Damage by the pine weevil Hylobius abietis to seedlings of two native and five introduced tree species in Sweden
Wallertz K., Nordenhem H., Nordlander G. (2014). Damage by the pine weevil Hylobius abietis to seedlings of two native and five introduced tree species in Sweden. Silva Fennica vol. 48 no. 4 article id 1188. https://doi.org/10.14214/sf.1188
Highlights
- Both native and introduced confer species in Sweden can be highly susceptible to damage by the pine weevil
- Douglas fir and Sitka spruce were generally the most damaged among six studied conifer species
- The results highlight some of the risks in establishing exotic tree species for forest production.
Abstract
There is increasing interest in using introduced species in Swedish forestry in response to climate change, but it is important to assess their resistance to native pests. Thus, we compared the extent of pine weevil feeding on two dominant native conifers, Scots pine (Pinus sylvestris L.) and Norway spruce (Picea abies (L.) Karst.), the non-host deciduous broadleaf hybrid aspen (Populus × wettsteinii Hämet-Ahti) and four introduced conifers: Douglas fir (Pseudotsuga menziesii (Mirb.) Franco), hybrid larch (Larix × marschlinsii Coaz), Sitka spruce (Picea sitchensis (Bong.) Carriére) and lodgepole pine (Pinus contorta Douglas ex Loudon). The extent of feeding damage on seedlings and its effect on their vitality were examined in a field study in south-central Sweden and a laboratory experiment, which gave largely consistent results. Generally, the species most heavily attacked by the pine weevil, in both experiments, were Douglas fir and Sitka spruce. In the field experiment pine weevils killed or severely damaged significantly higher proportions of Douglas fir and Sitka spruce seedlings (60%) than any other species except Norway spruce (49%). Among conifer seedlings the proportions of killed or severely damaged seedlings were lowest for Scots pine and hybrid larch (27%) and Lodgepole pine (36%). The results indicate that most conifer species planted on young clear-cuttings in Sweden need some kind of pine weevil protection, and the possibility that introducing new tree species might increase damage caused by pests must be considered. For instance, widespread use of hybrid aspen could reduce damage by pine weevils, but increase damage by other, untested pests or pathogens.
Keywords
forest regeneration;
conifer seedlings;
defence;
feeding damage;
forest plantation;
resistance
-
Wallertz,
Unit for Field-based Forest Research, Swedish University of Agricultural Sciences (SLU), Asa Research Station, SE-36030 Lammhult, Sweden
E-mail
kristina.wallertz@slu.se

- Nordenhem, Department of Ecology, Swedish University of Agricultural Sciences (SLU), P.O. Box 7044, SE-75007 Uppsala, Sweden E-mail henrik.nordenhem@slu.se
- Nordlander, Department of Ecology, Swedish University of Agricultural Sciences (SLU), P.O. Box 7044, SE-75007 Uppsala, Sweden E-mail goran.nordlander@slu.se
Received 9 May 2014 Accepted 25 August 2014 Published 13 October 2014
Views 147722
Available at https://doi.org/10.14214/sf.1188 | Download PDF

1 Introduction
Swedish forests are dominated by Norway spruce (Picea abies (L.) Karst.), Scots pine (Pinus sylvestris L.) and birches (Betula pendula Roth and Betula pubescens Ehrh.), which account for approximately 95% of the total growing stock (Skogsdata 2013). However, due to climate changes, including increases in mean temperatures and the length of growing seasons, there is increasing interest in introducing other tree species that might grow well in Sweden, but also species with desirable wood properties. Considered species include lodgepole pine (Pinus contorta) and Sitka spruce (Picea sitchensis), which may grow more rapidly than their native relatives Norway spruce and Scots pine, respectively (Elfving et al. 2001; Kristensen 2011; Gundale et al. 2013). Douglas fir (Pseudotsuga menziesii) may also grow well and produces strong, wood with numerous applications (Hermann and Lavender 1999; Karlsson 2007). Two other introduced species that grow rapidly in appropriate conditions are hybrid larch (a cross between Larix decidua Mill. and Larix kaempferi (Lamb) Carriére), which can also produce denser wood than native Swedish conifers (Larsson-Stern 2003), and hybrid aspen (a cross between Populus tremula and Populus tremuloides) (Tullus et al. 2012).
In addition to growth potential and wood properties it is also essential to consider the disease and pest resistance, or tolerance, of introduced species. A major pest of conifers in northern Europe is the pine weevil (Hylobius abietis), especially in areas where forests are mainly regenerated by planting after clearcutting old stands (Day and Leather 1997; Långström and Day 2004). The weevil’s feeding on the stem bark of young conifer seedlings often causes growth losses and high mortality (Örlander and Nilsson 1999; Day et al. 2004). Damage by the pine weevil is sometimes also recorded on seedlings of deciduous trees like birch even in the presence of conifer seedlings (Toivonen and Viiri 2006), but little feeding damage has been found on Populus spp. (Samuelsson 2001; Månsson and Schlyter 2004). However, since the demand for short-rotation plantation is increasing and hybrid aspen has proved to be one of the fastest growing deciduous tree species (Tullus et al. 2012) and therefore highly interesting for biomass production it is important to determine the level of damage caused by pine weevils to this tree species. Pine weevil feeding preferences among a number of different conifer species have mainly been investigated under laboratory conditions, and cut-off twigs or seedling stems have usually been used instead of living trees or seedlings (Leather et al. 1994; Manlove et al. 1997; Bratt et al. 2001; Olenici and Olenici 2003; Månsson and Schlyter 2004). This may give misleading results, since defence mechanisms may be induced in living seedlings that could strongly influence both feeding preferences and the amounts of bark consumed (Zas et al. 2011).
