Using thermal time models to predict germination of five provenances of silver birch (Betula pendula Roth) in southern England
Midmore E. K., McCartan S. A., Jinks R. L., Cahalan C. M. (2015). Using thermal time models to predict germination of five provenances of silver birch (Betula pendula Roth) in southern England. Silva Fennica vol. 49 no. 2 article id 1266. https://doi.org/10.14214/sf.1266
Highlights
- Using cumulative germination data, thermal time models were developed for Betula pendula
- Models indicated varying degrees of dormancy and pre-chill requirements among provenances
- Thermal time parameters were used with climatic data to predict germination times under mild and cold winters in southern England
- Predictions suggest that pre-chilled French seeds would germinate about six weeks later than the fastest germinating provenance.
Abstract
Climate predictions indicate that growing conditions may become unfavourable for certain tree species in parts of Britain. Guidelines suggest some planting of seed sources from regions between 2° and 5° south of those currently used as part of a climate change adaptation strategy. However, there has been little research on the benefits and risks associated with the use of planting stock from more southerly seed sources. Seeds of five provenances of the ‘relatively’ dormant Betula pendula were germinated over a range of temperatures both with and without a pre-chill. Subsequently, a thermal time model was used to predict the impact of migrating these provenances to southern England. Results identified geographical differences in germination response; those from higher latitude were more sensitive to pre-chill.
Keywords
climate change;
seed source;
assisted migration;
thermal time model
- Midmore, Forest Research Agency, Alice Holt, Surrey. Current: Dolwyddelan, Llandre, Ceredigion, Wales, SY24 5BZ E-mail emidmore@gmail.com
-
McCartan,
Forest Research, Alice Holt, Farnham, Surrey, GU10 4LH, UK
E-mail
shelagh.mccartan@forestry.gsi.gov.uk

- Jinks, Forest Research, Alice Holt Lodge, Farnham, Surrey, GU10 4LH, UK E-mail richard.jinks@forestry.gsi.gov.uk
- Cahalan, Bangor University, School of Environment, Natural Resources and Geography, Bangor, Gwynedd, Wales, LL57 2UW E-mail c.m.cahalan@bangor.ac.uk
Received 20 October 2014 Accepted 19 February 2015 Published 10 March 2015
Views 136633
Available at https://doi.org/10.14214/sf.1266 | Download PDF

1 Introduction
The effects of climate change on natural regeneration are likely to be complex and far-reaching (Fenner and Thompson 2005; Luna et al. 2012). According to the UK climate projections (UKCP09), average temperatures in southern England could increase by 4.2 °C in summer and 3.5 °C in winter by 2080 (Jenkins et al. 2009). Trees planted today could, when mature, experience climates similar to those currently prevailing in regions two or three degrees further south (Hubert and Cottrell 2007). Climate change poses several threats to the diversity, productivity and resilience of woodlands (Ray et al. 2010). A widely-discussed, and sometimes controversial, approach to addressing these issues is assisted migration; i.e. planting provenances from a different (typically more southerly) region that may be pre-adapted to future climates (McLachlan et al. 2007; Vitt et al. 2010; Pedlar et al. 2012). The levels of risk associated with assisted migration can be influenced by transfer distance (Worrell et al. 2000; Williams and Dumroese 2013).
Temperature is a key environmental factor regulating dormancy and germination of seeds (Heydecker 1977; Bewley and Black 1994; Thompson 2006). A warmer climate with milder winters could impact on the natural regeneration of tree species whose seeds require a period of pre-chilling for germination (Broadmeadow et al. 2005; Gosling et al. 2009). Geographical differences in temperature requirements for germination of various species have been shown using methods such as analysis of variation, time-course and maximum germination curve analysis (Barclay and Crawford 1984; Bevington 1986; Probert 2000; Fenner and Thompson 2005). These methods, although useful, are limited when seeking to predict the potential effects of changing seasonal temperatures on natural regeneration. Thermal time models estimate the minimum temperature at which germination occurs (Tb), and the thermal time (θt) required for a given percentage of germination, usually 50% (Pritchard et al. 1999; Steadman and Pritchard 2004; Jinks et al. 2006). Climatic data and thermal time parameters can be combined to investigate the effects of assisted migration on the germination of a target species.
This study aims to develop thermal time models and predict the potential impacts of assisted migration on the germination of five provenances of silver birch (Betula pendula Roth) in southern England.
