From a rare inhabitant into a potential pest – status of the nun moth in Finland based on pheromone trapping
Melin M., Viiri H., Tikkanen O.-P., Elfving R., Neuvonen S. (2020). From a rare inhabitant into a potential pest – status of the nun moth in Finland based on pheromone trapping. Silva Fennica vol. 54 no. 1 article id 10262. https://doi.org/10.14214/sf.10262
Highlights
- The nun moth is a significant defoliator of coniferous forests in Central-Europe
- In Finland, the populations have grown and expanded northwards
- Pheromone trapping confirmed the species’ presence throughout central- and southern Finland
- The risk of the nun moth becoming a pest for Finland is real as the area offers endless habitats, and climatic conditions are becoming more favourable
- This note describes the results from the first nun moth surveys conducted in 2018 and 2019.
Abstract
Forests are affected by climate change in various ways. This includes abiotic factors such as droughts, but also biotic damage by pest insects. There are numerous examples from cases where pest insects have benefitted from longer growing seasons or from warmer summers. Similarly, new pest insects have been able to expand their range due to climatic conditions that have changed from hostile to tolerable. Such seems to be the case with the nun moth (Lymantria monacha), an important defoliator of coniferous trees in Europe. For centuries, the species has had massive outbreaks across Central-Europe, while it has been a rare inhabitant in Northern Europe. Recently, the nun moth population in Finland has not only expanded in range, but also grown more abundant. This research note describes the results from the first years (2018–2019) of a monitoring program that is being conducted with pheromone traps across central and southern Finland. So far, the northernmost individuals were trapped near the 64 N degrees. However, there were more southern locations where no moths were trapped. The species was present in every trapping site below the latitude of 62 N degrees. More importantly, at some sites the abundance of the nun moth suggested that local forest damage may already occur. Given the current climatic scenarios for Fennoscandia, it is likely that the nun moth populations will continue to grow, which is why systematic surveys on their abundance and range expansions will be topical.
Keywords
climate change;
forest health;
forestry;
Lymantria monacha;
forest damage;
insect;
range expansion
-
Melin,
Natural Resources Institute Finland, Yliopistokatu 6b, FI-80100 Joensuu, Finland
 https://orcid.org/0000-0001-7290-9203
E-mail
markus.melin@luke.fi
https://orcid.org/0000-0001-7290-9203
E-mail
markus.melin@luke.fi

- Viiri, Natural Resources Institute Finland, Yliopistokatu 6b, FI-80100 Joensuu, Finland; UPM-Kymmene Oyj, UPM Forest, Åkerlundinkatu 11 B, FI-33100 Tampere, Finland E-mail heli.viiri@upm.com
- Tikkanen, University of Eastern Finland, School of Forest Sciences, Yliopistokatu 6, FI-80100 Joensuu, Finland E-mail olli-pekka.tikkanen@uef.fi
- Elfving, Natural Resources Institute Finland, Yliopistokatu 6b, FI-80100 Joensuu, Finland; University of Oulu, Department of Biology, Pentti Kaiteran katu 1, FI-90014 Oulu, Finland E-mail riku.elfving@gmail.com
- Neuvonen, University of Turku, Biodiversity Unit, Kevo Subarctic Research Institute, FI-20014 Turku, Finland E-mail seppo.neuvonen@utu.fi
Received 24 October 2019 Accepted 27 January 2020 Published 27 January 2020
Views 67487
Available at https://doi.org/10.14214/sf.10262 | Download PDF

1 Introduction
Climate change has affected forest ecosystems across the globe. In addition to causing direct effect on forest health via e.g. droughts, the changes also affect forests in a severe indirect way – by benefitting their pest insects (La Porta et al. 2008; Björkman and Niemelä 2015; van Lierop et al. 2015). Not only does warming benefit native pests, it may also allow the range expansion and population growth of species never considered as potential pests. Such seems to be the case in Finland with the nun moth (Lymantria monacha L.), one of the most important defoliators of coniferous forests in Europe (Schwenke 1978; Bejer 1988; Sierota et al. 2019). Its potential range expansion was discussed already by Vanhanen et al. (2007), and recently Fält-Nardman et al. (2018a) reported that between 1961 and 2013, the northern distribution limit of the nun moth has shifted ca. 185 km northwards in Finland and ca. 310 km in Sweden. In Finland, the speed of the recent northward expansion has been approximately 20 km per year (Fäldt-Nardmann et al. 2018a). Severe damage by the nun moth has mostly occurred in Central- and Eastern Europe (Bejer 1988), but its recent northwards expansion suggests that Fennoscandian forests are becoming susceptible as well (Heino and Pouttu 2014; Fäldt-Nardmann et al. 2018a).