The severity of the damage caused by the pine weevil is strongly related to the size of the seedling and in particular root collar diameter (Thorsén et al. 2001). The diameter is negatively correlated with the risk of mortality, i.e. a sufficiently thick stem is crucial for survival. The same absolute area of bark consumed on seedlings with different diameters will affect the larger seedlings less because more bark has to be consumed before the seedling is mortally injured, commonly by girdling of the stem (Selander 1993; Örlander and Nilsson 1999; Nordlander et al. 2011). In order to make a fair comparison between different tree species the seedlings should preferably be of similar size and age, and should also have been subjected to the same conditions in the nursery. However, this might be difficult to achieve because of large variation in growth between tree species.
Plant defence mechanisms include both resistance and tolerance systems, which respectively reduce damage and the impact of damage caused by pests (Zas et al. 2011). Resistance can either be related to constitutive defence, which is permanently expressed regardless of the plant exposure to enemies or to induced defence, when the response is activated by herbivore attacks (Karban and Myers 1989). For the same level of damage Zas et al. (2011) found that the native Pinus pinaster was more tolerant to damage than the introduced Pinus radiata. Both the constitutive defences of seedlings and their responses to damage caused by native enemies might differ between tree species and thus warrant careful consideration before the widespread use of any candidate species.
An important defence mechanism in most conifers is their ability to produce resin (Phillips and Croteau 1999; Trap and Croteau 2001), but resin flows vary substantially between species (Wainhouse et al. 2009). Differences in the species’ resin defence are likely to influence the size and location of feeding scars and thus the risk of seedling mortality (commonly by girdling near the base). The defensive response can also be induced by previous attacks of pine weevil as shown by Moreira et al. (2008) who found twice as high resin canal density in the xylem of Pinus pinaster seedlings in an attacked stand compared to a nearby unattacked stand. Variation in the amount of feeding is also reportedly related to plants’ nitrogen (N) content, indicating that weevils are probably sensitive to both the nutritional and defence status of plants they are consuming (Wainhouse et al. 2004).
Because of the growing interest for introduced tree species in Swedish forestry, it is important to obtain detailed information on their susceptibility to the pine weevil. Thus, in the presented study we compared the extent of the pest’s feeding on various introduced tree species of commercial interest to the two most common native conifer species in Sweden, (in both a controlled laboratory experiment and a field study over a longer time period, with the same types of seedlings). We assessed the proportions of seedlings attacked by pine weevils, proportions they killed or severely damaged, the amount of bark consumed by the weevils, and locations of feeding scars on seedlings of the included tree species.
2 Materials and methods
2.1 Seedlings
The tree species included in the study were the two main native species Scots pine (Pinus sylvestris) and Norway spruce (Picea abies), and the five introduced species Douglas fir (Pseudotsuga menziesii), hybrid larch (Larix × marschlinsii), Sitka spruce (Picea sitchensis), lodgepole pine (Pinus contorta) and hybrid aspen (Populus × wettsteinii). All of these species are conifers except hybrid aspen, a deciduous broadleaf that is not considered a host species of the pine weevil (Månsson and Schlyter 2004).
For each tree species, the same type of seedlings was used in both the field and laboratory experiments. They were all delivered at the same time and had been grown in the same section of the nursery. All, except those of Scots pine were containerized seedlings grown in HIKO® containers (volume 93 cm3) or, in the case of lodgepole pine, in Starpot containers (volume 120 cm3). The Scots pine seedlings were of Plug+1 type (initially grown for 10 weeks in a container and then transplanted to a seed bed). The reason for not using containerized seedlings of Scots pine was a shortage of this seedling type in nurseries at the time. All the seedlings were delivered from Södra odlarna, Falkenberg, except the lodgepole pine seedlings, which were obtained from Skogforsk, Sävar, because commercial planting of this species is prohibited below latitude 60°N and is thus grown in nurseries in the central/north part of Sweden. Norway spruce, Scots pine, Sitka spruce, and hybrid larch originated from different seed orchards (Bredinge, Gottharsberg, Flensburg and Maglehem), Douglas fir was sown from seeds collected in British Colombia (provenance Larch hills) and hybrid aspen was a mixture of clones. All seedlings were grown for commercial production, e.g. fertilization and watering were following standard procedures in the nursery. Initially the mean root collar diameter differed between the species, and both Scots pine and hybrid larch seedlings had significantly larger diameters than those of the other species (Table 1). At the end of the first season the hybrid larch seedlings had the largest mean root collar diameter, followed by Scots pine.
