The population development of small trees and shrubs after 100 years of free succession of a wooded meadow in southern Sweden
Finndin M., Milberg P. (2024). The population development of small trees and shrubs after 100 years of free succession of a wooded meadow in southern Sweden. Silva Fennica vol. 58 no. 1 article id 23071. https://doi.org/10.14214/sf.23071
Highlights
- Using a unique map of trees and shrubs from 1937, we estimated the mortality of woody species typical of wooded meadows after management ceased in 1923
- Both population size and canopy cover of the studied species had decreased during the past 86 years
- On the other hand, several tree and shrub specimens endured for a century, pointing to the slow changes involved as well as the potential for restoration.
Abstract
Wooded meadows are characterised by traditional-historic human use. Deliberate selection of species, pollarding and haymaking has created a complex and biodiverse habitat where small trees and shrubs were prevalent. This study set out to document what happens to such trees and shrubs during succession to forest, the normal fate when wooded meadows are abandoned but also when other open to semi-open patches revert to forest. The study was conducted at a site in southern Sweden where traditional management was abandoned by 1923 when the area was protected for research and allowed to follow natural succession. The current study is a follow-up of a 1937-inventory of small trees and shrubs. The results show a decrease in both population size and canopy cover in the selected species during the past 86 years. Hence, we can expect a loss of these species when wooded meadow are abandoned and left to developed into forests. On the other hand, several tree and shrub specimens endured for a century, pointing to the slow changes involved as well as the potential for restoration.
Keywords
conservation;
land-use change;
nemoral forest;
plants;
regrowth;
wooded grassland
-
Finndin,
IFM Biology, Conservation Ecology Group, Linköping University, 581 83 Linköping, Sweden
 https://orcid.org/0009-0003-7620-7104
E-mail
mattef123@gmail.com
https://orcid.org/0009-0003-7620-7104
E-mail
mattef123@gmail.com
-
Milberg,
IFM Biology, Conservation Ecology Group, Linköping University, 581 83 Linköping, Sweden
 https://orcid.org/0000-0001-6128-1051
E-mail
permi@ifm.liu.se
https://orcid.org/0000-0001-6128-1051
E-mail
permi@ifm.liu.se

Received 12 November 2023 Accepted 8 February 2024 Published 19 February 2024
Views 36260
Available at https://doi.org/10.14214/sf.23071 | Download PDF

Supplementary Files
1 Introduction
Wooded meadows are one of the most diverse European habitats and they were still common in Scandinavia and in the Baltic 150 years ago (Hæggström 1983; Emanuelsson 2009; Plieninger et al. 2015; Eriksson 2020). Wooded meadows are characterised by traditional-historic use, intended to shape the nature after human needs (Sjöbeck 1932). Species of trees and shrubs with limited usefulness were eliminated and beneficial species as for example Malus sylvestris (L.) Mill., Rhamnus cathartica L. and species of the genus Ribes L. were kept, planted or allowed to reproduce (Sjöbeck 1932; Axelsson Linkowski and Svensson 2009). Hundreds of years of such selectivity among species, pollarding and haymaking created a unique human-shaped habitat with high complexity (Slotte 2001; Emanuelsson 2009) with a number of typical woody species under scrutiny in the current study.
Shift in agricultural strategies and abandonment of low-productive areas has led to wooded meadows becoming overgrown and converted into forests (Milberg 1995; Losvik 1999; Kana et al. 2008; Götmark 2013; Milberg et al. 2019), putting focus on their management and restoration (Milberg and Tälle 2023). Meadows and grazed areas dropped from 16% in the 1870s to 0% in the current study area (Bergstedt et al. 2017). In fact, meadows cover a mere 11.600 ha in Sweden (0.02%; SOS 2019). Hence, many species confined to open areas persist in grazed areas and sites adjacent to open areas (e.g. road verges and verges of arable fields).
Of particular interest is therefore how previously promoted trees and shrubs respond to becoming over-grown. As this group of species often carries berries, their value for wildlife is high and they were previously collected for human or livestock consumption (Guadilla-Sáez et al. 2019). Some species also had valuable wood properties (Austad and Hauge 1990). Finally, their occurrence in wooded meadows is part of our cultural legacy (Sjöbeck 1932; Peterson 2005).
