Spruce regeneration on woody microsites in a subalpine forest in the western Carpathians
Bujoczek L., Bujoczek M., Banaś J., Zięba S. (2015). Spruce regeneration on woody microsites in a subalpine forest in the western Carpathians. Silva Fennica vol. 49 no. 3 article id 1337. https://doi.org/10.14214/sf.1337
Highlights
- The occurrence probability of Picea abies seedlings on fallen deadwood was found to increase with diameter and decay stage of deadwood and with the volume of living trees, and to decrease with the density of living trees, sapling density, and land slope. It was also higher on stumps with greater diameter and in plots with higher sapling density, but decreased with increasing stump height.
Abstract
The density of Picea abies [L.] Karst. regeneration on different microsites, the quantity and quality of woody microsites, and seedling occurrence probability on stumps and fallen deadwood were studied in a subalpine forest that has been under protection for approximately 30–40 years (Gorce Mountains in the western Carpathians). Thirty percent of seedlings and 29% of saplings grew on stumps and fallen deadwood, while the remaining regeneration occurred on soil surface and mounds created by uprooted trees. The occurrence probability of Picea seedlings on fallen deadwood increased with deadwood diameter and decay stage and with the volume of living trees, and decreased with increased density of living trees, sapling density, and land slope. Furthermore, seedlings were more likely to grow on stumps with a greater diameter and in plots with higher sapling density, but less likely to grow on higher stumps. Stumps and fallen deadwood covered about 4% of the forest floor, but the material that is most important for promoting regeneration (strongly decomposed logs and those of a diameter exceeding 30 cm) took up only about 22 m2 ha-1. We have concluded that in a subalpine forest that has been protected for 30–40 years regeneration processes take place mostly on soil surface and stumps. The role of fallen deadwood increases over time as a greater number of suitable logs (in terms of size and decay stage) become available.
Keywords
Picea abies;
coarse woody debris;
stumps;
decomposition;
regression model;
fallen deadwood
Received 17 March 2015 Accepted 7 May 2015 Published 3 June 2015
Views 211867
Available at https://doi.org/10.14214/sf.1337 | Download PDF

1 Introduction
In areas with harsh climate the occurrence of suitable microsites for seedlings and saplings, providing better conditions for their survival and growth, is an important aspect of phytocoenosis regeneration (Harmon et al. 1986; Kuuluvainen 1994; Hörnberg et al. 1995; Kupferschmid and Bugmann 2005a). The presence of different types of microsites creates diverse environmental conditions in terms of temperature, moisture, and light. In a windthrow area of boreal old-growth forest, Kuuluvainen and Kalmari (2003) found that the spatial distribution of Picea abies [L.] Karst. regeneration establishment was nonrandom and varied between different microsite types. Picea seedlings were found in microsites created by windthrow disturbance, and particularly on advanced-decay wood and uprooted pits and mounds. Sixty-three percent of Picea seedlings occurred in these microsites, although they only covered approx. 28% of the study area. The results reported by Baier et al. (2007) from Bavarian mixed mountain forests also indicated a significant positive effect of several site parameters on Picea regeneration. More Picea saplings grew on rough surfaces and near hindrances, such as stumps, uprooted stumps, rocks, and fallen snags. In turn, high ground vegetation coverage and humus thickness negatively affected regeneration. In their study of the upper montane zone, Hunziker and Brang (2005) revealed a significant positive correlation with the presence of mosses of low thickness. Their results also pointed to microsites covered with litter and decaying wood as preferred ones.
In subalpine forests, the role of coarse woody debris (CWD) in the regeneration process is particularly pronounced (Kupferschmid and Bugmann 2005a). In the Carpathians, this mountain zone is dominated by Picea abies (Jaworski and Karczmarski 1995; Chwistek 2001; Holeksa et al. 2007). The forest floor with its lush vegetation provides appropriate conditions for Picea seed germination, while the sites most suitable for the growth of young Picea plants are logs, stumps, and mounds created by uprooted trees (Holeksa 1998). The importance of these microsites has been reported in numerous papers (e.g., Hytteborn and Packham 1987; Hofgaard 1993; Hörnberg et al. 1997; Zielonka and Piątek 2004). Moreover, the removal of CWD has been found to be disadvantageous for stand regeneration (Holeksa and Ciapała 1998).
An abundance of deadwood does not guarantee the formation of woody microsites suitable for the emergence and survival of Picea seedlings. Shortly after their death, fallen trees are inaccessible to vascular plants (Zielonka 2006a, Holeksa et al. 2008). The process of decomposition changes the physical and chemical properties of wood and therefore affects the conditions for the functioning of the organisms associated with it. The decomposition of Picea deadwood is accompanied by a decrease in its density and increased moisture. Its chemical composition becomes altered as well (Næsset 1999; Harmon et al. 2000; Bütler et al. 2007). Bače et al. (2012) found that the presence of the white-rot fungi Armillaria spp. and Phellinus nigrolimitatus was positively correlated with both seedling and sapling density, and conversely the presence of the brown-rot fungus Fomitopsis pinicola was negatively correlated with regeneration density. According to data from various regions of the world, the decomposition rates of Picea wood expressed as mean k coefficients range from 0.004 to 0.071, and therefore exhibit significant differences in terms of decomposition time (Rock et al. 2008). Numerous studies have shown the complexity of the process (Edmonds and Eglitis 1989; Busse 1994; Zhou et al. 2007). In the Carpathians, the mean total residence time of Picea logs was studied in reference to their diameter and amounted to 71 years for logs with a diameter of under 23 cm, 90 years for 23–35 cm, and 113 years for more than 35 cm (Holeksa et al. 2008). On an eight-level scale, the mean time to decay stage III is about 28 years, to stage V – 45 years, and to stage VII – 60 years (Zielonka 2006b).
The succession pattern of Picea regeneration on logs in relation to decay stage has been discussed in several publications (Narukawa et al. 2003; Mori et. al. 2004). In the western Carpathians, the first seedlings may germinate on a log during the second decade after tree death and survive for decades. The most abundant Picea recruitment occurs on logs 30–60 years after tree death, when the wood is in decay stages IV–VII (Zielonka 2006a). It has also been found that the optimum period for seedling emergence and survival occurs before mosses completely cover the logs (Iijima and Shibuya 2010; Iijima et al. 2007; Zielonka and Piątek 2004).
Studies conducted in the Alps have shown that microsites undergo dynamic changes. It has been reported that the succession of microsite types has a direct influence on the future rates of germination, mortality, and growth during regeneration, and an indirect influence on the future tree number and species composition (Kupferschmid and Bugmann 2005b; Kupferschmid et al. 2006). Of particular importance at the subalpine level are light conditions, as the demand of young Picea plants for light increases with altitude (Jaworski 1995). Light conditions depend on both large-scale disturbances and the occurrence of canopy gaps, whose size determines the amount of direct and diffuse radiation reaching the forest floor (Diaci et al. 2005).
Christensen et al. (2005) reported a positive correlation between the time an area has been under strict protection and the amount of CWD remaining in stands. For a long time, montane Picea forests in Central Europe have been used for timber production (Svoboda et al. 2010). However, recent concerns have shifted forest management objectives to address biodiversity conservation (Kräuchi et al. 2000). Woody microsites play an important role in the functioning of forest ecosystems, and their deficiency is considered to be one of the most important factors in the loss of forest biodiversity (Siitonen 2001; Stokland et al. 2012). Therefore, the quantity and quality of such microsites and their impact on regeneration are issues of great interest, and research in this field can be helpful in predicting the development of future stands. Therefore, the present paper addresses the following questions:
- Which microsite types are favorable / suitable for Picea regeneration in a subalpine forest that has been under protection for the past several dozen years?
- What are the quantity, size, structure, and decay stage of the woody microsites available for Picea regeneration in such stands?
- Which characteristics of stands and parameters of woody microsites have an effect on the occurrence of Picea seedlings on fallen deadwood and stumps?
2 Materials and methods
2.1 Study area
The study was conducted in the subalpine forest located in its entirety in Gorce National Park, in the south of Poland. The Gorce Mountains are situated in the western Carpathians and cover an area of approximately 550 km2, extending between 19°53´– 20°26´E and 49°26´– 49°40´N. A subalpine forest occurs between the isotherms of 4 and 2°C (Hess 1965), from an altitude of about 1100 m a.s.l. up to Turbacz (1310 m a. s. l.), which is the highest summit. The plant communities of the subalpine forest growing on Albic Podzols, locally gleyed and with overlying sandy and silty loam, are classified as Plagiothecio-Piceetum tatricum (Szaf., Pawł. et Kulcz. 1923) Br.-Bl., Vlieg. et Siss. 1939 em. J. Mat. 1977 (Matuszkiewicz 2008). The forest is dominated by Picea abies (93% of stand volume and 87% of tree number), with other species (albeit less abundant, particularly in lower locations) being Fagus sylvatica L. (6% and 11%, respectively) and Abies alba Mill (in both cases 1%). Single occurrences of Sorbus aucuparia L. and Acer pseudoplatanus L. have been recorded (Chwistek 2001). The mean stand volume recorded in the years 1992–2007 was 357–403 m3 ha–1 with a basal area of 32–35 m2 ha–1, and the number of trees being 408–479 per ha (Chwistek 2010).
