Increasing air humidity – a climate trend predicted for northern latitudes – alters the chemical composition of stemwood in silver birch and hybrid aspen
Tullus A., Sellin A., Kupper P., Lutter R., Pärn L., Jasinska A. K., Alber M., Kukk M., Tullus T., Tullus H., Lõhmus K., Sõber A. (2014). Increasing air humidity – a climate trend predicted for northern latitudes – alters the chemical composition of stemwood in silver birch and hybrid aspen. Silva Fennica vol. 48 no. 4 article id 1107. https://doi.org/10.14214/sf.1107
Highlights
- Hybrid aspen and silver birch trees grew more slowly under increased air humidity conditions and had higher concentrations of N and P and a lower K to N ratio in stemwood
- Minor species-specific changes were detected in stemwood concentrations of cellulose and hemicellulose
- Density, calorific value and concentrations of lignin and ash in stemwood were not affected by elevated humidity.
Abstract
We studied the physicochemical properties of stemwood in saplings of silver birch (Betula pendula Roth) and hybrid aspen (Populus tremula L. × P. tremuloides Michx.), grown for four years under artificially elevated relative air humidity (on average by 7%) in field conditions, using the Free Air Humidity Manipulation (FAHM) research facility in Estonia. Altogether 91 sample trees from three experimental plots with manipulated air humidity and from three control plots were cut in the dormant season and sampled for the analysis of cellulose, hemicellulose, acid detergent lignin, macronutrients (N, P, K), ash content, density, and calorific value of wood. The analysed trees grew significantly more slowly under elevated humidity conditions, with a more pronounced effect on aspens. Significantly higher concentrations of N and P were observed in the stemwood of both aspens and birches grown under elevated humidity. This could be the result of a change in the content of living parenchyma cells and/or enhanced retranslocation of nutrients into wood parenchyma. Additionally, humidification resulted in a significantly higher concentration of cellulose and a lower concentration of hemicellulose in aspen stemwood, and in significantly lower concentrations of cellulose and K in birch stemwood. Elevated humidity did not affect lignin concentration, ash content, basic density and calorific value of stemwood. Results from the FAHM experiment suggest that the increasing air humidity accompanying global warming at northern latitudes will affect the growth and functioning of deciduous trees and forests, with obvious consequences also for forest management and industry.
Keywords
climate change;
Betula;
Populus;
macronutrients;
atmospheric humidity;
wood characteristics;
structural carbohydrates
-
Tullus,
Department of Botany, Institute of Ecology and Earth Sciences, Faculty of Science and Technology, University of Tartu, Lai 40, Tartu 51005, Estonia
E-mail
arvo.tullus@ut.ee

- Sellin, Department of Botany, Institute of Ecology and Earth Sciences, Faculty of Science and Technology, University of Tartu, Lai 40, Tartu 51005, Estonia E-mail arne.sellin@ut.ee
- Kupper, Department of Botany, Institute of Ecology and Earth Sciences, Faculty of Science and Technology, University of Tartu, Lai 40, Tartu 51005, Estonia E-mail priit.kupper@ut.ee
- Lutter, Institute of Forestry and Rural Engineering, Estonian University of Life Sciences, Kreutzwaldi 5, Tartu 51014, Estonia E-mail reimo.lutter@emu.ee
- Pärn, Institute of Forestry and Rural Engineering, Estonian University of Life Sciences, Kreutzwaldi 5, Tartu 51014, Estonia E-mail linnar.parn@emu.ee
- Jasinska, Department of Botany, Institute of Ecology and Earth Sciences, Faculty of Science and Technology, University of Tartu, Lai 40, Tartu 51005, Estonia & Institute of Dendrology, Polish Academy of Sciences, Parkowa 5, 62-035 Kórnik, Poland E-mail jasiak9@wp.pl
- Alber, Department of Botany, Institute of Ecology and Earth Sciences, Faculty of Science and Technology, University of Tartu, Lai 40, Tartu 51005, Estonia E-mail meeli.alber@ut.ee
- Kukk, Department of Botany, Institute of Ecology and Earth Sciences, Faculty of Science and Technology, University of Tartu, Lai 40, Tartu 51005, Estonia E-mail maarja.kukk@ut.ee
- Tullus, Institute of Forestry and Rural Engineering, Estonian University of Life Sciences, Kreutzwaldi 5, Tartu 51014, Estonia E-mail tea.tullus@emu.ee
- Tullus, Institute of Forestry and Rural Engineering, Estonian University of Life Sciences, Kreutzwaldi 5, Tartu 51014, Estonia E-mail hardi.tullus@emu.ee
- Lõhmus, Department of Botany, Institute of Ecology and Earth Sciences, Faculty of Science and Technology, University of Tartu, Lai 40, Tartu 51005, Estonia E-mail krista.lohmus@ut.ee
- Sõber, Department of Botany, Institute of Ecology and Earth Sciences, Faculty of Science and Technology, University of Tartu, Lai 40, Tartu 51005, Estonia E-mail anu.sober@ut.ee
Received 3 February 2014 Accepted 19 May 2014 Published 21 November 2014
Views 210647
Available at https://doi.org/10.14214/sf.1107 | Download PDF

1 Introduction
The chemical build-up of wood varies between and within tree species, being controlled by genetic and environmental factors (Fukuda 1996; Plomion et al. 2001; Meerts 2002; Pallardy 2008). It is an essential issue for forestry, as it determines the industrial usability of the given species and nutrient removal with harvest, consequently affecting the biogeochemical balance of the forest ecosystems (Rochon et al. 1998; Helmisaari and Kaarakka 2013). The impact of nutrient removal with harvested biomass is especially considerable in short-rotation bioenergy production systems with fast-growing species (e.g. Salix and Populus spp.), where successive harvests are made in short intervals (Heilman and Norby 1998; Weih 2004; Dickmann 2006).
Knowledge about climate change effects on chemical composition of wood is scanty and mainly restricted to elevated temperature, CO2 and O3. Alterations in these environmental factors have been shown to cause significant changes in the wood chemistry of both Betula and Populus spp. (Kaakinen et al. 2004; Kostiainen et al. 2008; Richet et al. 2011). At the same time, the extent and direction of the change in a particular wood constituent in response to altered climate variables is not always consistent among different tree species (Kaakinen et al. 2004; Kostiainen et al. 2008; Luo and Polle 2009; Richet et al. 2011, 2012). Due to global warming, moister conditions (increasing precipitation and air humidity) are predicted in Northern Europe (IPCC 2013), including the Baltic region (Kont et al. 2003), however the effect of increasing humidity on wood formation has not been studied to date.
