Leaf photosynthetic capacity, trunk wood structure and stem xylem sap flow in 700-years old Quercus robur L.: a pilot study upon oak ‘Bartek’, a natural monument in Poland
Zajączkowska U., Dąbrowski P., Kowalczuk W., Tarwacki G. (2022). Leaf photosynthetic capacity, trunk wood structure and stem xylem sap flow in 700-years old Quercus robur L.: a pilot study upon oak ‘Bartek’, a natural monument in Poland. Silva Fennica vol. 56 no. 3 article id 10561. https://doi.org/10.14214/sf.10561
Highlights
- Photosynthetic and hydraulic capacity of a 700-year-old Quercus robur is comparable to reference values from the literature measured in younger oak trees.
Abstract
Physiological studies of long-lived trees are particularly important at this time, especially in light of the need for trees to adapt to global climate change. The results of the present studies were obtained on an approximately 700-year-old Quercus robur L. – the ‘Bartek’ oak. The tree has to adapt to changing climatic conditions, starting from the transition between the Medieval Warm Period and the Little Ice Age, up to the present time of rapid global climate change. Tomograph imaging showed decay of the tree trunk interior and revealed that undamaged wood forms a thin layer around the trunk perimeter. Two series of experiments were carried out to assess the physiological state of the tree. The first concerned measurements related to photosynthetic capacity: chlorophyll a fluorescence, gas exchange (CO2 assimilation, transpiration), stomatal conductance and leaf water potential. The second series concerned xylem sap flow velocity and anatomical studies of stem wood. Photosynthetic capacity was within the limits reported for young healthy trees. The diurnal pattern of velocity of xylem sap flow was also typical for young vigorous trees and flow velocity correlated positively with solar radiation and negatively with air relative humidity. Anatomical observations of the outermost wood showed relatively narrow annuals rings with large diameter earlywood vessels. The results indicate that the veteran tree does not show signs of water stress probably due to a good balance of water flow and that leaf area of the canopy needs only the current ring of wood to feed transpiration of the canopy.
Keywords
photosynthetic capacity;
‘Bartek’ oak;
tree stem tomography;
xylem sap flow
-
Zajączkowska,
Department of Forest Botany, Warsaw University of Life Sciences - SGGW, 159 Nowoursynowska Street, 02-776 Warsaw, Poland
 https://orcid.org/0000-0002-7119-7547
E-mail
urszula_zajaczkowska@sggw.edu.pl
https://orcid.org/0000-0002-7119-7547
E-mail
urszula_zajaczkowska@sggw.edu.pl

-
Dąbrowski,
Department of Environmental Development Warsaw University of Life Sciences - SGGW, Warsaw, Poland
 https://orcid.org/0000-0002-2867-8839
E-mail
piotr_dabrowski@sggw.edu.pl
https://orcid.org/0000-0002-2867-8839
E-mail
piotr_dabrowski@sggw.edu.pl
- Kowalczuk, Ekosystem Waldemar Kowalczuk Tomasz Kowalczuk, Otwock, Poland E-mail ekosystem@ekosystem.waw.pl
-
Tarwacki,
Forest Protection Department, Forest Research Institute, Sękocin Las, Poland
 https://orcid.org/0000-0001-5979-7788
E-mail
G.Tarwacki@ibles.waw.pl
https://orcid.org/0000-0001-5979-7788
E-mail
G.Tarwacki@ibles.waw.pl
Received 3 May 2021 Accepted 23 August 2022 Published 2 November 2022
Views 50619
Available at https://doi.org/10.14214/sf.10561 | Download PDF

Supplementary Files
1 Introduction
Studies of the physiological state of veteran trees are scarce, although such research creates unique opportunities to learn about the little known mechanisms of aging at the later stage of a tree’s life. Such iconic trees have grown for centuries and adapted to changing climatic conditions, starting from the transition between the Medieval Warm Period and the Little Ice Age, up to the present time of rapid global climate change (Hunt 2006; Crowley 2006). It should be noted that tree as long as the meristems are active, each year produces new tissues, in principle with the same genetic information during the whole several hundred years period, however, somatic mutations may occur in stem apical meristems (Plomion et al. 2018; Orr et al. 2020). Mutations in a meristematic cell can be passed on to all tissue derived from it and can potentially cause an entire branch (including the leaves) to have a different genotype than the rest of the tree. Mutations accumulate with age, which may explain how long-lived plants can adapt to changing environmental conditions. While long-term exposure to deleterious ultraviolet radiation contributes to mutation processes, however, Schmid-Siegert et al. (2017) indicate that the rate of somatic mutations in oak is relatively low due to protective shielding of meristems from ultraviolet radiation provided by Quercus bud morphology. It is commonly believed that trees do not have a strictly regulated age of senescence and the life span of trees is usually determined by biotic and abiotic stresses (Groover 2017). This opinion is supported by a recent work of Wang et al. (2020) on vascular cambial cells longevity mechanisms in Ginkgo biloba L. trees. The authors found that the vascular cambium of the very old (667 years) trees showed no signs of senescence and suggested that long-lived trees developed compensation mechanisms to maintain a balance between growth and aging processes. This involves continued cambial divisions, high expression of resistance-associated genes, and continued synthetic capacity of preformed protective secondary metabolites.