The density of seedlings (on average 9587 per ha) and saplings (1094 per ha) and their species composition in the entire subalpine forest were described in detail as of 2003 by Przybylska and Bujoczek (2006). Apart from Picea, whose share in seedlings and saplings was 88% and 34%, respectively, the other major tree species were Sorbus aucuparia (1% and 48% ), Fagus sylvatica (8% and 8%), and Abies alba (3% and 6%). Some individuals belonging to the species Larix decidua Mill., Betula pendula Roth., Acer pseudoplatanus, Salix caprea L., and Populus tremula L. were also found. The ground vegetation was dominated by Vaccinium myrtillus, Polytrichum attenuatum, Athyrium distentifolium, Calamagrostis sp., and Oxalis acetosella.
Due to the history of protection and silvicultural interventions, the studied subalpine forest is not homogeneous. Most of the forest (an area of approx. 1038 ha) has been under protection since 1979 (Fig. 1). That area consists of several smaller sub-areas. In the 1980s and 1990s, due to an outbreak of Cephalcia alpina Klug (= C. falleni Dalm.), the forest was under active protection (Capecki 1982), involving sanitation cutting and removal of dead trees. Currently, strict protection has been reestablished. The forest area is a mosaic of different developmental stages, from parts in the break-up stage and undergoing succession processes, through optimum stages, to old-growth forest.
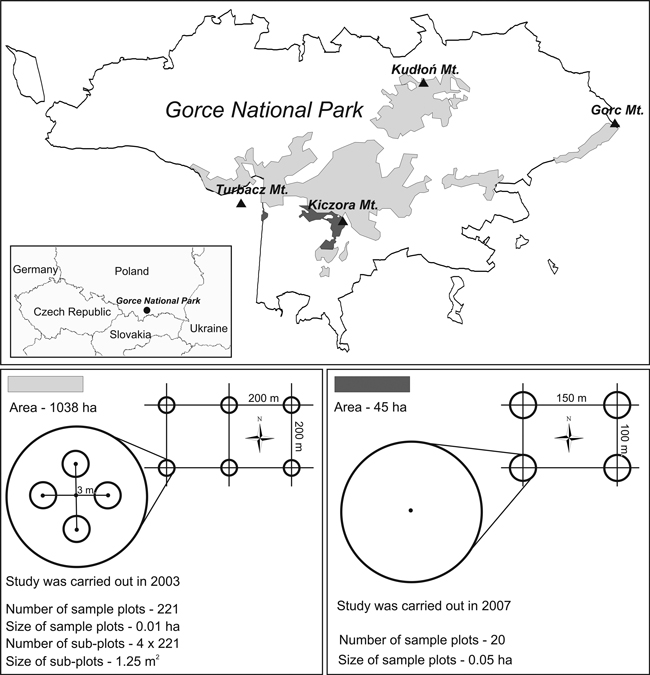
Fig. 1. Location of the study area and the arrangement and characteristics of sample plots.
The remaining part of the subalpine forest, consisting of two zones, with an area of 45 ha, has been under strict protection continuously since 1970 (some of the forest was included in the protection plan in 1979). It forms a mosaic of even-storied medium-aged stands and old-growth forest, marked by disturbances initiated in recent years.
These two forest areas were studied separately in the years 2003 and 2007.
2.2 Field methods
The first study was conducted in the larger part of the subalpine forest (an area of 1038 ha) in the summer of 2003. The size and number of seedlings and saplings and the types of microsites they grew on were recorded (Table 1). Data were collected from 221 circular sample plots, distributed regularly at the nodes of a grid formed by 200 × 200 m squares (Fig. 1). The size of sample plots was 100 m2. Due to high vegetation, it would have been difficult to identify all seedlings on such a large area, thus 4 subplots were delineated for counting seedlings, each of an area of 1.25 m2, with their centers located at a distance of 3 m towards N, W, S, and E from the center of the plot. In those areas, CWD was not quantified and the characteristics of stands were not determined.
| Table 1. Measurements taken on sample plots in 2003 and 2007. | |
| Measured characteristic | Rules for measurement and evaluation of characteristics |
| Measurements on an area of 1038 ha in 2003, on 221 sample plots | |
| Picea seedlings (two size classes, current-year seedlings were not included): 1. small: height < 26 cm 2. large: 26 cm ≤ height < 50 cm | Seedling height was measured and two types of microsites were distinguished: 1. forest floor (bare ground, ground with vegetation, litter and also the exposed mineral soil formed by tree fall) and mounds (formed by the root plates of uprooted trees) 2. decaying wood (stumps and fallen deadwood) |
| Picea saplings (eight classes): 1. 0.5 m ≤ height < 1.3 m 2. DBH1 < 1 cm 3. 1 ≤ DBH< 2 cm 4. 2 ≤ DBH< 3 cm 5. 3 ≤ DBH< 4 cm | Sapling height or DBH were measured and four types of microsites were distinguished: 1. forest floor 2. mounds 3. stumps 4. fallen deadwood |
| 6. 4 ≤ DBH< 5 cm 7. 5 ≤ DBH< 6 cm 8. 6 ≤ DBH< 7 cm | For last three classes of saplings, microsite categories were not given because identification was often uncertain. |
| Measurements on an area of 45 ha w 2007, on 20 sample plots | |
| Deadwood type | |
| Standing entire dead trees with DBH ≥ 7 cm | DBH and height of trees |
| Snags – standing snapped trees with stump height ≥ 1.3 m and DBH ≥ 7 cm) | Height and two diameters: under the ground and at the top of snag |
| Stumps with height < 1.3 m and diameter above the ground ≥ 10 cm | Height and two diameters: under the ground and at the top of stump |
| Fallen deadwood: • uprooted trees (windthrown trees with exposed root plates ) • fallen logs, trunks or branch fragments (pieces of a trunk or large branches etc.) Deadwood thinner than 7 cm in diameter was not taken into account. | Fallen deadwood was subdivided into smaller size categories during measurement, each category having a span of 8 cm in diameter: 7–14.9, 15–22.9, 23–30.9, etc. At the end of each deadwood piece, the width and height of the cross-sectional area were measured. The length of the piece and stage of decomposition were recorded on an 8 level scale (Table 2). |
| Regeneration growing on deadwood: | |
| Picea seedlings (two size classes, current-year seedlings were not included): 1. small: height < 26 cm 2. large: 26 cm ≤ height < 50 cm | Height of seedling and the type of microsite it grew on (stump or fallen deadwood). The size category of fallen deadwood and coarse woody debris decomposition were recorded. |
| Saplings on woody microsites were not taken into account because they occurred infrequently and in numbers too low to enable statistical analysis. | |
| Other characteristics measured on sample plots | |
| Living trees – all trees with DBH ≥ 7 cm (all species were included) | DBH and height of trees. |
| Tree losses – all trees that died over 15 years before the 2007 measurement | It was recorded which trees died relative to the 1992 measurement (tree coordinates and DBH were known from 1992). |
| Saplings – all individuals with height ≥ 0.5 m but with DBH < 7 cm (all species were included) | Number of saplings on the sample plot. |
| Slope of sample plot [°] | Slope of the land on which the sample plot was located. |
| Canopy closure [%] | Defined as the percentage of ground area shaded by overhead foliage and estimated visually. |
| 1DBH - diameter at breast height | |
The second study was carried out in the summer of 2007 in the second part of the subalpine forest, with an area of 45 ha. The amount of regeneration, CWD parameters, and stand characteristics were recorded (Table 1). Measurements were taken in 20 permanent circular sample plots, each with an area of 500 m2, distributed regularly at the nodes of a grid formed by 150 × 100 m rectangles (Fig. 1). In each sample plot, fallen deadwood and stumps were identified, measured, and evaluated for decay stage. Fallen deadwood was divided into size classes, each with a span of 8 cm in diameter: 7–14.9, 15–22.9, 23–30.9 cm, etc. Measurement was limited to inside the circumference of the circular sample plots, so if a piece of deadwood was situated across the plot border, only the part that lay within the plot was counted. The stage of CWD decomposition was recorded on an eight-level scale (Table 2). In total, 959 pieces of fallen deadwood and 193 stumps were examined. All fallen deadwood pieces and stumps were thoroughly checked for the occurrence of Picea seedlings (excluding current-year seedlings). No other species of seedlings grew on CWD. Saplings occurred infrequently on woody microsites and in numbers too low to enable statistical analysis. Additionally, in each plot, standing deadwood (snags and entire dead trees) and all live trees with diameter at breast height (DBH) ≥ 7 cm were measured, the slope of the sample plot was recorded, saplings of all species were counted, and canopy closure was determined (as a percentage of ground area shaded by overhead foliage and estimated visually). Moreover, based on 1992 data concerning the studied forest areas, the volume of trees that died over the past 15 years (tree losses) was estimated.