2 Materials and methods
2.1 Seed source and quality
The term provenance refers to the geographic locality of a stand of trees from where the seed was collected (Hubert and Cundall 2006). Three provenances of Betula pendula were selected from Great Britain (central England [UK403], the Lake District [UK301] and north-east Scotland [UK201]) and two from continental Europe, southern Finland and eastern France (Table 1). The five seedlots were purchased from commercial suppliers. Each seedlot contained varying proportions of winged seeds (technically achenes) and inert material, principally bracts and strobile particles. Moisture content (MC) was determined following standard protocols (ISTA 2009) on a fresh weight basis (Table 2).
| Table 1. Seed zone/region with coordinates in degrees latitude and longitude, approximate elevation and harvest year of provenances. | ||||
| Seedlot | Seed zone / Region | Approx. location | Approx. elevation | Harvest yr. |
| Finland | B. pendula – Region 1 (Lahti, Southern Finland) | 60° N; 25° E | 90 m | 2002 |
| Scotland | UK201 (Scottish Highlands, Grantown on Spey) | 57° N; 03° W | 275 m | 2001 |
| England | UK301 (England Keswick, Cumberland) | 55° N; 03° W | 100 m | 2009 |
| England | UK403 (Central England, Nesscliffe, Shropshire) | 53° N; 03° W | 75 m | 2010 |
| France | BPE 901-montagne (East France – Rhône-Alpes) | 47° N; 05° E | 230 m | 2010 |
| Table 2. Total number of seeds tested in each provenance, pure seed moisture content and average and standard deviation of number of filled and empty seeds in a 0.1 g sample. | ||||
| Provenance | Total number of seeds | Moisture content (%) | Filled seeds (Average ± SD) | Empty seeds (Average ± SD) |
| Finland | 8440 | 9.7 | 108 ± 11 | 43 ± 16 |
| UK201 | 10454 | 9.6 | 56 ± 13 | 130 ± 23 |
| UK301 | 9001 | 6.5 | 48 ± 11 | 112 ± 20 |
| UK403 | 10144 | 10 | 88 ± 14 | 94 ± 17 |
| France | 12966 | 7.2 | 79 ± 15 | 156 ± 24 |
2.2 Germination tests
The International Seed Testing Association (ISTA 2009) recommends using weighed replicates for germination tests of Betula pendula. Consequently, representative 0.1 g samples (containing seeds and inert matter) from each provenance were distributed across moistened filter paper (Ederol No. 187, 90 × 145 mm, D-330 g m–2). These were suspended above a reservoir of water within germination boxes (Gosling 1988). There were two treatments: a control without pre-chill (NPC); and a 21-day moist pre-chill (PC) at 4 ± 1 °C. Four pseudo-replicates per treatment were incubated at seven constant temperatures (10, 13, 17, 20, 25, 30 and 35 °C) for 21 days. During incubation, germination was systematically monitored under ambient light conditions. Germination was considered successful when seedlings measured 10 mm from radicle to cotyledon and had a balanced and healthy appearance. Seedlings displaying abnormal symptoms (e.g. stunted, necrotic and damaged radicles, or deformed hypocotyls) were discarded. After incubation un-germinated seeds within each replicate were stained with 0.5% 2,3,5 tetrazolium chloride for 24 hours at 30 °C to assess viability. Seeds were cut and scored as empty or filled. The proportions of filled and empty seeds varied among provenances (Table 2), and germination capacity was therefore calculated as the percentage of normal seedlings produced from filled seeds by the end of the incubation period. This permitted direct comparison between provenances whilst accounting for potential heterogeneity of samples. Generalised linear mixed models (GLMM) were fitted to the data for germination capacity (%) with a binomial error distribution and logit link function. Replicate was defined as a random effect.
