Variation in height and survival among northern populations of pedunculate oak (Quercus robur L.): results of a 13-year field study
Hautsalo J., Mathieu P., Elshibli S., Vakkari P., Raisio J., Pulkkinen P. (2015). Variation in height and survival among northern populations of pedunculate oak (Quercus robur L.): results of a 13-year field study. Silva Fennica vol. 49 no. 2 article id 1274. https://doi.org/10.14214/sf.1274
Highlights
- A height-sum function allowed us to compare the combined growth and survival of northernmost stands of pedunculate oak
- Individuals from Turku-Katariinanlaakso performed the best, although other families performed more consistently across trials, which should be considered in future conservation and breeding. Surprisingly, trees planted in a trial location beyond the natural northern limit of pedunculate oak showed the best performance.
Abstract
We analysed the adaptive potential of pedunculate oak (Quercus robur L.) in terms of variation in height and survival in five field trials located in southern and central Finland. The trials were established with Finnish native material from six different seed origins. Thirteen years after planting, the number of living trees was counted and measured for height. Analysis of height and survival revealed a significant effect of origin, i.e., a genetic basis to individual tree performance. Two origins from the Turku region (Ruissalo and Katariinanlaakso) performed the best while trees originating from Parainen (Lenholmen) performed the worst. In order to study the effects due to tree origin, a comparison of families (half-sibling trees, i.e. those sharing the same ‘mother’ tree) was made by combining height and survival through a height-sum equation (i.e., the product of mean survival and height of each family in each trial) and used to calculate family- and origin-level ecovalences. Ecovalence is a metric for performance consistency, and indicates how much each variable contributes to the total variation; the higher the value, the lower the consistency of trees across the trials based on their origin or family. Analysis of consistency showed similar results to growth and survival, with Turku families performing the best and families from Parainen performing the worst. Families in the Katariinanlaakso stand (Turku) generally had more stable ecovalence values and more dispersed height-sums, while Ruissalo (Turku) families had higher mean height-sum but higher variability in ecovalence values. These results suggest that seed origins (i.e., genotypes) can be optimized in terms of their suitability for commercial or ecological forest management.
Keywords
adaptation;
growth;
pedunculate oak;
northern stands
-
Hautsalo,
Natural Resources Institute Finland (Luke), Green technology, Antinniementie 1, FI-41330 Vihtavuori, Finland
E-mail
juho.hautsalo@luke.fi

- Mathieu, Agrocampus Ouest, 35000 Rennes, France E-mail pm@nn.fr
- Elshibli, University of Helsinki, Helsinki, Finland E-mail se@nn.fi
- Vakkari, Natural Resources Institute Finland (Luke), Vantaa, Finland E-mail pekka.vakkari@luke.fi
- Raisio, City of Helsinki, Helsinki, Finland E-mail jr@nn.fi
- Pulkkinen, Natural Resources Institute Finland (Luke), Vantaa, Finland E-mail pertti.pulkkinen@luke.fi
Received 4 November 2014 Accepted 20 February 2015 Published 23 March 2015
Views 115032
Available at https://doi.org/10.14214/sf.1274 | Download PDF

1 Introduction
Oaks of the genus Quercus are a diverse assemblage of polymorphic species that harbor a rich adaptive potential (Kremer and Petit 1993). Including hybrids, Quercus contains more than 450 species worldwide, of which 21 and three hybrids occur naturally within Europe (Kleinschmidt 1993). Pedunculate oak (Q. robur L.) and sessile oak (Q. petraea (Matt.) Liebl.) are commercially-important species and have been well studied (Gömöry et al. 2001). Although typically associated with more temperate climates, pedunculate oak has some production potential even at the northern limit of its distribution in Europe. Valkonen et al. (2002) estimated a high volume growth potential for pedunculate oak in Finland and stated that its growth and performance is influenced by local factors rather than climatic gradients operating over a wider scale. Previous studies have shown that although the demand for oak timber in Finland is rather low (ca. 33000 m3y–1 in 1993: Luona and Valkonen 1995), domestic production (<1000 ha) does not meet demand (Valkonen et al. 2002). However, Valkonen et al. (2002) suggested that if suitably managed, Finnish stands could meet domestic demand and produce high quality timber with few defects.
Quercus robur is found throughout mainland Europe and the British Isles, and extends as far north as the southwestern coasts of Norway and Finland. The species is continuously distributed in the central part of its natural range, but it becomes scattered in isolated stands at its southern and northern limits in the Mediterranean and southern Finland, respectively (Zanetto et al. 1994). Genetic diversity is a fundamental concern in species protection and management, and current levels of allelic variation provide the potential for species to respond and adapt to expected changes in future climates (Kremer et al. 2002). Several studies have analyzed the genetic diversity of pedunculate oak in order to help delimit populations and determine their interactions with those elsewhere (Yakovlevn and Kleinschmidt 2002; Kremer et al. 2002; Vakkari et al. 2006). Allelic diversity of Finnish stands is slightly lower than central European populations (Vakkari et al. 2006). If we accept that pedunculate oak populations of central Europe are only slightly differentiated (Kremer and Petit 1993), Finnish stands are as differentiated as German stands (Müller-Starck and Ziehe 1991), contain a number of rare alleles, and are more diverse than northern stands of Scots pine (Muona and Szmidt 1985).
