Contribution of poplar plantations to bird conservation in riparian landscapes
Martín-García J., Barbaro L., Diez J. J., Jactel H. (2013). Contribution of poplar plantations to bird conservation in riparian landscapes. Silva Fennica vol. 47 no. 4 article id 1043. https://doi.org/10.14214/sf.1043
Highlights
- Poplar plantations should not be used as surrogate habitat for native riparian forests with the aim of preserving bird species diversity
- Native riparian forests should be preserved or restored as far as possible
- Bird communities occurring in poplar plantations can still accommodate rich communities of forest bird species, providing that suitable management is applied at local and landscape levels.
Abstract
In Mediterranean areas, riparian zones are particularly important for maintaining biodiversity. Nevertheless, the native vegetation in these zones has been modified or lost at an alarming rate during the last decades. The main objective of this study was to investigate the influence of poplar plantations on bird diversity in riparian zones, in order to estimate the ecological implications of a substantial expansion of poplar plantations. Breeding birds were sampled by the point-count method in twenty-four poplar plantations of I-214 clone, according to a factorial design combining stand age and understory management. Furthermore, the three native riparian forests remaining in the study area were also surveyed. Explanatory variables included (1) dendrometric, (2) understory and (3) landscape variables within six different radii of circular buffers. The species richness and abundance index were higher in riparian forests than in poplar plantations. Landscape variables (percentage of poplar plantations in the surrounding landscape) strongly influenced bird diversity in poplar plantations. Furthermore, at the local scale, understory cover was also a key factor in shaping bird assemblages. This suggests that poplar plantations should not be used as surrogates for native forests. Nevertheless, poplar plantations can still accommodate rich communities of forest bird species, providing that suitable management is applied at local and landscape levels.
Keywords
management;
Populus x euramericana;
clone I-214;
hybrid;
native
-
Martín-García,
Sustainable Forest Management Research Institute, University of Valladolid – INIA, Avenida Madrid, 57, 34004 Palencia, Spain
E-mail
jorgemg@pvs.uva.es

- Barbaro, NRA, UMR 1202 BIOGECO, 69 Route d’Arcachon, F-33612 Cestas cedex, France E-mail luc@pierroton.inra.fr
- Diez, Sustainable Forest Management Research Institute, University of Valladolid – INIA, Avenida Madrid, 57, 34004 Palencia, Spain E-mail jdcasero@pvs.uva.es
- Jactel, NRA, UMR 1202 BIOGECO, 69 Route d’Arcachon, F-33612 Cestas cedex, France; Université de Bordeaux, UMR 1202, Bordeaux, F-33000 France E-mail herve.jactel@pierroton.inra.fr
Received 30 May 2013 Accepted 28 October 2013 Published 12 November 2013
Views 238184
Available at https://doi.org/10.14214/sf.1043 | Download PDF

1 Introduction
Riparian zones are the interfaces between terrestrial and aquatic environments. In arid regions, such as the Mediterranean area, these ecotones are particularly important for maintaining biodiversity because of sharp moisture gradients that determine ecological processes (Schnitzler 1994) and because they provide wildlife corridors (Gregory et al. 1991; Naiman et al. 1993; Machtans et al. 1996; Naiman and Décamps 1997). Nevertheless, riparian zones have been disturbed or lost at an alarming rate during the past five decades in many European countries, including Spain (e.g., in the river Duero basin). Native vegetation was almost entirely lost from riparian zones when stream flow was regulated by storage reservoir and canalizations in the middle of the 20th century (Schnitzler 1994; González and García 2007). Such regulation was followed by a major change from native vegetation (riparian forest) to crops or planted forests such as poplar plantations.
Current national and regional forest policies aim to increase the area occupied by plantation forests, since establishing plantations on degraded land or agricultural land may have multiple benefits, such as wood and biomass production and biodiversity restoration (Hartley 2002; Carnus et al. 2006; Loyn et al. 2007; Brockerhoff et al. 2008). However, if the effect on bird diversity of increasing area of planted forests has already been tested in Mediterranean landscapes (see Diaz et al. 1998; Reino et al. 2009), it has seldom been tested in landscapes including remnants of riparian forest, poplar plantations and agricultural land.
As the overall biodiversity in forest landscapes cannot be measured and quantified directly, the use of indicators may be helpful (Noss 1999). Biodiversity indicators can be based on species richness, indicator species or the functional diversity of one or several taxonomic groups. Birds are often considered as efficient indicators as they play an essential functional role in ecosystems at (or near) the top of the food chain (Ormerod and Watkinson 2000; Gregory et al. 2005; Gil-Tena et al. 2007). Moreover, it is well known that bird diversity can respond rapidly to forest management, such as timber harvesting (Hanowski et al. 2007; Vanderwel et al. 2007; Chizinski et al. 2011) and site preparation (Lane et al. 2011). Birds are also responsive to signals that accumulate across local and landscape scales, since bird communities typically select habitat features at multiple scales (MacFaden and Capen 2002; Warren et al. 2005; Mitchell et al. 2006; Barbaro et al. 2007). Nevertheless, the spatial scale at which birds select their habitat remains a matter of debate. Although several authors have pointed out that landscape is the most relevant scale accounting for turnover in bird communities (Christian et al. 1998; Saab 1999; Bennett et al. 2004; Barbaro et al. 2007), others have reported that bird communities are mainly influenced by habitat patch features (MacFaden and Capen 2002; Loyn et al. 2007; Styring et al. 2011) or that local and landscape variables are equally influential (Herrando and Brotons 2002; Moreira et al. 2005; Coreau and Martin 2007).
The main objective of this study was to investigate the influence of poplar plantations on biodiversity in riparian zones, at both local and landscape scales, in order to estimate the ecological implications of the expansion of poplar plantations. To achieve this objective, the following two questions were addressed:
- Can poplar plantations act as surrogate habitat for bird communities of native riparian forests?
- What are the respective influences of local and landscape-scale poplar plantation features on bird diversity?
2 Material and methods
2.1 Description of the study site and sampling
The present study was carried out in the Duero river basin, in the middle reach of the Carrión river (Castilla y León, NW Spain). The altitude in the study area ranges between 800 and 900 meters and, in most stands, the terrain is almost flat. The average annual precipitation varies between 496 and 630 mm, and the average annual temperature, between 9 and 11.4 °C (Ninyerola et al. 2005).