| Table 2. Characteristics of different decay stages of coarse woody debris (Holeksa 1998, modified). | ||||
| Decay stage | Surface | Shape | Depth of penetration of sharpened tool | Branches |
| I | Smooth | Round | Hard, fresh wood | All branches present, elevated above ground |
| II | Smooth | Round | Max. 1 cm or wood bends under the pressure of tool | Branches over 2 cm thick present |
| III | Crevices up to 0.5 cm deep | Round | Up to 2 cm | Branches over 3 cm thick present |
| IV | Crevices about 0.5 cm deep | Round | Up to 4 cm | Only base parts present |
| V | Crevices about 1 cm deep | Round | Up to 6 cm | Only thickest base parts present |
| VI | Several cm thick pieces torn off | Slightly flattened | Wood solid only in the central part of log | Only thickest base parts present |
| VII | Covered with furrows several cm deep | Distinctly flattened | Tool goes through | Lack of any remains |
| VIII | Often covered with vegetation | Flattened, covered with vegetation | Tool goes through | Lack of any remains |
The volume of living and standing dead trees, as well as tree losses, was estimated with the use of tariff tables (Przybylska and Przybylski 1994). The volume of stumps, fallen deadwood, and snags was calculated according to Smalian’s formula (Eq. 1):
where: sb,se – cross-sectional area at the beginning and end of a CWD piece and l – length of the piece. The cross-sectional area of each end of a CWD piece, depending on its shape, was calculated with the use of formulas for the areas of a circle, ellipse, trapezium, or a combination of a trapezium and semicircle, approximately conformable with the actual shapes of the deadwood ends.
The area of fallen deadwood covering the forest floor was calculated as the area of a trapezoid, while for stumps as the area of a circle.
2.3 Statistical analysis
The density of regeneration occurring on various microsites was calculated for the data from 2003 (221 sample plots). For saplings and seedlings, the unit of analysis was the plot. For seedlings, data from 4 subplots were aggregated and treated as one datum. Analysis of variance and Student’s t-test were used for testing density differences between microsites. The dependent variable was transformed by natural logarithm.
The data from 2007 (20 sample plots) were used to characterize the size structure and decay stages of woody microsites, seedling density according to the parameters of woody microsites, and the probability occurrence of Picea seedlings on fallen deadwood and stumps according to the characteristics of both the woody microsites and the stands.
The quantity of stumps and fallen deadwood were expressed in m2 ha–1. The areas of those microsites were compared according to CWD decay stage and size. The unit of analysis was the plot. Analysis of variance was used to test for differences. The dependent variable was transformed by natural logarithm.
Seedling density on woody microsites was analyzed according to CWD decay stage and size. Units of analysis were fallen deadwood pieces and stumps, with the density of seedlings calculated as the number of seedlings per 1 m2 of those sites. Pieces of fallen deadwood above 31 cm in diameter were aggregated into one group due to their low number in the sample plots. Seedlings were not found on most of those microsites, which made it difficult to conduct statistical analysis. Due to the excessive number of zeros (in each group the median was zero), 50% of the cases were rejected in each decay stage and size class during hypothesis testing. The Kruskal-Wallis test was used to test for differences.
Logistic regression analysis was used to determine the probability of Picea seedling occurrence on 959 fallen deadwood pieces and 193 stumps (Larose 2008). Each size class and decay stage of fallen deadwood and stumps was considered to be a separate component for which the presence or absence of Picea seedlings was indicated as 1 and 0, respectively. Along with the decay stage and size of CWD pieces, some independent variables characterizing the sample plots were used (Table 3). However, the model did not incorporate values obtained for 1 ha, but values that were 20 times lower, that is, obtained for the sample plots, as changes in the density or volume of trees by 1 unit calculated for 1 ha did not lead to perceptible changes in the odds ratio, which was determined for those variables which were significant in the model. An increase or decrease in independent variables by one unit increased or decreased Picea seedling probability by the odds ratio.
| Table 3. Characteristics of stands and coarse woody debris as measured on twenty 0.05 ha sample plots in 2007. | |||
| Variables | Mean | Min–Max | Standard deviation |
| Volume of living trees [m3 ha-1] | 375 | 131–724 | 170 |
| Density of living trees [trees ha-1] | 520 | 80–1205 | 287 |
| Basal area of living trees [m2 ha-1] | 37.9 | 16.4–63.2 | 13.4 |
| Sapling density [individuals ha-1] | 700 | 0–2600 | 741 |
| Total volume of coarse woody debris [m3 ha-1] | 180 | 45–422 | 104 |
| Volume of fallen deadwood and stumps [m3 ha-1] | 65 | 12–172 | 48 |
| Height of stumps [m] | 0.55 | 0.15–1.20 | 0.25 |
| Canopy closure [%] | 41.2 | 10–85 | 22.7 |
| Tree losses [m3 ha-1 year-1] (over the past 15 years) | 7.0 | 0.0–20.0 | 5.7 |
| Slope of sample plot [degrees] | 17.8 | 0–30 | 8.0 |
Interdependencies among the independent variables were tested by the existence of correlations, and additionally by multivariate regression analysis and the corresponding tolerance values. A model was built using the stepwise forward method. Initially, only one explanatory variable was applied, and subsequently other variables were included in the model. The new model was compared with the preceding one with the use of the likelihood ratio Chi-squared test. McFadden, Nagelkerke, and Cragg-Uhler R2 values were used as quality characteristics of the model (Larose 2008; Introduction to SAS 2007). Also a successful classification was important for the quality of the model. A classification test was carried out on the basis of those observations that were used to estimate the parameters of the model. In order to assess the validity of the model, standardized Pearson’s residuals were calculated (Menard 2001).
The Spearman rank correlation was used to assess the relationship between all independent variables in a given sample plot (volume, basal area, density of living trees, losses over 15 years prior to measurement, canopy closure, sapling density, slope of sample plot, volume of fallen deadwood and stumps, total volume of CWD).
3 Results
3.1 Density of Picea seedlings and saplings on different microsites
Based on data obtained from 221 sample plots in 2003, total Picea regeneration, that is, the total number of seedlings and saplings (excluding current-year seedlings) was 7587 ha-1. The average Picea seedling density was 7167 ha–1 (Fig. 2). The density of Picea saplings was 395 ha–1 for those with a DBH of less than 4 cm and 25 ha-1 for those with a DBH of 4–6.9 cm.
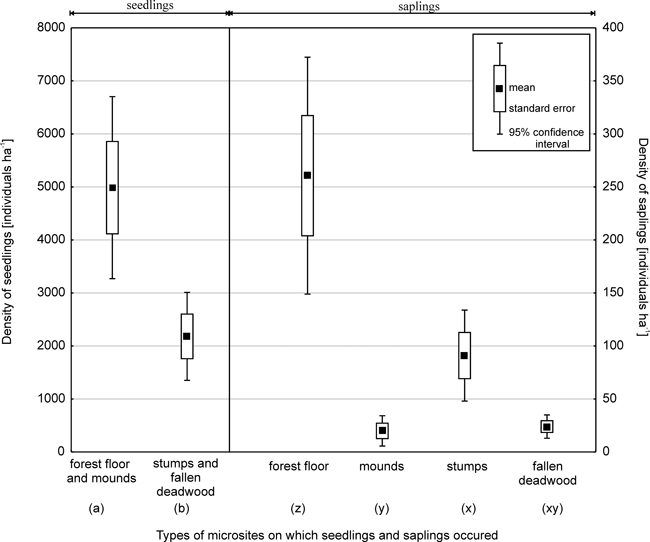
Fig. 2. Density of Picea seedling and saplings (with a diameter at breast height of less than 4 cm) on different microsites.
Note: Values with different letters differ significantly at p < 0.05 as evaluated using the Tukey post-hoc test. Two
separate analyses were conducted for seedlings and saplings.
Fewer seedlings grew on decaying wood (30%, Student’s t = 3.4; p < 0.001) than on the forest floor and mounds. In the case of small and large seedlings, the share of individuals found on stumps and fallen deadwood was 33% and 11%, respectively (Fig. 3).
