Impact of climate change on growth dynamics of Norway spruce in south-eastern Norway
Čermák P., Rybníček M., Žid T., Andreassen K., Børja I., Kolář T. (2017). Impact of climate change on growth dynamics of Norway spruce in south-eastern Norway. Silva Fennica vol. 51 no. 2 article id 1781. https://doi.org/10.14214/sf.1781
Highlights
- Correlations between tree-ring width and climate parameters showed temporal instability in their relationship during the period 1915–2012
- A statistically significant positive correlation of April–May precipitation on tree-ring growth was identified since the mid-1970s
- The concomitant temperature increase may have contributed to the changes of growth dynamics.
Abstract
The ongoing climate change may have a distinct effect on Norway spruce growth, one of the most important tree species in European forest management. Therefore, the understanding and assessment of climate-growth relationship can help to reveal relevant patterns in temporal variability that may result in lower tree vitality and decline. The main objective of our study was to evaluate the long-term climate-growth variability of Norway spruce in south-eastern Norway, at the northern edge of the temperate zone. We sampled in total 270 dominant and co-dominant trees from 18 plots in south-eastern Norway. We analysed stem cores and evaluated crown condition parameters to assess the retrospective tree growth and vitality. Despite considerable differences in the crown parameters, high similarity among tree-ring width (TRW) series allowed compiling the regional tree-ring width chronology. Correlations between TRW and climate parameters showed temporal instability in their relationship during the period 1915–2012. While we did not detect any significant relationships between TRW and climate parameters in the first half of the study period (1915–1963), a significant correlation between TRW and spring precipitation was observed for the period 1964–2012. This shift appeared concurrent with temperatures reaching above-average values compared to the average of the climate normal period 1961–1990.
Keywords
Picea abies;
precipitation;
crown condition;
decline;
tree-ring width;
Oslofjord
-
Čermák,
Faculty of Forestry and Wood Technology, Mendel University in Brno, Zemědělská 3, 613 00 Brno, Czech Republic
E-mail
cermacek@mendelu.cz

- Rybníček, Faculty of Forestry and Wood Technology, Mendel University in Brno, Zemědělská 3, 613 00 Brno, Czech Republic; Global Change Research Institute, The Czech Academy of Sciences, Bělidla 986/4a, 603 00 Brno, Czech Republic E-mail michalryb@post.cz
- Žid, Faculty of Forestry and Wood Technology, Mendel University in Brno, Zemědělská 3, 613 00 Brno, Czech Republic E-mail tom.z@centrum.cz
- Andreassen, Norwegian Institute of Bioeconomy Research (NIBIO), P.O. Box 115, NO-1431 Ås, Norway E-mail Kjell.Andressen@nibio.no
- Børja, Norwegian Institute of Bioeconomy Research (NIBIO), P.O. Box 115, NO-1431 Ås, Norway E-mail Isabella.Borja@nibio.no
- Kolář, Faculty of Forestry and Wood Technology, Mendel University in Brno, Zemědělská 3, 613 00 Brno, Czech Republic; Global Change Research Institute, The Czech Academy of Sciences, Bělidla 986/4a, 603 00 Brno, Czech Republic E-mail koldatom@gmail.com
Received 12 January 2017 Accepted 2 March 2017 Published 7 March 2017
Views 170869
Available at https://doi.org/10.14214/sf.1781 | Download PDF

1 Introduction
Norway spruce (Picea abies (L.) Karst.) is one of the economically important species in Central and Northern Europe. At the same time, it is a species most prone to biotic and abiotic damage. In the ongoing climate change, the growth and vitality of Norway spruce may be challenged. Indeed, the effect of climatic conditions on Norway spruce growth has been a major topic of numerous dendrochronological studies in Fennoscandia (Mäkinen et al. 2002, 2003; Solberg et al. 2002; Linderholm et al. 2003; Andreassen et al. 2006) as well as other European areas (Vitas 2004; Koprowski and Zielski 2006; Savva et al. 2006; Rybníček et al. 2010a, 2012a, b; Kolář et al. 2015). Summer temperature is generally identified as the dominant growth-determining factor (Mäkinen et al. 2001, 2002; Solberg et al. 2002; Andreassen et al. 2006). Also, while summer precipitation negatively correlates with radial growth mainly at higher altitudes and latitudes when wet summers are cool, it correlates positively with the growth in the lowlands of south-eastern Norway when warmer summers with lower precipitation lead to drying-out of the upper layer of soil (Mäkinen et al. 2002; Andreassen et al. 2006). Climatic changes, especially the increase in temperature during the 20th century, have been associated with drought stress in Norway spruce stands (Solberg 2004).
The common dendroclimatology premise is that tree-growth–climate relationships are stable over time so that we can reliably infer the nature of past climate from statistically derived tree-ring calibration in the recent past (Fritts 1976). However, several recent reports show that the responses of tree-ring growth to climate variation may be of non-stable nature (Biondi 2000; Solberg et al. 2002; Carrer et al. 2006; Dobrovolný et al. 2016). The high importance of the ongoing climate change for growth–climate relationship has been reported also for other woody species and territories (Jump et al. 2006; Weber et al. 2007; Büntgen et al. 2008; Huang et al. 2010; Rybníček et al. 2016).
Shifts in radial growth response of coastal Norway spruce induced by climatic change have been observed in central Norway. In periods when May and June temperatures were above their long-term means, the significance of other climate factors increased (Solberg et al. 2002). An increasing sensitivity of Picea abies, Larix decidua Mill. and Pinus sylvestris L. to precipitation or late-summer drought also was identified in the Alpine region (Büntgen et al. 2006; Schuster and Oberhuber 2013). The temperature-induced drought stress is probably an increasingly important limiting factor in the white spruce forests of Alaska (Lloyd and Fastie 2002). This means that the shift was even observed in areas where are good conditions in general – at high altitudes and cold climatic zones.