The aim of the current study was to evaluate how small trees and shrubs, characteristic of the former open agricultural landscape, are affected by succession of a wooded meadow into a forest. The study site was allowed to become overgrown 100 years ago when a no-access and no-touch policy was enforced (Sernander 1922), when it was protected. Fifteen years later, all trees and shrubs were carefully mapped (Julin 1948), and we used these maps to compare with 2023. We expected a general decrease in population sizes following abandonment, caused by a combination of increasing shade and competition and reduction in recruitment. However, species would vary in their sensitivity to over-growth with some being able to survive longer during succession (Kobe et al. 1995; Mason et al. 2004).
2 Materials and methods
2.1 Study area
Vessers Udde in Östergötland, Sweden is a promontory which previously was a wooded meadow probably since at least the late 16th century (Kardell and Fiskejö 1999). The 3.42 ha study area (Fig. 1) is currently part of the 7.4 ha Vessers Udde naturreservat which include also a part of the lake Stora Rängen and a small island. The reserve has been protected since 1923 when it was set aside with the purpose to enable the study of succession from a wooded meadow into a forest (Sernander 1922, 1925). It is worth noting that a parallel reserve was also set aside with a similar purpose, but this lacked the many shrub and small tree species present at Vessers Udde (Hytteborn et al. 2017). The area has been subjected to three studies that have been published. In 1921, Rutger Sernander placed a number of large permanent plots in different vegetation types (Sernander 1925). Between 1935 and 1941, and mainly in 1936–1937, Erik Julin conducted a very comprehensive study, eventually resulting in his PhD thesis (Julin 1948). In 1978–1979 and again in 1991–1992 Lars Kardell and Anne-Li Fiskesjö made another comprehensive study (Kardell and Fiskejö 1999). Their original data from 1978–1979 has been consulted to extract un-published numbers.
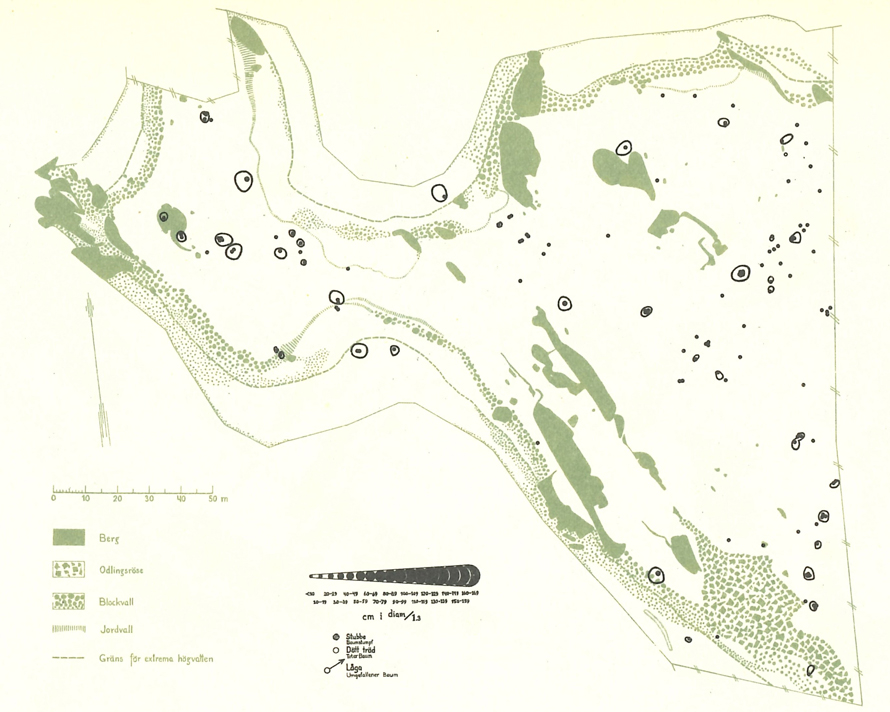
Fig. 1. The 1937 map of Malus sylvetris in Vessers Udde, southern Sweden (Julin 1948). The open, somewhat non-circular areas are tree canopy projections. The point is the stem of individuals tree, with the size of the points being proportional to dbh. View larger in new window/tab.
