Patterns of short-term vegetation recovery after a fire in protected Scots pine forests of hemiboreal Latvia
Liepa L., Rendenieks Z., Dubrovskis E., Freimane L., Straupe I., Jansons Ā. (2025). Patterns of short-term vegetation recovery after a fire in protected Scots pine forests of hemiboreal Latvia. Silva Fennica vol. 59 no. 1 article id 24046. https://doi.org/10.14214/sf.24046
Highlights
- We observed more rapid vegetation recovery at the ground level, but the dominance increased more steadily at herb and tree layers
- We found the highest species diversity at the herb layer during the third (middle-aged stands) and fourth (over-mature stands) years after fire disturbance
- From regenerating tree species, only Populus tremula in over-mature stands showed a decline in projective cover during the four studied years.
Abstract
Wildfires as natural disturbances have had important impacts on terrestrial ecosystems, including forests. We studied patterns of short-term vegetation recovery after surface fire in protected hemiboreal Pinus sylvestris L.-dominated forest. Our study was carried out near Stikli village in Western Latvia. Seven forest stands – middle-age and over-mature were sampled on nutrient-poor and mesic soils. Forest fire occurred in the summer of 2018 and covered 1440 ha of forested area. In each stand we established 16 sample plots (1 m × 1 m) in a radial pattern from the center. Every summer from 2019 till 2022 we surveyed these sample plots – recorded projective cover (%) and identified Ellenberg indicator values and species traits – plant strategy groups (C-S-R after Grime), Raunkiær life history forms and habitat types. Additionally, the occurrence of specialized fire-adapted plants was recorded. In total we identified 15 species in the ground layer, 47 species in the herbaceous layer, and 9 regenerating tree species. The colonization at the ground layer was the most rapid (projective cover increased overall by 67% in middle-aged stands and by 82% in over-mature stands). Species diversity was the highest at the herb layer during the third (middle-aged stands) and fourth (over-mature stands) after fire disturbance but showed overall declining trends. Betula spp. and Populus tremula L.-dominated regenerating tree species. The dominance of fire-adapted species declined rapidly after the fire except for moss Polytrichum spp. Overall, hemiboreal over-mature stands demonstrated higher vegetation cover and more rapid rate of initial colonization compared to middle-aged stands.
Keywords
succession;
fire disturbance;
plant functional traits;
post-fire regeneration;
protected forests
-
Liepa,
Latvian State Forest Research Institute “Silava”, Rīgas street 111, LV-2169, Salaspils, Latvia; Latvian University of Life Sciences and Technologies, Faculty of Forest and Environmental Sciences, Akadēmijas street 11, LV-3001, Jelgava, Latvia
 https://orcid.org/0000-0002-8270-6722
E-mail
liga.liepa@outlook.com
https://orcid.org/0000-0002-8270-6722
E-mail
liga.liepa@outlook.com

-
Rendenieks,
Latvian State Forest Research Institute “Silava”, Rīgas street 111, LV-2169, Salaspils, Latvia
 https://orcid.org/0000-0002-3511-1486
E-mail
zigmars.rendenieks@silava.lv
https://orcid.org/0000-0002-3511-1486
E-mail
zigmars.rendenieks@silava.lv
-
Dubrovskis,
Latvian University of Life Sciences and Technologies, Faculty of Forest and Environmental Sciences, Akadēmijas street 11, LV-3001, Jelgava, Latvia
 https://orcid.org/0000-0003-0810-5651
E-mail
edgars.dubrovskis@lbtu.lv
https://orcid.org/0000-0003-0810-5651
E-mail
edgars.dubrovskis@lbtu.lv
- Freimane, Latvian University of Life Sciences and Technologies, Faculty of Forest and Environmental Sciences, Akadēmijas street 11, LV-3001, Jelgava, Latvia E-mail lasma.freimane@lbtu.lv
-
Straupe,
Latvian University of Life Sciences and Technologies, Faculty of Forest and Environmental Sciences, Akadēmijas street 11, LV-3001, Jelgava, Latvia
 https://orcid.org/0000-0002-2098-7194
E-mail
inga.straupe@lbtu.lv
https://orcid.org/0000-0002-2098-7194
E-mail
inga.straupe@lbtu.lv
-
Jansons,
Latvian State Forest Research Institute “Silava”, Rīgas street 111, LV-2169, Salaspils, Latvia
 https://orcid.org/0000-0001-7981-4346
E-mail
aris.jansons@silava.lv
https://orcid.org/0000-0001-7981-4346
E-mail
aris.jansons@silava.lv