Moreover, changes in the climate, especially the extreme climatic events, are probably one of the causes of top dieback of Norway spruce in SE Norway. The top dieback has frequently occurred in these stands since the 1980s (Solberg 2004). Spruce trees have manifested needle yellowing, top drying and other unspecific symptoms of dying. The latest observed dieback in this area was most probably caused by the interaction between predisposing factors such as site, stand and tree genetic makeup in combination with the inciting climatic factors, such as summer drought (Børja et al. 2016; Hentschel et al. 2014; Rosner et al. 2016). In the same set of trees in SE Norway Rosner et al. (2016) showed that trees with dieback symptoms were anatomically predisposed to hydraulic failure before the drought period; Hentschel et al. (2014) detected less strict stomatal control in the symptomatic trees, while Børja et al. (2016) found that even trees without visual symptoms of top dieback manifested disruptions in their water transport system. In general, trees may survive infrequent extreme events but finally die if the frequency, intensity and duration of such events increase (Lebourgeios et al. 2010). Changes in frequency of drought events may shift the dynamics of tree growth (Breda et al. 2006).
Our experimental plots were Norway spruce stands in SE Norway, around Oslofjord, with the presence of nonspecific stress symptoms (defoliation, yellowing and formation of secondary shoots). Our aims were to i) identify possible relationships between crown condition and radial growth response, ii) determine which climate variables have influenced the radial growth of Norway spruce in SE Norway, and iii) investigate the temporal stability/variability of this relationship.
2 Materials and methods
2.1 Study area
South-eastern Norway is climatically highly diverse as it is divided into three bioclimatic zones: temperate oceanic, temperate continental and boreal subcontinental, and lies on the border between boreal and temperate forests (Rivas-Martínez et al. 2004). Therefore, this area is useful for the comparison with boreal forests of middle and north Fennoscandia as well as with temperate forests of continental Europe. Our study took place in 18 plots around Oslofjord in south-eastern Norway from 2012 to 2013 (Fig. 1). Ten of the selected plots were forest officer plots that had been monitored annually for ten years until the 2006. These discontinued monitoring plots were representative of forests in this area. The remaining eight plots had typical stress symptoms and were a part of a larger study designed to find out the causes of the spruce decline (Børja et al. 2016; Hentschel et al. 2014; Rosner et al. 2016).
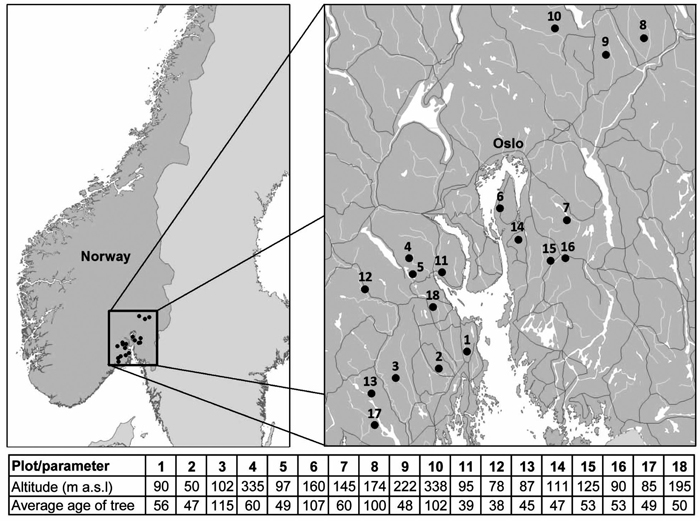
Fig. 1. Location and basic characteristics of the study plots.
The dominant understory vegetation type was the bilberry type (Eu-piceetum myrtilletosum). The altitude of the plots ranged from 50 to 338 m a.s.l. with a mean of 143 ± 83 m (median was 107 m). All plots were either Norway spruce monocultures or stands with spruce as a dominant tree species (> 90 %). There were closed canopy stands – all the trees are dominant or co-dominant (Kraft 1884). All stands were representative planted commercial forests with unknown stand history. The dominating soils were podzols, especially ferric podzols. All plots were situated on a plain or on a gentle slope. The average annual precipitation (for the period 1964–2012) was 849 mm, the average annual temperature was 4.9 °C (Climate Research Unit Time Series data, see below).
2.2 Crown condition assessment
We evaluated crowns visually by dividing them into three sections (based on Cudlín et al. 2001): the juvenile part, the production part, and the saturation part. In the production part of the crown, we observed the total defoliation, the proportion of secondary shoots, and the type of damage (Cudlín et al. 2001). Discoloration, i.e. yellowing and browning, was assessed as the percentage of the total volume of a crown with discoloration. All the parameters were estimated in intervals of 5%.
We classified all trees into categories of stress response based on basic parameters, i.e. total defoliation, defoliation of the primary shoots, proportion of secondary shoots (Cudlín et al. 2001). These categories describe stress tolerance and crown structure transformation stage (rate of substitution of original primary shoots by secondary shoots). According to this classification scheme, the resistant tree has total defoliation lower than 35% and the proportion of secondary shoots lower than 50%. The resilient tree has total defoliation lower than 35% and the proportion of secondary shoots higher than 50%. The slightly transformed damaged tree has total defoliation higher than 35% and the proportion of secondary shoots lower than 50%. The considerably transformed damaged tree has total defoliation higher than 35% and the proportion of secondary shoots higher than 50% (Cudlín et al. 2001).
2.3 Tree-ring sampling and chronology development
To analyse tree-rings we used the same trees as for the crown condition assessment. Among all the dominant and co-dominant assessed trees, fifteen were randomly selected within each plot (270 trees in total). We used the Pressler borer to extract all samples at the breast height. Because the between-tree variability within a plot is much higher than the within-tree variability around the stem (Bošeľa et al. 2014), we extracted one core per tree. We measured the samples using the VIAS TimeTable (Vienna Institute for Archaeological Science, Vienna, Austria) measuring system (with accuracy of 0.01 mm). We then synchronized and cross-dated the tree-ring sequences using software PAST4 (©SCIEM) and COFECHA (Grissino-Mayer 2001). Based on the crown condition assessment we divided all tree-ring width (TRW) series into categories of stress response and compiled three individual TRW chronologies. To assess the degree of similarity between the raw TRW series and individual raw TRW chronologies we used the T-test according to Hollstein (1980), the coefficient of agreement (so called Gleichläufigkeit) (Eckstein and Bauch 1969), and a visual comparison of TRW series, which is crucial for the final dating (Rybníček et al. 2010b). Additionally, statistically significant differences between basic characteristics of these individual TRW chronologies (mean segment length – MSL and average growth rate – AGR) were compared using the T-test. We removed the tree-age-related growth trends and the autocorrelation structures by the ARSTAN application (Grissino–Mayer et al. 1992) using a single detrending method (Holmes et al. 1986) and a 50yr spline function with power transformation (Fritts et al. 1969). This spline length corresponds to the mean segment length. We calculated indices as residuals after power transformation between the measured tree-ring widths and their corresponding values fitted by the function. To assess the quality of each chronology we calculated the Expressed Population Signal (EPS; Wigley et al. 1984) and inter-series correlation (Rbar).
