Seed soak-sorting prior to sowing affects the size and quality of 1.5-year-old containerized Picea abies seedlings
Himanen K., Nygren M. (2015). Seed soak-sorting prior to sowing affects the size and quality of 1.5-year-old containerized Picea abies seedlings. Silva Fennica vol. 49 no. 3 article id 1056. https://doi.org/10.14214/sf.1056
Highlights
- After soak-sorting all sunken seeds (bottom fraction) were full and viable, whereas floating seeds contained larvae-filled and immature seed
- Seedlings originating from the bottom fraction were greater in height and diameter than control seedlings or those originating from the floating seeds
- The proportion of saleable seedlings was four percentage points higher in the bottom fraction than in the other seedlings.
Abstract
We studied the effect of soak-sorting Norway spruce (Picea abies (L.) H. Karst.) seeds on emergence, development and quality of container seedlings in two commercial seed lots. The seeds, separated by soaking into bottom and surface fractions, were sown in June, and the seedlings were grown during two growing seasons under typical Finnish nursery conditions. The first summer seedlings were grown in a greenhouse and outdoors for the second, full growing season. All sunken seeds were full and viable according to radiography, whereas the floating seeds contained 2% and 13% larvae-filled and 8% and 11% anatomically immature seeds, depending on the seed lot. Seedlings grown from the bottom fraction seed emerged 2.5–3.5 days earlier than seedlings of storage dry (i.e. control) seed. Height, diameter, and shoot and root dry mass of the seedlings were affected by soaking after both the first and second growing seasons. The largest seedlings originated from the bottom fraction. The proportion of saleable seedlings was four percentage points higher in the bottom fraction than in the other seedlings. The effects of soaking found in this study are more notable than as previously reported for Norway spruce seedlings. This suggests that soaking and soak-sorting may be most useful when the growing conditions are stressful, i.e. when seeds are sown in summer rather than 1-year-old seedling crops sown in spring under the climate conditions typical of Finland.
Keywords
Norway spruce;
seed treatment;
seed radiography;
seedling emergence;
seedling morphology
-
Himanen,
Natural Resources Institute Finland (Luke), Green technology, Juntintie 154, FI-77600 Suonenjoki, Finland
E-mail
katri.himanen@luke.fi

- Nygren, Natural Resources Institute Finland (Luke), Management and Production of Renewable Resources, Juntintie 154, FI-77600 Suonenjoki, Finland E-mail markku.nygren@luke.fi
Received 26 November 2013 Accepted 9 March 2015 Published 10 June 2015
Views 95912
Available at https://doi.org/10.14214/sf.1056 | Download PDF

1 Introduction
The morphological features of tree seedlings affect their field performance after out-planting (Long and Carrier 1993; Ward et al. 2000; Aphalo and Rikala 2003; South et al. 2005; Rikala 2012). Therefore, seedlings that are too short and thin, as well as those with underdeveloped root systems or other developmental problems, are culled from seedling crops (Mexal and Fisher 1987; Puttonen 1996). Nursery growing measures aim at producing evenly sized seedling crops, and seed-soaking treatments are used to promote germination as a way to ensure a good start for the seedlings. The simplest soaking method used in conifers is to soak seeds as a first step in stratification aimed at breaking seed dormancy (Kolotelo et al. 2001) or to sow the seeds straight after the soak (Himanen and Nygren 2014). Since the seeds’ water content rises during soaking, germination can start immediately in the seed bed. Soaking can also be used to sort out poor-quality seeds by skimming the floating, presumably low-vigor seeds after soaking. This can be done after seed extraction and prior seed storage (Karrfalt 2008) or at a nursery before sowing (Himanen and Nygren 2014).
Soaking seeds in still or aerated water speeds up germination and seedling emergence of Norway spruce (Picea abies (L.) H. Karst.) by a few days (Himanen et al. 2010; 2013; Himanen and Nygren 2014). The longer growth period caused by the earlier emergence is believed to lead to an increased seedling size. Sorting out poor-quality, e.g. immature seed can also increase seedling size and decrease mortality. The positive effects of soak-sorting on the seedling size of 1-year-old Norway spruce seedlings have however been marginal (Himanen and Nygren 2014). This contrasts with of studies of other conifer species in which earlier seedling emergence is linked to increased seedling size and survival (Boyer et al. 1985, 1987; Mexal and Fisher 1987).
In Finland, around 110 million Norway spruce seedlings are used for forest regeneration annually (Finnish Forest Research Institute 2013). Of these, over 98% are containerized seedlings grown in Sphagnum peat. The two main seedling types are 1-year-old seedlings grown for an entire growing season and slightly larger seedlings grown during two growing seasons, called 1.5- or 2-year-old seedlings. Both types are out-planted mainly during spring and autumn. The larger seedlings are used at more fertile sites, where competition by surface vegetation is harsh.
The 1-year-old seedling crop is sown in the nursery in early spring (April), while the 1.5-year-old seedling crop is sown in late June, making the first summer only half a growing season. Here, we refer to both summers as growing seasons, although during the first year the seedlings experience only a partial thermal growing season. The 1.5-year-old seedlings are typically grown in larger containers to ensure balanced root system for sustaining the larger shoots, in contrast to the 1-year-old seedlings. For both seedling types, the containers are kept in plastic houses or greenhouses during the time of seedling emergence and early development to regulate temperature fluctuations. Temperatures are still typically higher at the seedling level during midsummer than in April and therefore early growing conditions are usually more stressful for the 1.5-year-old seedlings compared to the 1-year-old crop. Later in the season the seedlings are moved outdoors to ensure the development of cold hardiness.