This riparian zone was previously characterized by several vegetation strips between the river and the external zone (Lara et al. 2004). One vegetation strip, which was in direct contact with the watercourse, was reported to consist of plant species (mainly a shrub stratum) with a high water requirement and the ability to tolerate floods; a second vegetation strip, which was located in alluvial soils, was reported to consist of Salix, Alnus, Populus, Ulmus, and Fraxinus spp., which only required a temporal water table of accessible depth (Lara et al. 2004). Nevertheless, nowadays the first strip of vegetation is found to be very narrow (ca. 5–7 meters) and to consist of a mixed tree and shrub stratum (mainly species of genus Salix sp. and Alnus glutinosa, and to a lesser extent Populus spp.) because of a lack of drastic periodic floods. Moreover, wetland forest species in the second strip (mainly stands of alder, ash or poplar) have been replaced with agricultural crops or poplar plantations, and there are only occasional remnant patches of native forest.
Poplar plantations were initially located adjacent to the first strip of vegetation, where the land could not be cultivated because of the high moisture content of the soil; other adjacent alluvial meadow soils were able to be cultivated because the nutrient rich soils were irrigated. Nevertheless, poplar plantations are increasingly being established in the study area because of the high profitability of these trees (up to 2400 € ha–1 year–1; Díaz and Romero 2001). Poplar plantations are usually monoclonal and although several hybrids are used in Spain, Populus × euramericana (Dode) Guinier clone I-214 (P. deltoides Marsh. ♀ × P. nigra L. ♂) is the most common. This clone represents about 70 % of the total area covered by poplar plantations (Fernández and Hernanz 2004). Poplar plantations are managed intensively in short rotations (12–16 years), and weed control techniques (mainly surface ploughing) are used regularly during the first six years. The density of poplar plantations, which is kept constant during the whole rotation, is approximately 278–400 stems/ha (De Mier 2001; Fernández and Hernanz 2004).
2.2 Sampling design
Twenty four Populus × euramericana (clone I-214) stands were selected for the study by use of a factorial scheme with two factors: (i) stand stage, with two categories: young stands of 3–7 years old and adult stands of 8–14 years old, (ii) understory management, with two categories: harrowed or non-harrowed. Understory management was carried out using disc harrows, which broke up and smoothed out the soil surface. Harrowed stands were harrowed each year, and non-harrowed stands had not been harrowed for at least two years. Eight clonal plantations in the young stands and four plantations in the old stands were therefore selected as replicates for each combination of the previous two factors (young harrowed stands, young non-harrowed stands, adult harrowed stands and adult non-harrowed stands).
Furthermore, the three native riparian forests found in the study area were sampled to enable comparison of bird assemblages in poplar plantations and native forest. These stands consisted of an upper storey of alders (Alnus glutinosa), a scattered lower storey of elders (Sambucus nigra), common hawthorn (Crataegus monogyna), common dogwood (Cornus sanguinea) and a forb stratum. All sampled forests were located within the same landscape of ca 3500 ha.
Breeding birds were sampled by the point-count method with one visit in spring 2006 (Bibby et al. 2000). One observer recorded all bird individuals heard and seen within a stand during a 20 min period within 3 hours after sunrise. We avoided double counting the same individuals by drawing the approximate positions of birds in virtual concentric circles around the observer’s position (Prodon and Lebreton 1981). Abundance index of each stand was calculated as the sum of the heard and seen birds, using a semi-quantitative abundance index where a territorial male or pair was noted as 1 and a non-singing bird was noted as 0.5 (Bibby et al. 2000). Species richness was the number of different species represented in each stand. The sampling was not done on particularly windy and rainy days.
2.3 Habitat and landscape description
In each poplar plantation, four circular subplots of radius 15 m were established for measuring trees. The subplots were located 50 metres apart from each other, at the ends of a cross located in the middle of the stand. All trees within each subplot were marked and sampled. Diameter at breast height (dbh), crown height, crown diameter and total height were measured in an average of 84 trees per stand (ranging from 68 to 112 trees per stand).
Nine 2 × 2 m quadrats (36 m2 in total) were also established in the centre of each stand, for quantifying the understory vegetation. The species richness and percentage cover of all vascular plant species were visually estimated using the Braun-Blanquet (1964) scale during the spring.
Landscape mapping within the study area was performed, by photo-interpretation and field cross-validation, using a GIS (ArcGis 9.3, ESRI) and colour aerial orthophotographs of scale 1: 1500, dated from 2004. Eight land cover types were assigned to each landscape patch according to the following classification: young poplar plantation (canopy not closed), adult poplar plantation (closed canopy), riparian forest, pine forest, oak forest, hedgerow, agricultural land and roads. Landscape metrics were calculated using Fragstats 3.3 (raster version) and a cell size of 2.5 m (McGarigal et al. 2002). Circular buffers of six different radii (100, 200, 300, 400, 500 and 1000 m) were included around each sampled stand to capture landscape features at different spatial scales. The following metrics were calculated within each buffer to characterize the composition and configuration of the landscape: percentage cover of the eight land cover types, distance to river, edge density (in m ha–1) and Shannon index of habitat diversity. The procedures and metrics used are fully described in McGarigal et al. (2002).
2.4 Data analysis
Correspondence analysis (CA) was used to ordinate bird communities along a gradient of forest composition and structure ranging from riparian forests to poplar plantations. As there were only three riparian forests, which were mature and not ploughed, three adult non-harrowed poplar plantations were used in the analyses for comparative purposes. Analyses of variance (ANOVAs) and Tukey’s HSD post-hoc test were used to test for the effect of habitat type on species richness and abundance index in poplar plantations. Mann-Whitney tests were also used to detect whether species richness and abundance index differed in riparian forests and adult non-harrowed poplar plantations.
A two-step approach was used to evaluate the relative influence of habitat and landscape variables on bird communities in poplar plantations. First, a principal component analysis (PCA) was applied to the dendrometric, understory and landscape variables calculated for each of the six different radii of circular buffers. Six multiple regressions (with forward selection) were carried out with bird species richness or bird abundance index as dependent variables and coordinates of sampled poplar plantations on the significant axes of each PCA (using the broken-stick method) as predictor variables, to select the buffer radius at which bird variables were best explained. Subsequently, to test whether our multiple spatial approach may violate the assumption of spatial independence, for each buffer we assessed spatial autocorrelation patterns by calculating spatial correlograms (Moran’s I) of model residuals across increasing distance classes (Zuckerberg et al. 2012). At each distance class, 1.000 permutations were run to conduct a two-sided significant test of whether the value of Moran’s I differed from zero (α = 0.05). Analyses were carried out using the “ncf” package (Bjørnstad, 2013) implemented in the R software environment (R Foundation for Statistical Computing, Vienna, Austria).
Second, other multiple regressions were carried out to test the effects on bird species richness or bird abundance index of the single dendrometric, understory and landscape variables that were best correlated with the selected axes (using the broken-stick method) of PCA calculated for different buffer radii.
