Species diversity, biomass accumulation and carbon sequestration in the understorey of post-agricultural Scots pine forests
Woziwoda B., Parzych A., Kopeć D. (2014). Species diversity, biomass accumulation and carbon sequestration in the understorey of post-agricultural Scots pine forests. Silva Fennica vol. 48 no. 4 article id 1119. https://doi.org/10.14214/sf.1119
Highlights
- Understorey plant species diversity significantly increases with the age of a Scots pine stand
- Biomass of mosses decreases by a quarter, while biomass of herbs increases several times
- Total understorey’s carbon stock increases over three times. The highest amount of carbon is accumulated in understorey species like Vaccinium myrtillus and Dicranum polysetum
- The growing proportion of vascular plants in the understorey biomass results in an increase in the understorey C/N ratio.
Abstract
The purpose of this study was to examine how the age of a stand of post-agricultural Scots pine forests affects the species composition, biomass and the carbon stock of the forest understorey. The community structure and species composition were studied in 75 plots (100 m2 in size), the amount of biomass, organic carbon and total nitrogen were analysed in 75 subplots (1/16 m2 in size). The plots were located in 21 plantations with the stand age of 41–60, 61–80 and over 80-years. Results show that the understorey species diversity increased with the increasing age of Scots pine stands, and the structure and species composition of secondary forests (although managed for timber production) became similar to the fresh pine forest of the European temperate region (Leucobryo-Pinetum community). Despite the increasing species diversity, however, only six understorey vascular and moss species played an important role in the biomass accumulation and C sequestration. Due to the differences in the dominant species composition, the total amount of understorey biomass significantly differed among the forest stands. The mean moss biomass ranged from 3046 kg ha–1 in 41–60-year-old stands, trough 2686 kg ha–1 in 61–80-year-old stands to 2273 kg ha–1 in over 80-year-old stands, and the mean understorey vascular plant biomass amounted to 2 kg ha–1, 1924 kg ha–1 and 3508 kg ha–1, respectively. The concentration of organic C varied considerably between species; it was the highest in Vaccinium myrtillus (50.6%) and in Dicranum polysetum (49.5%). The total mass of C was nearly 800 kg ha–1 in the youngest forests, in the subsequent age series it was two times higher and 3.5 times higher in the oldest ones. Differences in the species composition and in the C/N ratio in different species (generally higher for vascular plants and lower for mosses) were expressed in an increase in the understorey C/N ratio, which was 39.5, 46.6 and 48.6, respectively.
Keywords
biodiversity;
Pinus sylvestris plantation;
overstorey-understorey interaction;
biotic homogenization;
Leucobryo-Pinetum community
-
Woziwoda,
Department of Geobotany and Plant Ecology, Faculty of Biology and Environmental Protection, University of Lodz, Banacha 12/16, 90-237 Lodz, Poland
E-mail
woziwoda@biol.uni.lodz.pl

- Parzych, Environmental Chemistry Research Unit, Institute of Biology and Environmental Protection, Pomeranian University in Słupsk, Arciszewskiego 22b, 76-200 Słupsk, Poland E-mail parzycha1@op.pl
- Kopeć, Department of Geobotany and Plant Ecology, Faculty of Biology and Environmental Protection, University of Lodz, Banacha 12/16, 90-237 Lodz, Poland E-mail domin@biol.uni.lodz.pl
Received 18 February 2014 Accepted 14 October 2014 Published 24 October 2014
Views 350429
Available at https://doi.org/10.14214/sf.1119 | Download PDF

1 Introduction
The increase in the forest cover and the reduction in the agricultural land area have been a common feature of land use changes in Central and Eastern Europe since the beginning of the 19th century (Wulf 2004; Taff et al. 2010; Prishchepov et al. 2012). Every year, another patches of agricultural lands, abandoned or unsuitable for cultivation, have been afforested, mainly by the Scots pine Pinus sylvestris L. Consequently, thousands of hectares of contemporary woodlands comprises of the mosaic of Scots pine monocultures at different ages (Zerbe 2002; Hermy and Verheyen 2007; EEA 2008). In Poland, post-agricultural forests dominated by P. sylvestris cover 1.47 million hectares and most of them are used for timber harvest (CSO 2012). Besides the high economic value, planted forests play an important role in enhancing the biodiversity and in carbon (C) sequestration, both at a regional and global scale (Cannel et al. 1992; Guo and Gifford 2002; EU ETS 2003; Stoate et al. 2009; Nagendra and Southworth 2010; Bernier et al. 2011; De Frenne et al. 2011; Pan et al. 2011; Coote et al. 2012). The tree stand structure and species composition of secondary forests is determined by forest management. Many silvicultural methods, including stand thinning and mixing of tree species, are used to modify a plantation in order to create conditions favouring the growth of commercial woody species (Mäkinen and Isomäki 2004; Ericksson 2006; Kelty 2006; Jacob et al. 2010; Morin et al. 2011; Nowak et al. 2011; Paquette and Messier 2011; Seidel et al. 2013). Every modification of forest canopy changes the microclimate within a forest and affects the soil properties (Messier et al. 1998; Augusto et al. 2002; Jandl et al. 2007; Jonczak and Parzych 2012), which in turn, strongly influence the ground vegetation (Kolström 1999; Dzwonko 2001; Graae et al. 2004; Bruelheide and Undelhoven 2005). In the case of post-agricultural forests, the community structure and composition have been also affected for many years by the site history (Hermy and Stieperaere 1981; Hermy et al. 1993; Flinn and Vellend 2005; Góras and Orczewska 2007; Hermy and Verheyen 2007; Matuszkiewicz et al. 2013; Ouyang et al. 2013). The moss, understorey vascular plant and shrub layers of secondary forests consist of both species which are resistant to an agricultural land use and species characteristic of forest communities (Dzwonko and Gawroński 1994; Dzwonko and Loster 1997; Majchrowska and Woziwoda 2009).
The species composition of plantations is important in terms of controlling the level of offsetting greenhouse gas emissions (Thornley and Cannell 2000; Nabuurs et al. 2008; Malmsheimer et al. 2011). High stock of carbon is sequestered in tree stand biomass (Körner 2003; FAO 2011; Thomas and Martin 2012; Wilson et al. 2013) and in the forest floor (including the soil and litter) (Dewar and Cannell 1992; Masera et al. 2003; Berg and McClaugherty 2008). It is noteworthy that the coniferous stands have higher stocks of carbon than hardwood stands (Raulund-Rasmussen and Vejre 1995; Gärdenäs 1998). Hansson et al. (2011) and Vesterdal et al. (2013) reported that also the forest floor C stock is higher for coniferous (Pinus) than for broadleaved (Betula and Populus species) forests growing in temperate regions. Thus, post-agricultural plantations of Scots pine contribute to climate change mitigation by C sequestration. The forest ground vegetation, however, also participate in C storage (Moore et al. 2007; Parzych 2010; Jagodziński et al. 2013). Usually the contribution of understorey plant biomass, expressed as a percentage of the total aboveground biomass of the forest community, is at the 2% level (Yarie 1980; Muller 2003; Gilliam 2007). The total annual production of the understorey may reach 20% of the total aboveground biomass if we consider the seasonal changes in the ground vegetation composition, particularly in deciduous forests (Kaźmierczakowa 1971; Kubiček and Jurko 1975; Tremblay and Larocque 2001; Jagodziński et al. 2013). The seasonal differences in the understorey cover and the species composition are much less pronounced in coniferous forests where mosses and perennial vascular plants (some of them even evergreen) dominate (Matuszkiewicz 2001). This relatively persistent ground vegetation produces a high amount of recalcitrant biomass and litter (Mälkönen 1974; Tuterski 2003). Due to the fact that carbon is sequestered in tissues of mosses and perennial vascular plants, these long-lived forest components contribute (together with trees) to climate change mitigation (Nabuurs et al. 2007).
The amount of biomass and the C stock differs between species, both in the case of trees and shrubs (Elias and Potvin 2003; Lamlom and Savidge 2003; Jagodziński et al. 2012), as well as herbs and mosses (Tutersky 2003; Parzych 2010). This variability should be considered in modelling of carbon storage in forest ecosystems. The ongoing temporal changes in the forest community structure and composition must also be accounted for (Baeten et al. 2010).
The aim of our study was to examine: i) how the stand age of the first generation of a post-agricultural Scots pine forest affects the understorey species composition; ii) what is the role of understorey vascular and moss species in the biomass accumulation in the successive stand-age series; and, iii) how much organic Carbon and total Nitrogen is sequestered in the biomass of dominant species.
2 Material and methods
2.1 Study area
The study was conducted in Central Poland (N: 51°51′–51°55′, E: 18°57′–19°09′; the area is managed by the Regional Directorate of the State Forests in Lodz), in Scots pine forests planted on lands which have been gradually excluded from agricultural use. The study area encompasses 1070 ha of woodlands and the studied post-agricultural forests constitute 17.4% of the total woodland area. The study sites are characterized by acid (pH = 4–5), well-drained but moist soils, mineral, with sandy-loams bedrock lying on sands (data obtained from the Information System of the Polish State Forests, SILP 2011). Such a habitat is suitable for the fresh pine forest type of the European temperate region and is naturally covered with the forest vegetation community of Leucobryo-Pinetum (W.Mat. 1962) W.Mat. & J.Mat. 1973 (Matuszkiewicz 2001). The Scots pine is a natural dominant component of the fresh pine forest community, so these tree monocultures at the sites described above are consistent with the type of potential forest vegetation. The studied secondary forests cover 91% of Leucobryo-Pinetum sites.