| Table 1. Mean diameters of living seedlings of the included tree species at the start of the field experiment and the end of the first season, analysed with a general linear model (Proc GLM, SAS) and separated by overall pair-wise comparisons using Tukey’s test, indicated with different letters, SE in brackets. | ||
| Tree species | Initial diameter (mm) | Diameter at the end of the first season (mm) |
| Norway spruce | 4.2 (0.10)b | 5.1 (0.17)de |
| Sitka spruce | 4.2 (0.12)b | 6.1 (0.20)c |
| Douglas fir | 4.5 (0.12) b | 5.7 (0.21)dc |
| Lodgepole pine | 3.9 (0.14)b | 5.9 (0.16)c |
| Scots pine | 7.3 (0.15)a | 8.5 (0.19)b |
| Hybrid larch | 7.5 (0.22)a | 9.5 (0.34)a |
| Hybrid aspen | 4.0 (0.14)b | 4.8 (0.16)e |
2.2 Field experiment
The field experiment was carried out in 2010 on three fresh clear-cuttings in the south-central part of Sweden (within 50 km of Asa Research Station; 57°10´N, 14°47´E). The sites were all characterized by a bilberry vegetation type, sandy silty till soil, and mesic soil moisture, and they were representative of relatively fertile forests (yield capacity around 10 m3 ha–1 year–1) with the original stands dominated by Norway spruce and Scots pine. At all three sites the previous stands, were harvested during the winter 2009/2010, slash was removed, and the sites were prepared for planting by disc trenching in early spring 2010. During the study year there were higher temperature and more precipitation during May to August compared with a long-term annual average from 1961–1990 (Ottosson Löfvenius 2010). The mean temperature was 14.1 °C (Asa Research Station´s climate station), which is more than 1 °C higher than normal. The average precipitation was higher than normal during the same period, with 95 mm compared with the reference normal of 52 mm. A randomized block design was used for the experiment including the seven tree species listed above. At each of the three sites five blocks with 70 seedlings each were planted, at 2m spacing, on 4–6 June. Each block consisted of 10 rows with one seedling of each tree species randomly distributed in each row. Thus, in total 1050 seedlings (10 seedlings of seven species x five blocks x three sites) were planted. If seedlings are entirely surrounded by pure mineral soil the risk of damage by the pine weevil is highly reduced (Petersson et al. 2005). Therefore, our site preparation and planting instructions were designed to ensure there was sufficient debarking by pine weevils to evaluate differences between tree species while promoting establishment of the seedlings. As a consequence, the seedlings were planted in mineral soil, but the distance to the humus layer was only about 2 cm instead of at least 10 cm as normally recommended.
Data on the status of each seedling were collected immediately after planting, two weeks after planting, and in October, at the end of the first season. Height and root collar diameter were measured at the time of planting and at the inventory in October, while pine weevil feeding was assessed two weeks after planting and in October. The area debarked by pine weevil on the main stem was recorded to within 0.1 cm2, and the severity of the damage was recorded as slightly damaged, severely damaged or killed by pine weevil (mortality due to other factors than pine weevil feeding was also recorded). In addition, in the October inventory the main stem of each seedling was visually divided into ten equally long sections (numbered from 1 at the base to 10 at the top) then the position of the main feeding damage (if any) was recorded, and the mean position of main damage was calculated for each species. Hybrid aspen was excluded in this analysis because few seedlings of the species were damaged.
2.3 Laboratory experiment
To obtain complementary information on pine weevil feeding preferences under controlled conditions we also compared the extent of feeding on seedlings of the included tree species in a non-choice laboratory experiment. Six seedlings of each species were individually planted in 1 L jars containing 0.75 L moist sand. A transparent plastic cylinder (height 33 cm, diameter 11.5 cm) was placed on top of each jar and four pine weevils (two females and two males) were released in each cylinder. The jars with seedlings were randomly placed on a uniformly illuminated bench in the centre of a chamber with a light-darkness regime of L18:D6 and a constant temperature of 22 °C. The experiment started on 21 June 2010, and after 1, 3, 7 and 9 days pine weevil feeding was recorded as the total area debarked on the main stem, to within 0.1 cm2.
2.4 Statistical analyses
SAS software (SAS Institute, Cary, NC, USA) was used for all analyses of the acquired data. The effect of differences in root collar diameter between tree species on seedling mortality and severe damage caused by pine weevil were analysed using a mixed model (Proc Mixed SAS), with initial seedling diameter as a covariate. Seedling mortality and severe seedling damage data were arc sine-transformed to improve normality and homoscedasticity. Site and block were treated as random factors and tree species as a fixed factor. For all tests, differences between means were deemed significant if P < 0.05. No significant effect of initial diameter or any interactions between tree species and diameter were detected, thus in the final models initial diameter was excluded.
In the field experiment, mean values of each response variable for each block and tree species were calculated before further analyses. When analysing species’ effects on proportions of “attacked seedlings” and “killed or severely damaged seedlings”, the data were arc sin-transformed to improve normality and homoscedasticity. The experiment was treated as a randomized block design with site and block as random factors, using the following (PROC GLM SAS) model (followed by Tukey’s tests to separate effects of individual factors when significant differences were identified):
![]()
where µ is the overall mean, αi the site effect (i = 1–3), βj the block effect (j = 1–5), γm the effect of tree species (m = 1–7) and εijm the experimental error.
In the laboratory experiment mean values of debarked area for each tree species were calculated for each time period. PROC GLM was used to test effects of considered variables over the whole period, and where significant differences were indicated means were separated by overall pair-wise comparisons using Tukey’s test.