Formation of wood (secondary xylem) includes cell division, cell expansion (elongation and radial enlargement), cell wall thickening (involving cellulose, hemicellulose, cell wall proteins, and lignin biosynthesis and deposition) and programmed cell death (Plomion et al. 2001). Usually this process (xylogenesis) takes up to three weeks in secondary xylem cells (Myburg et al. 2013). However, the parenchyma cells located in longitudinal and radial pith rays remain alive and functional for several years before they die (Plomion et al. 2001). After that a distinct inner zone forms in stems, containing only dead cells, known as heartwood. Heartwood formation starts when a tree reaches a certain age or size and in young unsuppressed trees it is relatively small or absent (Sellin 1994, 1996; Taylor et al. 2002). The outer wood zone, where living parenchyma cells are found, is known as sapwood. Sapwood conducts water and minerals from the roots to the leaves, offers mechanical support and acts as an important storage organ for water, nutrients and carbohydrates (Pallardy 2008; Myburg et al. 2013). For example the content of N doubles in young poplar stems between August and November during nutrient retranslocation from senescing leaves to other living tissues, with the main storage organ being ≥1 mm roots, followed by stems, branches and < 1 mm roots (Pregitzer et al. 1990).
Normally 40–50% of wood consists of cellulose (Plomion et al. 2001). Its fundamental structural units are the microfibrils – insoluble cable-like structures that are typically composed of approximately 36 hydrogen-bonded chains containing 500 to 14 000 β-1,4-linked glucose molecules (Somerville 2006). The water-insoluble cellulose microfibrils are associated with mixtures of soluble noncellulosic polysaccharides, the hemicelluloses, which account for about 25% of the dry weight of wood. They generally occur as a heteropolymer such as glucomannan, galactoglucomannan, arabinogalactan, and glucuronoxylan, or as a homopolymer like galactan, arabinan, and β-1,3-glucan (Plomion et al. 2001). Lignin is a complex polymer of aromatic phenolic monomers (mainly hydroxycinnamyl alcohols, coniferyl alcohol and sinapyl alcohol) that usually comprise approximately 20–30% of plant cell walls (Somerville 2006; Myburg et al. 2013). In both herbaceous and woody plants, the concentration of lignin may increase in response to environmental stress (Plomion et al. 2001; Moura et al. 2010). Lignin is predominantly deposited in the secondarily thickened cell walls, providing mechanical strength and protecting cell wall polysaccharides from microbial degradation, thus imparting decay resistance (Somerville 2006; Vanholme et al. 2010). Lignins also provide the rigidity and structural support that allow water transport in the vascular system (Novaes et al. 2010; Richet et al. 2012). At the same time it is one of the most important limiting factors in the conversion of plant biomass to pulp or liquid biofuels, since the removal of lignin from plant biomass is a costly process (Vanholme et al. 2010).
Altogether, chemical composition of wood cells and xylem anatomy determine several industrially significant wood characteristics, such as mechanical strength, wood density, ash content and calorific value (Kauter et al. 2003; Werkelin et al. 2005; Myburg et al. 2013). Calorific value (heating value) of wood is determined by the proportions of its main elemental constituents (carbon, hydrogen and oxygen), with the share of C and H increasing it and the share of O decreasing it. More energy-rich compounds include resins, lipids and lignin, while cellulose and hemicellulose are less energy-rich. However, calorific value of wood does not vary considerably between different species when expressed on a dry weight (not volume) basis (Nurmi 1993). Ash content of wood is generally low compared to other tree compartments e.g. bark and branches (Werkelin et al. 2005).
The current study was conducted in a unique Free Air Humidity Manipulation (FAHM) research facility, which was established from 2006–2007 in Estonia to clarify the effect of elevated air humidity on the functioning of deciduous forest ecosystems (Kupper et al. 2011). Decreased transpiration, macronutrient (N and P) uptake by foliage, and growth rate were observed in the FAHM experiment as short-term responses of trees to elevated air humidity (Kupper et al. 2011; Tullus et al. 2012; Sellin et al. 2013).
The aim of this study was to analyse the possible changes in stemwood chemistry in young silver birch (Betula pendula Roth) and hybrid aspen (Populus tremula L. × P. tremuloides Michx.) trees in response to artificially elevated air humidity during four years in the FAHM experiment. We clarified the effect of elevated humidity on: concentrations of cellulose, hemicellulose and acid-insoluble lignin, concentrations of macronutrients (N, P, K), calorific value, ash content, and basic wood density.
2 Material and methods
2.1 Study area
The study was conducted in the Free Air Humidity Manipulation (FAHM) experimental facility located in South-East Estonia (58°14´N, 27°18´E; http://www.lote.ut.ee/FAHM/in-english). The study area lies in the northern part of the temperate climate zone in the transition zone between maritime and continental climates. During the study period (2008–2012), the mean temperature sum (base temperature 10°C) was 950 degree days and mean annual precipitation was 760 mm. Soil is classified as fertile Endogleyic Planosol (Hansen et al. 2013). The FAHM site is a 2.7 ha fenced area on abandoned agricultural land, where nine circular experimental plots are situated (Fig. 1). Data were collected from the FAHM humidification experiment comprising three control plots (C treatment) and three plots with elevated air humidity (H treatment). The other three plots are open-tops with different experimental purposes. Inside the humidified plots a computer-controlled system elevates relative air humidity by 7%, on average, compared to the ambient, using misting technology (water is vaporized to ~10 μm droplets) and air blowers that homogenize humid air inside the plots; for a detailed technical description of the experimental facility see Kupper et al. (2011) and Tullus et al. (2012). Half of each experimental plot was planted with 1-yr-old silver birch (Betula pendula Roth) seedlings in the spring of 2006 and the other half with monoclonal hybrid aspens (Populus tremula L. × P. tremuloides Michx.) in the autumn of 2006. Humidification treatment was applied every year from 2008 onwards during the growing periods (May–October). Sample trees did not differ in initial height and basal diameter of stem between C and H plots before humidity manipulation was started.
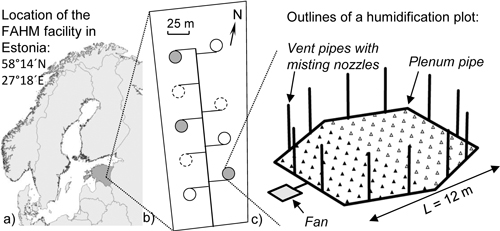
Fig. 1. a) Location of the FAHM facility, b) layout of the experimental area (empty circles are control plots, filled circles are humidification plots, empty circles with dashed outline are open-top plots not included in this study) and c) layout of the humidification plot (black triangles stand for hybrid aspens and empty triangles for silver birches).