Management at Vessers Udde continued up until protection (Julin 1948; i.e. the last mowing would have been in 1922), when the area consisted of a combination of open, sun-exposed grass-dominated areas and areas with shrubs and trees (Sernander 1925). This spatial pattern was apparent also in the 1930s (Julin 1948). Timber volume increased from 90 m3 ha–1 in 1937 to 288 m3 ha–1 in 1992 (Kardell and Fiskesjö 1999). Additional changes noted were the loss of species from the ground vegetation, the increase in cover of Corylus avellana L. and a substantial increase of deadwood (Kardell and Fiskesjö 1999). Today, after 100 years of free development and regrowth, the area consists of Sub-Atlantic and medio-European oak forests of the Carpinion betuli, with the Naura 2000 EU-code 9160 (Länsstyrelsen Östergötland 2018). The tree and Corylus canopies creates substantial shade.
As the reserve is only fenced on the landside, larger animals can access via the lake, e.g. in winter. Ungulates and wildboar may have visited. These were absent or very rare in 1937, but three species of ungulates and wildboar have increased since the 1980s (Bergstedt et al. 2017; Petersson et al. 2019).
2.2 Maps
During Julin’s field work in 1937, all trees and shrubs >100 cm in height in the reserve were mapped, in total 24 maps, some with multiple species (Julin 1948). For trees, their dbh size class is indicated by 17 different symbols (Fig. 1; <10 cm, 10–19 cm, 20–29 cm etc). Most of the maps indicate stem diameter, e.g. Malus sylvestris, Crataegus L. spp. and Rhamnus cathartica, while height was instead used for Juniperus communis L. (Julin 1948). For some species, maps show crown projections (Berberis vulgaris L., Crataegus ssp., Malus sylvestris, Ribes alpinum L., Rhamnus cathartica, Ribes spicatum E. Robson, Rosa L. spp., Ribes uva-crispa L.). We used magnifying glass to count stems, to allocate a tree to a size class, and used Adobe Photoshop (2021) to calculate the area covered by the canopy in 1937.
To establish the size of the mapped area (3.42 ha), we used an open digital map-service provided by the Swedish Mapping, Cadastral and Land Registration Authority.
2.3 Fieldwork 2023
The species searched in April 2023 were Berberis vulgaris, Crataegus ssp., Juniperus communis, Malus sylvestris, Prunus avium (L.) L., Rhamnus cathartica, Ribes alpinum, Ribes spicatum, Ribes uva-crispa and Rosa spp. N.b. that Prunus avium was not present in 1937 (Julin 1948) but it was later noted by Kardell and Fiskesjö (1999).
The methods followed that of Julin (1948) as closely as possible. Individuals of the species listed above marked in Julin’s maps were searched for carefully, together with a complete search for the species in the full study area. Every individual found in 2023 was positioned with GPS and individual notes and measurements were taken. Diameter at breast height (dbh) was measured on all individuals larger than 1.3 meters in height. Uneven stem diameter was taken in consideration by measuring a maximum and minimum diameter and using the average. A tree calliper was used to take the measurements.
The radius of the crown of the individuals was recorded using a tape measure to assess canopy cover. The height of all individuals was measured with a tape measure or a digital clinometer. Individuals with a dead top were measured up to the height of where living branches or stems were detected, in line with data collection guidelines of the Swedish National Forest Inventory (SLU 2023).
Dead individuals were included in the data collection if they could be identified to species, but were not included in the current analysis.
