Black spruce seedling growth response in controlled organic and organic-mineral substrates
Henneb M., Valeria O., Thiffault N., Fenton N. (2019). Black spruce seedling growth response in controlled organic and organic-mineral substrates. Silva Fennica vol. 53 no. 4 article id 10230. https://doi.org/10.14214/sf.10230
Highlights
- Seedling height and diameter were highest on clay and mesic substrates respectively
- Foliar nutrients were relatively high in seedlings that were established on mesic substrates
- We recommend the application of mechanical soil preparation techniques that promote the creation of organic-mesic substrates to support regeneration establishment.
Abstract
In the boreal forest of eastern Canada, a large proportion of black spruce (Picea mariana [Mill.] Britton, Sterns & Poggenb.) stands are affected by paludification. Edaphic conditions that are created by paludification processes, including an abundance of microsites with high moisture and low nutrient contents, hinder forest regeneration. Disturbance of paludified sites by mechanical soil preparation (MSP) reduces organic layer thickness, while generating a range of substrates for regeneration establishment. Yet, little information is available regarding the effects of these substrates on tree growth. Our objective was to determine the effect of organic, mineral and organo-mineral substrates that are created following MSP of a paludified site on the growth and root development of black spruce seedlings in a semi-controlled environment. We demonstrated that substrate exerted a significant effect on seedling growth and foliar concentrations of N, P and K. Increase in height and diameter were respectively greatest on clay (mineral) and mesic substrates. Substrate effects did not affect total biomass increases or final root biomass. Foliar nutrients (N, P, K) were relatively high in seedlings that were established on mesic substrates and relatively low for those established on clay substrates. To ensure successful seedling establishment, we recommend the application of MSP techniques that expose organic-mesic substrates on sites that are susceptible to paludification.
Keywords
Picea mariana;
paludification;
seedling growth;
seedling nutrition;
semi-controlled experiment;
substrate
-
Henneb,
Institut de recherche sur les forêts (IRF), Université du Québec en Abitibi-Témiscamingue, 445 boul. de l’Université, Rouyn-Noranda, QC J9X 5E4, Canada
 http://orcid.org/0000-0003-4507-1219
E-mail
mohammed.henneb@uqat.ca
http://orcid.org/0000-0003-4507-1219
E-mail
mohammed.henneb@uqat.ca

-
Valeria,
Institut de recherche sur les forêts (IRF), Université du Québec en Abitibi-Témiscamingue, 445 boul. de l’Université, Rouyn-Noranda, QC J9X 5E4, Canada
 http://orcid.org/0000-0002-9921-7474
E-mail
osvaldo.valeria@uqat.ca
http://orcid.org/0000-0002-9921-7474
E-mail
osvaldo.valeria@uqat.ca
-
Thiffault,
Institut de recherche sur les forêts (IRF), Université du Québec en Abitibi-Témiscamingue, 445 boul. de l’Université, Rouyn-Noranda, QC J9X 5E4, Canada; Natural Resources Canada, Canadian Wood Fibre Centre, 1055 rue du PEPS, P.O. Box 10380, Stn Sainte Foy, Quebec, QC G1V 4C7, Canada
 http://orcid.org/0000-0003-2017-6890
E-mail
nelson.thiffault@canada.ca
http://orcid.org/0000-0003-2017-6890
E-mail
nelson.thiffault@canada.ca
-
Fenton,
Institut de recherche sur les forêts (IRF), Université du Québec en Abitibi-Témiscamingue, 445 boul. de l’Université, Rouyn-Noranda, QC J9X 5E4, Canada
 http://orcid.org/0000-0002-3782-2361
E-mail
nicole.fenton@uqat.ca
http://orcid.org/0000-0002-3782-2361
E-mail
nicole.fenton@uqat.ca
Received 10 September 2019 Accepted 5 December 2019 Published 10 December 2019
Views 19361
Available at https://doi.org/10.14214/sf.10230 | Download PDF

1 Introduction
Picea mariana (Mill.) Britton, Sterns & Poggenb. (black spruce) is a common conifer that dominates the North American boreal forest (Farrar 1995). The intrinsic characteristics of its wood make black spruce a desirable source of fiber, especially favoured by the pulp and paper industry (Koubaa et al. 2007). Black spruce can grow under conditions of low nutrient availability (Viereck and Johnston 1990) and on a wide range of mineral and organic soils (Cauboue and Malenfant 1988; Sims et al. 1990). The species is also tolerant of low temperatures and excess moisture in the soil (Levan and Riha 1986; Bannister and Neuner 2001). Yet, its survival and growth are negatively affected by soil conditions that are encountered in paludified areas (Gower et al. 1996; Prescott et al. 2000; Lavoie et al. 2005; Bergeron et al. 2007).
Under paludification, increasing moisture saturation of the surface and underlying edaphic layers contributes to soil cooling, that in turns reduces microbial activity and limits the mineralization of nutrients and their subsequent uptake by plants (Gower et al. 1996; Prescott et al. 2000). With paludification, mortality of existing trees is incurred, the survival of natural or planted regeneration is reduced and tree growth decreases (Simard et al. 2007, 2009). On such sites, microsites that are available for regeneration have few nutrients within the surface horizons that are composed of living and dead mosses. The mineral soil and humified horizon, which are rich in available nutrients, become buried under a thick organic layer with low nutrient availability, thereby rendering nutrients inaccessible to regenerating trees.
