The greatest density of parenchyma inclusions in Karelian birch wood occurs at confluences of phloem flows
Novitskaya L., Nikolaeva N., Galibina N., Tarelkina T., Semenova L. (2016). The greatest density of parenchyma inclusions in Karelian birch wood occurs at confluences of phloem flows. Silva Fennica vol. 50 no. 3 article id 1461. https://doi.org/10.14214/sf.1461
Highlights
- Dark-colored inclusions creating the figured pattern in Karelian birch wood consist of storage parenchyma cells
- Their greatest density is formed above branch attachments and below forks
- In these zones, the sucrose content is elevated since photoassimilate flows of the trunk and branches merge into one pathway
- A high level of sucrose enhances the differentiation of parenchyma cells.
Abstract
The specific pattern of the wood of Karelian birch (Betula pendula Roth var. carelica (Merckl.) Hämet-Ahti), is created mainly by dark-coloured inclusions of parenchyma tissue. Our study revealed that the greatest density of parenchyma inclusions in Karelian birch wood is observed above branch attachments to the trunk and below forks. In the place of branch attachment, phloem flows of photoassimilates (sucrose) from the branch and along the trunk merge into one pathway, causing a rise in sucrose content in tissues there. In the area below the fork, sucrose flows from two (or more) trunk axes are combined. Many studies have demonstrated that elevated sucrose level is associated with the differentiation of parenchyma. We believe that where large phloem fluxes merge a high level of sucrose promotes mass differentiation of parenchyma cells instead of fibers and vessels. As a result, the density of the figured pattern in the wood increases. The obtained data have a practical value and can be used in developing recommendations for Karelian birch cultivation.
Keywords
Betula pendula;
patterned wood;
parenchyma;
branch attachments;
forks;
sucrose content
-
Novitskaya,
Forest Research Institute, Karelian Research Center, Russian Academy of Sciences, Pushkinskaya str. 11, 185910, Petrozavodsk, Russia
E-mail
ludnovits@rambler.ru

- Nikolaeva, Forest Research Institute, Karelian Research Center, Russian Academy of Sciences, Pushkinskaya str. 11, 185910, Petrozavodsk, Russia E-mail kar-birch@mail.ru
- Galibina, Forest Research Institute, Karelian Research Center, Russian Academy of Sciences, Pushkinskaya str. 11, 185910, Petrozavodsk, Russia E-mail galibina@krc.karelia.ru
- Tarelkina, Forest Research Institute, Karelian Research Center, Russian Academy of Sciences, Pushkinskaya str. 11, 185910, Petrozavodsk, Russia E-mail karelina.t.v@gmail.com
- Semenova, Forest Research Institute, Karelian Research Center, Russian Academy of Sciences, Pushkinskaya str. 11, 185910, Petrozavodsk, Russia E-mail mi7enova@gmail.com
Received 4 September 2015 Accepted 20 May 2016 Published 1 June 2016
Views 153636
Available at https://doi.org/10.14214/sf.1461 | Download PDF

1 Introduction
Patterned wood formation in Karelian birch, Betula pendula Roth var. carelica, is associated with the modification of cambial activity (Hintikka 1941; Barilskaya 1978; Korovin et al. 2003). Karelian birch trees may vary widely as to when and how patterned wood develops through the ontogeny. Wood figure usually begins to form at an age of 4–8 years (Yermakov 1986). In plants growing under shade of neighboring trees, the process may begin later (after thinning), e.g. at 25–30 years (Sokolov 1970; Novitskaya 2008). The formation of wood figure may sometimes abruptly stop, and wood with normal grain will form thereafter; in some trees periods of normal and abnormal cambial growth may take turns several times (Novitskaya 2008). Examples have been mentioned where figured wood formed only on one side of the trunk (Yermakov 1986; Kosonen 2004; Hagqvist and Mikkola 2008; Novitskaya 2008). It is widely known that wood structure of Karelian birch gradually tends to normal as the tree ages. All that taken together proves that the emergence of figured wood is determined not only by genetic factors, but also depends on the environmental conditions and physiological state of the tree. Opinions have been expressed that abnormal morphogenesis of trunk tissues in Karelian birch is associated with changes in their metabolic status. There are currently two hypotheses concerning the metabolic causes of abnormal cambial growth induction in Karelian birch. Some authors attribute this phenomenon mainly to a rise in the auxin level in trunk tissues (Kosichenko and Shchetinkin 1982; Shchetinkin 1987), while others to a rise in sucrose level (Novitskaya 1997, 2008; Novitskaya and Kushnir 2006; Galibina et al. 2015b).
It is known that (1) hormones are needed for differentiation of mechanical and water conducting elements of wood: fibers are induced by gibberellin (GA), tracheids are induced by a mixture of GA and auxin (IAA), and differentiation of vessel members is based on auxin signal alone (reviewed by Aloni 2015), (2) hormones are involved in plant developmental processes in the form of free hormones, (3) the action of hormones within a plant is regulated by their reversible transition to the conjugated (bound, inactive) state (reviewed by Ludwig-Müller 2011).
Supporters of the “hormonal” hypothesis of figured wood formation refer to the higher (1.8-fold) auxin content in the bark of the Karelian birch trunk as compared to silver birch (B. pendula var. pendula) (Shchetinkin 1987). However, what they leave without comment is that where the bulk of IAA in silver birch is free IAA (free/bound = 2.9), in Karelian birch it is bound IAA (free/bound = 0.5). Furthermore, areas where dense figured pattern was formed contained 4 times more bound IAA than areas with poor figure within the same Karelian birch trunk (Shchetinkin 1987).
The wood of Betula pendula consists of fibers, vessels and parenchyma cells (Barilskaya 1978; Luostarinen and Verkasalo 2000; Heräjärvi 2005). The specific texture of Karelian birch wood results from dark brown inclusions of various shapes. Electron microscopy showed these inclusions to be mainly made up of parenchyma cells (Barilskaya 1978). The large amount of parenchyma in the wood of Karelian birch indicates a disturbance in the differentiation of cambial derivatives: parenchyma cells are formed instead of fibers and vessels. This is evidence of a decline in active forms of GA and IAA. Thus, the anatomical peculiarities of Karelian birch wood correlate well with data on the high level of bound IAA in its tissues.
Conjugation of GA and IAA occurs when they interact with sugars (Michalczuk and Bandurski 1982; Sembdner et al. 1991). It was demonstrated that a high sucrose supply increases the conjugation of GA (Simko 1994; Xu et al. 1998). On the other hand, there are plenty of data showing that a rise in sucrose level induces storage metabolism and differentiation of storage parenchyma cells (Kursanov 1984; Simko 1994; Xu et al. 1998; Wobus and Weber 1999; Borisjuk et al. 2002, 2003).