Given that oak is a temperate species, it follows that an expected increase in mean annual temperature sum and milder winters (Jylhä et al. 2009) will improve growth conditions for oak in Finland. According to climate models based on the A1B scenario (IPCC 2007), temperatures will increase by up to 6 °C and rainfall will increase by up to 30% in winter and 10% in summer by 2100 (Jylhä et al. 2009). Relatively more rainfall during the winter months will also mean a greater mean temperature increase in comparison to future summers, and shift the winter average in southern Finland above 0°C (Jylhä et al. 2009). This may lead to better over-wintering of temperate species in the long run, although a predicted reduction in snow cover may cause serious frost damage when temperatures drop below 0°C and a milder winter climate might also facilitate the spread and establishment of new pests and pathogens.
At their northern limit in southwest Finland, pedunculate oaks occur in a few fragmented stands (Repo et al. 2008) experiencing limited gene flow (Lahtinen et al. 1997) and which collectively have higher genetic differentiation and lower diversity than populations in central Europe (Vakkari et al. 2006). It is generally accepted that isolated species with a relatively small gene pool are the most threatened by climate change (Aitken et al. 2008). High diversity within and among populations is important for a species to respond to changing environmental conditions (Hamrick 2004). Plasticity may also help populations respond to rapid changes (Aitken et al. 2008). Understanding the interaction between environmental change and genetic diversity is critical for effective forest management, and decision makers must plan wisely to ensure the highest probability of success in a dynamic and uncertain future climate. Furthermore, the range of variation observed for the entire species can be found within the Finnish stands. This is to be expected for wind-pollinated trees (Hamrick et al. 1992) and encourages an evaluation of Finnish populations of pedunculate oak and the influence of their mother trees as sources of variation for future conservation and breeding purposes.
The main objectives of this study were to determine (i) if differences in height and survival occur among pedunculate oak stands in Finland and (ii) to what extent they are genetically or environmentally determined. Answers to these questions give indication on the likely response of pedunculate oak to climatic change in Finland.
2 Material and methods
Seedling height and survival were studied in five field trials planted with seedlings taken from six native stands (Fig. 1). Although small and isolated, these stands represent the largest aggregations of pedunculate oaks in Finland, where they grow as the main tree species in mixed woodlands also containing Scots pine (Pinus sylvestris L.), Norway spruce (Picea abies [L.] H. Karst.) and birch (Betula spp.). Unfortunately, the origin or planting history of these protected oak forests is not known. Malmi is managed as a park where sick trees have been felled in the interests of public safety.
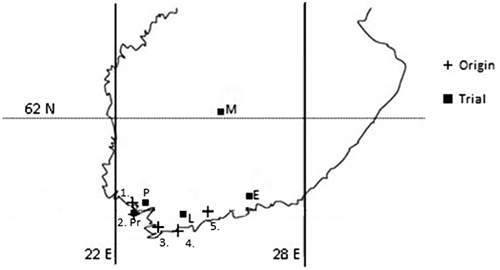
Fig. 1. Locations of natural stands (crosses) and trial sites (filled squares). Seedlings were taken from stands: 1: Ruissalo (60°26´N, 22°08´E, ETRS89) and Katariinanlaakso (60°25´N, 22°16´E) in Turku, 2: Parainen-Lenholmen (60°14´N, 22°12´E), 3: Tammisaari-Framnäs (60°01´N, 23°06´E), 4: Inkoo-Elisaari (59°58´N, 23°54´E) and 5: Helsinki-Malmi (60°14´N, 25°01´E). Trials were conducted at Elimäki (E), Lohja (L), Muurame (M), Paimio (P) and Parainen (Pr).
As an indication of the size and dominance of Finnish oak populations, the island of Ruissalo (Turku) is the largest stand in Finland where pedunculate oak dominates the woodland tree community but only occupies ca. 120 ha (22%) of the woods (City of Turku 2014). Parainen-Lenholmen, Katariinanlaakso and Ruissalo are recreational and conservation areas located less than 20 km apart in southwest Finland. The Helsinki-Malmi stand is a small population maintained by professional gardeners in a Helsinki cemetery. A location close to the city centre limits gene flow and the growth of this population. The Inkoo-Elisaari population is on an island a few hundred meters from the mainland and occupies a few dozen hectares. Tammisaari-Framnäs is a tiny population on the southern coast. Although stands were small, every stand comprised several hundred trees. Since a population can be defined as a breeding group with limited gene flow, we considered these stands as separate populations.