2.4 Growth-climate responses
To calculate the correlation between radial increments and climate characteristics (temperature, precipitation) we used the residual indexed tree-ring width chronology (TRWI) in the DendroClim2002 software (Biondi and Waikul 2004) for the period 1915–2012. We assessed different age and geographical tree groupings without detecting any differences between the age groups (data not presented). We calculated Pearson’s correlation coefficients for the seasonal window from January to September. Based on the monthly results, the significant seasonal means (Apr–May) of the current year (i.e. the year of ring formation) were additionally analysed. We averaged monthly and seasonal means of gridded (0.5° × 0.5°) meteorological measurements (Climate Research Unit Time Series, CRU TS3.23; via http://climexp.knmi.nl) from the 59–60.5°N and 9.5–11.5°E region, dry land. Given that almost no statistically significant correlations have been found for the entire period we divided the period into two halves (1915–1963 and 1964–2012) to assess the relationship before and after the distinct temperature increase. Furthermore, we calculated 21-year moving correlations to explore the expected temporal changes. This window length is a compromise between isolating signal changes with the highest possible temporal resolution and having enough data-points to estimate the signal (Friedrichs et al. 2009). The degree of spatial coherence of the TRWI chronology with April–May precipitation patterns in south-eastern Norway was estimated using the CRUTS3.23 precipitation gridded database (Harris et al. 2013) for the two periods. The statistical comparison of radial increment and climatic factor time-series allowed us to determine the average long-term influence of the studied climatic parameters on the increments. Long-term climatic changes that may also affect tree growth do not have to be demonstrated in the correlation analysis to a statistically significant degree (Kienast et al. 1987); to explore these effects, we analysed negative pointer years. A negative pointer year was defined as an extremely narrow TRW with growth reduction exceeding 40% compared with the average TRW in the previous four years; this strong increment reduction is found in at least 20% of the trees (Schweingruber et al. 1990). The negative pointer years were considered only for the period with the EPS value higher than the acceptable threshold 0.85 (Wigley et al. 1984).
3 Results
3.1 Crown condition assessment
The values of crown parameters widely fluctuated (Fig. 2). The minimum total defoliation of the crown production part was 10%, and the maximum value was 95%. The minimum proportion of secondary shoots in the crown production part was 5%, the maximum was 100%. Yellowing was present in 82 of 270 trees. The average yellow discoloured part of crown was 21%, the maximum was 70% (one tree, plot 16). Browning was rare – only 10 trees were affected. The average brown discoloured part of crown was 9.5%, the maximum was 20% (one tree, plot 10). The discoloration had no relationship with defoliation or the proportion of secondary shoots. There were no statistically significant relationships between tree age and crown condition parameters (Pearson correlation).
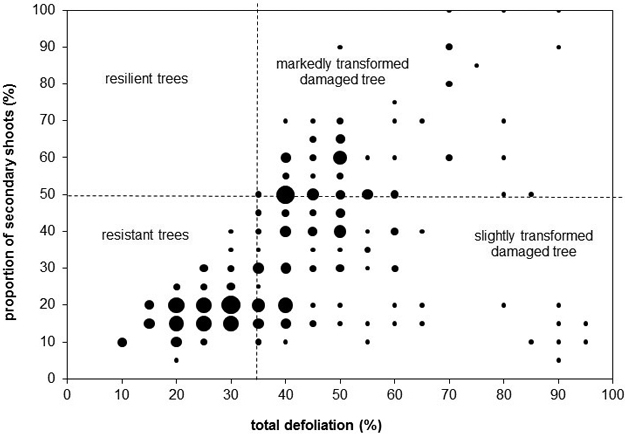
Fig. 2. Total defoliation, proportion of secondary shoots, and categories of stress response. The bubble size represents the number of trees with the given values of the total defoliation and proportion of secondary shoots – this is continuum from the smallest bubble representing one tree to the biggest bubble representing 15 trees.
Three categories of stress response were present (Fig. 2). The resilient trees were absent – all trees with total defoliation higher than 25% had the proportion of secondary shoots lower than 50%. Resistant trees dominated (117 trees), while both categories of damaged trees had very similar abundance (slightly transformed damaged trees: 77 trees, markedly transformed damaged trees: 76 trees).
3.2 Tree-ring width chronologies
TRW chronologies for the stress response categories were similar (Fig. 3a), not only by visual assessment but also by the statistical metrics frequently used in dendrochronology (Fig. 3c). T-values (varied from 7.6 to 12.1; p < 0.01) and Gleichläufigkeit (75–84.4; p < 0.05) showed sufficient coherence among the TRW chronologies; however, resistant and markedly transformed chronologies were less similar than the remaining two pairs. Basic characteristics of the TRW chronologies showed differences mainly in the cambial age expressed by mean segment length (MSL; t > 3.04, p < 0.01) and the average growth rate (AGR (t > 5.89, p < 0.01) of the investigated trees (Fig. 3b). The resistant trees were the youngest ones (40 ± 13 years old on average) with the highest tree-ring width caused by a high proportion of juvenile wood. On the other hand, the markedly transformed trees were older with significantly narrower tree-ring width and lower variability. Mean sensitivity was comparable for all TRW chronologies, as were first-order autocorrelations. The resistant trees had the highest signal strength expressed by the highest values of both the inter-series correlation (Rbar; 0.64) and the expressed population signal (EPS; 0.97).