Sucrose is the main transport form of photoassimilates delivered to acceptor tissues via phloem sieve tubes. For the phloem transport to function, the sucrose concentration in the phloem unloading zone must be kept relatively low. “Excessive” sucrose is utilized in the phloem through the synthesis of storage substances (Kursanov 1984; Evert 1990; Gamalei 2004). We found that in the period of high cambial activity the conducting phloem of Karelian birch, unlike silver birch, stored high amounts of starch, lipids and tannins (Novitskaya and Kushnir 2006). These compounds are synthesized as a result of intensive utilization of sucrose: total activity of sucrose-degrading enzymes in Karelian birch phloem is 2.5 times higher then that of silver birch (Galibina et al. 2015a,b).
Based on available data, we elaborated the hypothesis postulating that the formation of figured pattern in Karelian birch wood is related to appearance of sucrose excess in the conducting phloem. A rise in the sucrose level results in conjugation of GA and IAA. This, in turn, blocks the differentiation of fibres and vessels in the cambial zone. At the same time, excess of sucrose induces the differentiation of storage parenchyma cells. The result of above- mentioned processes is a shift in the percent ratio of wood structural elements towards the share of parenchyma.
It should be noted that our point of view does not downgrade the role of hormones in plant growth and development, but demonstrates the consequences of their temporary inactivation by certain changes in metabolism. This hypothesis is in good agreement with the results of numerous in vitro studies, which have shown that a rise in the sucrose content in the medium inhibits the formation of tracheids in callus cultures and pith explants (Wetmore and Rier 1963; Jeffs and Northcote 1967; Beslow and Rier 1969; Fadia and Mehta 1973; Sachs 1981; Warren Wilson 1984; Warren Wilson et al. 1994), and when reaching a certain level sucrose would stimulate the increase in mass of callus tissue, i.e. parenchyma (Warren Wilson et al. 1994).
In tree trunk tissues, a high level of sucrose may occur in zones where large phloem flows meet, such as places of branch attachment and forks. The aim of our research was to find a correlation between the increase in the density of parenchyma inclusions (wood figure density) and the junctions of large fluxes of sucrose transport along axial organs of Karelian birch.
2 Materials and methods
We examined Karelian birch, Betula pendula Roth var. carelica, trees aged 5- to 60-years-old grown in planted and natural stands. The surveyed Karelian birch stands were situated within: 64°N and 30°E in the north, 50°N and 26°E in the southwest, and 58°N and 49°E in the east.
Surveyed planted stands. Russia: Republic of Karelia, Kirov Region, Moscow Region, Pskov Region. Republic of Belarus: Gomel Region. Ukraine: Rovno Region. The plants were raised from seeds obtained by control-pollination.
Surveyed natural stands. Russia: Smolensk Region, Pskov Region. Republic of Belarus: Minsk Region.
In surveyed stands artificial pruning was not carried out and the tree crowns thus retained their natural structure.
Research methods included visual observations, as well as morphometric, microscopic and biochemical analyses.
The material was collected during 9 years, 2007–2015.
2.1 Choice of trees
Trees selected for the study varied in stages of ontogenetic development of the trunk structural abnormalities: young trees (5–10 yrs.) in which external signs of abnormal tissue development just became visible and adult trees (15–60 yrs.) in which the external trunk morphology allows to attribute them to certain type.
Adult trees were selected according to the classification suggested by Saarnio (1976). The author distinguishes four clearly different categories (types) of Karelian birch: with protuberances (P), with necks (K), with stripes (J), and with rings (R) (abbreviations are taken from Saarnio, they reflect names of the types in Finnish). Very many trees are mixed forms. On the basis of the structure of curly-grained figures, Saarnio divides the trees into two groups: (1) birches with clearly pronounced figures, in which the dark-brown color is dominating (types P and K) and (2) birches in which the dark-brown color is less dominating (type J), or is completely lacking (type R). The aim of our study was to reveal mechanisms of wood figure formation in Karelian birch that is why we selected the trees belonging to types K and P.
2.2 Biochemical research
2.2.1 Sampling
Samples were collected in July when intensive formation of trunk tissue structural abnormalities was underway in Karelian birch (Novitskaya 2008).
In the sampling area bark was separated from wood. The outer wood layers ca. 0.5 mm thick were cut off for analysis with a razor blade. The samples included the zone of xylem growth and differentiation. Sampling was monitored by light microscopy.
Surveys were carried out in two planted stands of Karelian birch: (1) with 7-year-old trees and (2) with 22-year-old trees.
7-year-old trees. Six trees of similar size and crown structure were chosen. The height of the trees was 3.1 ± 0.2 m (SE), and diameter at root collar zone was 6.8 ± 0.5 cm (SE). Sampling was made on 10 and 21 July (three trees on each date). Samples were taken at about 1.3 m above the ground: (1) in the area above the point of the skeletal branch attachment and (2) in the middle of the area between two branches. Samples from the areas (1) and (2) were collected from each of the three trees (3 replications in each variant).
22-year-old trees. The trees belonged to type K. Samples were taken from swollen trunk areas about 1.3 m above the ground. Trees with weak (w.f.), moderate (m.f.) and dense (d.f.) figure were selected. To this end, the technique proposed by Yermakov (1986) was applied. The technique is based on the known correlation between the intensity of figure in Karelian birch wood and the number of pits (depressions) on the wood surface exposed by debarking. A rectangular piece of bark 3 × 4 cm, with the longer side oriented along the trunk, was cut out with a grafting knife in the period of high cambial activity, when the bark can be easily separated from wood. After the bark had been removed, the number of pits in the exposed zone was counted, and then calculated per 1 cm2. Figure was considered very dense when there were 7 or more depressions per 1 cm2 (d.f. – densely figured wood), moderately dense with 4–6 depressions (m.f. – medium figured wood), sparse with 1–3 pits (w.f. – weakly figured wood). Material for sucrose determinations was collected from three trees of each type. Sampling was made on 2 July.
2.2.2 Determination of sucrose
After sampling the plant material was fixed in liquid nitrogen and freeze-dried at low temperature in high vacuum.