3 Results
3.1 Field experiment
3.1.1 Diameter
There were large variations in diameters among seedlings of the same species, especially hybrid larch, and both Scots pine and hybrid larch seedlings had significantly larger mean diameters than the other species (Table 1). There were still significant between-species differences (P < 0.01) in proportions of killed or severely damaged seedlings at the end of the first growing season when initial diameter was included as a covariate (Table 2). Initial diameter had no significant effect on proportions of killed or severely damaged seedlings (P = 0.92) and no interactions between tree species and diameter were detected.
| Table 2. Results of analysis on differences in root collar diameter between tree species on seedling mortality and severe damage caused by pine weevil using a mixed model (Proc Mixed SAS), with initial seedling diameter as a covariate. | ||||||
| Seedling killed | Killed or severely damaged | |||||
| Df | F-value | P-value | Df | F-value | P-value | |
| Tree species | 6 | 2.78 | 0.02 | 6 | 3.08 | 0.01 |
| Diameter | 1 | 0.15 | 0.70 | 1 | 0.06 | 0.82 |
| Diameter × tree species | 6 | 1.17 | 0.33 | 6 | 1.25 | 0.29 |
3.1.2 Attack rate
As shown in Fig. 1, two weeks after plantation the proportion of attacked seedlings varied among the seven species from 1% (hybrid aspen) to 38% (Douglas fir), and significantly more Douglas fir seedlings had been attacked than seedlings of all other species except Sitka spruce and lodgepole pine. After one season, proportions of seedlings of the conifer species that had been attacked ranged from 64% to 81%, and the proportion significantly differed between the most frequently attacked conifers (Sitka spruce and Douglas fir) and the least frequently attacked (hybrid larch). Extremely few hybrid aspen seedlings had still been attacked (4%) at this time.
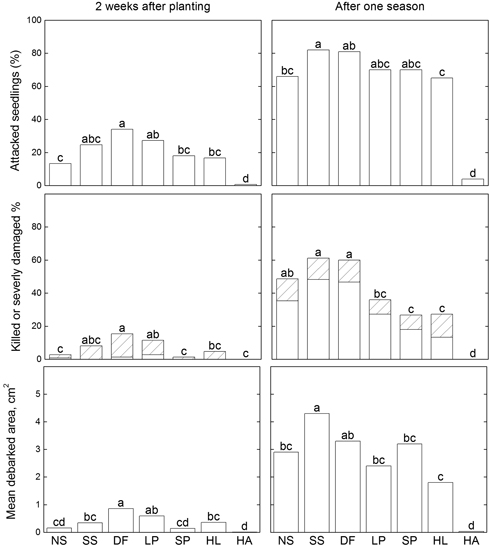
Fig. 1. Results of pine weevil damage to seedlings of different tree species; proportions of attacked seedlings (top diagram), killed, (unfilled bars) or severely damaged (lined bars) (middle diagram), and mean debarked area (bottom diagram) assessed after two weeks and after one season, using a general linear model (Proc GLM, SAS) followed by Tukey’s tests to separate effects of individual factors when significant differences were identified. Significant differences (p < 0.05) are indicated with different letters above the bars. Abbreviations of species names: NS = Norway spruce, SS = Sitka spruce, DF = Douglas fir, LP = lodgepole pine, SP = Scots pine, HL = Hybrid larch, HA = Hybrid aspen.
3.1.3 Proportions of killed or severely damaged seedlings
Seedling mortality caused by pine weevil was low two weeks after planting; only a few seedlings of Norway spruce, Douglas fir and lodgepole pine had died after this short period (Fig. 1). However, a significantly higher proportion of Douglas fir seedlings had been killed or severely damaged (14%) than Norway spruce, Scots pine, hybrid larch and hybrid aspen seedlings (Fig. 1). After one growing season significantly higher proportions of Douglas fir and Sitka spruce seedlings had been killed or severely damaged (60 and 61%, respectively) than those of all other species except Norway spruce (49%). Among the conifer seedlings, the proportions of killed or severely damaged seedlings were lowest for Scots pine, hybrid larch and lodgepole pine (27, 27 and 36%, respectively). No mortality or severe damage caused by pine weevil was recorded for hybrid aspen. Mortality due to factors other than pine weevil feeding was relatively low in all species except hybrid larch, 26% of seedlings of this species died from other, unknown causes.
3.1.4 Debarked area
Two weeks after planting, Douglas fir seedlings had a larger mean debarked area than all the other species except lodgepole pine (Fig. 1), although lodgepole pine seedlings did not significantly differ in this respect from Sitka spruce and hybrid larch seedlings. After one growing season Sitka spruce and Douglas fir seedlings had the largest debarked areas (4.3 cm2 and 3.3 cm2, respectively). However, the only significant difference in this variable among conifer species was between Douglas fir and hybrid larch. A few hybrid aspen seedlings were attacked by pine weevil, but the debarked area was very small, too small to be visible in the diagram.
3.1.5 Location of feeding on the seedlings
The highest main level recorded in this study was position 5 and most feeding on seedlings of all species was at position 1, the lowest part of the stem (Fig. 2). This pattern was most obvious in the Norway spruce, Sitka spruce and lodgepole pine seedlings (the main feeding scars on about 60% of attacked seedlings of these three species were at position 1). Higher proportions of the main feeding scars on Douglas fir, hybrid larch, and Scots pine seedlings were above position 1, and especially for Scots pine the feeding scars were more scattered. The mean value of the positions on Scots pine seedlings (2.4) was significantly higher than for the other species (1.4–1.9), which did not significantly differ in this respect.