Studies of very old trees could facilitate a better understanding of the complex physiological, anatomical and biomechanical mechanisms functioning in the terminal phases of tree life, as well as giving indications of the reasons for differences in the maximum age reached by trees of different species (Nicolotti and Gonthier 2004). There are many studies in the literature on the modification of the physiological processes and structure of wood related to the age and size of the tree, which indicate that old and large trees show physiological and morphological accumulation related to the increasing stem size and the mass of leaves (Ishii et al. 2000; Meinzer et al. 2011; Olson et al. 2014) and that rate of tree carbon accumulation increases continuously with tree size (Stephenson et al. 2014). Usually, however, most of the experiments were carried out on individuals that do not exceed the age of 100–200 years and can be considered as mature trees , while studies on ancient specimens that has passed beyond maturity (Lonsdale 2013) are very rare. Physiological and anatomical studies of such trees are difficult because commonly, due to being hundreds of years old and having heritage value, they have the status of natural monuments (Read 2000; Fay 2002). Therefore, any experimental manipulation causing even slight wounding or damage of such protected trees requires approval of the appropriate authorities, which can be difficult to obtain.
In Europe, two species of oak belong to the oldest deciduous trees Quercus robur L. and Q. petraea (Matt.) Liebl., whose maximum age is usually estimated at about 700–800 years, although some individuals reach an age of about 1000 years (Read 2000). The oldest specimens of these two species are distinguished by large spreading crowns, with very large stem diameter and greatly decayed interior of the tree stem. It should be noted that the both oak species have a ring-porous structure of wood (Jane 1956; Schweingruber 1990) and the water transport takes place predominantly in large-diameter early-wood vessels only in the current annual rings (Granier et al. 1994). It is known from the studies on younger specimens of other woody species that physiological performance of trees, specially net photosynthesis rate and stomatal conductance highly depend on xylem sap flow and the significantly greater xylem vessel area and vessel diameter can help to higher sap flow and good plant water status by maintaining the efficient leaf water potential of the plants (Bhusal et al. 2018). Until now, there was no research on such ancient oak trees regarding stem wood anatomy, xylem sap flow or photosynthetic capacity that would allow the assessment of the physiological state of such ancient trees. The results presented in this paper were obtained on one of the oldest specimens of oaks in Poland – the ‘Bartek’ oak (Quercus robur), a legendary tree, which from the middle of the twentieth century has been designated a natural monument (Pacyniak 2006; Zarzyński and Tomusiak 2009).
The hypothesis that in such ancient tree the vessel structure of the lastly formed annual rings of wood enables water transport the stem that ensures high leaf photosynthetic capacity was tested. In order to check this hypothesis we investigated selected physiological parameters that are used in research to determine the health condition of woody plants (Meinzer et al. 2011): chlorophyll a fluorescence, gas exchange (CO2 assimilation, transpiration), stomatal conductance and water potential of leaves as well as transport of the xylem sap in the trunk with respect to wood anatomy.
The experiments were made possible thanks to the consent of the Nature Conservator to allow collection of samples. However, due to restrictive regulations for the protection of this legendary tree, the collection of samples and testing were limited. Despite these restrictions we believe that the results presented in this paper provide new highly relevant information on the physiological state and structure of wood tissue in a very advanced stage of ageing of this oak species.
2 Materials and methods
The ‘Bartek’ oak (Quercus robur L.) tree, protected by law since 1954 as a natural monument, grows in the Zagnańsk Forest District of Central Poland (Supplementary file S1). Coordinates of the site are 50°59´N, 20°39´E. Climatic data were obtained from the Kielce-Suków meteorological station, 20 km away from the tree site (https://pl.climate-data.org/europa/polska/swietokrzyskie-voivodeship/kielce-764743/ ). The mean annual temperature in 1991–2021 was 7.8 °C; the mean temperature for the summer season (June–September) and winter season (December–March) season was 17.5 °C and –0.4 °C, respectively. Mean annual precipitation for the same period was estimated 725 mm; of which, 311 mm are for the summer and 182 for the winter. The mean annual numbers of hot days (tmax > 25 °C) and very hot days (tmax > 30 °C) was 37.1 and 5.5, respectively (Jarzyna 2016). The ‘Bartek’ tree is 28.5 meters tall with trunk circumference 9.80 m (at a height of 1.30 m) and crown spread of about 20 × 40 m. The living sapwood in the trunk is reportedly very thin and decay of the interior of the tree trunk makes it difficult to accurately determine tree age by coring. According to Pacyniak (2006), the ‘Bartek’ oak was estimated to be about 650 years old in 1992. The evaluation was done on the basis of measurements of the outermost rings by the Pressler drill and approximate estimation of age inside the decayed stem interior. In the 1920s, the cavity inside the trunk was filled with cement; the concrete was removed in 1978, and the cavity filled with fir wood that was preserved with synthetic resin and masked with bark patches on the eastern side of the trunk. In order to determine the degree of decay of the tree stem, a PICUS 3 Q74EXP sonic tomograph (Argus Electronic GmbH) was used to assess trunk structure at six heights above ground level: 25, 75, 125, 175, 225 and 275 cm . This type of tomograph, which was earlier tested on the old oak trunks by Wang et al. (2007, 2008), detects differences in the ability to transmit acoustic waves by the examined wood, which are related to its density and elasticity modulus. The outcome is a tomogram that depicts the sound velocity distribution in the stem cross-section. The values of sound velocity are depicted by a color scale with relative ranges: (i) the highest speed is set to v: 100% – it corresponds to the darkest shade of brown, (ii) the slowest speed for all six measurements is v: 50% – it corresponds to the lightest shade of blue. The color definitions are part of the PICUS Expert Software.