Use of mechanical soil preparation (MSP) in paludified areas, including scarification, reduces the thickness and disrupts the structure of the organic matter layer (Henneb et al. 2015). MSP yields a range of substrates that are available for the establishment of natural or planted regeneration. Substrates consisting of organic soil types (fibric, mesic, humic or mixtures of the three) or mixtures with mineral soil (organo-mineral) promote or hinder the survival and growth of conifer regeneration (Sutherland and Foreman 1995; Schmidt et al. 1996; Sutherland and Foreman 2000; Prévost 2004). For example, decomposed or burned Pleurozium schreberi (Brid.) Mitt. (red-stem feather moss) is rich in nutrients and better for black spruce growth than purely mineral substrates (Lavoie et al. 2007a,b). However, little research has documented the characteristics of substrates that are derived from MSP on paludified sites, together with their effects on the growth of black spruce plantations (Lavoie et al. 2007a).
In this context, our objective was to determine the effects of organic, mineral and organo-mineral substrates that were exposed following MSP of a paludified site on the growth and root development of black spruce seedlings under semi-controlled environmental conditions. We conducted a six-month-long greenhouse experiment to test the following hypotheses: 1) organo-mineral mixtures promote growth and root development of black spruce seedlings relative to other types of substrates; and 2) organo-mineral mixtures offer greater nutrient availability (N, P, K, Ca, Mg) than do other types of substrates, which results in higher concentrations of foliar nutrients.
2 Materials and methods
2.1 Collection of substrates
In autumn of 2016, four substrates were collected from a paludified forest site that had been subjected to MSP. This spruce-feather moss site was located in the Clay Belt of Quebec (Canada), about 80 km north of the municipality of Villebois (49°06´N, 79°08´W). The material consisted of three organic substrates (fibric, mesic and humic) and a mineral substrate that was typical of the clayey lacustrine deposits of the proglacial lakes Barlow and Ojibway. This mineral substrate is low in carbonates (2%) and characterized by its fine grain size (Ballivy et al. 1971; Locat et al. 1984). Physico-chemical characteristics of the substrates are summarized in Table 1.
| Table 1. Physico-chemical characteristics of organic and clay substrates collected on a spruce-feather moss site located in the Clay Belt of Quebec (Canada) for use in the greenhouse, transplanting experiment. | ||||||||
| Substrates | Degree of decomposition | pH | CEC (meq 100g–1) | N (g kg–1) | P (g kg–1) | K (g kg–1) | Mg (g kg–1) | Ca (g kg–1) |
| Clay a | - | 5.7 | 25.9 | 0.9 | 0.005 | 0.08 | 0.28 | 1.57 |
| Fibric b | Low | 3.2–4 | 124 | 5–10 | 0.1 | 0.08 | 1 | 0.9 |
| Mesic b | Moderate | 4–7 | 116 | 8–11 | 3.5 | 5.7 | 4.4 | 2.4 |
| Humic b | High | 3.5–8 | 160 | 9–19 | 8 | 12.5 | 4.9 | 6 |
| a: sampled clay (60 samples) on the prepared site (Clay-Belt). b: (Henneb et al. 2019; Soil Classification Working Group 1998). | ||||||||
2.2 Experimental design and monitoring
A greenhouse experiment was undertaken in Rouyn-Noranda (Quebec), from late January 2017 to late July 2017. The six-month period was equivalent to one growing season for root and shoot growth in this region. The average ambient daytime temperature was set to 25 °C, while nighttime temperatures were maintained at 18 °C. Photoperiod was set to 15 h per day, without controlling relative humidity of the air. Greenhouse conditions were used to stimulate seedling growth, in order to rapidly detect the growth responses to substrates.
Six groups of rooting substrates (Table 2), which were representative of a paludified site that had been subjected to mechanical soil preparation (Henneb et al. 2019), were prepared from the field-harvested material. The six experimental groups were: Group 1) 100% clay substrate (control); Group 2) 100% fibric substrate; Group 3) 100% mesic substrate; Group 4) 100% humic substrate; Group 5) mixed organic substrates, where fibric, mesic or humic material each dominated the mixture; and Group 6), which was organo-clay mixtures. Mixtures from Group 5) were blended in the following volumetric proportions: 2/3 fibric + 1/3 mesic; 1/2 fibric + 1/2 mesic; 1/3 fibric + 2/3 mesic; 2/3 mesic + 1/3 humic; 1/2 mesic + 1/2 humic; and 1/3 mesic + 2/3 humic. Substrates from Group 6) were composed of the following volumetric mixtures: 1/2 clay + 1/2 fibric; 1/2 clay + 1/2 mesic; and 1/2 clay + 1/2 humic. Each of the prepared substrates was placed in a sterilized cylindrical PVC pot (diameter, 20 cm; height, 20 cm; volume, 6.28 dm3) and replicated 10 times, for a total of 130 pots that were distributed over the six groups. Pots were arranged randomly on the greenhouse bench (Fig. 1).

Fig. 1. Randomized distribution of 130 potted black spruce seedlings on the greenhouse bench. The pots contained black spruce seedlings transplanted into six substrates prepared from field-harvested material collected in a spruce-feather moss site located on the Clay Belt of Quebec and Ontario (Canada).
At the end of November 2016, we obtained 200 container-grown 2-year-old black spruce seedlings, which were produced in containers of 45-cavities of 110 cm3 each, in a governmental nursery (Pépinière forestière de Trécesson, Amos, Québec). The 2+0 seedlings were dormant at the beginning of the experiment. We stored the plants inside the greenhouse from the end of November to January 2017. Our aim was to gradually acclimatize seedlings to greenhouse temperature and lift off dormancy.