Canonical correspondence analysis (CCA) was applied to study the influence of the habitat and landscape variables (buffer radius selected by the two-step approach) on the bird assemblages in poplar plantations. A forward selection procedure with Monte Carlo tests was then used to determine the significance of the results, with 499 permutations.
3 Results
Comparison between riparian forests and poplar plantations (adult non-harrowed) revealed differences in bird species richness and abundance index (N = 6, Z = 1.99, p = 0.046 and N = 6, Z = 1.96, p = 0.049, respectively). In particular, the number of species and abundance index were higher in riparian forests (18.3 ± 1.5 and 39.0 ± 0.3, respectively) than in poplar plantations (11.7 ± 0.7 and 22.2 ± 2.9, respectively). Correspondence analysis (CA) of the abundance of bird species indicated a complete turnover of bird assemblages from poplar plantations to riparian forests (Fig. 1). Although riparian forests were characterized by a greater abundance of several bird species, such as Aegithalos caudatus, Cettia cetti, Garrulus glandarius, Parus major, Phylloscopus collybita and Regulus ignicapillus, poplar plantations were not characterized by any particular species (Table 1, Fig. 1).
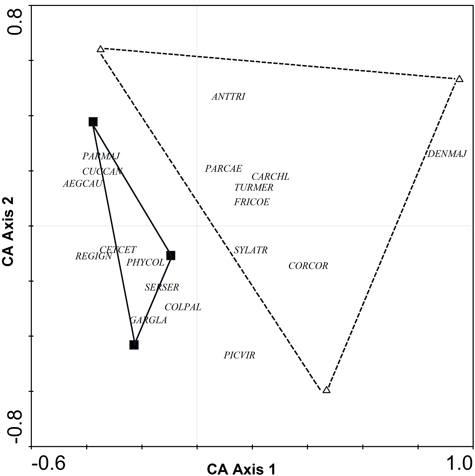
Fig. 1. CA ordination biplot for bird species and forest stands. Riparian forests are represented by black squares and poplar plantations (adult non-harrowed stands) by white triangles. Abbreviations of species names AEGCAU: Aegithalos caudatus; ANTTRI: Anthus trivialis; CARCHL: Carduelis chloris; CETCET: Cettia cetti; COLPAL: Columba palumbus; CORCOR: Corvus corone; CUCCAN: Cuculus canorus; DENMAJ: Dendrocopos major; FRICOE: Fringilla coelebs; GARGLA: Garrulus glandarius; PARCAE: Parus caeruleus; PARMAJ: Parus major; PHYCOL: Phylloscopus collybita; REGIGN: Regulus ignicapillus; SERSER: Serinus serinus; SYLATR: Sylvia atricapila; TURMER: Turdus merula.
| Table 1. List of bird species recorded in riparian forests and in the three non-harrowed poplar stands under study. Aerial, game and urban species were excluded. A six-letter abbreviation (first three letters of genus and species names) was used for species codes. | |||||
| Bird species | Species code | Riparian | Poplar | ||
| Number of stands | Abundance index | Number of stands | Abundance index | ||
| Fringilla coelebs | FRICOE | 3 | 17 | 3 | 17 |
| Phylloscopus collybita | PHYCOL | 3 | 11 | 2 | 3 |
| Cuculus canorus | CUCCAN | 3 | 8.5 | 1 | 2 |
| Columba palumbus | COLPAL | 3 | 8 | 1 | 2 |
| Aegithalos caudatus | AEGCAU | 2 | 7.5 | 0 | 0 |
| Serinus serinus | SERSER | 3 | 7 | 1 | 1 |
| Turdus merula | TURMER | 3 | 6 | 3 | 7 |
| Regulus ignicapillus | REGIGN | 2 | 5 | 0 | 0 |
| Cettia cetti | CETCET | 2 | 4 | 1 | 1 |
| Corvus corone | CORCOR | 2 | 4 | 2 | 5 |
| Parus caeruleus | PARCAE | 2 | 4 | 1 | 1 |
| Parus major | PARMAJ | 2 | 4 | 1 | 1 |
| Sylvia atricapilla | SYLATR | 3 | 4 | 2 | 2 |
| Carduelis chloris | CARCHL | 3 | 3 | 3 | 4 |
| Garrulus glandarius | GARGLA | 2 | 3 | 0 | 0 |
| Picus viridis | PICVIR | 2 | 2 | 1 | 3 |
| Troglodytes troglodytes | TROTRO | 1 | 2 | 0 | 0 |
| Anthus trivialis | ANTTRI | 1 | 1 | 1 | 0.5 |
| Carduelis carduelis | CARCAR | 1 | 1 | 1 | 1 |
| Certhia brachydactyla | CERBRA | 1 | 1 | 0 | 0 |
| Coccothraustes coccothraustes | COCCOC | 1 | 1 | 1 | 1 |
| Emberiza cirlus | EMBCIR | 1 | 1 | 1 | 1 |
| Emberiza citrinella | EMBCIT | 1 | 1 | 0 | 0 |
| Erithacus rubecula | ERIRUB | 1 | 1 | 1 | 1 |
| Hippolais polyglotta | HIPPOL | 1 | 1 | 0 | 0 |
| Jynx torquilla | JYNTOR | 1 | 1 | 1 | 1 |
| Luscinia megarhynchos | LUSMEG | 1 | 1 | 0 | 0 |
| Sylvia borin | SYLBOR | 1 | 1 | 0 | 0 |
| Sylvia melanocephala | SYLMEL | 1 | 1 | 0 | 0 |
| Turdus viscivorus | TURVIS | 1 | 1 | 0 | 0 |
| Carduelis cannabina | CARCAN | 0 | 0 | 1 | 1 |
| Dendrocopus major | DENMAJ | 0 | 0 | 2 | 2.5 |
| Parus ater | PARATE | 0 | 0 | 1 | 2 |
| Phylloscopus trochilus | PHYTRO | 0 | 0 | 1 | 1 |
| Sylvia communis | SYLCOM | 0 | 0 | 1 | 1 |
| Abundance index: sum of the heard and seen birds, using a semi-quantitative abundance index where a territorial male or pair was noted as 1 and a non-singing bird was noted as 0.5. | |||||
Examination of the dataset corresponding to the 24 poplar plantations revealed that a total of 45 species were recorded, of which 16 occurred in only one stand. The most abundant species were as follows, in decreasing order: Fringilla coelebs, Corvus corone, Turdus merula, Anthus trivialis, Phylloscopus collybita, Carduelis chloris and Columba palumbus. Analysis of variance for mean species richness per stand (N = 24) revealed a significant interaction between age and management (N = 24, F = 5.35, p = 0.03). Tukey’s HSD test revealed that species richness was lowest in the adult harrowed stands and highest in the young harrowed stands. By contrast, bird abundance index did not differ according to the type of poplar plantations (Fig. 2).