2.1.1 Characteristics of the studied Scots pine plantations and description of management practices
The oldest plantations with mature stands at the age of over 80 years cover 27 ha of post-agricultural lands and they are distributed in ten forest patches from 0.3 ha to 5.5 ha in area, 2.7 ha on average (SILP data). Plantations with pre-mature stands at the age of 61–80 years were established in 13 forest fragments in the area of 2.5 ha on average (from 0.1 to 6.8 ha) and they cover 33 ha in total. The youngest studied plantations at the age between 41 and 60 years are distributed in 51 forests patches of 1.7 ha on average (from 0.1 to 6.4 ha) and they cover in total 89 ha of post-agricultural lands. Tree stand characteristics of the studied post-agricultural forests are described in Table 1.
| Table 1. Tree stand characteristics of the studied post-agricultural sites. | |||
| Age series: | 41–60 | 61–80 | > 80 |
| On average ± standard deviation | |||
| Pinus sylvestris | |||
| No. of stems ha–1 | 1900 ± 212 | 1000 ± 158 | 820 ± 130 |
| Stems DBH [cm] | 15.9 ± 3.4 | 22.7 ± 5.1 | 30.1 ± 5.9 |
| Height of trees [m] | 13.3 ± 1.5 | 14.5 ± 2.0 | 18.7 ± 2.3 |
| Betula pendula | |||
| No. of stems ha–1 | 80 ± 109 | 62 ± 91 | 40 ± 55 |
| Stems DBH [cm] | 9.2 ± 2.7 | 19.1 ± 3.9 | 29.1 ± 3.5 |
| Height of trees [m] | 8.8 ± 1.9 | 11.3 ± 1.1 | 15.6 ± 1.9 |
| Mean volume of stand [m3·ha–1] * | 210 ± 57 | 247 ± 31 | 295 ± 37 |
| Data estimated in own field inventories and *SILP data | |||
The management practises of post-agricultural Scots pine forests (State Forests) following the thinning pattern have not changed considerably since the introduction of planned forest management in the interwar period (Forest Research Institute 1998; Bernadzki et al. 1999), so the management practises were possibly similar in all studied plantations. A total of 5–7(9) thousand of 1–2-year-old Scots pine seedlings per one hectare are planted on the poorest post-agricultural soils (Skolud 2006; The State Forests... 2012). The recommended spacing for P. sylvestris is 1.5 x 0.7–0.5 m. The contribution of Scots pine in the species composition amounts to 80–90%. The remaining 20(10)% is represented by silver birch Betula pendula L. which is planted in groups, each composed of several seedlings, or it is used to afforest the edges of Scots pine stands. The dense young stands are pre-commercially thinned (“cleaned”) twice: the early pre-commercial thinning is performed in 3–10-year-old cultivations, and the late pre-commercial thinning − in 11–20-year-old stands (the thicket phase). Pre-commercial thinning involves mainly negative selection, which consists in removing undesirable trees without canopy interruption (The State Forests... 2012). The weakest and undesirable trees are cut down and the biomass is left to decompose naturally or removed. Stands older than 20 years are commercially thinned with positive selection applied – trees of high quality (the tallest ones, with a straight stem and high increment) and relatively evenly spaced in a stand, are selected and promoted (The State Forests... 2012). The early commercial thinning is performed in intensively growing 21–40-year-old stands (the small pool phase) to favour the stem diameter growth. The number of Scots pine trees is reduced up to 1–3 thousand per hectare. The late commercial thinning starts with the age of 40 years. In the studied forests, the number of stems in 41–60-year-old stands amounted to around 2 thousand trees per hectare (Table 1). In this age series, the number of stems was reduced to around 1 thousand trees per hectare. Further 200 trees (on average) were harvested per hectare in 61–80-year-old stands. As a result, the mean number of trees in stands at the age of over 80 years amounted to about 800 per hectare. The first generation of Scots pine trees planted on post-agricultural soils are cleared when a stand reaches the age of 100 years (SILP data). However, once a post-agricultural land is afforested, it remains in forestry use and the next generation of trees is obligatorily planted in a clear-cutting area during next 3 years (The State Forests... 2012).
2.2 Data collection
2.2.1 Community structure and composition
Field sampling of forest vegetation was conducted using phytosociological relevés (= sample plots) located in homogenous patches of vegetation in the area of Scots pine plantations, within three age-series of stands: 41–60, 61–80 and over 80-year-old (younger stands were not included in the study due to the lack of such Scots pine plantations established at a fresh pine forest site in the last 40 years). The research plots were located in 21 Scots pine stands (seven in each age series), each larger than 1.5 ha. Altogether, 75 sample plots (25 within each age series) located within homogenous patches of vegetation, at least 50 m from the plantation edge, were established in August 2012. The size of a sample plot was 100 m2 (10 x 10 m). In each plot, the percentage cover of species in the upper (a1) and lower (a2) tree stand layers, and in the shrub (b), understorey vascular plant (c) and moss (d) layers was visually estimated. The cover of each vascular plant species (trees, shrubs and herbs) and mosses was estimated using a six-degree cover-abundance scale (from “+”: a few specimens covering less than 1%; to “5”: plant species covering more than 75% of the plot area; according to Braun-Blanquet 1964), which allowed us to estimate both the number of species and the proportion of the area covered by species. The species nomenclature followed Mirek et al. (2002) for vascular plants and Ochyra et al. (2003) for mosses.
2.2.2 Biomass
Samples of plants for analysis of biomass of understorey vascular plants and mosses were also collected in August 2012. Sampling in late summer aimed at estimating the maximum biomass accumulation of all species during the growing season, characteristic of temperate coniferous forest (vascular plants – grasses and semi-shrubs with seasonal leaves – had the largest dimensions at that time). The study was conducted in 75 subplots (25 in each age series) selected randomly (five subplots in each five plots) within Scots pine monocultures, 25 × 25 cm (= 1/16 m2) in size. The list of vascular and bryophyte species was compiled in every plot, including tree seedlings and saplings (with a height below 90 cm). Furthermore, the percentage cover (within an accuracy of 10%, and the value of 5% for the rarest species) of all species was estimated in each plot. All plants, including woody species of a height up to 0.9 m, which grew within a plot were collected manually by cutting them flush with the ground surface using scissors, and sorted (before drying) by species. Plant samples were dried at +65oC for 48 hours to the constant weight and weighed to the nearest 0.01 g.
2.2.3 The amount of organic C and total N
Samples of vascular plants and mosses, the dry mass of which was above 1.5 g were crushed in a grinder for chemical analysis. Due to the fact that the collection of plant material composed of different species with different abundance in plots located in different age series, the number of vascular plant and moss samples (by species) ranged from 7 to 45. Finally, the amount of organic carbon (C) and total nitrogen (N) was analysed for six species which were dominant in the studied forest communities, including three moss species: Pleurozium schreberi (Willd. ex Brid.) Mitt. (45 samples), Dicranum polysetum Sw. ex anon. (15) and Sciuro-hypnum oedipodium (Mitt.) Ignatov & Huttunen (15), and three vascular plant species: Vaccinium myrtillus L. (30), Vaccinium vitis-idaea L. (30) and Calluna vulgaris (L.) Hull (15). The amount of C was determined with the Alten method (Bednarek et al. 2005), which is used when plant samples contain more than 10% of this component. It is based on strong C oxidation with 0.34 mol.dm–3 solution of K2Cr2O7 and titration of Mohr salt. Total N was determined with the Kjeldahl method upon mineralization in the mixture of H2SO4 and 30% H2O2 (CMA 1973) using a BÜCHI K-350 distilling unit.
2.3 Data analysis
Detrended correspondence analysis (DCA) was performed using CANOCO version 4.5 software (ter Braak and Šmilauer 2002) to describe general patterns in the variation of species composition within phytosociological relevés, divided on the basis of age-series of Scots pine stands. Before the DCA analysis, original species cover values were transformed into percentage cover values (+ = 1.0; 1 = 5.0; 2 = 17.5; 3 = 37.5; 4 = 62.5; 5 = 87.5; according to Braun-Blanquet 1964).
The biomass of 6 dominant species was calculated for each phytosociological relevés (100 m2 in area). A linear regression model was used to assess the relationship between the percentage cover of herbs and mosses and their biomass in subplots for each species. Next, the coefficient of determination (r2) for species was used to calculate the biomass for phytosociological relevés from the percentage of species cover. For this calculation, the “a” parameter from the linear regression equation (y = ax) was used. The biomass of herbs and mosses was expressed in kg per hectare [kg ha–1].
Statistical differences in the percentage cover for each forest layer, moss biomass, and understorey vascular plant biomass, and the mass of organic carbon and total nitrogen sequestered in dry mass of species were assessed with One-Way ANOVA (analysis of variance) with the significance level set at 0.05. The normality was checked with the Kolmogorov-Smirnov test, and homogeneity of variance with Levene’s test. Due to the lack of normal distribution of percentage cover values, the Box-Cox transformation was used. The Tukey post-hoc test was used following one-way ANOVA for multiple comparisons of individual groups. The above comparisons were carried out using STATISTICA software (version 10.0, StatSoft Inc., Tulsa).
3 Results
3.1 Community structure and species composition
The post-agricultural Scots pine forests established at different times (40, 60 and over 80 years ago) varied considerably in the community structure and species composition (Fig. 1; Fig. 2).
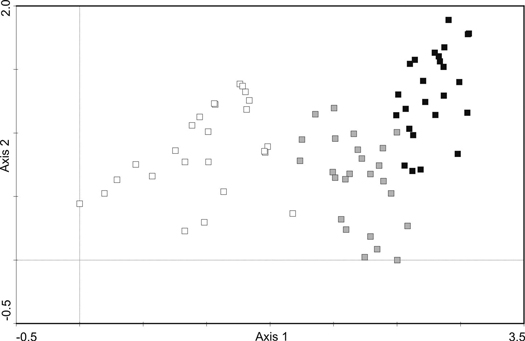
Fig. 1. DCA analysis of phytosociological samples from the Scots pine forests. The colour of symbols indicates three age-series of stands: white – 41–60, gray – 61–80, black – over 80-year-old. The first two axes explain 33.2% of the species cover variability.

Fig. 2. Post-agricultural forests with Scots pine stands at the age of 41–60, 61–80 and over 80-years.