Previously we studied the effects of soaking Norway spruce seeds on germination under laboratory conditions (Himanen et al. 2010) and the effect of soaking on the microbial community in seeds and on disease risk in young germinants (Himanen et al. 2013). The effect of soak-sorting on emergence uniformity, seedling size, and eventual crop size uniformity was studied in 1-year-old seedlings (Himanen and Nygren 2014). However, it is unclear how the soak-sorting affects seedling emergence and development of 1.5-year-old seedlings. Due to the different conditions during early development of these seedlings compared with the 1-year-old seedlings, in addition to the longer cultivation period, we hypothesize that soak-sorting may give different results for 1.5-year-old seedlings, possibly resembling those observed in other tree species (Boyer et al. 1985; 1987; Mexal and Fisher 1987). Despite the regulation of temperature (heating and ventilation) in the plastic houses, weather conditions play a leading role during the growth of the seedlings. To evaluate the functionality and usefulness of soak-sorting, the method needs to be evaluated over several years and under varying conditions.
In the present study, we therefore investigated under typical nursery conditions used for growing 1.5-year-old containerized Norway spruce seedlings, how seed soak-sorting influences the mean time of seedling emergence, seedling morphology after the first and second growing seasons, and the proportion of saleable seedlings in the final crop. The consistency of the sorting effect was also examined, and therefore the same seed lots used in our previous study (Himanen and Nygren 2014) were chosen for this experiment. We hypothesize that soak-sorting hastens seedling emergence and that seedlings grown from bottom fraction seed have the greatest height and stem diameter. We also predict that the number of saleable seedlings will be largest in those originating from the bottom fraction.
2 Materials and methods
2.1 Seed material
Two commercial Norway spruce stand seed lots, i.e. seed collected after final felling from a stand, were used in the study (Table 1). Germinability prior to the study was determined under laboratory conditions using 400 seeds per lot (16 replications of 25 seeds). The seeds were germinated for 21 days in a Flohr GC 10/11 germination cabinet (Flohr Instruments, Nieuwegein, The Netherlands;
20 °C, 16h day, relative humidity (HD) 98%, 1000/1500 lux) on Petri dishes (Ø = 85 mm) on two layers of blotter paper (Munktell no. 1701; Munktell Filted AB, Falun, Sweden) moistened with 5 ml of tap water. A seed was considered as germinated when the radicle was at least four times the length of the seed coat (International Seed Testing Association 2005).
| Table 1. Norway spruce seed lots (stand seed) used in the study, their origin and germination percentages under laboratory conditions. | ||||||
| Seedlot | Year of maturation | Municipality | Co-ordinates | 1000 seed weight (g) | Germination, % (±SE) | |
| Day 7 | Day 14 | |||||
| A T03-06-0414 | 2006 | Suonenjoki | 62°27´N - 62°51´N, 26°50´E - 27°32´E | 6.0 | 22 (± 2.4) | 97 (± 0.6) |
| B T03-06-0421 | 2006 | Maaninka and Siilinjärvi | 62°57´N - 63°20´N, 26°57´E - 27°57´E | 5.8 | 20 (± 2.1) | 99 (± 0.5) |
2.2 Experimental design and seedling measurements
For both seed lots (A and B) 100 g of seed were soaked and 8 g samples were used as un-soaked controls. All samples were taken randomly from 5 kg seed lots, using a sleeve type- sampling stick (Kruse 2004). The seeds were soaked for 15h in tap water aerated with Sera 301 aquarium pumps (Sera, PA, USA) in plastic tubs in Snijders ECD01E incubator cabinets (Snijders Scientific B.V., Tillburg, The Netherlands) at 15 °C under fluorescent light (irradiance 17 Wm–2, red 4.9
µmolm–2s–1, far-red 1.0 µmolm–2s–1). After soaking, the floating seeds (surface fraction) were skimmed off the water surface and the sunken seeds (bottom fraction) were poured into a sieve. The fractions were from then on kept separate. The seeds were surface-dried on stainless metal sieves in a Termaks TS8430 heating cabinet (Termaks AS, Bergen, Norway) at + 24 °C for 4 h and occasionally stirred with a plastic spoon during the drying. After the surface drying, 3 × 0.5 g seed samples were drawn randomly from un-soaked control seeds and from the bottom fraction for water content analysis. The water content was measured on a fresh weight basis, using the constant temperature oven method (103 °C, 17 h) (International Seed Testing Association 2007). No samples were drawn from the surface fractions, due to the small number of seeds contained within them.
Following the water content analysis, six samples of 64 seeds from both soak fractions and the dry control for both seed lots were counted with an Elmor Unit Model 600 electronic seed counter (Elmor Ltd, Schwyz, Switzerland). These samples were x-rayed, using Faxitron MX-20 (Faxitron Bioptics LLC, AZ, USA) and CEA orthochromatic mammography film (Agfa Healthcare NV, Mortsel, Belgium) exposure 18 kV, 4 s, to detect empty, insect infested (larvae-filled) or otherwise damaged seeds. The insect species were identified according to Simak (1955) and Wiersma (1973). Seeds in which the megagametophyte did not fill the seed coat entirely or in which the embryo did not completely fill the embryo cavity were considered viable, but anatomically immature.
