Height increment of hybrid aspen Populus tremuloides x P. tremula as a function of weather conditions in central part of Latvia
Jansons Ā., Zeps M., Rieksts-Riekstiņš J., Matisons R., Krišāns O. (2014). Height increment of hybrid aspen Populus tremuloides x P. tremula as a function of weather conditions in central part of Latvia. Silva Fennica vol. 48 no. 5 article id 1124. https://doi.org/10.14214/sf.1124
Highlights
- Intra-annual height growth of hybrid aspen was monitored
- Clones with early leaf flushing dates showed faster height growth
- Height growth was generally controlled by temperature
- Fast-growing hybrids were more robust to weather conditions than slow-growing ones
- Potential evapotranspiration (moisture regime) negatively affected height growth of clones with delayed phenology.
Abstract
Height growth of young hybrid aspen (Populus tremula L. × P. tremuloides Michx.) was studied in relation to weather conditions. Height of clones with different leaf flushing phenology (early, intermediate and late) was monitored during the growing periods of 2010 and 2011 in a plantation established on former agricultural land. Mean daily height increment (HI) was calculated. Multiple linear regression was used to determine which weather factors (variables) had significant effect on HI. Mean seasonal height growth (mean seasonal HI) between clones (groups) was compared by ANOVA. In both years, HI was significantly higher for clones with early and intermediate leaf flushing compared to clones with late leaf flushing. The effect of weather factors also differed between clones according to their leaf flushing phenology; it was the weakest for HI of clones with early leaf flushing compared to clones with intermediate and late leaf flushing. Mean temperature was the main factor, which positively affected HI of all clones, suggesting that warmer climate might be beneficial for height growth of young hybrid aspen in Latvia. Nevertheless, significant negative relationship between HI and potential evapotranspiration (PET) was observed for clones with delayed leaf flushing, suggesting negative effect of increasing variability of precipitation on growth. Thus, the differences in height growth intensity might be related to growth sensitivity to weather conditions. On the other hand, such differences in height growth between clones might be caused by competition (i.e. with herbs), as trees with early leaf flushing might conquer more resources and become more robust against the environmental fluctuation.
Keywords
height growth;
energy wood;
tree growth;
temperature;
intra-annual dynamics;
plantation silviculture
- Jansons, LSFRI „SILAVA”, Rigas Str. 111, Salaspils, Latvia, LV2169 E-mail aris.jansons@silava.lv
- Zeps, LSFRI „SILAVA”, Rigas Str. 111, Salaspils, Latvia, LV2169 E-mail martins.zeps@silava.lv
- Rieksts-Riekstiņš, LSFRI „SILAVA”, Rigas Str. 111, Salaspils, Latvia, LV2169 E-mail Juris.Riekstins@silava.lv
-
Matisons,
LSFRI „SILAVA”, Rigas Str. 111, Salaspils, Latvia, LV2169
E-mail
robism@inbox.lv

- Krišāns, LSFRI „SILAVA”, Rigas Str. 111, Salaspils, Latvia, LV2169 E-mail oskars.krisans@silava.lv
Received 24 February 2014 Accepted 21 August 2014 Published 5 December 2014
Views 156710
Available at https://doi.org/10.14214/sf.1124 | Download PDF

1 Introduction
Growing demand for renewable energy is increasing the importance of energy crops and tree plantations (Schueler et al. 2013). In the Baltic Sea region, hybrid aspen (Populus tremula L. × P. tremuloides Michx.) is one of the most promising trees (hybrids) for biomass production due to high growth rates (Yu et al. 2001b; Tullus et al. 2011). Growth of hybrid aspen is rapid, significantly exceeding wood volume increment of its parent species due to longer period of assimilation (Yu et al. 2001b; Rytter and Stener 2005). Nevertheless, significant differences in onset and termination dates, productivity and dynamics of growth between various clones and families of Populus species have been shown (Ceulemans et al. 1987; Orlovic et al. 1998; Yu et al. 2001a), thus breeding and selection of appropriate progeny traits might be used to improve the yields from hybrid aspen plantations (stands).
The length of growing period of trees in northern latitudes is limited by photoperiod; however, temperature affects growth onset and particularly cessation dates, causing annual variation of length of growing period (Sarvas 1972; Rohde et al. 2011). Under changing climate, vegetation period in Europe has extended (Menzel and Fabian 1999) increasing productivity of tree growth in northern latitudes (Boisvenue and Running 2006; Lindner et al. 2010) that seems advantageous for forestry. On the other hand, the fit of environmental conditions throughout the growing season to the physiological demands (i.e. optimal temperature or moisture regime) of trees has been shown to maximize growth (Kolari et al. 2007). An extension of growing (vegetation) season due to climate changes can have negative effect on growth via increase of susceptibility to spring and autumn frosts (Gu et al. 2008). Additionally, raised temperature in summer intensifies evapotranspiration (Trajkovic 2005) that might cause drought stress (Pallardy 2008; Berry and Downtown 2012). Although this is usual in arid regions (i.e. Mediterranean region) (Rozas 2001; Andreu et al. 2007), similar effects have recently been observed also for trees growing in boreal regions (Wilmking et al. 2004). Thus, knowledge on climate growth relationships, considering regional variability (Andreu et al. 2007), is necessary.