Sugars were extracted using a standard technique (Glyad 1999). A weighed unit of a dry sample was crushed by laboratory crushing machine, placed into an evaporation dish and double extracted in 80% ethyl alcohol at 50 °С for 30 minutes. The ethanolic extracts were joined together and evaporated over water bath at 35–40 °С. The dry residue with mono-, di- and oligosaccharides was dissolved in 3–5 ml (depending on the expected amount of carbohydrates) of double distilled water, and filtered through paper filters. The filtrate was thoroughly purified by method of solid phase extraction (SPE) to remove the superfluous components such as pigments, polysaccharides, various salts, and organic acids. To achieve this, solutions of samples were passed through membrane filters (d = 25 mm, 0.45 µm, Nylon) (ProFill, Germany), and then through SPE cartridges (NH2, 500 mg/6 ml, 55 um, 70 A) (Phenomemex Strata, USA). The content of soluble carbohydrates in the filtrates was analysed on Stayer (Aquilon, Russia) high-performance liquid chromatography (HPLC) system with the following conditions: Rezex RCM-Monosaccharide column, double distilled water as the eluent, eluent flow rate of 0.6 ml min–1, refractometer as the detector. The criterion for identification of peaks was the retention time of reference substance, sucrose (Panreac, Spain). The sucrose content was expressed as mg of sucrose per gram of wood dry weight. Biochemical analyses were performed with the equipment of the Center for collective use (Analytical laboratory) of Forest Research Institute of Karelian Research Center of RAS.
2.3 Wood microstructure
Wood was sampled from trees of type P and type K (4 trees of each type). The trees were aged 25 years. Cubes (side length 3 mm) were cut from outer layers of wood approximately at 1.3 m above the ground (in trees of type K – from the swollen area of the trunk). Samples were cut out of two opposite sides of a trunk (8 trees, 2 cubes from each tree, total of 16 cubes).
The material was fixed following the technique used in electron microscopy (Hunter 1993). Samples were fixed in 3% glutaraldehyde: 0.1 M sucrose: 0.1 M phosphate buffer (pH 7.4) at room temperature for 6 h. After rinsing in buffer, postfixation was carried out in 2% OsO4 : 0.1 M phosphate buffer (pH 8.0) at 6 °C for 13 h. After washing in distilled water, the material was dehydrated in an ethanol series (30%, 50%, 70%, 85%, 96%, 100%) and embedded in epoxy resin. Cross and tangential sections (square 3 × 3 mm, 2 µm thick) were cut on an LKB-Ultrotome IV (Sweden), using glass knives. Eight cubes were used for transverse sections, and eight cubes for tangential sections.
Sections were stained with 1% aqueous safranin which is a general stain for lignified cell walls of the wood (Srebotnik and Messner 1994). The second stain used was OsO4, which works as a stain, as well as a fixative. Osmium tetroxide reacts with fatty acids of cell membranes (Thiéry et al. 1995) and with phenols, such as tannin in vacuoles (Shnawa et al. 2011). Vacuoles of parenchyma cells in Karelian birch trunk tissues contain very high amounts of tannins, which are detected any time around the year (Barilskaya 1978; Novitskaya and Kushnir 2006). Reduction of Os produces dark brown color in processed tissue (Kuwajima 2011). That is why osmium stained parenchyma cells in birch wood stand out distinctly from the other xylem elements (Barilskaya 1978; Novitskaya and Kushnir 2006).
Stained sections were mounted in Canada balsam and examined with an AxioImager A1 light microscope (Carl Zeiss, Germany) equipped with a ProgRess C10plus camera (Jenoptic, Germany) at X 100 magnification.
2.4 Wood macrostructure
Wood figure density was assessed in sawn section of tree trunks of types K and Р. A total of 47 trees aged 20–40 years were sawn down.
A segment 50 cm long was sawn out from the middle of the lower half of the trunk. The segment was sawn into sections transversely or tangentially. The surfaces of cuts were polished and covered with varnish for better distinguishing of wood texture.
Cross-sections. A segment was sawn transversely at 4 points: 2 sections from branch attachment points and 2 sections from areas with “no branches”/“traces of fallen off branches” (total of 27 trees: type K – 21 trees, type Р – 6 trees; 4 cross-sections from a trunk segment, total of 108 cross-sections).
Tangential sections. A segment was sawn tangentially into boards around 2 cm thick (total of 20 trees: type K – 13 trees, type Р – 7 trees; 4 boards from a segment, total of 80 boards).
Nine of the sawn down trees (type K) had a bifurcated trunk. In these trees transverse and tangential sections were additionally taken immediately below the fork. Cross-sections: 5 trees, 1 cross-section from a tree, total of 5 cross-sections. Tangential sections: 4 trees, 3 boards 50 cm long from a tree, total of 12 boards).
The samples were studied to identify potential correlations between areas with the greatest density of figure in wood and areas of branch attachment or traces of fallen off branches (where phloem flows of axial organs merge into a united pathway). Assessments were done both visually (in all samples) and quantitatively (selectively).
Quantitative analysis of figure density was carried out using boards. They allow for a more objective assessment of the character of figured pattern distribution in the trunk. Some of the sampled trees had poor figure, sometimes observed only in branch attachment areas (Figs. 1 D,E,G). In these cases it was difficult, if at all possible, to comparatively assess figure density in areas with and without traces of branches. The boards were therefore selected to conform to two requirements: (1) well pronounced wood figure present all along the trunk segment, and (2) traces of branches (knots) present in the segment. The first requirement was fulfilled by trees of type Р (Fig. 1 B). Among the 7 trees of this type that were sawn into boards we chose 3 trees (3 trunk segments) with the richest wood figure. Three boards with easily distinguishable knots were taken from each tree (total of 9 boards). Two areas were visually selected on each board: (1) the area with densest figure in the zone without knots and (2) the area with densest figure in the zone directly above a knot. In the selected areas 3 × 3 cm square frames were drawn. The frames were photographed. The photos were run through image processing software (VideoTest Morphology 5.0, Russia): dark-coloured inclusions inside the frames were stained and the area they occupied was determined. This area was expressed as % of the area enclosed in the frame. Measurements were carried out in 9 replications (9 boards, one measurement in each of “area 1” and “area 2” on each board).