To mimic the typical growing procedure, the seeds were then sown in Plantek PL64F plastic nursery containers (64 cells per tray, cell volume 115 cm3, growing density 432 cells per m2; BCC Oy, Säkylä, Finland) filled with base-fertilized and limed (pH 4.5), low-humified (H 1–3) Sphagnum peat (Novagro M1L; Biolan Oy, Eura, Finland). Each sample of 64 seeds was sown in a single container, one seed in each cell. Each seed was placed in a shallow depression made in the peat surface and then covered with a thin layer of sand. A total of 2304 seeds ( = 2 seed lots × 3 treatments × 64 seeds per tray × 6 blocks) were sown.
The containers were placed in a nursery greenhouse at the Finnish Forest Research Institute in Suonenjoki, Finland (62°39´N, 27°03´E, altitude 142 m above sea level) using a randomized block design on June 14, 2011, with one container from each seed lot (A and B) × treatment (bottom, surface, and control) combination in each of the six blocks. The blocks were surrounded by containers with commercial seedlings so that the experimental seedlings were not affected by conditions such as draft, typical of edges at the end of the seedling bed, etc. The seedlings were grown until late September of the following year (2012), following normal Finnish nursery practice of growing 1.5-year-old Norway spruce seedlings among a commercial seedling crop.
During the first growing season, temperature and relative humidity at seedling level were monitored on an hourly basis (DS1923-F5 Hygrochron, iButton; Maxim Integrated, CA, USA). Emergence and the condition (death, development of disease symptoms) of each germinant were examined and recorded on days 14, 21, 28 and 49 after sowing. At the end of the growing season, the height (from the base of the seedling to the uppermost needle tip) and stem diameter (1 cm from the surface of the sand) of all living seedlings was measured. Seedlings that had died after emergence were also recorded. In addition to these measurements, 20 live seedlings from each container were drawn randomly to determine the dry mass of the shoots and roots and to calculate the shoot/root -ratio. The shoot was cut from the surface of the sand and the root plug was rinsed under running water until all the peat was washed off. The shoots and roots were placed in paper envelopes and dried in a Binder FED 720 heating cabinet (Binder GmbH, Tuttlingen, Germany) at 60 °C for 72 h, after which they were placed in a desiccator for a minimum of 30 min prior to weighing (Sartorius LC 1200S; Sartorius Corp., NY, USA).
After these first measurements (early October 2011), the containers were moved to an outdoor area, fenced to protect against mammal herbivory, to overwinter under natural snow cover. In May 2012, the seedling containers were moved to a field outdoors in the nursery to grow, as the commercial seedling crop. The seedlings used for determining shoot and root dry masses the previous autumn were replaced by supplemental seedlings grown from seeds of seed lot A to keep the growing density equal in both growing seasons. These supplemental seedlings were marked and not included in the final measurements.
At the end of the second growing season, the height and stem diameter of all the original seedlings were measured. In addition, a set of 20 seedlings from each container was drawn randomly from the original seedlings to determine the final root and shoot dry mass. Again, the dead seedlings were recorded. In case the seedling drawn for sampling of mass had died, the next available original seedling was chosen. In some containers this led to a sample size smaller than 20 seedlings, but a minimum of 15 seedlings was sampled from all containers. The final quality of all original seedlings (saleable or culled) was also assessed, using standard nursery production criteria (Rikala 2012). A seedling was considered for culling if it was very short (<15 cm), if the shoot had branched into several tops of equal height, if there was some other type of growth disturbance in the seedling (apical dominance missing, stem very crooked, etc.), if the shoot or root system was damaged so that recovery of the plant was unlikely, or if the root plug was not intact.
2.3 Calculations and statistical analyses
Relative seedling height growth was calculated as the relationship between the final height of each individual seedling and the height of the same seedling after the first growing season. Relative diameter growth was calculated similarly.
Seedling height, stem diameter, shoot and root dry mass after both the first and second growing seasons, as well as relative height and diameter growth were analyzed using linear mixed models. Seed lot (A and B), treatment (control, bottom fraction, and surface fraction), and their interaction were used as fixed model terms, and seedling nested inside the block was used as a random term. The experimental unit was an individual, emerged seedling. The goodness-of-fit of the various models was evaluated based on the deviance and normality of standardized residuals. The individual model terms were assessed by comparing the log-likelihood statistics of two nested, candidate models, as well as the Akaike information criterion and Wald-significance tests for parameter coefficients. The fixed model terms were considered statistically significant at p ≤ 0.05. The time of emergence was analyzed using non-parametric ANOVA (Friedman’s test), since the assumption of normality of the residuals was not met. This analysis was done separately for the two seed lots.
The effect of the soaking treatments and seed lot on the percentage of emerged seedlings and the proportion of saleable seedlings at the end of the second growing season was analyzed using generalized linear mixed models. The emergence data were grouped binary, where experimental unit was a batch of seeds and the counts of emerged seedlings were based on either the total number or the number of viable seeds sown in a container. The data on the proportion of saleable seedlings were binary, and the experimental unit was an individual emerged seedling. Goodness-of-fit and the statistical significance of the fixed terms were evaluated, as mentioned above. All analyses were carried out with Genstat 15 (VSN International, 2012).