2.3 Thermal time model and predictions
The thermal time analysis used a generalised linear model (GLM) which relates time-to-germination of each provenance to a thermal time (θt) parameter (Jinks et al. 2006). A logit link function was used in the model, which assumes that base temperature (Tb) is constant for a seed population at sub-optimal temperatures. The model was based on the generalisation and re-parameterisation of a calculation (Eq. 1) used to determine θ50 by repeat probit regression (Ellis et al. 1986).
 and the thermal time requirement for 50% germination (Eq. 4) as:
and the thermal time requirement for 50% germination (Eq. 4) as:  The model assumes a linear relationship between temperature and germination rate (Finch-Savage and Whalley 2006). Thermal time analysis was applied to cumulative germination data for temperatures 13 °C to 30 °C inclusive. Data for upper asymptotes were removed, as these false plateaux were due to dormancy, particularly in the NPC seeds. At 35 °C germination decreased significantly due to thermo-inhibition; these data were excluded from thermal time analysis. Using the thermal time parameters and climatic data, germination in southern England (50°75´N, 1°75´W) was predicted for each provenance. The climatic data were sourced from the CRUTS v3.10 database for a thirty-year period (1980–2009) (Mitchell and Jones 2005). The predictions assume that provenances received either no pre-chill (NPC, mild winter scenario) or the equivalent of a three-week pre-chill (PC, colder winter scenario). The 1st of March was used as a starting point for thermal time accumulation; heat accumulation and germination occurs when the ambient temperature exceeds predicted Tb.
The model assumes a linear relationship between temperature and germination rate (Finch-Savage and Whalley 2006). Thermal time analysis was applied to cumulative germination data for temperatures 13 °C to 30 °C inclusive. Data for upper asymptotes were removed, as these false plateaux were due to dormancy, particularly in the NPC seeds. At 35 °C germination decreased significantly due to thermo-inhibition; these data were excluded from thermal time analysis. Using the thermal time parameters and climatic data, germination in southern England (50°75´N, 1°75´W) was predicted for each provenance. The climatic data were sourced from the CRUTS v3.10 database for a thirty-year period (1980–2009) (Mitchell and Jones 2005). The predictions assume that provenances received either no pre-chill (NPC, mild winter scenario) or the equivalent of a three-week pre-chill (PC, colder winter scenario). The 1st of March was used as a starting point for thermal time accumulation; heat accumulation and germination occurs when the ambient temperature exceeds predicted Tb. 3 Results
Seedlot quality varied considerably. Each 0.1 g replicate contained over 100 seeds with the average number of filled seed ranging from 48 (UK201) to 108 (Finland). The Finnish seeds had the highest purity (ratio of seed to inert matter) of 64%, while other provenances ranged between 38% and 44%.
3.1 Germination
For all provenances, maximum-germination plots (Fig. 1) show that control seeds (NPC) germinated over a narrower temperature range than pre-chilled seeds (PC). In control seeds, no germination occurred at 10 °C or 13 °C. Above 13 °C, germination capacity increased with increasing temperature, reaching a maximum at 30 °C. Maximum germination capacity varied among provenances, with 54% of French seeds germinating compared with 91% of Finnish seeds. Following pre-chilling, germination occurred rapidly over a wider temperature range. For all provenances, germination was thermo-inhibited at 35 °C (a supra-optimal temperature), a common trait in wild species (Dürr et al. 2015). The French seeds were the most dormant while the Finnish seeds were the least dormant (Fig. 1). The GLMM analyses showed that all the main effects (temperature, pre-chilling and provenance) were significant (all p < 0.001). Seedlots were of different ages, and seedlot age was tested by replacing provenance with each of the co-variates seedlot age and latitude in turn. Replacing provenance with latitude resulted in a highly significant main effect of latitude (p < 0.001); there were no significant interactions between latitude, germination temperature and pre-chilling. When provenance was replaced with seedlot age, the main effect of seedlot age was not significant (p = 0.34), and there were no significant interactions between seedlot age and temperature or between seedlot age and pre-chilling.
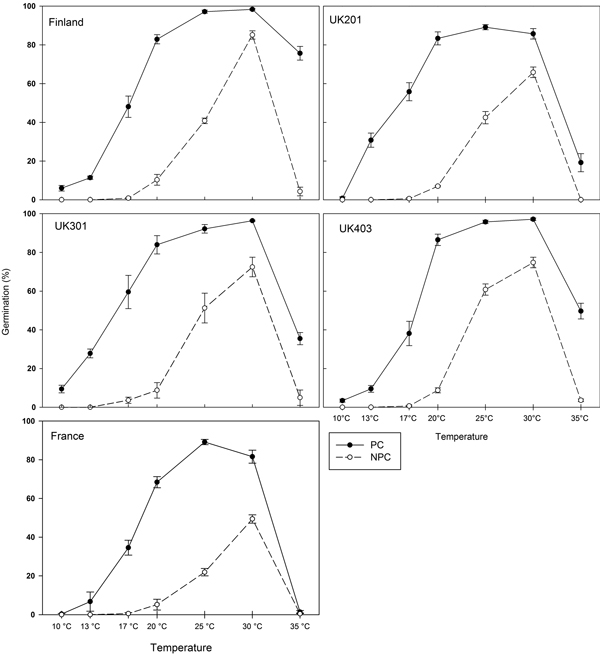
Fig. 1. Maximum germination capacity (at day 21) for five provenances of Betula pendula expressed as a percentage of filled seed. Closed symbols: germination following pre-chilling (PC). Open symbols: germination of control seeds (NPC). Error-bars: Standard error.
