | 13.7 ± 9.5 | 12.4 ± 6.2 |
| Aspen | 15.9 ± 5.7 | 12.8 ± 4.5 | 14.5 ± 6.1 | 14.4 ± 5.7 |
| Rowan | 12.6 ± 3.6 | 11.6 ± 4.0 | 12.6 ± 4.5 | 12 0 ± 3.9 |
| Willow | 12.3 ± 3.2 | 9.7 ± 3.2 | 12.1 ± 6.7 | 11.3 ± 4.3 |
| Stump height (cm) | 34.9 ± 15.4 | 28.7 ± 10.7 | 32.2 ± 11.7 | 31.6 ± 12.8 |
| Birch | 34.8 ± 14.5 | 29.8 ± 9.8 | 33.2 ± 14.5 | 32.0 ± 12.4 |
| Aspen | 32.6 ± 12.8 | 28.7 ± 12.8 | 33.8 ± 10.7 | 32.3 ± 11.8 |
| Rowan | 32.9 ± 19.9 | 28.8 ± 11.4 | 29.8 ± 9.4 | 30.0 ± 14.1 |
| Willow | 37.9 ± 14.8 | 25.4 ± 8.8 | 27.8 ± 11.8 | 31.7 ± 12.8 |
| Density (ha–1) | ||||
| Saplings (before) | 12 591 ± 6543 | 15 420 ± 10 729 | 16 824 ± 7251 | 13 896 ± 8403 |
| Stumps | 7851 ± 6635 | 10 610 ± 9693 | 7234 ± 4089 | 8575 ± 7641 |
| Saplings (after) | 4739 ± 3693 | 4810 ± 3755 | 6438 ± 5717 | 5321 ± 3899 |
| * Year 2018 value in parentheses (2/3 of blocks were destroyed due to land use changes in the area). | ||||
3.1 Mortality
Mortality was higher for the fungal treatment stumps done by Tehojätkä or Mense than on the control stumps (cutting only) one, two, and three years after the treatments (Table 2, Fig. 2a). Mortality increased slightly with time (year 2016 < 2017 < 2018) being 34.0%, 41.5%, and 13.4% (predicted mean values based on GLMM) in birch stumps three years after the fungal treatments performed by Tehojätkä and Mense, and the control (cutting only), respectively (note: in the final inventory, 2/3 of the experimental area for Tehojätkä was destroyed). There were differences in mortality between the investigated tree species. Compared to birch, the mortality of rowan stumps was lower (2.1%, 2.9%, and 0.7% for Tehojätkä, Mense, and the control, respectively), similarly as in willow stumps (indicative differences were detected) (17.4%. 22.5%, and 6.0% for Tehojätkä, Mense, and the control, respectively) but compared to aspen no differences were found (38.7%, 46.5%, and 16.0% for Tehojätkä, Mense, and the control, respectively). Higher stand density (cut stumps and saplings) was associated with higher mortality one year after the treatments, and larger stumps were associated with higher mortality two years after the treatments, but neither influenced mortality three years after the treatments.