The number of mother trees and thus families varied among origins: Helsinki-Malmi had 11, Inkoo-Elisaari and Parainen-Lenholmen had five, Tammisaari-Framnäs had 17, Turku-Katariinanlaakso had 13 and Turku-Ruissalo had 18 families. Twenty-five seedlings from each family were planted in each trial. Trial seedlings were grown from acorns in a greenhouse during the spring and early summer, and were later moved outside to the nursery at Haapastensyrjä (60°37´N, 24°26´E). In 1998, one-year-old bare-rooted seedlings were planted in five field trials in southern and central Finland (60°14´–62°11´N, 22°25´–26°23´E: Fig. 1, Table 1). Seedling material used in the trials represented a total of 69 open-pollinated matrilineages (hereafter referred to as “families”).
Soil type (agricultural loam) and climate were similar in Elimäki, Lohja, Paimio and Parainen sites during the study period (Table 1). These sites were established on old fields to which herbicides and mechanical treatments (e.g., ploughing and harrowing) had been applied as required (Table 1). The trial site at Muurame was on rich forest soil and located near Jyväskylä, ca. 250 km north of the natural northern limit of pedunculate oak in Finland (Solantie 1983). Seedlings were planted in mounds at Muurame, a typical method applied in deciduous forestry. All five trials took place within a mixed landscape of agriculture and forest. The terrain was flat or slightly sloping (<5%) in all trial sites except at Muurame, which had greater slope (Table 1).
| Table 1. Basic descriptive data concerning the pedunculate oak trials. DD = day degrees, OMT = Oxalis acetosella -Vaccinium myrtillus type (Cajander 1949), RBD = randomized block design. | |||||
| Parameters | Trial location | ||||
| Elimäki | Lohja | Muurame | Paimio | Parainen | |
| Planting date | 25/05/1998 | 29/05/1998 | 02/06/1998 | 28/05/1998 | 12/06/1998 |
| Longitude (E) | 26°23´ | 24°08´ | 25°32´ | 22°45´ | 22°25´ |
| Latitude (N) | 60°43´ | 60°14´ | 62°11´ | 60°27´ | 60°17´ |
| DD | 1297 °C | 1315 °C | 1160 °C | 1298 °C | 1339 °C |
| Slope (%) | 0−1 | 0−1 | 7 | 0−5 | 0−2 |
| Orientation | - | - | South West | - | North West |
| Elevation (m) (mean sea level) | 45 | 40 | 160 | 32 | 13 |
| Soil type | Arable | Arable | OMT | Arable | Arable |
| Plantation density (trees/ha) | 500 | 500 | 500 | 500 | 500 |
| Preparations | herbicide treatment, ploughing, harrowing | ploughing | mounding | ploughing, harrowing | herbicide treatment, ploughing |
| Plantation design | RBD | RBD | RBD | RBD | RBD |
| Number of blocks | 25 | 25 | 25 | 25 | 25 |
| Spacing (m) | 4.5 × 4.5 | 4.5 × 4.5 | 4.5 × 4.5 | 4.5 × 4.5 | 4.5 × 4.5 |
| Total planting area (ha) | 5.06 | 5 | 4.5 | 5 | 5.1 |
Trials contained 25 blocks, each consisting of 80–100 randomized single tree plots. Planting distance was 4.5 m, and all trials except Elimäki were fenced to prevent damage from local deer and moose. Seedlings in all trials were protected by 1.2 m long polypropylene tubes (Tubex ®) to prevent damage from hares and rodents. Seedling height and survival were determined in 2010 after 13 growing seasons. Survival was assessed by classifying whether each tree was alive or dead. To evaluate overall growth performance, we calculated height-sums (living tree height-sum):

where HSik = height-sum of the ith family in the kth trial, Nik = total number of trees from the ith family in the kth trial, nik = the number of trees alive from the ith family in the kth trial, hijk = height of jth tree from the ith family in the kth trial, ![]() = mean height of the ith family in the kth trial, and
= mean height of the ith family in the kth trial, and ![]() survival of the ith family in the kth trial.
survival of the ith family in the kth trial.
In order to evaluate the importance of seed origin, family trial site and genotype-environment interactions, an analysis of variance GLM-procedure using a nested model was employed with blocks and trials as random factors and all variables fixed. Statistical analyses and graphs were made with SYSTAT (v. 13 2009).