The 41–60-year-old forests were characterized by the highest percentage values of the canopy cover, which amounted to 70–80%. The forest canopy cover was intentionally decreased by tree stand thinning. In the 61–80-year-old forests, it was decreased to 50–70%. In the next stand development stage (over 80 years), the canopy cover was left at the 50–70% level (Table 2). However, the mean percentage cover of dominant P. sylvestris decreased gradually with the tree stand age, and the canopy of older forest series was built also of broadleaved B. pendula crowns (Table 3). The mean percentage cover of the shrub layer built mainly of B. pendula and Quercus robur L. increased several times in the successive stand-age series. The highest increase in the mean percentage cover was noted for the understorey vascular plant layer (Table 2).
| Table 2. The mean percentage cover (calculated from the total cover values of layers noted in 25 research plots in each age series) and the mean number of species (with ± standard error) for the layers of trees, shrubs, understorey vascular plants and mosses in the stand age series of 41–60, 61–80- and over 80-year-old in the studied secondary Scots pine forests. For each variable (= within a row), stand age series marked with different letters are significantly different (P < 0.05). | |||||
| Forest layer | ANOVA | Multiple comparisons | |||
| F | P | 41–60 (a) | 61–80 (b) | > 80 (c) | |
| Mean cover ± standard error | |||||
| Tree layer | 15.65 | <0.0001 | 74.4 ± 1.01a | 66.8 ± 1.25b | 66.4 ± 1.14b |
| Shrub layer | 37.14 | <0.0001 | 1.4 ± 0.61a | 9.8 ±1.93b | 22.0 ± 2.14c |
| Understorey vascular plant layer | 293.97 | <0.0001 | 3.0 ± 0.42a | 40.8 ± 2.76b | 81.2 ± 2.79c |
| Moss layer | 14.76 | <0.0001 | 74.4 ± 2.39a | 90.8 ± 1.52b | 75.2 ± 3.06a |
| Mean number of species ± standard error | |||||
| Tree layer | 2.69 | ns | 1.24 ± 0.08 | 1.12 ± 0.06 | 1.40 ± 0.10 |
| Shrub layer | 68.52 | <0.0001 | 0.76 ± 0.19a | 2.88 ± 0.23b | 4.12 ± 0.19c |
| Understorey vascular plant layer | 348.34 | <0.0001 | 0.44 ± 0.10a | 6.32 ± 0.28b | 8.60 ± 0.26c |
| Moss layer | 4.80 | 0.0109 | 3.28 ± 0.14a | 3.28 ± 0.19a | 4.08 ± 0.28b |
The total number of species noted in the studied secondary forests was 38; 19 species were found in the youngest stands, 30 species in the 61–80-year-old stands and 32 species in the oldest one. The mean total number of species increased with the age of a stand and it amounted to 5.6 for research plots located in 41–60-year-old forests, 13.1 in 61–80-year-old forests and 17.1 for plots with the stand age of over 80 years. Significant increases in the mean number of species were noted only for the shrub layer and the understorey vascular plant layer (Table 2).
The well-developed understorey vascular plant and/or moss layers, however, were dominated by two or three species, while most of the species reached very low mean percentage cover (< 1) despite their high frequency (Table 3).
| Table 3. Frequency and the mean cover (calculated from the original species cover values transformed to percentage cover values: + = 1.0; 1 = 5.0; 2 = 17.5; 3 = 37.5; 4 = 62.5; 5 = 87.5, according to Braun-Blanquet 1964; n = 25 in each age series) of plant species noted in Scots pine stands at the age of 41–60, 61–80 and over 80 years. Species characteristic of forest communities from the class: VP – Vaccinio-Piceetea, NC – Nardo-Callunetea, Ea – Epilobietea angustifolii, Qrp – Quercetea robori-petreae; found (+) in coniferous forests (CF) and/or in mixed coniferous-deciduous forests (MF), infiltrating from one forest type into (►) another one (according to Matuszkiewicz 2001); “-“ – species was not found; alien – species not native to flora of Poland. | |||||||||
| Species names by forest communities | Age series: | 41–60 | 61–80 | > 80 | 41–60 | 61–80 | > 80 | ||
| Naturally occurs in | |||||||||
| CF | MF | Frequency [%] | Mean cover | ||||||
| Tree stand layer | |||||||||
| Pinus sylvestris VP | + | + | 100 | 100 | 100 | 73.50 | 60.50 | 55.50 | |
| Betula pendula | + | + | 24 | 12 | 40 | 0.48 | 1.10 | 3.50 | |
| Shrub layer | |||||||||
| Betula pendula | + | + | 12 | 36 | 60 | 0.42 | 2.12 | 6.88 | |
| Frangula alnus | + | + | 12 | 28 | 32 | 0.24 | 1.86 | 0.70 | |
| Quercus robur | + | ◄ | + | - | 32 | 68 | 0.00 | 1.38 | 8.80 |
| Sorbus aucuparia | + | ◄ | + | - | 24 | 24 | - | 1.02 | 0.66 |
| Pinus sylvestris VP | + | + | - | 20 | 20 | 0.00 | 0.28 | 2.00 | |
| Juniperus communis | + | + | <10 | <10 | <10 | 0.40 | 0.02 | 0.02 | |
| Padus serotina | alien | - | - | <10 | - | - | 0.22 | ||
| Shrubs and trees up to 0.9 m height | |||||||||
| Betula pendula | + | + | 20 | 56 | 72 | 0.10 | 1.00 | 3.34 | |
| Frangula alnus | + | + | 12 | 48 | 52 | 0.06 | 0.78 | 1.34 | |
| Quercus robur | + | ◄ | + | <10 | 52 | 84 | 0.02 | 1.16 | 4.26 |
| Sorbus aucuparia | + | ◄ | + | <10 | 40 | 52 | 0.02 | 0.56 | 0.80 |
| Pinus sylvestris VP | + | + | - | 32 | 80 | - | 0.70 | 3.02 | |
| Quercus rubra | alien | - | - | 16 | - | - | 0.26 | ||
| Padus serotina | alien | - | - | 16 | - | - | 0.08 | ||
| Juniperus communis | + | + | 12 | <10 | - | 0.06 | 0.04 | - | |
| Understorey vascular plant layer | |||||||||
| Vaccinium myrtillus VP | + | + | <10 | 84 | 100 | 0.04 | 8.40 | 70.50 | |
| Vaccinium vitis-idaea VP | + | ► | + | <10 | 88 | 100 | 0.02 | 17.40 | 9.92 |
| Festuca ovina | + | + | 20 | 100 | 100 | 0.28 | 8.06 | 12.00 | |
| Melampyrum pratense VP | + | ► | + | <10 | 84 | 76 | 0.04 | 3.08 | 7.88 |
| Calluna vulgaris NC | + | + | - | 76 | 72 | - | 4.36 | 3.48 | |
| Luzula pilosa | + | ◄ | + | - | 24 | 88 | - | 0.30 | 1.52 |
| Pteridium aquilinum | + | ◄ | + | - | 16 | 52 | - | 0.44 | 4.24 |
| Antoxantum odoratum | + | - | 16 | 48 | - | 0.08 | 0.78 | ||
| Deschampsia caespitosa | ◄ | + | - | 12 | <10 | - | 0.06 | 0.02 | |
| Carex ovalis | + | + | - | 40 | 76 | - | 1.10 | 1.82 | |
| Calamagrostis epigejos Ea | + | + | - | 16 | 32 | - | 0.94 | 1.02 | |
| Deschampsia flexuosa | + | ► | + | - | 16 | 40 | - | 0.08 | 0.74 |
| Dryopteris carthusiana | + | + | - | <10 | 40 | - | 0.02 | 0.56 | |
| Carex hirta | + | + | - | 20 | <10 | - | 0.28 | 0.02 | |
| Trientalis europaea VP | + | + | - | <10 | <10 | - | 0.04 | 0.04 | |
| Juncus effusus | + | - | 12 | - | - | 0.06 | - | ||
| Hieracium pilosella NC | + | + | - | 16 | - | - | 0.44 | - | |
| Hieracium murorum Qrp | + | ◄ | + | - | - | 12 | - | - | 0.06 |
| Scorzonera humilis | + | + | - | - | <10 | - | - | 0.04 | |
| Astragalus arenarius | - | - | <10 | - | - | 0.02 | - | - | |
| Moss layer | |||||||||
| Pleurozium schreberi VP | + | ► | + | 100 | 100 | 100 | 32.84 | 53.30 | 63.50 |
| Dicranum polysetum VP | + | ► | + | 88 | 100 | 96 | 6.58 | 36.40 | 14.20 |
| Hypnum cupressiforme Qrp | ◄ | + | 16 | 52 | 68 | 0.26 | 0.98 | 1.60 | |
| Sciuro-hypnum oedipodium | + | 100 | 40 | - | 19.42 | 0.74 | 0.00 | ||
| Dicranum scoparium VP | + | ► | + | <10 | <10 | 56 | 0.02 | 0.02 | 2.72 |
| Pohlia nutans | + | + | <10 | 16 | 48 | 0.04 | 0.26 | 0.96 | |
| Leucobryum glaucum VP | + | - | 16 | 16 | - | 0.94 | 0.26 | ||
| Thuidium tamariscinum | + | + | - | - | 24 | - | - | 0.48 | |
| Polytrichum piliferum | - | - | <10 | - | - | 0.04 | - | - | |
| Ptilium crista-castrensis VP | + | ► | <10 | - | - | 0.02 | - | - | |
The moss layer of 41–60-year-old forests was dominated by S. oedipodium and by P. schreberi. The latter had the highest mean cover in the moss layer in forests of all three age series (Table 3). The understorey of the 61–80-year-old community was built mainly of D. polysetum − co-dominant in the moss layer, and V. vitis-idaea, V. myrtillus and C. vulgaris − dominant in the vascular plant layer. The above-mentioned ericaceous species occurred with the high frequency also in the oldest stands, however, V. myrtillus dominated over other species, while the cover of V. vitis-idaea decreased. The species associated with mixed coniferous/deciduous forests were more frequently encountered; also Melampyrum pratense L. and Pteridium aquilinum (L.) Kuhn reached high cover (Table 3).
3.2 Aboveground biomass of the understorey vascular plant and moss layers
The mean understorey biomass increased with the age of a stand and it was 1.5 times higher in 61–80-year-old forests and almost two times higher in the > 80-year-old forests as compared to the youngest ones; it amounted to 3048 kg ha–1, 4610 kg ha–1 and 5781 kg ha–1, respectively. The successive stand-age-series were characterized by significant differences in the biomass of understorey vascular plants and mosses (Fig. 3ab).
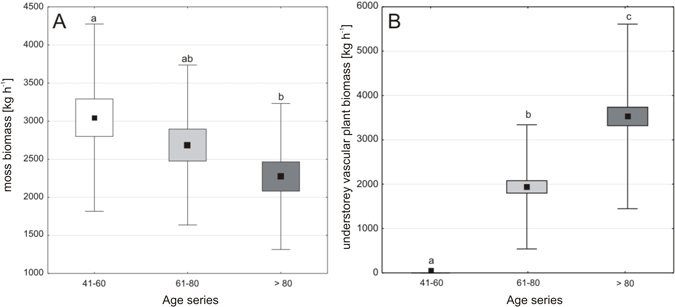
Fig. 3. Comparison of the moss biomass (A) and understorey vascular plant biomass (B) for the three age-series of forest stands; forest stands at the age of 41–60 (a), 61–80 (b) and over 80 years (c); dot – mean, box – interquartile range, whiskers – range of variability (min-max). Means with different letters above whiskers differ at P < 0.05 in post-hoc comparisons.