Prior to planting, we randomly selected 10 seedlings to measure their initial oven-dry biomass after drying at 65 °C for 48 h and foliar concentrations of macronutrients (N, P, K, Ca, Mg). Foliar concentrations were determined on 2 g-subsamples of dried needles. The tissues were ground (Pulverisette 0, Fritsch, Idar-Oberstein, Germany) prior to analyses. Nitrogen was quantified following high-temperature dry combustion followed by thermo-conductometric detection (TruMAC, LECO Corp., St Joseph, MI). Tissues were digested in hot H2SO4/H2O2 prior to determining P, K, Ca and Mg by plasma atomic emission spectroscopy (Thermo Jarrel-Ash-ICAP 61E, Thermo Fisher Scientific, Waltham, MA).
At the end of January 2017, we transplanted 130 seedlings into pots containing one of the growth substrate groups (one seedling per pot) and measured their initial size. The means ± standard deviations (SD) were: 23.29 cm ± 2.04 cm for height and 2.45 mm ± 0.3 mm for root collar diameter. Seedlings were watered twice a day until saturation during the experiment to avoid drying of the substrates. All seedlings received the same amount of water.
We carried out weekly measurements of seedling height (cm) and root collar diameter (mm) throughout the experiment. We also measured substrate temperature in the root zone hourly using miniature data loggers (iBWetland 22L, Alpha Mach iButton®, Bombardier, Ste-Julie, QC), which were buried at the centers of 28 pots that had been randomly selected and that were representative of the six substrate groups. These measurements verified whether greenhouse temperatures were sufficiently controlled (i.e., minimal temperature variation among substrates groups) during the experiment.
At the end of the experiment, five seedlings were randomly selected from each substrate group for foliar nutrient analysis using the aforementioned methods. Results of foliar nutrient analysis were compared to the foliar concentrations (g kg–1) that have been suggested for optimal growth of black spruce. These same diagnostic concentrations were used by Lavoie et al. (2007a,b), and were reported by Swan (1970), as critical concentrations corresponding to deficiency and sufficiency, respectively: N (12–15 g kg–1); P (1.4–1.8 g kg–1); K (3.0–4.0 g kg–1); Ca (1.0–1.5 g kg–1); Mg (0.9–1.2 g kg–1). The remaining seedlings (3 to 5 per substrate, due to mortality) were used to determine final total shoot and root biomass after oven-drying at 65 °C for 48 h.
2.3 Statistical analyses
All analyses were conducted in the R statistical environment (v. 3.5.1; R Core Team 2018). In order to verify if the temperature was adequately controlled in the greenhouse, one-way analyses of variance (ANOVA) and multiple Tukey tests (multcomp library) were used to compare the average temperatures that were recorded in each substrate during the experimental period. We then subjected growth and nutrient data to one-way ANOVAs, followed by multiple means comparisons (lsmeans library, emmeans), to evaluate: 1) the effect of substrate on the relative increases (RI) in height, root-collar diameter, and total biomass. The RI (Eq. 1) were computed as follows:

where RI is relative increases (%), F and I are, respectively, the final and initial values of height (cm), or root-collar diameter (cm), or total biomass (g); 2) the effect of substrate on final root biomass (g); and 3) the effect of substrate type on needle nutrient concentrations of N, P, K, Ca, and Mg. Log transformations were applied to the data to respect normality and homoscedasticity assumptions. Effects were reported as significant using a threshold α of 0.05.
Finally, we used a principal component analysis (PCA; ade4 library) to explore the relationships and correlations among the variables that we studied, including substrate, growth and seedling nutrition.
3 Results
3.1 Greenhouse conditions
We noted no significant differences in average substrate temperatures among the groups during the growth period (Table 2). Thus, substrates and seedling roots were maintained under the same temperatures, confirming that greenhouse conditions were controlled sufficiently during the experiment.
| Table 2. Mean temperatures (°C) of the greenhouse and substrates over the 6-month duration of an experiment looking at black spruce seedling growth and nutrition after transplantation into six substrates prepared from field-harvested material collected in a spruce-feather moss site located on the Clay Belt of Quebec and Ontario (Canada). Means followed by the same letter are not statistically different (P ≥ 0.05). | ||
| Mean temperature (°C) | ||
| Greenhouse | 22.6 (0.151) b | |
| Substrates | Fibric | 20.1 (0.151) ab |
| Mesic | 19.7 (0.151) a | |
| Humic | 19.6 (0.151) a | |
| Clay | 20.0 (0.151) a | |
| Organic-mix | 20.0 (0.062) a | |
| Organic-Clay | 20.0 (0.087) a | |
| The values in parentheses represent the standard deviation. | ||
3.2 Seedling mortality and growth
We observed seedling mortality on most substrates (cf. fibric substrate, 0%) over the course of the experiment (Table 3). Mortality was highest on the humic material (40%) and high on clay (30%) compared to the remaining substrates.