The caterpillars of this univoltine nocturnal moth are the ones causing the actual damage by feeding on needles, leaves and flowering parts of trees. The species is specifically a pest of coniferous species [Norway spruce (Picea abies (L.) H. Karst.), Scots pine (Pinus sylvestris L.) and larch (Larix sp.)], but it can also feed on broadleaved species especially during mass outbreaks (Bejer 1988; Lipa 1996; Nakládal and Brinkeová 2015). The timing of larvae hatching (and the feeding) takes place in late spring or early summer, but the exact timing is dependent on temperature (von Majunke et al. 2004). After hatching, the larvae climb up to the tree canopy and begin their feeding. The larvae can also spread to neighbouring trees via wind by using a silk thread, which means that they can move in search for food. It has been suggested that ca. 600–1000 larvae can completely defoliate a mature Scots pine (Schönherr 1985).
By early July, the larvae have pupated and around mid-July, they emerge as flying moths (the duration and timing of this development stage is also dependent on temperature). Breeding takes place between mid-July and mid-August, but flying during this time is halted if temperatures drop below ca. 12 °C (Jensen and Nielsen 1984; Skuhravy 1987). The females do not fly actively; they rest on tree trunks and attract males with sexual pheromones. After successful copulation, the females place their eggs in crevices of the tree bark, typically in more than one tree and most often on the lower parts of the trunk (Zubrik et al. 2013). The eggs overwinter (as almost fully developed larvae) and the cycle begins again next spring.
While any healthy forest ecosystem hosts several moth species whose larvae feed on vegetation, the nun moth is of high interest due to its ability to form mass outbreaks associated with exponential population growth. Such outbreaks have led to considerable damage in many parts of Central- and Eastern Europe. Poland has seen numerous outbreaks during the past centuries, including an outbreak that between 1978–1983 caused damage in over 2 million hectares of coniferous forests, which at the time accounted for nearly 25% of the country’s total forest area. The damage was worst in stands of Norway spruce and in stands of Scots pine growing on poor dry soils (Schönherr et al. 1985). In Czech Republic, Nakládal and Brinkeová (2015) reviewed numerous outbreaks that had occurred between 1784 and 2010 and found that defoliation during the outbreaks was more severe in larch stands that were pure or mixed with pine and spruce. Bejer (1988) reviewed nun moth outbreaks in Denmark (1971–1982), where the majority of the outbreaks and damage occurred in stands of Norway spruce. In the Danish cases, the area of forest under the threat of total defoliation ranged annually between 20 to 1000 hectares, but the rapid use of insecticides halted the outbreaks before they grew out of control.
To assess the species’ expansion and study the related dynamics, monitoring of the nun moth populations in Finland was started by researchers from Natural Resources Institute Finland and University of Eastern Finland (the authors). The trapping methods were piloted in the summer of 2018, and the first extensive survey was conducted in the summer of 2019. The aims of the 2019 survey were to detect a northern latitude after which no nun moths would be observed, and to gain information about the areas with population levels high enough to warrant further actions (Fält-Nardmann et al. 2018a). This research note describes the applied methodology, presents the gained results and discusses the future of the species, and its damage potential, in Finland.