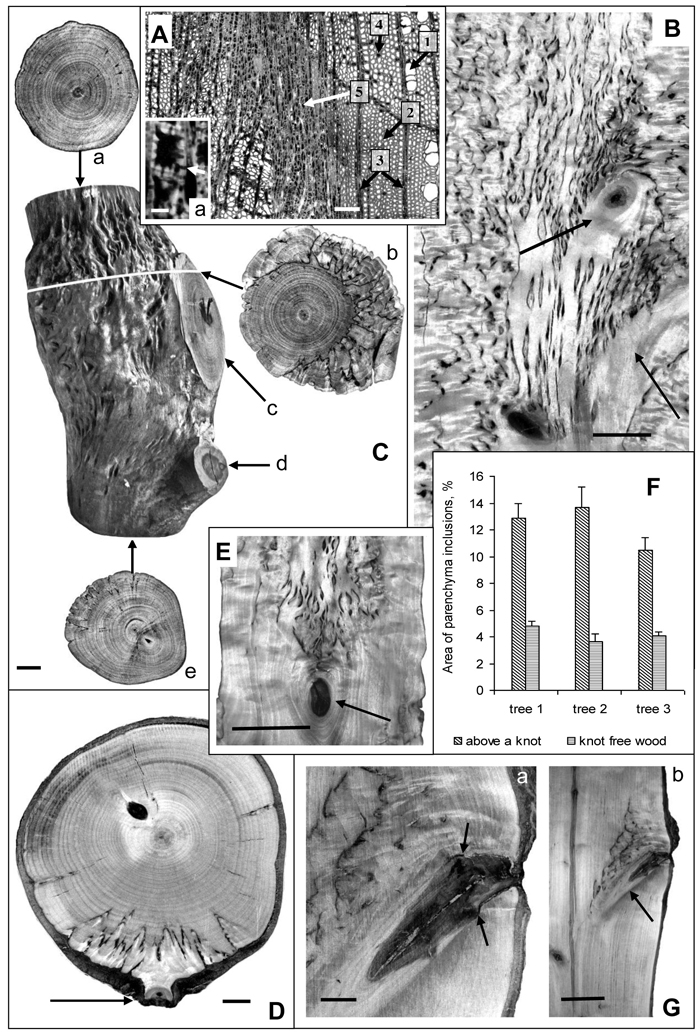
Fig. 1. Karelian birch wood at branch attachment sites.
А – microphotograph of a wood cross-section. 1 – group of three vessels, 2 – fibres, 3 – radial ray parenchyma, 4 – axial parenchyma, 5 – abnormal aggregation of parenchyma cells. a – a group of cells from the zone of parenchyma aggregation; arrow points to a pore in the walls of neighboring cells. Bar: A – 100 μm, a – 5 μm.
B – longitudinal section of a trunk. Arrows point to knots left of nearly perpendicular branches. The density of parenchyma inclusions above the knots is higher than in the rest of the trunk wood. Bar – 2.5 cm.
С – a debarked trunk segment of a 20-year-old tree: a – the wood at the top end of the trunk segment which displays only slight signs of figure; b – a transverse saw cut of wood in the zone of large branch attachment. The most valuable figure is situated on the side adjoining the branch. The darker portion of bark inside the wood indicates the border between branchwood and trunkwood; c – the wood of a large branch with no signs of figure, d – the figureless wood of a thin branch, e – the wood at the bottom end of the trunk segment with no figure below the zone of small brunch attachment to the trunk. Bar – 1 cm.
D – the surface of a trunk cross-cut of a 30-year-old Karelian birch tree. Parenchyma inclusions (figured wood) is found only in the narrow sector associated with the branch-trunk junction. The arrow points to the location of a thin branch attachment. Bar – 1 cm.
E – longitudinal trunk section. Parenchyma inclusions are confined to area above knot (indicated by arrow). Bar – 5 cm.
F – The area of parenchyma inclusions (%) in the middle part of the Karelian birch trunks (trees N 1–3). In each case knot-free figured wood zones were compared with figured wood above knots. M ± SE.
G – longitudinal trunk section. Arrows point to the site where the branch had been removed, causing the formation of parenchyma inclusions to stop. Bar: a – 1 cm, b – 5 cm.
2.5 Trunk external morphology
External traits of the trunk were used to obtain data from a large number of trees without damaging them. Adult (20–60 yrs.) and young (5–15 yrs.) trees were surveyed.
Underlying the methodology is the known fact that trees of type K have richer figure in trunk swellings than in necks (Sokolov 1950; Saarnio 1976; Lyubavskaya 1978; Hagqvist and Mikkola 2008). We monitored from the trunks whether the zones of branch attachments were at trunk swellings or in necks. Thus, one can rely on external morphology of the trunk to evaluate correlations between areas differing in wood figure density and branch attachment sites (where large phloem flows are merged).
All in all, 493 adult trees with globular swellings/muffs on the trunk were examined. In 109 of these trees the trunk was bifurcated. In this case special attention was paid to the external traits of the trunk immediately below the fork.
In young trees the locations of areas with emerging signs of trunk swelling were determined, and stages of the structural anomaly spreading through the trunk were studied until the formation of the globular bulge. Observations covered trees at the age of 5, 10, and 15 years, 10 trees for each age class.
2.6 Statistical treatment
The data obtained through biochemical (sucrose concentration) and morphometric (density of parenchyma inclusions) analyses were statistically processed using the Statgraphics software package for Windows 7.0. Sucrose concentration: for each sampling date and each sampling point the analysis was done in 3 biological (3 trees) and 3 analytical replications (each extract from one biological replicate was measured three times). Density of parenchyma inclusions: in each of the studied wood areas 9 replications were carried out (3 trees, 3 boards from each tree) Measurements are presented as mean values and SE. The confidence intervals for all values were estimated according to Student’s t-distribution.
3 Results
3.1 Sucrose concentration
7-year-old trees. Biochemical analysis showed that sucrose level above the site of branch attachment was higher than in the trunk area between branches (Fig. 2 A).
22-year-old trees. In trees with different wood figure density the sucrose level was twice higher in the case of high figure density compared to poor figure (Fig. 2 B).
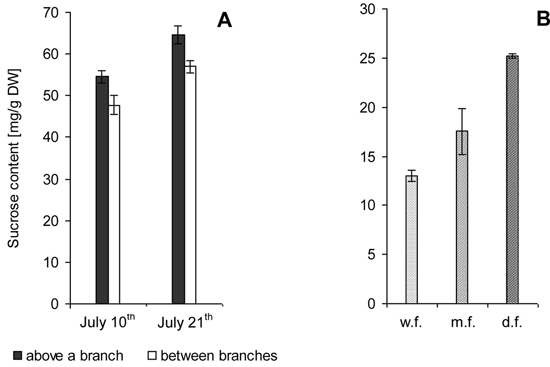
Fig. 2. Sucrose content in the zone of xylem growth and differentiation on the trunks of Karelian birch trees. M ± SE.
A – 7-year-old trees. Samples were taken from branch attachment sites and along the trunk between branches during active formation of wood structural abnormalities.
B – 22-year-old trees. Trunks with weakly figured wood (w.f.), medium figured wood (m.f.) and densely figured wood (d.f.).
Sucrose concentrations were significantly higher in adult than in young trees.
The confidence intervals for the data obtained through biochemical analysis meet 95% reliability according to Student’s t-distribution.
3.2 Wood microstructure
Microscopic sections of Karelian birch wood contain areas with large aggregations of cells with cell content, in contrast to fibres and vessels, which consist of cell walls only (Fig. 1 A). These cells look similar in transverse and tangential sections. Cell walls are perforated by a large number of simple pores connecting the cells to each other (Fig. 1 A-a). All these features are typical of parenchyma. After contact with osmium, the inside of these cells appears almost black. Hence, they can be identified as cells with high tannin content. The high level of tannins in vacuoles is a specific feature of Karelian birch parenchyma cells. We have concluded that aggregations of cells with dark contents are aggregations of parenchyma cells.