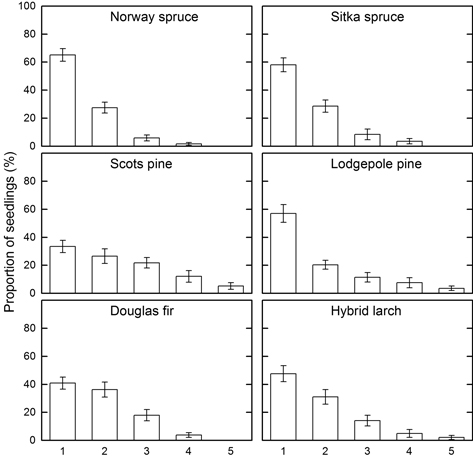
Fig. 2. Results of the distribution of the main feeding on the stem of seedlings of indicated tree species using a vertical scale from 1 at the base to 10 at the top of the seedling, shown as mean proportion of seedlings (± SE) on different positions.
3.2 Laboratory experiment
As shown in Fig. 3, the species with the largest mean debarked areas after 9 days in the laboratory were Sitka spruce (9.7 cm2), Douglas fir (9.6 cm2), and Norway spruce (8.1 cm2). The mean debarked area on Scots pine seedlings was 7.5 cm2, significantly lower than the area on Sitka spruce seedlings, but not Norway spruce and Douglas fir seedlings. Pine weevil feeding resulted in smaller mean debarked areas on lodgepole pine and hybrid larch seedlings (5.1 and 4.9 cm2, respectively) than on the other conifer seedlings, but the mean area debarked by pine weevil on hybrid aspen seedlings was far smaller (1.3 cm2).
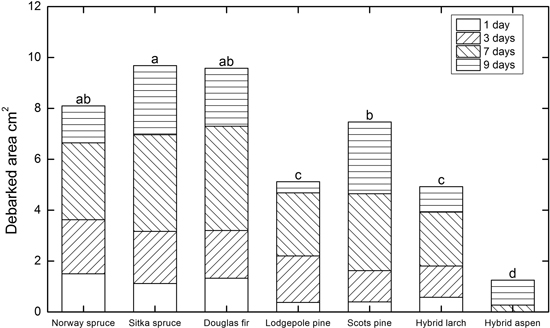
Fig. 3. Mean debarked area on seedlings of the indicated species after 1, 3, 7, and 9 days in the laboratory experiment. Significant differences after 9 days were tested with a general linear model (Proc GLM, SAS) and were separated by overall pair-wise comparisons using Tukey’s test, indicated in the diagram with different letters above the bars when (p < 0.05).
4 Discussion
Results of this study show that the introduced conifer tree species are highly attractive food sources for the pine weevil. The attack rate, amount of bark consumed and proportions of killed or severely damaged seedlings were generally highest for Douglas fir and Sitka spruce, although they were not always significantly higher than those of the other tree species. These results were consistent in both the long-term field study and laboratory feeding experiment.
Differences in damage between tree species were evident despite differences in diameter. Using diameter as a covariate showed that differences in proportions of killed or severely damaged seedlings between tree species were significant, although hybrid larch and Scots pine seedlings had larger mean initial diameters than the other species. Large seedlings are generally more tolerant to pine weevil feeding because of their lower likelihood of being girdled (Selander 1993; Örlander and Nilsson 1999; Thorsén et al. 2001). Moreover, the size of resin ducts, which contribute to weevil resistance in young conifers, is correlated to bark thickness and stem base diameter (Wainhouse et al. 2005). For high survival rates of seedlings in areas infested with pine weevils, threshold root collar diameters of 8–9 mm for Norway spruce and 12 mm for Scots pine have been suggested (Thorsén et al. 2001; Wallertz et al. 2005). If these thresholds are generally applicable larch may have significant advantages, because young hybrid larch can grow rapidly (Ekö 2009; Larsson-Stern 2003). Accordingly, the mean root collar diameter of the larch seedlings at the end of the first growing season was 9.5 mm, and more than 50% had a root collar diameter > 8 mm. Thus, the time that larch seedlings are vulnerable to pine weevil feeding may be shorter than for other more slowly growing species.
By the end of the first season on average more than 70% of the conifer seedlings had been attacked by pine weevil. Björklund et al. (2003) concluded that most seedlings on fresh clear-cuttings are approached by weevils, but decide whether or not to feed on a seedling when they are very close to it. Douglas fir was more frequently attacked by pine weevils than the native species during the first two weeks after planting, and by the end of the season they had fed upon most Douglas fir and Sitka spruce seedlings. Thus the bark of these species was presumably more chemically attractive than the bark of the other species. Moreover, pine weevils are more likely to find wounded seedlings than uninjured seedlings (Nordlander 1991), further increasing attacks on initially attractive seedlings.
The result from the laboratory experiment was largely consistent with the field study; Douglas fir and Sitka spruce were the species generally most consumed by the pine weevil. However, in the laboratory higher proportions of Norway spruce seedlings, and lower proportions of lodgepole seedlings, were attacked than in the field experiment. Moreover, the weevils fed more frequently on hybrid aspen in the laboratory test, which allowed no choice of food source, but still significantly less frequently than on any of the other species. So even though pine weevils sometimes attacks broadleaves trees like birch, hybrid aspen seems to be out of danger for severe attacks by the pine weevil and could be recommend to be planted without protection against the insect.