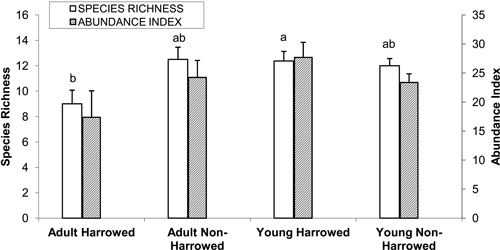
Fig. 2. Mean (± S.E.) bird species richness and abundance index. Bars with different letters indicate significantly different means (Tukey’s post hoc test, α = 0.05). Species richness: number of different species represented in each stand. Abundance index: sum of the heard and seen birds, using a semi-quantitative abundance index where a territorial male or pair was noted as 1 and a non-singing bird was noted as 0.5.
Principal component analyses (PCAs) and multiple regressions were used to test the first assumption of the two-step approach, i.e. whether the effect of landscape may vary according to the spatial scale. The broken-stick method retained the first three axes in all buffers but the 100 m-radius one where the first four axes were retained. The first three axes of PCAs clearly separated the effect of landscape, dendrometric and understory variables, respectively (Table 2). Multiple regressions only retained the axis related to landscape variables across all the buffers, and the strongest correlation between bird diversity and explanatory variables was obtained with a buffer size of 400 meters (Fig. 3). Spatial analyses demonstrated that there was hardly any autocorrelation in landscape buffer data, except for the smallest buffers, in which the overlapping was almost not significant (Fig. 4).
| Table 2. Coefficients of correlation between dendrometric, understory and landscape variables and the significant axes (D) of each principal component analysis (PCA) carried out for six different buffer radii. The highest correlations are shown in bold type. View in new window/tab. |
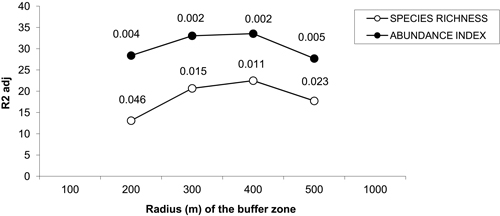
Fig. 3. Percentage of variance of bird species richness and abundance index explained by the multiple linear model in which the coordinates of sampled poplar stands along the significance axes from PCAs are included as explanatory variables. The p-values for each radius buffer are shown above each line for each buffer size. No values were represented for buffers of radius 100 and 1000 m because the respective models were not significant. Species richness: number of different species represented in each stand. Abundance index: sum of the heard and seen birds, using a semi-quantitative abundance index where a territorial male or pair was noted as 1 and a non-singing bird was noted as 0.5.
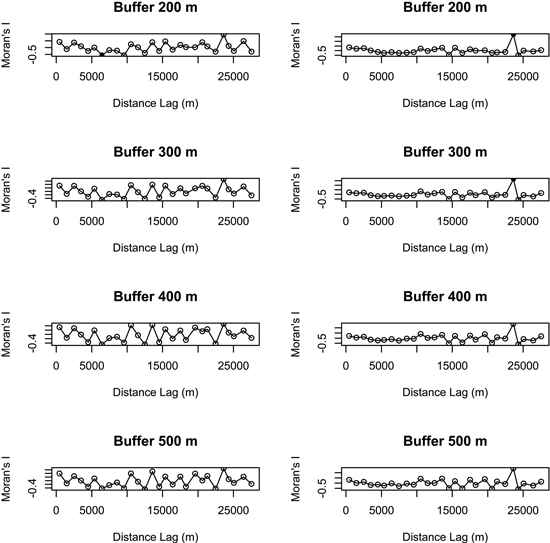
Fig. 4. Spatial correlograms (Moran’s I values) of model residuals against distance classes for bird species richness (on the left) and abundance index (on the right) in different buffers (no values were represented for buffers of 100 m and 1000 m radius because the respective models were not significant). Values significant at a nominal (two-sided) 5 % level are represented by black dots and non-significant values by open circles. Species richness: number of different species represented in each stand. Abundance index: sum of the heard and seen birds, using a semi-quantitative abundance index where a territorial male or pair was noted as 1 and a non-singing bird was noted as 0.5.
Moreover, the multiple regressions of variables calculated for a buffer size of 400 m showed that bird species richness and abundance index were only related to the percentage of poplar plantations (excluding the area of the sampled stand) in the surrounding landscape (N = 24, F = 15.45, p < 0.001, R2 = 0.413 and N = 24, F = 19.92, p < 0.001, R2 = 0.475, respectively) (Fig. 5).
Only two variables were retained in the CCA: understory cover (local level) and percentage of adult poplar plantations (excluding the area of the sampled stand) in the surrounding landscape (landscape level) (Fig. 6). The eigenvalues (λ) for axes 1 and 2 were 0.106 and 0.079, respectively, and the model was significant according to the results of the Monte Carlo test (F = 1.834, p = 0.008, 499 permutations). The CCA biplot showed a clear gradient according to forest cover, since the percentage of adult poplar plantations was positively correlated with axis 1 and, to a lesser extent, negatively correlated with axis 2. Although high percentages of adult poplar plantations were associated with Regulus ignicapillus, Aegithalos caudatus and Cettia cetti. Carduelis cannabina, Carduelis carduelis and, to a lesser extent, Corvus corone, Miliaria calandra occurred at low plantation cover. Furthermore, understory cover also determined the bird community, with a high understory cover associated with a typical species assemblage including Cettia cetti, Parus major, Carduelis carduelis and, to lesser extent, Aegithalos caudatus, Erithacus rubecula, Lullula arborea, Phylloscopus collybita and Sylvia atricapilla.
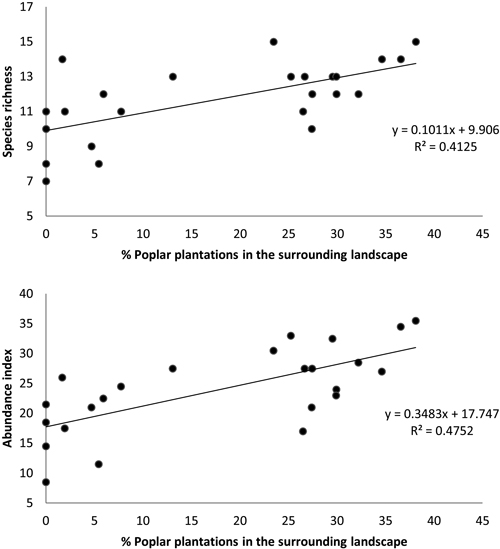
Fig. 5. Relationship between (a) bird species richness and (b) bird abundance index and the percent cover of poplar plantations (excluding area of sampled stand) within a circular buffer of 400 m radius. Species richness: number of different species represented in each stand. Abundance index: sum of the heard and seen birds, using a semi-quantitative abundance index where a territorial male or pair was noted as 1 and a non-singing bird was noted as 0.5.