Received 28 July 2024 Accepted 19 February 2025 Published 26 March 2025
Views 40317
Available at https://doi.org/10.14214/sf.24046 | Download PDF

Supplementary Files
1 Introduction
Fire is one of the most fundamental agents of ecological disturbances globally (Krebs et al. 2010; Vilà-Cabrera et al. 2012; Yin et al. 2024). Wildfires as natural disturbances have impacted terrestrial ecosystems where they shape ecological and evolutionary changes. However, wildfire regimes have been changing all over the world (Doerr and Santín 2016; Pausas and Keeley 2019) and wildfires occur more often, affected by human activity. Wildfires in forest ecosystems are increasing due to climatic changes (through longer fire seasons, reduced rainfall and increased drought frequencies), with greater numbers and intensity expected in more locations (Pausas and Keeley 2019; Gajendiran et al. 2024). For instance, boreal forests are largely dependent on severe wildfires to shape natural disturbance regimes (Johnson et al. 1998; Hart et al. 2019). In natural ecosystems where fire is the main disturbance agent, it is expected to see changes in vegetation structure, composition, and ecosystem processes (Bond and Keeley 2005). The lack of fire disturbance may cause the accumulation of fuel, thus altering the existing fire regime (Johnson et al. 2001; Thompson et al. 2017).
During the post-fire vegetation recovery, succession changes the species composition and structure at all levels of vegetation vertical structure (Ryan 2002; Lydersen et al. 2016). The succession after disturbances is largely dependent on local environmental conditions or on the interaction between disturbances other than fire (Peeler and Smithwick 2021). In such places species are occupying distinct niches in a post-fire succession regime, appearing at specific spatial and temporal scales. It is also known that the lack of fire in the longer term might cause extinction of species dependent on fire disturbance, for instance particular tree species (Tingley et al. 2016; Karavani et al. 2018). However, the number and size of fires, and forest type in landscape constitute the frequency of fires (Angelstam 1998), but stand structural changes, species composition and tree mortality are characteristics of fire intensity (Bessie and Johnsson 1995).
Studies have shown that in boreal forests the early phase of post-fire succession determines the trajectory for vegetation recovery in the long term, and the first three years are critically important (Boulanger et al. 2018; Dawe et al. 2022). Immediately after the wildfire, the competition in forest plant communities is altered due to the mobilization of nutrients, which support plant growth (Khapugin et al. 2016). Specific trajectories of vegetation recovery are influenced by the persistence of soil seed bank, nutrients retained in soil after fire, climatic conditions, and other factors (Brown and Johnstone 2012).
Initial conditions for the regrowth of vegetation after fire disturbances are determined by forest type, frequency, and severity of disturbances (Hislop et al. 2019). However, there is no strong link between fire regime and forest biodiversity (Granström 2001) and local conditions are important in post-fire succession (Driscoll et al. 2010). Early successional post-fire vegetation is generally less fire-prone compared to the later seral stages and thus provides negative feedback for the build-up of the new fire cycle (Tepley et al. 2018). The typical boreal species are well adapted and highly resistant to the dynamics of natural disturbances in the forests. Pinus sylvestris L., for example, has an evolutionary adaptation to low- and medium intensity fires in the form of thick bark on the lower trunk (Kuuluvainen 2002).
Vegetation recovery after fires in hemiboreal and boreal forests have been studied mostly over longer periods using chronosequences (Parro et al. 2015; Orumaa et al. 2023). Studies of short-term dynamics of vegetation recovery mostly utilize remote sensing methods (Meng et al. 2014; Fernández-Guisuraga et al. 2023), however, only on-field vegetation survey can provide adequate data on rapid developments after fire disturbance and characterize the patterns of succession in more detail (Erdős 2014; Kibler et al. 2019) and allows to understand species and community dynamics (Clarke et al. 2014).