Two weeks after planting in the field experiment Douglas fir seedlings had a larger mean debarked area than seedlings of most other species, and by the end of the season Sitka spruce had the largest mean debarked area followed by Douglas fir. Accordingly, in a comparison of effects of site preparation Wallertz and Malmqvist (2013) found that larger areas of Douglas fir seedlings were debarked than Norway spruce seedlings, on average, although Zumr (1989) reported that Douglas fir is less attractive than pine and spruce as a food source for the pine weevil. Other studies also suggest that Scots pine is preferred before spruce (Sylvén, 1927; Leather et al. 1994; Manlove et al. 1997) although von Sydow and Örlander (1994) found no differences between these species in feeding rate or damage. In the present study the mean debarked area did not differ between Norway spruce and Scots pine seedlings, but significantly more feeding damage was found on Sitka spruce, showing that there are also differences within the genus Picea. The pine weevil consumed less bark on lodgepole pine than on Scots pine seedlings, possibly because antifeedant compounds that have been identified in lodgepole pine but not in Scots pine influence weevil feeding preferences (Bratt et al. 2001). Several field and laboratory experiments have also shown that larch is a less attractive food source than Norway spruce and Scots pine (Löf et al. 2004; Olenci and Olenci, 2003), and billets of Sitka spruce reportedly attract more pine weevils than larch billets (Wilson and Day 1995).
The introduced species Douglas fir and Sitka spruce were the most damaged of all tree species, around 60% of seedlings of these species were killed or severely damaged. In addition, the mean debarked area of the native species Norway spruce was significantly lower than that of the introduced congeneric Sitka spruce, but weevil-induced mortality rates of the two species were more similar. Thus, Sitka spruce appeared to have greater tolerance to damage by the weevils. These results are partially consistent with findings presented by Zas et al. (2011), who examined resistance and tolerance to pine weevil feeding in a native (Pinus pinaster) and an introduced (Pinus radiata) pine species. They observed more damage and much lower stem resin contents after exposure to weevils in the latter, suggesting that stronger defences are induced in the native species. However, in that experiment, the native species reportedly had higher tolerance to the damage. The differences between tree species in the extent of feeding damage are probably related to variation in chemical composition, influencing the quantity of phloem tissue consumed by the pine weevil (Bratt et al. 2001; Carillo-Gavilán 2012; Moreira et al. 2013). Induction of resin in the stem and phloem after wounding as well as nitrogen concentration has been found to vary between tree species, and may contribute to variation in the amount of pine weevil feeding (Wainhouse et al. 2004; Zas et al. 2011). The optimal defence theory (ODT) predicts, because defence is costly (Herms and Mattson 1992), that defence will be allocated specifically to the tissues with highest value to the plant or to tissues with greatest risk for attacks from herbivores (Zangerl and Bazzaz 1992 ). The greater inducibility of phloem resin observed in the lower part of the stem of Pinus radiata found by Moreira et al. (2012) seem to agree with the latter part of this prediction.
The greater inducibility of phloem resin observed in the lower part of the stem of Pinus radiata found by Moreira et al. (2012) seem to agree with the latter part of the theory. In relation to feeding damage by the pine weevil, a defence forcing the weevils to feed more scattered on the stem and less concentrated to the lower part of the seedling will reduce the risk of girdling and thereby death of the seedling.
In the present study, the location of feeding on the stem differed somewhat among the conifer species; notably the feeding scars on Scots pine seedlings were more scattered than on those of the other species, and consequently the mortality was low in relation to the total amount of bark consumed on the Scots pine seedlings. Allocation of chemicals in the plant may have resulted in feeding scars spreading more over pine seedlings. Furthermore, the Plug+1 Scots pine seedlings were larger and older than the others, and growth and the strength of resin-based defences in pine are positively correlated (Wainhouse et al. 2009). In this study we did not make any measurements of resin flow or other chemical analyses but the abovementioned factors most likely contributed to the differences in feeding patterns between the seedling types.
The results of this study show that seedlings of most conifer species planted on young clear-cuttings in Sweden need some kind of protection against pine weevil, and that introduced species such as Douglas fir and Sitka spruce may be even more vulnerable to pine weevil feeding than native species. Such serious threats by native pest species highlight some of the risks in establishing new tree species for forest production.
Acknowledgements
We would like to thank the staff at Asa Research Station, SLU, for their help with the field measurements. Special thanks are due to Helena Bylund for statistical advice. This study was part of the Swedish Hylobius Research Program funded by the Swedish forestry sector.
References
Björklund N., Nordlander G., Bylund H. (2003). Host-plant acceptance on mineral soil and humus by the pine weevil Hylobius abietis (L.). Agricultural and Forest Entomology 5: 61–65. http://dx.doi.org/10.1046/j.1461-9563.2003.00163.x.
Bratt K., Sunnerheim K., Nordenhem H., Nordlander G., Långström B. (2001). Pine weevil (Hylobius abietis) antifeedants from lodgepole pine (Pinus contorta). Journal of Chemical Ecology 27: 2253–2262. http://dx.doi.org/10.1023/a:1012231020944.