3 Results
3.1 Seed treatment and size of seedling after the first growing season
The water content of both seed lots (A and B) was initially similar and increased as a result of the soaking (Table 2). Anatomically immature, larvae-infested, empty, and damaged seeds were more common in the surface fraction than in the bottom fraction or among the control seeds in both seed lots. As a result of the soak-sorting, the bottom fraction included only full seed. The proportion of insect-infested seeds was higher in the surface fraction of seed lot A than in seed lot B. The seeds were infested by Megastigmus strobilobius Ratzeburg (Hymenoptera: Torymidae) larvae.
| Table 2. Percentage of different quality seeds according to radiography (n = 384) and the water content of the un-soaked control and bottom fraction seeds after a 15h soaking and surface drying. The class “immature” includes anatomically immature, but viable seeds. Seeds with mechanical or undetermined damage were classified as “other”. | |||||||
| Seed lot | Treatment | Full | Larva | Immature | Empty | Other | Water content (± SD) |
| A | Control | 96.1 | 0.5 | 3.1 | 0 | 0.3 | 5.0 (± 0.1) |
| A | Bottom fraction | 100 | 0 | 0 | 0 | 0 | 17.4 (± 0.2) |
| A | Surface fraction | 74.7 | 13.3 | 8.3 | 0.8 | 2.9 | not measured |
| B | Control | 97.9 | 0.5 | 1.6 | 0 | 0 | 5.26 (± 0.1) |
| B | Bottom fraction | 100 | 0 | 0 | 0 | 0 | 17.8 (± 0.4) |
| B | Surface fraction | 86.7 | 1.8 | 10.7 | 0.5 | 0.3 | not measured |
The temperature in the nursery at the seedling level varied between 7.6 and 38.7 °C during the first 49 days (Fig.1). The highest temperature was measured 2.5 weeks after sowing. The relative humidity varied between 35.9% and 100% during the 49 days. More seedlings emerged from the seeds in the bottom fraction than in the other treatments (Table 3). When the emergence percentage was calculated based on all seeds sown, over 20% of the surface seeds of lot A were unable to emerge, while the number was just over 8% in seed lot B, indicating statistically significant interaction between seed lot and treatment (p = 0.032). When the number of subjects was determined as the number of viable seeds according to radiography (full and immature seed), the emergence percentage was again higher in the bottom fraction than in the control and surface seeds. The treatment effect was therefore significant (p = 0.004) but the seed lots did not differ from each other (p = 0.645). The interaction term (seed lot × treatment) was also not significant (p = 0.253).
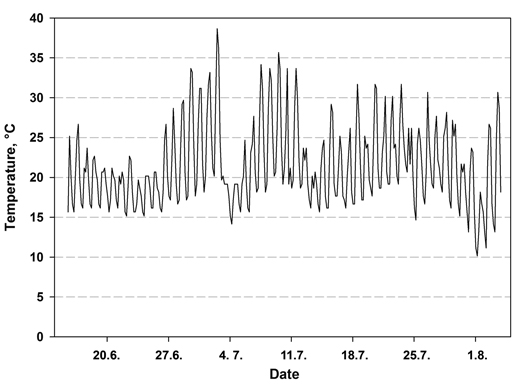
Fig. 1. Air temperature at the seedling level during the first 49 days after sowing in a nursery greenhouse measured at 1h interval.
Almost all the seedlings from both soaked fractions emerged during the first 2 weeks, while seedling emergence extended over a longer period of time in the control seeds (Fig. 2).
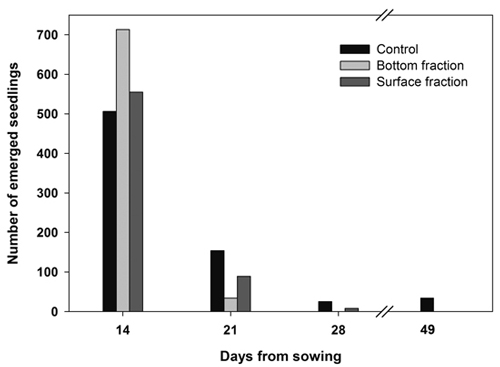
Fig. 2. Number of new emerged seedlings on each day of observation. Containerized Norway spruce seedlings originating from soaked (bottom and surface fraction) and control seeds sown on June 14.
The mean time of emergence was 2.5–3.5 days shorter in seeds of the bottom fraction than in the control seeds, while the difference between the surface and control seeds was smaller (Table 3). The treatment effect to time of emergence was statistically significant in both seed lot A (approximate p-value p = 0.016) and in seed lot B (approximate p = 0.002).
| Table 3. Percentage of emerged Norway spruce seedlings 49 days after sowing in peat-filled containers and the mean time of emergence. The emergence percentage is calculated in relation to both all seeds sown (n = 64) and on the based on viable seed (full and anatomically immature) according to radiography (n = viable). | |||
| Emergence percentage (± SE) | Mean time of emergence, days (± SE) | ||
| n = 64 | n = viable | ||
| Seed lot A | |||
| Control | 92.4 (± 1.7) | 94.2 (± 1.4) | 16.9 (± 0.4) |
| Bottom fraction | 97.7 (± 0.8) | 97.4 (± 0.8) | 14.3 (± 0.1) |
| Surface fraction | 79.1 (± 3.3) | 94.0 (± 1.5) | 15.4 (± 0.2) |
| Seed lot B | |||
| Control | 95.2 (± 1.3) | 94.7 (± 1.3) | 18.0 (± 0.5) |
| Bottom fraction | 97.2 (± 0.9) | 97.6 (± 0.7) | 14.4 (± 0.1) |
| Surface fraction | 91.4 (± 1.9) | 94.5 (± 1.3) | 15.1 (± 0.2) |
Seedlings originating from the bottom fraction were greater in height and had a larger stem diameter after the first growing season than the control seedlings and seedlings grown from the surface fraction (Fig. 3A and Table 4). The seed lots also differed from each other, with seedlings of seed lot A being greater in height and having a larger stem diameter than those in seed lot B (Table 4). The interaction term treatment × seed lot was statistically not significant in both cases (Fig. 3B and Table 4).