Analysis of secondary growth (radial increment) is commonly used for assessment of tree-growth-climate relationships (Speer 2010); however, height growth of trees is a crucial parameter that influences productivity of stands (primary growth and stem volume increment) and quality of wood (i.e. knottiness), which are important for multiple use plantations (Savill et al. 1997; Burton 2012). Height growth rates have been also related to the development of tree species in mixed stands due to differences in competitive ability (Scott and Loehle 1998). Considering that hybrid aspen is intended for biomass production and tree height is used for assessment of biomass (Zabek and Prescott 2006), knowledge on the effect of weather on height growth is necessary for assessment of potential yields under changing climate. Still, information on the climate-growth interaction of hybrid aspen in the Baltic Sea region is insufficient. In this regard, the aim of this study was to determine the effect of weather factors (variables) on intra-annual height increment of young hybrid aspen in Latvia. The development of equations describing height growth of hybrid aspen within growing season based on weather factors (mean daily temperature and precipitation) was a subordinate objective.
2 Material and methods
2.1 Study site
The studied site was a plantation of hybrid aspen located in south-western part of Latvia on the eastern side of Eastern Courland upland (Fig. 1). The absolute elevation of site was ~ 90 m a.s.l. and the topography was flat; plantation was growing on well-drained arenosoil. The plantation was established in 2007 on former agricultural land using one-year old containerized seedlings, which were produced by a microclonal propagation method. Planted material was clones of the most productive 15 progenies from 10 control-crossed families (grown in national forest research station in eastern part of Latvia, near Kalsnava) of Populus tremuloides (growing in botanical garden in central part of Latvia, but no information on its origin was available) and 10 local Populus tremula plus trees (growing in plantations in central and eastern region of Latvia) (Smilga 1970). Initial spacing of the plantation was 3 x 3 m; no thinning was performed prior to sampling (height measurements). Ramets of 15 clones were randomly distributed in 25 replications (single tree plots). The proportion of damaged trees before the sampling in 2010 (at the age of five years) was rather high, reaching ~ 30% of all planted trees; these trees, however, were not sampled.
Fig. 1. Location of the study site (black square, 56°27´N, 22°54´E).
Climate in the study area is mild and oceanic; determined by dominant western winds, which bring warm and moist air masses from the Baltic Sea and the Atlantic; however, a considerable part of precipitation is intercepted by western Courland uplands (Temņikova 1975). According to data from Latvian Environment, Geology and Meteorology Centre (LEGMC), mean annual temperature is about 6 °C, the warmest month is July (mean temperature is 16 °C) and the coldest month is January (mean temperature is –3.7 °C). The length of the growing period (mean daily temperature > 5 °C) ranges from 185 to 195 days. The amount of annual precipitation is ~ 580 mm, but the distribution of precipitation is uneven; most of the precipitation (~ 380 mm) falls during summer (June–August). Precipitation in winter is low (< 100 mm), the depth of snow layer rarely exceeds 0.2 m due to frequent thaws.
Weather (climatic) conditions during March–October of 2010 and 2011 (years of sampling) were generally similar (Fig. 2). Mean temperature during May–October period in these years was 10.7 and 11.2 °C, respectively, and precipitation sums were 540 and 498 mm, respectively. Seasonal changes of mean monthly temperature were similar; however, increased variability of temperature (increased differences between monthly minimal and maximal temperature) was observed in March and April of 2010 and in May 2011. The distribution of precipitation was more heterogeneous in 2011, when spring and autumn were relatively drier and the proportion of precipitation that fell in July and August was higher (45 and 58% of annual precipitation in 2010 and 2011, respectively).
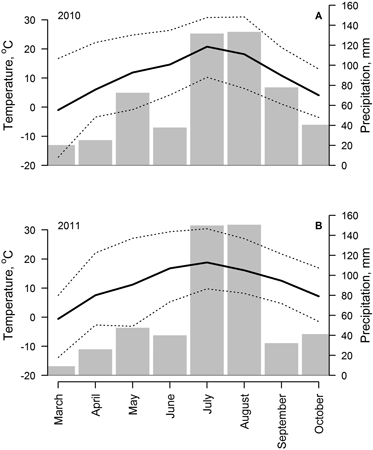
Fig. 2. Weather conditions (monthly mean (solid line), absolute minimum and maximum (dotted lines) temperature and precipitation sums (bars)) from March to November of 2010 (A) and 2011 (B), recorded in meteorological station located at 2 km distance from the study site.
The nearest meteorological station (Davis Vintage Pro2) was located at ~ 2 km distance from the sampling site. Measurements of weather parameters were recorded with hourly resolution. Data on temperature, precipitation, relative air humidity, potential evapotranspiration (PET) and solar radiation intensity with daily resolution, covering period from May to October in 2010 and 2011, were obtained. Additionally, sums of active temperatures (mean daily temperature > + 5 °C) (Sarvas 1972) were calculated.