Ecovalence was used to identify the seed origin with the most consistent performance across all trial locations. The concept is defined as the sum of squares contributed by a genotype to a genotype-environment interaction (Kang 2003). Ecovalence and consistency are inversely related, i.e., a low ecovalence means a more consistent performance and vice-versa (Sharma 1998). A generalized estimate of performance consistency in terms of ‘ecovalence’ was given by Wricke (1962) as follows: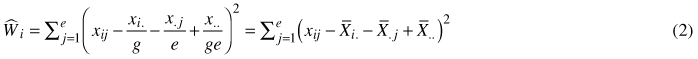
where ![]() = ecovalence of ith genotype, xij = mean of the ith genotype in jth environment,
= ecovalence of ith genotype, xij = mean of the ith genotype in jth environment, ![]() = total of ith genotype over all environments (e),
= total of ith genotype over all environments (e), ![]() = total of jth environment over all genotypes (g),
= total of jth environment over all genotypes (g), ![]() = mean performance of all genotypes in all environments. In this study, genotypes are represented by different seed origins and environments by trials, thus g = 6, e = 5, and ge = 30, and the corresponding values for family-level ecovalence are g = 69, e = 5 and ge = 345.
= mean performance of all genotypes in all environments. In this study, genotypes are represented by different seed origins and environments by trials, thus g = 6, e = 5, and ge = 30, and the corresponding values for family-level ecovalence are g = 69, e = 5 and ge = 345.
3 Results
3.1 Survival and height
Mean survival after 13 years was 71.3 % (across all sites and seed origins), being highest in seedlings from Turku-Katariinanlaakso (mean ± SD, 78.5 ± 41.1 %) and lowest in seedlings from Tammisaari-Framnäs (67.3 ± 46.9 %) (Fig. 2a). Turku-Katariinanlaakso trees were also the tallest (mean height ± SD, 267.8 ± 101.1 cm) and the smallest trees were from Parainen-Lenholmen origin (235.9 ± 95.4 cm) (Fig. 2b). The overall mean height of trial seedlings was 255.7 ± 100.1 cm.
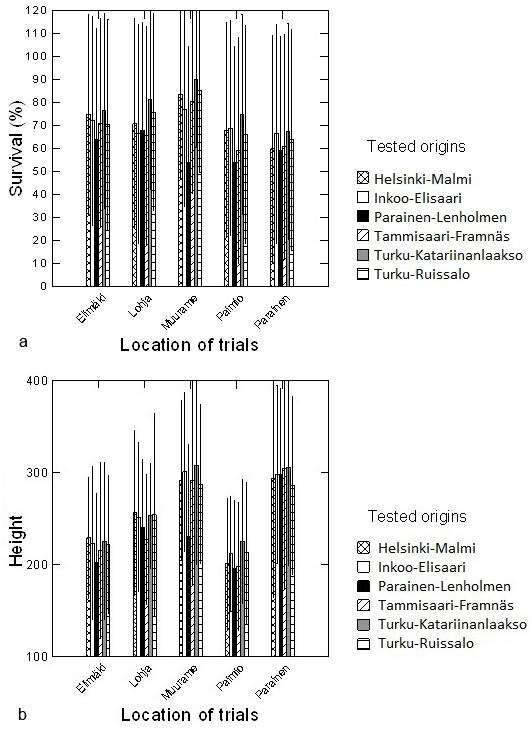
Fig. 2. Mean percent survival (2a) and height (2b) (cm) with standard deviations of pedunculate oak seedlings with different origins after 13 years of growth in several trial sites.
Mean survival rate (Fig. 2a) and height (Fig. 2b) differed among trial locations. The tallest trees (mean ± SD, 297.7 ± 106.8 cm) were in Parainen, where the lowest survival rate was observed (62.9 ± 13.2 %). The shortest trees were in Paimio (210.0 ± 71.4 cm), and the highest survival rate was in Muurame (81.6 ± 14.8 %) (Fig. 2a).
Seed origin, family, trial location, blocks within trials and all interactions were found to contribute significantly to variation in survival (Table 2). All sources of variation except the interaction family and trial explained a significant portion of the variation observed in seedling height. Overall, trial location was found to be the main factor influencing height and survival (Table 2).
| Table 2. Analysis of variance. Effects of seedling origin, family and trial site on the survival and height of six pedunculate oak populations. | |||||
| Factors | Sources of variation | d.f. | M.S. | F | P |
| Survival | Trial | 4 | 49 684.2 | 27.5 | 0.000 |
| Origin | 5 | 38 354.8 | 21.2 | 0.000 | |
| Trial × origin | 20 | 4167.8 | 2.3 | 0.001 | |
| Block(trial) | 120 | 7590.0 | 4.2 | 0.000 | |
| Family(origin) | 63 | 8438.3 | 4.7 | 0.000 | |
| Family(origin) × trial | 252 | 3432.1 | 1.9 | 0.000 | |
| Error | 8586 | 1807.9 | |||
| Height | Trial | 4 | 1 181 149.9 | 166.4 | 0.000 |
| Origin | 5 | 65 671.0 | 9.2 | 0.000 | |
| Trial × origin | 20 | 18 077.9 | 2.5 | 0.000 | |
| Block(trial) | 120 | 79 080.5 | 11.1 | 0.000 | |
| Family(origin) | 63 | 21 919.7 | 3.1 | 0.000 | |
| Family(origin) × trial | 252 | 7717.7 | 1.1 | 0.169 | |
| Error | 5987 | 7100.3 | |||
3.2 Consistency of performance
Seedlings from Inkoo-Elisaari (![]() (%) = 3.88) and Turku-Ruissalo (
(%) = 3.88) and Turku-Ruissalo (![]() (%) = 4.39) had the lowest ecovalence values for height-sum and those from Parainen-Lenholmen had the highest (
(%) = 4.39) had the lowest ecovalence values for height-sum and those from Parainen-Lenholmen had the highest (![]() (%) = 66.66) (Fig. 3), contributing more than half of the sum of squares for the interaction. Seedlings from Turku-Katariinanlaakso had the highest average sum of heights together with relatively low ecovalence, and those from Parainen-Lenholmen had the lowest mean height-sum (Fig. 3).