2.2 Sample trees and determination of wood properties
From each experimental plot, seven to eight sample trees of both species were randomly selected at the end of the first FAHM study period (silver birches: autumn 2011, hybrid aspens: autumn 2012), when all trees were harvested from the plots. Altogether 43 birch and 48 aspen trees were analysed. Height and diameter at breast height (DBH) of all trees were measured. From each tree, the stem section that had formed and grown during the four years under treatment before harvesting (birches: 2008–2011, aspens: 2009–2012), was identified and two stem disks were cut from this section. Fresh dimensions (diameter over bark in two perpendicular directions at both disk ends, and length) of one disk were recorded before drying at 65°C to constant weight to estimate basic stem density (dry weight per fresh volume approximated to the shape of a truncated cone). Bark, phloem and cambium were removed from the other stem disk before drying, and afterwards it was milled with SM 300 cutting mill (Retsch GmbH, Haan, Germany) for chemical analysis.
In the milled wood samples the concentration of total N ([N], %) was determined by standard Kjeldahl procedure using a Kjeltec Auto 1030 Analyzer (FOSS Tecator AB, Höganäs, Sweden); P ([P], %) was determined spectrophotometrically from Kjeldahl digest using a FIAstar 5000 Analyzer (FOSS Tecator AB). Concentration of K ([K], %) was determined flame photometrically.
The ash content was determined according to standard CEN/TS 14775 “Solid biofuels –Method for the determination of ash content.” For the determination of upper calorimetric heating values (hereafter called ‘calorific value’, kJ g–1), the sub-samples were analysed with the C 5003 calorimeter (IKA-Werke GmbH & Co. KG, Staufen, Germany) using adiabatic measurement mode.
Three 1 g air-dried and ground (< 1mm) wood samples were analysed for the Acid Detergent Fibre (ADF), Neutral Detergent Fibre (NDF), and Acid Detergent Lignin (ADL). For the determination of fibres, the Fibertec I&M Systems (Foss AB, Hillerød, Denmark), which apply methods elaborated by Van Soest (1987), were used. ADF and ADL were analysed in accordance with EN ISO 13906:2008 standard and NDF in accordance with EN ISO 16472:2006 standard. From the first sample, NDF was determined after treatment with a neutral detergent solution (sodium lauryl sulphate and EDTA), the residue consisting of cellulose, hemicellulose and lignin. From the second sample, ADF was determined after the treatment of residues with an acid detergent solution (cetyl trimethylammonium bromide, in sulphur acid solution). The residue contained cellulose and lignin. From the third sample, ADL (hereafter called ‘lignin’) was determined after the initial treatment for ADF measurement, followed by the removal of the cellulose fraction through extraction with 72% H2SO4. A fraction of acid-soluble lignin and cellulose could be lost during this procedure (Van Soest, 1987; Monties, 1989). Cellulose content was calculated as ADF minus ADL, and hemicellulose, as NDF minus ADF. The results for lignin, hemicellulose and cellulose were expressed as a percentage of the dry mass of plant material.
The physicochemical analyses were performed at the Laboratory of Biochemistry and the Laboratory of Wood Properties of the Estonian University of Life Sciences.
2.3 Statistical analyses
The effect of humidification treatment on wood characteristics was analysed separately for birch and aspen data with the following two-way nested ANOVA model:
![]()
where yijk is the studied wood characteristic of the kth sample tree from plot j in treatment i, µ is the overall mean, αi is the effect of treatment (control or humidification), βj(i) is the effect of experimental plot within treatment and εijk is an error term.
Student’s t-test (two-sided, assuming equal variances) was used to compare the average wood and growth characteristics between the two tree species separately within control and humidification plots. In order to express the differences in trait means (x) between the reference (i.e. control, C) and humidified (H) plots, relative changes (Δr) were calculated:

Relative differences (dr) were calculated between the trait means of the two tree species (a, b):

Pearson’s correlation coefficients were used to estimate pairwise relations among growth and wood characteristics. Two outliers were excluded from the wood chemistry data set in order to meet the assumptions for tests. As significant relationships were observed between growth (height, DBH) and wood characteristics, partial correlation coefficients controlling for the effect of DBH were used to characterize interrelations among wood characteristics. Statistical analyses were performed with STATISTICA ver. 7.1 software (StatSoft, Inc. 2005); level of significance α = 0.05 was accepted in all cases.
3 Results
3.1 Growth characteristics of analysed trees
Analysed hybrid aspen and silver birch trees had grown for six years at the FAHM site before harvest and there were no differences in average heights and stem diameters at breast height (DBH) between the two species (Table 1, Fig. 2). At harvest, hybrid aspens from experimental plots with elevated air humidity (H plots) had significantly lower average height (Δr = –9.4%) and DBH (Δr = –16.4%) than the trees from control plots (C plots). Average height of silver birches was also smaller (Δr = –11.1%) in H plots, but the difference in their DBH was not significant (p > 0.05). Growth characteristics were not affected by replication (i.e. experimental plot; Table 1).
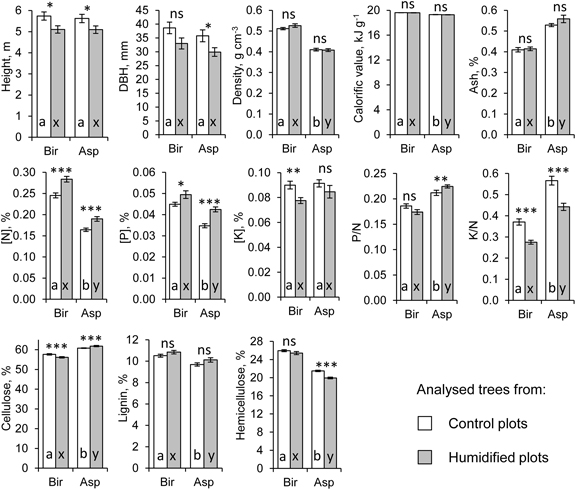
Fig. 2. Average growth and wood characteristics of the analysed silver birch (Bir) and hybrid aspen (Asp) sample trees (n = 91). Error bars show standard error, asterisks show the significance of treatment effect (p < 0.05*, p < 0.01**, p < 0.001***, ns: not significant) based on ANOVA model (Eq. 1), letters on the columns denote differences between tree species separately for control (a, b) and humidified plots (x, y).