2.2 Sampling
Height growth of 278 undamaged trees higher than one metre in April 2010 and representing 13 clones (each with 12–23 ramets), was monitored from mid-May until cessation of growth (beginning of October). Measurements of tree height were done periodically throughout the growing seasons (mid-May–October) of 2010 and 2011 (5th and 6th growing season); 18 observations per season were done. Length of the period between measurements ranged from four to 22 days in the beginning and in the middle of growing period, respectively, and from seven to 18 days in the later part of the season (nine days on average). Height of trees was measured with accuracy of one centimetre. Permanent marking of trees (ramets) was made prior to height measurements to ensure correct recognition of the study material. Dates of leaf flushing for the same trees were obtained from measurements during the spring of 2010 and 2011 when phenophases were monitored daily.
2.3 Data analysis
Considering that clones with different phenology can have different growth patterns (Ceulemans et al. 1987), studied trees were divided into three groups (early, intermediate and late) according to differences in leaf flushing phenology. For that reason, range of dates (day of year) of leaf flushing was divided into three equal parts and clones were grouped accordingly. Similar results were obtained based on data from 2010 and 2011; the groups differed in mean leaf flushing dates (day of year) by ~ 3.5 days and there were only slight overlaps between consecutive groups. Groups with early, intermediate and late leaf flushing consisted of 117, 102 and 59 trees (representing seven, six and three clones), respectively. There were also differences in tree height between groups at the beginning and at the end of studied period; clones with early and intermediate leaf flushing phenology were higher (Fig. 3). Considering that periods between measurements of tree height differed, mean daily height increment (HI) was calculated and used for further analysis.
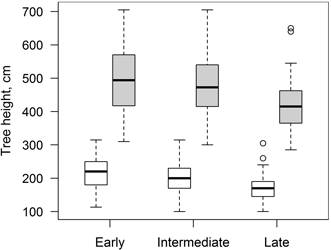
Fig. 3. Mean height of clones of hybrid aspen with early, intermediate and late leaf flushing phenology in the beginning (17.05.2010, white boxes) and at the end (10.10.2011, grey boxes) of the increment monitoring period. Median is shown by the bold line, box corresponds to lower and upper quartile, whiskers show minimum and maximum values (within 150% of interquartile range from the median) and circles represent outliers of the datasets.
The differences in HI between groups of clones in each period were assessed by ANOVA and Tukey’s HSD test (Sokal and Rohlf 1995). The effect of climatic variables on HI of each group of clones was assessed by multiple linear regression (McGullagh and Nelder 1989); all parameters were tested for normality by Shapiro-Wilk test (Sokal and Rohlf 1995). In the initial model, 10 parameters (1) solar radiation intensity, 2) sum of temperature, 3) sum of active temperatures, 4) mean temperature, 5) mean daily precipitation, 6) precipitation sum, 7) relative humidity, 8) potential evapotranspiration (PET) (summarized according to the length of the period between height measurements), 9) length of period between measurements and 10) day of growth (number of days after growth initiation)) were included as independent variables. Stepwise bidirectional elimination was used for the selection of model (equation) variables (variables were eliminated until all remaining variables were significant). The selection of the best-describing models was based on Akaike information criterion (AIC) (McGullagh and Nelder 1989), R-squared (multiple and adjusted) values (Sokal and Rohlf 1995), F-statistic and residual standard errors, which describe the explanatory power of models. In cases, when two or more variables were included in the model, multicolinearity, which causes overestimation of model, was assessed by Variance Inflation factor (Lin et al. 2011). Residuals were tested for normality and heteroscedasticity (preconditions for linear models) by Shapiro-Wilk test and Breusch-Pagan test, respectively, and additionally by graphical assessment. Comparison of different models for the same response variable(s) was done using ANOVA. Data analysis was conducted in program R v. 3.0.1 (R Core Team 2013) using package “gvlma” (Pena and Slate 2012).
3 Results
3.1 Intra-annual height growth pattern
Significant differences in height growth (annual HI) were observed between the groups of clones with diverse leaf flushing phenology (Fig. 4); HI was significantly (p-value < 0.01) lower for clones with late leave flushing, while the differences between the other two groups were insignificant (p-value > 0.05) (Fig. 4). Height growth (HI) of hybrid aspen had different intensity during the growing period in both of studied years but it culminated in July. Considering that growth was monitored since mid-May, the onset of height growth was not documented by height measurements (Fig. 5), explaining increased HI in the first intervals, particularly in 2010. Although height increment of all three groups of clones was higher in 2011 (Fig. 4), HI showed steeper decrease at the cessation of growth at the end of growing season of 2011. In contrast, mean annual HI in 2010 was lower and the changes of HI during the growing season were smoother.