| Table 2. The effects of (1) the treatment (the control vs. the fungal treatments done by Tehojätkä or Mense), (2) tree species (birch vs. aspen, rowan or willow), (3) the number of saplings and stumps on a plot, and (4) the diameter of an investigated stump (mm) on the mortality of stumps (0 = alive, 1 = dead) one (2016, n = 361), two (2017, n = 361), and three years (2018, n = 249) after the early pre-commercial thinning, as well as on the number of sprouts in a stump one (2016, n = 282), two (2017, n = 282), and three years (2018, n = 194) after the early pre-commercial thinning (generalized linear mixed models). Statistically significant p-values (p < 0.05) are in bold and indicative results have been underlined (0.05 ≤ p ≤ 0.10). See also Fig. 2. | ||||||
| Explanatory variables | Year 2016 | Year 2017 | Year 2018 | |||
| Coeff. ± SE | p | Coeff. ± SE | p | Coeff. ± SE | p | |
| Mortality | ||||||
| Intercept | –3.469 ± 0.765 | <0.001 | –3.628 ± 0.833 | <0.001 | –3.112 ± 0.985 | 0.002 |
| Treatment (compared to the control) | ||||||
| Tehojätkä | 1.298 ± 0.446 | 0.004 | 1.551 ± 0.472 | 0.001 | 1.200 ± 0.646 | 0.006 |
| Mense | 1.455 ± 0.445 | 0.001 | 1.601 ± 0.462 | <0.001 | 1.521 ± 0.469 | 0.001 |
| Tree species (compared to birch) | ||||||
| Aspen | 0.047 ± 0.356 | 0.894 | –0.216 ± 0.362 | 0.550 | 0.206 ± 0.450 | 0.647 |
| Rowan | –1.954 ± 0.574 | <0.001 | –2.504 ± 0.654 | <0.001 | –3.153 ± 1.08 | 0.003 |
| Willow | –0.585 ± 0.407 | 0.150 | –0.806 ± 0.412 | 0.050 | –0.895 ± 0.492 | 0.069 |
| Density (saplings per plot) | 0.139 ± 0.056 | 0.013 | 0.068 ± 0.057 | 0.233 | 0.123 ± 0.102 | 0.226 |
| Stump diameter (mm) | 0.040 ± 0.028 | 0.146 | 0.087 ± 0.030 | 0.003 | 0.036 ± 0.032 | 0.252 |
| Number of stump sprouts | ||||||
| Intercept | 0.783 ± 0.199 | <0.001 | 0.797 ± 0.182 | <0.001 | 0.703 ± 0.230 | 0.002 |
| Treatment (compared to the control) | ||||||
| Tehojätkä | –0.107 ± 0.132 | 0.416 | –0.071 ± 0.106 | 0.500 | 0.046 ± 0.153 | 0.763 |
| Mense | 0.107 ± 0.131 | 0.416 | 0.138 ± 0.104 | 0.185 | 0.126 ± 0.109 | 0.249 |
| Tree species (compared to birch) | ||||||
| Aspen | –0.189 ± 0.114 | 0.097 | –0.088 ± 0.110 | 0.425 | 0.084 ± 0.141 | 0.552 |
| Rowan | 0.887 ± 0.100 | <0.001 | 0.795 ± 0.100 | <0.001 | 0.871 ± 0.131 | <0.001 |
| Willow | 0.434 ± 0.101 | <0.001 | 0.434 ± 0.103 | <0.001 | 0.418 ± 0.133 | 0.002 |
| Density (saplings per plot) | –0.031 ± 0.019 | 0.103 | –0.037 ± 0.017 | 0.028 | –0.051 ± 0.025 | 0.041 |
| Stump diameter (mm) | 0.038 ± 0.007 | <0.001 | 0.034 ± 0.007 | <0.001 | 0.026 ± 0.008 | 0.001 |
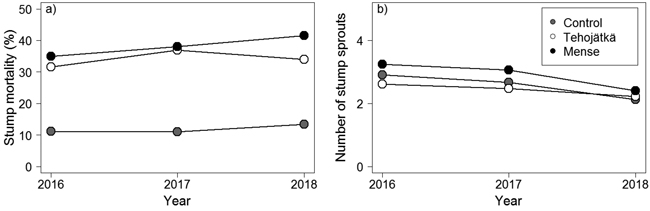
Fig. 2. The effects of the control (cutting only) and the fungal treatments done by Tehojätkä and Mense on a) the mortality of birch stumps (%), and b) the number of stump sprouts per stump one, two and three years after the early pre-commercial thinning. Figures have been drawn based on the predicted values of the generalized linear mixed models for birch, see Table 2.
One year after the treatments, the occurrence of basidiomata was 15.3% and 12.5% for the fungal treatments performed by Tehojätkä and Mense, and only 4.5%, and 3.6% after two years, respectively. There were more basidiomata on birch stumps than on the stumps of the other tree species. Of birch stumps, 26.0% had basidiomata, whereas only 5.3%, 1.3%, and 10.6% of aspen, rowan, and willow stumps had basidiomata, respectively. Basidiomata were not found on control stumps.