Two series of measurements were carried out to assess the physiological state of the ‘Bartek’ oak, with emphasis on assessing its potential susceptibility to water stress. The first series was performed on June 15, 2018, using five leaves from each of two sub-branches from one main lateral branch; the branching point of the main tree stem was about 3 meters above the ground and the two sub-branches, located at similar height, were about 6 meters distant from the branching point. Measurements of chlorophyll a fluorescence, gas exchange and leaf water potential were carried out. Chlorophyll a fluorescence measurements were performed using an OS5p+ Fluorimeter (Delta – T Device, UK) on leaves that were not cut off. Both ambient light and dark adaptation parameters were determined, including Fo (initial fluorescence), Fm (maximal fluorescence) and Fv/Fm (where Fv = Fm – Fo) . A modulated measurement of chlorophyll fluorescence was also made that allowed determination of two non-photochemical fluorescence quenching parameters: qN [qN = 1 − Fm × Fo/(Fm − Fo)2 × (1 − ΦD)2/ΦD, where ΦD – the quantum rate of absorbed light energy in PSII allocated to ‘Dissipation’ in the Demmig-Adams model] and NPQ [NPQ = (Fm − Fo)/Fo × ΦD/(1 − ΦD) – 1 ] (Ishida et al. 2011). Gas exchange (CO2 assimilation, transpiration) and stomatal conductance were measured with the LCpro + gas analyzer (ADC Bioscentific Ltd., UK). During the measurements, the air temperature was 20–21 °C, and the Photosynthetic Photon Flux Density (PPFD) was about 1000 µmol m–2 s–1 . Leaf water potential was estimated using a portable Plant Water Status Console (PWSC 3000, Soilmoisture Equipment Corp., USA. The measurements were made on the leaves that were cut off and the measurement procedure was in line with described by Turner (1988). The results of the measurements of gas exchange and water potential in the leaves were statistically analyzed using the Student’s t-test at a significance level of 0.05 using the Statistica 10.0 program (Statsoft, Inc. Tulsa, USA).The second series of experiments, which concerned assessment of stem xylem sap flo velocity, was carried out on 5–7 September 2018, on three sides of the tree trunk (S – southern, N – northern and W – western) at heights 3.70, 4.20 and 2.60 m above ground for S, N and W, respectively. Choice of measurement location was made to avoid branching regions in the main trunk that could disturb the measurement of water flow. Due to the cavity on the eastern side of the stem, this cardinal direction of the trunk was not tested. Measurements were carried out using a SFM1 Sap Flow Meter (ICT International). In order to perform the measurements, the outer bark was removed to a depth of about 3 mm above the surface of the living secondary phloem, on an area of about 4 cm2. Three holes along a vertical line at distances of 5 mm, with a diameter of 1.25 mm and a depth of 35 mm, were drilled on the surface. Standard SFM1 35 mm long needles, which served as thermal conductivity probes, were inserted into the holes. The results of the xylem sap flow velocity measurements were compared with solar radiation and relative humidity of the air at a height of 2 m, obtained from the meteorological station in Kielce-Suków. After completing xylem sap flow measurements, samples for anatomical study were collected from the locations where the xylem sap flow measurements probes were placed. Wood samples containing about 20 annual rings were taken with a hammer-driven cylindrical punch, 1.2 cm in diameter, to a depth of about 2.5 cm from the cambial boundary. Wood samples were boiled in a solution of water with glycerol in a ratio of 3:1 v/v to soften and deaerate the tissues. Anatomical sections of wood were made in a sliding microtome and observed under an Olympus BX-61 optical microscope. Measurements of ring widths and xylem vessel diameters were obtained using Fiji1 image analysis software (Schindelin et al. 2012).
3 Results
Sonic tomograph assessments of the interior trunk tissues of the ‘Bartek’ tree taken up to a height of 2.75 m above ground (Fig. 1) show that the trunk is most damaged from 1.25 –1.75 m above the ground. The undamaged wood forms a layer 2.2 to 38.2 centimeters thick around the trunk perimeter and share of undamaged wood is about 5 per cent of the oak trunk wood cross-sections (Table 1).
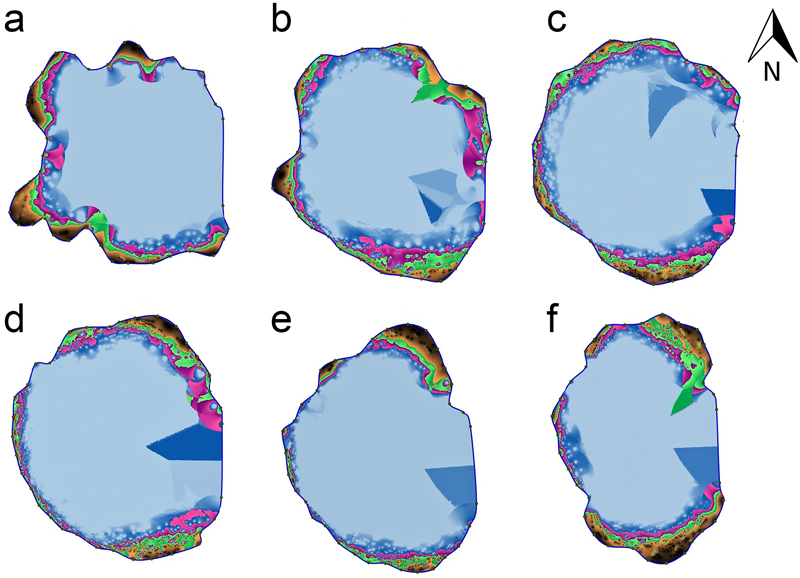
Fig. 1. Tomograms of the stem cross-sections at six heights above the ground: 25 (a), 75 (b), 125 (c), 175 (d) 225 (e) and 275 (f) cm of the ‘Bartek’ oak tree in Poland. Areas marked in black and brown indicate layers of undamaged wood and the green indicates slightly damaged wood; purple, pink, blue and white are areas with the weakest wood structure or where the trunk cavity is sealed with fir wood preserved with synthetic resin.