| Table 1. Mean growth and wood characteristics of the analysed silver birch and hybrid aspen trees (n = 91) in control (C) and humidified (H) plots, factor effects based on ANOVA model (Eq. 1), superscript letters (a, b) denote significant differences between tree species within treatment according to t-test. | |||||||
| Characteristic | Tree species | Mean ± st. error | Factor effects | ||||
| Treatment | Plot(Treatment) | ||||||
| C plots | H plots | F | p | F | p | ||
| Height, m | Birch | 5.74 ± 0.19a | 5.10 ± 0.18a | 6.6 | 0.015 | 2.5 | 0.056 |
| Aspen | 5.62 ± 0.20a | 5.09 ± 0.18a | 4.3 | 0.044 | 2.3 | 0.077 | |
| DBH, mm | Birch | 38.6 ± 2.1a | 33.0 ± 2.0a | 3.6 | 0.066 | 0.9 | 0.483 |
| Aspen | 35.8 ± 2.1a | 29.9 ± 1.6a | 4.7 | 0.036 | 0.8 | 0.508 | |
| Cellulose, % | Birch | 57.6 ± 0.35a | 56.1 ± 0.34a | 13.2 | <0.001 | 5.6 | <0.001 |
| Aspen | 60.8 ± 0.15b | 61.8 ± 0.32b | 12.9 | <0.001 | 6.9 | <0.001 | |
| Lignin, % | Birch | 10.5 ± 0.14a | 10.8 ± 0.17a | 2.2 | 0.142 | 0.9 | 0.480 |
| Aspen | 9.7 ± 0.15b | 10.1 ± 0.21b | 3.0 | 0.089 | 2.7 | 0.045 | |
| Hemicellulose, % | Birch | 25.9 ± 0.21a | 25.4 ± 0.33a | 2.1 | 0.154 | 2.4 | 0.065 |
| Aspen | 21.5 ± 0.16b | 19.9 ± 0.19b | 42.3 | <0.001 | 1.8 | 0.150 | |
| [N], % | Birch | 0.25 ± 0.006a | 0.28 ± 0.007a | 20.9 | <0.001 | 2.6 | 0.052 |
| Aspen | 0.16 ± 0.004b | 0.19 ± 0.006b | 17.0 | <0.001 | 5.2 | 0.002 | |
| [P], % | Birch | 0.045 ± 0.001a | 0.049 ± 0.002a | 5.5 | 0.024 | 3.0 | 0.030 |
| Aspen | 0.035 ± 0.001b | 0.042 ± 0.001b | 32.1 | 0.000 | 3.7 | 0.012 | |
| [K], % | Birch | 0.090 ± 0.003a | 0.078 ± 0.002a | 10.4 | 0.003 | 1.5 | 0.215 |
| Aspen | 0.091 ± 0.003a | 0.085 ± 0.005a | 1.2 | 0.290 | 0.6 | 0.668 | |
| P/N | Birch | 0.19 ± 0.004a | 0.17 ± 0.005a | 3.3 | 0.078 | 1.5 | 0.227 |
| Aspen | 0.21 ± 0.005b | 0.22 ± 0.003b | 8.6 | 0.006 | 6.0 | <0.001 | |
| K/N | Birch | 0.37 ± 0.015a | 0.28 ± 0.009a | 30.4 | <0.001 | 1.8 | 0.144 |
| Aspen | 0.57 ± 0.021b | 0.44 ± 0.017b | 23.6 | <0.001 | 3.6 | 0.014 | |
| Ash, % | Birch | 0.41 ± 0.010a | 0.41 ± 0.009a | 0.1 | 0.749 | 2.0 | 0.118 |
| Aspen | 0.53 ± 0.008b | 0.56 ± 0.018b | 2.4 | 0.133 | 1.9 | 0.126 | |
| Calorific value, kJ g–1 | Birch | 19.62 ± 0.016a | 19.60 ± 0.018a | 1.0 | 0.309 | 1.0 | 0.275 |
| Aspen | 19.30 ± 0.026b | 19.29 ± 0.024b | 0.0 | 0.747 | 12.0 | <0.001 | |
| Density, g cm–3 | Birch | 0.51 ± 0.005a | 0.53 ± 0.009a | 2.0 | 0.165 | 0.4 | 0.779 |
| Aspen | 0.41 ± 0.007b | 0.41 ± 0.007b | 0.1 | 0.768 | 3.0 | 0.031 | |
3.2 Macronutrient (NPK) contents in stemwood
Concentrations of N ([N]) and P ([P]) in stemwood harvested in the dormant season differed significantly between the treatments and tree species. Both [N] (dr = 33%) and [P] (dr = 14.1 to 22.6%) were higher in the wood of birch compared to aspen (Table 1, Fig. 2). In H plots, [N] and [P] were significantly higher in both birch (Δ[N]r = 15.7%, Δ[P]r = 10.1%) and aspen wood (Δ[N]r = 15.6%, Δ[P]r = 22.2%) compared to C plots, while the concentration of K ([K]) was lower in aspen wood in H plots (Δr = –13.8%, Table 1). K to N ratio was substantially lower in both birch (Δr = –25.8%) and aspen wood (Δr = –21.9%) in H plots, whereas P to N ratio in aspen wood was higher in H plots (Δr = 5.9%). Both [N] and [P] were negatively correlated with height and DBH of aspen stems, while the respective relations were not significant for birch (Table 2). [K] was negatively correlated with stem dimensions only in aspens from H plots. Macronutrients varied to a certain extent also among plots within treatment, being more pronounced in aspen (Table 1).
| Table 2. Pairwise Pearson’s correlation coefficients among wood and tree growth characteristics in control (C) and humidified (H) plots, bold indicates significant (p < 0.05) relations. | |||||
| Wood characteristic | Tree species | Height | DBH | ||
| C | H | C | H | ||
| Cellulose | Aspen | –0.41 | 0.45 | –0.44 | 0.29 |
| Birch | –0.03 | 0.03 | –0.41 | –0.23 | |
| Lignin | Aspen | –0.61 | –0.47 | –0.50 | –0.45 |
| Birch | 0.38 | –0.14 | 0.41 | –0.20 | |
| Hemicellulose | Aspen | 0.75 | 0.01 | 0.68 | 0.20 |
| Birch | 0.17 | 0.35 | 0.35 | 0.63 | |
| [N] | Aspen | –0.65 | –0.54 | –0.53 | –0.44 |
| Birch | –0.35 | –0.63 | –0.24 | –0.28 | |
| [P] | Aspen | –0.60 | –0.58 | –0.39 | –0.48 |
| Birch | –0.01 | –0.57 | –0.29 | –0.41 | |
| [K] | Aspen | –0.40 | –0.51 | –0.35 | –0.45 |
| Birch | 0.21 | –0.02 | –0.09 | 0.06 | |
| Calorific value | Aspen | 0.12 | –0.26 | 0.26 | –0.23 |
| Birch | 0.40 | –0.17 | 0.64 | –0.26 | |
| Density | Aspen | –0.83 | –0.41 | –0.76 | –0.51 |
| Birch | 0.10 | 0.06 | –0.13 | 0.31 | |
| Ash content | Aspen | –0.44 | –0.53 | –0.41 | –0.38 |
| Birch | 0.15 | –0.41 | –0.09 | –0.22 | |
3.3 Cellulose, hemicellulose and lignin in stemwood
Under elevated humidity, aspen stemwood had a significantly higher (Δr = 1.7%) and birch stemwood a lower (Δr = –2.6%) concentration of cellulose (Table 1, Fig. 2). Concentration of cellulose was significantly higher in aspen wood (dr = 5.2 to 9.2%), while birch wood had higher concentrations of lignin (dr = 6.7 to 7.9%) and hemicellulose (dr = 17.2 to 21.6%). Concentration of hemicellulose in aspen wood was lower in H plots (Δr = –7.3%). Content of structural carbohydrates and lignin was not correlated with birch stem size, except a significant positive relation between height and hemicellulose concentration in H plots (Table 2). In aspen, concentration of lignin was always negatively correlated with stem size, while concentration of hemicellulose was positively related to tree size in C plots. Concentration of cellulose had negative correlation coefficients with [N], lignin and hemicellulose, although these relations were not always significant (Table 3). Concentration of cellulose varied significantly also among the plots (Table 1).