(%) = 66.66) (Fig. 3), contributing more than half of the sum of squares for the interaction. Seedlings from Turku-Katariinanlaakso had the highest average sum of heights together with relatively low ecovalence, and those from Parainen-Lenholmen had the lowest mean height-sum (Fig. 3).
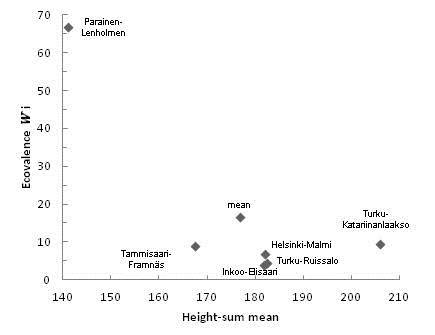
Fig. 3. Ecovalences and mean height-sums of the six seedling origins. Ecovalence values are shown as the proportion contributed by each origin to the total sum of squares.
Ecovalence was also calculated for each family and there was considerable variation in ecovalence values for the 69 families (0.1–5.3), and in height-sums ranging from 106 to 252 (Fig. 4). Families with high and consistent performance across trial locations can be found in the lower right corner of Fig. 4. Within populations, ecovalence variation among families was also high, even within the same stand (e.g., families 66 and 81 of Turku-Katariinanlaakso; Fig. 4). Ideal seedling families with ecovalence values < 1 and height-sum > 200 can be found in Turku-Ruissalo (five families), Turku-Katariinanlaakso (three families), Inkoo-Elisaari (one family) and Tammisaari (one family).
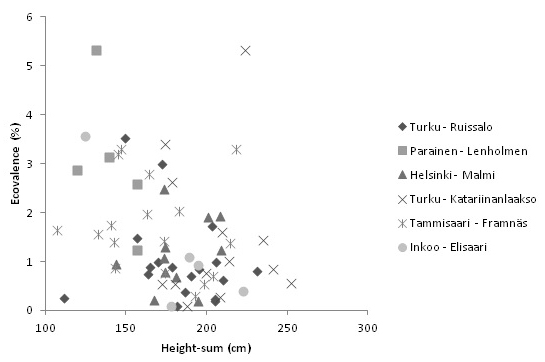
Fig. 4. Open-pollinated family level ecovalences and height-sums for the six seedling origins. Each symbol represents one family in the experiment.
4 Discussion
This study gathered information about variation in growth and survival of seedlings drawn from five Finnish populations of pedunculate oak in six trial locations. A similar study of pedunculate oak in several locations across southern Finland stressed the importance of protecting seedlings from rodents during the early stages of growth (Valkonen 2008). Valkonen (2008) noted that many damaged tubes and trees (up to 2% of protected trees had injuries), twisted label tags as well as animal tracks in the grass and snow suggest that protection measures such as tubes and fences are an imperfect solution. Thus, although injuries from herbivores were not quantified in our trials, they may have affected survival and growth differently based on local abundance of cervids and rodents.
In a boreal climate, populations tend to differ in terms of survival more than growth (Savolainen et al. 2007). The phenomenon was observed here when survival varied more than height among seed origins. Seedlings from Turku-Katariinanlaakso scored the highest in terms of survival and growth, revealing this population to be a promising source of germplasm for oak forestry in Finland. When compared to the growth and survival of southern seedlings in southern trials, growth was understandably weaker in Finland. For example, Jensen (2000) studied variation shown by seedlings from several European populations from Eastern Europe to the Netherlands and Norway in several locations in Denmark and reported heights after 13 growing seasons that were 0.5–2.0 m taller than the best average heights in our trials. Jensen (2000) also found height and volume to be highly heritable traits (h2 = 0.87). However, when the overall height and survival of seedlings in our study are compared to similarly planted and protected Finnish seedlings in Finland (Valkonen 2008), growth and survival are similar (e.g., average survival in that experiment was 82.4% after 5 years on the field).