2.4 Variables analysed
Julin (1948) made two decisions during field work that has complicated follow-ups and comparison with other studies. First, Julin’s smallest stem diameter size class included individuals of 100–130 cm height, i.e. also those that had not yet reached breast height. Tree data from other studies, including the 1978–1979 and 1991–1992 follow-ups (Kardell and Fiskesjö 1999), are not comparable as they followed the current convention of excluding trees below breast height. Second, Juniperus communis was treated differently in two ways: all specimens >50 cm were recorded, and hight classes were used rather than dbh classes. These inconsistencies have precluded some comparisons and meant that the number of variables analysed per species varied. Hence, number of individuals ha–1 was calculated for 1937 and 2023 for all species >100 cm (>50 cm for Juniperus communis), and canopy cover m2 was calculated for 1937 and 2023, whenever possible. Finally, basal area at bh (m2 ha–1) was calculated only for Malus sylvestris. The smallest dbh size class in 1937 was assigned the basal area 39.2 cm2, and the following intervals were given a value based on the median diameter of each class. Data from 1979 were extracted from the original field notes (SLUs centralarkiv, Institutionen för skoglig landskapsvård, volym 59: ”Torrgrenar, torrträd, döda småträd, småträd och buskar, kuberingslistor, trädmätningar, trädmaterial, trädmätningsprotokoll, årsringsanalys, kartor med trädförekomst”).
3 Results
3.1 Species composition
In 1937, Julin recorded nine species of shrubs and low-grown trees typical of the old agricultural landscape: Juniperus communis, Malus sylvestris, Rosa spp., Crataegus spp., Rhamnus cathartica, Berberis vulgaris, Ribes alpinum, Ribes spicatum and Ribes uva-crispa. N.b. that he only considered specimens taller than 100 cm (or 50 cm regarding Juniperus communis). Later in the 1970s, a new species was added (Prunus avium; Kardell and Fiskesjö 1999). In 2023, there was no trace of Rhamnus cathartica, Berberis vulgaris or Ribes spicatum (all rare in 1937) while the other species were still present although all Ribes uva-crispa were below the 100 cm cut-off (Table 1). So, the number of species had dropped from nine in 1937 to seven in 2023, despite the addition of Prunus avium.
| Table 1. Populations density (number ha–1) of smaller tree and shrub species in Vessers Udde (southern Sweden) at different points in time, and estimated annual mortality (percentage year–1). | |||||
| 1937 | 1978–1979 | 2023 | Loss since 1937 | Annual mortality | |
| SMALL TREES | |||||
| Malus sylvestris | 36.8 | 27.8 | 7.0 | 81.0% | 1.91% |
| Crataegus spp. | 21.9 | 7.6 | 65.3% | ||
| Prunus avium | 0 | 1.2 | 0.3 | ||
| Rhamnus cathartica | 3.2 | 0 | 0 | 100% | |
| SHRUBS | |||||
| Juniperus communis | 77.8 | 4.7 | 94.0% | 2.63% | |
| Berberis vulgaris | 0.9 | 0 | 100% | ||
| Ribes alpinum | 21.9 | 9.4 | 57.3% | ||
| Ribes spicatum | 0.6 | 0 | 100% | ||
| Ribes uva-crispa | 0.3 | 0 | 100% | ||
| Rosa spp. | 24.3 | 2.0 | 91.6% | ||
| TOTAL | 187.7 | 31.0 | 83.5% | ||
3.2 Population density
Population densities were based only on those trees and shrubs >100 cm in height (or >50 cm in the case of Juniperus communis), and hence underestimate the true densities, especially for low-grown shrubs where a substantial proportion of the existing population in 2023 was excluded (personal observation).
The total population of small trees and shrubs had dropped by 83% from 1937 to 2023. Population densities had dropped for all ten species, but to varying degree (Table 1). Of the five species in 1937 still present 2023, densities today varied from 2.0 ha–1 (Rosa spp.) to 9.4 ha–1 (Ribes alpinum).
Losses in population density, calculated as percentages, was highest in Juniperus communis (94%), followed by Rosa spp. (92%), Malus sylvestris (81%), Crataegus spp. (65%) and Ribes alpinum (57%).
Prunus avium was not originally present in 1937, but four individuals >130 cm was found in 1991–1992 (Kardell and Fiskesjö 1999), and 1 in 2023 (Table 1).