| Table 3. Black spruce seedling mortality as a function of substrate type, over the 6-month duration of an experiment looking at seedling responses after transplantation into six substrates prepared from field-harvested material collected in a spruce-feather moss site located on the Clay Belt of Quebec and Ontario (Canada). | ||
| Substrates groups | Mortality (number of seedlings) | Mortality rate (%) |
| 1) Fibric | 0 | 0 |
| 2) Mesic | 1 | 10 |
| 3) Humic | 4 | 40 |
| 4) Clay | 3 | 30 |
| 5) Organic-mix | 8 | 13.3 |
| 6) Organic-Clay mix | 1 | 3.3 |
Substrate exerted a significant effect on seedling growth (ANOVA, Table 4), in terms of increases in height and collar diameter. Increases in height were greater for seedlings that were planted on clay compared to those on humic substrates and organic mixtures (Table 5; P < 0.05). Differences in height increases were not observed (P > 0.05) among fibric, mesic, humic, and organic and organo-mineral substrates (Table 5). However, greater mean seedling diameter increases were observed on mesic compared to humic substrates (120% vs 85%, respectively; Table 5). We further noted no differences among substrates in total biomass or final root biomass (Table 4).
| Table 4. ANOVA summary of substrate effects (degrees of freedom = 5) on black spruce seedling growth, biomass, and foliar nutrient concentrations at the end of the 6-month duration of an experiment looking at seedling responses after transplantation into six substrates prepared from field-harvested material collected in a spruce-feather moss site located on the Clay Belt of Quebec and Ontario (Canada). | ||
| Response variable | F-Value | P-Value |
| Seedling height increase (%) | 2.266 | 0.014 |
| Seedling diameter increase (%) | 1.894 | 0.044 |
| Total biomass increase (%) | 0.695 | 0.630 |
| Root biomass (g) | 1.189 | 0.330 |
| Foliar total N (g kg–1) | 4.525 | < 0.001 |
| Foliar P (g kg–1) | 2.659 | 0.001 |
| Foliar K (g kg–1) | 2.227 | 0.021 |
| Foliar Mg (g kg–1) | 1.145 | 0.339 |
| Foliar Ca (g kg–1) | 1.075 | 0.401 |
| Values in bold type indicate significance at P = 0.05. | ||
| Table 5. Summary of multiple means comparisons concerning substrate effects on black spruce seedling growth and foliar nutrient concentrations at the end of the 6-month duration of an experiment looking at seedling responses after transplantation into six substrates prepared from field-harvested material collected in a spruce-feather moss site located on the Clay Belt of Quebec and Ontario (Canada). For each variable (column), means followed by the same letter do not differ at P = 0.05. | |||||||
| Substrates | Height increase (%) | Diameter increase (%) | N (g kg–1) | P (g kg–1) | K (g kg–1) | Ca (g kg–1) | Mg (g kg–1) |
| Fibric | 41.8 ab | 88.7 ab | 8.4 ab | 1.4 ab | 4.3 ab | 14.1 a | 1.9 a |
| Mesic | 40.8 ab | 119.9 b | 11.4 c | 1.8 b | 4.6 ab | 18.7 a | 2.1 a |
| Humic | 31.8 a | 85.2 a | 9.9 bc | 1.7 b | 4.6 ab | 17 a | 1.9 a |
| Clay | 50.8 b | 92.6 ab | 6.3 a | 1.2 a | 4.0 a | 15 a | 2.0 a |
| Organic-mix | 39.6 a | 108.4 ab | 9.2 b | 1.8 b | 5.1 b | 15.8 a | 2.0 a |
| Organic-Clay mix | 39.9 ab | 94.4 ab | 6.9 a | 1.7 b | 5.2 b | 16.4 a | 2.0 a |
3.3 Seedling nutrition
Substrate significantly influenced (P < 0.05) foliar concentrations of N, P and K (Table 4), but not those of Ca or Mg. Foliar N was higher in seedlings that were planted on mesic substrate compared to those planted on fibric, clay, organic and organo-clay substrates (Table 5). No significant differences in foliar N were detected between seedlings planted in mesic and humic substrates. Yet, seedling growth on all substrates resulted in foliar N concentrations below the critical threshold of 12 g kg–1 (Swan 1970). In contrast, foliar P (Table 5) exceeded the critical upper threshold of 1.4 g kg–1 (Swan 1970) in most substrates, except for clay. Seedling growth on clay resulted in the lowest concentrations of foliar P, when compared with those measured in mesic, humic, organic and organo-clay substrates. No differences in foliar P concentration were observed between clay and fibric substrates (Table 5). All seedlings showed foliar K concentrations that were below the critical deficiency level of 3 g kg–1 (Swan 1970). Seedlings planted in organic and organo-clay mixtures had higher foliar K concentrations than those measured in the clay substrate. No significant differences in foliar K concentration were observed among clay, fibric, mesic and humic substrates (Table 5). Foliar concentrations of Ca and Mg were above the critical threshold (1 g kg–1, Ca; 0.9 g kg–1, Mg) for all planted seedlings, with no difference among treatments (Table 5).
The first two axes of the PCA explained 53.8% of the total variation in the data (Fig. 2). Fig. 3 shows the contributions (%) of the variables in explaining the variance on the first two axes. Foliar concentrations of nutrients (N, P, Ca) were mainly associated with variation on Axis 1, while diameter growth, substrate type and foliar K were associated with Axis 2.
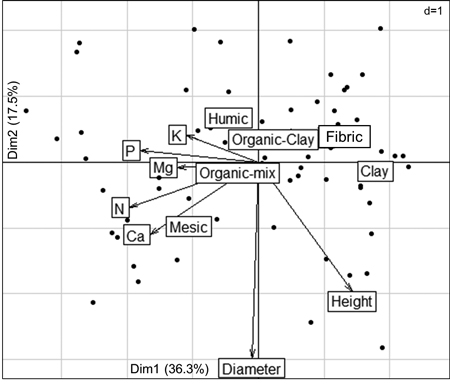
Fig. 2. Principal component analysis (PCA) summarizing associations that exist between the substrates, black spruce seedling growth and nutrition at the end of the 6-month duration of an experiment looking at seedling responses after transplantation into six substrates prepared from field-harvested material collected in a spruce-feather moss site located on the Clay Belt of Quebec and Ontario (Canada). PCA explains 53.8% of the variation.