In all the examined microscopic sections vessels disappeared, and fibers were replaced by parenchyma cells either gradually (Fig. 1 A, left) or quite abruptly (Fig. 1 A, right) towards parenchyma inclusions in the wood.
3.3 Wood macrostructure
At the macrostructural level abnormal parenchyma inclusions in wood form a distinctive pattern. The examination of transverse and tangential cuts revealed certain regularity in the localization of areas with richer wood figure: in all variants a denser pattern was observed above branch attachments and knots as compared to other parts of the trunk (Figs. 1 В–E, G). Sometimes, where wood figure was weak or practically absent in the trunk, it could still be quite rich immediately above such areas (Figs. 1 С–E, G). An interesting illustration is Fig. 1 G, which shows that after a branch had been removed the formation of figured pattern in the area ceased. Another feature that was detected is that above a relatively large branch the density of parenchyma inclusions in the wood was considerably higher (Fig. 1 C-b) than above the point of small branch attachment (Fig. 1 D).
Quantitative estimation of figure density in the sections with figured pattern present all along the trunk segment (Fig. 1 B) confirmed that the density of parenchyma inclusions above knots was higher than in other parts of the trunk (Fig. 1 F). The confidence intervals for the data meet 95% reliability according to Student’s t-distribution.
Another zone where dense figured wood can be found is the zone of trunk bifurcation (Figs. 3 A–E). Figures 3 C–E show saw cuts of three trunks made at the zone where two approximately equivalent lead branches were connected. In one case, a sample was taken from a Karelian birch tree with very poor wood figure in the trunk, but even in this case a few large parenchyma inclusions were found between two divergent branches (Fig. 3 D). Two other samples were taken from Karelian birch trees with relatively dense figure. These trees had rich figure in the zone of trunk bifurcation (Figs. 3 C,E).
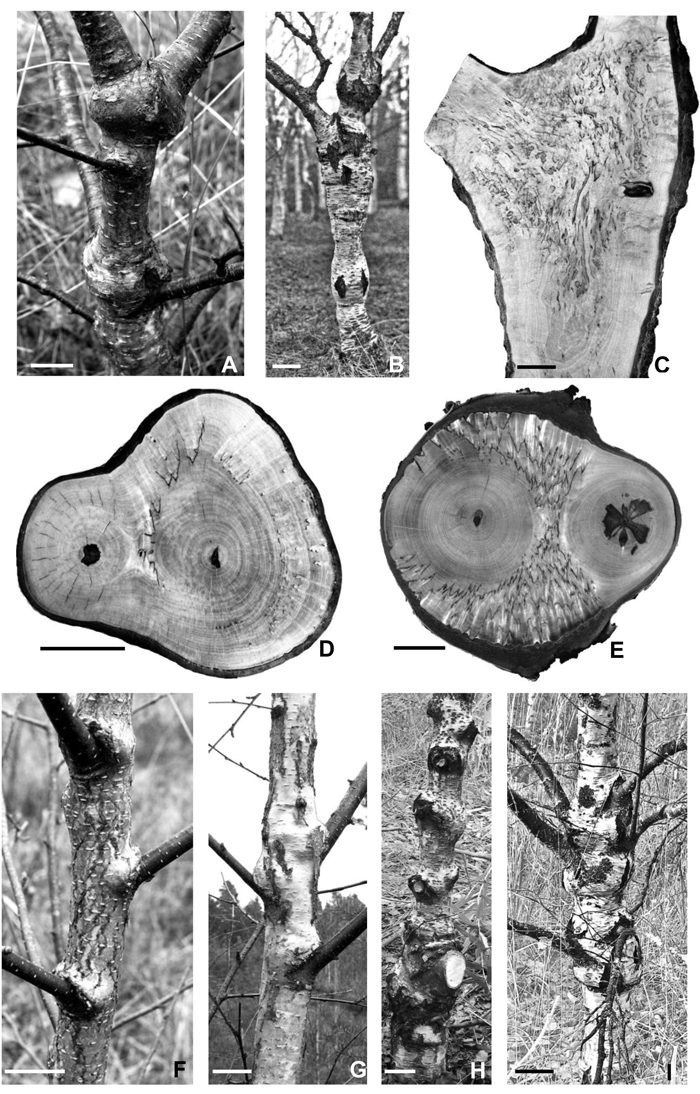
Fig. 3. Fork zones and different stages of globular bulges development on the trunks of Karelian birch trees.
A – a fragment of a 7-year-old stem with a well developed globular bulge located below the fork. Bar – 3 cm.
B – massive globular bulge below the fork in the trunk of a 35-year-old tree. Bar – 10 cm.
C – longitudinal section of a “figured” Karelian birch trunk at the branch junction site; sawn along the edge of a globular bulge, avoiding branchwood. Bar – 5 cm.
D, E – transverse saw cuts in the zone where two branches join. The wood of branches demonstrates no signs of figure. D – a few large parenchyma inclusions were found only in the trunk bifurcation zone. Bar – 5 cm. E – figured wood of the highest decorative value is situated in the branch attachment site. Bar – 3 cm.
F – the appearance of so-called “cheeks” over the sites of branch attachment detected on the trunk of a 5-year-old tree. Bar – 3 cm.
G – expansion of the swellings around the circumference of the trunk in a 10-year-old tree. Bar – 4 cm.
H – the formation of globular bulges at branch-trunk junctions on the trunk of a 17-year-old tree. Bar – 7 cm.
I – massive bulge formation in the zone of the attachment of a few large branches to the trunk of a 27-year-old tree. Bar – 10 cm.
3.4 External morphology of the trunk
The study of the swellings development showed that the zones of their formation coincide with branch attachment points on the trunk (Figs. 3 F–I). In 5-year-old saplings we detected explicit swellings above the places of branch attachment (Fig. 3 F). Older trees demonstrated that as the tree was growing, the swelling was spreading around the trunk circumferentially (Fig. 3 G). Gradually, the swelling developed into a globular bulge (Fig. 3 H). Massive globular bulges were separated by constriction zones (necks), which coincided with branch-free parts of the trunk (Fig. 3 I).
In all the examined adult Karelian birch trees (493 trees) areas of globular swellings of the trunk (where wood figure density was higher) coincided with branch attachment areas or contained knot traces. Neck areas usually lacked branches/traces of fallen off branches. This dependence was sometimes difficult to discern in the lower part of the trunk. The reason was the natural die-back and fall-off of lower branches, with thick cork covering the site afterwards. Further up the trunk however correlation between swollen trunk areas and branch attachment was observed in nearly all trees. Often, several large branches were attached to the trunk in swelling zones (Fig. 3 I).