3.2 Thermal time model
Table 3 shows the estimated base temperature (Tb) and the thermal time required for 50% germination (θ50) for each provenance. For control seeds (NPC), Tb was highest for Finnish seeds and lowest for northern English (UK301) seeds. With the exception of UK301, Tb appeared to increase with increasing latitude. In addition, the predicted thermal time required for 50% germination (θ50) for the French seeds was approximately double that of the Finnish seeds. After pre-chilling (PC), the estimated base temperature (Tb) for germination and thermal time required for 50% germination (θ50) decreased in each provenance. Differences in the relative magnitude of the change in thermal time parameters indicate varying degrees of dormancy and pre-chill requirements among provenances.
| Table 3. Thermal time parameters (with standard errors) of Betula pendula: estimated base temperature (Tb) in °C; thermal time to 50% germination (θ50) in degree-weeks for both treatments (NPC and PC); relative change in parameters after pre-chilling (PC – NPC). | ||||||||||
| Provenance | Control seeds (NPC) | Pre-chilled seeds (PC) | PC – NPC | |||||||
| Tb | S.E. | θ50 | S.E. | Tb | S.E. | θ50 | S.E. | Tb | θ50 | |
| Finland | 15.8 | 0.8 | 21.2 | 1.4 | 10.3 | 0.9 | 13.4 | 1.2 | 5.6 | 7.8 |
| UK201 | 13.1 | 2.2 | 37.9 | 5.0 | 6.8 | 1.1 | 23.6 | 2.0 | 6.3 | 14.3 |
| UK301 | 9.4 | 1.8 | 37.4 | 3.7 | 7.5 | 0.9 | 16.8 | 1.3 | 1.9 | 20.6 |
| UK403 | 12.6 | 1.0 | 28.1 | 2.0 | 10.2 | 1.0 | 14.8 | 1.4 | 2.4 | 13.2 |
| France | 12.3 | 1.9 | 42.5 | 4.8 | 9.9 | 1.1 | 19.6 | 2.0 | 2.5 | 23.0 |
3.3 Predictions
The predictions show germination of seeds from different provenances after a mild winter (NPC) compared to a colder winter (PC) in southern England (Fig. 2). Under mild conditions, seeds from northern England (UK301) reach a predicted maximum germination of 99% by mid-Aug. Seeds from central England (UK403) reach 40% germination but at a slower rate. Maximum germination for Scottish and French seeds is predicted to be 10% and 12% respectively, and practically zero (1%) for Finnish seeds. After a colder winter (equivalent to a three-week pre-chilling [PC]), all provenances are predicted to reach 100% germination by July, central English and French seeds having the slowest rate.
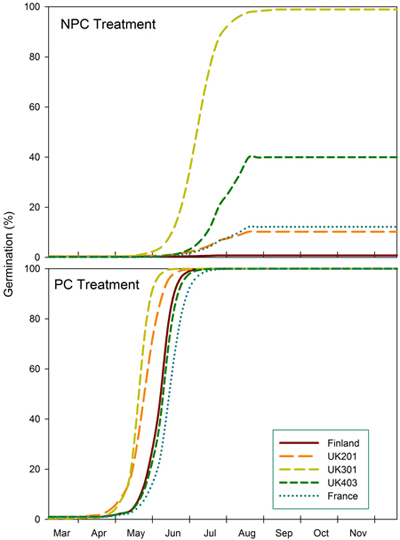
Fig. 2. Predicted germination of seeds from Finland (FIN), Scotland (UK201), north England (UK301), central England (UK403) and Eastern France (FRA) provenances under conditions that simulate a mild (NPC) and slightly colder winter (PC) in southern England.