With respect to the variation described here, trial location is a critical factor influencing height and survival. This emphasizes the importance of considering not only the expected growth of seedling stock, but also its consistency and adaptive potential. Results from the Parainen trial (Fig. 2) show that growth and survival do not always correlate positively and this supports the use of sum of heights as a cumulative trait as a long-term index of the environment at each trial location, and justification of its use in the analysis of performance consistency.
Intriguingly, when both height and survival are considered, the best trial location for all seedlings (except those from Parainen-Lenholmen) was Muurame, which is located ca. 250 km north of the most northern natural stand. In addition to being much further north, Muurame differed from other trial locations in its soil type, slope and orientation. However, oak is undemanding provided the soil is moderately fertile and free-draining (Valkonen et al. 1996), and the site is relatively warm to limit freezing injuries. Therefore, the south-facing slope of the trial site likely led to a slightly warmer microclimate that made the site more similar to conditions found further south (Ollinmaa 1952), and this may explain these unexpected results.
Jensen and Deans (2004) demonstrated considerable variation in frost tolerance of pedunculate oak with respect to north-south and maritime-continental axes. Parainen-Lenholmen was the only Finnish stand included in that study and found to be one of the most frost tolerant of those tested (Jensen and Deans 2004), suggesting that northern populations of oak are adapted to a colder climate. However, Repo et al. (2008) could not support the hypothesis that frost damage during hardening is a major factor preventing oak from spreading northwards when they studied the performance of oak seedlings from Helsinki (Malmi) in a trial located ca. 300 km to the north at Joensuu. Although, its natural dispersal there would require it pass through sites with a harsh microclimate, these findings give some support to the idea of growing oak at more northern latitudes. However, the experiment of Repo et al. (2008) covered only a single growing season, and it is premature to make predictions concerning performance over the long term.
Although not the best overall, seedlings from Parainen had their best performance at the Parainen trial, suggesting some local adaptation of the oak stand there. This observation is supported by strong frost-tolerance (Jensen and Deans 2004) and that Parainen-Lenholmen is located more remotely in the archipelago, where selective forces have likely shaped local adaptation in an old stand containing 350–450 year-old trees (Metsähallitus 2015). The significant trial×origin interaction for both height and survival also suggests that Finnish populations are adapted to local conditions. In contrast, a similar type of reciprocal-transplantation study conducted in the UK found no evidence of local-adaptation when height, diameter and volume growth among eight populations of ash (Fraxinus excelsior L.) were compared in native and unfamiliar soil types (Boshier and Steward 2005). However, the British study was conducted in nursery conditions with different soil types representing different field conditions and was conducted for only a single growing season, which makes a comparison with our long-term field study difficult and may explain their contrary results.
In terms of performance consistency (ecovalence) across trial locations, seedlings from Turku-Katariinanlaakso, Turku-Ruissalo and Inkoo-Elisaari were the best, although we note that the number of families was considerably smaller for Inkoo, which may have biased the results. Helsinki-Malmi seedlings also performed relatively well and had low ecovalence values. Seedlings from both Turku stands performed well but in slightly different ways; Katariinanlaakso seedlings achieved a slightly higher mean height-sum, indicating better survival and growth on the one hand, but seedlings from Ruissalo performed more consistently over all trial locations. These differences should be considered in the conservation of natural oak stands in Finland, which becomes increasingly important in light of expected climate change and European oak decline (Hertel and Zaspel 1995). Oak stands that give constantly well growing and surviving seedlings should be favored if everything cannot be saved.
Baliuckas and Pliura (2003) used the same type of stability analysis to determine the adaptive potential of pedunculate oak in Lithuania by estimating variation within and among populations and the interaction between genotype and environment for juvenile growth and growth rhythm in half-sib families. They compared ecovalence values for height among Lithuanian populations and these were of a similar range to those we observed for height-sums in our trials except Parainen-Lenholmen, which had low stability and poor performance at Muurame while performing well at other locations. Pliura et al. (2009) measured adaptive traits and reported considerable genetic variation within and between Lithuanian oak populations based on chloroplast DNA haplotyping. An inference from these studies is that any realistic conservation program must consider many local populations and selective breeding can be employed to develop improved strains for forestry purposes (Baliuckas and Pliura 2003; Pliura et al. 2009). Height is a highly heritable trait in pedunculate oak (Jensen 2000; Barzdajn 2008) and positive effects of selective breeding are proportional to selection intensity (Barzdajn 2008). Therefore, knowledge of the differences between families and native populations is of critical importance for breeding programs.
No study has definitively shown a positive or negative adaptative response of pedunculate oak to climate change. In the present study, the family (origin)*trial interaction was significant for survival which suggests that some families differ in this respect. The lack of a significant interaction for growth indicates limited local adaptation of this trait, if any. Thus, our results show significant differences in the adaptive plasticity of different oak stands that may help the species to adapt to a future climate.