| Table 1. The results of sonic tomograph measurements of wood on trunk cross-sections at six heights above the ground at the ‘Bartek’ oak tree in Poland. | ||
| Height above ground level [cm] | Thickness of layer of undamaged wood min–max [cm] | Share of undamaged wood [per cent] |
| 25 | 4.2–36.3 | 9 |
| 75 | 3.5–29.9 | 7 |
| 125 | 2.2–19.7 | 5 |
| 175 | 3.3–23.0 | 5 |
| 225 | 2.4–33.5 | 6 |
| 275 | 3.2–38.2 | 11 |
The photosynthetic capacity in the ‘Bartek’ oak was assessed using chlorophyll a fluorescence, gas exchange (CO2 assimilation, transpiration) and stomatal conductance. The results of both ambient light and dark adaptation chlorophyll a fluorescence of leaves from two branches are presented in Table 2. The initial fluorescence (Fo) of chlorophyll a averaged 145.4 and 141.6 relative units for the first and second branches, respectively, and statistical analysis did not indicate a significant difference between branches. The maximal fluorescence (Fm) measured on the first branch was 705.8 relative units, and on the second was 579.4. Measurements of Fo and Fm were used to calculate the parameter Fv/Fm (where Fv = Fm – Fo). The values of Fv/Fm were 0.79 and 0.75 for the first and second branches, respectively, with no statistically significant difference between branches. Based on pulse amplitude-modulated measurements of chlorophyll a fluorescence, PAM (Pulse – Amplitude – Modulation), a chlorophyll fluorescence curve was drawn for leaves from both branches (Suppl. file S2). The measurement of chlorophyll a fluorescence allowed us to also determine two non-photochemical fluorescence quenching parameters: qN and NPQ. The value of qN for the first branch was 0.68, and for the second was 0.70 and the NPQ values amounted to 2.14 and 1.98 for the first and second branch, respectively.
| Table 2. Chlorophyll a fluorescence parameters (Fo,, Fm, Fv/Fm, non-photochemical fluorescence quenching parameters (qN, NPQ), gas exchange parameters (A, E, WUE), stomatal conductivity [Gs] ) and water potential [Ψ] measured on leaves from two branches of ‘Bartek’ oak tree in Poland. Averages of 5 leaves, ±SD. | ||
| Branch | ||
| 1 | 2 | |
| Chlorophyll a fluorescence parameters | ||
| Fo | 145.4 ± 7.8 | 141.6 ± 7.7 |
| Fm | 705.8 ± 123.5 | 579.4 ± 93.4 |
| Fv/Fm | 0.79 ± 0.03 | 0.75 ± 0.03 |
| qN | 0.68 ± 0.03 | 0.70 ± 0.15 |
| NPQ | 2.14 ± 0.34 | 1.98 ± 0.50 |
| Gas exchange parameters | ||
| A [µmol CO2 m–2 s–1] | 16.9 ± 1.8 | 18.5 ± 2.1 |
| E [mmol H2O m–2 s–1] | 6.1 ± 0.6 | 7.3 ± 0.8 |
| WUE [A/E] | 2.8 | 2.5 |
| Stomatal conductivity | ||
| Gs [mol H2O m –2 s–1] | 0.15 ± 0.02 | 0.16 ± 0.02 |
| Leaf water potential | ||
| Ψ [–MPa] | –1.4 ± 0.3 | –1.1 ± 0.2 |
| [Fo], initial fluorescence, [Fm], maximal fluorescence, [Fv/Fm] = (Fm – Fo)/ Fm, qN = 1 − Fm × Fo/(Fm − Fo)2 × (1 − ΦD)2/ΦD, where [ΦD] quantum rate of absorbed light energy in PSII allocated to ‘Dissipation’ in the Demmig-Adams model; NPQ = (Fm − Fo)/Fo × ΦD/(1 − ΦD) – 1 ]; [A] assimilation; [E] transpiration [E]; [WUE] water use efficiency; [Ψ] leaf water potential. | ||
The physiological state of green plants can be reflected by the rate of photosynthesis, which is very sensitive to stress (Ashraf and Harris 2013). Table 2 summarizes measurements of gas exchange made on leaves from two branches of the ‘Bartek’ oak. CO2 assimilation (A) were 16.9 and 18.5 μmol CO2 m–2 s–1 for leaves from the first and second branches, respectively. The transpiration rate (E) of leaves from the first branch was 6.1 compared to 7.3 mmol H2O m–2 s–1 for leaves from the second branch. In both cases, no statistically significant differences were found between values measured on the two branches. The ratio of (A) to (E) was used to calculate photosynthetic water use efficiency (WUE), which is an indicator of the physiological state of the plant (Medrano et al. 2012). On branch one, this ratio was 2.8, and on the second branch, 2.5. Leaf water potential of the first branch was –1.4 MPa, while for the second branch it was –1.1 MPa.
Values of xylem sap flow velocity in the trunk of the ‘Bartek’ oak are summarized in Fig. 2A. The diurnal pattern of sap velocity was similar in all three probes, regardless of being on the south, north or west side of the trunk. Comparison of xylem sap flow with meteorological data indicates a high degree of responsiveness to changes in solar radiation and relative humidity. Xylem sap flow resulting from transpiration is correlated positively with solar radiation and negatively with relative humidity. It can also be noticed that the SFM1 Sap Flow Meter registered relatively high values of the sap flow during the night.