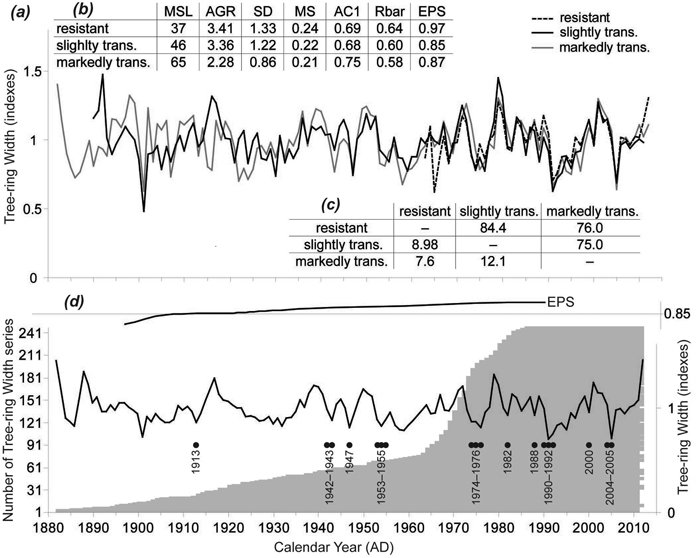
Fig. 3. (a) Tree-ring width (TRW) chronologies of the three stress response categories. (b) Basic characteristics of the three TRW chronologies: MSL – mean segment length; AGR – average growth rate (mm); SD – standard deviation; MS – mean sensitivity; AC1 – first order autocorrelation; Rbar – inter-series correlation; EPS – expressed population signal. (c) Coherence of the TRW chronologies over the common period (1963–2012) expressed on the left down side by THO (THO: T-value after Hollstein (1980)) and on the right upper side by GL (Gleichläufigkeit [“coefficient of agreement”] (Eckstein and Bauch 1969). (d) TRW chronology of all the trees, number of TRW series and negative pointer years.
3.3 Tree-ring width response to climate variability
TRW responses of the three stress response categories to climate parameters were very similar. Therefore, we have compiled one TRW chronology from all trees representing south-eastern Norway (Fig. 3d). The EPS value of the TRW chronology gradually decreased in the past and dropped under the threshold 0.85 (Wigley et al. 1984) in 1915. For that reason, correlations between radial growth and climatic parameters were calculated for the period 1915–2012. Significant correlations for previous years were not identified. Correlations for the current year were significant only for the April precipitation for the whole period. We divided the whole period into two identically long periods: 1915–1963 and 1964–2012). For the first period, we did not detect any significant relationships between TRW and climate parameters. In the second period, April and April–May precipitation sums positively correlated with TRW. Conversely, the average April temperature and January precipitation negatively correlated with the TRW (Fig. 4).
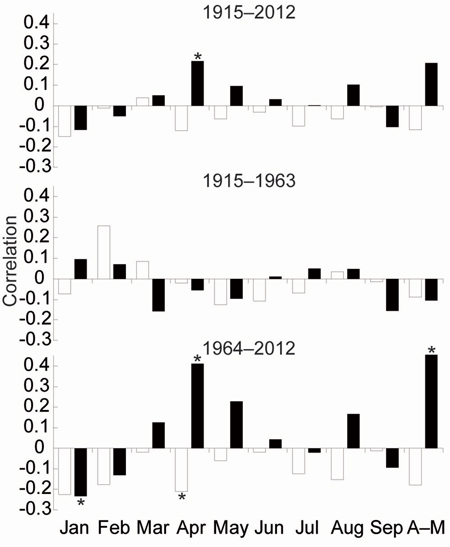
Fig. 4. Means of the Pearson correlations between growth indices and the monthly climate variables temperature (white bar), precipitation (black bar). * indicate significant level (p < 0.05).
In total, 18 negative pointer years were identified during the study period (Fig. 3d). Twelve of them were years when markedly subnormal precipitation (less than 50% of climate normal period 1961–1990 average) was observed in one or more months during the vegetation period. In 2005 we recorded the greatest decline in the TRW when 82% of trees responded negatively. Additionally, we identified other considerable TRW reductions in 1991 (71% of trees) and 1992 (45%).
The average monthly temperatures in the growing seasons increased during the period 1915–2012, with the highest temperature increase in the April–May period (Fig. 5a). The 1915–1963 mean April–May temperature was 6.23 °C while the 1964–2012 mean April–May temperature was 7.11 °C. Although the interannual variability of precipitation was high, the means for the same spring period increased only slightly (Fig. 5b). The 1915–1963 mean April–May precipitation sum was 106 mm while the 1964–2012 mean April–May precipitation sum was 115 mm. However, 21-year moving correlations have revealed a significant change in the relationship between TRW and April–May precipitation (Fig. 5c). Purely negative correlations in the period before 1949 gradually turned into only positive correlations after 1964. The correlations exceeded the critical value for statistical significance at the time when the April–May temperatures reached above-average values compared to the climate normal period 1961–1990 mean. We also recorded an obvious change in the relationship between TRW and April–May temperature during the monitored period. We detected only negative correlations since 1960 and these even approached the critical value for statistical significance in the 1970s and the 1980s.
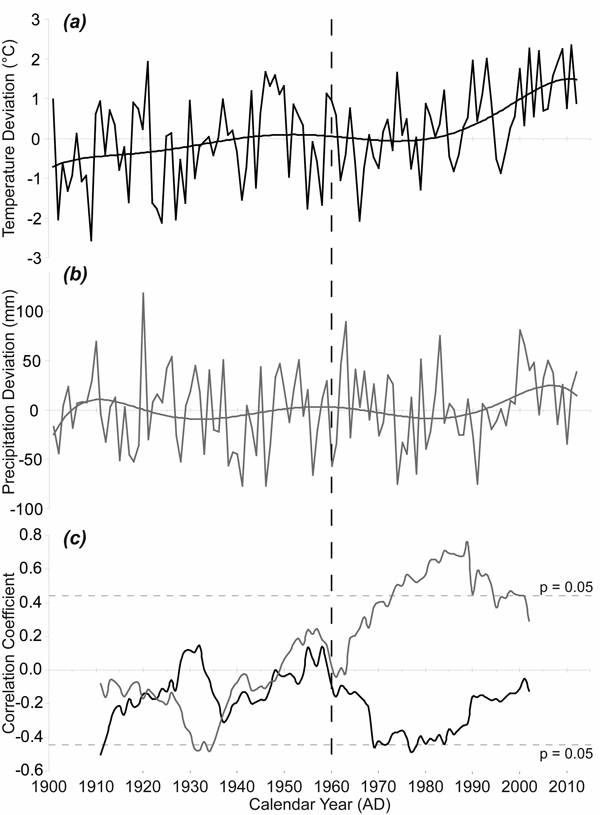
Fig. 5. April–May temperature and precipitation and their effects on radial growth. (a) April–May temperature given as absolute deviation relative to the climate normal period 1961–1990 (°C); (b) April–May precipitation given as absolute deviation relative to the climate normal period 1961–1990 (mm); (c) 21-year moving correlation windows: between tree-ring width index (TRWI) and April–May precipitation (grey); between TRWI and April–May temperature (black). The dotted horizontal lines indicate significant level (p < 0.05).