The high performance of seedlings in the Muurame trial indicates that the natural distribution of oak is short of any physiological limit imposed by latitude, and one might expect oak to gradually spread north as climate becomes increasingly favorable. However, the mean dispersal distance for oak pollen is 300–350 m (Streiff et al. 1999) and even less in the north (Lahtinen et al. 1997). Given the isolation of the northernmost populations, the effective management and long-term health of pedunculate oak in Finland must involve a selective breeding program.
Acknowledgements
We would like to thank Raimo Jaatinen and Sirkku Pöykkö for the valuable information they provided on the trials, and Raimo for his help in organizing logistics and conducting the field measurements. We also thank Raija Viirros for technical assistance with the manuscript and Dr. Michael Hardman for checking the English.
References
Aitken S.N., Yeaman S., Holliday J.A., Wang T., Curtis-McLane S. (2008). Adaptation, migration or extirpation: climate change outcomes for tree populations. Evolutionary Applications 1: 95−111. http://dx.doi.org/10.1111/j.1752-4571.2007.00013.x.
Baliuckas V., Pliura A. (2003). Genetic variation and phenotypic plasticity of Quercus robur populations and open-pollinated families in Lithuania. Scandinavian Journal of Forest Research 18: 305−319. http://dx.doi.org/10.1080/02827580310005153.
Barzdajn W. (2008). Comparison of provenance, family and individual heritability of growth traits in pedunculate oak (Quercus robur L.) in the family-provenance trial in the Milicz Forest District. Sylwan 152(5): 52−59.
Boshier D., Steward J. (2005). How local is local? Identifying the scale of adaptive variation in ash (Fraxinus excelsior L.): results from the nursery. Forestry 78: 135−143. http://dx.doi.org/10.1093/forestry/cpi013.
Cajander A.K. (1949). Forest types and their significance. Acta Forestalia Fennica 56(5). 71 p.
City of Turku. Description of the maintenance activities in Ruissalo and other oak forests in Turku. [In Finnish]. http://www.turku.fi/public/default.aspx?contentid=111323&nodeid=4910. [Cited 20.5.2014].
Gömöry D., Yakovlev I., Ahelev P., Jedináková J., Paule L. (2001). Genetic differentiation of oak populations within the Quercus robur/Quercus petraea complex in Central and Eastern Europe. Heredity 86: 557−563. http://dx.doi.org/10.1046/j.1365-2540.2001.00874.x.
Hamrick J.L. (2004). Response of forest trees to global environmental changes. Forest Ecology and Management 197(1): 323−335. http://dx.doi.org/10.1016/j.foreco.2004.05.023.
Hamrick J.L., Godt M.J.W., Sherman-Broyles S.L. (1992). Factors influencing levels of genetic diversity in woody plant species. New Forests 6: 95−124. http://dx.doi.org/10.1007/978-94-011-2815-5_7.
Hertel H., Zaspel I. (1995). Investigations on the vitality and genetic structure in oak stands. Annals of Forest Science 53: 761−773. http://dx.doi.org/10.1051/forest:19960252.
IPCC (2007). Climate change 2007. The physical science basis. In: Solomon S., Qin D., Manning M., Chen Z., Marquis M., Averyt K.B., Tignor M., Miller H.L. (eds.). Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA. 996 p.
Jensen J. (2000). Provenance variation in phenotypic traits in Quercus robur and Quercus petraea in Danish provenance trials. Scandinavian Journal of Forest Research 15: 297−308. http://dx.doi.org/10.1080/028275800447922.
Jensen J., Deans J. (2004). Late autumn frost resistance of twelve north European provenances of Quercus species. Scandinavian Journal of Forest Research 19: 390−399. http://dx.doi.org/10.1080/02827580410019391.
Jylhä K., Ruosteenoja K., Räisänen J., Venäläinen A., Tuomenvirta H., Ruokolainen L., Saku S., Seitola T. (2009). Arvioita Suomen muuttuvasta ilmastosta sopeutumistutkimuksia varten. ACCLIM-hankkeen raportti 2009. Abstract: The changing climate in Finland: estimates for adaptation studies. ACCLIM project report 2009. Ilmatieteen laitos, Raportteja 2009:4, 102. [In Finnish, abstract, extended abstract and captions for figures and tables also in English].
Kang M.S. (2003). Ecovalence and stability variance. In: Kang M.S. (ed.). Handbook of formulas and software for plant geneticists and breeders. The Haworth Press, Inc, Binghamton, NY. ISBN 1-56022-948-9. p. 123−128.
Kleinschmit J. (1993). Intraspecific variation of growth and adaptive traits in European oak species. Annals of Forest Science 53: 181−196. http://dx.doi.org/10.1051/forest:19930716.
Kremer A., Petit R.J. (1993). Gene diversity in natural populations of oak species. Annals of Forest Science 50: 186−202. http://dx.doi.org/10.1051/forest:19930717.
Kremer A., Petit R.J., Ducousso A. (2002). Biologie évolutive et diversité génétique des chênes sessile et pédonculé. Biologie et écologie. Revue Forestière Française 2: 111−130. http://dx.doi.org/10.4267/2042/4907.