2 Materials and methods
2.1 Study area and selection of trapping sites
Trapping was mostly conducted in government owned lands managed by Metsähallitus, but some traps were also placed in private- and urban forests. In each case, permission from the land owner was secured. Trapping was conducted in stands dominated by Norway spruce or Scots pine, or where the two species were mixed. The target forests were mostly mature where the average diameter at breast height was over 20 cm. The forests were located on both, mineral- and peatland soils. Trapping was not conducted in stands that were stressed or in otherwise poor condition. That is, the traps were placed on “normal” forests that did not differ from the structure of the wider landscape.
The year 2018 was, in essence, a pilot year during which the trapping method was tested and preliminary information about the moth’s abundance was collected to guide the setup for the larger survey in 2019. Based on the results from 2018, the northernmost trapping latitude for the 2019 survey was dropped from 65 N degrees to ca. 64 N (Fig. 1). In 2019, the area between the south coast and the 64 N degrees was covered from east to west. The aim was to set the traps so that the distance between neighbouring traps would be no more than 50 km. Due to the location of state owned lands, this criteria was not reached in all regions, whilst in others, traps were located even more densely (Fig. 1).
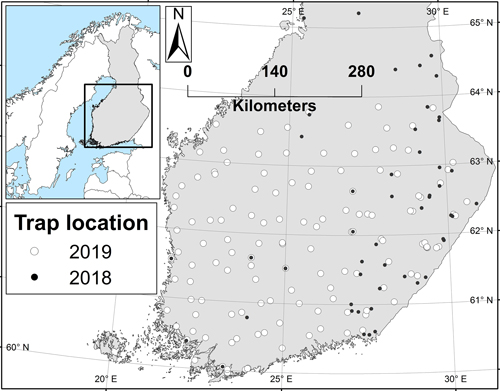
Fig. 1. The locations of the nun moth trapping sites in 2018 and 2019. Trapping in the 2019 locations is planned to be repeated in the future surveys.
2.2 Trapping method
The survey of 2019 was conducted solely with funnel traps (WitaTrap Fallenlampion, Witasek PflanzenSchutz Gmbh ) that attract male nun moths with a pheromone (Lymowit, Witasek PflanzenSchutz Gmbh) that mimics the one released by females during breeding. This pheromone is known to lure males from a distance of at least 200 m (Wang et al. 2017), and each trap was equipped with one pheromone lure. In 2018, an additional set of glue-based traps with pheromone was also used in the northernmost locations.
The funnel trap, unlike a glue-based one, maintains the same trapping power regardless of the size of the catch (Morewood et al. 2000). The funnel traps were set to hang from a branch of a tree (Fig. 2). In the absence of branches, the trap was tied firmly around the tree trunk.

Fig. 2. The type of pheromone trap used in the survey installed on a Scots pine. The nun moths are trapped in the lower container part of the trap (the zoomed image). In the image, the typical black-white colouring has been lost from the wings of the densely packed moths.
The traps were placed at a height of 1.5–3 meters from ground, which has been found to be optimal for pheromone trapping of this species (Skuhravy 1987; Wang et al. 2017). Total number of traps was 58 in 2018 (+ 13 glue-based traps) and 137 in 2019 (funnel traps only). Based on results from 2018 as well as from other studies (Hielscher and Engelmann 2012), the 2019 trapping was conducted predominantly between 10th of July and 30th of August. This is the main period when male nun moths fly actively in search of the breeding-ready females. Also, a period of this length ensured that the pheromones maintained their activity (estimated at 6–8 weeks) throughout the survey period. The traps were checked and emptied at ca. two-week intervals. The final data consisted of information on caught nun moths per trap.
3 Results
3.1 Abundance of nun moths in the study area
During the pilot trappings in 2018, a total of 939 moths were caught. In 2019, the total number of caught moths increased to 16 776. The median and mean catches per trap were 2 and 16 moths in 2018, and 40 and 125 moths in 2019. Due to the smaller sample size and spatial coverage of the 2018 survey, only the results from the 2019 survey are reported in more detail. The first checks on the first placed traps were done between 14th and 15th of July during which no moths were observed. During the second inspection done two weeks later, the majority of the traps had successfully caught male nun moths. The moths across the study area were flying at least until the 6th of September, but a clear majority of the total catch (>95%) was collected between July 22nd and August 23rd.