4 Discussion
Betula pendula is a masting species that is characterised by its wide variation in the quantity and quality of seed produced annually (Atkinson 1992). This monoecious species produces male catkins, which release vast quantities of wind-dispersed pollen in spring (Atkinson 1992). Subsequently, female catkins develop into cylindrical strobiles that disintegrate at the end of summer/autumn releasing wind and water-dispersed seeds (Suszka et al. 1996). Upon dispersal, seeds are relatively (or conditionally) dormant; this is regulated by an interaction between photoperiod and temperature (Vaartaja 1952; Black and Wareing 1954, 1955; Vanhatalo et al. 1996). Under natural conditions some seeds may germinate in autumn but seedlings are unlikely to survive (Vanhatalo et al. 1996). During winter, seeds are exposed to a moist-chilling condition resulting in a widening of the range of temperatures suitable for germination.
The beneficial effect of moist pre-chilling on germination of Betula pendula is well documented (Atkinson 1992; Vanhatalo et al. 1996; De Atrip and O’Reilly 2007; Tylkowski 2012). This study confirmed that a pre-chill improved the germination capacity and germination rate, and widened the thermal thresholds for germination in all provenances. Results revealed differences in degree of dormancy among the provenances (indicated by the area between the NPC and PC plots in Fig. 1) and therefore in pre-chill sensitivity, particularly at sub-optimal temperatures. In a similar study, Bevington (1986) compared North American provenances of paper birch (Betula papyrifera). He found that northerly seeds germinated over a wider range of temperatures and showed greater sensitivity to pre-chilling. Seeds also had consistently thinner, more translucent pericarps than their southern counterparts. Bevington (1986) proposed that the thinner pericarps of northerly seeds could facilitate germination at lower temperatures, possibly being an adaptation to a shorter growing season. In this study, the Finnish seeds also appeared to have thinner pericarps than other provenances.
Temperature is a key environmental factor in the regulation of dormancy and germination, which in turn synchronise regeneration with seasons. This is demonstrated by the base temperature (Tb) for germination of provenances which, with the exception of UK301, appeared to increase with latitude. In addition, provenances with a lower Tb often had a higher θ50. This negative correlation between Tb and the thermal time required for fifty percent germination mirrors a general trend described in a recent review (Dürr et al. 2015). In this study, the greatest effect of pre-chilling (PC – NPC) on base temperature for germination was seen in northerly provenances (Finland and Scotland). Germination, therefore, becomes constrained to a safe window when the threat of winter damage has passed. Generally, an increase in pre-chill duration results in a corresponding decrease in Tb (Pritchard et al. 1999; Necajeva and Probert 2011). In some cases, secondary dormancy can develop following excessive pre-chill duration (Bevington and Hoyle 1981). Pre-chill temperature is also important; Vanhatalo et al. (1996) found that dormancy was released at 2.4 °C and 5.5 °C but imposed at 12.4 °C. Thus, adaptations to cope with or avoid low winter temperatures become more important towards the poles where favourable growing seasons are shorter (Saikkonen et al. 2012). As near-surface temperatures decrease with increasing altitude (Blandford et al. 2008), this dynamic may contribute to the increased dormancy observed in provenances of higher altitude (UK201 and France). Indeed elevation has been shown to influence optimal pre-chill duration in certain tree species, e.g. Sorbus aucuparia (Barclay and Crawford 1984).
In this study, statistical analyses showed that pre-chilling, temperature and provenance significantly influenced germination capacity. Furthermore, the provenance effect was due to latitude rather than seed age. Seeds of Betula species are orthodox, and following a reduction in moisture content can be stored successfully without loss of germinability for several years (Clausen 1975; Suszka et al. 1996). There are even reports of unexplained increases in germinability following extended storage (Clausen 1975; Tylkowski 2012). All seedlots had high maximum germination capacity, regardless of seed age and particularly after pre-chilling. In this study, however, latitude was confounded with maternal effects. Maternal effect refers to the ecological environment of the maternal parent and its influence on the phenotype of its progeny (Donohue 2009). The impact of latitude is two-fold; firstly, it influences photoperiod, which remains constant for a specific time of the year at a specific latitude (Saikkonen et al. 2012). Thus, seeds of the more northerly provenances (Finland) were exposed to shorter photoperiods during seed development and maturation than those of the southerly provenances (France). In Betula pendula, relative dormancy is regulated by an interaction between photoperiod and temperature, in which light can substitute for pre-chilling (Vaartaja 1952; Black and Wareing 1954, 1955; Vanhatalo et al. 1996). Donohue (2009) reported that seeds of Arabidopsis thaliana matured under short photoperiods were more responsive to pre-chilling than those matured under longer photoperiods. Secondly, latitude influences the temperature during seed maturation and post-dispersal; temperature also varies among years. Kelly et al. (2003) examined amplified fragment length polymorphisms in 15 individuals of Betula pendula in northern England. Results showed individuals clearly grouped in relation to mean temperature of establishment year (between 8.5 and 10.4 °C). This suggests that ‘pre-prepared’ genotypes, which germinate preferentially in cool or warm years, are an important feature of the adaptive capacity of Betula pendula (Kelly et al. 2003).