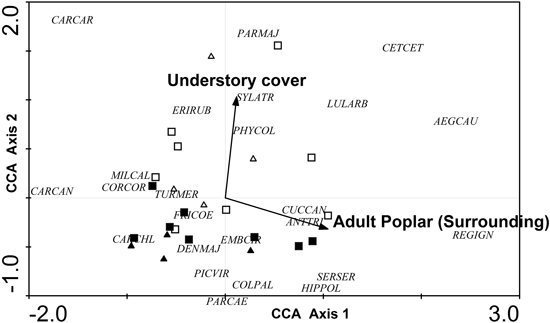
Fig. 6. CCA ordination biplot for bird species, forest stands and the significant environmental variables (dendrometric, understory and landscape variables with a 400 m radius buffer). Type of forest: adult harrowed stands are represented by black triangles, young harrowed stands by black squares, adult non-harrowed stands by white triangles and young non- harrowed stands by white squares. Abbreviations of species names AEGCAU: Aegithalos caudatus; ANTTRI: Anthus trivialis; CARCAN: Carduelis cannabina; CARCAR: Carduelis carduelis; CARCHL: Carduelis chloris; CETCET: Cettia cetti; COLPAL: Columba palumbus; CORCOR: Corvus corone; CUCCAN: Cuculus canorus; DENMAJ: Dendrocopos major; EMBCIR: Emberiza cirlus; ERIRUB: Erithacus rubecula; FRICOE: Fringilla coelebs; GARGLA: Garrulus glandarius; HIPPOL: Hippolais polyglotta; LULARB: Lullula arborea; MILCAL: Miliaria calandra; PARCAE: Parus caeruleus; PARMAJ: Parus major; PHYCOL: Phylloscopus collybita; PICVIR: Picus viridis; REGIGN: Regulus ignicapillus; SERSER: Serinus serinus; SYLATR: Sylvia atricapila; TURMER: Turdus merula.
4 Discussion
The results of this study confirm that both the type and the characteristics of forest habitats influence bird diversity in riparian landscapes. Although the number of riparian forest stands in the study area was very low (three), these stands still hosted typical bird communities of natural riparian forests, which were clearly different from the bird communities in poplar plantations. Species richness and abundance index were significantly lower in adult poplar plantations than in riparian forests, which is consistent with findings in other riparian landscapes of North America, Australia and Europe (Hanowski et al. 1997; Christian et al. 1998; Twedt et al. 1999; Palmer and Bennett 2006; Archaux and Martin 2009). Bird assemblages also differed in riparian forest and poplar plantations. The poplar plantations mainly hosted generalist species, such as Fringilla coelebs, Turdus merula and Corvus corone, whereas riparian forests were associated with bird species favouring well preserved riparian forest areas with a dense and unmanaged understory, such as Cettia cetti, Phylloscopus collybita, Aegithalos caudatus, Garrulus glandarius, Parus major and Regulus ignicapillus (Jubete 1997).
Furthermore, analysis of the data from the 24 poplar plantations revealed significant differences between poplar plantation types, with bird species richness being lowest in adult and harrowed stands and highest in the young harrowed stands, suggesting an inverse effect of stand age in harrowed stands. This contrasts with the findings of other studies conducted in tree plantations worldwide, which reported an increase in bird species richness and abundance with increasing stand age (Lance et al. 1996; Hanowski et al. 1997; Vanhinsbergh et al. 2002; Barbaro et al. 2005; Styring et al. 2011). Here, this contrasting pattern could result from an indirect effect of understorey vegetation which is known to benefit forest birds (Mills et al. 1991). While in harrowed stands the understorey cover decreased with increasing stand age (N = 12, F = 5.63, p = 0.03), no significant differences were found in not-harrowed stands (N = 12, F = 0.04, p = 0.84). Harrowing young poplar stands may have brought plant seeds to the soil surface where they were better exposed to sunlight and therefore more able to germinate and grow (Decocq et al. 2004). On the opposite, older poplar stands display higher canopy closure thus limiting the amount of light reaching understorey layer and then the development of the vegetation. Furthermore even in the oldest poplar plantations (14 years old), trees are not mature and cannot provide birds with suitable microhabitats such as nesting cavities (Villard and Taylor 1994).
Moreover, understory vegetation was also a key factor determining bird assemblages in poplar plantations in the study area. Only poplar stands with high understory cover (non-harrowed stands) were actually associated with typical bird species of natural riparian forests, such as Cettia cetti, Aegithalos caudatus, Parus major and Regulus ignicapillus (Jubete 1997). We also found that landscape variables had a strong influence on species richness, abundance index and bird assemblages in poplar plantations. This may be due to a lack of suitable habitats at local scale as a result of the small size of poplar plantations (average 6.37 ha, ranging between 0.97–19.71 ha) and a high level of fragmentation (Andrén 1994; Warren et al. 2005).
Although most studies select a single landscape scale on the basis of assumptions or previous research, a priori selection of the most influential scale in a specific landscape matrix is controversial. One of the greatest constraints on multiple scales approach is actually the violation of the assumption of spatial independence. While some researchers suggest avoiding overlapping landscape buffers (Koper and Schmiegelow 2006; Eigenbrod et al. 2011), others have demonstrated that such an overlap does not always result in violation of spatial independency, pointing out that this statement represents an oversimplification of the statistical and ecological issues about spatial autocorrelation (Zuckerberg et al. 2012). Our study also demonstrated a lack of spatial autocorrelation of data calculated in the larger buffers (400 m and 500 m) where a greater overlap occurs.
This study shows that the scale at which landscape matrix has the greatest influence on bird diversity is about 50 ha (buffer radius of 400 m). This is rather smaller than the scales recommended in other studies carried out in forest areas (e.g. 1 × 1 km cells used by Gil-Tena et al. 2007 and the 10 × 10 km cells used by Oja et al. 2005). However, this scale is consistent with findings in mixed farmland where landscape composition effect was stronger at smaller scales (200 - 400 m) than at larger scales (2 - 3 km) (Deconchat et al. 2009). This buffer range is also similar to breeding dispersal distances of several birds, such as Turdus migratorius and Toxostoma rufum (ca. 200 m within a breeding season), in agricultural landscape with wooded patches (Haas 1995), or other territorial passerines that have territories covering ca. 3–30 ha in riparian areas (Paradis et al. 1998; Pearson and Manuwal 2001). The mean distance of breeding bird dispersal varies according to landscape composition and structure, and a complex landscape matrix is more likely to supply several types of habitat for foraging, nesting and sheltering in multi-habitat species through a mechanism of habitat complementation (Dunning et al. 1992).