In 2019, the northernmost nun moth was caught near 64 N degrees – from the northernmost trap (Fig. 3). In 2018, however, six traps were placed also further north from this latitude (Fig. 1), but no nun moths were caught from any of those traps. In general, male nun moths were found in all traps below 62 N degrees, and in most of the traps below 63 N degrees as well (Fig. 3).
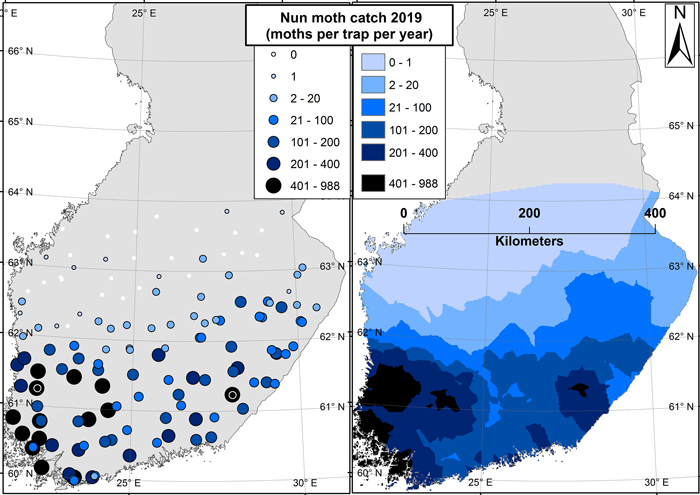
Fig. 3. Results from the 2019 nun moth survey. Image on the left shows the trap locations symbolized by catch size (moths per trap per season). The image on the right shows an interpolated surface based on the results of the left-hand side image. The two sites in east and west with the highest catches are marked with black-white spheres on the left-hand side image.
The highest catches of the 2019 trapping season came from south-east (988 individuals per trap) and south-west (899 individuals per trap) of Finland. In 2018, the maximum catch was 239 moths per trap (south-west of Finland). The catches along the west coast of Finland were generally higher than those at the eastern border along the same latitude. Still, a latitudinal gradient existed where the number of nun moths caught generally decreased towards the north (Fig. 3).
4 Discussion
In Finland, the earlier nun moth observations came from random catches of lepidopterists using light traps or visual sightings (www.laji.fi, ca. 4200 total observations), and also from the Nocturna moth monitoring program of the Finnish Environment Institute (Leinonen et al. 2016). While these data show that the nun moth populations have been growing consistently in the 21st century, they do not provide systematic information on how the abundance varies across the country. Therefore, the presented survey network and the 2019 catch of nearly 17 000 individuals brought vital new information about the species’ current range and abundance.
Importance to the issues was added by Fält-Nardmann et al. (2018c), who discovered that the Finnish populations seems to have already adapted to the shorter northern summers: larvae from northern populations pupated, and adults emerged, at lower temperature sums than their German counterparts. This, given the fact that the species has been present in Finland for less than a century (Grönblom and Suomalainen 1950), suggests that it is capable of adapting to changing climatic conditions. Fält-Nardmann et al. (2018b) also discovered that exposure to a temperature of –29 °C kills nun moth eggs. This temperature is commonly reached in most parts of continental Finland during most winters. However, as these temperatures are predicted to be less frequent especially in southern Finland (Asikainen et al. 2019; Ruosteenoja et al. 2020), the risk of nun moth becoming a local pest is high.
The situation is also made more severe by the probable secondary damage. In a massive historical nun moth outbreak in parts of modern Poland and Russia, the nun moth did not kill all of the forests it attacked, but it weakened them enough for the spruce bark beetle (Ips typographus L.) to do so (Bejer 1988). In Fennoscandia, the spruce bark beetle has equally benefitted from recent warming (Öhrn et al. 2014; Økland et al. 2015), but it is still dependent on a primary damage (storm etc.) before it is able to cause an epidemic. Therefore, even though the nun moth might not cause mass death of e.g. Norway spruce in Finland, it can make the forests more susceptible to follow-up damage by the spruce bark beetle. In the most recent and the largest spruce bark beetle epidemic of Finland (2012–2013), the highest population densities were observed in the same regions where the nun moth catches of 2019 were also the highest (Neuvonen et al. 2014).