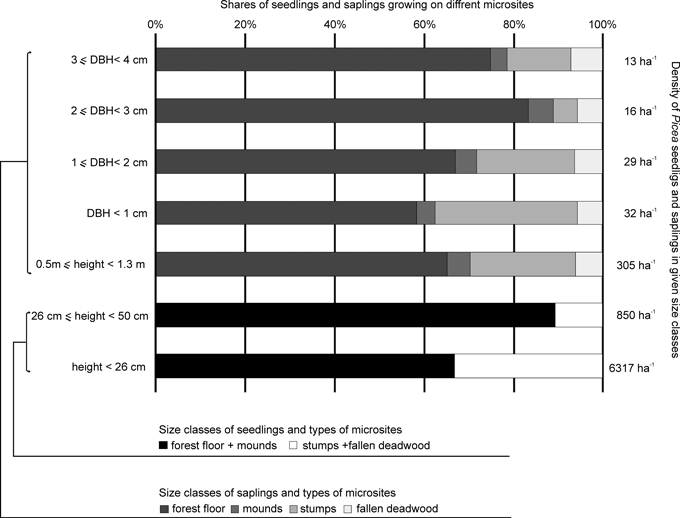
Fig. 3. Density and shares of the various size classes of Picea seedlings and saplings with a DBH (diameter at breast height) of less than 4 cm on different microsites.
Microsite type was also important for Picea saplings with a DBH of less than 4 cm (ANOVA; F = 23.8; p < 0.001) (Fig. 2, 3). Most saplings (66%) grew on the forest floor, while 5% were found on mounds created by uprooted trees. In terms of regeneration on decaying wood, the most numerous group of saplings was associated with stumps (23%), while those growing on fallen deadwood amounted to 6%. Significant differences in sapling density were found between the forest floor and other microsites (Tukey test p < 0.001), and between stumps and mounds (p < 0.01). In turn, no significant differences were found between stumps and fallen deadwood (p = 0.08), or between mounds and fallen deadwood.
3.2 Quantity and quality of woody microsites
According to data obtained from 20 sample plots in 2007, the fallen deadwood and stumps covered about 4% of the forest floor (408 m2 ha–1). The area occupied by fallen deadwood of different size classes on the forest floor differed significantly (ANOVA; F = 11.1; p < 0.001) (Fig. 4). Fallen deadwood of small dimensions occupied the largest area, amounting to 166 m2 ha–1 for pieces 7–14.9 cm in diameter (aggregate value for decay stages I–VIII). The next size class, 15–22.9 cm in diameter, covered an area of 109 m2 ha–1. The two largest size classes occupied approximately 60 m2 ha–1 each. The total area of stumps was 12 m2 ha-1.
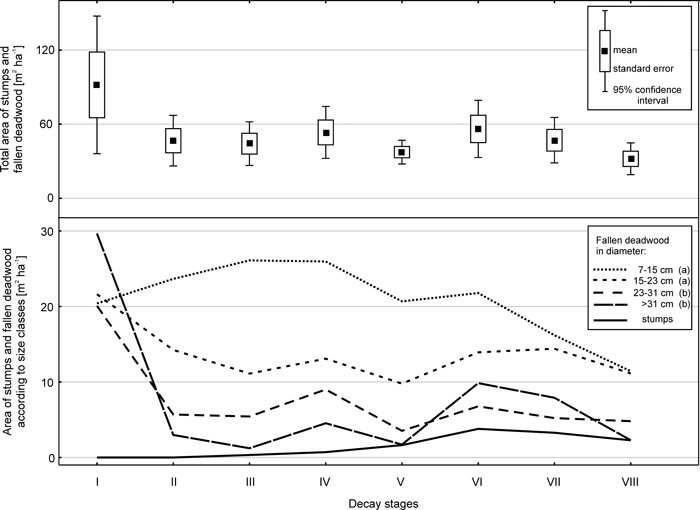
Fig. 4. Total area of stumps and fallen deadwood in the various decay stages (top chart) and areas of those microsites depending on size class and decay stage (bottom chart). Results of the Tukey test are given next to the size classes of fallen deadwood in the chart legend.
In terms of decay stages, the area of deadwood in decay stage I was 92 m2 ha–1 due to the accumulation of fresh wood (Fig. 4). Decay stages (II–VII) showed similar values of approximately 40–50 m2 ha–1. The most decomposed wood occupied the smallest area (32 m2 ha–1). The differences were not significant, because the area of fallen deadwood in decay stage I varied considerably between the sample plots.
The volume of woody microsites (fallen deadwood, stumps) in the sample plots was significantly positively correlated with the volume of tree losses and total volume of CWD and negatively correlated with the density of living trees and canopy closure (Table 4).
| Table 4. Spearman correlation matrix of independent variables (characteristics of 20 sample plots). | |||||||||
| Basal area of living trees | Density of living trees | Volume of living trees | Tree losses over 15 years | Canopy closure | Sapling density (all species) | Slope of sample plot | Volume of fallen deadwood and stumps | Total volume of coarse woody debris | |
| Basal area of living trees | 1.000 | ||||||||
| Density of living trees | 0.245 | 1.000 | |||||||
| Volume of living trees | 0.932*** | 0.084 | 1.000 | ||||||
| Tree losses over 15 years | -0.414 | -0.545* | -0.313 | 1.000 | |||||
| Canopy closure | 0.430 | 0.724*** | 0.288 | -0.612** | 1.000 | ||||
| Sapling density (all species) | -0.242 | -0.378 | -0.165 | 0.096 | -0.169 | 1.000 | |||
| Slope of sample plot | 0.242 | -0.141 | 0.225 | 0.006 | -0.100 | 0.541* | 1.000 | ||
| Volume of fallen deadwood and stumps | -0.206 | -0.673** | -0.134 | 0.597** | -0.716*** | 0.265 | 0.260 | 1.000 | |
| Total volume of coarse woody debris | -0.223 | -0.658** | -0.104 | 0.765*** | -0.650** | 0.224 | 0.238 | 0.853*** | 1.000 |
| Significance: *p < 0.05; **p < 0.01, ***p < 0.001. | |||||||||
3.3 Picea seedling occurrence on woody microsites
Picea seedlings growing on woody microsites occurred in 19 out of 20 sample plots, with a mean of 21.1 individuals per sample plot (SD = 22.7, maximum = 70). They were found in low numbers on 10% of the total number of stumps and fallen deadwood pieces; 92% of the seedlings grew either individually or in groups of up to 9 per 1 stump or piece of fallen deadwood. The highest number of individuals on a single deadwood piece was 23. The mean seedling density on decaying wood was 1.2 individuals per m2, with 0.6 individuals per m2 for fallen deadwood and 4.6 individuals per m2 for stumps.
Seedling density depended on CWD decay stage (Kruskal-Wallis, H = 51.7; p < 0.001). The greatest number of seedlings were found on wood in decay stage VIII, and their density gradually decreased to wood in decay stage III (Table 5). No seedlings were found on wood in the two first stages of decay. More seedlings were found on thicker deadwood pieces (23–30.9 cm and above 31 cm in diameter) than on pieces in the two smaller size classes (deadwood in decay stages I–II was excluded from this analysis) (Kruskal-Wallis H = 149.2; p < 0.001). Furthermore, a higher amount of regeneration was found on stumps with a diameter of over 31 cm than on stumps in the other three size classes (Kruskal-Wallis H = 53.7; p < 0.001). Seedlings above 26 cm in height occurred on deadwood that was at least in decay stage V, and with a diameter above 15 cm.