Carrillo-Gavilán A., Moreira X., Zas R., Vilà M., Sampedro L. (2012). Early resistance of alien and native pines against two native generalist insect herbivores: no support for the natural enemy hypothesis. Functional Ecology 26: 283–293. http://dx.doi.org/10.1111/j.1365-2435.2011.01931.x.
Day K.R., Nordlander G., Kenis M., Halldórsson G. (2004). General biology and life cycles of bark weevils. Chapter 14 in: Lieutier F., Day K.R., Battisti A. Grégoire J.-C., Evans H.F. (eds.). Bark and wood boring insects in living trees in Europe, a synthesis. Kluwer Academic Publishers, Dordrecht. p. 331–349.
Ekö P.M., Larsson-Stern M., Albrektson A. (2004). Growth and yield of hybrid larch (Larix × eurolepis A. Henry) in southern Sweden. Scandinavian Journal of Forest Research 19: 320–328. http://dx.doi.org/10.1080/02827580410024151.
Elfving B., Ericsson T., Rosvall O. (2001). The introduction of lodgepole pine for wood production in Sweden – a review. Forest Ecology and Management 141: 15–29.
Gundale M.J, Kardol P., Nilsson, M-C., Nilsson U., Lucas R.W., Wardle D.A. (2014). Interactions with soil biota shift from negative to positive when a tree species is moved outside its native range. New Phytologist. http://dx.doi.org/10.1111/nph.12699.
Hermann R.K., Lavender D.P. (1999). Douglas-fir planted forests. New Forests 17: 53–70.
Herms D.A., Mattson W.J. (1992). The dilemma of plants – to grow or defend. Quarterly Review of Biology 67(3): 283–335.
Karban R., Myers J.H. (1989). Induced plant-responses to herbivory. Annual Review of Ecology and Systematics 20: 331–348.
Karlsson B. (2007). Sitka- och douglasgran-alternativ för ett nytt klimat. Resultat från SkogForsk 17. [In Swedish].
Kristensen E. (2011). Survival, growth and damages in plantations of Sitka spruce (Picea sitchensis), after the storm Gudrun. Sveriges lantbruksuniversitet, Institutionen för sydsvensk skogsvetenskap, Master’s thesis 181.
Larsson-Stern M. (2003). Aspects of hybrid larch (Larix × eurolepis Henry) as a potential tree species in southern Swedish forestry. Licentiate thesis. Acta Universitatis Agriculturae Sueciae, Silvestria.
Leather S.R., Ahmed S.I., Hogan L. (1994). Adult feeding preferences of the large pine weevil, Hylobius abietis (Coleoptera Curculionidae). European Journal of Entomology 91: 385–389.
Löf M., Isacsson G., Rydberg D., Welander T.N. (2004). Herbivory by the pine weevil (Hylobius abietis L.) and short-snouted weevils (Strophosoma melanogrammum Forst. and Otiorhynchus scaber L.) during the conversion of a wind-thrown Norway spruce forest into a mixed-species plantation. Forest Ecology and Management 190: 281–290. http://dx.doi.org/10.1016/j.foreco.2003.10.027.
Manlove J.D., Styles J., Leather S.R. (1997). Feeding of the adults of the large pine weevil, Hylobius abietis (Coleoptera Curculionidae). European Journal of Entomology 94: 153–156.
Månsson P.E., Schlyter F. (2004). Hylobius pine weevils adult host selection and antifeedants: feeding behaviour on host and non-host woody Scandinavian plants. Agricultural and Forest Entomology 6: 165–171. http://dx.doi.org/10.1111/j.1461-9563.2004.00217.x.
Moreira X., Zas R., Sampedro L.(2012). Differential allocation of constitutive and induced chemical defenses in pine tree juveniles: a test of the optimal defense theory. Plos One 7(3). http://dx.doi.org/10.1371/journal.pone.0034006.
Moreira X., Zas R., Sampedro L. (2013). Inducibility of chemical defences by two chewing insect herbivores in pine trees is specific to targeted plant tissue, particular herbivore and defensive trait. Phytochemistry 94: 113–122. http://dx.doi.org/10.1016/j.phytochem.2013.05.008.
Nordlander G. (1991). Host finding in the pine weevil Hylobius abietis – effects of conifer volatiles and added limonene. Entomologia Experimentalis et Applicata 59: 229–237.
Nordlander G., Hellqvist C., Johansson K. (2011). Regeneration of European boreal forests: effectiveness of measures against seedling mortality caused by the pine weevil Hylobius abietis. Forest Ecology and Management 262: 2354–2363. http://dx.doi.org/10.1016/j.foreco.2011.08.033.
Olenici N., Olenici V. (2003). Feeding preferences of Hylobius abietis based on different species of conifers utilized as feed source. Revista Padurilor 118: 25–34.
Örlander G., Nilsson U. (1999). Effect of reforestation methods on pine weevil (Hylobius abietis) damage and seedling survival. Scandinavian Journal of Forest Research 14: 341–354.
Ottosson Löfvenius. M. (2011) Referensmätning av klimat vid skogliga försöksparkerna. Årsrapport 2010. Swedish University of Agricultural Sciences, Unit for Field-based Forest Research. [In Swedish].