For the two species, Malus sylvestris and Juniperus communis, there had been no new recruitment, so we could calculate the annual mortality rate as 1.91% year–1 and 2.63% year–1, respectively since 1937.
Finally, for the record we like to note that 42 specimens of Ribes uva-crispa <100 cm were present in 2023, compared with the single shrub found in 1937 (Julin 1948).
3.3 Canopy cover and basal area
Canopy cover had also decreased, but only modestly so. The total cover of the studies trees had dropped from 321 to 201 m2 ha–1 from 1937 to 2023 (Table 2), hence a percentage cover from 3.2% to 2.0%.
| Table 2. Canopy cover (m2 ha–1) of small tree and shrub species in Vessers udde (southern Sweden) at two points in time; and basal area of Malus sylvestris (m2 ha–1) | ||||
| 1937 | 1978–1979 | 2023 | Loss since 1937 | |
| BASAL AREA | ||||
| Malus sylvestris | 0.26 | 0.14 | 0.10 | 61.5% |
| SMALL TREES | ||||
| Malus sylvestris | 172.6 | 142.6 | 17.4% | |
| Crataegus spp. | 50.2 | 34.1 | 32.1% | |
| Prunus avium | 0.0 | 5.8 | ||
| Rhamnus cathartica | 12.4 | 0 | 100% | |
| SHRUBS | ||||
| Berberis vulgaris | 1.9 | 0 | 100% | |
| Ribes alpinum | 40.0 | 12.4 | 69.0% | |
| Ribes spicatum | 1.0 | 0 | 100% | |
| Rosa spp. | 42.9 | 6.2 | 85.7% | |
| Ribes uva-crispa | 0.7 | 0 | 100% | |
| TOTAL | 321.6 | 201.0 | 37.5% | |
Surprisingly, canopy cover of Malus sylvestris – that had lost 81% of its specimens (Table 1) – had decreased least (17%) suggesting that the few specimens remaining had grown considerably. This was confirmed by the average dbh increasing from 9.6 to 13.6 cm between 1937 and 2023.
Canopy cover of Crataegus spp., Ribes alpinum and Rosa spp. had dropped by 32, 69 and 86%, respectively. N.b. that these species were dominated by specimens <100 cm in 2023, and presumably also in 1937.
4 Discussion
All populations of the inventoried species in 1937 had decreased or disappeared completely after 86 years.
4.1 The biggest loser?
The largest drop in population size was shown by Juniperus communis (94% since 1937), confirming its sensitivity towards shade (Grubb et al. 1996; Thomas et al. 2007). Next in line was Rosa spp. with 92% reduction, also known to be sensitive to shade (Grubb et al. 1996).
Malus sylvestris had dropped by 81%, and all 24 trees present in 2023 were on Julin’s maps, meaning that there had been no recruitment since then. This too, is a species known to be sensitive to shade (Schnitzler et al. 2014; Walentowski et al. 2018), but the relative importance of lack of reproduction and shade remains to be investigated.
Population density of Crataegus spp. had dropped by 65% in the 86 years. A characteristic feature of this light sensitive taxon (Grubb et al. 1996) is its ability to asexually reproduce by suckers, forming dense stands under browsing. In our study, four individuals of Crataegus spp. had a dead top and more than 50 were noted with browsing damages, stresses that might affect the height of the individuals and trigger asexual reproduction (Bellingham and Sparrow 2000; Grubb et al. 1996). Most prominently, this could be seen in the nine square meters large area in the northeast part of the reserve containing 50 individuals all under the height of 20 cm and the majority characterized by browsing (a factor near-absent in 1937).
Another species displaying clear signs of browsing was Ribes alpinum that remained an abundant species despite a 57% reduction.
No individuals of Ribes spicatum were identified in the current study (it remains unclear whether it was seen by Kardell and Fiskesjö (1999)). Other lost species, that were no longer present with specimens >100 cm, were Berberis vulgaris, Ribes uva-crispa and Rhamnus cathartica.