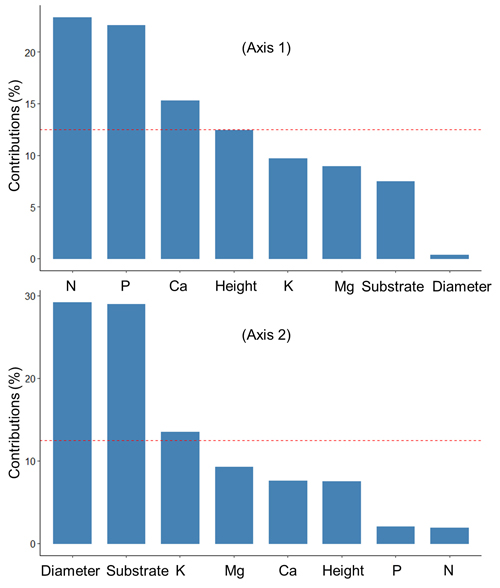
Fig. 3. Contribution (%) of the variables to the axes 1 and 2 of the principal component analysis that summarized the relationships between the substrates, growth and nutrition of black spruce seedling at the end of the 6-month duration of an experiment looking at seedling responses after transplantation into six substrates prepared from field-harvested material collected in a spruce-feather moss site located on the Clay Belt of Quebec and Ontario (Canada). The red dotted line indicates the expected average contribution for each axis. A variable whose contribution is greater than this limit can be considered important in its contribution to the variance on each axis.
The PCA analysis further showed that foliar N and diameter growth were positively correlated, especially for seedlings that were growing in mesic substrate. Further, the highest foliar concentrations of N, Ca, and Mg were more closely associated with mesic substrate than with other substrates. Height growth was more associated with clay substrates than with other substrates, and was negatively correlated with foliar concentrations of P and K, which were higher in humic substrates than in other substrates.
4 Discussion
The mortality that we observed was probably due to the lack of acclimatization of some seedlings to greenhouse temperature and the new substrates after transplantation. Lavoie et al. (2007a) made similar observations. Future work should aim at increasing the number of replicas per substrate to consider potential plant mortality.
The rooting substrates had a significant effect on height and diameter growth of the black spruce seedlings. Similar responses in the greenhouse (Lavoie et al. 2007a) and in field conditions (i.e., a prepared paludified site; Lavoie et al. 2007b; Henneb et al. 2019) have been previously observed. Seedling diameter growth was greatest on the mesic substrate, which is rich in nutrients (Lavoie et al. 2007a; Lafleur et al. 2010) (Table 1). This is particularly true at greenhouse temperatures that were conducive to black spruce growth (18–25 °C, Table 2) (Lopushinsky and Max 1990; Lahti et al. 2005). At cold temperatures, nutrients become less limiting as plants growth and nutrient requirements are low (Pregitzer et al. 2000; Alvarez-Uria and Körner 2007). However, provided that the establishment phase is completed and that planting shock associated with water stress has been alleviated (Grossnickle 2005), seedling growth is promoted by increasing soil temperature and nutrient availability (Londo and Mroz 2001; Kabrick et al. 2005; Löf and Birkedal 2009). In the boreal forest, an increase in root zone temperature stimulates microbial activity in mesic organic soils, compared to mineral soils and other underlying organic horizons (Kähkönen et al. 2001; Dioumaeva et al. 2002; Li et al. 2012). Increased microbial activity, in turn, increases the availability of nutrients (especially N), which has a positive effect on seedling growth (Van Cleve et al. 1983a,b; Li et al. 2012).
In contrast, height growth was greatest on clay substrates. However, seedlings had relatively low foliar nutrient concentrations with clay substrate compared to the mesic substrate, especially for N and P. In response to low nutrient availability, seedlings tend to reorganize their growth patterns for more efficient use of available nutrients (Madgwick 1971; Farmer 1975; Immel et al. 1978; Farmer 1980). Indeed, seedlings that were established on clay, which was nutrient-poor particularly with respect to N (Lavoie et al. 2007a,b), likely favoured height growth over root growth in response to low nutrient supplies (Boivin et al. 2002; Munson and Bernier 1993; Rikala et al. 2004; Heiskanen 2005). Also, these seedlings likely depleted foliar nutrient reserves to optimize height growth at the expense of root growth (Van den Driessche 1985; Thiffault and Jobidon 2006).
Other studies have reported that short-term seedling growth is better on clay substrates than on organic or organo-mineral substrates following mechanical soil preparation of paludified sites (Henneb et al. 2019). In paludified soils mechanically prepared by scarification or plowing, seedlings favor access to light and water over other resources (e.g., nutrients) in order to maximize growth (Haase and Rose 1993; Lamhamedi and Bernier 1994; Johnstone and Chapin 2006). Under these conditions, access to water is more reliable on clay substrates that were exposed by disturbance of the surface soil, characterized by high water-retention capacity (Bruand and Tessier 2000; Boivin et al. 2004). During our short experiment under semi-controlled conditions, all seedlings had unlimited access to water. Nutrient availability of the substrates has thus emerged as the principal factor limiting seedling nutrition and growth.