Spatial correlations confirm the significance of the April–May precipitation on TRW and the differences between the two periods 1915–1963 and 1964–2012 (Fig. 6). The significant correlation between TRW and spring precipitation started at the beginning of the 1960s and the highest correlations (> 0.4) were observed for a limited area of SE Norway.
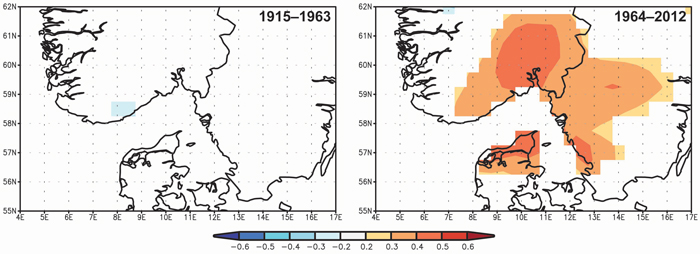
Fig. 6. Spatial correlations between tree-ring width indices and April–May precipitation from Climate Research Unit Time Series CRU TS3.23 (Harris et al. 2013) for the periods 1915–1963 and 1964–2012. View larger in new window/tab
4 Discussion
The variability of crown parameters in Norway spruce stands in south-eastern Norway was wider than the variability of stands investigated in Central Europe (Rybníček et al. 2010a, 2012a, 2012b). The present spruce forests in southern Scandinavia are mixtures of naturally regenerated forests and plantations of both native and non-native origins. Some of the trees are of central European provenance (Aarrestad et al. 2014). It can be one of the reasons for the crown response variability – different provenances can differently react to the same change of conditions, for example southern provenances are less adapted to extreme frosts. Also, many of the south-eastern forest plots in Norway are mosaics of different microsites where the conditions vary from elevated, dry, stony sites with shallow soil layers to depressed, moister microsites. The microsite variability is an important factor especially for Norway spruce, which has a shallow root system (Kalliokoski 2011) with most of the fine root biomass located in the upper soil layers (Børja et al. 2008). During warm periods, the water content in elevated stony microsites may become depleted to the point of causing the dieback of fine roots, thus limiting the water and nutrient uptake of the trees. Together with other mechanisms induced after periods of drought, such as embolism in tree stems, these may be reflected in the crown condition responses. However, the crown condition had no effect on tree growth. The reason can be that crown condition reflects only a relatively recent period and also that the assessed crown damage was not high.
Although the crown parameters were markedly differentiated, TRW chronologies for the stress response categories resembled each other. Tree growth processes can be ranked by order of importance: foliage growth, root growth, bud growth, storage tissue growth, stem growth and growth of defence compounds, and reproductive growth (Waring 1987). While drought affects tree stem growth almost immediately, foliage reduction occurs either late in the season or not at all (Dobbertin 2005). It is probable that each important stress event led to the radial growth reduction in most of the assessed trees but only some of the trees reacted to the stress by displaying the crown symptoms and the reactions were of a variable strength.
In general, short-term stress reaction may not coincide with a long-term change in tree vitality. Growth changes must therefore be interpreted with a long-term perspective in mind (Dobbertin 2005). However, depending on the time of the stress and the tree compartments affected by the stress, crown parameters and tree growth may complement each other as reliable stress indicators.
The most intense climate-growth relationships at our study area were identified for April–May. However, this period was significant only after 1960, specifically, in a few cases between the 1960s and the 1980s for temperature and between1973 and 2001 except 1995–1997 for precipitation (Fig. 4, 5c). The positive significant correlations between TRW and April or May precipitation have also been identified in some Continental European areas (Wilson and Hopfmueller 2001; Bouriaud and Popa 2009; Rybníček et al. 2012a, b). The average precipitation sum in SE Norway was similar to areas of the Czech Republic (Rybníček et al. 2012a, b) during the growing season (April–August; less than 400 mm) but also during the April–May period (about 100 mm). Therefore, we calculated spatial correlations to show a geographical significance of the effect (Fig. 6). The effect of April–May precipitation on TRW seems to be limited to a small area around Oslofjord and the adjacent coast of Skagerrak strait.
Our findings differ from many Fennoscandia tree-ring studies in which summer (especially June temperature) is the most important period for TRW (Børset 1985; Tveite 1990; Mäkinen et al. 2001, 2002; Solberg et al. 2002; Andreassen et al. 2006). The correlation of TRW with temperatures is generally more significant in the north as well as at high altitudes, while the significance of precipitation increases in the south and at low altitudes (Mäkinen et al. 2002). Mäkinen et al. (2003) underlined the importance of May temperatures and Solberg et al. (2002) commented that in the periods when May and June temperatures were above their long-term means, the importance of other climate factors, especially the precipitation, increased.