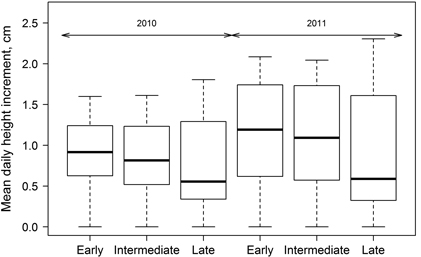
Fig. 4. Mean daily height increment for growing seasons of 2010 and 2011 for clones of hybrid aspen with early, intermediate and late leaf flushing. Median is shown by the bold line, box corresponds to lower and upper quartile, whiskers show minimum and maximum values (within 150% of interquartile range from the median) and circles represent outliers of the datasets.
Differences in HI variation patterns were observed between groups of clones (Fig. 5) as there were only a few periods in both growing seasons when differences in HI between groups (particularly between late flushing and the other two groups) were insignificant. Generally, clones with early and intermediate leaf flushing showed higher HI in the beginning and in the ending of growing periods (particularly in 2011). Clones with late leaf flushing showed the lowest HI in the beginning and the ending of growing season and the highest HI in the mid-season (July); however, height of these clones was the lowest during the whole monitored period (Fig. 3). Clones with intermediate leaf flushing phenology showed the highest HI among all groups in late August of 2011.
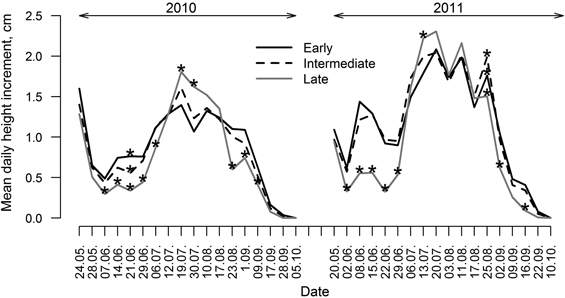
Fig. 5. Mean daily height increment of clones of hybrid aspen with early, intermediate and late leaf flushing phenology, calculated for periods between measurements for growing seasons of 2010 and 2011. Asterisks indicate significance (p-value < 0.05) of differences of height increment among groups of clones with differing phenology of leaf flushing.
3.2 Effect of weather on height growth
The developed models, describing HI based on weather variables, were significant (p-values < 0.001) and model residuals corresponded to normal distribution; the use of collinear variables was omitted. The regression equations (models) differed according to groups of clones and between studied periods (2010, 2011 or both years together) (Table 1) as suggested by their fit to data (R-squared and F-statistic values and residual standard errors). HI was better described by the tested climatic factors (variables) in 2010 than in 2011 for all three groups of clones as suggested by higher R-squared and F-statistic values and lower values of residual standard errors. Equations based on data from both seasons showed lower R-squared values than when years were used separately. The fit of equations slightly differed also between the groups of clones. Equations developed for trees with early leaf flushing showed slightly lower standard errors compared with the other two groups.
| Table 1. Significant variables, their coefficients and significance, and main statistics for developed equations of height increment of hybrid aspen based on weather variables and duration of growth (as HI = ax1 + bx2 +...+ intercept, where x1, x2 etc. are the significant parameters and a, b, etc. are coefficient values) showing the best fit to HI. Equations for data of 2010, 2011 and both years are shown. PET – potential evapotranspiration. | ||||||||
| Early leaf flushing | ||||||||
| 2010 | 2011 | Both years | ||||||
| Variable | Coeff. | p-value | Variable | Coeff. | p-value | Variable | Coeff. | p-value |
| Intercept | 0.142 | 0.719 | Intercept | –1.908 | 0.023 | Intercept | –0.782 | 0.060 |
| Mean temp. | 0.116 | <0.001 | Mean temp. | 0.274 | <0.001 | Mean temp. | 0.192 | <0.001 |
| Temp. sum ( > 5 °C) | –0.003 | 0.033 | Temp. sum ( > 5 °C) | –0.007 | 0.038 | Temp. sum ( > 5 °C) | –0.007 | <0.001 |
| Day of growth | –0.006 | 0.032 | ||||||
| AIC | –29.2 | –19.97 | –38.69 | |||||
| F-statistic | 16.51 | 15.94 | 24.15 | |||||
| R-squared | 0.81 | 0.69 | 0.62 | |||||
| Adjuster R-squared | 0.76 | 0.65 | 0.59 | |||||
| Residual standard error | 0.34 | 0.53 | 0.53 | |||||
| Intermediate leaf flushing | ||||||||
| 2010 | 2011 | Both years | ||||||
| Variable | Coeff. | p-value | Variable | Coeff. | p-value | Variable | Coeff. | p-value |
| Intercept | –0.684 | 0.061 | Intercept | –1.201 | 0.186 | Intercept | –1 | 0.026 |
| Mean temp. | 0.159 | <0.001 | Mean temp. | 0.396 | <0.001 | Mean temp. | 0.208 | <0.001 |
| Temp. sum ( > 5 °C) | –0.005 | 0.002 | Solar radiation | 1.165 | 0.004 | Temp. sum ( > 5 °C) | –0.007 | <0.001 |
| PET | –3.409 | 0.003 | ||||||
| AIC | –27.62 | –20.66 | –34.62 | |||||
| F-statistic | 21.89 | 15.47 | 24.74 | |||||
| R-squared | 0.78 | 0.77 | 0.62 | |||||
| Adjuster R-squared | 0.75 | 0.72 | 0.6 | |||||
| Residual standard error | 0.36 | 0.51 | 0.57 | |||||
| Late leaf flushing | ||||||||
| 2010 | 2011 | Both years | ||||||