5 Conclusion
The objective of our study was to determine the effects of organic, mineral and organo-mineral substrates on the growth and root development of black spruce seedlings in a semi-controlled environment. Temperature, soil moisture and light conditions were controlled to highlight effects of substrate on growth and nutrition of black spruce of the 6-month duration experiment. Contrary to our initial hypotheses, black spruce seedlings planted on mesic substrates exhibited the best results, both in terms of diameter growth (119.9% increase) and nutrient foliar concentrations (N, P, K). Black spruce seedlings planted on Clay substrates promoted the greatest height growth (50.8% increase) with low nutrient contents. In mechanically prepared paludified soil, we have previously reported that seedling growth in clay substrates was better than those planted in organic or organo-mineral substrates because of high water retention that is maintained in the former during dry summer periods (Henneb et al. 2019). To ensure the success of seedling establishment in the short-term, we recommend the use of MSP techniques that can expose clay and organic-mesic substrates on sites with limited and unlimited access to water, respectively, to guide silviculturists in planning effective actions for regenerating paludified forest stands. Longer term monitoring will be necessary to understand nutrition and growth impacts of mechanical soil preparation on paludified soils.
Acknowledgements
We thank the personnel of the Laboratoire de chimie organique et inorganique of the Direction de la recherche forestière du Ministère des Forêts, de la Faune et des Parcs du Québec (DRF–MFFP), who performed the chemical analyses. We are equally indebted to Evelyne Gaillard (DRF–MFFP) for her assistance in managing the samples. We also thank the Cégep de l’Abitibi-Témiscamingue that facilitate the installation of greenhouse to conduct the experiment. This project was funded by a grant (RDCPJ 478742-15) from the Natural Sciences and Engineering Research of Canada (NSERC), in collaboration with the DRF–MFFP (former employer of N. Thiffault, project 142332106). Finally, we thank W.F.J. Parsons for English translation of an earlier version of the work.
References
Alvarez‐Uria P., Körner C. (2007). Low temperature limits of root growth in deciduous and evergreen temperate tree species. Functional Ecology 21(2): 211–218. https://doi.org/10.1111/j.1365-2435.2007.01231.x.
Ballivy G., Pouliot G., Loiselle A. (1971). Quelques Caractéristiques Géologiques et Minéralogiques des Dépôts d’Argile du Nord-Ouest du Québec. Canadian Journal of Earth Sciences 8(12): 1525–1541. https://doi.org/10.1139/e71-142.
Bannister P., Neuner G. (2001). Frost resistance and the distribution of conifers. In: Bigras F.J., Colombo S.J. (eds.). Conifer cold hardiness. p. 3–21. https://doi.org/10.1007/978-94-015-9650-3_1.
Bergeron Y., Drapeau P., Gauthier S., Lecomte N. (2007). Using knowledge of natural disturbances to support sustainable forest management in the northern Clay Belt. The Forestry Chronicle 83(3): 326–337. https://doi.org/10.5558/tfc83326-3.
Boivin J.R., Miller B.D., Timmer V.R. (2002). Late-season fertilization of Picea mariana seedlings under greenhouse culture: biomass and nutrient dynamics. Annals of Forest Science 59(3): 255–264. https://doi.org/10.1051/forest:2002021.
Boivin P., Garnier P., Tessier D. (2004). Relationship between clay content, clay type, and shrinkage properties of soil samples. Soil Science Society of America Journal 68(4): 1145–1153. https://doi.org/10.2136/sssaj2004.1145.
Bruand A., Tessier D. (2000). Water retention properties of the clay in soils developed on clayey sediments: significance of parent material and soil history. European Journal of Soil Science 51(4): 679–688. https://doi.org/10.1111/j.1365-2389.2000.00338.x.
Cauboue M., Malenfant D. (1988). Exigences écologiques des épinettes (Picea), des pins (Pinus) et des mélèzes (Larix) plantés au Québec (Ministère de l’Énergie et des Ressources). Les publications du Québec, Québec. 90 p.
Dioumaeva I., Trumbore S., Schuur E.A.G., Goulden M.L., Litvak M., Hirsch A.I. (2002). Decomposition of peat from upland boreal forest: temperature dependence and sources of respired carbon. Journal of Geophysical Research: Atmospheres 107(D3): WFX 3-1-WFX 3-12. https://doi.org/10.1029/2001JD000848.
Ericsson T. (1994). Nutrient dynamics and requirements of forest crops. New Zealand Journal of Forestry Science 24(2/3): 133–168.
Farmer R.E. Jr. (1975). Growth and assimilation rate of juvenile northern red oak: effects of light and temperature. Forest Science 21(4): 373–381. https://doi.org/10.1093/forestscience/21.4.373.
Farmer R.E. Jr. (1980). Comparative analysis of 1st-year growth in six deciduous tree species. Canadian Journal of Forest Research 10(1): 35–41. https://doi.org/10.1139/x80-007.
Farrar J.L. (1995). Trees in Canada. (1st ed.). Fitzhenry & Whiteside, Ottawa. 502 p. https://www.cfs.nrcan.gc.ca/publications?id=10185.
Foster N.W., Bhatti J.S. (2006). Forest ecosystems: nutrient cycling. Encyclopedia of soil science. Taylor & Francis Group, New York. p. 718–721.
Gower S.T., McMurtrie R.E., Murty D. (1996). Aboveground net primary production decline with stand age: potential causes. Trends in Ecology & Evolution 11(9): 378–382. https://doi.org/10.1016/0169-5347(96)10042-2.