These two examples highlight the challenges faced when selecting seed sources for assisted migration as part of a climate change adaptation strategy. Under projected climate change scenarios, seeds are likely to receive less pre-chill during milder winters, and therefore may be unable to accumulate sufficient thermal time for germination within a safe window. Some provenances may germinate poorly and erratically and others not at all. In addition, trees are long-lived, and selected provenances therefore need to be well adapted to both present and future climates. In this study, predictions suggest that pre-chilled French seeds would germinate about six weeks later than the fastest germinating provenance (UK 301), with the risk that seedlings would suffer water stress under the present climate in southern England. Currently, the UK guidelines for Betula pendula recommend using seeds from British breeding programmes and avoiding seed sources from continental Europe (Forestry Commission 2015). In Finland, the recommended transfer distance is 150 km north or south to avoid potential damage by both late spring and early autumn frosts (Vakkari 2009).
5 Conclusion
Using thermal time models, germination parameters (base temperature and thermal time) were derived for five provenances of Betula pendula (Finland to France). The models showed that there were significant differences in the degree of dormancy and pre-chill sensitivity of the provenances. These differences were due to latitude, suggesting local adaptation to different regions. Thermal time models provide a useful means of predicting the germination capacity and germination rate of seeds under different climate change scenarios, and therefore for assessing the potential risks associated with assisted migration.
Acknowledgements
The authors acknowledge Andy Peace (Forest Research) for statistical help. This project was conducted by E. K. Midmore for his BSc (Hons) dissertation at Bangor University, Wales. The project was funded by the Forestry Commission.
References
Atkinson M.D. (1992). Betula pendula Roth (B. verrucosa Ehrh.) and B. pubescens Ehrh. Journal of Ecology 80: 837–870. http://dx.doi.org/10.2307/2260870.
Barclay A.M., Crawford R.M.M. (1984). Seedling emergence in the rowan (Sorbus aucuparia) from an altitudinal gradient. Journal of Ecology 72: 627–636. http://dx.doi.org/10.2307/2260072.
Bevington J. (1986). Geographical differences in the seed germination of paper birch (Betula papyrifera). American Journal of Botany 73 (4): 564–573. http://dx.doi.org/10.2307/2444262.
Bevington J., Hoyle M. (1981). Phytochrome action during prechilling induced germination of Betula papyrifera. Marsh Plant Physiology 67: 705–710. http://dx.doi.org/10.1104/pp.67.4.705.
Bewley J.D., Black M. (1994). Seeds, physiology of development and germination. 2nd ed. Plenum Press, New York and London. 445 p.
Black M., Wareing P.F. (1955). Growth studies in woody species (VII). Photoperiodic control of germination in Betula pubescens Ehrh. Physiologia Plantarum 8: 300–316. http://dx.doi.org/10.1111/j.1399-3054.1955.tb08977.x.
Black M., Wareing P.F. (1954). Photoperiodic control of germination in seeds of birch (Betula pubescens Ehrh.). Nature 174: 705–706. http://dx.doi.org/10.1038/174705a0.
Blandford T.R., Humes K.S., Harshburger B.J., Moore B.C., Walden V.P. (2008). Seasonal and synoptic variations in near-surface air temperature lapse rates in a mountainous basin. Journal of Applied Meteorology and Climatology 47: 249–261. http://dx.doi.org/10.1175/2007jamc1565.1.
Broadmeadow M.S.J., Ray D., Samuel C.J.A. (2005). Climate change and the future for broadleaved tree species in Britain. Forestry 78(2): 145–161. http://dx.doi.org/10.1093/forestry/cpi014.