Furthermore, a landscape matrix with open areas may limit bird movement outside poplar plantations as a result of gap-crossing decision, a process influenced by travel costs due to predation risk or energy expenses (Desrochers and Fortin 2000; Bélisle and Desrochers 2002). Size of open areas in our study is not a physical barrier for songbirds, therefore it may reflect a behavioural response, which was already demonstrated in other matrix types such as a mixture of conifer plantations and deciduous forests (Villard and Haché 2012).
The most important variable explaining species richness, abundance index and the structure of bird assemblages was the percentage of poplar plantations in the surrounding landscape (within a 400m range). This probably involves a mechanism of landscape supplementation (Dunning et al. 1992; Tubelis et al. 2004), since the small size of poplar plantations in the study area may not support species-rich bird communities. Furthermore, other poplar plantations in the surroundings could be used by birds as corridors or stepping stones for dispersal across complex landscape matrix with habitats as diverse as riparian forest and agricultural crops, thus acting like hedgerow networks in other areas (Parish et al. 1994; Baudry et al. 2000; Hinsley and Bellamy 2000; Fuller et al. 2001). Indeed, only poplar stands with a high cover of surrounding plantations were visited by bird species typical of natural riparian forests, such as Cettia cetti, Aegithalos caudatus, Parus major and Regulus ignicapillus (Jubete 1997), whereas poplar plantations within predominantly agricultural landscapes (i.e. with a lower percentage of surrounding poplar plantations) were mainly visited by bird species associated with open areas, such as Carduelis cannabina, Carduelis carduelis and Miliaria calandra (Jubete 1997).
5 Conclusions
The study results indicated that poplar plantations should not be used as surrogate habitat for native riparian forests with the aim of preserving bird species diversity and that native riparian forests should be preserved or restored as far as possible. Nevertheless, bird communities occurring in poplar plantations can still accommodate rich communities of forest bird species, providing that suitable management is applied at local and landscape levels. The landscape matrix should include a high percentage of poplar plantations, with a distance between the plantations of less than 400 m, to provide forest bird species with well connected supplementary forest habitats. However, as the effect of large scale forest plantations in agricultural areas may also adversely affect threatened bird communities of open habitats (Diaz et al. 1998; Reino et al. 2009), we suggest that future poplar plantations might be considered within a global landscape planning perspective taking into account all relevant bird habitats such as grasslands and riparian forests.
Acknowledgements
We thank Carlos García Talegón for their technical assistance for the bird sampling. Financial support for this study was provided by European Union and Regional Government of Castile and Leon, through the INTERREG IIIB Atlantic Area programme (FORSEE project).
References
Andrén H. (1994). Effects of habitat fragmentation on birds and mammals in landscapes with different proportions of suitable habitat: a review. Oikos 71: 355–366.
Archaux F., Martin H. (2009). Hybrid poplar plantations in a floodplain have balanced impacts on farmland and woodland birds. Forest Ecology and Management 257: 1474–1479. http://dx.doi.org/10.1016/j.foreco.2008.12.021.
Barbaro L., Pontcharraud L., Vetillard F., Guyon D., Jactel H. (2005). Comparative responses of bird, carabid, and spider assemblages to stand and landscape diversity in maritime pine plantation forests. Ecoscience 12: 110–121. http://dx.doi.org/10.2980/i1195-6860-12-1-110.1.
Barbaro L., Rossi J-P., Vetillard F., Nezan J., Jactel H. (2007). The spatial distribution of birds and carabid beetles in pine plantation forests: the role of landscape composition and structure. Journal of Biogeography 34: 652–664. http://dx.doi.org/10.1111/j.1365-2699.2006.01656.x.
Baudry J., Bunce R.G.H., Burel F. (2000). Hedgerows: an international perspective on their origin, function and management. Journal of Environmental Management 60: 7–22. http://dx.doi.org/10.1006/jema.2000.0358.
Bélisle M., Desrochers A. (2002). Gap-crossing decisions by forest birds: an empirical basis for parameterizing spatially-explicit, individual-based models. Landscape Ecology 17: 219–231. http://dx.doi.org/10.1023/A:1020260326889.
Bennett A.F., Hinsley S.A., Bellamy P.E., Swetnam R.D., MacNally R. (2004). Do regional gradients in land-use influence richness, composition and turnover of bird assemblages in small woods? Biological Conservation 119: 191–206. http://dx.doi.org/10.1016/j.biocon.2003.11.003.
Bibby C.J., Burgess N.D., Hill D.A., Mustoe S.H. (2000). Bird census techniques. 2nd edition. Academic Press.
Bjørnstad O.N. (2013). ncf: spatial nonparametric covariance functions. http://cran.r-project.org/web/packages/ncf/index.html.
Braun-Blanquet J. (1964). Pflanzensoziologie. 3rd edition. Springer, New York.
Brockerhoff E., Jactel H., Parrotta J.A., Quine C.P., Sayer J. (2008). Plantation forests and biodiversity: oxymoron or opportunity? Biodiversity and Conservation 17: 925–951. http://dx.doi.org/10.1007/s10531-008-9380-x.
Carnus J-M., Parrotta J., Brockerhoff E., Arbez M., Jactel H., Kremer A., Lamb D., O’Hara K., Walters B. (2006). Planted forests and biodiversity. Journal of Forestry 104(2): 65–77.
Chizinski C.J., Peterson A., Hanowski J., Blinn C.R., Vondracek B., Niemi G. (2011). Breeding bird response to partially harvested riparian management zones. Forest Ecology and Management 261: 1892–1900. http://dx.doi.org/10.1016/j.foreco.2011.02.012.
Christian D.P., Hoffman W., Hanowski J.M., Niemi G.J., Beyea J. (1998). Bird and mammal diversity on woody biomass plantations in North America. Biomass and Bioenergy 14(4): 395–402. http://dx.doi.org/10.1016/S0961-9534(97)10076-9.
Coreau A., Martin J-L. (2007). Multi-scale study of bird species distribution and of their response to vegetation change: a Mediterranean example. Landscape Ecology 22(5): 747–764. http://dx.doi.org/10.1007/s10980-006-9074-2.
Decocq G., Valentin B., Toussaint B., Hendoux F., Saguez R., Bardat J. (2004). Soil seed bank composition and diversity in a managed temperate deciduous forest. Biodiversity and Conservation 13: 2485–2509. http://dx.doi.org/10.1023/B:BIOC.0000048454.08438.c6.
Deconchat M., Brockerhoff E.G., Barbaro L. (2009). Effects of surrounding landscape composition on the conservation value of native and exotic habitats for native forest birds. Forest Ecology and Management 258: 196–204. http://dx.doi.org/10.1016/j.foreco.2009.08.003.