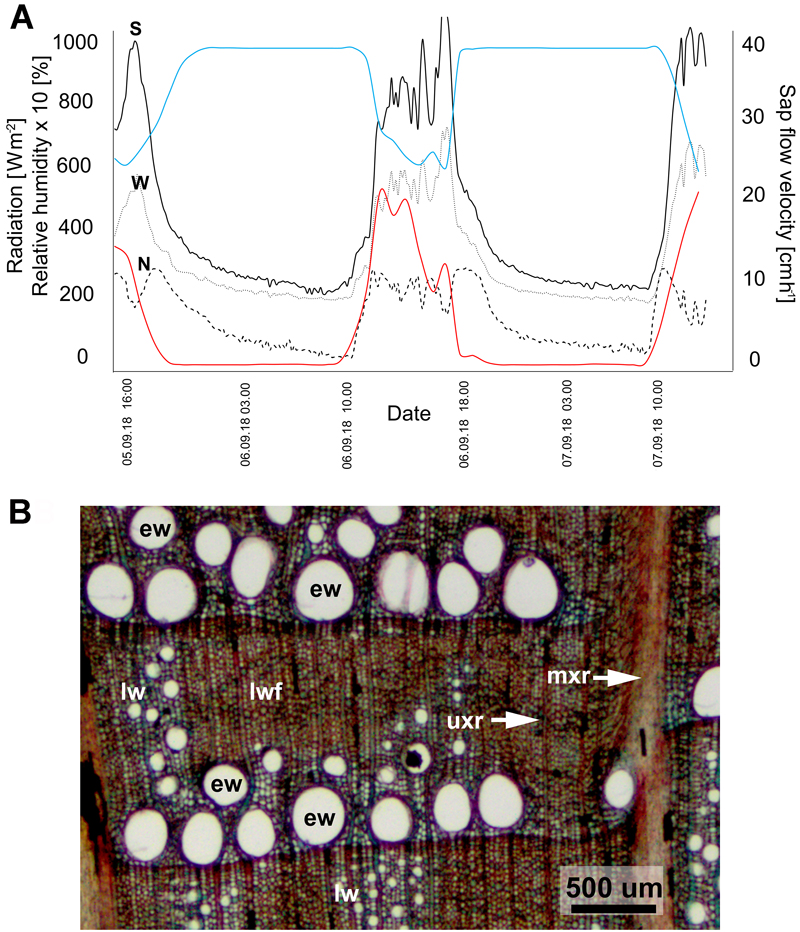
Fig. 2. Xylem sap flow (A) and wood anatomy (B) in the trunk of ‘Bartek’ oak tree in Poland. A: Dynamics of xylem sap flow velocity (black lines) on three sides (S – southern, N – northern, W – western) of the trunk of in comparison with solar radiation (red lines) and relative humidity (blue lines). Measurements were made from September 5, 2018 at 15:00 to September 7, 2018 at 12:00. B: Transverse section through the outermost annual rings of wood from the base of the tree. Earlywood with large diameter vessels (ew) form a layer aligned to the annual ring boundary. The radial arrangement of small diameter latewood vessels (lw) in the layer of latewood fibers (lwf) in wider rings is visible. Unilateral (uxr) and multilateral xylem rays (mxr) are present.
Microscopic measurements of stem xylem tissue showed that the width of the ten outermost annual rings was on average 1310 ± 120, 1650 ± 170 and 1470 ± 140 μm for samples from the south, north and west sides of the stem, respectively. The share of earlywood in the annual ring averaged 55, 30 and 45 percent for the S, N and W sides of the trunk, respectively, zone of earlywood at the base of the stem has characteristic cell structure for this tree species, with large diameter vessels (up to approx. 300 μm diameter) (Fig. 2B, Suppl. file S3).
4 Discussion
The results of sonic tomograph assessments of the ‘Bartek’ oak are in accordance with previous reports that ancient oak trees in Europe show significant decay of internal trunk tissues and that the undamaged wood forms a thin layer in the outermost part of the tree stem (Read 2000). Comparing the obtained data on the examined traits features of the photosynthetic capacity of the leaves of monumental oak with the data reported in the literature it can be seen that they do not differ from the values for healthy young trees. The Fv/Fm value which for the leaves of two branches of oak was 0,79 and 0.75. For the majority of plants, normal healthy leaves in stress-free conditions have the Fv/Fm value of around 0.8 (Björkman and Demmig 1987). The values of qN and NPQ parameters were within the limits of values reported in the literature. The value of qN ranges between 0and 1 (Fracheboud and Leipner 2003) and NPQ for healthy plants is usually between 0.5 and 3.5, although there may be significant differences depending on species (Maxwell and Johnson 2000; Lichtenthaler et al. 2004). The values of CO2 assimilation, transpiration rate and stomatal conductance for ‘Bartek’ oak leaves did not differ from typical values reported in the literature for young plants of this species kept in growth chambers or in greenhouse conditions (Hajji et al. 2009; Roussel et al. 2009). Although single leaf measurements are of limited value for assessing the health of an entire tree (Medrano et al. 2015), leaf WUE measured on the ‘Bartek’ oak was similar to values for non-stressed trees (e.g. Renninger et al. 2015; Ferrini and Nicese 2002). Thus, based on the measurements of chlorophyll a fluorescence and gas exchange, we conclude that this ancient veteran tree does not show obvious symptoms of photosynthetic and water stress.
In addition, values reported in the literature show that leaf water potential in plants not exposed to water stress should not be less than –2 MPa (Boyer 1966; Turner 1988) and the results from the ‘Bartek’ oak (–1.1 and –1.4 MPa ) may also indicate that the tree does not suffer from the water stress . This conclusion can be accepted, however, with some reservation as this type of fixed plant water stress are not widely used in literature currently. Instead, xylem or leaf hydraulic traits, such as turgor loss point, leaf/xylem 50% hydraulic conductance loss, provide more physiologically meaningful thresholds (Choat et al. 2012), but this kind of destructive sampling could not be applied to the very old protected tree. Moreover, the water potential of the leaves measured in lateral branches of the ‘Bartek’ oak at a height of 3 m from the ground and a distance of 6 m from the branching poin tof the main stem, may not reflect the most stressed leaves in the canopy and that the leaves at the top of the canopy should have a much lower water potential. Unfortunately, we were unable to directly measure the water potential of the leaves from the top of the tree crown, which were located at a height of about 28 m.