| Variable | Coeff. | p-value | Variable | Coeff. | p-value | Variable | Coeff. | p-value |
| Intercept | –1.082 | 0.009 | Intercept | –1.169 | 0.265 | Intercept | –0.312 | 0.617 |
| Mean temp. | 0.174 | <0.001 | Mean temp. | 0.489 | <0.001 | Mean temp. | 0.337 | <0.001 |
| Temp. sum ( > 5 °C) | –0.005 | 0.006 | PET | –4.475 | 0.002 | PET | –3.354 | 0.003 |
| Solar radiation | 1.389 | 0.006 | Solar radiation | 1.038 | 0.005 | |||
| AIC | –26.02 | –12.76 | –22.75 | |||||
| F-statistic | 23.6 | 12.18 | 12.81 | |||||
| R-squared | 0.8 | 0.72 | 0.57 | |||||
| Adjuster R-squared | 0.76 | 0.66 | 0.53 | |||||
| Residual standard error | 0.38 | 0.64 | 0.67 | |||||
The developed equations combining two or three (weather) variables showed the best explanatory power (fit to data). Mean temperature was the only variable significant (p-values < 0.001) in all equations (Table 1); its coefficient values varied between the seasons (highest values observed in 2011) and groups of clones (highest for trees with late leaf flushing). PET was the only precipitation related variable, which showed a significant effect on HI (except trees with early leaf flushing) when the whole growing period was analysed. In addition to mean temperature, HI of trees with early leaf flushing was also affected by sum of temperatures > 5 °C and day of growth; however, their coefficient values and significance were lower (p-value above 0.01) (Table 1). Regarding clones with intermediate and late leaf flushing, the sets of climatic variables significant for HI clearly differed between studied years. Shift of additional (to mean temperature) variables from temperature sum in 2010 to solar radiation and PET in 2011 was observed for HI of clones with intermediate leaf flushing; still, temperature sum was significant, when both seasons were used together. In equations developed for clones with late leaf flushing, solar radiation and PET was significant in 2011 and when both seasons were used together, while temperature sum was significant only in 2010, suggesting stronger effect of PET and solar radiation for these clones.
4 Discussion
Although studied clones of hybrid aspen showed common trends of HI (Fig. 5), their productivity differed according to leaf flushing phenology (Figs. 3, 4) as shown for poplar by Ceulemans et al. (1987) and such differences have been related to the differences in length of the growing period (Yu et al. 2001b). Clones with earlier leaf flushing apparently had longer growing period, which allows to assimilate more nutrients and thus to increase growth (Barbaroux and Breda 2002; Pallardy 2008), as observed for boreal forests (Boisvenue and Running 2006; Lindner et al. 2010). Considering that the length of growing season was insignificant for growth of hybrid aspen in Finland (Yu et al. 2001b), the observed differences in height growth (Fig. 5) might have coincided with differences in leaf flushing phenology. In such case, differences in HI patterns might be related to distinct functional traits (Geber and Griffen 2003; Reich et al. 2003), represented in the studied clones. Nevertheless, the differences of HI variation patterns between the groups of clones (Figs. 5)) implied that differences in sensitivity of height growth to environmental factors were expectable (McCarroll et al. 2003; Speer 2010).
Considering that climatic conditions are essential for growth of trees (Salminen and Jalkanen 2007; Lindner et al. 2010), the established equations linking HI of hybrid aspen with weather factors (variables) were statistically significant (Table 1). The fit of the equations to data (adjusted R-squared values) ranged from 0.53 to 0.76, suggesting that more than 50% of HI variation might be explained by weather conditions. The sets of variables significant for HI in addition to mean temperature (which was significant in all equations) differed among the groups of clones (Table 1), suggesting differences in climate-growth sensitivity. Still, the differences of significant variables between years (Table 1) suggested that the tested climatic variables might not be truly universal for the estimation of HI throughout several seasons. Probably, sensitivity of growth might have been altered by temporal distribution of precipitation, as equation coefficients were higher for 2011 (Table 1), when precipitation was distributed more heterogeneously (Fig. 2). Changes in sets of significant climatic variables between consecutive years (Table 1) might be also related to the ageing of trees (Carrer and Urbinati 2006).