The dry mass of understorey vascular plants was very low in the youngest Scots pine forests − on average 2 kg ha–1, which constituted only 0.1% of the total understorey biomass, while 99.9% of the total understorey biomass (3046 kg ha–1) was accumulated in mosses. The mean understorey vascular plant biomass value was significantly higher in older stands: almost 1924 kg ha–1 in the 61–80-year-old forests and 3508 kg ha–1 in the oldest ones (Fig. 3b). Variation in moss biomass values was smaller; the biomass value was lower by a quarter in the oldest Scots pine forests compared to the youngest ones (Fig. 3a) and, the mean value of moss biomass was significantly different in 41–60-year-old and over 80-year-old forests.
Only a few understorey vascular plant and moss species (Table 4) reached high biomass values. The biomass of six dominant plant species varied in three age-series, and the differences were statistically significant (Table 4).
| Table 4. The coefficient of determination (r2) and a-slope parameter from the linear regression equation (y = ax + b) for the biomass and the species percentage covers of six species which dominate in the understory of 41–60- (a), 61–80- (b) and > 80- (c) year-old Scots pine forests and their significance (One-Way ANOVA). For each variable (= within a row), stand age series with different letters are significantly different (P < 0.05). | |||||||||
| Species name | Biomass calculation | ANOVA | Multiple comparisons | ||||||
| 41–60 | 61–80 | > 80 | |||||||
| r² | a | F | P | Mean biomass [kg ha–1] ± standard error | |||||
| Pleurozium schreberi | 0.6347 | 0.2672 | 13.28 | <0.0001 | 877.5 ± 110.2a | 424.2 ± 132.4b | 696.7 ± 98.18b | ||
| Dicranum polysetum | 0.7301 | 0.4153 | 31.51 | <0.0001 | 273.3 ± 76.4a | 1511.7 ± 137.2b | 589.7 ± 121.4a | ||
| Sciuro-hypnum oedipodium | 0.8955 | 0.2988 | 36.73 | <0.0001 | 580.3 ± 93.5a | 22.1 ± 9.7b | 0.0b | ||
| Vaccinium myrtillus | 0.8640 | 0.4058 | 194.60 | <0.0001 | 1.6 ± 1.1a | 340.9 ± 87.8a | 2860.9 ± 173.0b | ||
| Vaccinium vitis-idaea | 0.8035 | 0.3710 | 18.82 | <0.0001 | 0.7 ± 0.7a | 645.5 ± 90.2b | 368.0 ± 92.4c | ||
| Calluna vulgaris | 0.8248 | 0.2645 | 5.65 | 0.005 | 0.0a | 115.3 ± 32.9b | 92.0 ± 29.92b | ||
P. schreberi reached the highest biomass value in the youngest plantations, while that of D. polysetum was the highest in 61–80-year-old Scots pine stands. The biomass of S. oedipodium reached the highest value in the 41–60-year-old stands; it decreased significantly in the 61–80-year-old stands, and, in the oldest Scots pine forests the species was absent.
The highest biomass of V. vitis-idaea and C. vulgaris was in the 61–80-year-old forests. V. myrtillus reached the highest biomass in the oldest age-series of Scots pine forest and it was several times higher than the total biomass of any of those other understorey vascular plant species.
3.3 Sequestration of organic C and total N in dominant understorey species
The mean total concentration of organic C in dry mass of the six studied understorey components amounted to 46.96%. However, the proportion of C accumulated in those six species varied significantly (P < 0.05) as follows: V. myrtillus – 50.6%, D. polysetum – 49.5%, C. vulgaris – 46.9%, P. schreberi and S. oedipodium – 45.1%, and V. vitis-idaea – 44.5%. The mean of the total nitrogen concentration in all studied species was 0.98%. However, it was higher for mosses than for vascular plants: 1.2% for S. oedipodium and P. schreberi, 1% for D. polysetum, and 0.9%, 0.8% and 0.7% for V. myrtillus, C. vulgaris and V. vitis-idaea, respectively. Consequently, the C/N ratio was higher for vascular plants than for mosses: 60.0 for V. vitis-idaea, 56.1 for C. vulgaris, 54.6 for V. myrtillus, 48.8 for D. polysetum, 38.8 for P. schreberi and 37.0 for S. oedipodium.
Significantly the highest mass of organic C and total N was sequestered in the biomass of V. myrtillus occurring in the oldest stand age series of the Scots pine plantations (Table 5).
| Table 5. The mass of organic carbon (C) and total nitrogen (N) sequestered by six dominant species in 41–60- (a), 61–80- (b) and > 80-year-old (c) Scots pine forests. For each variable (= within a row), stand age series with different letters are significantly different (P < 0.05). | |||||||
| Species name | ANOVA | Multiple comparisons | |||||
| 41–60 | 61–80 | > 80 | |||||
| F | P | [kg ha–1] ± standard error | |||||
| Pleurozium schreberi | 13.28 | <0.0001 | C | 395.75 ± 49.69a | 642.30 ± 59.72b | 765.22 ± 44.28b | |
| N | 10.22 ± 1.28a | 16.59 ± 1.54b | 19.77 ± 1.14b | ||||
| Dicranum polysetum | 31.51 | <0.0001 | C | 135.27 ± 37.83a | 748.29 ± 67.93b | 291.91 ± 60.10a | |
| N | 2.77 ± 0.77a | 15.33 ± 1.39b | 5.98 ± 1.23a | ||||
| Sciuro-hypnum oedipodium | 36.73 | <0.0001 | C | 261.70 ± 42.16a | 9.97 ± 4.37b | 0.0b | |
| N | 7.08 ± 1.14a | 0.27 ± 0.12b | 0.0b | ||||
| Vaccinium myrtillus | 194.60 | <0.0001 | C | 0.8 ± 0.57a | 172.48 ± 44.40a | 1447.61 ± 87.52b | |
| N | 0.02 ± 0.01a | 3.16 ± 0.81a | 26.55 ± 1.61b | ||||
| Vaccinium vitis-idaea | 18.82 | <0.0001 | C | 0.33 ± 0.33a | 287.27 ± 40.12b | 163.77 ± 41.13c | |
| N | 0.005 ± 0.005a | 4.78 ± 0.66b | 2.73 ± 0.68c | ||||
| Calluna vulgaris | 5.65 | 0.005 | C | 0.0a | 54.09 ± 15.42b | 43.17 ± 14.04b | |
| N | 0.0a | 0.96 ± 0.27b | 0.58 ± 0.13b | ||||
| In total: | |||||||
| C | 262.72 | <0.0001 | 793.87 ± 40.45a | 1914.39 ± 333.65b | 2711.70 ± 335.78c | ||
| N | 207.27 | <0.0001 | 20.09 ± 0.97a | 41.10 ± 1.34b | 55.79 ± 1.38c | ||
In the youngest Scots pine stands, the mass of C concentrated in mosses was many times higher than in the understorey vascular plants. It was also nearly 3 times higher than in 61–80-year-old stands. In the oldest forests, however, organic C was accumulated more in the understorey vascular plants than in mosses (Table 5). The total mass of C sequestered in the biomass of six understorey plant species increased with the age of Scots pine stands, and the observed differences between the stand age-series were statistically significant. Almost 800 kg ha–1 of C was concentrated in the biomass of six studied understorey species of 41–60-year-old Scots pine forests. However, this value was 2.4 times lower than the value recorded in the 61–80-year-old forests and 3.4 times lower than the value recorded in the oldest forests.
The mass of total N was much lower in all age-series compared to the mass of total C, but it was also positively correlated with the age of planted stands and the differences were statistically significant (Table 5). The C/N ratio calculated as a proportion of the total C mass and the total N mass accumulated in six understorey components increased from 39.5 in the 41–60-year-old forests, through 46.6 in the 61–80-year-old forests to 48.6 in the oldest ones.
4 Discussion
4.1 Transformation of post-agricultural Scots pine forests into natural-like community
The successive development of forest vegetation in woodlands artificially planted on a former agricultural land is a long-lasting and dynamic process (Dzwonko 2001; Zerbe 2002; Góras and Orczewska 2009). The results of our study showed that the species composition considerably varies in the post-agricultural Scots pine forests of different ages. The understorey vegetation (not studied in this paper) of initial and early stages of Scots pine stands growing on sandy acid soils is usually poorly developed and/or it is dominated by mosses and lichens (Olaczek 1972; Woziwoda and Ambrożkiewicz 2011; Stefańska-Krzaczek 2011a). The high proportion of non-forest species is also evident (Tolunay 2009; Woziwoda and Ambrożkiewicz 2011). The dominance of grasses lasting for years after planting the trees, reported by Uotila et al. (2005) and Matuszkiewicz et al. (2013), is characteristic of sites with more humid and more fertile soils than in this study. The growth of grasses in Scots pine plantations is likely prevented by strong closure of the canopy of trees in initial stages of stand development (Bernadzki et al. 1999), which effectively excludes also non-forest species (Olaczek 1972). The gradual pre-commercial and commercial thinning favours the development of the moss ground cover (Bernadzki et al. 1999). Species which are characteristic of forest ecosystems occur successively along with the ageing of woodlands (Góras and Orczewska 2009; Matuszkiewicz et al. 2013). The understorey plant species composition differs significantly in the studied 41–60, 61–80 and over 80-year-old forests. Generally, the older the Scots pine trees, the higher was the plant species richness and diversity. The differences in the ground vegetation are correlated with the differences in the overstory structure and composition which are strongly affected by the forest management, mainly by the thinning treatments. The spreading, establishment and survival of mosses and acidophilic ericaceous species in the studied Scots pine forests probably resulted from the acid litterfall of P. sylvestris (Dzwonko 2001; Astel et al. 2009). However, the dominance of these plants in forests planted on abandoned agricultural lands can also be explained by the post-agricultural soil condition (Alriksson and Olsson 1995; Dupouey et al. 2002; Wall and Hytönen 2005; Falkengren-Grerup et al. 2006; Flin and Marks 2007; Baeten et al. 2011). For example, ploughing of cultivated crop lands in pre-afforestation times causes the homogenization of soils (Fraterrigo et al. 2005; Flinn and Marks 2007), which destroys the microsites and consequently, reduces the possibility of acid-sensitive flora establishment, particularly in the early stages of forest community development (Flin 2011).
The temporary dominance of light demanding V. vitis-idaea and C. vulgaris in the 61–80-year-old forests (Table 3 and 4) was the ecosystem’s response to the increased understorey insolation after the pre-commercial stand thinning conducted previously in 41–60-year-old stands. These plants could survive in open micro-habitats preserved in the oldest forests, or their occurrence may be also connected with forest management disturbances, e.g. to selective logging (Wulf 2004; Maciejewski and Zubel 2010).