The diurnal pattern of sap velocity in the trunk of the ‘Bartek’ oak recorded daily fluctuations in sap transport characteristic of healthy young trees (Montague and Kjelgren 2006) and showed a high degree of responsiveness to changes in solar radiation and relative humidity. Very high nocturnal sap flow values (about 25 to 30 percent of diurnal values) recorded during the experiment apparently do to not reflect the actual process and are likely related with nocturnal capacitance refilling or nocturnal leaf transpiration or it is an artifact caused by probe misalignment (Burgess et al. 2001). It should be noted, however that the main goal of our xylem sap flow measurements was not to determine the absolute values of the flow velocity, but to check whether the dynamics of this flow was sensitive to fluctuations in the values of meteorological factors. The decrease in the velocity of trunk xylem sap flow on September 6 at about 15.00 h, when solar radiation decreased, may indicate sensitivity of transpiration by the ‘Bartek’ oak to cloudiness, similar to that reported for healthy trees (Čermák et al. 1984; Montague and Kjelgren 2006). The values of the outermost annual rings widths in the ‘Bartek’ trunk can be considered as quite small as compared to those in young healthy Q. robur trees (Tulik 2014) and the share of earlywood zone higher than is typically observed in young trees, where the share usually is about 30 percent (Vavrčík and Gryc 2012). It should be noted, however, that Q. robur belongs to ring porous hardwoods where decreasing with age annual ring width is associated with decreasing share of the latewood (Tsoumis 1991). In the outermost annual rings of the veteran tree stem, the diameter of the earlywood vessels, which are mainly responsible for the effectiveness of stem xylem sap flow (Zimmermann 1983; Granier et al. 1994) was typical for this tree species. Thus our results may indicate that the ‘Bartek’ oak does not show signs of water stress, probably due to a good balance of water. The results are in line with the studies on leaf photosynthetic traits and plant-water relations of two apple cultivars grown as bi-leader trees under well-irrigated field conditions and long-term waterlogging condition (Bhusal et al. 2018, 2020).
5 Conclusion
Our measurements of the leaf photosynthetic capacity, stem water transport and the anatomical structure annual rings of wood in a 700-year-old Quercus robur indicate that the veteran tree shows no signs of water stress. It is due to a good balance of water flow, despite the fact that ancient oak shows considerable decay inside the trunk and undamaged wood appears as a thin layer around the perimeter of the trunk. Our results indicate that leaf area of the canopy needs only the current annual ring with large diameter early wood vessels to feed transpiration of the canopy.
Declaration of openness of research materials
The research material used during the current study is available from the corresponding author on reasonable request.
Author contribution statement
UZ designed and performed stem xylem sap flow experiments, made microscopic observations of wood anatomy, analyzed the data and wrote the manuscript. PD conducted measurements and analyzed data of chlorophyll a fluorescence, gas exchange and leaf water potential. WK made tomographic measurements of the trunk interior. GT supervised the research. All authors read and approved the manuscript.
Funding
This work was supported by a grant from Directorate General of State Forests in Poland [No. 500451] and coordinated by the Forest Research Institute.
Conflict of interest
The authors declare they have no conflict of interest.
References
Ashraf M, Harris PJC (2013) Photosynthesis under stressful environments: an overview. Photosynthetica 51: 163–190. https://doi org/10.1007/s11099-013-0021-6.
Björkman O, Demmig B (1987) Photon yield of O2 evolution and chlorophyll fluorescence characteristics at 77 K among vascular plants of diverse origins. Planta 170: 489–504. https://doi.10.1007/BF00402983.
Bhusal N, Bhusal SJ, Yoon T-E (2018) Comparisons of physiological and anatomical characteristics between two cultivars in bi-leader apple trees (Malus x domestica Borkh.). Sci Horic 231: 73–81. https://doi.org/10.1016/j.scienta.2017.12.006.
Bhusal N, Kim HS, Han S-G, Yoo T-M (2020) Photosynthetic traits and plant-water relations of two apple cultivars grown as bi-leader trees under long-term waterlogging conditions. Environ Exp Bot 176, article id 104111. https://doi.org/10.1016/j.envexpbot.2020.104111.
Boyer JS (1966) Leaf water potential measured with a pressure chamber. Plant Physiol 42: 133–137. https://doi.org/10.1104/pp.42.1.133.
Burgess SS, Adams MA, Turner NC, Beverly CR, Ong CK, Khan AAH, Bleby TM (2001) An improved heat pulse method to measure low and reverse rates of sap flow in woody plants. Tree Physiol 21: 589–598. https://doi.org/10.1093/treephys/21.9.589.
Čermák J, Jeník J, Kučera J, Žídek V (1984) Xylem water flow in a crack willow tree (Salix fragilis L.) in relation to diurnal changes of environment. Oecologia 64:145–151. https://doi.org/10.1007/BF00376862.
Choat B, Jansen S, Brodribb TJ, Cochard H, B, Delzon S, Bhaskar R, Bucci SJ, Feild TS, Gleason SM, Hacke UG, Jacobsen AL, Lens F, Maherali H, Martínez-Vilalta J, Mayr S, Mencuccini M, Mitchell PJ, Nardini A, Pittermann J, Pratt RB, Sperry JS, Westoby M, Wright IJ, Zanne AE (2012) Global convergence in the vulnerability of forests to drought. Nature 491: 752–755. https://doi.org/10.1038/nature11688.
Crowley TJ (2000) Causes of climate change over the past 1000 years. Science 289: 270–277. https://doi.org/10.1126/science.289.5477.270.
Fay N (2002) Environmental arboriculture, tree ecology & veteran tree management. Arboric J 26: 213–238. https://doi.org/10.1080/03071375.2002.9747336.
Ferrini F, Nicese FP (2002) Response of English oak (Quercus robur L.) trees to biostimulants applicaction in the urban environment. J Arboric 28: 70–75. https://doi.org/10.48044/jauf.2002.009.