It is apparent that the shading tree and hazel canopy that has developed since 1923 exert a negative influence on the species studied here (Einarsson and Milberg 1999; Siemann and Rogers 2003; Sarlöv Herlin and Fry 2000). Between 1937 and 1991 the canopy cover of Corylus avellana increased from 30% to 80% (Kardell and Fiskejsö 1999), a number likely higher today.
4.2 The winners are …?
Despite a full century of overgrowth, in a habitat changing from a wooded meadow to a dense mixed forest, certain specimens of shrubs and trees of the open agricultural landscape still survive. So, if considering to restore previously semi-open sites abandoned in the 1950s and 60s (a period when many small-scale farms closed down and many meadows were abandoned; SOS 2011), prospects are promising. If managing semi-open sites with occurrences of the species studied here, it might also be re-assuring to know that changed are relatively slow.
Julin (1948) specifically noted that he found only a single Ribes uva-crispa while we found 42 in 2023, all <100 cm in height. This non-native shrub, introduced for horticulture several centuries ago, seems to be doing relatively well, also under the shade of a dense canopy in our and other studies (von Oheimb and Brunet 2007).
4.3 Concluding remark
We believe our study area has great scientific value and should be kept this way for future studies. It was set aside to develop as a “reference areas” for deciduous forests in southern Sweden. Although much acreage of similar landuse had been abandoned since then, these have eventually become subjected to forestry, leaving Vessers Udde as a special case with older data available. However, despite being a nature reserve, with its original protection decided a century ago, threats loom at the horizon. To preserve important ecological structures in form of several old and large oaks in the area the regional government has suggested clearing of vegetation around these oaks in the reserve (Länsstyrelsen Östergötland 2018). This is in line with the conservation plan of the Nature 2000 guidelines, and the current paradigm that biodiversity should be promoted in reserves. But it would remove the areas scientific value as an area with free succession and be at odds with the stated purpose when the reserve was set aside a century ago by a generous landowner.
Acknowledgement
We thank the landowner and the county administration of Östergötland for permission to collect data in Vessers Udde nature reserve. Professor Lars Kardell provided inspiration, and the archive of the Swedish University of Agricultural Sciences helpfully provided Kardell’s field notes.
Author’s contribution
P.M. defined the research question and design of the work. M.F conducted the fieldwork and drafted the manuscript while both authors carried out numerical analyses, revising the manuscript and approved the final version to be published.
References
Austad I, Hauge L (1990) Juniper fields in Sogn, Western Norway, a man-made vegetation type. Nordic J Bot 9: 665–683. https://doi.org/10.1111/j.1756-1051.1990.tb00559.x.
Axelsson Linkowski W, Svensson R (2009) Träd och buskar i jordbrukslandskapet. Värden och hot: en litteraturgenomgång. [Trees and shrubs in the agricultural landscape. Values and threats: a review of the literature]. CBM:s skriftserie 24. Centrum för biologisk mångfald, Uppsala. https://www.slu.se/globalassets/ew/org/centrb/cbm/dokument/publikationer-cbm/cbm-skriftserie/skrift24.pdf.
Bellingham PJ, Sparrow AD (2000) Resprouting as a life history strategy in woody plant communities. Oikos 89: 409–416. https://doi.org/10.1034/j.1600-0706.2000.890224.x.
Bergstedt J, Axelsson A-L, Karlsson J, Lönander J, Törnqvist L, Milberg P (2017) Förändringar i Eklandskapet 1927 till 2013: i den första riksskogstaxeringens fotspår. [The first Swedish National Forest Inventory revisited: changes between 1927 and 2013]. Svensk Bot Tidskr 331–343. https://urn.kb.se/resolve?urn=urn%3Anbn%3Ase%3Aliu%3Adiva-143221.
Einarsson A, Milberg P (1999) Species richness and distribution in relation to light in wooded meadows and pastures in southern Sweden. Annales Bot Fenn 36: 99–107. https://www.sekj.org/PDF/anbf36/anbf36-099p.pdf.
Emanuelsson U (2009) The rural landscapes of Europe: how man has shaped European nature. The Swedish Research Council Formas, Stockholm. ISBN 9789154060351.