The results shown in this paper were based on pheromone trapping data. Although the pheromone-based method attracts only males, a sufficient catch of males (~1000 moths per trap per season) has still been proven to be indicative of potential outbreaks and damage (Hielscher and Engelmann 2012). This figure was nearly reached in the trapping sites of south-west and south-east with the highest catches, indicating that local damage in these areas is likely already present (Fig. 3). The situation is different in the northern areas: adult nun moth males are known to stay alive for weeks after breeding, and during this time they can fly far outside of the actual breeding territory (Skuhravy 1987). Therefore, the presence of individual males (such as the northernmost catches in Fig. 3) is never indicative of forest damage or even of the presence of females (Jensen and Nielsen 1984).
To assess the relationships between nun moth abundance and different temperature or landscape structure variables, or to create predictions about future distribution ranges, time-series data is a pre-requisite. Here, we chose not to focus on these issues as results based on a limited dataset would have been more questionable than informative. In general, the temperatures during the summer of 2019 were close to the long-term averages apart from late July that was cooler than average (Finnish Meteorological Institute 2020), but the amount of data is yet not sufficient for more detailed analysis. The planned future field campaigns will eventually provide more data for more in-depth analyses. Presently, we wish to draw attention to the species as it is clearly present throughout southern and central Finland, and further range expansion is likely. More importantly, based on the number of caught males at certain sites, observable visual damage is likely to become more common as well. Given the species’ known preference for Norway spruce and Scots pine, it is clear that in its northernmost range the limiting factor will be thermal conditions, as the coniferous landscape of Finland will provide the nun moth with practically endless habitats.
Fält-Nardmann (2018) concludes that developing a regionally adapted monitoring programme would serve as a first preventive measure, which is what the participating researchers aim to do. Another future task is to create a link with the population density (e.g. measured as moths per pheromone traps) and actual defoliation levels in the surrounding forests – a task requiring more data, similarly to the issues regarding relationships between nun moth abundance and variables of weather and landscape structure. Such information is crucial for hazard risk mapping (i.e. proper modelling on where the most susceptible areas to nun moth damage are) and for predicting how the hazard risk develops in the future, given the current climatic scenarios for Finland (Ruosteenoja et al. 2016; Neuvonen et al. 2018; Ruosteenoja et al.2020).
Acknowledgments
We would like to thank all of the volunteers within and outside of Luke. They helped in organizing the survey by managing some of the funnel traps in 2018 and 2019. Their participation allowed us to extend the 2019 network into its current range. Work by the Luke researchers was funded by Natural Resources Institute Finland’s project 41007-00090300, Climate and pest insects. BSc Riku Elfving’s work was funded also by a state-sponsored trainee grant for university students.
References
Asikainen A., Viiri H., Neuvonen S., Nevalainen S., Lintunen J., Laturi J., Uusivuori J., Venäläinen A., Lehtonen I., Ruosteenoja K. (2019). Climate change and forest damage. The Finnish Climate Change Panel 1/2019.
Bejer B. (1988). The nun moth in European spruce forests. In: Berrymann A.A. (ed.). Dynamics of forest insect populations, Springer Science + Business Media, New York. p. 211–231. https://doi.org/10.1007/978-1-4899-0789-9_11.
Björkman C., Niemelä P. (eds.) (2015). Climate change and insect pests. CABI climate change series nr 7. CAB International, Wallingford, United Kingdom. 279 p. https://doi.org/10.1079/9781780643786.0000.
Fält-Nardmann J.J.J. (2018). Lepidopteran forest defoliators in a changing climate: performance in different life-history stages, and range expansion. PhD thesis. Annales Universitatis Turkuensis 347. ISBN 978-951-29-7389-7.