Lahtinen M.-L., Pulkkinen P., Helander M. (1997). Potential gene flow by pollen between English oak (Quercus robur L.) stands in Finland. Proc. Nordic Meeting for Forest Geneticists and Tree Breeders, Estonia, June 3.−7. Estonian Agricultural University, Faculty of Forestry, Forestry Studies XXVIII. p. 46−50.
Louna T., Valkonen S. (1995). Kotimaisen raaka-aineen aseman lehtipuiden teollisessa käytössä. Metsäntutkimuslaitoksen julkaisuja 553. 38 p. [In Finnish].
Metsähallitus 2015. Description of Lenholmen conservation area. [In Finnish]. http://www.luontoon.fi/lenholmen/luonto?inheritRedirect=true) [Cited 14 Jan 2015].
Muona O., Szmidt A.E. (1985). A multilocus study of natural populations of Pinus sylvestris. In: Gregorious H.-R. (ed.). Population genetics in forest trees. Springer Verlag, Berlin. p. 226−240.
Müller-Starck G., Ziehe M. (1991). Genetic variation in populations of Fagus sylvatica L., Quercus robur L., and Q. petraea Liebl. in Germany. In: Müller-Starck G., Ziehe M. (eds.). Genetic variation in European populations of forest trees. Sauerländer’s Verlag, Frankfurt am Main. p. 20−37.
Ollinmaa P. (1952). Jalot lehtipuumme luontaisina ja viljeltyinä. Silva Fennica 77: 73. [In Finnish].
Pliura A., Rungis D., Baliuckas V. (2009). Population structure of pedunculate oak (Quercus robur L.) in Lithuania based on analysis of chloroplast DNA haplotypes and adaptive traits. Baltic Forestry 15(1): 2−12.
Repo T., Mononen K., Alvila L., Pakkanen T.T., Hänninen H. (2008). Cold acclimation of pedunculate oak (Quercus robur L.) at its northernmost distribution range. Environmental and Experimental Botany 63: 59−70. http://dx.doi.org/10.1016/j.envexpbot.2007.10.023.
Savolainen O., Pyhäjärvi T., Knürr T. (2007). Gene flow and local adaptation in trees. Annual Review of Ecology, Evolution and Systematics 38: 595−619. http://dx.doi.org/10.1146/annurev.ecolsys.38.091206.095646.
Sharma J.R. (1998). Part three: Analysis of G × E interactions and stability parameters. In: Statistical and biometrical techniques in plant breeding. New Age International (P) Limited Publishers, New Delhi. ISBN 81-224-0888-5. p. 77−92.
Solantie R. (1983). “Mereisyyden - mantereisuuden” ja “humidisuuden” käsitteistä erityisesti tammen luontaisen levinneisyyden perusteella. Silva Fennica 17(1): 91−99. [In Finnish].
Streiff R., Ducousso A., Lexer C., Steinkellner H., Gloessl J., Kremer A. (1999). Pollen dispersal inferred from paternity analysis in a mixed oak stand of Quercus robur L. and Q. petraea (Matt.) Liebl. Molecular Ecology 8(5): 831−841. http://dx.doi.org/10.1046/j.1365-294x.1999.00637.x.
Vakkari P., Blom A., Rusanen M., Raisio J., Toivonen H. (2006). Genetic variability of fragmented stands of pedunculate oak (Quercus robur) in Finland. Genetica 127: 231−241. http://dx.doi.org/10.1007/s10709-005-4014-7.
Valkonen S. (2008). Survival and growth of planted and seeded oak (Quercus robur L.) seedlings with and without shelters on field afforestation sites in Finland. Forest Ecology and Management 255: 1085−1094. http://dx.doi.org/10.1016/j.foreco.2007.10.038.
Valkonen S., Rantala S., Sipilä A. (1996). Jalopuiden ja tervalepän viljely ja kasvattaminen. Metsäntutkimuslaitoksen tiedonantoja 575. [In Finnish].
Valkonen S., Urpelainen P., Virkki A. (2002). Yield potential of Quercus robur stands in Finland. Scandinavian Journal of Forest Research 17: 248−255. http://dx.doi.org/10.1080/028275802753742918.
Wricke G. (1962). On a method of understanding the biological diversity in field research. Zeitschrift für Pflanzenzüchtung 47: 92–146.
Yakovlev I.A., Kleinschmidt J. (2002). Genetic differentiation of pedunculate oak Quercus robur L. in the European part of Russia based on RAPD markers. Russian Journal of Genetics 38(2): 148−155. http://dx.doi.org/10.1023/A:1014330010233.
Zanetto A., Roussel G., Kremer A. (1994). Geographic variation of inter-specific differentiation between Quercus robur L. and Quercus petraea (Matt.) Liebl. Forest Genetics 1(2): 111−123.
Total of 37 references