Although Latvia is located in central part of distribution area of aspen (EUFORGEN 2009), temperature showed strong positive effect on HI (lowest p-values for all equations) (Table 1) as observed for wood increment of trees growing in northern regions of their distribution (Wilmking et al. 2004; Salminen and Jalkanen 2007). The effect of temperature on HI might be explained by the direct influence on meristematic activity (i.e. speed of cell division, differentiation and elongation) (Pallardy 2008) and assimilation (Berry and Downtown 2012), which affects growth, particularly in the second part of growing period (Barbaroux and Breda 2002). The amount of precipitation, which is often significant for tree growth in southern and central parts of specie’s distribution area (Rozas 2001; Kelly et al. 2002; Jansons et al. 2013), did not show significant effect on HI of hybrid aspen (Table 1), suggesting that the availability of water was not limiting height growth throughout the entire vegetation season. Probably, precipitation might be significant for HI in June–August, when temperature is the highest (Fig. 2); but temporal resolution of our data was too coarse to analyse changes in climate-growth relationships within a season. Nevertheless, significant effect of PET was also observed, indicating that moisture regime (Trajkovic 2005) affected height growth of clones with intermediate and late leaf flushing.
Weather conditions showed the weakest effect on HI of clones with early leaf flush as suggested by the lowest equation coefficients (i.e. to mean temperature) (Table 1) compared with the other two groups. Robustness to weather variability has been shown to increase growth (Speer 2010; Burton 2012) thus explaining larger HI (Fig. 4). Positive effect of temperature on HI also implies that increased productivity might be obtained with warming of climate, thus the use of these clones might be considered for reduction of weather related hazards. However, earlier leaf flushing is also increasing susceptibility to late frosts and chill damage (Yu et al. 2001b; Gu et al. 2008), that might significantly reduce growth. Clones with intermediate leaf flushing were also productive (Fig. 4), but higher equation coefficient values (Table 1) suggested them as more susceptible to weather conditions. Additionally, significant negative effects of PET in 2011 (Table 1), when precipitation was more heterogeneous (Fig. 2), suggest that the increasing variability in temperature and precipitation (Avotniece et al. 2010) might affect growth. Slow growing clones (late leaf flushing) showed the strongest effect of the tested weather variables as suggested by the highest equation coefficient values (Table 1). The negative effect of PET on slow growing clones was observed in 2011 and when both years were analysed together, supporting the significance of moisture regime on HI. Thus, increasing heterogeneity of summer precipitation and temperature (Avotniece et al. 2010) is likely to result in reduction of height growth. Positive effect of solar radiation on HI suggested that HI was enhanced by sunny days; however, sunny days in summer are usually warmer than cloudy days (LEGMC) thus facilitating PET (Trajkovic 2005) and potentially forming a negative feedback loop. Nevertheless, reasonable effect of non-tested factors on slow growing clones was still apparent as suggested by the lowest R-squared values (0.53) when both seasons were analysed together. Alternatively, differences in mean HI and sensitivity to weather variables might be explained by competition (i.e. with herbs) (McMurtrie and Wolf 1983; Coomes and Grubb 2000). Trees starting growth earlier might be more advantageous in competition for resources (Rathcke and Lacey 1985), than trees with later onset of growth, thus providing alternative explanation to differences in growth rates and sensitivity to climatic factors (Martin-Benito et al. 2008). Although clones with early leaf flushing showed the fastest height growth and robustness to weather conditions during the growing period, long-term tests in various conditions should be applied to determine, which clones are the most suitable for commercial use.
5 Conclusions
Mean temperature was the main weather parameter that positively affected height growth of all studied trees. Increased growth of young hybrid aspen might therefore be expected due to warming of climate. Differences in set of significant weather factors and height growth intensity were found among clones of hybrid aspen with differing phenology of leaf flushing. Clones with the earliest leaf flushing showed the lowest sensitivity to temperature and the highest growth rates, proposing their suitability for commercial use, while clones with late leaf flushing showed the opposite. Clones with delayed leaf flushing were also affected by moisture regime, thus altered distribution of summer precipitation, as forecasted for Northern Europe, might impede their growth. On the other hand, differences in growth intensity between clones with distinct phenology might also be related to altered competition (i.e. with herbs). Still, considering that changes in climate-growth sensitivity occur with ageing of trees, growth of older hybrid aspen should be investigated to clarify potential climatic hazards.
Acknowledgements
Financial support for the initial study (measurements in first season) was provided by ESF project «Importance of Genetic Factors in Formation of Forest Stands with High Adaptability and Qualitative Wood Properties» (No. 2009/0200/1DP/1.1.1.2.0/09/APIA/VIAA/146), second year measurements and analysis was carried out in frame of Forest Competence Centre (ERAF) project «Methods and technologies for increasing forest capital value» (No. L-KC-11-0004). Raitis Rieksts-Riekstiņš, Jānis Jansons and Mārtiņš Puriņš helped much during the sampling.
References
Andreu L., Guiterrez E., Macias M., Ribas M., Bosch O., Camarero J.J. (2007). Climate increases regional tree-growth variability in Iberian pine forests. Global Change Biology 13: 804–815. http://dx.doi.org/10.1111/j.1365-2486.2007.01322.x.
Avotniece Z., Rodinov V., Lizuma L., Briede A., Kļaviņš M. (2010). Trends in frequency of extreme climate events in Latvia. Baltica 23: 135–148.
Barbaroux C., Breda N. (2002). Contrasting distribution and seasonal dynamics of carbohydrate reserves in stem wood of adult ring-porous sessile oak and diffuse-porous beech trees. Tree Physiology 22: 1201–1210. http://dx.doi.org/10.1093/treephys/22.17.1201.