Fält-Nardmann J.J.J., Tikkanen O.-P., Ruohomäki K., Otto L.-F., Leinonen R., Pöyry J., Saikkonen K., Neuvonen S. (2018a). The recent northwards expansion of Lymantria monacha in relation to realised change in temperatures of different seasons. Forest Ecology and Management 427: 96–105. https://doi.org/10.1016/j.foreco.2018.05.053.
Fält-Nardmann J.J.J., Ruohomäki K., Tikkanen O.-P., Neuvonen S. (2018b). Cold hardiness of Lymantria monacha and L. dispar (Lepidoptera: Erebidae) eggs to extreme winter temperatures: implications for predicting climate change impacts. Ecological Entomology 43(4): 422–430. https://doi.org/10.1111/een.12515.
Fält-Nardmann J.J.J., Klemola T., Ruohomäki K., Niemelä P., Roth M. Saikkonen K. (2018c). Local adaptations and phenotypic plasticity may render gypsy moth and nun moth future pests in northern European boreal forests. Canadian Journal of Forest Research 48(3): 265–275. https://doi.org/10.1139/cjfr-2016-0481.
Finnish Meteorological Institute (2020). Monthly weather statistics. https://ilmatieteenlaitos.fi/kuukausitilastot. [Cited 21 Jan 2020].
Grönblom T., Suomalainen E. (1950). Über das Vorkommen der Nonne, Lymantria monacha L. in Finnland. [On the occurrence of the nun moth in Finland]. Annales Entomologici Fennici 16: 178–181.
Heino E., Pouttu A. (2014). Metsätuhot vuonna 2013. [Forest damage in 2013]. Metlan työraportteja 295. 28 p. http://urn.fi/URN:ISBN:978-951-40-2474-0.
Hielscher K., Engelmann A. (2012). Operational monitoring of the nun moth Lymantria monacha L. (Lepidoptera: Lymantriidae) using pheromone-baited traps – a rationalization proposal. Journal of Forest Science 58(5): 225–233. https://doi.org/10.17221/52/2011-JFS.
Jensen T.S., Nielsen B.O. (1984). Evaluation of pheromone catches of the nun moth, Lymantria monacha L. Effect of habitat heterogeneity and weather conditions in the flight period. Zeitschrift für Angewandte Entomologie 98(1–5): 399–413. https://doi.org/10.1111/j.1439-0418.1984.tb02728.x.
La Porta N., Capretti P., Thomsen I.M., Kasanen R., Hietala A.M., Weissenberg K. (2008). Forest pathogens with higher damage potential due to climate change in Europe. Canadian Journal of Plant Pathology 30(2): 177–195. https://doi.org/10.1080/07060661.2008.10540534.
Leinonen R., Pöyry J,. Söderman G., Tuominen-Roto L. (2016). Suomen yöperhosseuranta (Nocturna) 1993–2012. [Finnish moth monitoring program Nocturna]. Suomen ympäristökeskuksen raportteja 15/2016. http://hdl.handle.net/10138/161221.
Lipa J.J. (1996). Present status of noxious Lymantriidae in Europe and Poland. In: Proceedings of the International Conference Integrated Management of Forest Lymantridae. Warsaw-Sekocin, 27.–29.9.1996. p.13–31.
Morewood P., Gries G., Liška J., Kapitola P., Häussler D., Möller K., Bogenschütz H. (2000). Towards pheromone‐based monitoring of nun moth, Lymantria monacha (L.) (Lep. Lymantriidae) populations. Journal of Applied Entomology 124(2): 77–85. https://doi.org/10.1046/j.1439-0418.2000.00444.x.
Nakládal O., Brinkeová H. (2015). Review of historical outbreaks of the nun moth (Lymantria monacha) with respect to tree host species. Journal of Forest Science 61(1): 18–26. https://doi.org/10.17221/94/2014-JFS.
Neuvonen S., Tikkanen O.-P., Viiri H. (2014). Kirjanpainajatilanne Suomessa 2012–2013 feromoniseurantojen perusteella. [Status of the spruce bark beetle in Finland based on pheremonot trapping, 2012–2013]. In: Heino E., Pouttu A. (eds.). Metsätuhot vuonna 2013. Metlan työraportteja 295. p. 11–18. http://urn.fi/URN:ISBN:978-951-40-2474-0.