| Table 5. Density of seedlings on woody microsites in different decay stages, according to diameter of fallen deadwood and stumps (wood in decay stages I–II was not taken into account). | |||||||||||
| Number of coarse woody debris pieces | Height of Picea seedlings < 26 cm | 26 cm ≤ height of Picea seedlings < 50 cm | |||||||||
| Mean | Median | Top quartile | Max | Mean | Median | Top quartile | Max | ||||
| individuals / m2 | |||||||||||
| Decay stage of stumps and fallen deadwood | III | (a) | 137 | 0.06 | 0.0 | 0.0 | 7.6 | 0.00 | 0.0 | 0.0 | 0.0 |
| IV | (ab) | 162 | 0.48 | 0.0 | 0.0 | 24.9 | 0.00 | 0.0 | 0.0 | 0.0 | |
| V | (ab) | 162 | 1.23 | 0.0 | 0.0 | 35.3 | 0.28 | 0.0 | 0.0 | 35.3 | |
| VI | (bc) | 189 | 1.90 | 0.0 | 0.0 | 52.6 | 0.27 | 0.0 | 0.0 | 20.8 | |
| VII | (bc) | 144 | 2.09 | 0.0 | 0.0 | 46.8 | 0.04 | 0.0 | 0.0 | 5.4 | |
| VIII | (c) | 98 | 3.77 | 0.0 | 0.0 | 65.1 | 0.22 | 0.0 | 0.0 | 10.0 | |
| Size class of fallen deadwood | 7–14.9 cm | (a) | 449 | 0.22 | 0.0 | 0.0 | 15.8 | 0.00 | 0.0 | 0.0 | 0.0 |
| 15–22.9 cm | (b) | 150 | 0.95 | 0.0 | 0.0 | 49.7 | 0.03 | 0.0 | 0.0 | 4.7 | |
| 23–30.9 cm | (c) | 59 | 2.71 | 0.0 | 1.9 | 36.0 | 0.28 | 0.0 | 0.0 | 10.0 | |
| ≥ 31 cm | (c) | 41 | 3.51 | 0.0 | 4.7 | 22.2 | 0.29 | 0.0 | 0.0 | 5.4 | |
| Diameter of stumps | 10–14.9 cm | (a) | 51 | 0.00 | 0.0 | 0.0 | 0.0 | 0.00 | 0.0 | 0.0 | 0.0 |
| 15–22.9 cm | (a) | 50 | 2.81 | 0.0 | 0.0 | 52.6 | 0.71 | 0.0 | 0.0 | 35.3 | |
| 23–30.9 cm | (a) | 33 | 3.56 | 0.0 | 0.0 | 32.5 | 0.00 | 0.0 | 0.0 | 0.0 | |
| ≥ 31 cm | (b) | 59 | 8.69 | 0.0 | 9.3 | 65.1 | 0.92 | 0.0 | 0.0 | 20.8 | |
| Note: Values designated with different letters differ significantly at p < 0.05 as evaluated by the nonparametric Kruskal–Wallis test corrected with a post hoc test for the number of comparisons. Analyses were conducted for total number of seedlings (small and large). | |||||||||||
In the model of Picea seedling occurrence on fallen deadwood, six independent values were significant (Table 6). The probability of seedling occurrence increased with the decay stage and diameter of fallen deadwood, as well as the volume of living trees in the sample plot. In turn, this probability decreased with increasing density of living trees, sapling density, and the slope of the sample plot. The odds ratio for stand parameters, saplings, deadwood diameter, and slope of land amounted to about 1 (0.90–1.09). The odds ratio for decay stage amounted to 2.02. This model explained more variability than the one obtained for stumps, in which some other variables were significant, as there were no stumps in initial stages of decay (Table 7). The probability of the occurrence of Picea seedlings on stumps significantly rose with stump diameter and sapling density (odds ratios of 1.14 and 1.03, respectively), but decreased with increasing stump height (odds ratio of 0.94). The slope of sample plots and the variables associated with the stand did not significantly improve the model.
| Table 6. Results of logistic regression analysis of the probability of Picea seedling occurrence on fallen deadwood on sample plots. | ||||
| Independent variable | Evaluation (standard error) | Wald chi-square | Odds ratio (95% confidence interval) | p |
| Decay stage [1–8] | 0.705 (0.082) | 73.7 | 2.02 (1.72-2.38) | 0.001 |
| Diameter of deadwood [cm] | 0.085 (0.016) | 27.3 | 1.09 (1.05-1.12) | 0.001 |
| Volume of living trees [m3 0.05 ha-1] | 0.047 (0.018) | 6.9 | 1.05 (1.01-1.09) | 0.01 |
| Number of living trees [trees 0.05 ha-1] | -0.105 (0.018) | 33.8 | 0.90 (0.87-0.93) | 0.001 |
| Sapling density [trees 0.05 ha-1] | -0.012 (0.006) | 3.9 | 0.99 (0.98-1.00) | 0.05 |
| Land slope [°] | -0.063 (0.028) | 4.9 | 0.94 (0.89-0.99) | 0.05 |
| Constant | -4.327 (0.779) | 30.9 | 0.001 | |
| Quality characteristics of the model: Likelihood-ratio test: χ2 = 217; p < 0.0001. | ||||
| Coefficients: McFadden’s R2 – 0.409, Nagelkerke R2 – 0.344, Cragg-Uhler (Nagelkerke) R2 – 0.477. | ||||
| The model correctly predicted results in 95% of the cases. Tolerance values for explanatory variables exceeded 0.25. The mean value for standardized Pearson’s residuals was -0.005, standard deviation: 0.884; more than 95% of results were within 2 standard deviations. | ||||
| Table 7. Results of logistic regression analysis of the probability of Picea seedling occurrence on stumps on sample plots. | ||||
| Independent variable | Evaluation (standard error) | Wald chi-square | Odds ratio (95% confidence interval) | p |
| Diameter of stump [cm] | 0.128 (0.024) | 27.9 | 1.14 (1.08–1.19) | 0.001 |
| Height of stump [cm] | -0.066 (0.019) | 12.1 | 0.94 (0.90–0.97) | 0.001 |
| Sapling density [individuals 0.05 ha-1] | 0.026 (0.009) | 8.1 | 1.03 (1.00–1.04) | 0.01 |
| Constant | -3.066 (0.988) | 9.6 | 0.01 | |
| Quality characteristics of the model: Likelihood-ratio test: χ2 = 73; p < 0.0001. | ||||
| Coefficients: McFadden’s R2 – 0.399, Nagelkerke R2 – 0.291, Cragg-Uhler (Nagelkerke) R2 – 0.514. | ||||
| The model correctly predicted the results in 88% of the cases. Tolerance values for explanatory variables exceeded 0.25. The mean value of standardized Pearson’s residuals was -0.033, standard deviation: 0.889; 95% of the results were within 2 standard deviations. | ||||
4 Discussion
4.1 Types of microsites for Picea regeneration in the subalpine forest
In 2003, in the Gorce subalpine forest Picea seedlings and saplings accounted for 88% and 34% of the total amount of regeneration, respectively (Przybylska and Bujoczek 2006). Apart from Picea regeneration, large densities, especially in open areas, were found for Sorbus aucuparia, but that species rarely occurs in the tree layer. Due to the fact that in the Gorce subalpine forest Picea abies accounts for more than 90% of stand volume (Chwistek 2001), special attention was given to this species and the microsites it occupies.
In the study area regeneration occurred mainly on the forest floor, which was the most prevalent type of microsite. Due to ground vegetation in the field layer, the conditions for seedling establishment and growth on the forest floor may vary considerably (Eis 1981; Peltzer et. al 2000; Nilsson and Wardle 2005). Such phytocoenoses are very dynamic. A simulation of frequencies of the most important microsites types in snag stands in the Alps showed large changes in ground vegetation over several years following a disturbance linked to insect outbreaks (Kupferschmid and Bugmann 2005b). In turn, Holeksa’s study (1998) carried out in the Babia Góra massif in the Carpathians, reported that the highest mortality of Picea seedlings in the subalpine forest occurred on the forest floor. At the same time, Holeksa distinguished 19 different synusia in the field layer. The variability and spatial distribution of the field layer was related to land slope, canopy gaps, and the stage of development of the tree stand. Polytrichum formosum and vascular plants of the field layer strongly influenced Picea regeneration. In patches dominated by Polytrichum sp. and Athyrium distentifolium, Picea found suitable conditions for germination and growth for up to one year, but further development was strongly restricted. In contrast, in Vaccinium myrtillus patches germination was limited, but the survivorship of young Picea plants was relatively high. In the Gorce Mountains, a weak but significantly positive correlation was found between the occurrence of Picea seedlings and both Polytrichum sp. and Vaccinium myrtillus (Przybylska and Bujoczek 2006). In turn, Picea sapling density was positively correlated with the presence of Vaccinium myrtillus, but negatively with Polytrichum sp.
Picea plants growing on decaying wood also significantly contributed to regeneration. In this study, 30% of seedlings and 29% of saplings occurred on stumps or fallen deadwood. Other authors have reported that in a subalpine forest the population of Picea occupying woody microsites accounts for 20–80% of total regeneration (Holeksa et al. 2007; Svoboda and Pouska 2008; Svoboda et al. 2010). Several dozen years ago dead trees were still being removed from the forest in the Gorce Mountains, while active protection over the last three decades has also had an impact on the quantity and quality of different microsites. Therefore, we interpreted the high number of saplings on stumps as a residue of past forest management practices. Stumps may thus play a major role in regeneration establishment in subalpine forest stands. This was corroborated by a study carried out by Motta et al. (2006) in the Alps, where regeneration occurred on 43% of stumps.
4.2 Quantity and quality of woody microsites
Previous studies from Carpathian forests reported that the area of forest floor covered by stumps and logs amounted to approximately 4–10% (Szewczyk and Szwagrzyk 1996; Holeksa 1998; Holeksa et al. 2007; Svoboda and Pouska 2008; Svoboda et al. 2010). The area of woody microsites found in this study was low. On the other hand, the total volume of CWD was rather high (180 m3 ha–1), but almost two thirds of the volume was represented by snags and standing entire dead trees.