4 Discussion
In areas with dark-coloured inclusions forming in Karelian birch wood the programmes of differentiation of xylem mother cells are modified: parenchyma cells form there instead of vessels and fibres (Fig. 1 A). Our previous studies have demonstrated that this type of abnormal morphogenesis is connected with excess of sucrose in the conducting phloem (Novitskaya 1997; Novitskaya and Kushnir 2006; Novitskaya 2008; Galibina et al. 2015а,b).
Branch attachments and trunk bifurcations are very special zones from the point of view of sucrose transport. In the lower part of a branch attachment site the conducting phloem of a branch is organically connected to the conducting phloem of the trunk (Fig. 4 A). In the upper part there is an obstacle to the flows of sucrose moving in the basipetal direction (Figs. 4 A–C). In addition, two sucrose streams meet there: flows that move down the trunk and flows from the branch (Figs. 4 A,B). All together, the conditions are created for an increased sucrose content above the branch. Figures 4 B,C schematically show the paths of the phloem flows of the trunk and the branch and their fusion into a common pathway which runs around the branch. The merging of the flows causes a rise in the sucrose level in the tissues located immediately above and on the sides of the branch. The trunk bifurcation is another zone where phloem flows merge (Fig. 4 D). In this case, the greatest amounts of sucrose have to accumulate in the place of axes junction.
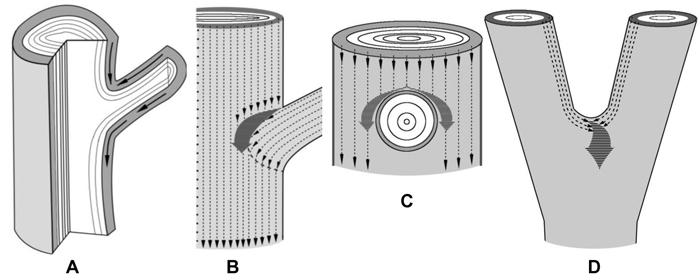
Fig. 4. Schematic representation of the confluence of sucrose phloem streams in the zones of branch attachment and the trunk fork. The xylem is white, the phloem is gray.
A – a fragment of a longitudinal section of the branch attachment area. Arrows indicate the pathways of phloem streams. On the lower side of branch attachment phloem flows move unobstructedly, whereas on the upper side of the branch-trunk junction there is an obstacle to phloem transport.
B, C – fragments of trunk surface with the zone of branch attachment. B – the scheme shows how the phloem flows of a branch and the trunk (thin dotted arrows) meet and merge into a common pathway (thick dark gray arrow). The resulting phloem pathway is reoriented to circumvent the branch. It provides for a rise in the sucrose level in tissues located sideways from the branch, inducing the formation of swellings. C – phloem flows of the trunk circumvent a branch.
D – a fragment of a trunk in the fork zone. Thin dotted arrows indicate the phloem flows of two axes which merge into a common pathway (thick dark gray arrow) at the top of the fork. It creates the conditions for a rise in sucrose content there, which induces abnormal tissue formation.
The conducting phloem is in direct contact with the cambial zone, where determination of the development pathway of the xylem structural elements occurs. The metabolism of the cambial zone is controlled by the influx of assimilates from the phloem (Kursanov 1984; Warren Wilson 1984; Gamalei 2004). A higher level of sucrose at the junctions of phloem flows of the trunk and the branch has to result in an increase in sucrose content in the cambial zone in the respective segment of the trunk. Biochemical analysis showed that in the xylem growth and differentiation zone sucrose content above a branch was higher than between branches (Fig. 2 A). Tissues were sampled from young Karelian birch plants (7 years of age). The rationale was that at this stage of the tree ontogeny the structural anomalies are just beginning to develop, and trunk areas with morphological signs of abnormal growth are still very compact and located immediately above branches (Fig. 3 F).
According to our understanding of the role of sucrose in wood figure formation, a rise in disaccharide level at branch attachments and below trunk forks can be expected to cause an increase in the number of parenchyma inclusions (increased figure density) in these zones. Empirical results have supported this assumption: the greatest density of parenchyma inclusions in the wood of Karelian birch is formed above branch attachments (Fig. 1) and below forks (Figs. 3 C–E).
The fact that sucrose content and the number of parenchyma cells in wood are related is evidenced also by differences in sucrose levels between adult Karelian birch trees with different degrees of figure density: in the xylem formation zone the sucrose level showed an upward trend from trees with slightly figured wood to trees with densely figured wood (Fig. 2 B).
Noteworthy is the higher sucrose concentration in the tissues of young plants (Fig. 2 A) as compared to adult trees (Fig. 2 B). The reasons for that could potentially be the different stages in the development of the trunk structural abnormalities. The formation of parenchyma inclusions in young trees just begins, whereas in adult trees parenchyma constitutes a substantial part of the xylem. As mentioned above, sucrose in parenchyma cells is actively utilized for the synthesis of storage substances. Hence, xylem parenchyma in adult trees is generally able to utilize greater amounts of sucrose, and this fact will be reflected in the lower disaccharide content in the tissue. This approach agrees with the concept that a high level of sucrose induces the differentiation of parenchyma cells, and their metabolism effectively reduces the sucrose concentration in the tissue.
The questions arising in connection with the subject matter are: do structural abnormalities at the confluence of large phloem flows appear in the wood of common silver birch and other woody species?; if they do, are they structurally similar to those in Karelian birch? Until recently, the anatomy of the xylem tissues that are formed at a junction in a tree had not received much scientific attention, no illustration of wood anatomy of a junction at the tissue or cellular level was provided. The first study of this kind was carried out by Slater et al. (2014), involving 20 species, including Betula pendula. The authors demonstrated that wood anatomy and grain pattern in the forks and other junctions within a tree differed markedly from the outer section of the join. With Corylus avellana L. (Betulaceae) as the example it was found that the wood at the junctions contained only 37% of the number of vessels contained in wood within the adjacent stem (Slater et al. 2014). A survey of rays (i.e. parenchyma) showed them to be 58% more abundant in fork wood. A conclusion from these data is that in these zones the wood of many woody species has some structural features similar to Karelian birch wood. The main distinctions of Karelian birch are the following: (1) the wood is more parenchymatous, parenchyma cells forms large aggregations visible to the naked eye; (2) where in other tree species this type of structural abnormalities is confined to a junction apex, in Karelian birch such abnormalities usually occur throughout the trunk, being more intensive at the junctions of phloem flows.
We believe the reason for these distinctions is the peculiar metabolic status of Karelian birch trunk tissues. According to our results, the conducting phloem in Karelian birch functions under conditions of sucrose excess, which is withdrawn from metabolism through the synthesis of storage substances. The conducting phloem of silver birch contains almost no such compounds (Novitskaya and Kushnir 2006). Hence, the conducting phloem cells in silver birch can, if necessary, utilize great amounts of sucrose. That is why structural abnormalities of conducting tissues at the confluence of phloem flows are much weaker in silver birch and remain confined within this zone.