3.4 Density, ash content and calorific value of wood
We did not detect any significant effect from elevated air humidity on density, ash content or calorific value of wood (Table 1, Fig. 2). Wood density was significantly higher in birch compared to aspen (dr = 19.8 to 22.4%). Aspen wood density was negatively correlated with stem size, and the respective correlations were stronger in C plots (Table 2). As a rule, wood density was not correlated with other wood characteristics (Table 3). Ash content was higher (dr = 22.5 to 25.8%) in aspen wood compared to birch (Table 1). It was positively correlated with concentrations of macronutrients, and these relations were usually stronger in H plots (Table 3). Calorific value was slightly higher (dr = 1.6%) in birch wood compared to aspen (Table 1). Usually calorific value was not correlated with stem size (Table 2); in birch wood it was positively associated with concentration of lignin (Table 3). Calorific value and density of aspen wood showed some variability also among the experimental plots (Table 1).
| Table 3. Pairwise partial correlations (controlling for the effect of DBH) among wood characteristics in control (C) and humidified (H) plots, bold indicates significant (p < 0.05) relations. View in new window/tab. |
4 Discussion
Our results suggest that if future climate predictions for Northern Europe (Kont et al. 2003; IPCC 2013) hold true, then increasing air humidity will be an additional climate variable that will alter wood characteristics of economically important deciduous tree species in addition to the already known effects of increasing CO2 and O3 (Kaakinen et al. 2004; Kostiainen et al. 2008; Richet et al. 2011).
Elevated humidity had a negative effect on height and DBH of 6-yr-old aspen stems. This is in agreement with our earlier study with younger (4-yr-old) aspens in the FAHM experiment (Tullus et al. 2012). However in birches, the negative effect was observed only in height growth, and DBH did not differ significantly; at a younger age both characteristics had been significantly suppressed (Sellin et al. 2013). This confirms the better acclimation of birches to elevated humidity as also observed in alterations in their root morphological characteristics (Parts et al. 2013) and delayed leaf fall (Godbold et al. 2014). Possible causes of reduced growth have been discussed as part of other studies. Tree leaves were smaller in H plots, contained less N and P, and were photosynthetically less active, which was partly attributed to the decreased transpirational flux from roots to leaves and transpiration-driven mass flow of nutrients in the soil (Tullus et al. 2012; Sellin et al. 2013). However, reduced photosynthetic capacity under elevated air humidity was recorded only in rainy summers (Niglas et al. 2014). Lower atmospheric evaporative demand in H plots resulted in higher soil water potential, this change being especially distinct in dry summers (Hansen et al. 2013).
We observed considerable differences in macronutrient content of stemwood between trees grown under elevated and ambient humidity conditions. Among the above-ground parts of a tree, stemwood has lower concentrations of nutrients compared to leaves and bark, however total nutrient content accumulated in wood is still considerable due to its large biomass. For example in the aboveground tree compartments (stemwood, stembark, branches) of 7-yr-old winter-cut hybrid aspens, ca 20% of N and 26% of P is located in stemwood (Tullus et al. 2009). Considering that we observed a 1.16 times higher concentration of N and a 1.22 times higher concentration of P in the stemwood of hybrid aspen under elevated humidity, this could mean ca 3–6% higher removal of N and P with potential whole-tree harvest in winter (e.g. as energy wood). Nutrient content varies in wood, being generally higher in sapwood than in heartwood, although this differs among elements and species (Meerts 2002). Our study comprised 6-yr-old trees, in which heartwood formation has not yet started and the whole woody part of the stem comprised sapwood. Macronutrients in sapwood can be found in different forms in living parenchyma cells as enzymes and structural and storage proteins. The storage proteins are formed during leaf senescence as a result of nutrient retranslocation from leaves to the bark parenchyma and the xylem ray cells of the main stem, branches and roots of the tree (Cooke and Weih 2005). Macronutrient content in aspen and birch leaves was generally smaller in H plots during the first study years (Tullus et al. 2012; Sellin et al. 2013), however it depended on overall weather conditions, and in some years the opposite outcome has also been detected (Tullus et al. 2012; K. Lõhmus, unpubl. data). Thus, enhanced retranslocation of nutrients in H plots from leaves into wood parenchyma cannot be ruled out and this requires further investigation. This is consistent with the longer leaf retention of birches in H plots (Godbold et al. 2014), which could enhance N resorption (Marty et al. 2009). Although on average only 10% of cells in sapwood are alive (Pallardy 2008), it has recently been demonstrated that the amount of ray parenchyma in annual rings can be associated with climate variables such as temperature and precipitation during certain periods within the growing season (Olano et al. 2013). The preliminary analysis of 24 cross-sectional hybrid aspen wood images gave evidence of a 5% greater proportion of pith rays in trees grown in H plots as compared to the control, although the change was statistically insignificant (A.K. Jasińska, unpubl. data). Maintenance respiration of living parenchyma cells in sapwood correlates with sapwood amount, and wood nutrient (N, P) concentration can be regarded as an indirect measure of that (Maier et al. 1998; Penninckx et al. 1999). However, in some studies, such a relation has not proved significant (Lavigne and Ryan 1997). Nevertheless, if our interpretation holds true, then wood maintenance respiration costs should be higher in humidified trees, which could alternatively explain the lower growth rate of stems.