The cause of death is important with regard to both the proportions of fallen and standing deadwood and the regeneration process. Bače et al. (2012) found that tree death as a result of wind uprooting was positively related to sapling density; conversely, tree death as a result of bark beetle attack was negatively correlated with regeneration density. In the study area, we observed that wind had a small effect on the amount of fallen deadwood, while tree death as a result of bark beetle attack was common. A dead Picea tree can remain standing for a long time and in the third decade after dying even 40% of such trees may still be found erect (Holeksa et al. 2006, 2008). Wind, being an important factor in Picea stands, may increase the amount of fallen deadwood in a short time (Faliński 1978). In such stands, an important role may be played by fungal decomposition of snags. In the Alps, the probability of snag breakage was reported to be independent of either tree diameter or time since tree death. Wood degradation was probably equally advanced in thicker and thinner snags and the likelihood of their breakage was therefore similar four and eight years after tree death (Kupferschmid et al. 2003).
Given the high amount of existing standing deadwood, the area of woody microsites in the study area is going to increase if new deadwood is left in place. Such changes were described in a dynamic model of snag decay for Picea snag stands in Switzerland, where almost all trees were killed by bark beetles (Kupferschmid and Bugmann 2005b). The model predicted that more than 20% of the area would be covered by deadwood 20 years after tree death. The simulations also showed that 30 years after the death of the Picea stand, CWD would become one of the most important microsite types for Picea regeneration, both at the montane and subalpine levels (Kupferschmid et al. 2006). In the Gorce Mountains, the increase in the area of fallen deadwood is not going to be so high due to the fact that only some of the trees are dead.
According to Holeksa (1998), the most important factors for promoting regeneration are strongly decomposed logs and those of a diameter exceeding 30 cm. Zielonka (2006b) determined that it takes 48 years for Picea deadwood to reach decay stage IV. Within the examined area, pieces exceeding 30 cm in diameter and in a decay stage higher than IV covered only about 0.2% of the forest floor (22 m2 ha–1). This shows that an increase in the area of suitable woody microsites depends on both the appearance of new thick dead trees and on the rate of decomposition of new dead trees on the ground. In the case of trees that fall immediately after death, decomposition starts without a lag time (Zielonka 2006b). However, it should be noted that even among fallen trees a large part of the log may not have direct contact with the ground. In the Swiss Alps 73% of the log length sampled was on average 85 cm above the soil surface (Kupferschmid et al. 2003).
There are many factors affecting the creation of woody microsites. We have concluded that forests that have been protected for 30–40 years do not have a sufficient number of suitable logs, in contrast to natural or “pristine” forests (Jaworski and Karczmarski 1995; Zielonka and Niklasson 2001). In such forest regeneration processes take place mainly on the forest floor and stumps left from previous treatments.
4.3 Picea occurrence on woody microsites
The mean regeneration density on woody microsites in the study area was relatively low (about 1 individual per m2). While about 5–6 individuals per m2 were reported in a study from Central Europe (Zielonka 2006a; Bače et al. 2012), in the Gorce Mountains the area of suitable woody microsites (in terms of size and decay stage) was low. We did not find any other species than Picea abies growing on CWD.
The diameter of CWD pieces appeared to be significant in our study. A similar conclusion was reported by Bače et al. (2012), who found that both seedling and sapling density were positively related to the diameter of the wider end of the log. This is probably explained by the fact that thin logs are slightly elevated above the ground, and seedlings become shaded by the tall plants of ground vegetation, but this is not always true. Harmon and Franklin (1989) did not find a relationship between increased elevation of deadwood above ground level and seedling density. Greater diameters of CWD pieces also imply their greater areas. Besides, the density of seedlings per 1 m2 calculated for each CWD size class showed that thicker pieces were of greater importance, which was most probably associated with a higher stability of habitat conditions on larger pieces of decaying wood (Renvall 1995).
The stage of decay was significant for the occurrence of Picea seedlings on fallen deadwood. The examined stands included numerous strongly decomposed stumps, while fresh ones were rather rare, as tree cutting ceased several dozen years ago. Therefore, this variable was not significant in the model developed for Picea occurrence on stumps. Apart from the physical properties of decaying wood enabling the stabilization of young Picea, their greater density may be promoted by deadwood that remains in advanced decay stages for a prolonged period of time, which was shown in a study of beech debris (Müller-Using and Bartsch 2009). It was also reported that thicker deadwood pieces remained in the final stages of decomposition longer than pieces of a smaller diameter (Marra and Edmonds 1994; Montes and Cañellas 2006; Holeksa et al. 2008). Wood remaining on the ground for a longer time increases the likelihood of seeds falling and germinating on such substrates. Our results did not corroborate previous findings that seedling density decreases due to intraspecific and interspecific competition with herbs and dwarf shrubs on the most decayed wood (in stage VIII) (Nakagawa et al. 2003; Mori et al. 2004; Zielonka 2006a). In the Gorce Mountains, seedling density increased with decomposition of woody microsites. This suggests that on those sites competition was not as intense as in other studies.
Apart from CWD parameters, land slope appeared to be significant for the occurrence of Picea seedlings on fallen deadwood – its probability decreased with increasing slope. The slope of individual CWD pieces was not taken into consideration, but the mean value for a given plot was. Nevertheless, it may be expected that seedling establishment on steep and smooth logs should be delayed, as inclined wood surfaces hinder the retention of seeds. For instance, it was observed that seeds are more easily blown away from the surface of logs elevated above their surroundings (Beatty and Stone 1986; Harmon 1989). Furthermore, Baier et al. (2007) noted that the natural regeneration of Picea on steep slopes may be hampered by drifting snow, and under such circumstances hindrances such as stumps or fallen deadwood are of great importance for the recruitment of a new Picea generation. In the case of logs lying on steep mountainsides, one may expect that this process is similar and regeneration may not be initiated before the wood surface becomes rougher due to decomposition. Although most logs become softer and covered with crevices several millimeters deep after 10 years (Holeksa et al. 2008), deadwood is inaccessible to regeneration within 20 years after the death of the tree. It takes about 30 years to improve habitat conditions for the development of a young generation of Picea on woody microsites (Holeksa 1998). It may be supposed that deep crevices are more important for Picea seed establishment on steep slopes. Furthermore, the herbs growing on logs may play a slightly different role in the retention of seeds on steep surfaces (Zielonka and Piątek 2004).
In terms of the occurrence of Picea seedlings on stumps, stump height was found to be significant, in contrast to land slope. Such an observation may result from the fact that regardless of land slope, the upper surface of a stump is horizontal after cutting, or strongly ragged after natural tree fall, which facilitates the retention of humus and seeds. In turn, litter and humus improve nutrient and moisture conditions for the survival and growth of seedlings on woody microsites (Harmon 1987; Takahashi et al. 2000). As far as stump height is concerned, the higher likelihood of Picea seedling occurrence on lower stumps may also be attributable to more beneficial humidity conditions closer to the forest floor. Higher stumps are also more exposed to wind, which may blow seeds away from their top surface. Furthermore, as they decompose, their upper parts tend to fall off together with the humus and plants growing on them.
In addition to a suitable substrate, conditions conducive to Picea germination and survival must be provided. Stand parameters were described based on the mean values of selected characteristics of sample plots. The probability of the occurrence of Picea seedlings on fallen deadwood was significantly positively correlated with the volume of living trees and negatively correlated with the density of living trees and sapling density. A high volume and low density of living trees are characteristic of older stands, which provide favorable conditions for regeneration and seed supply. Older stands also exhibited better light conditions, and it should be borne in mind that Picea seedlings need direct light in the subalpine zone (Brang 1998). In younger stands in Gorce National Park, instances of several-year-old Picea regeneration have also been recorded, but with lower density and a lower survival rate attributable to disadvantageous light conditions (Przybylska and Bujoczek 2006). The period of survival under a dense canopy is limited (Jaworski 1995). While canopy closure was insignificant in our model, it should be noted that it was negatively correlated with the volume of tree losses over the past 15 years. The considerable amount of fresh deadwood indicates that light conditions have changed in the years leading up to the study. This is supported by the fact that the volume of trees that died from 1992 to 2007 was 106 m3 ha–1, while in the period 1982–1992 it was only about 14 m3 ha–1 (unpublished results). We suppose that better light conditions on sample plots helped the regeneration process.
In the case of stumps, stand characteristics were of minor importance for Picea occurrence. The parameter of sapling density was significant, but in contrast to fallen deadwood, increased density led to a higher probability of Picea regeneration. The effect of sapling competition on seedlings differed between stumps and fallen deadwood. This can be connected to their distribution, but it was not studied.
5 Conclusions
In the years 2003–2007, regeneration processes in the subalpine forest in the Gorce Mountains, part of the western Carpathians, took place mostly on the forest floor and on the stumps resulting from cuttings conducted in previous decades. The role of fallen deadwood as a substrate for Picea seed germination, survival, and growth is going to increase over the years. On steeper slopes, regeneration on logs may occur later than on fallen deadwood located in level or slightly inclined areas due to difficulty with seed retention and the absence of deep crevices in deadwood in the initial stage of decomposition. As a result of the dynamic changes occurring in the subalpine forest, stand characteristics such as light condition are more difficult to capture, and so this issue requires long-term observation in conjunction with analysis of the parameters of woody microsites.