In addition to studying wood grain pattern in sawn sections we investigated correlations between increased figure density and branch attachment sites relying on the external morphology of Karelian birch trunks. We applied this approach to Karelian birch trees with large globular bulges, muffs, on the trunk alternating with characteristic constriction zones, necks. It has been ascertained that figure density is always higher in such bulges than in necks (Sokolov 1970; Saarnio 1976; Lyubavskaya 2006). Examination of a big sample set (493 trees) showed that branches (or traces of branches) were present in areas of swelling and absent from neck areas. Comparing these features of trunk morphology with the distribution of wood figure densities one can see that richer wood figure is found at branch attachment sites, i.e. where large phloem flows merge. The ontogeny of these swellings was studied, showing that they emerged and evolved in the sites of branch attachment to the trunk.
Importantly, the information presented in this paper has applied value. Firstly, it provides scientific grounds for recommendations on thinnings meant to improve light conditions in Karelian birch stands, since abundant supply of photoassimilates (sucrose) to the trunk is possible only with a well-developed crown and a high rate of photosynthesis. Secondly, the results can be used to update pruning recommendations. In Finland Karelian birch is often artificially pruned in order to get knot free logs for rotary peeling (Peltonen 2012; Leminen 2013). Proceeding from our data, two stand tending alternatives can be suggested: if forest owners aim for knot free wood with milder figure, they are to clear the trunk of branches, and if they want to get timber with densely figured areas, they should leave some branches grow. Preference should be given to the branches carrying many well-lit leaves.
5 Conclusions
It was ascertained that the densest figure in Karelian birch wood occurs above branch attachments and immediately below forks. Wood figure density in this case grows owing to the increase in the amount of parenchyma. In these areas phloem fluxes of the trunk and the branch (branch attachments) or of two or more trunk axes (forks) merge together, resulting in an increase in sucrose concentration. Relying on the data on the relationship between the level of sucrose and the differentiation of parenchyma cells the conclusion was drawn that the higher density of wood figure in Karelian birch at the confluences of large phloem flows is due to the increase in sucrose content in the trunk tissues.
Acknowledgements
One part of the study was carried out within the framework of the state-ordered assignment for the Forest Research Institute of KarRC RAS for 2014–2016. The other part (biochemical and microscopic analyzes) was funded by the Russian Foundation for Basic Research, grant N 16-04-01191_a.
References
Aloni R. (2015). Ecophysiological implications of vascular differentiation and plant evolution. Trees 29(1): 1–16. http://dx.doi.org/10.1007/s00468-014-1070-6.
Barilskaya L.A. (1978). Structural analysis of decorative wood of Karelian birch. Botanicheskiy zhurnal 63: 805–811. [In Russian].
Beslow D.T., Rier J.P. (1969). Sucrose concentration and xylem regeneration in Coleus internodes in vitro. Plant and Cell Physiology 10: 69–77.
Borisjuk L., Walenta S., Rolletschek H., Mueller-Klieser W., Wobus U., Weber H. (2002). Spatial analysis of plant metabolism: sucrose imaging within Vicia faba cotyledons reveals specific developmental patterns. The Plant Journal 29(4): 521–530. http://dx.doi.org/10.1046/j.1365-313x.2002.01222.x.
Borisjuk L., Rolletschek H., Wobus U., Weber H. (2003). Differentiation of legume cotyledons as related to metabolic gradients and assimilate transport into seeds. Journal of Experimental Botany 54(382): 503–512. http://dx.doi.org/10.1093/jxb/erg051.
Evert R.F. (1990). “Dicotyledons”. In: Behnke H., Sjlund R. (eds.). Sieve Elements. Comparative structure, induction and development. Springer-Verlag, Berlin, Heidelberg, Germany. p. 103–137. http://dx.doi.org/10.1007/978-3-642-74445-7_6.
Fadia V.P., Mehta A.R. (1973). Tissue culture studies on cucurbits: the effect of NAA, sucrose and kinetin on tracheal differentiation in Cucumis tissues cultured in vitro. Phytomorphology 23: 212–215.
Galibina N.A., Novitskaya L.L., Krasavina M.S., Moshchenskaya Yu.L. (2015a). Activity of sucrose synthase in trunk tissues of Karelian birch during cambial growth. Russian Journal of Plant Physiology 62(3): 381–389. http://dx.doi.org/10.1134/S102144371503005X.
Galibina N.A., Novitskaya L.L., Krasavina M.S., Moshchenskaya Yu.L. (2015b). Invertase activity in trunk tissues of Karelian birch. Russian Journal of Plant Physiology 62(6): 753–760. http://dx.doi.org/10.1134/S1021443715060060.
Gamalei Yu.V. (2004). Transport system of vascular plants. Publishing House of Saint-Petersburg State University. 424 p. [In Russian].
Glyad V.M. (1999). Determination of mono- and oligosaccharides in plants by normal-phase high-performance chromatography. Publishing House Komi Research Centre of RAS, Syktyvkar. 14 p. [In Russian].
Hagqvist R., Mikkola A. (2008). Visakoivun kasvatus ja käyttö. [Development and use of curly birch]. Paino Karisto Oy, Hämeenlinna. 168 p.
Heräjärvi H. (2005). Birch – properties and utilisation. A presentation given at COST E42 workshop, Thessaloniki, Greece. Finnish Forest Research Institute (Metla), Finland. 22 p. http://www.valbro.uni-freiburg.de/pdf/birch_prop_and_util.pdf.
Hintikka T.J. (1941). Visakoivusta ja niiden anatomista. [Curly birch and its anatomy]. Suomalaisen kirjallisuuden seuran kirjapainon Oy, Helsinki. 346 p. [In Finnish].
Hunter E.E. (1993). Practical electron microscopy. A beginner’s illustrated guide. 2nd edition. Cambridge University Press, New York. 173 p. http://dx.doi.org/10.1017/cbo9781139087131.
Jeffs R.A., Northcote D.H. (1967). The influence of indol-3yl acetic acid and sugar on the pattern of induced differentiation in plant tissue culture. Journal of Cell Science 2: 77–88.
Korovin V.V., Novitskaya L.L., Kurnosov G.A. (2003). Structural abnormalities of the stem in woody plants. Moscow State Forest University, Moscow. 280 p. [In Russian].
Kosichenko N.E., Shchetinkin S.V. (1982). Structural aspects of the hormone dependency of cambial activity disruptions. Abstracts of the USSR Conference “Problems of Woody Plants Physiology and Biochemistry”. Krasnoyarsk. 124 p. [In Russian].