Clausen K.E. (1975). Long-term storage of yellow and paper birch seed. USDA Forest Service, North Central Forest Experimental Station, Research Note NC-183. St. Paul MN, USA. 3 p.
De Atrip N., O’Reilly C. (2007). Germination response of alder and birch seeds to applied gibberellic acid and priming treatments in combination with chilling. Annals of Forest Science 64(4): 385–394. http://dx.doi.org/10.1051/forest:2007015.
Donohue K. (2009). Completing the cycle: maternal effects as the missing link in plant life histories. Philosophical Transactions of the Royal Society of London, Series B 364: 1059–1074. http://dx.doi.org/10.1098/rstb.2008.0291.
Dürr C., Dickie J.B., Yang X.-Y., Pritchard H.W. (2015). Ranges of critical temperature and water potential values for the germination of species worldwide: contribution to a seed trait database. Agricultural and Forest Meteorology 200: 222–232. http://dx.doi.org/10.1016/j.agrformet.2014.09.024.
Ellis R.H., Covell S., Roberts E.H., Summerfield R.J. (1986). The influence of temperature on seed germination rate in grain legumes. II. Intraspecific variation in chickpea (Cicer arietinum L.) at constant temperatures. Journal of Experimental Botany 37: 1503–1515. http://dx.doi.org/10.1093/jxb/37.10.1503.
Fenner. M., Thompson K. (2005). The ecology of seeds. Cambridge University Press, Edinburgh House, Cambridge. 250 p.
Finch-Savage W., Whalley R. (2006). Germination – field emergence models. In: Black M., Bewley J.D., Halmer P. (eds.). The encyclopedia of seeds: science, technology and uses. Cromwell Press, Trowbridge. 260 p.
Forestry Commission (2015). Silver birch (SBI). http://www.forestry.gov.uk/website/forestresearch.nsf/byunique/INFD-8CYKGL!OpenDocument&Click=. [Cited 24 Jan 2015].
Gosling P.G. (1988). The effect of moist chilling on the subsequent germination of some temperate conifer seeds over a range of temperatures. Journal of Seed Technology 12(1): 90–98.
Gosling P.G., McCartan S.A., Peace A.J. (2009). Seed dormancy and germination characteristics of common alder (Alnus glutinosa L.) indicate some potential to adapt to climate change in Britain. Forestry 82: 573–582. http://dx.doi.org/10.1093/forestry/cpp024.
Heydecker W. (1977). Stress and seed germination: an agronomic view. In: Khan A.A. (ed.). The physiology and biochemistry of seed dormancy and germination. Oxford Biochemical Press, Oxford. p. 237–277.
Hubert J., Cottrell J. (2007). The role of forest genetic resources in helping British forests respond to climate change. FCIN086. Forestry Commission, Edinburgh. 12 p.
Hubert J., Cundall E. (2006). Choosing provenance in broadleaved trees. FCIN82. Forestry Commission, Edinburgh. p. 1–12.
IPCC (2007). Climate change 2007: synthesis report. Contribution of Working Groups I, II and III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. [Core Writing Team, Pachauri R.K., Reisinger A. (eds.)]. IPCC, Geneva, Switzerland. 104 p.
ISTA (2009). International rules of seed testing. International Seed Testing Association, Bassersdorf, Switzerland.
Jenkins G.J., Murphy J.M., Sexton D.M.H., Lowe J.A., Jones P., Kilsby C.G. (2009). UK climate projections: briefing report. Meteorolocigal Office Hadley Centre, Exeter, UK. 59 p.
Jinks R.L., Willoughby I., Baker C. (2006). Direct seeding of ash (Fraxinus excelsior L.) and sycamore (Acer pseudoplatanus L.): the effects of sowing date, pre-emergent herbicides, cultivation, and protection on seedling emergence and survival. Forest Ecology and Management 237: 373–386. http://dx.doi.org/10.1016/j.foreco.2006.09.060.
Kelly C.K., Chase M.W., de Bruijn A., Fay M.F., Woodward F.I. (2003). Temperature-based population segregation in birch. Ecology Letters 6: 1–3. http://dx.doi.org/10.1046/j.1461-0248.2003.00402.x.
Luna B., Pérez B., Torres I., Moreno J.M. (2012). Effects of incubation temperature on seed germination of Mediterranean plants with different geographical distribution ranges. Folia Geobotanica 47: 17–27. http://dx.doi.org/10.1007/s12224-011-9110-0.