Petersson M., Örlander G., Nordlander G. (2005). Soil features affecting damage to conifer seedlings by the pine weevil Hylobius abietis. Forestry 78(1): 83–92. http://dx.doi.org/10.1093/forestry/cps062.
Phillips M.A., Croteau R.B. (1999). Resin-based defenses in conifers. Trends in Plant Science 4: 184–190.
Samuelsson F. (2001). Damage caused by the pine weevil to deciduous seedlings. Sveriges lantbruksuniversitet, Institutionen för sydsvensk skogsvetenskap, Examensarbete 23.
Selander J. (1993). Survival model for Pinus sylvestris seedlings at risk from Hylobius abietis. Scandinavian Journal of Forest Research 8: 66–72.
Skogsdata 2013. Forest statistics 2013. (2013). Official Statistics of Sweden. Swedish University of Agricultural Sciences, Umeå.
Sylvén H. (1927). Snytbaggarna. Skogsvårdsföreningens Tidskrift 3: 521–555. [In Swedish].
Thorsén Å., Mattson S., Weslien J. (2001). Influence of stem diameter on the survival and growth of containerized Norway spruce seedlings attacked by pine weevils (Hylobius spp.) Scandinavian Journal of Forest Research 16: 54–66. http://dx.doi.org/10.1080/028275801300004415.
Toivonen R., Viiri H. (2006). Adult large pine weevils Hylobius abietis feed on silver birch Betula pendula even in the presence of conifer seedlings. Agricultural and Forest Entomology 8(2): 121–128. http://dx.doi.org/10.1111/j.1461-9563.2006.00290.x.
Trapp S., Croteau R. (2001). Defensive resin biosynthesis in conifers. Annual Review of Plant Physiology and Plant Molecular Biology 52: 689–724.
Tullus A., Rytter L., Tullus T., Weih M., Tullus H. (2012). Short-rotation forestry with hybrid aspen (Populus tremula L. × P. tremuloides Michx.) in Northern Europe. Scandinavian Journal of Forest Research 27: 10–29. http://dx.doi.org/10.1080/02827581.2011.628949.
Von Sydow F., Örlander G. (1994). The influence of shelterwood density on Hylobius abietis (L.) occurrence and feeding on planted conifers. Scandinavian Journal of Forest Research 9: 367–375.
Wainhouse D. (2004). Hylobius abietis – host utilization and resistance. In: Lieutier F., Day K.R., Battisti A., Grégoire J.-C., Evans H.F. (eds.). Bark and wood boring insects in living trees in Europe, a synthesis. Kluwer Academic Publishers, Dordrecht. p. 365–379.
Wainhouse D., Boswell R., Ashburner R. (2004). Maturation feeding and reproductive development in adult pine weevil, Hylobius abietis (Coleoptera Curculionidae). Bulletin of Entomological Research 94: 81–87. http://dx.doi.org/10.1079/ber2003283.
Wainhouse D., Staley J.T., Johnston J., Boswell R. (2005). The effect of environmentally induced changes in the bark of young conifers on feeding behaviour and reproductive development of adult Hylobius abietis (Coleoptera Curculionidae). Bulletin of Entomological Research 95:151–159. http://dx.doi.org/10.1079/ber2004344.
Wainhouse D., Jinks R., Morgan G. (2009). Growth and defence in young pine and spruce and the expression of resistance to a stem-feeding weevil. Oecologia 158: 641–650. http://dx.doi.org/10.1007/s00442-008-1173-0.
Wallertz K., Malmqvist C. (2013). The effect of mechanical site preparation methods on the establishment of Norway spruce (Picea abies (L.) Karst.) and Douglas fir (Pseudotsuga menziesii (Mirb.) Franco) in southern Sweden. Forestry 86(1): 71–78. http://dx.doi.org/10.1093/forestry/cps065.
Wallertz K., Petersson M. (2011). Pine weevil damage to Norway spruce seedlings: effects of nutrient-loading, soil inversion and physical protection during seedling establishment. Agricultural and Forest Entomology 13: 413–421. http://dx.doi.org/10.1111/j.1461-9563.2011.00536.
Wallertz K., Orlander G., Luoranen J. (2005). Damage by pine weevil Hylobius abietis to conifer seedlings after shelterwood removal. Scandinavian Journal of Forest Research 20(5): 412–420. http://dx.doi.org/10.1080/02827580500306954.
Wilson W.L., Day K.R. (1995). The comparative effectiveness of chemical traps, and fir, spruce and larch billets, for the estimations of pine weevil (Hylobius abietis L.) (Col., Curculionidae) density indexes. Journal of Applied Entomology–Zeitschrift Fur Angewandte Entomologie 119(2): 157–160.
Zangerl A.R., Bazzaz F.A. (1992). Theory and pattern in plant defense allocation. In: Fritz R., Simms E. (eds.). Plant resistance to herbivores and pathogens, ecology, evolution and genetics. University of Chicago Press, Chicago. p. 363–391.
Zas R., Moreira X., Sampredo L. (2011). Tolerance and induced resistance in a native and an exotic pine species: relevant traits for invasion ecology. Journal of Ecology 99: 1316–1326. http://dx.doi.org/10.1111/j.1365-2745.2011.01872.x.
Zumr V. (1989). Responses of the large pine weevil Hylobius abietis L. Coleoptera Curculionidae to different food attractants. Lesnictvi (Prague) 35: 607–620.
Total of 46 references