Eriksson O (2020) Origin and development of managed meadows in Sweden: a review. Rural Landsc Soc Env Hist 7: 1–23. https://doi.org/10.16993/rl.51.
Götmark F (2013) Habitat management alternatives for conservation forests in the temperate zone: Review, synthesis, and implications. For Ecol Manag 306: 292–307. https://doi.org/10.1016/j.foreco.2013.06.014.
Grubb PJ, Lee WG, Kollmann J, Wilson JB (1996) Interaction of irradiance and soil nutrient supply on growth of seedlings of ten European tall-shrub species and Fagus sylvatica. J Ecol 84: 827–840. https://doi.org/10.2307/2960555.
Guadilla-Sáez S, Pardo-de-Santayana M, Reyes-García V (2019) The role of traditional management practices in shaping a diverse habitat mosaic in a mountain region of Northern Spain. Land Use Policy 89, article id 104235. https://doi.org/10.1016/j.landusepol.2019.104235.
Hæggström C-A (1983) Vegetation and soil of the wooded meadows in Nåtö, Åland. Acta Bot Fenn 120. ISBN 951-9469-16-8.
Hytteborn H, Svensson B, Kempe K, Press A, Rydin H (2017) Century-long tree population dynamics in a deciduous forest stand in central Sweden. J Veg Sci 28: 1057–1069. https://doi.org/10.1111/jvs.12556.
Julin E (1948) Vessers Udde: Mark och vegetation i en igenväxande löväng vid Bjärka-Säby. Acta Phytogeogr Suecica 23. Svenska Växtgeografiska Sällskapet, Uppsala. https://urn.kb.se/resolve?urn=urn%3Anbn%3Ase%3Auu%3Adiva-184385.
Kardell L, Fiskesjö A-L (1999) Vessers udde 1921–1992: Skog, vegetation och mark efter 70 års fridlysning. [Vessers Udde 1921–1992: forest, vegetation and soil after 70 years of protection]. Sveriges lantbruksuniversitet, Institutionen för skoglig landskapsvård, Rapport 83.
Kobe RK, Pacala SW, Silander Jr, JA, Canham CD (1995) Juvenile tree survivorship as a component of shade tolerance. Ecol Appl 5: 517–532. https://doi.org/10.2307/1942040.
Länsstyrelsen Östergötland (2018) Bevarandeplan för Natura 2000-området Vessers udde SE0230267. [Preservation plan for the Natura 2000 area Vessers Udde]. https://geodata.naturvardsverket.se/handlingar/rest/dokument/284206.
Losvik MH (1999) Plant species diversity in an old, traditionally managed hay meadow compared to abandoned hay meadows in southwest Norway. Nordic J Bot 19: 473–487. https://doi.org/10.1111/j.1756-1051.1999.tb01231.x.
Mason WL, Edwards C, Hale SE (2004) Survival and early seedling growth of conifers with different shade tolerance in a Sitka spruce spacing trial and relationship to understorey light climate. Silva Fenn 38: 357–370. https://doi.org/10.14214/sf.404.
Milberg P (1995) Seed bank after eighteen years of succession from grassland to forest. Oikos 72: 3–13. https://doi.org/10.2307/3546031.
Milberg P, Tälle M (2023) Maintaining an open landscape: a 15-year multi-site experiment comparing seven management methods for semi-natural grasslands. Global Ecol Conserv 48, article id e02721. https://doi.org/10.1016/j.gecco.2023.e02721.
Milberg P, Bergman K-O, Jonason D, Karlsson J, Westerberg L (2019) Land-use history influence the vegetation in coniferous production forests in southern Sweden. For Ecol Manag 440: 23–30. https://doi.org/10.1016/j.foreco.2019.03.005.
Peterson A (2005) Has the generalisation regarding conservation of trees and shrubs in Swedish agricultural landscapes gone too far? Landsc Urban Plann 70: 97–109. https://doi.org/10.1016/j.landurbplan.2003.10.007.