One of the possible interpretations is that the observed considerable increase in April and May mean temperatures (more than 1 °C) can be the main reason for the proven significant correlation between TRW and April–May climate in the last thirty years of the 20th century (Fig. 5, 6). Positive correlations between TRW and April and May precipitation (Fig. 4, 6) generally indicate the importance of water supply during the first part of the growing season. A combination of normal precipitation and higher temperature leading to higher evaporation can markedly limit the radial growth in this period (Fritts 1976). Spring is also a very important period of root growth (Puhe 2003). Low water content during April–May can lead to lower fine root biomass and, consequently, to a radical decrease in the radial growth. Schuster and Oberhuber (2013) also indicated that sensitivity of Norway spruce to precipitation increased in recent decades, although a decreasing trend in April–June precipitation or increasing aridity were not obvious. However, higher evaporation evoked by higher temperatures dries out the upper soil layer more quickly. The upper layer drying-out markedly decreases the water availability for spruce, especially for young trees. The high proportion of middle-aged trees can be an important factor for the observed change of sensitivity to spring precipitation (Fig. 5, 6). Voelker (2011) reported age dependence of radial growth rate on precipitation for trees less than 50 years old, which the author also explained by changes in the rooting depth during the first few decades of a tree’s life. Moreover, Merian and Lebourgeois (2011) suggested that, for shade tolerant species (including Norway spruce), big trees could be more sensitive to climate change. The differences of sensitivity were observed especially in areas with xeric climate, but it is possible that the increasing tree size influenced also changes of relationships between climate parameters and TRW observed in our study.
5 Conclusions
Despite considerable differences in the crown parameters, high similarity among TRW series allowed compiling a regional TRW chronology. Correlations between tree-ring width and climate parameters showed temporal instability in their relationship. Lack of significant correlations in the period 1915–1963 indicate that tree-ring growth could have been controlled by other factors. The chronology reflects a significant positive correlation of tree-ring growth with April–May precipitation since the mid-1970s. Our results suggest that the concomitant temperature increase may have contributed to this shift. This phenomenon is already quite common in some areas at low and middle altitudes of Continental Europe (Bouriaud and Popa 2009; Rybníček et al. 2012a, b) and now it can also be observed in the forests of southern Scandinavia.
Acknowledgements
The paper was supported by the EEA Grants project “Frameworks and possibilities of forest adaptation measures and strategies connected with Climate change” (no. EHP-CZ02-OV-1-019-2014); grant number LO1415 of the National Sustainability Program I (NPU I), the Ministry of Education, Youth and Sports of CR and the grant numbered 13-04291S of the Czech Republic Grant Agency.
References
Aarrestad P.A., Myking T., Stabbetorp O.E., Tollefsrud M.M. (2014). Foreign Norway spruce (Picea abies) provenances in Norway and effects on biodiversity. NINA report 1075. 39 p. http://www.nina.no/archive/nina/PppBasePdf/rapport%5C2014%5C1075.pdf. [Cited 20 Sept 2016].
Andreassen K., Solberg S., Tveito O.A., Lystad S.L. (2006). Regional differences in climatic responses of Norway spruce (Picea abies L. Karst) growth in Norway. Forest Ecology and Management 222(1–3): 211–221. https://doi.org/10.1016/j.foreco.2005.10.029.
Biondi F. (2000). Are climate-tree growth relationships changing in North-Central Idaho, USA? Arctic, Antarctic and Alpine Research 32(2): 111–116. https://doi.org/10.2307/1552442.
Biondi F., Waikul K. (2004). DendroClim2002: AC++ program for statistical calibration of climate signals in tree ring chronologies. Computers and Geosciences 30(3): 303–311. https://doi.org/10.1016/j.cageo.2003.11.004.
Børja I., Svĕtlík J., Nadezhdin V., Čermák J., Rosner S., Nadezhdina N. (2016). Sap flux – a real time assessment of health status in Norway spruce. Scandinavian Journal of Forest Research 31(5): 450–457. https://doi.org/10.1080/02827581.2015.1130851.
Børja I., de Wit H.A., Steffenrem A., Majdi H. (2008). Stand age and fine root biomass, distribution and morphology in a Norway spruce chronosequence in southeast Norway. Tree Physiology 28(5): 773–784. https://doi.org/10.1093/treephys/28.5.773.
Børset O. (1985). Skogskjøtsel I. Skogøkologi. [Forest management I. Forest ecology]. Landbruksforlaget, Oslo.
Bošeľa M., Sedmák R., Marušák R., Sedmáková D., Petráš R., Barna M. (2014). Evaluating similarity of radial increments around tree stem circumference of European beech and Norway spruce from Central Europe. Geochronometria 41(2): 136–146. https://doi.org/10.2478/s13386-013-0152-3.
Bouriaud O., Popa I. (2009). Comparative dendroclimatic study of Scots pine, Norway spruce, and silver fir in the Vrancea Range, Eastern Carpathian Mountains. Trees 23(1):95–106. https://doi.org/10.1007/s00468-008-0258-z.
Bréda N., Huc R., Granier A., Dreyer E. (2006). Temperate forest trees and stands under severe drought: a review of ecophysiological responses, adaptation processes and long-term consequences. Annals of Forest Science 63(6): 625–644. https://doi.org/10.1051/forest:2006042.
Büntgen U., Frank D.C., Schmidhalter M., Neuwirth B, Seifert M., Esper J. (2006). Growth/climate response shift in a long subalpine spruce chronology. Trees 20(1): 99–110. https://doi.org/10.1007/s00468-005-0017-3.
Büntgen U., Frank D.C., Kaczka R.J., Verstege A., Zwijacz-Kozica T., Esper J. (2007). Growth responses to climate in a multi-species tree-ring network in the Western Carpathian Tatra Mountains, Poland and Slovakia. Tree Physiology 27(5): 689–702. https://doi.org/10.1093/treephys/27.5.689.
Büntgen U., Frank D.C., Wilson R., Carrer M., Urbinati C., Esper J. (2008). Testing for tree-ring divergence in the European Alps. Global Change Biology 14(10): 2443–2453. https://doi.org/10.1111/j.1365-2486.2008.01640.x.
Carrer M., Urbinati C. (2006). Long-term change in the sensitivity of tree-ring growth to climate forcing in Larix decidua. New Phytologist 170(4): 861–872. https://doi.org/10.1111/j.1469-8137.2006.01703.x.
Cudlín P., Novotný R., Moravec I., Chmelíková E. (2001). Retrospective evaluation of the response of montane forest ecosystems to multiple stress. Ekológia 20: 108–124.
Dobbertin M. (2005). Tree growth as indicator of tree vitality and of tree reaction to environmental stress: a review. European Journal of Forest Research 124(4): 319–333. https://doi.org/10.1007/s10342-005-0085-3.