Berry J.A., Downtown W.J.S. (2012). Environmental regulation of photosynthesis. In: Govindjee (ed.). Photosynthesis: development, carbon metabolism and plant productivity. Vol. 2. Elsevier, Amsterdam. p. 265–345.
Boisvenue C., Running S.W. (2006). Impacts of climate change on natural forest productivity – evidence since the middle of the 20th century. Global Change Biology 12: 862–882. http://dx.doi.org/10.1111/j.1365-2486.2006.01134.x.
Burton L. (2012). Introduction to forestry science. 3rd ed. Cengage Learning, New York. 554 p.
Carrer M., Urbinati C. (2006). Long-term changes in the sensitivity of tree-ring width growth to climate forcing in Larix decidua. New Phytologist 170: 861–872. http://dx.doi.org/10.1111/j.1469-8137.2006.01703.x.
Ceulemans R., Impens I., Steeneckers V. (1987). Variation in photosynthetic, anatomical, and enzymatic leaf traits and correlation with growth in recently selected Populus hybrids. Canadian Journal of Forests Research 17: 273–283. http://dx.doi.org/10.1139/x87-047.
Coomes D.A., Grubb P. (2000). Impacts of root competition in forests and woodlands: a theoretical framework and review of experiments. Ecological Monographs 70: 171–207. http://dx.doi.org/10.1890/0012-9615(2000)070[0171:IORCIF]2.0.CO;2.
EUFORGEN (2009). Distribution maps: Populus tremula. http://www.euforgen.org/fileadmin/www.euforgen.org/Documents/Maps/JPG/Populus_tremula.jpg. [Cited 12 Feb 2014].
Geber M.A., Griffen L.R. (2003). Inheritance and natural selection of functional traits. International Journal of Plant Science 164: 21–42. http://dx.doi.org/10.1086/368233.
Gu L., Hanson P.J., Post W.M., Kaiser D.P., Yang B., Enami R., Pallardy S.G., Meyers T. (2008). The 2007 Eastern US spring freeze: increased cold damage in a warming world? BioScience 58: 253–262. http://dx.doi.org/10.1641/B580311.
Jansons Ā., Matisons R., Baumanis I., Puriņa L. (2013). Effect of climatic factors on height increment of Scots pine in experimental plantation in Kalsnava, Latvia. Forest Ecology and Management 306: 185–191. http://dx.doi.org/10.1016/j.foreco.2013.06.039.
Kelly P.M., Leuschner H.H., Briffa K.R., Harris I.C. (2002). The climatic interpretation of pan-European signature years in oak ring-width series. Holocene 12: 689–694. http://dx.doi.org/10.1191/0959683602hl582rp.
Kolari P., Lappalainen H., Hänninen H., Hari P. (2007). Relationship between temperature and the seasonal course of photosynthesis in Scots pine at northern timberline and in southern boreal zone. Tellus 59: 542–552. http://dx.doi.org/10.1111/j.1600-0889.2007.00262.x.
Lin D., Foster D.P., Ungar L.H. (2011). VIF-regression: a fast regression algorithm for large data. Journal of the American Statistical Association 106: 232–247. http://dx.doi.org/10.1198/jasa.2011.tm10113.
Lindner M., Maroschek M., Netherer S., Kremer A., Barbati A., Garcia-Gonzalo J., Seidl R., Delzon S., Corona P., Kolström M., Lexer M.J., Marchetti M. (2010). Climate change impacts, adaptive capacity, and vulnerability of European forest ecosystems. Forest Ecology and Management 259: 698–709. http://dx.doi.org/10.1016/j.foreco.2009.09.023.
Martin-Benito D., Cherubini P., del Rio M., Canellas I. (2008). Growth response to climate and drought in Pinus nigra Arn. trees of different crown classes. Trees 22: 363–373. http://dx.doi.org/10.1007/s00468-007-0191-6.
McCarroll D., Jalkanen R., Hicks S., Tuovinen M., Gagen M., Pawellek F., Eckstein D., Schmitt U., Autio J., Heikkinen O. (2003). Multiproxy dendroclimatology: a pilot study in northern Finland. Holocene 13: 826–838. http://dx.doi.org/10.1191/0959683603hl668rp.
McGullagh P., Nelder J.A. (1989). Generalized linear models. 2nd ed. CRC Press, New York. 532 p.
McMurtrie R., Wolf L. (1983). A model of competition between trees and grass for radiation, water and nutrients. Annals of Ecology 52: 449–458.
Menzel A., Fabian P. (1999). Growing season extended in Europe. Nature 397: 659–659. http://dx.doi.org/10.1038/17709.
Orlovic S., Guzina V., Krstic B., Merkulov L. (1998). Genetic variability in anatomical, physiological and growth characteristics of hybrids poplar (Populus × euramericana Dode (Guinier)) and eastern cottonwood (Populus deltoides Bartr.) clones. Silvae Genetica 47: 383–397.
Pallardy S.G. (2008). Physiology of woody plants. 3rd ed. Elsevier, London. 454 p.