Acknowledgements
The project was supported by the State Committee for Scientific Research – grant No. N N309 192837. We are grateful to anonymous reviewers for their critical and helpful comments, as well as the staff members of the Gorce National Park, for making the object available to studies.
References
Baier R., Meyer J., Göttlein A. (2007). Regeneration niches of Norway spruce (Picea abies [L.] Karst.) saplings in small canopy gaps in mixed mountain forests of the Bavarian Limestone Alps. European Journal of Forest Research 126: 11–22. http://dx.doi.org/10.1007/s10342-005-0091-5.
Bače R., Svoboda M, Pouska V, Janda P., Červenka J. (2012). Natural regeneration in Central-European subalpine spruce forests: which logs are suitable for seedling recruitment? Forest Ecology and Management 266: 254–262. http://dx.doi.org/10.1016/j.foreco.2011.11.025.
Beatty S.W., Stone E.L. (1986). The variety of soil microsites created by tree falls. Canadian Journal of Forest Research 16: 539–548. http://dx.doi.org/10.1139/x86-094.
Brang P. (1998). Early seedling establishment of Picea abies in small forest gaps in the Swiss Alps. Canadian Journal of Forest Research 28: 626–639. http://dx.doi.org/10.1139/x98-035.
Busse M.D. (1994). Downed bole-wood decomposition in lodgepole pine forests of central Oregon. Soil Science Society of America Journal 58: 221–227. http://dx.doi.org/10.2136/sssaj1994.03615995005800010033x.
Bütler R., Patty L., Le Bayon R.C., Guenat C., Schlaepfer R. (2007). Log decay of Picea abies in the Swiss Jura Mountains of central Europe. Forest Ecology and Management 242: 791–799. http://dx.doi.org/10.1016/j.foreco.2007.02.017.
Capecki Z. (1982). Masowe wystąpienie zasnui wysokogórskiej Cephalcia falleni (Dalm.), (Pamphiliidae; Hymenoptera) w Gorcach. Sylwan 126: 41–50.
Christensen M., Hahn K., Mountford E.P., Ódor P., Standovár T., Rozenbergar D., Diaci J., Wijdeven S., Meyer P., Winter S., Vrska T. (2005). Dead wood in European beech (Fagus sylvatica) forest reserves. Forest Ecology and Management 210: 267–282. http://dx.doi.org/10.1016/j.foreco.2005.02.032.
Chwistek K. (2001). Dynamics of tree stands in the Gorce National Park during the period 1992–1997. Nature Coservation 58: 15–30.
Chwistek K. (2010). Zmiany składu gatunkowego i struktury drzewostanów Gorczańskiego Parku Narodowego. Ochrona Beskidów Zachodnich 3: 79–92.
Diaci J., Pisek R., Boncina A. (2005). Regeneration in experimental gaps of subalpine Picea abies forest in the Slovenian Alps. European Journal of Forest Research. 124: 29–36. http://dx.doi.org/10.1007/s10342-005-0057-7.
Edmonds R.L., Eglitis A. (1989). The role of the Douglas-fir beetle and wood borers in the decomposition of and nutrient release from Douglas-fir logs. Canadian Journal of Forest Research 19: 853–859. http://dx.doi.org/10.1139/x89-130.
Eis S. (1981). Effect of vegetative competition on regeneration of white spruce. Canadian Journal of Forest Research 11(1): 1–8. http://dx.doi.org/10.1139/x81-001.
Faliński J.B. (1978). Uprooted trees, their distribution and influence in the primeval forest biotope. Vegetatio 38: 175–183. http://dx.doi.org/10.1007/BF00123268.
Harmon M.E. (1987). The influence of litter and humus accumulations and canopy openness on Picea sitchensis (Bong.) Carr. and Tsuga heterophylla (Raf.) Sarg. seedlings growing on logs. Canadian Journal of Forest Research 17: 1475–1479. http://dx.doi.org/10.1139/x87-229.
Harmon M.E. (1989). Retention of needles and seeds on logs in Picea sitchensis-Tsuga heterophylla forest of costal Oregon and Washington. Canadian Journal of Botany 67: 1833–1837. http://dx.doi.org/10.1139/b89-231.
Harmon M.E., Franklin J.F. (1989). Tree seedlings on logs in Picea–Tsuga forests of Oregon and Washington. Ecology 70: 48–59.
Harmon M.E., Franklin J.F., Swanson F.J., Sollins P., Gregory S.V., Lattin J.D., Anderson N.H., Cline S.P., Aumen N.G., Sedell J.R., Lienkaempfer G.W., Cromack K., Cummins J.R., Cummins K.W. (1986). Ecology of coarse woody debris in temperate ecosystems. Advances in Ecological Research 15: 133–302. http://dx.doi.org/10.1016/S0065-2504(03)34002-4.
Harmon M.E., Krankina O.N., Sexton J. (2000). Decomposition vectors: a new approach to estimating woody detritus decomposition dynamics. Canadian Journal of Forest Research 30: 76–84. http://dx.doi.org/10.1139/x99-187.
Hess M. (1965). Piętra klimatyczne w polskich Karpatach Zachodnich. Zeszyty Naukowe Uniwersytetu Jagiellońskiego 115. Prace Geograficzne 11: 1–267.
Hofgaard A. (1993). Structure and regeneration patterns in virgin Picea abies in northern Sweden. Journal of Vegetation Science 4: 601–608. http://dx.doi.org/10.2307/3236125.
Holeksa J. (1998). Rozpad drzewostanu i odnowienie świerka a struktura i dynamika karpackiego boru górnoreglowego. Monographiae Botanicae 82. 208 p.
Holeksa J., Ciapała S. (1998). Usuwanie martwych drzew a naturalne odnowienie świerka w górnoreglowych borach świerkowych Beskidu Wysokiego. Zeszyty naukowe AR w Krakowie 332. Sesja naukowa 56: 161–175.
Holeksa J., Barć A., Hyla A., Krawczyk B. (2006). Changes in coarse woody debris of a West Carpathian subalpine spruce forest over ten years. Polish Botanical Studies 22: 231–240.
Holeksa J., Saniga M., Szwagrzyk J., Dziedzic T., Ferenc S., Wodka M. (2007). Altitudinal variability of stand structure and regeneration in the subalpine spruce forests of the Pol’ana biosphere reserve, Central Slovakia. European Journal of Forest Research 126: 303–313. http://dx.doi.org/10.1007/s10342-006-0149-z.
Holeksa J., Zielonka T., Żywiec M. (2008). Modeling the decay of coarse woody debris in a subalpine Norway spruce forest of the West Carpathians, Poland. Canadian Journal of Forest Research 38: 415–428. http://dx.doi.org/10.1139/X07-139.
Hörnberg G., Ohlson M., Zackrisson O. (1995). Stand dynamics, regeneration pattern and long-term continuity in boreal old-growth Picea abies swamp forest. Journal of Vegetation Science. 6: 291–298. http://dx.doi.org/10.2307/3236224.
Hörnberg G., Ohlson M., Zackrisson O. (1997). Influence of bryophytes and microrelief conditions on Picea abies seed regeneration patterns in boreal old-growth swamp forests. Canadian Journal of Forest Research 27: 1015–1023. http://dx.doi.org/ 10.1139/x97-045.
Hunziker U., Brang P. (2005). Microsite patterns of conifer seedling establishment and growth in a mixed stands in the southern Alps. Forest Ecology and Management 210: 67–79. http://dx.doi.org/10.1016/j.foreco.2005.02.019.
Hytteborn H., Packham J.R. (1987). Decay rate of Picea abies logs and the storm gap theory: a re-examination of Sernander plot III, Fiby urskog, central Sweden. Arboricultural Journal 11: 299–311. http://dx.doi.org/10.1080/03071375.1987.9756362.
Iijima H., Shibuya M. (2010). Evaluation of suitable conditions for natural regeneration of Picea jezoensis on fallen logs. Journal of forest research 15(1): 46–54. http://dx.doi.org/10.1007/s10310-009-0133-9.
Iijima H., Shibuya M., Saito H. (2007). Effects of surface and light conditions of fallen logs on the emergence and survival of coniferous seedlings and saplings. Journal of forest research 12(4): 262–269. http://dx.doi.org/ 10.1007/s10310-007-0012-1.
Introduction to SAS (2007). UCLA: Academic Technology Services, Statistical Consulting Group. http://www.ats.ucla.edu/stat/mult_pkg/faq/general/psuedo_rsquareds.htm. [Cited 24 November 2007].