Dobrovolný P., Rybníček M., Buentgen U., Trnka M., Brázdil R., Stachoň Z., Prokop O., Kolář T. (2016). Recent growth coherence in long-term oak (Quercus spp.) ring width chronologies in the Czech Republic. Climate Research 70(2–3): 133–141. https://doi.org/10.3354/cr01402.
Eckstein D., Bauch J. (1969). Beitrag zur Rationalisierung eines dendrochronologischen Verfahrens und zur Analyse seiner Aussagesicherheit. [Contribution to the rationalization of a dendrochronological procedure and the analysis of its reliability]. Forstwiss. Centralblatt 88: 230–250.
Friedrichs D.A., Trouet V., Büntgen U., Frank D.C., Esper J., Neuwirth B., Löffler J. (2009). Species-specific climate sensitivity of tree growth in central-west Germany. Trees – Structure and Function 2: 729–739.
Fritts H.C., Mosimann J.E., Bottorff C.P. (1969). A revised computer program for standardizing tree – ring series. Tree Ring Bulletin 29: 15–20.
Fritts H.C. (1976). Tree ring and climate. Academic Press, London, New York, San Francisco.
Grissino-Mayer H.D., Holmes R., Fritts H.C. (1992). International tree-ring data bank program library. Version 1.1. Laboratory of Tree-Ring Research, University of Arizona, Tucson.
Grissino-Mayer H.D. (2001). Evaluating crossdating accuracy: a manual and tutorial for the computer program COFECHA. Tree-Ring Research 57(2): 205–221.
Harris I., Jones P.D., Osborn T.J., Lister D.H. (2013). Updated high-resolution grids of monthly climatic observations – the CRU TS3.10 dataset. International Journal of Climatology 34(3): 623–642. https://doi.org/10.1002/joc.3711.
Hentschel R., Rosner S., Kayler Z.E., Andreassen K., Borja I., Solberg S., Tveito O.E., Priesack E., Gessler A. (2014). Norway spruce physiological and anatomical predisposition to dieback. Forest Ecology and Management 322: 27–36. https://doi.org/10.1016/j.foreco.2014.03.007.
Hollstein E. (1980). Mitteleuropäische Eichenchronologie: Trierer dendrochronologische Forschungen zur Archäologie und Kunstgeschichte. [Central European oak chronology: Trier dendrochronological studies on archeology and art history]. Trierer Grabungen und Forschungen. Mainz am Rhein.
Holmes R.L., Adams R.K., Fritts H.C. (1986). Tree-ring chronologies of Western North America: California, Eastern Oregon and Northern Great Basin with procedures used in the chronology development work including users manuals for computer programs Cofecha and Arstan. Chronology series VI. Laboratory of Tree, Ring Research, University of Arizona, Tuscon, AZ.
Huang J., Tardif J.C., Bergeron Y., Denneler B., Berninger F., Girardin M.P. (2010). Radial growth response of four dominant boreal tree species to climate along a latitudinal gradient in the eastern Canadian boreal forest. Global Change Biology 16(2): 711–731. https://doi.org/10.1111/j.1365-2486.2009.01990.x.
Jump A., Hunt J.M., Peñuelas J. (2006). Rapid climate change-related growth decline at the southern range edge of Fagus sylvatica. Global Change Biology 12(11): 2163–2174. https://doi.org/10.1111/j.1365-2486.2006.01250.x.
Kalliokoski T. (2011). Root system traits of Norway spruce, Scots pine, and silver birch in mixed boreal forests: an analysis of root architecture, morphology, and anatomy. Dissertationes Forestales 121. 67 p. https://doi.org/10.14214/df.121.
Kienast F., Schweingruber F.H., Bräker O.U., Schär E. (1987). Tree ring studies on conifers along ecological gradients and the potential of single-year analyses. Canadian Journal of Forest Research 17(7): 683–696. https://doi.org/10.1139/x87-111.
Kolář T., Čermák P., Oulehle F., Trnka M., Štěpánek P., Cudlín P., Hruška J., Büntgen U., Rybníček M. (2015). Pollution control enhanced spruce growth in the “Black Triangle” near the Czech–Polish border. Science of the Total Environment 538: 703–711. https://doi.org/10.1016/j.scitotenv.2015.08.105.
Koprowski M., Zielski A. (2006). Dendrochronology of Norway spruce (Picea abies (L.) Karst.) from two range centres in lowland Poland. Trees – Structure and Function. 20(3): 383–390. https://doi.org/10.1007/s00468-006-0051-9.
Kraft G. (1884). Zur Lehre von den Durch Forstungen. [What we have learned about thinning]. Schlagstellungen und Lichtungshieben, Hanover, Germany.
Lebourgeois F., Rathgeber C.B.K., Ulrich E. (2010). Sensitivity of French temperate coniferous forests to climate variability and extreme events (Abies alba, Picea abies and Pinus sylvestris). Journal of Vegetation Science 21(2): 364–376. https://doi.org/10.1111/j.1654-1103.2009.01148.x.
Lesinski J.A., Landman G. (1995). Crown and branch malformation in conifers related to forest decline. In: Cape J.N., Mathy P. (eds.). Scientific basis of forest decline symptomatology. Air Pollution Research Report 15: 95–105.
Linderholm H.W., Solberg B.Ø., Lindholm M. (2003). Tree-ring records from central Fennoscandia: the relationship between tree growth and climate along a west–east transect. The Holocene 13(6): 887–895. https://doi.org/10.1191/0959683603hl671rp.
Lloyd A.H., Fastie C.L. (2002). Spatial and temporal variability in the growth and climate response of treeline trees in Alaska. Climatic Change 52(4): 481–509. https://doi.org/10.1023/A:1014278819094.
Mäkinen H., Nöjd P., Kahleb H.P., Neumann U., Tveite B., Mielikäinen K., Röhle H., Spiecker H. (2002). Radial growth variation of Norway spruce (Picea abies (L.) Karst.) across latitudinal and altitudinal gradients in central and northern Europe. Forest Ecology and Management 171(3): 243–259. https://doi.org/10.1016/S0378-1127(01)00786-1.
Mäkinen H., Nöjd P., Kahle H.P., Neumann U., Tveite B., Mielikäinen K., Röhle H., Spiecker H. (2003). Large-scale climatic variability and radial increment variation Picea abies (L.) Karst.) in central and northern Europe. Trees 17: 173–184.