De Mier A. (2001). Optimización de los sistemas de plantación y producción de chopo. Libro de actas del I Simposio del Chopo, Zamora (Spain). p. 97–105.
Desrochers A., Fortin M-J. (2000). Understanding avian responses to boundaries: a case study with Chikadee winter flocks. Oikos 91: 376–384. http://dx.doi.org/10.1034/j.1600-0706.2000.910218.x.
Diaz M., Carbonell R., Santos T., Telleria J.S. (1998). Breeding bird communities in pine plantations of the Spanish plateaux: biogeography, landscape and vegetation effects. Journal of Applied Ecology 35: 562–574. http://dx.doi.org/10.1046/j.1365-2664.1998.3540562.x.
Díaz L., Romero C. (2001). Caracterización económica de las choperas en Castilla y León: Rentabilidad y turnos óptimos. Libro de actas del I Simposio del Chopo, Zamora (Spain). p. 489–500.
Dunning J.B., Danielson B.J., Pulliam H.R. (1992). Ecological processes that affect populations in complex landscapes. Oikos 65(1): 169–175.
Eigenbrod F., Hecnar S.J., Fahrig L. (2011). Sub-optimal study design has major impacts on landscape-scale inference. Biological Conservation 144:298–305. http://dx.doi.org/10.1016/j.biocon.2010.09.007.
Fernández A., Hernanz G. (2004). El chopo (Populus sp.) Manual de gestión forestal sostenible. Junta de Castilla y León. España.
Fuller R.J., Chamberlain D.E., Burton N.H.K., Gough S.J. (2001). Distributions of birds in lowland agricultural landscapes of England and Wales: How distinctive are bird communities of hedgerows and woodland? Agriculture, Ecosystems & Environment 84: 79–92. http://dx.doi.org/10.1016/S0167-8809(00)00194-8.
Gil-Tena A., Saura S., Brotons L. (2007). Effects of forest composition and structure on bird species richness in a Mediterranean context: implications for forest ecosystem management. Forest Ecology and Management 242(2007): 470–476. http://dx.doi.org/10.1016/j.foreco.2007.01.080.
González M., García D. (2007). Restauración de ríos. Guía metodológica para la elaboración de proyectos. Ed. Secretaria General Técnica. Centro de publicaciones. Ministerio de Medio Ambiente, Madrid. ISBN 978-84-8320-413-9. 318 p.
Gregory R.D., Swanson F.J., Mckee W.A., Cummins K.W. (1991). An ecosystem perspective of riparian zones. BioScience 41(8): 540–551.
Gregory R.D., Van Strien A., Vorisek P., Gmelig Meyling A.W., Noble D.G., Foppen R.P.B., Gibbon D.W. (2005). Developing indicators for European birds. Philosophical Transactions of the Royal Society Biological Sciences 360: 269–288. http://dx.doi.org/10.1098/rstb.2004.1602.
Hanowski J.M., Niemi G.J., Christian D.C. (1997). Influence of within-plantation heterogeneity and surrounding landscape composition on avian communities in hybrid poplar plantations. Conservation Biology 11(4): 936–944. http://dx.doi.org/10.1046/j.1523-1739.1997.96173.x.
Hanowski J.M., Danz N., Lind J. (2007). Breeding bird response to riparian forest management: 9 years post-harvest. Forest Ecology and Management 241: 272–277. http://dx.doi.org/10.1016/j.foreco.2007.01.006.
Hartley M.J. (2002). Rationale and methods for conserving biodiversity in plantation forests. Forest Ecology and Management 155: 81–95. http://dx.doi.org/10.1016/S0378-1127(01)00549-7.
Haas C.A. (1995). Dispersal and use of corridors by birds in wooded patches on an agricultural landscape. Conservation Biology 9(4): 845–854. http://dx.doi.org/10.1046/j.1523-1739.1995.09040845.x.
Herrando S., Brotons L. (2002). Forest bird diversity in Mediterranean areas affected by wildfires: a multi-scale approach. Ecography 25: 161–172. http://dx.doi.org/10.1034/j.1600-0587.2002.250204.x.
Hinsley S.A., Bellamy P.E. (2000). The influence of hedge structure, management and landscape context on the value of hedgerows to birds: a review. Journal of Environmental Management 60: 33–49. http://dx.doi.org/10.1006/jema.2000.0360.
Jubete F. (1997). Atlas de las Aves nidificantes de la provincia de Palencia. Ed. Asociación de Naturalistas Palentinos. 384 p.
Koper N., Schmiegelow, F.K.A. (2006). A multi-scaled analysis of avian response to habitat amount and fragmentation in the Canadian dry mixedgrass prairie. Landscape Ecology 21: 1045–1059. http://dx.doi.org/10.1007/s10980-006-0004-0.
Lance A., Pojar R., Phinney M. (1996). Bird diversity and abundance in aspen forests in northern British Columbia. In: P.G. Comeau, G.J. Harper, M. Blache, J.O. Boateng, K.D. Thomas (eds.). Ecology and management of British Columbia hardwoods. Dec. 1–2, 1993, Richmond, B.C. B.C. Min. For. and For. Can. Victoria, B.C. FRDA Rep.
Lane V.R., Miller K.V., Castleberry S.B., Cooper R.J., Miller D.A. (2011). Bird community responses to a gradient of site preparation intensities in pine plantations in the Coastal Plain of North Carolina. Forest Ecology and Management 262: 1668–1678. http://dx.doi.org/10.1016/j.foreco.2011.07.029.
Lara F., Garilleti R., Calleja JA. (2004). La vegetación de ribera de la mitad norte española. Centro de Estudios de Técnicas Aplicadas del CEDEX. Serie Monografías 81. Madrid. 536 p.
Loyn R., Mcnabb EG., Macak P., Noble P. (2007). Eucalypt plantations as habitat for birds on previously cleared farmland in south-eastern Australia. Biodiversity and Conservation 137: 533–548. http://dx.doi.org/10.1016/j.biocon.2007.03.012.
Machtans C.S., Villard M-A., Hannon S.J. (1996). Use of riparian buffer strips as movement corridors by forest birds. Conservation Biology 10(5): 1366–1379. http://dx.doi.org/10.1046/j.1523-1739.1996.10051366.x.
McFaden S.W., Capen D.E. (2002). Avian habitat relationships at multiple scales in a New England forest. Forest Science 48: 243–253.
McGarigal K., Cushman S.A., Neel M.C., Ene E. (2002). FRAGSTATS: spatial pattern analysis program for categorical maps. University of Massachusetts, Amherst, MA.
Mills G.S., Dunning Jr. J.B., Bates J.M. (1991). The relationship between breeding bird density and vegetation volume. Wilson Bulletin 103: 468–479.