Grossnickle S.C. (2005). Importance of root growth in overcoming planting stress. New Forests 30(2–3): 273–294. https://doi.org/10.1007/s11056-004-8303-2.
Haase D.L., Rose R. (1993). Soil moisture stress induces transplant shock in stored and unstored 2 + 0 Douglas-fir seedlings of varying root volumes. Forest Science 39(2): 275–294.
Heiskanen J. (2005). Effect of nitrate and ammonium on growth of transplanted Norway spruce seedlings: a greenhouse study. Annales Botanici Fennici 42(1): 1–9.
Henneb M., Valeria O., Fenton N.J., Thiffault N., Bergeron Y. (2015). Mechanical site preparation: key to microsite creation success on Clay Belt paludified sites. The Forestry Chronicle 91(02): 187–196. https://doi.org/10.5558/tfc2015-030.
Henneb M., Valeria O., Thiffault N., Fenton N.J., Bergeron Y. (2019). Effects of mechanical site preparation on microsite availability and growth of planted black spruce in Canadian paludified forests. Forests 10(8) article 670. https://doi.org/10.3390/f10080670.
Immel M.J., Rumsey R.L., Carpenter S.B. (1978). Comparative growth responses of northern red oak and chestnut oak seedlings to varying photoperiods. Forest Science 24(4): 554–560. https://doi.org/10.1093/forestscience/24.4.554.
Johnstone J.F., Chapin F.S. (2006). Effects of soil burn severity on post-fire tree recruitment in boreal forest. Ecosystems 9(1): 14–31. https://doi.org/10.1007/s10021-004-0042-x.
Kabrick J.M., Dey D.C., Sambeek J.W.V., Wallendorf M., Gold M.A. (2005). Soil properties and growth of swamp white oak and pin oak on bedded soils in the lower Missouri River floodplain. Forest Ecology and Management 204(2): 315–327. https://doi.org/10.1016/j.foreco.2004.09.014.
Kähkönen M.A., Wittmann C., Kurola J., Ilvesniemi H., Salkinoja-Salonen M.S. (2001). Microbial activity of boreal forest soil in a cold climate. Boreal environment research 6(1): 19–28.
Koubaa A., Isabel N., Zhang S.Y., Beaulieu J., Bousquet J. (2007). Transition from juvenile to mature wood in black spruce (Picea mariana (Mill.) B.S.P.). Wood and Fiber Science 37(3): 445–455. https://wfs.swst.org/index.php/wfs/article/view/367.
Lafleur B., Fenton N.J., Paré D., Simard M., Bergeron Y. (2010). Contrasting effects of season and method of harvest on soil properties and the growth of black spruce regeneration in the boreal forested peatlands of eastern Canada. Silva Fennica 44(5): 799–813. https://doi.org/10.14214/sf.122.
Lahti M., Aphalo P.J., Finér L., Ryyppö A., Lehto T., Mannerkoski H. (2005). Effects of soil temperature on shoot and root growth and nutrient uptake of 5-year-old Norway spruce seedlings. Tree Physiology 25(1): 115–122. https://doi.org/10.1093/treephys/25.1.115.
Lamhamedi M.S., Bernier P.Y. (1994). Ecophysiology and field performance of black spruce (Picea mariana): a review. Annales Des Sciences Forestières 51(6): 529–551. https://doi.org/10.1051/forest:19940601.
Lavoie M., Paré D., Fenton N., Groot A., Taylor K. (2005). Paludification and management of forested peatlands in Canada: a literature review. Environmental Reviews 13(2): 21–50. https://doi.org/10.1139/a05-006.
Lavoie M., Paré D., Bergeron Y. (2007a). Quality of growth substrates of post-disturbed lowland black spruce sites for black spruce (Picea mariana) seedling growth. New Forests 33(2): 207–216. https://doi.org/10.1007/s11056-006-9024-5.
Lavoie M., Paré D., Bergeron Y. (2007b). Relationships between microsite type and the growth and nutrition of young black spruce on post-disturbed lowland black spruce sites in eastern Canada. Canadian Journal of Forest Research 37(1): 62–73. https://doi.org/10.1139/x06-196.
Levan M.A., Riha S.J. (1986). Response of root systems of northern conifer transplants to flooding. Canadian Journal of Forest Research 16(1): 42–46. https://doi.org/10.1139/x86-008.
Li J., Ziegler S., Lane C.S., Billings S.A. (2012). Warming-enhanced preferential microbial mineralization of humified boreal forest soil organic matter: interpretation of soil profiles along a climate transect using laboratory incubations. Journal of Geophysical Research: Biogeosciences 117(G2): 1–13. https://doi.org/10.1029/2011JG001769.
Locat J., Ballivy G., Lefebvre G. (1984). Notes sur la minéralogie des sédiments fins du lac Ojibway, en particulier ceux de la région de Matagami, Québec. Géographie physique et Quaternaire 38(1): 49–57. https://doi.org/10.7202/032535ar.
Löf M., Birkedal M. (2009). Direct seeding of Quercus robur L. for reforestation: the influence of mechanical site preparation and sowing date on early growth of seedlings. Forest Ecology and Management 258(5): 704–711. https://doi.org/10.1016/j.foreco.2009.05.008.
Londo A.J., Mroz G.D. (2001). Bucket mounding as a mechanical site preparation technique in wetlands. Northern Journal of Applied Forestry 18(1): 7–13. https://doi.org/10.1093/njaf/18.1.7.
Lopushinsky W., Max T.A. (1990). Effect of soil temperature on root and shoot growth and on budburst timing in conifer seedling transplants. New Forests 4(2): 107–124. https://doi.org/10.1007/BF00119004.