Petersson LK, Milberg P, Bergstedt J, Dahlgren J, Felton AM, Götmark F, Salk C, Lööf M (2019) Changing land use and increasing abundance of deer cause natural regeneration failure of oaks: six decades of landscape-scale evidence. For Ecol Manag 444: 299–307. https://doi.org/10.1016/j.foreco.2019.04.037.
Plieninger T, Hartel T, Martín-López B, Beaufoy G, Bergmeier E, Kirby J, Montero MJ, Moreno G, Oteros-Rozas E, Van Uytvanck J (2015) Wood-pastures of Europe: geographic coverage, social-ecological values, conservation management, and policy implications. Biol Conserv 190: 70–79. https://doi.org/10.1016/j.biocon.2015.05.014.
Sarlöv Herlin IL, Fry GLA (2000) Dispersal of woody plants in forest edges and hedgerows in a Southern Swedish agricultural area: the role of site and landscape structure. Landsc Ecol 15: 229–242. https://doi.org/10.1023/A:1008170220639.
Schnitzler A, Arnold C, Cornille A, Bachmann O, Schnitzler C (2014) Wild European apple (Malus sylvestris (L.) Mill.) population dynamics: insight from genetics and ecology in the Rhine Valley. Priorities for a future conservation programme. PLOS ONE 9, article id e96596. https://doi.org/10.1371/journal.pone.0096596.
Sernander R (1922) Nya naturskyddsförvärv på enskild mark. [New protected areas on private land]. Svenska Naturskyddsföreningens Årsskrift 1922: 71–83, Sveriges Natur.
Sernander R (1925) Lövängen i Bjärka-Säby bebyggelsehistoria. [Wooded meadows in the habitation history of Bjärka-Säby]. Bjärka-Säby i monografier. Svenska kyrkans diakonistyrelses förlag, Stockholm.
Siemann E, Rogers WE (2003) Changes in light and nitrogen availability under pioneer trees may indirectly facilitate tree invasions of grasslands. J Ecol 91: 923–931. https://doi.org/10.1046/j.1365-2745.2003.00822.x.
Sjöbeck M (1932) Löväng och trädgård: Några förutsättningar för den svenska trädgårdens utveckling. [Wooded meadows and gardens: some prerequisites for the development of Swedish gardening]. Fataburen 1932: 59–74. Nordiska museet och Skansens Årsbok.
Slotte H (2001) Harvesting of leaf-hay shaped the Swedish landscape. Landsc Ecol 16: 691–702. https://doi.org/10.1023/A:1014486331464.
SLU (2023) Fältinstruktion 2023, Riksinventeringen av skog. [Instruction for fieldwork 2023, The Swedish National Forest Inventory.] https://www.slu.se/globalassets/ew/org/centrb/rt/dokument/faltinst/ris_fin_2023.pdf.
SOS (2011) Agriculture in figures years 1866–2007. Official Statistics of Sweden. https://jordbruksverket.se/download/18.7d191a0317b2dcce63388e18/1628781102867/Jordbr.
SOS (2019) Jordbruksstatistisk: sammanställning 2019 med data om livsmedel – tabeller. [Agricultural statistics 2019 including food statistics: tables]. Sveriges officiella statistik. https://www2.jordbruksverket.se/download/18.2532524316cca0df48ab2548/1566885388130/JS_2019v2.pdf.
Thomas PA, El-Barghathi M, Polwart A (2007) Biological flora of the British Isles: Juniperus communis L. J Ecol 95: 1404–1440. https://doi.org/10.1111/j.1365-2745.2007.01308.x.
von Oheimb G, Brunet J (2007) Dalby Söderskog revisited: long-term vegetation changes in a south Swedish deciduous forest. Acta Oecol 31: 229–242. https://doi.org/10.1016/j.actao.2006.12.001.
Walentowski H, Aas G, Göllner A, Ahl L, Feulner M (2018) Phytosociological studies of Malus sylvestris in North Hesse and Upper Franconia, Germany. Tuexenia 38: 97–110. https://doi.org/10.14471/2018.38.010.
Total of 37 references.