Lower [K] and especially lower K/N ratio in H trees could reflect lower need for osmotic constituents (osmolytes) to maintain the turgor of living cells (Wang et al. 2013) in H plots, where transpirational flux was generally lower in rainy summers, and soil water availability was not limiting in control plots (Kupper et al. 2011; Tullus et al. 2012). Smaller transpiration of the trees was caused by a ca 20% reduction in the water vapour pressure difference between the leaf interior and the surrounding atmosphere, a primary driving force of transpiration (Kupper et al. 2011). As K+ concentration in xylem sap was slightly lower in H treatment (A. Sellin unpubl. data), the uptake of K by roots was probably smaller at lower sap flux densities. Accordingly, higher [K] in trees from control plots could be necessary for upregulation of osmotic pressure and turgor of living xylem cells, but also for xylem hydraulic conductivity to support higher water fluxes through the stem (Zwieniecki et al. 2001; Aasamaa and Sõber 2010; Sellin et al. 2010; Oddo et al. 2011; Aasamaa et al. 2014). The observed relatively high cellulose and low lignin concentrations in the wood of young hybrid aspens and silver birches can be explained by the method used for estimation of lignin. As we determined acid detergent lignin (ADL), the acid-soluble fraction of lignin was not estimated (Van Soest 1987; Monties 1989) and the total lignin content might be slightly underestimated. It has been shown that ADL gives systematically lower estimates of lignin compared to Klason lignin method (Jung et al. 1999). This difference is especially pronounced when lignin has high syringyl:guaiacyl ratio (like in aspen wood) and it is argued that the syringyl-rich proportions of the lignin are more susceptible to acid detergent solubilization (Fukushima and Hatfield 2004). Consequently, we could have overestimated cellulose concentration, which was calculated as acid detergent fibre minus ADL. The analysis of wood images did not reveal the notable occurrence of tension wood with typical thick wood cells due to cellulose-rich gelatinous layer and we may rule out tension wood as the reason for high cellulose concentration.
In H plots, birch wood had significantly lower, and aspen wood, higher cellulose concentration compared to the control, however, in relative terms, the differences were very small, not exceeding 2.6%. Thus, there appear also to be species-specific differences in acclimation of wood formation and deposition of structural carbohydrates and lignin in wood cell walls. Lignin concentration showed a similar although statistically insignificant trend in both species, being higher in H plots, which could be a result of more stressful conditions (Plomion et al. 2001; Moura et al. 2010). In aspen, lignin concentration was clearly negatively related to tree size, which is a commonly observed association in fast-growing trees such as Eucalyptus and Populus spp. (Novaes et al. 2010). Hemicellulose concentration in aspen wood was positively correlated with growth rate, which is in accordance with its significantly lower average content in H plots.
We did not detect any humidity effect on wood density, the latter being linked rather with anatomical properties than the chemical composition of wood. Our study relied on 6-yr-old sample trees, the analysed wood of which had formed during the last four years under humidity manipulation. Previous results with birches grown for two years under elevated humidity indicated significantly lower wood density in H plots, which was explained by possible alterations in xylem anatomy (Sellin et al. 2013). Differences have also been observed between short- and long-term responses of wood properties of Betula and Populus spp. to altered CO2 and O3 concentrations (Kostiainen et al. 2008).
Ash content was generally low in wood (0.4–0.5%), being slightly higher in aspen than in birch wood, which is in agreement with previous studies with these species (Werkelin et al. 2005; Tullus et al. 2009). Mean calorific values of birch and aspen wood (19.3–19.6 kJ g–1) were within the range of 18.9–19.7 kJ g–1 reported for these species in other studies (Lõhmus et al. 2000; Tullus et al. 2009). Similarly to the effect of humidity, it has been observed that elevated CO2 alters the chemical composition of poplar wood but not its calorific value (Luo and Polle 2009).
Besides the humidification treatment, the significant plot (i.e. replication) effect was detected on many characteristics, especially in aspen trees (Table 1). Apparently, this reflects certain heterogeneity in microrelief and physicochemical soil properties within the FAHM site (Tullus et al. 2012a; Hansen et al. 2013), which however did not diminish the main effect of the humidification treatment. Lower variability in wood characteristics among birch subplots was probably due to better adaptability of birch owing to higher genetic diversity of seed-originated trees compared to monoclonal aspens.
5 Conclusions
Elevated air humidity caused significant changes in chemical properties of wood in young silver birch and hybrid aspen trees. The most conspicuous effect, altered macronutrient content in wood of dormant-season-harvested stems, was detected in both species, with minor changes in cellulose and hemicellulose. Elevated humidity also suppressed the growth rate of trees, with hybrid aspen being more strongly affected in this respect. No significant changes were found in lignin content, wood density, calorific value and ash content. Altogether the observed shifts in wood chemical and physical properties have been driven by direct (via changes in atmospheric evaporative demand) and/or indirect (via changes in soil water potential) effects of air humidity.
Although we detected some significant changes in industrially significant wood characteristics (cellulose and hemicellulose contents) in response to elevated humidity, these changes were relatively small. As wood density was not affected, the reduced quantity of above-ground biomass (due to decreased growth rate) and its higher macronutrient content in the dormant season can be regarded as most important consequences for forestry. In short-rotation forestry using these species, one could expect an increase in nutrient removal per unit of winter-harvested biomass under future climate conditions in Northern Europe. Further studies are required to verify the role of increased parenchyma proportion of wood tissue versus enhanced nutrient retranslocation into wood as causes of higher nutrient content in stemwood.
The observed growth responses of trees during the first 5-year study period of the FAHM experiment suggest that the expected climate-change-induced increase in the growth rate of trees at northern latitudes (boreal areas) due to the earlier start of the growing season in spring or higher carbon assimilation rate could be smaller than expected if temperature rise is accompanied by a rise in atmospheric humidity, but also that acclimation with higher humidity varies among species.
Acknowledgements
This study was supported by the Ministry of Education and Science of Estonia (grants SF0180025s12 and IUT21-4), by the Estonian Science Foundation (grants Nos. 8333 and 9186) and by the EU through the European Social Fund (Mobilitas postdoctoral grants MJD 257 and MJD 398) and the European Regional Development Fund [project No. 3.2.0802.11-0043 (BioAtmos) and Centre of Excellence ENVIRON]. We acknowledge Olaf Räim and Ingmar Tulva for their help in field work and sample preparation and Ilmar Part for English proofreading.
The article is based on a presentation given at the Workshop on “Climate Change and Forestry in Northern Europe” held in Uppsala, Sweden, 11–12 November, 2013. The workshop was organized by Future Forests at the Swedish University of Agricultural Sciences (SLU), EFI-Nord, and the Finnish Forest Research Institute (Metla).
References
Aasamaa K., Sõber A. (2010). Sensitivity of stem and petiole hydraulic conductance of deciduous trees to xylem sap ion concentration. Biologia Plantarum 54(2): 299–307. http://dx.doi.org/10.1007/s10535-010-0052-9.
Aasamaa K., Kõivik K., Kupper P., Sõber A. (2014). Growth environment determines light sensitivity of shoot hydraulic conductance. Ecological Research 29: 143–151. http://dx.doi.org/10.1007/s11284-013-1104-3.
Cooke J.E.K., Weih M. (2005). Nitrogen storage and seasonal nitrogen cycling in Populus: bridging molecular physiology and ecophysiology. New Phytologist 167: 19–30. http://dx.doi.org/10.1111/j.1469-8137.2005.01451.x.
Dickmann D.I. (2006). Silviculture and biology of short-rotation woody crops in temperate regions: Then and now. Biomass and Bioenergy 30: 696–705. http://dx.doi.org/10.1016/j.biombioe.2005.02.008.