Pena E.A., Slate E.H. (2012). gvlma: global validation of linear models assumptions. R package version 1.0.0.1. http://CRAN.R-project.org/package=gvlma. [Cited 12 Feb 2014].
R Core Team (2013). R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/. [Cited 12 Feb 2014].
Rathcke B., Lacey E. (1985). Phenological patterns of terrestrial plants. Annual Review of Ecology and Systematics 16: 179–214. http://dx.doi.org/10.1146/annurev.es.16.110185.001143.
Reich P.B., Wright I.J., Cavender-Bares J., Craine M.J., Oleksyn J., Westoby M., Walter M.B. (2003). The evolution of plant functional variations: traits, spectra, and strategies. International Journal of Plant Science 164: 143–164. http://dx.doi.org/10.1086/374368.
Rohde A., Bastien C., Boerjan W. (2011). Temperature signals contribute to the timing of photoperiodic growth cessation and bud set in poplar. Tree Physiology 31: 472–482. http://dx.doi.org/10.1093/treephys/tpr038.
Rozas V. (2001). Detecting the impact of climate and disturbances on tree-rings of Fagus sylvatica L. and Quercus robur L. in a lowland forest in Cantabria, Northern Spain. Annals of Forest Science 58: 237–251. http://dx.doi.org/10.1051/forest:2001123.
Rytter L., Stener L.G. (2005). Productivity and thinning effects in hybrid aspen (Populus tremula L. × P. Tremuloides Michx.) stands in southern Sweden. Forestry 78: 285–295. http://dx.doi.org/10.1093/forestry/cpi026.
Salminen H., Jalkanen R. (2007). Intra-annual height increment of Pinus sylvestris at high latitudes in Finland. Tree Physiology 27: 1347–1353. http://dx.doi.org/10.1093/treephys/27.9.1347.
Sarvas R. (1972). Investigations on the annual cycle of development of forest trees. Active period. Communicationes Instituti Forestalis Fenniae 76. 110 p.
Savill P., Evans J., Auclair D., Falck J. (1997). Plantation silviculture in Europe. Oxford University Press, Oxford. 308 p.
Schueler V., Weddige U., Beringer T., Gamba L., Lamers P. (2013). Global biomass potentials under sustainability restrictions defined by the European Renewable Energy Directive 2009/28/ EC. GCB Bioenergy 5: 652–663. http://dx.doi.org/10.1111/gcbb.12036.
Scott P., Loehle C. (1998). Height growth tradeoffs determine northern and southern range limits of trees. Journal of Biogeography 25: 735–742. http://dx.doi.org/10.1046/j.1365-2699.1998.2540735.x.
Smilga J. (1970). Growth and morphology of aspen progeny. Jaunākais mežsaimniecībā 12: 3–19. [In Latvian].
Sokal R.R., Rohlf F.J. (1995). Biometry. 3rd ed. Freeman and Company, New York. 887 p.
Speer J.H. (2010). Fundamentals of tree-ring research. The University of Arizona Press, Tucson. 333 p.
Temņikova N. (1975). Klimats. [Climate]. In: Pūriņš V. (ed.). Latvijas PSR ģeogrāfija. [Geography of SSR of Latvia]. Zinātne, Riga. p. 45–54.
Trajkovic S. (2005). Temperature-based approaches for estimating reference evapotranspiration. Journal of Irrigation and Drainage Engineering-ASCE 131: 316–323. http://dx.doi.org/10.1061/(ASCE)0733-9437(2005)131:4(316).
Tullus A., Rytter L., Tullus T., Weih M., Tullus H. (2011). Short-rotation forestry with hybrid aspen (Populus tremula L. × P. tremuloides Michx.) in Northern Europe. Scandinavian Journal of Forest Research 27: 10–29. http://dx.doi.org/10.1080/02827581.2011.628949.
Wilmking M., Juday G.P., Barber V.A., Zald H.J. (2004). Recent climate warming forces contrasting growth responses of white spruce at treeline in Alaska through temperature thresholds. Global Change Biology 10: 1724–1736. http://dx.doi.org/10.1111/j.1365-2486.2004.00826.x.
Yu Q., Pulkkinen P., Rautio M., Haapanen M., Alen R, Stener E., Beuker E., Tigerstedt P.M.A. (2001a). Genetic control of wood physiochemical properties, growth, and phenology in hybrid aspen clones. Canadian Journal of Forest Research 31: 1348–1356. http://dx.doi.org/10.1139/x01-066.
Yu Q., Tigerstedt P.M.A., Haapanen M. (2001b). Growth and phenology of hybrid aspen clones (Populus tremula L. × Populus tremuloides Michx.). Silva Fennica 35: 15–25. http://dx.doi.org/10.14214/sf.600.
Zabek L.M., Prescott C.E. (2006). Biomass equations and carbon content of aboveground leafless biomass of hybrid poplar in Coastal British Columbia. Forest Ecology and Management 223: 291–302. http://dx.doi.org/10.1016/j.foreco.2005.11.009.
Total of 46 references