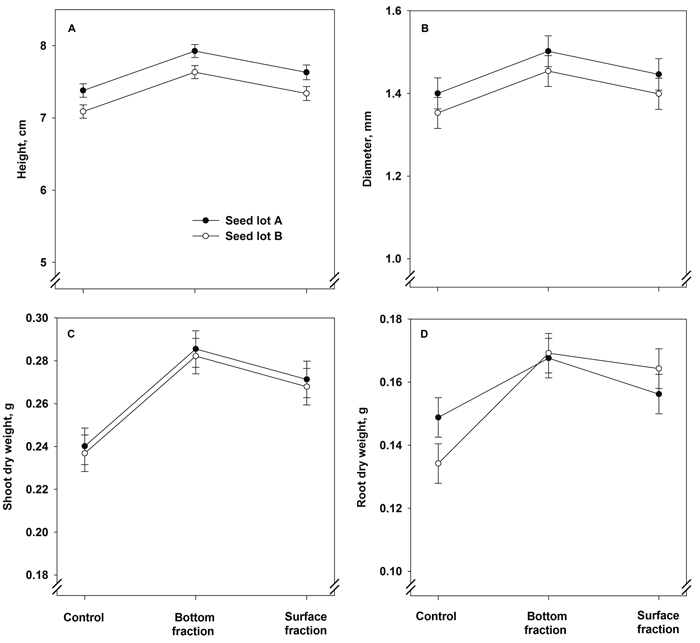
Fig. 3. Model predictions for height, stem diameter, and shoot and root dry mass (± SE) of containerized 1.5-year-old Norway spruce seedlings at the end of the first growing season. The seedlings were grown in the first year from June onwards.
| Table 4. Tests for fixed effects for measurements of 1.5-year-old containerized Norway spruce seedlings after the first growing season according to linear mixed models. The seedlings were grown in the first year from June onwards. A block was set as a random term (test not shown). Denominator degrees of freedom for approximate F-tests are calculated using algebraic derivatives. | ||||||
| Response variate | Fixed model term | Wald statistic | df nominator | F statistic | df denominator | p |
| Height, 1st year | Treatment | 24.56 | 2 | 12.32 | 1343.3 | <0.001 |
| Seed lot | 9.07 | 1 | 9.07 | 1356.3 | 0.003 | |
| Treatment × seed lot | 2.52 | 2 | 1.26 | 1352.4 | 0.284 | |
| Diameter, 1st year | Treatment | 38.23 | 2 | 19.12 | 1229.9 | <0.001 |
| Seed lot | 11.62 | 1 | 11.62 | 1208.6 | <0.001 | |
| Treatment × seed lot | 0.14 | 2 | 0.07 | 1203.5 | 0.930 | |
| Shoot, 1st year | Treatment | 30.37 | 2 | 15.18 | 678.6 | <0.001 |
| Seed lot | 0.23 | 1 | 0.23 | 658.0 | 0.632 | |
| Treatment × seed lot | 5.10 | 2 | 2.55 | 650.4 | 0.079 | |
| Root, 1st year | Treatment | 31.88 | 2 | 15.94 | 678.7 | <0.001 |
| Seed lot | 0.17 | 1 | 0.17 | 658.5 | 0.683 | |
| Treatment × seed lot | 5.70 | 2 | 2.85 | 651.1 | 0.058 | |
The shoot dry mass after the first growing season was affected solely by treatment (Fig. 3C and Table 4). Both soaking treatments increased the shoot dry mass compared to control. The root dry mass was also higher in the bottom and surface fraction seedlings than in the control (Fig. 3D and Table 4). The seed lot effect was statistically not significant, but the treatment × seed lot interaction term was almost significant: in control seedlings, those of seed lot A had heavier root system than those of seed lot B, but the differences between the seed lots were non-existent in the bottom and surface fraction seedlings.
3.2 Second growing season
The bottom fraction seeds produced the highest proportion of saleable seedlings, 94.0% (SE ± 1.2%), calculated as the number of saleable seedlings in relation to all emerged original seedlings. The proportion of saleable seedlings was similar in seedlings originating from the control and surface fraction seed, 88.9% (± 1.8%) and 90.1% (± 1.7%) respectively. The treatment effect was hence statistically significant (p = 0.014), while the seed lot effect was not significant (p = 0.70).
The treatment × seed lot interaction term had a statistically significant effect on the final height of the seedlings (Fig. 4A, Table 5). Seedlings from the bottom fractions showed the greatest height. Seedlings from seed lot A were greater in height than those from lot B in the control and bottom seedlings. Stem diameter, however, was affected solely by the treatment (Fig. 4B, Table 5): seedlings originating from the bottom fraction had the largest final stem diameter.