3.2 Number of sprouts
The fungal treatment done by Tehojätkä or Mense did not lower the number of sprouts per living stump compared to the control (cutting only) (Table 2, Fig. 2b). In stumps, the number of sprouts decreased every year (2016 > 2017 > 2018) being 2.2, 2.4, and 2.1 sprouts per stump (predicted mean values based on GLMM) three years after the treatments for Tehojätkä, Mense, and the control, respectively. There were differences in the number of sprouts per stump between tree species: compared to birch, rowan and willow had more sprouts (Table 2). The density of cut stumps and saplings (per plot) and the diameter of a stump affected the number of sprouts per birch stump. The higher the number of cut stumps and saplings in a plot, the lower the number of sprouts per stump, and larger diameter of a stump was associated with an increase in the number of stump sprouts.
4 Discussion
This study shows that in mechanized pre-commercial thinning, the mortality of stumps was higher for the fungal treatment compared to the control (cutting only) but concerning the number of stump sprouts there were no differences between the treatments. Our results indicate that there were technical problems with the accuracy of the spreading mechanism, causing lower mortalities e.g., in birch (34.0% and 41.5% for Tehojätkä and Mense, respectively) than observed after three growing seasons in an earlier study (78%) with similar stump diameters as in the present study (Hamberg et al. 2015). C. purpureum produces basidiomata only on dying and recently dead trees, and formation of basidiomata can be expected only in the first two years after the treatment (Wall 1990). Thus, in birch, the low occurrence of basidiomata after the first year (26%) compared to earlier research (56%, Hamberg et al. 2015) indicates that C. purpureum inoculum did not reach the surface of the stumps as well as in manual applications. In aspen, mortality did not differ from the mortality of birch but in rowan and willow, mortality was lower. Also, for these tree species mortalities were lower than in earlier studies (Lygis et al. 2012; Hamberg and Hantula 2016; Hamberg et al. 2017). In earlier studies, it has been shown that C. purpureum can utilize the woody material of birch and aspen better than that of the other species (Roy et al. 2010; Hamberg and Hantula 2016; Hamberg et al. 2015; Hamberg et al. 2017) thus verifying our results. It would have been valuable to study also effects on root suckers since C. purpureum has been proved to be effective in causing both mortality of aspen stumps and in preventing the development of root suckers (Hamberg and Hantula 2016). However, we believe that our investigations on stump mortality gave valuable information on the efficacy of the tested machines.
Due to the small number of observations, these results should be interpreted cautiously. However, these results verify that the fungal treatment was more effective than the control (cutting only) after mechanized pre-commercial thinning. In an earlier study, similar mortality values have been reported for Tehojätkä as in the present study (Laine et al. submitted manuscript), but concerning Mense this was the first study where its efficacy in cutting and spreading C. purpureum inoculum has been tested. Based on our results, Mense’s method also seems promising. These machines can easily carry C. purpureum inoculum, and spread it on stump surfaces immediately after cutting, and therefore it is worth continuing the development of mechanized applications. In the future, mechanized application may provide a promising alternative in sprout control and improve the cost-efficiency of young stand management by eliminating the need of repeated cuttings. To meet this, the accuracy of the spreading mechanism needs to be improved and the dosage should be minimized, so that the inoculum reaches the stump surface but is not spread elsewhere.
Acknowledgements
This work was supported by the EU, through the EFFORTE (Efficient forestry by precision planning and management for sustainable environment and cost competitive bio-based industry) project (Grant agreement number: 720712 — EFFORTE — H2020-BBI-PPP-2015-02/H2020-BBI-PPP-2015-2-1). Authors thank UPM Forest for arranging the machines and the research stand needed in the field experiments. We also thank Mr. Raimo Jaatinen and Mr. Aulis Leppänen for their efforts in data collection.
References
Äijälä O., Koistinen A., Sved J., Vanhatalo K., Väisänen P. (eds.) (2014). Metsänhoidon suositukset. [Finnish forest management practice recommendations]. Forestry Development Centre Tapio. 179 p. [In Finnish].
Bates D., Maechler M., Bolker B., Walker S. (2015). Fitting linear mixed-effects models using lme4. Journal of Statistical Software 67(1): 1–48. https://doi.org/10.18637/jss.v067.i01.
Gobakken T., Næsset E. (2002). Spruce diameter growth in young mixed stands of Norway spruce (Picea abies (L.) Karst.) and birch (Betula pedula Roth B. pubescens Ehrh.). Forest ecology and management 171(3): 297–308. https://doi.org/10.1016/S0378-1127(01)00790-3.