Mitchell M.S., Rutzmoser S.H., Wigley T.B., Loehle C., Gerwin JA., Keyser P.D., Lancia R.A., Perry R.W., Reynolds C.J., Thill R.E., Weih R., White D., Wood P.B. (2006). Relationships between avian richness and landscape structure at multiple scales using multiple landscapes. Forest Ecology and Management 221: 155–169. http://dx.doi.org/10.1016/j.foreco.2005.09.023.
Moreira F., Beja P., Morgado R., Reino L., Gordinho L., Delgado A., Borralho R. (2005). Effects of field management and landscape context on grassland wintering birds in Southern Portugal. Agriculture, Ecosystems & Environment 109: 59–74. http://dx.doi.org/10.1016/j.agee.2005.02.011.
Naiman R.J., Decamps H., Pollock M. (1993). The role of riparian corridors in maintaining regional biodiversity. Ecological Applications 3(2): 209–212. http://dx.doi.org/10.2307/1941822.
Naiman R.J., Decamps H. (1997). The ecology of interfaces: Riparian zones. Annual Review of Ecology and Systematics 28: 621–658.
Ninyerola M., Pons X., Roure JM. (2005). Atlas Climático Digital de la Península Ibérica. Metodología y aplicaciones en bioclimatología y geobotánica. ISBN 932860-8-7. Universidad Autónoma de Barcelona, Bellaterra.
Noss R.F. (1999). Assessing and monitoring forest biodiversity: a suggested framework and indicators. Forest Ecology and Management 115: 135–146. http://dx.doi.org/10.1016/S0378-1127(98)00394-6.
Oja T., Alamets K., Pärnamets H. (2005). Modelling bird habitat suitability based on landscape parameters at different scales. Ecological Indicators 5: 314–321. http://dx.doi.org/10.1016/j.ecolind.2005.03.008.
Ormerod S.J., Watkinson A.R. (2000). Editors’ introduction: birds and agriculture. Journal of Applied Ecology 37: 699–705. http://dx.doi.org/10.1046/j.1365-2664.2000.00576.x.
Palmer G.C., Bennett A.F. (2006). Riparian zones provide for distinct bird assemblages in forest mosaics of south-east Australia. Biodiversity and Conservation 130: 447–457. http://dx.doi.org/10.1016/j.biocon.2006.01.006.
Paradis E., Baillie S.R., Sutherland W.J., Gregory R.D. (1998). Patterns of natal and breeding dispersal in birds. Journal of Animal Ecology 67: 518–536. http://dx.doi.org/10.1046/j.1365-2656.1998.00215.x.
Parish T., Lakhani K.H., Sparks T.H. (1994). Modelling the relationship between bird population variables and hedgerow and other field margin attributes. I. Species richness of winter, summer and breeding birds. Journal of Applied Ecology 31: 764–775.
Pearson S.F., Manuwal D.A. (2001). Breeding bird response to riparian buffer width in managed pacific northwest Douglas-fir forests. Ecological Applications 11(3): 840–853. http://dx.doi.org/10.1890/1051-0761(2001)011[0840:BBRTRB]2.0.CO;2.
Prodon R., Lebreton J.D. (1981). Breeding avifauna of a mediterranean succession: the holm oak series in the Eastern Pyrénées, 1. Analysis and modeling of the structure gradient. Oikos 37: 21–38.
Reino L., Beja P., Osborne P.E., Morgado R., Fabiao A., Rotenberry J.T. (2009). Distance to edges, edge contrast and landscape fragmentation: interactions affecting farmland birds around forest plantations. Biological Conservation 142: 824–838. http://dx.doi.org/10.1016/j.biocon.2008.12.011.
Saab V. (1999). Importance of spatial scale to habitat use by breeding birds in riparian forests: a hierarchical analysis. Ecological Applications 9(1): 135–151. http://dx.doi.org/10.1890/1051-0761(1999)009[0135:IOSSTH]2.0.CO;2.
Schnitzler A. (1994). Conservation of biodiversity in alluvial hardwood forests of the temperate zone. The example of the Rhine valley. Forest Ecology and Management 68: 385–398. http://dx.doi.org/10.1016/0378-1127(94)90059-0.
Styring A.R., Ragai R., Unggang J., Stuebing R., Hosner P.A., Sheldon F.H. (2011). Bird community assembly in Bornean industrial tree plantations: effects of forest age and structure. Forest Ecology and Management 261: 531–544. http://dx.doi.org/10.1016/j.foreco.2010.11.003.
Tubelis D.P., Cowling A., Donnelly C. (2004). Landscape supplementation in adjacent savannas and its implications for the design of corridors for forest birds in the central Cerrado, Brazil. Biological Conservation 118: 353–364. http://dx.doi.org/10.1016/j.biocon.2003.09.014.
Twedt D.J., Wilson R.R., Henne-Kerr J.L., Hamilton R.B. (1999). Impact of forest type and management strategy on avian densities in the Mississippi Alluvial Valley, USA. Forest Ecology and Management 123: 261–274. http://dx.doi.org/10.1016/S0378-1127(99)00043-2.
Vanderwel M.C., Malcolm J.R., Mills S.C. (2007). A meta-analysis of bird responses to uniform partial harvesting across North America. Conservation Biology 21(5): 1230–1240. http://dx.doi.org/10.1111/j.1523-1739.2007.00756.x.
Vanhinsbergh D., Gough S., Fuller R.J., Brierley E.D.R. (2002). Summer and winter bird communities in recently established farm woodlands in lowland England. Agriculture, Ecosystems & Environment 92: 123–136. http://dx.doi.org/10.1016/S0167-8809(01)00301-2.
Villard M-A., Haché S. (2012). Conifer plantations consistently act as barriers to movement in a deciduous forest songbird: a translocation experiment. Biological Conservation 155:33–37. http://dx.doi.org/10.1016/j.biocon.2012.06.007.
Villard M-A., Taylor P.D. (1994). Tolerance to habitat fragmentation influences the colonization of new habitat by forest birds. Oecologia 98: 393–401. http://dx.doi.org/10.1007/BF00324229.
Warren T.L., Bett M.G., Diamond A.W., Forbes G.J. (2005). The influence of local habitat and landscape composition on cavity-nesting birds in a forested mosaic. Forest Ecology and Management 214: 331–343. http://dx.doi.org/10.1016/j.foreco.2005.04.017.
Zuckerberg B., Desrochers A., Hochachka W.M., Fink D., Koening W.D., Dickinson J.L. (2012). Overlapping landscapes: a persistent, but misdirected concern collecting and analyzing ecological data. The Journal of Wildlife Management 76(5): 1072–1080. http://dx.doi.org/10.1002/jwmg.326.
Total of 70 references