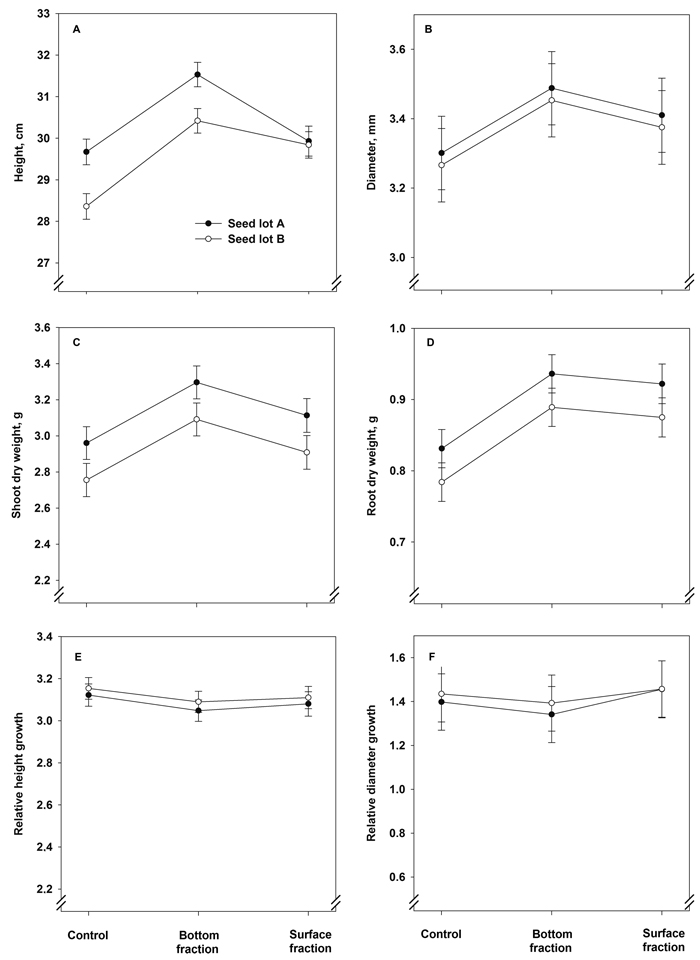
Fig. 4. Model predictions for height, stem diameter, shoot and root dry mass and relative height and stem diameter growth (± SE) of containerized 1.5-year-old Norway spruce seedlings at the end of the second, final growing season.
| Table 5. Tests for fixed effects for measurements of 1.5-year-old containerized Norway spruce seedlings after the second, final growing season according to linear mixed models. A block was set as a random term (test not shown). Denominator degrees of freedom for approximate F-tests are calculated, using algebraic derivatives. | ||||||
| Response variate | Fixed model term | Wald statistic | n.d.f. | F statistic | d.d.f. | F pr |
| Height, 2nd year | Treatment | 96985.46 | 2 | 48492.73 | 1383.0 | <0.001 |
| Seed lot | 27131.88 | 1 | 27131.88 | 1383.0 | <0.001 | |
| Treatment × seed lot | 9808.60 | 2 | 4904.30 | 1383.0 | <0.001 | |
| Diameter, 2nd year | Treatment | 21.62 | 2 | 10.81 | 1342.6 | <0.001 |
| Seed lot | 1.06 | 1 | 1.06 | 1341.6 | 0.304 | |
| Treatment × seed lot | 0.87 | 2 | 0.44 | 1340.7 | 0.647 | |
| Shoot dry mass, 2nd year | Treatment | 11.31 | 2 | 5.65 | 654.8 | 0.004 |
| Seed lot | 6.24 | 1 | 6.24 | 656.7 | 0.013 | |
| Treatment × seed lot | 0.37 | 2 | 0.18 | 655.6 | 0.833 | |
| Root dry mass, 2nd year | Treatment | 13.86 | 2 | 6.93 | 654.7 | 0.001 |
| Seed lot | 3.57 | 1 | 3.57 | 656.6 | 0.059 | |
| Treatment × seed lot | 0.47 | 2 | 0.24 | 655.2 | 0.789 | |
| Relative height growth | Treatment | 2.41 | 2 | 1.20 | 1224.4 | 0.300 |
| Seed lot | 0.87 | 1 | 0.87 | 1203.2 | 0.352 | |
| Treatment × seed lot | 0.02 | 2 | 0.01 | 1195.9 | 0.990 | |
| Relative diameter growth | Treatment | 5.46 | 2 | 2.73 | 1205.6 | 0.066 |
| Seed lot | 1.04 | 1 | 1.04 | 1182.7 | 0.308 | |
| Treatment × seed lot | 0.43 | 2 | 0.22 | 1177.0 | 0.806 | |
The final shoot dry mass showed a statistically significant increase as a result of the soaking treatments: the shoots were heaviest in the bottom fraction seedlings (Fig. 4C and Table 5). The shoot dry mass was also higher in seedlings of seed lot A than in those of lot B. The root dry mass was heavier in seedlings of both soaked fractions than in the control seedlings (Fig. 4D and Table 5). The seed lot effect was almost statistically significant, with heavier root systems in seedlings of seed lot A (Table 5). The relative height and diameter growth were influenced neither by treatment nor seed lot (Fig. 4E and 4F and Table 5).
4 Discussion
4.1 Seed sorting and time of emergence
In this study, we investigated the effects of soak-sorting on the development of 1.5-year-old Norway spruce seedlings. We previously tested this seed treatment method under laboratory conditions as well as under nursery conditions typical for 1-year-old seedlings, but not in the conditions typical for this seedling type, most commonly used in forest regeneration in Finland. This seedling type is sown during midsummer, when temperatures in the greenhouse are typically higher than in spring, when 1-year-old seedlings are sown.
Soaking resulted in viable and full seeds in the bottom fraction while floating surface fraction contained larvae-infested, anatomically immature and empty seeds in both seed lots (Table 2). However, the surface fraction also contained a large number of viable seeds, similar to our previously reported results (Himanen and Nygren 2014). Fractioning during aerated soaking can therefore be expected to give a rather consistent result in Norway spruce within a seed lot.