The highest species richness was observed in the forest with the oldest P. sylvestris stands. The post-agricultural forest community with trees at the age of over 80 years became similar to natural fresh pine forests described on the basis of contemporary studies conducted in Poland by Matuszkiewicz (2007), where understorey vascular vegetation consisted of acid-tolerant species and was dominated by V. myrtillus, V. vitis-idaea, C. vulgaris, Festuca ovina L. and Deschampsia flexuosa (L.) Trin. The moss layer covers 80–100% of the ground and is composed of the same species as those found in the studied forests (Matuszkiewicz 2001).
Many authors report that long after the restoration of the tree layer in secondary forests, the abundance of understorey vascular plants and mosses significantly differs from that observed in ancient forests (i.e. forests growing in areas where the land use has probably never been changed) (review in: Verheyen et al. 2003 and Hermy and Verheyen 2007; e.g. Góras and Orczewska 2007; Orczewska and Fernes 2011). At present, however, the process of convergence of planted Scots pine forests to natural-like forests is accelerated by a decrease in the floristic specificity in the Leucobryo-Pinetum community. This is reflected in the decline of species characteristic of fresh pine forests and in the increase of species characteristic of mixed coniferous-deciduous forests, e.g. Maianthemum bifolium (L.) F.W. Schmidt, Oxalis acetosella L., Trientalis europaea L., Solidago virgaurea L., Fragaria vesca L. and Veronica officinalis L. (Matuszkiewicz 2007). Changes in the composition of “ancient-like” coniferous forests are due to the site eutrophication and an increase in the proportion of deciduous woody species (Matuszkiewicz 2007; Matuszkiewicz et al. 2013). The post-agricultural coniferous plantations are easily colonized by broadleaved plants (Wulf and Heinken 2008). Their admixture favours an increase in the understorey vascular species diversity (Kaźmierczakowa 1971; Kubiček and Jurko 1975; Barbier et al. 2008; Jagodziński et al. 2013) and accelerates the development of ground vegetation. The increasing moss and understorey vascular species richness and understorey vascular plant biomass correlated with the growing contribution of broadleaved trees, which was also observed in our study in the two older age-series of the Scots pine plantation (Table 2, Fig. 3b). The establishment of more heterogeneous and species-rich flora was favoured by the increasing diversity of micro-sites under the coniferous-deciduous canopy (Hill 1992). The increase in the forest soil fertility, usually observed under deciduous trees (Paré and Bergeron 1996; Gilliam 2007; Gilliam and Roberts 2003), caused a significant increase in the understorey vascular plant biomass.
At present, the intentional mixing of coniferous and deciduous stands or deciduous subcanopy development is promoted in timber Scots pine forests to enhance the forest biodiversity and productivity (Simmons and Buckley 1992; Felton et al. 2012; Seidel et al. 2013). However, Matuszkiewicz (2007) reported that an increase in the deciduous species admixture in coniferous forests is the main cause of oligotrophic and acidophilic species disappearance. Moreover, according to the critical review of studies on the effects of tree species mixture in the understorey vegetation, the maximum understorey diversity is in many cases observed not in the mixed coniferous-deciduous stands, but in the pure coniferous or pure deciduous stands with sparse canopies (Barbier et al. 2008). In the studied forests, the progressive subcanopy development probably caused the gradual retreat of S. oedipodium, V. vitis-idaea and C. vulgaris, expressed as a decrease in the frequency, cover and biomass in the oldest age series (Table 2 and 3). Simultaneously, these light demanding species were replaced and dominated by V. myrtillus – a more shade-tolerant species. The multifold increase in the cover and biomass of the common bilberry in mature stands is also caused by high competiveness of this plant (Góras and Orczewska 2007; Matuszkiewicz et al. 2013).
The observed gradual loss of the Leucobryo-Pinetum community indicators, and the increased proportion of common, competitive and already widespread species, may cause the biotic homogenization (sensu Olden and Rooney 2006; Vellend et al. 2007), not only in the post-agricultural and natural fresh pine forests as reported by Matuszkiewicz (2007), but also in fresh (pine) coniferous (Leucobryo-Pinetum community) and mixed coniferous-deciduous forests (Querco robori-Pinetum community). The convergence of post-agricultural and natural forests was already reported from Poland by Olaczek in the 1970s (Olaczek 1972). It was also described in the Belgian forest ecosystems by Beaten et al. (2010). However, other factors (not studied in this paper), such as species dispersal mechanisms, habitat requirements or forest soil development (Honney et al. 2002; Brunet 2007; Hermy and Verheyen 2007), might be also important in the recovery of fresh pine forest on former agricultural lands.
Special attention should be paid to mosses. Bryophytes are important understorey components of the studied post-agricultural Scots pine forests at all stages of the community development (Tables 2 and 3). High cover and frequency of ground mosses and their dominance in the forest understorey was also reported by Stefańska-Krzaczek (2011a, 2011b) in timber forests with the second generation of P. sylvestris stands planted in clearing areas. The presence of bryophytes is a characteristic feature of natural forests with pine-dominated stands, accounting for 70–100 % of the ground cover (Matuszkiewicz 2001; Mills and MacDonald 2004).
4.2 Growing biomass amounts
Bryophytes are the major contributor to the productivity of coniferous forests, which in this study was expressed in their high biomass (Fig. 3a, Table 4). Also the research of Parzych and Sobisz (2010) conducted in the costal Scots pine forest (Empetro nigri-Pinetum community) showed the significant role of mosses in forest productivity – they constituted 29% of the total (3393 kg ha–1) understorey biomass. However, we found that the moss biomass decreased, while the understorey vascular plant biomass increased with the age of Scots pine stands (Fig. 3a). It is noteworthy that despite the growing understorey species diversity of successive stand-age-series, only a few dominant moss species – P. schreberi, D. polysetum and S. oedipodium, as well as a few understorey vascular plant species – V. myrtillus, V. vitis-idaea and C. vulgaris played an important role in the biomass accumulation. The decreasing cover of mosses in relation to understorey vascular plants in the studied coniferous forests may be also explained by the negative impact of broadleaved species (Glime 2007; Stratsev et al. 2008; Maciejewski and Zubel 2009). The increase in the proportion of B. pendula, Q. robur and/or Frangula alnus Mill. in the forest subcanopy results in the increased leaf litter amount, and according to Beatty and Scholes (1988) and Glime (2007), dense deciduous litter, which periodically or permanently covers the ground, can be an important factor limiting the growth of mosses. Studies of Stratsev et al. (2008) have shown that the growth of bryophytes is even more strongly suppressed by the leaf litter than the abundant pine litterfall. Furthermore, changes in soil properties caused by deciduous trees (Paré and Bergeron 1996; Saetre et al. 1997) and the increasing advantage of understorey vascular plants, which easily outcompete slower growing bryophytes (Frego and Carleton 1995; Tuterski 2003) may also reduce the cover and the growth of mosses.
4.3 Carbon and total Nitrogen stock and increase in the understorey C/N ratio
Mosses reach high biomass in post-agricultural Scots pine forests, so bryophytes play an important role in organic carbon and nitrogen sequestration in the understorey (Table 5). Moreover, C and N fixed by bryophytes from atmospheric pools are transformed into recalcitrant organic matter (Tuterski 2003), which favours their long-term accumulation. We observed that the total N sequestered in the moss biomass in 41–60- and 61–80-year-old forests was even higher than the total N stock in the biomass of understorey vascular plants. According to Tutersky (2003), bryophytes reduce the N availability for vascular plants and in this way they control the understorey vascular plants development. Due to the multiple increase in the understorey vascular plant cover and the biomass following the increasing age of stands, vascular plants were the major contributor in the organic C accumulation, as observed in the oldest series of forest (Table 5). Consequently, the growing proportion of vascular plants in the understorey biomass resulted in the increased understorey C/N ratio. It indicates a decrease in the decomposition rate and an increase in the organic matter accumulation in forest soils. The C/N ratio is primarily the result of the presence of lignin (63–66% of C) and hemicellulose (42–46% of C) in the understorey vascular plant species (Raven et al. 2004; Bert and Danjon 2006; Zheng 2009). A similar relationship in the C/N ratio in the undergrowth of 45-, 75- and 120-year-old Scots pine forests was described by Moszyńska (1991). Moreover, the calculated values of the C/N ratio in the studied pine forests are typical for undergrowth vegetation (Gifford 2000; Gifford et al. 2000; McGroddy et al. 2004; Silva et al. 2008).
The results clearly indicate that despite the changing species composition, the ground vegetation of planted woodlands play an important role in the biomass production and in the carbon sequestration.
5 Conclusions
The structure and the species composition of post-agricultural Scots pine forests within pre-mature (41–60- and 61–80-year-old) or mature (over 80-year-old) stands vary considerably. The forest overstorey is affected by forest management. However, spontaneous changes such as broadleaved species encroachment into tree and shrub layers occurs as well. These changes have a strong and long-lasting effect on the ground vegetation. The (planted) Scots pine favours the growth of bryophytes and light-demanding ericaceous plants, whereas oak and silver birch (colonising the plantation in the process of secondary vegetation succession) support the establishment of shade-tolerant vascular plant species, including species characteristic of mixed coniferous-deciduous forests. This successive colonisation of post-agricultural Scots pine plantations by mosses and vascular plants is a continuous process, which is reflected in the increasing species richness and diversity following the increasing age of stands. However, the gradual increase in the proportion of broadleaved trees and in the understorey vascular plant cover causes a decrease in the moss cover and the moss biomass in the post-agricultural Scots pine forest communities.
The organic C and total N content in dry mass of individual moss and understorey vascular plant species varies considerably; the N stock in the analysed vascular plant tissue is lower than N stock accumulated in the moss tissue, while the C stock is higher. Consequently, due to the differences in the understorey composition of the Scots pine forests in different age series, the total amount of understorey C stock also varies considerably. In general: the older Scots pine stands, the higher the amount of C accumulated in the ground vegetation.
The obtained empirical data on the relationships between the overstorey and understorey structure and composition, the correlation between the understorey composition and biomass, and the species carbon and nitrogen content, are essential for understanding the impact of forest planting on the former agricultural land in enhancing the biodiversity and carbon sequestration in the course of time. The assessment of the C content in the biomass of individual understorey components will allow us to predict more precisely the amount of C stock in the fresh pine forests of the European temperate region.
Acknowledgements
The authors acknowledge the Regional Directorate of the State Forests in Lodz, Poland, for sharing of data on the post-agricultural Scots pine forests from the SILP databases. We are also grateful to Anonymous Reviewers and Editors for their valuable comments and suggestions during the preparation of this manuscript.