Neuvonen S., Kullberg J., Kämäräinen M., Lehtonen I., Nevalainen S., Siljamo P., Venäläinen A. (2018). Havununna ja lehtinunna – tulevaisuuden metsätuholaisiin on syytä varautua ennakolta. [The nun- and gypsy moth – pre-emptive measures for future pests should be considered]. In: Nevalainen S., Heino E., Pouttu A. (eds.). Metsätuhot vuonna 2017. [Forest Damage in 2017]. Luonnonvara- ja biotalouden tutkimus 44/2018. p. 30–39. http://urn.fi/URN:ISBN:978-952-326-622-3.
Öhrn P., Långström B., Lindelöw Å., Björklund N. (2014). Seasonal flight patterns of Ips typographus in southern Sweden and thermal sums required for emergence. Agricultural and Forest Entomology 16(2): 147–157. https://doi.org/10.1111/afe.12044.
Økland B., Netherer S., Marini L. (2015). The Eurasian spruce bark beetle – role of climate. In: Björkman C., Niemelä P. (eds.). Climate change and insect pests. CABI climate change series no.7. CAB International, Wallingford, United Kingdom. p. 202–219. https://doi.org/10.1079/9781780643786.0202.
Ruosteenoja K., Jylhä K., Kämäräinen M. (2016). Climate projections for Finland under the RCP forcing scenarios. Geophysica 51(1): 17–50.
Ruosteenoja K., Markkanen T., Räisänen J. (2020). Thermal seasons in northern Europe in projected future climate. International Journal of Climatology. https://doi.org/10.1002/joc.6466.
Schönherr J. (1985). Nun moth outbreak in Poland 1978–1984. Zeitschrift für angewandte Entomologie 99(1–5): 73–76. https://doi.org/10.1111/j.1439-0418.1985.tb01963.x.
Schwenke W. (1978). Die Forstschädlinge Europas: 3. Band Schmetterlinge. [Europe’s Forest Pests 3. Ribbon butterflies]. Verlag Paul Parey. 468 p.
Sierota Z., Grodzki W., Szczepkowski A. (2019). Abiotic and biotic disturbances affecting forest health in Poland over the past 30 years: Impacts of climate and forest management. Forests 10(1) article 75. 17 p. https://doi.org/10.3390/f10010075.
Skuhravý V. (1987). A review of research on the nun moth (Lymantria monacha L.) conducted with pheromone traps in Czechoslovakia, 1973–1984. Anzeiger für Schädlingskunde, Pflanzenschutz, Umweltschutz 60(5): 96–98. https://doi.org/10.1007/BF01906038.
Vanhanen H., Veteli T.O., Päivinen S., Kellomäki S., Niemelä P. (2007). Climate change and range shifts in two insect defoliators: gypsy moth and nun moth – a model study. Silva Fennica 41(4): 621–638. https://doi.org/10.14214/sf.469.
van Lierop P., Lindquist E., Sathyapala S., Franceschini G. (2015). Global forest area disturbance from fire, insect pests, diseases and severe weather events. Forest Ecology and Management 352: 78–88. https://doi.org/10.1016/j.foreco.2015.06.010.
von Majunke C., Möller K., Funke M. (2004). Die Nonne – Lymantria monacha L. Waldschutz-Merkblatt 52.
Wang P., Chen G.F., Zhang J.S., Xue Q., Zhang J.H. Chen C., Zhang Q.H. (2017). Pheromone‐trapping the nun moth, Lymantria monacha (Lepidoptera: Lymantriidae) in Inner Mongolia, China. Insect science 24(4): 631–639. https://doi.org/10.1111/1744-7917.12350.
Zubrik M., Kunca A., Csóka G. (eds.) (2013). Insects and diseases damaging trees and shrubs of Europe. NAP Editions. ISBN 978-2-913688-18-6.
Total of 32 references.