Lupi C., Morin H., Deslauriers A., Rossi S., Houle D. (2013). Role of soil nitrogen for the conifers of the boreal forest: a critical review. International Journal of Plant & Soil Science 2(2): 155–189. https://doi.org/10.9734/IJPSS/2013/4233.
Madgwick H.a.I. (1971). Growth of Liriodendron tulipifera seedlings with different levels of nitrogen supply. Forest Science 17(3): 287–292.
Morris D.M., Reid D.E.B., Kwiaton M., Hunt S.L., Gordon A.M. (2014). Comparing growth patterns of jack pine and black spruce in mixed natural stands and plantations. Ecoscience 21(1): 1–10. https://doi.org/10.2980/21-1-3646.
Munson A.D., Bernier P.Y. (1993). Comparing natural and planted black spruce seedlings. II. Nutrient uptake and efficiency of use. Canadian Journal of Forest Research 23(11): 2435–2442. https://doi.org/10.1139/x93-301.
Pregitzer K.S., King J.S., Burton A.J., Brown S.E. (2000). Responses of tree fine roots to temperature. The New Phytologist 147(1): 105–115. https://doi.org/10.1046/j.1469-8137.2000.00689.x.
Prescott C.E., Maynard D.G., Laiho R. (2000). Humus in northern forests: friend or foe? Forest Ecology and Management 133(1): 23–36. https://doi.org/10.1016/S0378-1127(99)00295-9.
Prévost M. (2004). Predicting Soil properties from organic matter content following mechanical site preparation of forest soils. Soil Science Society of America Journal 68(3): 943–949. https://doi.org/10.2136/sssaj2004.9430.
R Core Team. (2018). R: a language and environment for statistical computing. R Fondation for Statistical Computing, Vienna, Austria. https://www.R-project.org/.
Rikala R., Heiskanen J., Lahti M. (2004). Autumn fertilization in the nursery affects growth of Picea abies container seedlings after transplanting. Scandinavian Journal of Forest Research 19(5): 409–414. https://doi.org/10.1080/02827580410030190.
Schmidt M.G., Macdonald S.E., Rothwell R.L. (1996). Impacts of harvesting and mechanical site preparation on soil chemical properties of mixed-wood boreal forest sites in Alberta. Canadian Journal of Soil Science 76(4): 531–540. https://doi.org/10.4141/cjss96-066.
Simard M., Lecomte N., Bergeron Y., Bernier P.Y., Paré D. (2007). Forest productivity decline caused by successional paludification of boreal soils. Ecological Applications 17(6): 1619–1637. https://doi.org/10.1890/06-1795.1.
Simard M., Bernier P.Y., Bergeron Y., Paré D., Guérine L. (2009). Paludification dynamics in the boreal forest of the James Bay Lowlands: effect of time since fire and topography. Canadian Journal of Forest Research 39(3): 546–552. https://doi.org/10.1139/X08-195.
Sims R.A., Kershaw H.M., Wickware G.M. (1990). The autecology of major tree species in the North Central region of Ontario. COFRDA Report no. 3302. 126 p. https://cfs.nrcan.gc.ca/publications?id=22213.
Soil Classification Working Group; Canadian Agricultural Services Coordinating Committee; National Research Council Canada; Canada; Agriculture and Agri-Food Canada; Research Branch (1998). The Canadian system of soil classification. NRC Research Press, Ottawa. ISBN 978-0-660-17404-4.
Sutherland B., Foreman F.F. (2000). Black spruce and vegetation response to chemical and mechanical site preparation on a boreal mixedwood site. Canadian Journal of Forest Research 30(10): 1561–1570. https://doi.org/10.1139/x00-087.
Sutherland B.J., Foreman F.F. (1995). Guide to the use of mechanical site preparation equipment in northwestern Ontario. https://cfs.nrcan.gc.ca/publications?id=9279.
Swan H.S.D. (1970). Relationship between nutrient supply, growth and nutrient concentrations in the foliage of black spruce and jack pine. Woodlands Paper, Pulp and Paper Research Institute of Canada. No. 19. 46 p. https://www.cabdirect.org/cabdirect/abstract/19700604058.
Thiffault N., Jobidon R. (2006). How to shift unproductive Kalmia angustifolia – Rhododendron groenlandicum heath to productive conifer plantation. Canadian Journal of Forest Research 36(10): 2364–2376. https://doi.org/10.1139/x06-090.
Van Cleve K., Dyrness C.T., Viereck L.A., Fox J., Chapin F.S., Oechel W. (1983a). Taiga ecosystems in interior Alaska. BioScience 33(1): 39–44. https://doi.org/10.2307/1309243.
Van Cleve K., Oliver L., Schlentner R., Viereck L.A., Dyrness C.T. (1983b). Productivity and nutrient cycling in taiga forest ecosystems. Canadian Journal of Forest Research 13(5): 747–766. https://doi.org/10.1139/x83-105.
Van den Driessche R. (1985). Late-season fertilization, mineral nutrient reserves, and retranslocation in planted Douglas-fir (Pseudotsuga menziesii (Mirb.) Franco) seedlings. Forest Science 31(2): 485–496.
Viereck L.A., Johnston W.F. (1990). Black spruce. In: Silvics of North America. United States Department of Agriculture (USDA), Forest Service. Agriculture Handbook 654(1): 227–237. https://www.srs.fs.usda.gov/pubs/misc/ag_654_vol1.pdf.
Total of 59 references.
Send to email