References
Alriksson A., Olsson M.T. (1995). Soil changes in different age classes of Norway spruce (Picea abies (L.) Karst.) on afforested farmland. Plant and Soil 168–169: 103–110. http://dx.doi.org/10.1007/BF00029319.
Astel A., Parzych A., Trojanowski J. (2009). Comparison of litterfall and nutrient return in a Vaccinio uliginosi-Betuletum pubescentis and an Empetro nigri-Pinetum forest stands in northern Poland. Forest Ecology and Management 257: 2331–2341. http://dx.doi.org/10.1016/j.foreco.2009.03.026.
Augusto L., Ranger J., Binkley D., Rothe A. (2002). Impact of several common tree species of European temperate forests on soil fertility. Annals of Forest Sciences 59: 233–253. http://dx.doi.org/10.1051/forest:2002020.
Baeten L., Hermy M., Van Daele S., Verheyen K. (2010). Unexpected understorey community development after 30 years in ancient and post-agricultural forests. Journal of Ecology 98: 1447–1453. http://dx.doi.org/10.1111/j.1365-2745.2010.01711.x.
Baeten L., Verstraeten G., De Frenne P., Vanhellemont M., Wuyts K., Hermy M., Verheyen K. (2011). Former land use affects the nitrogen and phosphorus concentration and biomass of forest herbs. Plant Ecology 21: 901–909. http://dx.doi.org/10.1007/s11258-010-9876-9.
Barbier S., Gosselin F., Balandier P. (2008). Influence of tree species on understory vegetation diversity and mechanisms involved – a critical review for temperate and boreal forests. Forest Ecology and Management 254: 1–15. http://dx.doi.org/10.1016/j.foreco.2007.09.038.
Beatty S.W., Scholes O.D.V. (1988). Leaf litter effect on plant species composition of deciduous forest treefall pits. Canadian Journal of Forest Research 18: 553–559.
Bednarek R., Dziadowiec H., Pokojska U., Prusinkiewicz Z. (2005). Badania ekologiczno-gleboznawcze. [Ecological soil researches]. Wyd. Nauk. PWN, Warszawa. [In Polish].
Berg B., McClaugherty C. (2008). Plant litter: decomposition, humus formation, carbon sequestration. 2nd edition. Springer Verlag, Berlin–Heidelberg.
Bernadzki E., Ilmurzyński E., Szymański S. (1999). Trzebieże. Poradnik Leśniczego. [Thinnings. The foresters’ guide]. PWRiL, Warszawa. [In Polish].
Bernier P., Braatz S., Felicani-Robles F., Mollicone D. (2011). The role of forests in climate change adaptation and mitigation. In: Flejzor L., Higman S., Ink G. (eds.). State of the world’s forests. FAO, Rome, Italy.
Bert D., Danjon F. (2006). Carbon concentration variations in the roots, stem and crown of mature Pinus pinaster (Ait.). Forest Ecology and Management 222: 279–295. http://dx.doi.org/10.1016/j.foreco.2005.10.030.
Braun-Blanquet J. (1964). Pflanzensoziologie. Grundzüge der Vegetationskunde. 3rd ed. Springer Verlag, Wien.
Bruelheide H., Undelhoven P. (2005). Correspondence of the fine-scale spatial variation in soil chemistry and the herb layer vegetation in beech forests. Forest Ecology and Management 210: 205–223. http://dx.doi.org/10.1016/j.foreco.2005.02.050.
Brunet J. (2007). Plant colonization in heterogeneous landscapes: an 80-year perspective on restoration of broadleaved forest vegetation. Journal of Applied Ecology 44: 563–572. http://dx.doi.org/10.1111/j.1365-2664.2007.01297.x.
Cannell M.G.R., Dewar R.C., Thornley J.H.M. (1992). Carbon flux storage in European forests. In: Teller A., Mathy A., Jeffers J.N.R. (eds.). Responses of forest ecosystems to environmental changes. Elsevier, Amsterdam. p. 256–271.
CMA del INEA. (1973). Determinaciones analiticas en suelo. Normalización de metódos. I. pH, materia orgánica y nitrógeno. Anales de Edafologia y Agronómica 32: 1153–1172.
Coote L., French L.J., Moore K.M., Mitchell F.J.G., Kelly D.L. (2012). Can plantation forests support plant species and communities of semi-natural woodland? Forest Ecology and Management 283: 86–95. http://dx.doi.org/10.1016/j.foreco.2012.07.013.
CSO. (2012). Forestry. Statistical information and elaboration. Central Statistical Office, Warsaw.
De Frenne P., Beaten L., Graae B.J., Brunet J., Wulf M., Orczewska A., Kolb A., Jansen I., Jamoneau A., Jacquemyn H., Hermy M., Diekmann M., De Schrijver A., De Sanctis M., Decocq G., Cousins S.A.O., Verheyen K. (2011). Interregional variation in the floristic recovery of post-agricultural forests. Journal of Ecology 99: 600-609. http://dx.doi.org/10.1111/j.1365-2745.2010.01768.x.
Dewar R.C., Cannell M.G.R. (1992). Carbon sequestration in the trees, products and soils of forest plantations: an analysis using U.K. examples. Tree Physiology 11: 49−71.
Dupouey J.L., Dambrine E., Laffite J.D., Moares C. (2002). Irreversible impact of past land use on forest soil and biodiversity. Ecology 83: 2978–2984.
Dzwonko Z. (2001). Assessment of light and soil conditions in ancient and recent woodlands by Ellenberg indicator values. Journal of Applied Ecology 38: 942–951.
Dzwonko Z., Gawroński S. (1994). The role of woodland fragments, soil types, and dominant species in secondary succession on the western Carpathian foothills. Vegetatio 111: 149–160.
Dzwonko Z., Loster S. (1997). Effects of dominant trees and anthropogenic disturbances on species richness and floristic composition of secondary communities in southern Poland. Journal of Applied Ecology 34: 861–870.
EEA. (2008). EEA Report 3/2008: European forests – ecosystem conditions and sustainable use. European Environment Agency, Copenhagen. http://dx.doi.org/10.2800/3601.
Elias M., Potvin C. (2003). Assessing inter- and intra-specific variation in trunk carbon concentration for 32 neotropical tree species. Canadian Journal of Forest Research 33: 1039−1045. http://dx.doi.org/10.1139/X03-018.
Ericksson E. (2006). Thinning operation and their impact on biomass production in stands of Norway spruce and Scots pine. Biomass and Bioenergy 30: 848–854. http://dx.doi.org/10.1016/j.biombioe.2006.04.001.
EU ETS. (2003). European emissions trading scheme. Directive 2003/87/EC of the European Parliament and of the Council establishing a scheme for greenhouse gas emission allowance trading within the Community. http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CELEX:32003L0087:en:NOT.
Falkengren-Grerup U., Ten Brink D.J., Brunet J. (2006). Land use effects on soil N, P, C and pH persist over 40–80 years of forest growth on agricultural soils. Forest Ecology and Management 225: 74–81. http://dx.doi.org/10.1016/j.foreco.2005.12.027.
FAO. (2011). State of the world’s forests 2011. Food and Agriculture Organization of the United Nations, Rome.
Felton A., Lindbladh M., Brunet J., Fritz O. (2010). Replacing coniferous monocultures with mixed-species production stands: an assessment of the potential benefits for forest biodiversity in northern Europe. Forest Ecology and Management 260(6): 939–947. http://dx.doi.org/10.1016/j.foreco.2010.06.011.
Flinn K.M. (2011). Why are acidophilic plants abundant in post agricultural forests? The Journal of the Torrey Botanical Society 138(1): 73–76. http://dx.doi.org/10.3159/10-RA-017.1.
Flinn K.M., Marks P.L. (2007). Agricultural legacies in forest environments: tree communities, soil properties and light availability. Ecological Applications 17: 452–463. http://dx.doi.org/10.1890/05-1963.
Flinn K., Vellend M. (2005). Recovery of forests plant communities in post-agricultural landscapes. Frontiers in Ecology and the Environment 3: 243–250. http://www.jstor.org/stable/3868486.
Fraterrigo J.M., Turner M.G., Pearson S.M., Dixon P. (2005). Effects of past land use on spatial heterogeneity of soil nutrients in southern Appalachian forests. Ecological Monographs 75: 215–230. http://dx.doi.org/10.1890/03-0475.
Forest Research Institute. (1998). Complex principles of agricultural land utilisation for forestry. Warsaw. [In Polish].
Frego K.A., Carleton T.J. (1995). Microsite tolerance of four bryophytes in a mature black spruce stand: reciprocal transplants. The Bryologist 98(4): 452–458.
Gärdenäs A.I. (1998). Soil organic matter in European forest floors in relation to stand characteristics and environmental factors. Scandinavian Journal of Forest Research 13: 274–283.
Gifford R.M. (2000). Carbon contents of aboveground tissues of forest and woodland trees. National Carbon Accounting System Technical Report 22. Australian Greenhouse Office, Canberra.
Gifford R.M., Barrett D.J., Lutze J.L. (2000). The effects of elevated [CO2] on the C:N and C:P mass ratios of plants tissues. Plant and Soil 224: 1–14.
Gilliam F.S. (2007). The ecological significance of the herbaceous layer in temperate forest ecosystems. BioScience 57(10): 845–858. http://dx.doi.org/10.1641/B571007.
Gilliam F.S., Roberts M.R. (2003). The herbaceous layer in forests of eastern North America. Oxford University Press, New York.
Glime J.M. (2007). Bryophyte ecology. Vol. 1. Physiological ecology. E-book sponsored by Michigan Technological University and the International Association of Bryologists. http://www.bryoecol.mtu.edu/.
Góras P., Orczewska A. (2007). Zróżnicowanie runa w lasach sosnowych posadzonych na gruntach porolnych i w starych lasach sosnowych na siedlisku boru mieszanego świeżego. [Differentiation of the herbaceous layer of the post-arable and ancient pine forests developing on a habitat of recent mixed pine forests]. Przegląd Przyrodniczy 18(1–2): 227–241.
Góras P., Orczewska A. (2009). Species composition of the herb layer of spatially isolated, post-agricultural pine plantations. In: Holeksa J., Babczyńska-Sendek B., Wika S. (eds.). The role of geobotany in biodiversity conservation. University of Silesia, Katowice. p. 89–100.
Graae B.J., Okland R.H., Petersen P.M., Jensen K., Fritzboger B. (2004). Influence of historical, geographical and environmental variables on understorey composition and richness in Danish forests. Journal of Vegetation Science 15: 465–474.