Kosonen M. (2004). Visakoivu. [Curly birch]. Metsälehti Kustannus. 208 p. [In Finnish].
Kursanov A.L. (1984). Assimilate transport in plants. Elsevier, Amsterdam, New York and Oxford. 660 p.
Kuwajima M. (2011). Cross-linking fixatives. SynapseWeb. Kristen M. Harris/principal investigator. http://synapses.clm.utexas.edu/. [Cited 25 April 2015].
Leminen J. (2013). Visakoivun kehittyvä kasvattaminen. [Curly birch nascent breeding]. Opinnäytetyö. [Bachelor’s thesis]. Mikkelin Ammattikorkeakoulu. [Mikkeli University of Applied Sciences]. 38 p. [In Finnish].
Ludwig-Müller J. (2011). Auxin conjugates: their role for plant development and in the evolution of land plants. Journal of Experimental Botany 62(6): 1757–1773. http://dx.doi.org/10.1093/jxb/erq412.
Luostarinen K., Verkasalo E. (2000). Birch as sawn timber and in mechanical further processing in Finland. A literature study. Silva Fennica Monographs 1. 40 p. http://www.silvafennica.fi/pdf/smf001.pdf.
Lyubavskaya A.Y. (2006). Karelskaja berjoza. Moscow State Forest University, Moscow. 128 p. [In Russian].
Michalczuk L., Bandurski R.S. (1982). Enzymic synthesis of 1-O-indol-3-ylacetyl-β-D-glucose and indol-3-ylacetyl-myo-inositol. Biochemical Journal 207(2): 273–281. http://dx.doi.org/10.1042/bj2070273.
Novitskaya L.L. (1997). About a possible cause of formation of Karelian birch structural abnormalities. Botanicheskiy zhurnal 82: 61–66. [In Russian].
Novitskaya L.L. (2008). Karelian birch: mechanisms of growth and development of structural abnormalities. Publishing House Verso, Petrozavodsk. 144 p. [In Russian].
Novitskaya L.L., Kushnir F.V. (2006). The role of sucrose in regulation of trunk tissue development in Betula pendula Roth. Journal of Plant Growth Regulation 25(1): 18–29. http://dx.doi.org/10.1007/s00344-004-0419-2.
Peltonen M. (2012). Visakoivikoiden hoidon taso Hartolan ja Sysmän alueella. [Care level of curly birch in the area of Hartola and Sysmä]. Opinnäytetyö. [Bachelor’s thesis]. Kymenlaakson Ammattikorkeakoulu. [University of Applied Sciences]. 31 p. http://www.theseus.fi/xmlui/bitstream/handle/10024/44787/Opinnaytetyo.pdf?sequence=1. [In Finnish, Cited 25 April 2015].
Saarnio R. (1976). Viljeltyjen visakoivikoiden laatu ja kehitys Etelä-Suomessa. [The quality and development of cultivated curly-birch (Betula verrucosa f. carelica Sok.) stands in southern Finland]. Folia Forestalia 263. 29 p.
Sachs T. (1981). The control of the patterned differentiation of vascular tissues. Advances in Botanical Research 9: 151–262. http://dx.doi.org/10.1016/S0065-2296(08)60351-1.
Sembdner G., Schliemann W., Schneider G. (1991). Biochemical and physiological aspects of gibberellin conjugation. In: Takahasi N., Phinney B.O., MacMillan J. (eds.). Gibberellins. Springer-Verlag, New York. p. 249–263. http://dx.doi.org/10.1007/978-1-4612-3002-1_24.
Shchetinkin S.V. (1987). Histogenesis of figured wood in birch (Betula pendula Roth var. carelica Merkl. and Betula pendula Roth). Cand. of Biology (PhD) Thesis. Voronezh. 169 p. [In Russian].
Shnawa H.A., Muhsen M.G., Aldaeem D.A., Ibraheem I.K., Gumaa F.M., Saleh A.I. (2011). Synthesis of barium tannate from eucalyptus bark and its use as a thermal stabilizer for poly(vinyl chloride). BioResources 6(1): 700–706.
Simko I. (1994). Sucrose application causes hormonal changes associated with potato tuber induction. Journal of Plant Growth Regulation 13: 73–77. http://dx.doi.org/10.1007/BF00210950.
Slater D., Bradely R.S., Withers P.J., Ennos A.R. (2014). The anatomy and grain pattern in forks of hazel (Corylus avellana L.) and other tree species. Trees 28(5): 1437–1448. http://dx.doi.org/10.1007/s00468-014-1047-5.
Sokolov N.O. (1950). Karelian birch. Petrozavodsk. 115 p. [In Russian].
Sokolov N.O. (1970). Selection and cultivation of Karelian birch in the Leningrad Region using natural seeding. In: Yermakov V.I. (ed.). Forest genetics, selection and seed production. Publishing House Karelia, Petrozavodsk. p. 277–281. [In Russian].
Srebotnik E., Messner K. (1994). A simple method that uses differential staining and light microscopy to assess the selectivity of wood delignification by white rot fungi. Applied and environmental microbiology 60: 1383–1386.
Thiéry G., Bernier J., Bergeron M. (1995). A simple technique for staining of cell membranes with imidazole and osmium tetroxide. The Journal of Histochemistry and Cytochemistry 43(10): 1079–1084. http://dx.doi.org/10.1177/43.10.7560886.
Warren Wilson J. (1984). Control of tissue patterns in normal development and in regeneration. In: Positional controls in plant development. Cambridge University Press, UK. p. 225–280.
Warren Wilson J., Roberts L.W., Warren Wilson P.M., Gresshoff P.M. (1994). Stimulatory and inhibitory effects of sucrose concentration on xylogenesis in lettuce pith explants; possible mediation by ethylene biosynthesis. Annals of Botany 73(1): 65–73. http://dx.doi.org/10.1006/anbo.1994.1008.
Wetmore R.H., Rier J.P. (1963). Experimental induction of vascular tissues in callus of angiosperms. American Journal of Botany 50(5): 410–429. http://dx.doi.org/10.2307/2440311.
Wobus U., Weber H. (1999). Sugars as signal molecules in plant seed development. Biological Chemistry 380(7–8): 937–944. http://dx.doi.org/10.1515/BC.1999.116.
Xu X., van Lammeren A.A.M., Vermeer E., Vreugdenhil D. (1998). The role of gibberellin abscisic acid and sucrose in the regulation of potato tuber formation in vitro. Plant Physiology 117(2): 575–584. http://dx.doi.org/10.1104/pp.117.2.575.
Yermakov V.I. (1986). Mechanisms of adaptation of birch in the North. Publishing House Nauka, Leningrad. 144 p. [In Russian].
Total of 47 references.