Jaworski A. (1995). Charakterystyka hodowlana drzew leśnych. Gutenberg. Kraków. p. 26–51. ISBN-83-86310-03-0.
Jaworski A., Karczmarski J. (1995). Budowa, struktura, dynamika i możliwości produkcyjne górnoreglowych borów świerkowych w Babiogórskim Parku Narodowym. Acta Agraria et Silvestria. Series Silvestria 33: 75–113.
Kräuchi N., Brang P., Schönenberger W. (2000). Forests of mountainous regions: gaps in knowledge and research needs. Forest Ecology and Management 132(1): 73–82. http://dx.doi.org/10.1016/S0378-1127(00)00382-0.
Kupferschmid A.D., Bugmann H. (2005a). Effect of microsites, logs and ungulate browsing on Picea abies regeneration in a mountain forest. Forest Ecology and Management 205: 251–265. http://dx.doi.org/10.1016/j.foreco.2004.10.008.
Kupferschmid A.D., Bugmann H. (2005b). Predicting decay and ground vegetation development in Picea abies snag stands. Plant Ecology 179(2): 247–268. http://dx.doi.org/10.1007/s11258-005-0903-1.
Kupferschmid A.D., Brang P., Schönenberger W., Bugmann H. (2006). Predicting tree regeneration in Picea abies snag stands. European Journal of Forest Research 125(2): 163–179. http://dx.doi.org/10.1007/s10342-005-0080-8.
Kupferschmid Albisetti A.D., Brang P., Schönenberger W., Bugmann H. (2003). Decay of Picea abies snag stands on steep mountain slopes. The forestry chronicle 79(2): 247–252. http://dx.doi.org/10.5558/tfc79247-2.
Kuuluvainen T. (1994). Gap disturbance, ground microtopography, and regeneration dynamics of boreal coniferous forests in Finland: a review. Annales Zoologici Fennici 31: 35–51.
Kuuluvainen T., Kalmari R. (2003). Regeneration microsites of Picea abies seedlings in a windthrow area of a boreal old-growth forest in southern Finland. In: Finnish Zoological and Botanical Publishing Board. Annales Botanici Fennici 40: 401–413.
Larose D.T. (2008). Metody i modele eksploracji danych. [Data mining methods and models]. Wydawnictwo Naukowe PWN, Warszawa. 340 p. ISBN 978-83-01-15467-7.
Marra J.L., Edmonds R.L. (1994). Coarse woody debris and forest floor respiration in an old-growth coniferous forest on the Olympic Peninsula, Washington, Canadian Journal of Forest Research 24: 1811–1817. http://dx.doi.org/10.1139/x94-234.
Matuszkiewicz J.M. (2008). Zespoły leśne Polski. Wydawnictwo Naukowe PWN, Warszawa. 376 p. ISBN 978-83-01-14555-2.
Menard S. (2001). Applied logistic regression analysis. Sage University Paper Series on Quantitative Applications in the Social Sciences, 07–106. Sage,Thousand Oaks, CA.
Montes F., Cañellas I. (2006). Modelling coarse woody debris dynamics in even-aged Scots pine forests. Forest Ecology and Management 221: 220–232. http://dx.doi.org/10.1016/j.foreco.2005.10.019.
Mori A., Mizumachi E., Osono T., Doi Y. (2004). Substrate-associated seedling recruitment and establishment of major conifer species in an old-growth subalpine forest in central Japan. Forest Ecology and Management 196(2): 287–297. http://dx.doi.org/10.1016/j.foreco.2004.03.027.
Motta R., Berretti R., Lingua E., Piussi P. (2006). Coarse woody debris, forest structure and regeneration in the Valbona Forest Reserve, Paneveggio, Italian Alps. Forest Ecology and Management 235: 155–163. http://dx.doi.org/10.1016/j.foreco.2006.08.007.
Müller-Using S., Bartsch N. (2009). Decay dynamic of coarse and fine woody debris of a beech (Fagus sylvatica L.) forest in Central Germany. European Journal of Forest Research 128: 287–296. http://dx.doi.org/10.1007/s10342-009-0264-8.
Næsset E. (1999). Decomposition rate constants of Picea abies logs in southeastern Norway. Canadian Journal of Forest Research 29: 372–381. http://dx.doi.org/10.1139/x99-005.
Nakagawa M., Kurahashi A., Hogetsu T. (2003). The regeneration characteristics of Picea jezoensis and Abies sachalinensis on cut stumps in the sub-boreal forests of Hokkaido Tokyo University Forest. Forest ecology and management 180(1): 353–359. http://dx.doi.org/10.1016/S0378-1127(02)00654-0.
Narukawa Y., Iida S., Tanouchi H., Abe S., Yamamoto S.I. (2003). State of fallen logs and the occurrence of conifer seedlings and saplings in boreal and subalpine old-growth forests in Japan. Ecological Research 18(3): 267–277. http://dx.doi.org/10.1046/j.1440-1703.2003.00553.x.
Nilsson M.C., Wardle D.A. (2005). Understory vegetation as a forest ecosystem driver: evidence from the northern Swedish boreal forest. Frontiers in Ecology and the Environment 3(8): 421–428. http://dx.doi.org/10.1890/1540-9295(2005)003[0421:UVAAFE]2.0.CO;2.
Peltzer D.A., Bast M.L., Wilson S.D., Gerry A.K. (2000). Plant diversity and tree responses following contrasting disturbances in boreal forest. Forest Ecology and Management 127(1): 191–203. http://dx.doi.org/10.1016/S0378-1127(99)00130-9.
Przybylska K., Bujoczek L. (2006). Procesy odnowieniowe w reglu górnym. Ochrona Beskidów Zachodnich 1: 109–123.
Przybylska K., Przybylski P. (1994). Zastosowanie metod numerycznych do aproksymacji krzywych miąższości. Sylwan 138: 63–70.
Renvall P. (1995). Community structure and dynamics of wood-rotting Basidiomycetes on decomposing conifer trunks in northern Finland. Karstenia 35: 1–51.
Rock J., Badeck F.W., Harmon M.E. (2008). Estimating decomposition rate constants for European tree species from literature sources. European Journal of Forest Research 127: 301–313. http://dx.doi.org/10.1007/s10342-008-0206-x.
Siitonen J. (2001). Forest management, coarse woody debris and saproxylic organisms: Fennoscandian boreal forests as an example. Ecological bulletins 49: 11–41.
Stokland J.N., Siitonen J., Jonsson B.G. (2012). Biodiversity in Dead Wood. Cambridge University Press, Cambridge, UK. p. 248–274. ISBN 978-0-521-88873-8.
Svoboda M., Pouska V. (2008). Structure of a Central-European mountain spruce old-growth forest with respect to historical development. Forest Ecology and Management 255: 2177–2188. http://dx.doi.org/10.1016/j.foreco.2007.12.031.
Svoboda M., Fraver S., Janda P., Bače R., Zenáhlíková J. (2010). Natural development and regeneration of a Central European montane spruce forest. Forest Ecology and Management 260: 707–714. http://dx.doi.org/10.1016/j.foreco.2010.05.027.
Szewczyk J., Szwagrzyk J. (1996). Tree regeneration on rotten wood and on soil in old-growth stand. Vegetatio 122: 37–46. http://dx.doi.org/10.1007/BF00052814.
Takahashi M., Sakai Y., Ootomo R., Shiozaki M. (2000). Establishment of tree seedlings and water-soluble nutrients in coarse woody debris in an old-growth Picea-Abies forest in Hokkaido, northern Japan. Canadian Journal of Forest Research 30(7): 1148–1155. http://dx.doi.org/ 10.1139/x00-042.
Zielonka T. (2006a). When does dead wood turn into a substrate for spruce replacement? Journal of Vegetation Science 17: 739–746. http://dx.doi.org/10.1111/j.1654-1103.2006.tb02497.x.
Zielonka T. (2006b). Quantity and decay stages of coarse woody debris in old-growth subalpine spruce forests of the western Carpathians, Poland. Canadian Journal of Forest Research 36: 2614–2622. http://dx.doi.org/10.1139/x06-149.
Zielonka T., Niklasson M. (2001). Dynamics of dead wood and regeneration pattern in natural spruce forest in the Tatra Mountains, Poland. Ecological Bulletin 49: 159–163.
Zielonka T., Piątek G. (2004). The herb and dwarf shrub colonization of decaying logs in subalpine forest in the Polish Tatra Mountains. Plant Ecology 172: 63–72. http://dx.doi.org/10.1023/B:VEGE.0000026037.03716.fc.
Zhou Li, Dal Li-min, Gu Hui-yan, Zhong Lei. (2007). Review on the decomposition and influence factors of coarse woody debris in forest ecosystem. Journal of Forestry Research 18: 48–54. http://dx.doi.org/10.1007/s11676-007-0009-9.
Total of 70 references