Guo L.B., Gifford R.M. (2002). Soil carbon stocks and land use changes: a meta analysis. Global Change Biology 8: 345–360.
Hansson K., Olsson B.A., Olsson M., Johansson U., Kleja D.B. (2011). Differences in soil properties in adjacent stands of Scots pine, Norway spruce and silver birch in SW Sweden. Forest Ecology and Management 262: 522–530.
Hermy M., Stieperaere H. (1981). An indirect gradient analysis of ecological relationships between ancient and recent forest riverine woodlands to the south of Burges (Flanders, Belgium). Vegetatio 44: 43–49.
Hermy M., van den Bremt P., Tack G. (1993). Effects of site history on woodland vegetation. In: Broekmeyer M.E.A., Vos W., Koop H. (eds.). European forest reserves. Pudoc, Wageningen. p. 219–232.
Hermy M., Verheyen K. (2007). Legacies of the past in the present-day forest biodiversity: review of past lad-use effects on forest plant species composition and diversity. Ecological Resources 22: 361–371. http://dx.doi.org/10.1007/s11284-007-0354-3.
Hill M.O. (1992). Mixtures as habitat for plants. In: Cannell M.G.R., Malcolm D.C., Atterson J. (eds.). The ecology of mixed species stands and trees. Blackwell Scientific Publications, Oxford. p. 301−302.
Honney O., Bossuyt B., Verheyen K., Butaye J., Jacquemyn H., Hermy M. (2002). Ecological perspectives for the restoration of plant communities in European temperate forests. Biodiversity and Conservation 11: 213–242.
Jacob M., Leuschner C., Thomas F.M. (2010). Productivity of temperate broad-leaved forest stands differing in tree species diversity. Annals of Forest Science 67: 503. http://dx.doi.org/10.1051/forest/2010005.
Jagodziński A.M., Jarosiewicz G., Karolewski P., Oleksyn J. (2012). Zawartość węgla w biomasie pospolitych gatunków krzewów podszycia leśnego. [Carbon concentration in the biomass of common species of understory shrubs. Sylwan 156(9): 650−662. [In Polish].
Jagodziński A.M., Pietrusiak K., Rawlik M., Janyszek S. (2013). Seasonal changes in the understorey biomass of an oak-hornbeam forest Galio sylvatici-Carpinetum betuli. Forest Research Papers 74(1): 35–47. http://dx.doi.org/10.2478/frp-2013-0005.
Jandl R., Lindner M., Vesterdal L., Bauwens B., Baritz R., Hagedorn F., Johnson D.W., Minkkinen K., Byrne K.A. (2007). How strongly can forest management influence soil carbon sequestration? Geoderma 137: 253–268. http://dx.doi.org/10.1016/j.geoderma.2006.09.003.
Jonczak J., Parzych A. (2012). Impact of Scots pine admixture in European beech stand on dissolved organic carbon and nitrogen leaching from organic and humic horizons of Dystric Arenosols in Northern Poland. Journal of Forest Science 58(6): 278–286. http://www.agriculturejournals.cz/publicFiles/66813.pdf.
Kaźmierczakowa R. (1971). Ekologia i produkcja runa świetlistej dąbrowy i grądu w rezerwatach Kwiatkówka i Lipny Dół na Wyżynie Małopolskiej. Studia Naturae 15: 1–107. [In Polish].
Kelty M.J. (2006). The role of species mixtures in plantation forestry. Forest Ecology and Management 233: 195–204. http://dx.doi.org/10.1016/j.foreco.2006.05.011.
Kolström M. (1999). Effect of forest management on biodiversity in boreal forests: a model approach. University of Joensuu, Faculty of Forestry, Research Notes 86: 1–29.
Körner C. (2003). Carbon limitation in trees. Journal of Ecology 91: 4–17. http://dx.doi.org/10.1046/j.1365-2745.2003.00742.x.
Kubiček F., Jurko A. (1975). Estimation of the above-ground biomass of the herb layer in forest communities. Folia Geobotanica et Phytotaxonomica 10: 113–129.
Lamlom S.H., Savidge R.A. (2003). A reassessment of carbon content in wood: variation within and between 41 North American species. Biomass Bioenergy 25: 381–388. http://dx.doi.org/10.1016/S0961-9534(03)00033-3.
Maciejewski Z., Zubel R. (2009). Long-term changes in the Leucobryo-Pinetum community: interactions between the tree-stand, understorey and moss layer. Annales Universitatis Mariae-Curie Skłodowska, Lublin–Polonia, Sectio C, 64(2): 23–34. http://dx.doi.org/10.2478/v10067-010-0011-z.
Majchrowska A., Woziwoda B. (2009). Effects of forest history on the biodiversity of vascular plants flora. In: Holeksa J., Babczyńska-Sendek B., Wika S. (eds.). The role of geobotany in biodiversity conservation. University of Silesia, Katowice, p. 165–174.
Mäkinen H., Isomäki A. (2004). Thinning intensity and growth of Scots pine stands in Finland. Forest Ecology and Management 201: 311–325. http://dx.doi.org/10.1016/j.foreco.2004.07.016.
Mälkönen E. (1974). Annual primary production and nutrient cycle in some Scots pine stands. Communicationes Instituti Forestalias Fenniae 84(5). 87 p.
Malmsheimer R.W., Bowyer J.L., Fried J.S., Gee E., Izlar R., Reid R.A., Munn I.A., Oneil E., Stewart W.C. (2011). Managing forests because carbon matters: integrating energy, products, and land management policy. Journal of Forestry 109: S7–S50. http://www.safnet.org/documents/JOFSupplement.pdf.
Masera O.R., Garza-Caligaris J.F., Kanninen M., Karjalainen T., Liski J., Nabuurs G.J., Pussinen A., de Jong B.H.J., Mohren G.M.J. (2003). Modelling carbon sequestration in afforestation, agroforestry and forest management projects: the CO2FIX V.2 approach. Ecological Modelling 164(2–3): 177−199. http://dx.doi.org/10.1016/S0304-3800(02)00419-2.
Matuszkiewicz J.M. (ed.). (2007). Geobotanical identification of the development tendencies in forest associations in the regions of Poland. PAN, IGiPZ im. S. Leszczyckiego, Warszawa.
Matuszkiewicz J.M., Kowalska A., Kozłowska A., Roo-Zielińska E., Solon J. (2013). Differences in plant-species composition, richness and community structure in ancient and post-agricultural pine forests in central Poland. Forest Ecology and Management 310: 567–576. http://dx.doi.org/10.1016/j.foreco.2013.08.060.
Matuszkiewicz W. (2001). Przewodnik do oznaczania zbiorowisk roślinnych Polski. [A guide for the identification of Polish plant communieties. Wydawnictwo Naukowe PWN, Warszawa. [In Polish].
McGroddy M.E., Daufresne T., Hedin L.D. (2004). Scaling of C:N:P stechiometry in forests worldwide: implications of terrestrial redfield – type ratios. Ecology 85(9): 2390–2401. http://dx.doi.org/10.1890/03-0351.
Messier C., Parent S., Bergeron Y. (1998). Effects of overstory and understory vegetation on the understory light environment in mixed boreal forest. Journal of Vegetation Science 9: 511–520.
Mills S.E., MacDonald S.E. (2004). Predictors of moss and liverwort species diversity of micro-sites in conifer-dominated boreal forest. Journal of Vegetation Science 15: 189–198. http://dx.doi.org/10.1658/1100-9233(2004)015[0189:POMALS]2.0.CO;2.
Mirek Z., Piękoś-Mirkowa H., Zając A., Zając M. (2002). Flowering plants and pteridophytes of Poland. A checklist. W. Szafer Institute of Botany, Polish Academy of Science, Kraków.
Moore P.T., van Miegroet H., Nicholas N.S. (2007). Relative role of understory and overstory in carbon and nitrogen cycling in a southern Appalachian spruce-fir forest. Canadian Journal of Forest Research 37: 2689–2700. http://dx.doi.org/10.1139/X07-115.
Morin X., Fahse L., Scherer-Lorenzen M., Bugmann H. (2011). Tree species richness promotes productivity in temperate forests through strong complementarity between species. Ecology Letters 14: 1211–1219. http://dx.doi.org/10.1111/j.1461-0248.2011.01691.
Moszyńska B. (1991). The regulation of matter transfer from plants to soil during primary forest succession on blown-out areas on Dutch drift sands. De Dorschkamp Instituut voor Bosbouw en Groenbeheer, Wageningen. 23 p.
Muller R.N. (2003). Nutrient relations of the herbaceous layer in deciduous forest ecosystems. In: Gilliam F.S., Roberts M.R. (eds.). The herbaceous layer in forests of eastern North America. Oxford University Press, New York. p. 15–37.
Nabuurs G.J., Masera O., Andrasko K., Benitez-Ponce P., Boer R., Dutschke M., Elsiddig E., Ford-Robertson J., Frumhoff P., Karjalainen T., Krankina O., Kurz W.A., Matsumoto M., Oyhancabal W., Ravindranath N.H., Sanz Sanchez M.J., Zhang X. (2007). Forestry. In: Metz B., Davidson O.R., Bosch P.R., Dave R., Meyer L.A. (eds.). Climate change 2007: mitigation. Contribution of Working Group III to the fourth assessment report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA. p. 543–578. http://www.ipcc.ch/pdf/assessment-report/ar4/wg3/ar4-wg3-chapter9.pdf.
Nabuurs G.J., Thürig E., Heidema N., Armolaitis K., Biber P., Cienciala E., Kaufmann E., Mäkipää R., Nilsen P., Petritsch R., Pristova T., Rock J., Schelhaas M.J., Sievanen R., Somogyi Z., Vallet P. (2008). Hotspots of the European forests carbon cycle. Forest Ecology and Management 256: 194–200. http://dx.doi.org/10.1016/j.foreco.2008.04.009.
Nagendra H., Southworth J. (eds.). (2010). Reforesting landscapes: linking pattern and process. Landscape Series 10: 1–396.
Nowak J., Slodicak M., Dusek D. (2011). Thinning effects on forest productivity and site characteristics in stands of Pinus sylvestris in the Czech Republic. Forest Systems 20(3): 464–474. http://dx.doi.org/10.5424/fs/20112003-11074.
Ochyra R., Żarnowiec J., Bednarek-Ochyra H. (2003). Census catalogue of Polish mosses. W. Szafer Institute of Botany, Polish Academy of Sciences, Kraków.
Olaczek R. (1972). Formy antropogenicznej degeneracji leśnych zbiorowisk roślinnych w krajobrazie rolniczym Polski niżowej. [The anthropogenic forms of degeneration of forest communities in rural landscape of lowland Poland]. Wydawnictwo Uniwersytetu Łódzkiego, Łódź. [In Polish].