Gosselin L., Jobidon R., Bernier L. (1999). Biological control of stump sprouting of broadleaf species in rights-of-way with Chondrostereum purpureum: incidence of the disease on nontarget hosts. Biological Control 16(1): 60–67. https://doi.org/10.1006/bcon.1999.0736.
Hamberg L., Hantula J. (2016). The efficacy of six elite isolates of the fungus Chondrostereum purpureum against the sprouting of European aspen. Journal of Environmental Management 171: 217–224. https://doi.org/10.1016/j.jenvman.2016.02.016.
Hamberg L., Vartiamäki H., Hantula J. (2015). Breeding increases the efficacy of Chondrostereum purpureum in the sprout control of birch. PLoS ONE 10(2): e0117381. https://doi.org/10.1371/journal.pone.0117381.
Hamberg L., Lemola J., Hantula J. (2017). The potential of the decay fungus Chondrostereum purpureum in the biocontrol of broadleaved tree species. Fungal Ecology 30: 67–75. https://doi.org/10.1016/j.funeco.2017.09.001.
Huuskonen S., Hynynen J. (2006). Timing and intensity of precommercial thinning and their effects on the first commercial thinning in Scots pine stands. Silva Fennica 40(4): 645–662. https://doi.org/10.14214/sf.320.
Johansson T. (2008). Sprouting ability and biomass production of downy and silver birch stumps of different diameters. Biomass and Bioenergy 32(10): 944–951. https://doi.org/10.1016/j.biombioe.2008.01.009.
Kauppi A., Rinne P., Ferm A. (1987). Initiation, structure and sprouting of dormant basal buds in Betula pubescens. Flora 179(1): 55–83. https://doi.org/10.1016/S0367-2530(17)30217-7.
Laine T., Hamberg L., Saarinen V.-M., Saksa T. (2019). The efficacy of Chondrostereum purpureum in the sprout control of birch in mechanized pre-commercial thinnings. Submitted manuscript.
[Luke] Natural resources institute Finland (2018). Statistical services. Silvicultural and forest improvement work 2017. http://stat.luke.fi/en/node/6967. [Cited 26 April 2018].
Lygis V., Bakys R., Burokienė D., Vasiliauskaitė I. (2012). Chondrostereum purpureum-based control of stump sprouting of seven hardwood species in Lithuania. Baltic Forestry 18(1): 41–55.
MenSe (2018). MenSe Oy, Leikkuri RP6L metsäkoneisiin. https://www.mense.fi/product/361/leikkuri-rp6l-metsakoneisiin. [Cited 26 October 2018].
[PEFC] The Programme for the Endorsement of Forest Certification (2017). Criteria for PEFC Forest Certification. PEFC FI 1002:2014. 49 p. http://pefc.fi/wp-content/uploads/2016/09/PEFC_FI_1002_2014_Criteria_for_Forest_Certification_20141027.pdf. [Cited 4 April 2018].
R Core Team (2018). R: a language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria. https://www.R-project.org/.
Roy V., Dubeau D., Auger I. (2010). Biological control of intolerant hardwood competition: Silvicultural efficacy of Chondrostereum purpureum and worker productivity in conifer plantations. Forest Ecology and Management 259(8): 1571–1579. https://doi.org/10.1016/j.foreco.2010.01.033.
Setliff E.C. (2002). The wound pathogen Chondrostereum purpureum, its history and incidence on trees in North America. Australian Journal of Botany 50(5): 645 – 651. https://doi.org/10.1071/BT01058.
Uotila K. (2017). Optimization of early cleaning and precommercial thinning methods in juvenile stand management of Norway spruce stands. Disserationes Forestales 231. http://dx.doi.org/10.14214/df.231.
Usewood (2018). Usewood Forest Tec Oy, UW40. http://www.usewood.fi/index.php/en/products. [Cited 11 June 2018].
Vartiamäki H. (2009). The efficacy and potential risks of controlling sprouting in Finnish birches (Betula spp.) with fungal decomposer Chondrostereum purpureum. Dissertationes Forestales 93. https://doi.org/10.14214/df.93.
Wall R.E. (1990). The Fungus Chondrostereum purpureum as a Silvicide to Control Stump Sprouting in Hardwoods. Northern Journal of Applied Forestry 7(1): 17–19. https://doi.org/10.1093/njaf/7.1.17.
Total of 22 references.
Send to email