The mean time of emergence was 2.5–3.5 days shorter in the bottom fraction than in the un-soaked control seed (Table 3). This is more than we have previously reported in laboratory conditions (Himanen et al. 2010; 2013) and in nursery (Himanen and Nygren 2014). The larger decrease of time of emergence may have been due to the harsh germination temperatures, which occurred approximately 2 weeks after sowing (Fig. 1). Most seed had emerged by this time, but those that had not were faced with high temperatures, reaching over 35 °C. The proportion of non-germinated seed at this point was greater in the control seed than in the soaked seed. Temperatures such as this delay germination, since the germination rate of Norway spruce decreases at temperatures above 23 °C (Leinonen et al. 1993). Our previous studies of Norway spruce were conducted in more favorable and temperature-stable conditions (Himanen et al. 2010, 2013; Himanen and Nygren 2014). The effect of soaking on the germination or emergence rate is therefore likely to vary according to temperature conditions. Since the small number of seed in the surface fractions did not allow us to take moisture samples (Table 2), we cannot speculate on whether the slower emergence of the surface fraction compared with the bottom seeds was at least in part simply due to its lower moisture content.
4.2 Seedling morphology and quality
Seedlings originating from the bottom fraction were greatest in height and had the largest stem diameter and the highest shoot and root biomass. Seedlings originating from the surface fraction performed equally well or better than the control seedlings, depending on the morphological feature measured. This differs from our results for 1-year-old seedlings (sown in spring) grown from the same seed lots (Himanen and Nygren 2014). In our previous study, the shoot and root dry masses were unaffected by the same soak-sorting and the effects on height and diameter varied. The present results are, however, similar to those of studies in other conifer species. Boyer et al. (1985) found that seed treatments shortened the germination time and subsequently increased the average seedling diameter of loblolly pine (Pinus taeda L.). In another study, loblolly pine seedlings that germinated earliest achieved the largest stem diameter (Boyer et al. 1987). Mexal and Fisher (1987) also reported a decrease in shoot biomass in relation to emergence time in loblolly and ponderosa pine (Pinus ponderosa Dougl. ex Laws.). As a result of the decreased emergence time, the first growing season was 2.3–3.5 days longer for the bottom fraction seedlings than for the control seedlings in the present study. This lengthening of the growing season may have been sufficient to cause and increase in seedling size, whereas the 1.5–2 day gained in previous studies of Norway spruce were too short to cause major changes in seedling morphology.
The increase observed in final seedling height between the control and bottom fraction seedlings was 1.9–2.1 cm, depending on the seed lot. The stem diameter increased 0.19 mm in both lots. Although these differences are statistically significant, their biological significance can be debated. However, since soak-sorting increased all the attributes of seedling size, including the mass of both the shoot and root system, the size increase observed can be interpreted as a beneficial change, especially that of large stem diameter which predicts high survival and good growth in tree seedlings (Rikala and Aphalo 1998; Aphalo and Rikala 2003; Grossnickle 2012). Large size (Nordlander et al. 2011), and especially large stem diameter, also decreases mortality caused by pine weevils (Hylobius abietis (L.)) (Thorsén et al. 2001) and other pests in Norway spruce seedlings after out-planting. As the differences in size were observed after both the first and second growing season between the treatments, it can be concluded that the effect of soak-sorting is not entirely short-term, but possibly influences growth or survival after out-planting.
The bottom fraction seeds produced the largest number of saleable seedlings. The earlier seedling emergence in the bottom fraction likely explains the better quality of its seedlings. A decrease in germination time decreases mortality (Boyer et al. 1987; Mexal and Fisher 1987; Himanen and Nygren 2014) and the number of cull seedlings (Himanen and Nygren 2014) in conifer seedling crops. Removal of the immature and otherwise damaged seeds with the surface fraction may also explain the decrease in proportion of cull seedlings and the increase in size: the better seeds left after sorting produce better seedlings.
The simple soaking method studied is effective in separating poor-quality seeds to the surface fraction in commercial Norway spruce seed lots. Bottom fraction separated by the soaking produces more saleable seedlings than do untreated seed. However, it must be noted that a number of viable seed is lost if the surface fraction is always discarded. In the case of having a large surface fraction in a seed lot of reasonable germination capacity, further analysis of the floating seed may be advisable. Under nursery conditions the quality of the floaters can be evaluated by cutting the seeds, if radiography is not available.
In comparison to our previous studies on soaking and soak-sorting of Norway spruce seeds (Himanen et al. 2013; Himanen and Nygren 2014), the key finding of this study was that soak-sorting may give varying results in terms of its effects on emergence time and subsequent seedling development, depending on the growing conditions and seedling type. For 1.5-year-old seedlings, sown in midsummer, the environmental conditions are typically harsher than for 1-year-old seedling crops sown in early spring. Based on our results, we suggest that the beneficial effects of this simple method on seedling size and survival can last at least the duration of seedling cultivation in the nursery. Our present and previous work further show that the responses of varying seed lots to soak-sorting are similar, although differences in quality of the floating seeds and in the final morphology of the seedlings may be expected.
Acknowledgements
We thank Ms. Sirpa Kolehmainen for her assistance in gathering the study material. Dr. Pekka Helenius provided valuable comments to the manuscript. The study was financially supported by Graduate School in Forest Sciences (GSForest), Metsämiesten säätiö foundation and Metsänjalostussäätiö foundation.
References
Aphalo P., Rikala R. (2003). Field performance of silver-birch planting-stock grown at different spacing and in containers of different volume. New Forests 25: 93–108. http://dx.doi.org/10.1023/A:1022618810937.
Boyer J.N., Duba S.E., South D.B. (1987). Emergence timing affects root-collar diameter and mortality in loblolly pine seedbeds. New Forests 1: 135–140. http://dx.doi.org/10.1007/BF00030057.