Olden J.D., Rooney T.P. (2006). On defining and quantifying biotic homogenization. Global Ecology and Biogeography 15: 113–120. http://dx.doi.org/10.1111/j.1466-822x.2006.00214.x.
Orczewska A., Fernes M. (2011). Migration of herb layer species into the poorest post-agricultural pine woods adjacent to ancient pine forests. Polish Journal of Ecology 59(1): 75–85. http://www.pol.j.ecol.cbe-pan.pl/article/ar59_1_07.pdf.
Ouyang W., Xu Y., Hao F., Wang X., Siyang C., Lin C. (2013). Effect of long-term agricultural cultivation and land use conversion on soil nutrient contents in the Sanjiang Plain. Catena 104: 243–250. http://dx.doi.org/10.1016/j.catena.2012.12.002.
Pan Y., Birdsey R.A., Fang J., Houghton R., Kauppi P.E., Kurz W.A., Phillips O.L., Shvidenko A., Lewis S.L., Canadell J.G., Ciais P., Jackson R.B., Pacala S.W., McGuire A.D., Piao S., Rautiainen A., Sitch S., Hayes D. (2011). A large and persistent carbon sink in the world’s forests. Science 333: 988–993. http://dx.doi.org/10.1126/science.1201609.
Paquette A., Messier C. (2011). The effect of biodiversity on tree productivity: from temperate to boreal forests. Global Ecology and Biogeography 20: 170–180. http://dx.doi.org/10.1111/j.1466-8238.2010.00592.x.
Paré D., Bergeron Y. (1996). Effect of colonizing tree species on soil nutrient availability in a clay soil of the boreal mixedwood. Canadian Journal of Forest Research 26: 1022–1031.
Parzych A. (2010). Azot, fosfor i węgiel w roślinności leśnej Słowińskiego Parku Narodowego w latach 2002–2005. [Nitrogen, phosphorus and carbon in forest plants in the Słowiński National Park in 2002–2005]. Ochrona Środowiska i Zasobów Naturalnych 43: 45–64. [In Polish].
Parzych A., Sobisz Z. (2010). Biomasa i produkcja pierwotna netto roślin runa w wybranych zespołach leśnych Słowińskiego Parku Narodowego. [Biomass and net primary production of herbaceous plants in chosen forest associations in the Słowiński National Park]. Ochrona środowiska i Zasobów Naturalnych 42: 72–83. [In Polish].
Prishchepov A.V., Radeloff V.C., Baumann M., Kuemmerle T., Müller D. (2012). Effects of institutional changes on land use: agricultural land abandonment during the transition from state-command to market-driven economies in post-Soviet Eastern Europe. Environmental Research Letters 7: 1–13. http://dx.doi.org/10.1088/1748-9326/7/2/024021.
Raulund-Rasmussen K., Vejre H. (1995). Effect of tree species and soil properties on nutrient immobilization in the forest floor. Plants and Soil 168–169: 345–352.
Raven J.A., Handley L.L., Andrews M. (2004). Global aspects of C/N interactions determining plant-environment interactions. Journal of Experimental Botany 55: 11–25. http://dx.doi.org/10.1093/jxb/erh011.
Saetre P., Saetre L.S., Brantberg P.O., Lundkvist H., Bengtsson J. (1997). Ground vegetation composition and heterogeneity in pure Norway spruce and mixed Norway spruce–birch stands. Canadian Journal of Forest Research 27: 2034–2042.
Seidel D., Leuschner C., Scherber C., Beyer F., Wommelsdorf T., Cashman M.J., Fehrmann L. (2013). The relationship between tree species richness, canopy space exploration and productivity in a temperate broad-leaf mixed forest. Forest Ecology and Management 310: 366–374. http://dx.doi.org/10.1016/j.foreco.2013.08.058.
Silva G.T.A., Matos L.V., Nobrega P.O., Campello E.F.C., Resende A.S. (2008). Chemical composition and decomposition rate of plants used as green manure. Scientia Agricola 65(3): 298–305. http://www.scielo.br/pdf/sa/v65n3/a10v65n3.
Simmons E.A., Buckley G.P. (1992). Ground vegetation under planted mixtures of trees. In: Cannell M.G.R., Malcolm D.C., Atterson J. (eds.). The ecology of mixed species stands and trees. Blackwell Scientific Publications, Oxford. p. 211−231.
Skolud P. (2006). [Afforestation of arable lands and wastelands – the owner guide]. CILP, Warsaw. [In Polish].
Stefańska-Krzaczek E. (2011a). Przemiany ubogich siedlisk borowych a aktualny stan roślinności runa w drzewostanach sosnowych kolejnych klas wieku w Nadleśnictwie Bolesławiec. [Consistency in the classification of oligotrophic forest sites and forest vegetation in Scots pine stands of successive age classes in Bolesławiec Forest]. Forest Research Papers 72(1): 53–64. [In Polish]. http://dx.doi.org/10.2478/v10111-011-0007-8.
Stefańska-Krzaczek E. (2011b). Plant communities of Scots pine stands in the south-eastern part of the Bory Dolnośląskie forest (SW Poland). Acta Botanica Silesiaca, Monographiae 6. 98 p.
Stoate C., Báldi A., Beja P., Boatman N.D., Herzon I., van Doorn A., de Snoo G.R., Rakosy L., Ramwell C. (2009). Ecological impacts of early 21st century agricultural change in Europe – a review. Journal of Environmental Management 91: 22–46.
Stratsev N., Lieffers V.J., Landhäusser S.M. (2008). Effects of leaf litter on the growth of boreal feather mosses: implications for forest floor development. Journal of Vegetation Science 19: 253–260.
Taff G.N., Müller D., Kuemmerle T., Ozdeneral E., Walsh S.J. (2010). Reforestation in Central and Eastern Europe after the breakdown of socialism. In: Nagendra H., Southworth J. (eds.). Reforesting landscapes: linking pattern and process. Landscape Series 10: 121–147.
ter Braak C.J.F., Šmilauer P. (2002). CANOCO reference manual and CanoDraw for Windows. User’s guide: software for canonical community ordination (version 4.5). Microcomputer Power, Ithaca NY, USA.
The State Forests National Forests Holding. (2012). Zasady Hodowli Lasu obowiązujące w Państwowym Gospodarstwie Leśnym Lasy Państwowe. [Silvicultural principles binding in the State Forests National Forests Holding]. CILP, Warszawa. [In Polish].
Thomas S.C., Martin A.R. (2012). Carbon content of tree tissue: a synthesis. Forests 3: 332–352. http://dx.doi.org/10.3390/f3020332.
Thornley J.H.M., Cannell M.G.R. (2000). Managing forests for wood yield and carbon storage: a theoretical study. Tree Physiology 20: 477–84.
Tolunay D. (2009). Carbon concentrations of tree components, forest floor and understorey in young Pinus sylvestris stands in north-western Turkey. Scandinavian Journal of Forest Research 24: 394–402. http://dx.doi.org/10.1080/02827580903164471.
Tremblay N.O., Larocque G.R. (2001). Seasonal dynamics of understorey vegetation in four eastern Canadian forest types. International Journal of Plant Science 162(2): 271–286. http://www.jstor.org/stable/10.1086/319582.
Tutersky M.R. (2003). The role of bryophytes in carbon and nitrogen cycling. The Bryologist 106(3): 395–409. http://www.jstor.org/discover/10.2307/3244721.
Uotila A., Hotanen J.-P., Kouki J. (2005). Succession of understory vegetation in managed and seminatural Scots pine forests in eastern Finland and Russian Karelia. Canadian Journal of Forest Research 35(6): 1422–144. http://dx.doi.org/10.1139/x05-063.
Vellend M., Verheyen K., Flinn K.M., Jacquemyn H., Kolb A., Van Calster H., Peterken G., Graae B.J., Bellemare J., Honnay O., Brunet J., Wulf M., Gerhardt F., Hermy M. (2007). Homogenization of forest plant communities and weakening of species-environment relationships via agricultural land use. Journal of Ecology 95: 565–573. http://dx.doi.org/10.1111/j.1365-2745.2007.01233.x.
Verheyen K., Honnay O., Motzkin G., Hermy M., Foster D. (2003). Response of forest plant species to land-use change: a life-history based approach. Journal of Ecology 91: 563–577. http://hdl.handle.net/1854/LU-312592. [Cited 9 June 2005].
Vesterdal L., Clarke N., Sigurdsson B.D., Gundersen P. (2013). Do tree species influence soil carbon stocks in temperate and boreal forests? Forest Ecology and Management 309: 4–18. http://dx.doi.org/10.1016/j.foreco.2013.01.017.
Wall A., Hytönen J. (2005). Soil fertility of afforested arable land compared to continuously forested sites. Plant and Soil 275(1–2): 245–258.
Wilson B.T., Woodall C.W, Griffith D.M. (2013). Imputing forest carbon stock estimates from inventory plots to a nationally continuous coverage. Carbon Balance and Management 8(1): 1–15. http://dx.doi.org/10.1186/1750-0680-8-1.
Woziwoda B., Ambrożkiewicz K. (2011). Diversity of forest and shrub communities as a result of site history and of extensive and intensive forest management (Glinno Ługi case study). Acta Universitatis Lodziensis, Folia Biologica et Oecologica 7: 149–162. http://dx.doi.org/10.2478/v10107-009-0022-1.
Wulf M. (2004). Plant species richness of afforestation with different former use and habitat continuity. Forest Ecology and Management 195: 191–204. http://dx.doi.org/10.1016/j.foreco.2004.02.046.
Wulf M., Heinken T. (2008). Colonization of recent coniferous versus deciduous forest stands by vascular plants at the local scale. Applied Vegetation Science 11: 307–316. http://dx.doi.org/10.3170/2008-7-18432.
Yarie J. (1980). The role of understorey vegetation in the nutrient cycle of forested ecosystems in the mountain hemlock biogeoclimatic zone. Ecology 61: 1498–1514. http://dx.doi.org/10.2307/1939057.
Zerbe S. (2002). Restoration of natural broad-leaved woodland in Central Europe on sites with coniferous forest plantations. Forest Ecology and Management 167: 27–42. http://dx.doi. org/10.1016/S0378-1127(01)00686-7.
Zheng Z.-L. (2009). Carbon and nitrogen nutrient balance signaling in plants. A review. Plant Signaling & Behavior 4(7): 584–591. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2710548/pdf/psb0407_0584.pdf.
Total of 128 references