Fracheboud Y, Leipner J (2003) The application of chlorophyll fluorescence to study light, temperature, and drought stress. In: DeEll JR, Toivonen PMA (eds) Practical applications of chlorophyll fluorescence in plant biology. Springer, Boston, MA, pp 125–150. https://doi.org/10.1007/978-1-4615-0415-3_4.
Granier A, Andofillo T, Sabattl M, Cochard H, Dreyer E, Tomasl M, Valentini R, Breda N (1994) Axial and radial water flow in trunks of trees: a quantitative and qualitative analysis. Tree Physiol 14: 1383–1396. https://doi.org/10.1093/treephys/14.12.1383.
Groover A (2017) Age related changes in tree growth and physiology. In: John Wiley & Sons, Ltd, Chichester. https://doi.org/10.1002/9780470015902.a0023924.
Hajji M, Dreyer E, Marçais B (2009) Impact of Erysiphe alphitoides on transpiration and photosynthesis in Quercus robur leaves. Eur J Plant Pathol 125: 63–72. https://doi.org/10.1007/s10658-009-9458-7.
Hunt BG (2006) The Medieval Warm Period, the Little Ice Age and simulated climatic variability. Clim Dyn 27: 677–694. https://doi.org/10.1007/s00382-006-0153-5.
Ishida S, Morita K-i, Kishine M, Takabayashi A, Murakami R, Takeda S, Shimamoto K, Sato F, Endo T (2011) Allocation of absorbed light energy in PSII to thermal dissipations in the presence or absence of PsbS subunits of rice. Plant Cell Physiol 52: 1822–1831. https://doi.org/10.1093/pcp/pcr119.
Ishii HR, Azuma W, Kuroda K, Sillett SC (2000) Pushing the limits to tree height: could foliar water storage compensate for hydraulic constraints in Sequoia sempervirens? Funct Ecol 28: 1087–1093. https://doi.org/10.1111/1365-2435.12284.
Jane FW (1956) The structure of wood. A. & C. Black, London.
Jarzyna K (2016) Ekstrema termiczne w Górach Świętokrzyskich na przełomie XX i XXI wieku. [Thermal extremes in the Świętokrzyskie Mts (central Poland) at the turn of the 21st century]. Prace Geograficzne 147: 99–118. https://doi.org/10.4467/20833113PG.16.024.6086.
Lichtenthaler H, Buschmann C, Knapp M (2004) Measurement of chlorophyll fluorescence kinetics (Kautsky effect) and the chlorophyll fluorescence decrease ratio (RFd-values) with the PAM fluorometer. In: Filek M, Biesaga-Kościelniak J, Marcińska I (eds) Analytical methods in plant stress biology. The Franciszek Górski Institute of Plant Physiology, Polish Academy of Sciences, Kraków, Poland, pp 93–111.
Lonsdale D (ed) (2013) Ancient and other veteran trees: further guidance on management. The Tree Council, UK.
Maxwell K, Johnson NG (2000) Chlorophyll fluorescence – a practical guide. J Exp Bot 51: 659–668. https://doi.org/10.1093/jexbot/51.345.659.
Medrano H, Gulías J, Chaves M, Galmés J, Flexas J (2012) Photosynthesis water-use efficiency. In: Flexas J, Loreto F, Medrano H (eds) Terrestrial photosynthesis in a changing environment. A molecular, physiological and ecological approach. Cambridge University Press, Cambridge, pp. 529–543. https://doi.org/10.1017/CBO9781139051477.040.
Medrano H, Tomás M, Martorell S, Flexas J, Hernández E, Rosselló J, Pou A, Escalona J-M, Bota J (2015) From leaf to whole-plant water use efficiency (WUE) in complex canopies: limitations of leaf WUE as a selection target. Crop J 3: 220–228. https://doi.org/10.1016/j.cj.2015.04.002.
Meinzer FC, Lachenbruch B, Dawson TE (eds) (2011) Size and age-related changes in tree structure and Function. Springer, Heidelberg. https://doi.org/10.1007/978-94-007-1242-3.
Montague T, Kjelgren R (2006) Use of thermal dissipation probes to estimate water loss of containerized landscape trees. J Environ Hortic 24: 95–104. https://doi.org/10.24266/0738-2898-24.2.95.
Nicolotti G, Gonthier P (eds) (2004) The trees of history. Protection and exploitation of veteran trees. Proc Int Congress Torino, Italy, April 1st–2nd, 2004, Università di Torino, Torino.
Olson ME, Anfodillo T, Rosell JA, Petit G, Crivellaro A, Isnard S, León-Gómez C, Alvarado-Cárdenas LO, Castorena M (2014) Universal hydraulics of the flowering plants: vessel diameter scales with stem length across angiosperm lineages, habits and climates. Ecol Lett 17: 988–997. https://doi.org/10.1111/ele.12302.
Orr AJ, Padovan A, Keiner D, Külheim C, Bromhan L, Bustos-Segura C, FoleyW, Haff T, Hsieh J-F, Morales-Suarez A, Cartwright Reed A, Lanfear R (2020) A phylogenomic approach reveals a low somatic mutation rate in a long-lived plant. Proc R Soc B 287, article id 20192364. https://doi.org/10.1098/rspb.2019.2364.
Pacyniak C (2006) Wiek najstarszych i niektórych pomnikowych dębów w Polsce. [The age of the oldest and some monumental oaks in Poland]. In: Boratyński A (ed) Dęby. [Oaks]. Bogucki Wyd. Naukowe. [Bogucki Scientific Publishing House], Poznań-Kórnik, pp 850–876.