Fukuda H. (1996). Xylogenesis: initiation, progression, and cell death. Annual Review of Plant Physiology and Plant Molecular Biology 47: 299–325. http://dx.doi.org/10.1146/annurev.arplant.47.1.299.
Fukushima R.S., Hatfield R.D. (2004). Comparison of the acetyl bromide spectrophotometric method with other analytical lignin methods for determining lignin concentration in forage samples. Journal of Agricultural and Food Chemistry 52: 3713–3720. http://dx.doi.org/10.1021/jf035497l.
Godbold D., Tullus A., Kupper P., Sõber J., Ostonen I., Godbold J.A., Lukac M., Ahmed I.U., Smith A.R. (2014). Elevated atmospheric CO2 and humidity delay leaf fall in Betula pendula, but not in Alnus glutinosa or Populus tremula × tremuloides. Annals of Forest Science. [In press]. http://dx.doi.org/10.1007/s13595-014-0382-4.
Hansen R., Mander Ü., Soosaar K., Maddison M., Lõhmus K., Kupper P., Kanal A., Sõber J. (2013). Greenhouse gas fluxes in an open air humidity manipulation experiment. Landscape Ecology 28(4): 637–649. http://dx.doi.org/10.1007/s10980-012-9775-7.
Heilman P., Norby R. (1998). Nutrient cycling and fertility management in temperate short rotation forest systems. Biomass and Bioenergy 14(4): 361–70. http://dx.doi.org/10.1016/S0961-9534(97)10072-1.
Helmisaari, H-S., Kaarakka L. (2013). Nutrient management for sustainable production of energy biomass in boreal forests. In: Kellomäki S., Kilpeläinen A., Alam A. (eds.). Forest bioenergy production: management, carbon sequestration and adaptation. Springer, New York. p. 81–94. http://dx.doi.org/10.1007/978-1-4614-8391-5_5.
IPCC. (2013). Climate change 2013: the physical science basis. Cambridge University Press, Cambridge.
Jung H.-J.G., Varel V.H., Weimer P.J., Ralph J. (1999). Accuracy of klason lignin and acid detergent lignin methods as assessed by bomb calorimetry. Journal of Agricultural and Food Chemistry 47: 2005–2008. http://dx.doi.org/10.1021/jf981250q.
Kaakinen S., Kostiainen K., Ek F., Saranpaa P., Kubiske M.E., Sõber J., Karnosky D.F., Vapaavuori E. (2004). Stem wood properties of Populus tremuloides, Betula papyrifera and Acer saccharum saplings after 3 years of treatments to elevated carbon dioxide and ozone. Global Change Biology 10: 1513–1525. http://dx.doi.org/10.1111/j.1365-2486.2004.00814.x.
Kauter D., Lewandowski I., Claupein W. (2003). Quantity and quality of harvestable biomass from Populus short rotation coppice for solid fuel use—a review of the physiological basis and management influences. Biomass and Bioenergy 24: 411–27. http://dx.doi.org/10.1016/S0961-9534(02)00177-0.
Kont A., Jaagus J., Aunap R. (2003). Climate change scenarios and the effect of sea-level rise for Estonia. Global and Planetary Change 36: 1–15. http://dx.doi.org/10.1016/S0921-8181(02)00149-2.
Kostiainen K., Kaakinen S., Warsta E., Kubiske M.E., Nelson N.D., Sõber J., Karnosky D.F., Saranpaa P., Vapaavuori E. (2008). Wood properties of trembling aspen and paper birch after 5 years of exposure to elevated concentrations of CO2 and O3. Tree Physiology 28: 805–813. http://dx.doi.org/10.1093/treephys/28.5.805.
Kupper P., Sõber J., Sellin A., Lõhmus K., Tullus A., Räim O., Lubenets K., Tulva I., Uri V., Zobel M., Kull O., Sõber A. (2011). An experimental facility for Free Air Humidity Manipulation (FAHM) can alter water flux through deciduous tree canopy. Environmental and Experimental Botany 72 (3): 432–438. http://dx.doi.org/10.1016/j.envexpbot.2010.09.003.
Lavigne M.B., Ryan M.G. (1997). Growth and maintenance respiration rates of aspen, black spruce and jack pine stems at northern and southern BOREAS sites. Tree Physiology 17: 543–551. http://dx.doi.org/10.1093/treephys/17.8-9.543.
Lõhmus K., Ivask M., Tamm Ü., Vares A., Tamm U. (2000). The caloric value of stem of silver birch (Betula pendula Roth.), downy birch (Betula pubescens Ehrh.), black alder (Alnus glutinosa (L.) Gaertn.) and aspen (Populus tremula L.) in Estonia. Forestry Studies | Metsanduslikud Uurimused 32: 113–120.
Luo Z.B., Polle A. (2009). Wood composition and energy content in a poplar short rotation plantation on fertilized agricultural land in a future CO2 atmosphere. Global Change Biology 15: 38–47. http://dx.doi.org/10.1111/j.1365-2486.2008.01768.x.
Maier C.A., Zarnoch S.J., Dougherty P.M. (1998). Effects of temperature and tissue nitrogen on dormant season stem and branch maintenance respiration in a young loblolly pine (Pinus taeda) plantation. Tree Physiology 18: 11–20. http://dx.doi.org/10.1093/treephys/18.1.11.
Marty C., Lamaze T., Pornon A. (2009). Endogenous sink-source interactions and soil nitrogen regulate leaf life-span in an evergreen shrub. New Phytologist 183: 1114–1123. http://dx.doi.org/10.1111/j.1469-8137.2009.02893.x.
Meerts P. (2002). Mineral nutrient concentrations in sapwood and heartwood: a literature review. Annals of Forest Science 59: 713–722. http://dx.doi.org/10.1051/forest:2002059.
Monties B. (1989). Lignins. In: Dey P.M., Harborne J.B. (eds.). Methods in plant biochemistry. Plant phenolics. Vol. 1. Academic Press, London. p. 113–157.
Moura J.C.M.S., Bonine C.A.V., Viana J.O.F., Dornelas M.C., Mazzafera P. (2010). Abiotic and biotic stresses and changes in the lignin content and composition in plants. Journal of Integrative Plant Biology 52(4): 360–376. http://dx.doi.org/10.1111/j.1744-7909.2010.00892.x.
Myburg A.A., Lev-Yadun S., Sederoff R.R. (2013). Xylem structure and function. eLS. John Wiley & Sons, Ltd, Chichester. http://dx.doi.org/10.1002/9780470015902.a0001302.pub2.
Niglas A., Kupper P., Tullus A., Sellin A. (2014). Responses of sap flow, leaf gas exchange and growth of hybrid aspen to elevated atmospheric humidity under field conditions. AoB PLANTS 6: plu021. http://dx.doi.org/10.1093/aobpla/plu021.