Mäkinen H., Nöjd P., Mielikäinen K. (2001). Climatic signal in annual growth variation in damaged and healthy stands of Norway spruce [Picea abies (L.) Karst.] in southern Finland. Trees 15(3): 177–185. https://doi.org/10.1007/s004680100089.
Mérian P., Lebourgeois F. (2011). Size-mediated climate-growth relationships in temperate forests: a multi-species analysis. Forest Ecology and Management 261(8): 1382–1391. https://doi.org/10.1016/j.foreco.2011.01.019.
Prokop O., Kolář T., Büntgen U., Kyncl J., Kyncl T., Bošeľa M., Choma M., Barta P., Rybníček M. (2016). On the paleoclimatic potential of a millennium-long oak ring width chronology from Slovakia. Dendrochronologia 40: 93 – 101. https://doi.org/10.1016/j.dendro.2016.08.001.
Puhe J. (2003). Growth and development of the root system of Norway spruce (Picea abies) in forest stands – a review. Forest Ecology and Management 175(1–3): 253–273. https://doi.org/10.1016/S0378-1127(02)00134-2.
Rivas-Martínez S., Penas A., Díaz T.E. (2004). Bioclimatic map of Europe, bioclimates. Cartographic Service, University of Léon, Spain. http://www.globalbioclimatics.org/form/maps.htm. [Cited 2016 Sept 20].
Rosner S., Svĕtlík J., Andreassen K., Børja I., Dalsgaard L., Evans R., Luss S., Tveito O.E., Solberg S. (2016). Novel hydraulic vulnerability proxies for boreal conifer species reveal that opportunists may have lower survival prospects under extreme climatic events. Frontiers in Plant Science 7(831). https://doi.org/10.3389/fpls.2016.00831.
Rybníček M., Čermák P., Kolář T., Žid T. (2010a). Radial growth and health condition of Norway spruce (Picea abies (L.) Karst.) stands in relation to climate (Silesian Beskids, Czech Republic). Geochronometria 36(1): 9–16. https://doi.org/10.2478/v10003-010-0017-1.
Rybníček M., Čermák P., Hadaš P., Kolář T., Žid T. (2012a). Dendrochronological analysis and habitual stress diagnostic assessment of Norway spruce (Picea abies) stands in the Drahany Highlands. Wood Research 57(2): 189–206. https://doi.org/10.2478/s13386-012-0003-7.
Rybníček M., Koňas P., Kolář T. (2010b). The benefits of tree-ring curves detrending for dating archaeological wood. Geochronometria 35(1): 85–90. https://doi.org/10.2478/v10003-010-0004-6.
Rybníček M., Čermák P., Prokop O., Žid T., Trnka M., Kolář T. (2016). Oak (Quercus spp.) response to climate differs more among sites than among species in central Czech Republic. Dendrobiology 75: 55–65. https://doi.org/10.12657/denbio.075.006.
Rybníček M., Čermák P., Žid T., Kolář T. (2012b). Growth responses of Norway spruce (Picea abies (L.) Karst.) to the climate in the south-eastern part of the Českomoravská Upland (Czech Republic). Geochronometria 39(2): 101–109. https://doi.org/10.2478/s13386-012-0003-7.
Savva J., Oleksyn J., Reich P.B., Tjoelker M.G., Vaganov E.A., Modrzynski J. (2006). Interannual growth response of Norway spruce to climate along an altitudinal gradient in the Tatra Mountains, Poland. Trees – Structure and Function 20(6): 735–746. https://doi.org/10.1007/s00468-006-0088-9.
Schuster R., Oberhuber W. (2013). Drought sensitivity of three co-occurring conifers within a dry inner Alpine environment. Trees 27(1): 61–69. https://doi.org/10.1007/s00468-012-0768-6.
Schweingruber F.H., Eckstein D., Serre-Bachet F., Bräker O.U. (1990). Identification, presentation and interpretation of event years and pointer years in dendrochronology. Dendrochronologia 8: 9–38.
Solberg B.Ø., Hofgaard A., Hytteborn H. (2002). Shifts in radial growth responses of coastal Picea abies induced by climatic change during the 20th century, central Norway. Écoscience 9(1): 79–88. https://doi.org/10.1080/11956860.2002.11682693.
Solberg S. (2004). Summer drought: a driver for crown condition and mortality of Norway spruce in Norway. Forest Pathology 34(2): 93–104. https://doi.org/10.1111/j.1439-0329.2004.00351.x.
Tveite B. (1990). Klima og vekst. [Climate and growth]. Norwegian Forest Research Institute, Aktuelt 5: 12–17. [In Norwegian].
Vitas A. (2004). Tree rings of Norway spruce (Picea abies (L.) Karst.) in Lithuania as drought indicators: dendroecological approach. Polish Journal of Ecology 52(2): 201–210.
Voelker S.L. (2011). Age-dependent changes in environmental influences on tree growth and their implications for forest responses to climate change. In: Meinzer F.C., Lachenbruch B., Dawson T.E. (eds.). Size- and age-related changes in tree structure and function. Tree physiology 4: 455–479. https://doi.org/10.1007/978-94-007-1242-3_17.
Waring R.H. (1987). Characteristics of trees predisposed to die. Bioscience 37(8): 569–577. https://doi.org/10.2307/1310667.
Weber P., Bugmann H., Rigling A. (2007). Radial growth responses to drought of Pinus sylvestris and Quercus pubescens in an inner-Alpine dry valley. Journal of Vegetation Science 18 (6): 777–792. https://doi.org/10.1111/j.1654-1103.2007.tb02594.x.
Wigley T.M.L, Briffa K.R., Jones P.D. (1984). On the average value of correlated time series, with applications in dendroclimatology and hydrometeorology. Journal of Climate and Applied Meteorology 23: 201–213. https://doi.org/10.1175/1520-0450(1984)023%3C0201:OTAVOC%3E2.0.CO;2.
Wilson R.J.S, Hopfmueller M. (2001). Dendrochronological investigations of Norway spruce along an elevational transect in the Bavarian Forest, Germany. Dendrochronologia 19(1): 67–79.
Total of 63 references.