Plomion C, Aury J-M, Amselem J, Leroy T, Murat F, Duplessis S, Faye S, Francillonne N, Labadie K, Le Provost G, Lesur I, Bartholomé J, Faivre-Rampant P, Kohler A,Leplé J-C, Chantret N, Jun C, Diévart A, Alaeitabar T, Barbe V, Belser C, Bergès H, Bodénès C, Bogeat-Triboulot M-B, Bouffaud M-L, Brachi B, Chancerel E, Cohen D, Couloux A, Da Silva C, Dossat C, Ehrenmann F, Gaspin C, Grima-Pettenati J, Guichoux E, Arnaud Hecker A, Herrmann S, Hugueney P, Irène Hummel I, Klopp C, Lalanne C, Lascoux M, Lasserre E, Lemainque A, Desprez-Loustau M-L, Luyten I, Madoui M-A, Mangenot S, Marchal C, Maumus F, Mercier J, Michotey C, Panaud O, Picault N, Rouhier N, Rué O, Rustenholz C, Salin F, Soler M, Tarkka M, Velt A, Zanne AE, Martin F, Wincker P, Quesneville H, Kremer A, Salse J (2018) Oak genome reveals facets of long lifespan. Nature Plants 4: 440–452. https://doi.org/10.1038/s41477-018-0172-3.
Read H (2000) Veteran trees: a guide to good management. Natural England, Peterborough.
Renninger HJ, Carlo NJ, Clark KL, Schäfer KVR (2015) Resource use and efficiency, and stomatal responses to environmental drivers of oak and pine species in an Atlantic coastal plain forest. Front Plant Sci 6, article id 297. https://doi.org/10.3389/fpls.2015.00297.
Roussel M, Le Thiec D, Montpied P, Ningre N, Guehl J-M, Brendel O (2009) Diversity of water use efficiency among Quercus robur genotypes: contribution of related leaf traits. Ann For Sci 66: 408–418. https://doi.org/10.1051/forest/2009010.
Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez J-Y, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A (2012) Fiji: an open-source platform for biological-image analysis. Nat Methods 9: 676–682. https://doi.org/10.1038/nmeth.2019.
Schmid-Siegert E, Namrata Sarkar N, Christian Iseli C, Sandra Calderon S, Gouhier-Darimont C, Chrast J, Cattaneo P, Schütz F, Farinelli L, Pagni M, Schneider M, Voumard J, Jaboyedoff M, Fankhauser C, Hardtke CS, Keller L, Pannell JR, Reymond A, Robinson-Rechavi M, Xenarios I, Reymond P(2017) Low number of fixed somatic mutations in a long-lived oak tree. Nature Plants 3: 926–929. https://doi.org/10.1038/s41477-017-0066-9.
Schweingruber FH (1990) Anatomy of European woods: an atlas for the identification of European trees, shrubs and dwarf shrubs / Anatomie Europäischer Hölzer: ein atlas zur Bestimmung Europäischer Baum-, Strauch- und Zwergstrauchhölzer. Verlag Paul Haupt, Bern.
Stephenson NL, Das AJ, Condit R, Russo SE, Baker PJ, Beckman NG, Coomes DA, Lines ER, Morris WK, Rüger N, Álvarez E, Blundo C, Bunyavejchewin S, Chuyong G, Davies SJ, Duque Á, Ewango CN, Flores O, Franklin JF, Grau HR, Hao Z, Harmon ME, Hubbell SP, Kenfack D, Lin Y, Makana J-R, Malizia A, Malizia LR, Pabst RJ, Pongpattananurak N, Su S-H, Sun IF, Tan S, Thomas D, van Mantgem PJ, Wang X, Wiser SK, Zavala MA (2014) Rate of tree carbon accumulation increases continuously with tree size. Nature 507: 90–93. https://doi.org/10.1038/nature12914.
Tsoumis GT (1991) Science and technology of wood: structure, properties, utilization. Chapman & Hall, New York.
Tulik M (2014) The anatomical traits of trunk wood and their relevance to oak (Quercus robur L.) vitality. Eur J Forest Res 133: 845–855. https://doi.org/10.1007/s10342-014-0801-y.
Turner NC (1988) Measurement of plant water status by the pressure chamber technique. Irrig Sci 9: 289–308. https://doi.org/10.1007/BF00296704.
Vavrčík H, Gryc V (2012) Analysis of the annual ring structure and wood density relations in English oak and sessile oak. Wood Res 57: 573–580.
Wang L, Cui J, Jin B, Zhao J, Xu H, Lu Z, Li W, Li X, Li L, Liang E, Rao X, Wang S, Fu C, Cao F, Dixon RA, Lin J (2020) Multifeature analyses of vascular cambial cells reveal longevity mechanisms in old Ginkgo biloba trees. PNAS 117: 2201–2210. https://doi.org/10.1073/pnas.1916548117.
Wang X, Allison RB (2008) Decay detection in red oak trees using a combination of visual inspection, acoustic testing, and resistance microdrilling. Arboric Urban For 34: 1–4. https://doi.org/10.48044/jauf.2008.001.
Wang X, Allison RB, Wang L, Ross RJ (2007). Acoustic tomography for decay detection in red oak trees. Research Paper FPL-RP-642. U.S. Department of Agriculture, Forest Service, Forest Products Laboratory, Madison, WI. https://doi.org/10.2737/FPL-RP-642.
Zarzyński P, Tomusiak R (2009) Dwanaście najgrubszych dębów szypułkowych (Quercus robur L) Polski. [The twelve largest girthed Common Oak trees (Quercus robur L.) in Poland]. Rocznik Polskiego Towarzystwa Dendrologicznego 57: 117–127.
Zimmermann MH (1983) Xylem structure and the ascent of sap. Springer-Verlag, Berlin, Heidelberg, New York, Tokyo. https://doi.org/10.1007/978-3-662-22627-8.
Total of 48 references.