Novaes E., Kirst M., Chiang V., Winter-Sederoff H., Sederoff R. (2010). Lignin and biomass: a negative correlation for wood formation and lignin content in trees. Plant Physiology 154: 555–561. http://dx.doi.org/10.1104/pp.110.161281.
Nurmi J. (1993). Heating values of the above ground biomass of small-sized trees. Acta Forestalia Fennica 236. 30 p.
Oddo E., Inzerillo S., La Bella F., Grisafi F., Salleo S., Nardini A. (2011). Short-term effects of potassium fertilization on the hydraulic conductance of Laurus nobilis L. Tree Physiology 31: 131–138. http://dx.doi.org/10.1093/treephys/tpq115.
Olano J.M., Arzac A., García-Cervigón A.I., von Arx G., Rozas V. (2013). New star on the stage: amount of ray parenchyma in tree rings shows a link to climate. New Phytologist 198: 486–495. http://dx.doi.org/10.1111/nph.12113.
Pallardy S.G. (2008). Physiology of woody plants. 3rd ed. Academic Press, USA. 454 p.
Parts K., Tedersoo L., Lõhmus K., Kupper P., Rosenvald K., Sõber A., Ostonen I. (2013). Increased air humidity and understory composition shape short root traits and the colonizing ectomycorrhizal fungal community in silver birch stand. Forest Ecology and Management 310: 720–728. http://dx.doi.org/10.1016/j.foreco.2013.09.017.
Penninckx V., Meerts P., Herbauts J., Gruber W. (1999). Ring width and element concentrations in beech (Fagus sylvatica L.) from a periurban forest in central Belgium. Forest Ecology and Management 113: 23–33. http://dx.doi.org/10.1016/S0378-1127(98)00412-5.
Plomion C., Leprovost G., Stokes A. (2001). Wood formation in trees. Plant Physiology 127: 1513–1523. http://dx.doi.org/10.1104/pp.010816.
Pregitzer K.S., Dickmann D.I., Hendrick R., Nguyen P.V. (1990). Whole-tree carbon and nitrogen partitioning in young hybrid poplars. Tree Physiology 7: 79–93. http://dx.doi.org/10.1093/treephys/7.1-2-3-4.79.
Richet N., Afif D., Huber F., Pollet B., Banvoy J., El Zein R., Lapierre C., Dizengremel P., Perré P., Cabané M. (2011). Cellulose and lignin biosynthesis is altered by ozone in wood of hybrid poplar (Populus tremula × alba). Journal of Experimental Botany 62: 3575–3586. http://dx.doi.org/10.1093/jxb/err047.
Richet N., Afif D., Tozo K., Pollet B., Maillard P., Huber F., Priault P.,Banvoy J., Gross P., Dizengremel P., Lapierre C., Perré P., Cabané M. (2012). Elevated CO2 and/or ozone modify lignification in the wood of poplars (Populus tremula × alba). Journal of Experimental Botany 63 (11): 4291–4301. http://dx.doi.org/10.1093/jxb/ers118.
Rochon P., Paré D., Messier C. (1998). Development of an improved model estimating the nutrient content of the bole for four boreal tree species. Canadian Journal of Forest Research 28: 37–43. http://dx.doi.org/10.1139/x97-176.
Sellin A. (1994). Sapwood heartwood proportion related to tree diameter, age, and growth-rate in picea-abies. Canadian Journal of Forest Research-Revue Canadienne de Recherche Forestiere 24(5): 1022–1028. http://dx.doi.org/10.1139/x94-133.
Sellin A. (1996). Sapwood amount in Picea abies (L.) Karst. determined by tree age and radial growth rate. Holzforschung 50: 291–296. http://dx.doi.org/10.1515/hfsg.1996.50.4.291.
Sellin,A., Õunapuu E., Karusion A. (2010). Experimental evidence supporting the concept of light-mediated modulation of stem hydraulic conductance. Tree Physiology 30: 1528–1535. http://dx.doi.org/10.1093/treephys/tpq091.
Sellin A., Tullus A., Niglas A., Õunapuu E., Karusion A., Lõhmus K. (2013). Humidity-driven changes in growth rate, photosynthetic capacity, hydraulic properties and other functional traits in silver birch (Betula pendula). Ecological Research 28: 523–535. http://dx.doi.org/10.1007/s11284-013-1041-1.
Somerville C. (2006). Cellulose synthesis in higher plants. Annual Review of Cell and Developmental Biology 22: 53–78. http://dx.doi.org/10.1146/annurev.cellbio.22.022206.160206.
StatSoft, Inc. (2005). STATISTICA (data analysis software system), version 7.1. http://www.statsoft.com.
Taylor A.M., Gartner B.L., Morrell J.J. (2002). Heartwood formation and natural durability – a review. Wood and Fibre Science 34(4): 587–611.
Tullus A., Tullus H., Soo T., Pärn L. (2009). Above-ground biomass characteristics of young hybrid aspen (Populus tremula L. × P. tremuloides Michx.) plantations on former agricultural land in Estonia. Biomass and Bioenergy 33: 1617–1625. http://dx.doi.org/10.1016/j.biombioe.2009.08.001.
Tullus A., Kupper P., Sellin A., Parts L., Sõber J., Tullus T., Lõhmus K., Sõber A., Tullus H. (2012). Climate change at northern latitudes: rising atmospheric humidity decreases transpiration, N-uptake and growth rate of hybrid aspen. PLoS ONE 7(8):e42648. http://dx.doi.org/10.1371/journal.pone.0042648.
Van Soest P.J. (1987). Nutritional ecology of the ruminant. Ruminant metabolism, nutritional strategy, the cellulytic fermentation and the chemistry of forages and plant fibers. Cornell University Press, Ithaca, London.
Vanholme R., Demedts B., Morreel K., Ralph J., Boerjan W. (2010). Lignin biosynthesis and structure. Plant Physiology 153: 895–905. http://dx.doi.org/10.1104/pp.110.155119.
Wang M., Zheng Q., Shen Q., Guo S. (2013). The critical role of potassium in plant stress response. International Journal of Molecular Sciences 14: 7370–7390. http://dx.doi.org/10.3390/ijms14047370.
Weih M. (2004). Intensive short rotation forestry in boreal climates: present and future perspectives. Canadian Journal of Forest Research 34: 1369–1378. http://dx.doi.org/10.1139/x04-090.
Werkelin J., Skrifvars B.-J., Hupa M. (2005). Ash-forming elements in four Scandinavian wood species. Part 1: Summer harvest. Biomass and Bioenergy 29: 451–66. http://dx.doi.org/10.1016/j.biombioe.2005.06.005.
Zwieniecki M.A., Melcher P.J., Holbrook N.M. (2001). Hydrogel control of xylem hydraulic resistance in plants. Science 291: 1059. http://dx.doi.org/10.1126/science.1057175.
Total of 54 references