Boyer J.N., South D.B., Muller C., Vanderveer H., Chapman W., Rayfield W. (1985). Speed of germination affects diameter at lifting of nursery-grown loblolly pine seedlings. Southern Journal of Applied Forestry 9(10): 243–247.
Finnish Forest Research Institute (2013). Finnish statistical yearbook of foresty. Official statistics of Finland. Agriculture, forestry and fishery. 450 p. ISBN 978-951-40-2449-8.
Grossnickle S.C. (2012). Why seedlings survive: influence of plant attributes. New Forests 43: 711–738. http://dx.doi.org/10.1007/s11056-012-9336-6.
Himanen K., Nygren M. (2014). Effects of seed pre-soaking on the emergence and early growth of containerized Norway spruce seedlings. New Forests 45(1): 71–82. http://dx.doi.org/10.1007/s11056-013-9392-6.
Himanen K., Helenius P., Nygren M. (2010). Liotuskäsittelyiden vaikutus kuusen siementen itämiseen. [Effect of pre-sowing soaking treatments on germination of Norway spruce seeds] Metsätieteen aikakauskirja 2/2010: 103–114. [In Finnish].
Himanen K., Lilja A., Rytkönen A., Nygren M. (2013). Soaking effect on seed germination and fungal infection in Picea abies. Scandinavian Journal of Forest Research 28(1): 1–7. http://dx.doi.org/10.1080/02827581.2012.683037.
International seed testing association (2005). International rules for seed testing, 2nd edition. Bassersdorf: International Seed Testing Association.
International seed testing association (2007). ISTA handbook on moisture determination, 1st edition. International Seed Testing Association. Bassersdorf, Switzerland.
Karrfalt RP. (2008). Seed harvesting and conditioning. In: Bonner, FT., Karrfalt, RP. (eds.). The woody plant seed manual. United States Department of Agriculture, Forest Service. Agricultural Handbook 727. p. 57–84.
Kolotelo D., Van Steenis E., Peterson M., Bennett R., Trotter D., Dennis J. (2001). Seed handling guidebook. Tree Improvement Branch, Ministry of Forests, British Columbia. 151 p.
Kruse M. (ed.). (2004). ISTA handbook on seed sampling. Second edition. International Seed Testing Association. ISBN 3-906549-02-X.
Leinonen K., Nygren M., Rita H. (1993). Temperature control of germination in seeds of Picea abies. Scandinavian Journal of Forest Research 8: 107–117. http://dx.doi.org/10.1080/02827589309382759.
Long AJ., Carrier BD. (1993). Effects of Douglas-fir 2+0 seedling morphology on field performance. New Forests 7: 19–32. http://dx.doi.org/10.1007/BF00037469.
Mexal J.G., Fisher J.T. (1987). Size hierarchy in conifer seedbeds. I. Time of emergence. New Forests 3: 187–196. http://dx.doi.org/10.1007/BF00118756.
Nordlander G., Hellqvist C., Johansson K., Nordenhem H. (2011). Regeneration of European boreal forests: effectiveness of measures against seedling mortality caused by the pine weevil Hylobius abietis. Forest Ecology and Management 262(12): 2354–2363. http://dx.doi.org/10.1016/j.foreco.2011.08.033.
Puttonen P. (1996). Looking for the “silver bullet” – can one test do it all? New Forests 13: 9–27. http://dx.doi.org/10.1023/A:1006557502326.
Rikala R. (2012). Metsäpuiden paakkutaimien kasvatusopas. [A guide for growing container tree seedlings]. Metsäkustannus Oy and Finnish Forest Research Institute. [In Finnish].
Rikala R., Aphalo P. (1998). Kasvatustiheyden ja paakkukoon vaikutus taimien ominaisuuksiin taimitarhalla ja menestymiseen istutuksen jälkeen. [The effect of growing density and container cell volume to the properties of seedlings in nursery and after outplanting]. In: Poteri M. (ed.). Taimitarhatutkimuksen vuosikirja 1998. Metsäntutkimuslaitoksen tiedonantoja 696. p. 21–35.
Simak M. (1955). Insect damages on seeds of Norway spruce determined by X-ray photography. Meddelanden från statens skogsforskningsinstitut. Serien uppsatser nr 41.
South D.B., Harris S.W., Barnett J.P., Hainds M.J., Gjerstad D.H. (2005). Effects of container type and seedling size on survival and early height growth of Pinus palustris seedlings in Alabama, U.S.A. Forest Ecology and Management 204: 385–398. http://dx.doi.org/10.1016/j.foreco.2004.09.016.
Thorsén Å., Mattson S., Weslien J. (2001). Influence of stem diameter on the survival and growth containerized Norway spruce seedlings attacked by pine weevils (Hylobius spp.). Scandinavian Journal of Forest Research 16: 54–66. http://dx.doi.org/10.1080/028275801300004415.
VSN International. (2012). GenStat for Windows 15th Edition. VSN International, Hemel Hempstead, UK.
Ward J.S., Gent M.P.N., Stephens G.R. (2000). Effects of planting stock quality and browse protection-type on height growth of northern red oak and eastern white pine. Forest Ecology and Management 127: 205–216. http://dx.doi.org/10.1016/S0378-1127(99)00132-2.
Wiersma N. (1973). The importance of insect damage to Norway spruce (Picea abies L.) cones for seed processing. In: Machaníšek J. (ed.). Economic collection of cones of forest conifers on the basis of preceding estimation of cone crop. IUFRO Working party: S2.01.06. p. 119–122.
Total of 26 references