McLachlan J.S., Hellmann J.J., Schwartz M.W. (2007). A framework for debate of assisted migration in an era of climate change. Conservation Biology 21(2): 297–302. http://dx.doi.org/10.1111/j.1523-1739.2007.00676.x.
Mitchell T.D., Jones P.D. (2005). An improved method of constructing a database of monthly climate observations and associated high-resolution grids. International Journal of Climatology 25: 693–712. http://dx.doi.org/10.1002/joc.1181.
Necajeva J., Probert R.J. (2011). Effect of cold stratification and germination temperature on seed germination of two ecologically distinct species, Linaria loeselii and L. vulgaris (Scrophulariaceae). Polish Botanical Journal 56: 261–266.
Pedlar J.H., McKenney D.W., Aubin I., Beardmore T., Beaulieu J., Iverson L., O’Neill G.A., Winder R.S., Ste-Marie C. (2012). Placing forestry in the assisted migration debate. BioScience 62: 835–842. http://dx.doi.org/10.1525/bio.2012.62.9.10.
Probert R.J. (2000). The role of temperature in the regulation of seed dormancy and germination. In: Fenner M. (ed.). Seeds: the ecology of regeneration in plant communities. CABI Publishing, Wallingford. p. 261–292.
Pritchard H.W., Steadman J.K., Nash J.V., Jones C. (1999). Kinetics of dormancy release and the high temperature germination response in Aesculus hippocastanum seeds. Journal of Experimental Botany 50(338): 1507–1514. http://dx.doi.org/10.1093/jxb/50.338.1507.
Ray D., Morison J., Broadmeadow M. (2010). Climate change: impacts and adaptation in England’s woodlands. Forestry Commission (England), Research Note FCRN 201. Forestry Commission, Edinburgh. 16 p.
Saikkonen K., Taulavuori K., Hyvönen T., Gundel P.E., Hamilton C.E., Vänninen I., Nissinen, A, Helander M. (2012). Climate change-driven species’ range shifts filtered by photoperiodism. Nature Climate Change 2: 239–242. http://dx.doi.org/10.1038/nclimate1430.
Steadman K.J., Pritchard H.W. (2004). Germination of Aesculus hippocastanum seeds following cold-induced dormancy loss can be described in relation to a temperature-dependent reduction in base temperature (Tb) and thermal time. New Phytologist 161(2): 415–425. http://dx.doi.org/10.1046/j.1469-8137.2003.00940.x.
Suszka B., Muller C., Bonnet-Masimbert M. (1996). Seeds of forest broadleaves, from harvest to sowing, techniques and practices. INRA, Paris. 294 p.
Thompson K. (2006). Seasonal cycles of dormancy. In: The encyclopedia of seeds: science, technology and uses. Black M., Bewley J.D., Halmer P. (eds.). Cromwell Press, USA. 187 p.
Tylkowski T. (2012). Betula pendula seed storage and sowing pre-treatment: effect on germination and seedling emergence in container cultivation. Dendrobiology 67: 49–58.
Vaartaja O. (1952). Forest humus quality and light conditions as factors influencing damping-off. Phytopathology 42: 501–506.
Vakkari P. (2009). EUFORGEN technical guidelines for genetic conservation and use of silver birch (Betula pendula). Bioversity International, Rome, Italy. 6 p.
Vanhatalo V., Leinonen K., Rita H., Nygren M. (1996). Effect of prechilling on the dormancy of Betula pendula seeds. Canadian Journal of Forest Research 26: 1203–1208. http://dx.doi.org/10.1139/x26-134.
Vitt, P, Havens K., Kramer A.T., Sollenberger D., Yates E. (2010). Assisted migration of plants: changes in latitudes, changes in attitudes. Biological Conservation 143: 18–27. http://dx.doi.org/10.1016/j.biocon.2009.08.015.
Williams M.I., Dumroese R.K. (2013). Preparing for climate change: forestry and assisted migration. Journal of Forestry 111(4): 287–297. http://dx.doi.org/10.5849/jof.13-016.
Worrell R., Cundall E.P., Malcolm D.C., Ennos R.A. (2000). Variation among seed sources of silver birch in Scotland. Forestry 73(5): 419–535. http://dx.doi.org/10.1093/forestry/73.5.419.
Total of 46 references