Despite fire suppression measures, forest fires still occur, including inside protected areas (Ananyev et al. 2022; Wang et al. 2022). The occurrence of wildfires in the Nordic-Baltic region has decreased steadily during the last century due to suppression and large-scale atmospheric circulation patterns (Wallenius 2011; Drobyshev et al. 2012; Donis et al. 2017). For instance, the annual burned area in Latvia for past 10 years have been just 730 ha (State Forest Service 2021), and, on average 75% of wildfires were initiated as results of unattended actions (approx. 75% cases), intentional burning (approx. 12% of cases), while 10% of cases the origin of wildfire is unknown (Central Statistics Bureau 2023). The largest wildfire for past 25 years in the territory of Latvia occurred in the Northern part of Latvia in 2018 when the burnt area was 1550 ha. The largest proportion of affected area falls into the territory of nature reserve “Stikli Mires”, where in some places fire severity (deep burning or crown fire) was very high on peat and mineral soils.
Our aim was to analyse patterns of natural post-fire vegetation recovery in hemiboreal forests on mineral soils in protected forests. This entailed several objectives: 1) collect vegetation survey data across four years in 112 sample plots; 2) extract patterns of vegetation recovery at ground and herb layers and for regenerating tree species; and 3) analyse differences between mature and over-mature Pinus sylvestris-dominated stands. In this study, we examine initial, short-term vegetation regeneration, focusing on species dynamics and using projective cover and plant functional traits as our main variables. Our established permanent study plots inside protected forest were unaffected by salvage logging.
2 Materials and methods
2.1 Study area
Our study area is located in the Northern part of Latvia (Fig. 1), which is a part of the hemiboreal vegetation zone (Ahti et al. 1968). The large wildfire occurred here in mid-late July 2018 when the area of 1550 ha burnt out of which approx. 1440 ha were forests and approx. 110 ha were mires and raised bogs. The largest part of the burnt area falls inside the Stikli Mires nature reserve, which is also a part of the Natura 2000 site of the same name. In the wildfire episode, a severe ground fire killed most trees, leaving only some live trees, mainly Pinus sylvestris. The total area of Stikli Mires nature reserve is 7244 ha; the reserve also contains boreal forests on peat and mineral soils, mires, raised bogs, lakes and small streams. The mean annual temperature of the area is 6 °C for the 2013–2023, and the mean annual precipitation for this period was approx. 683 mm with the majority of it during the summer months (Briede 2024).
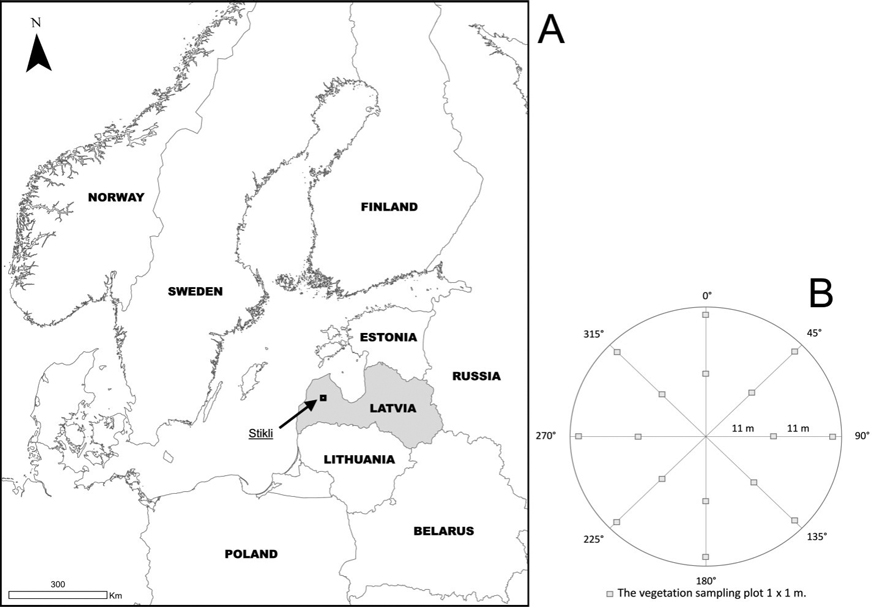
Fig. 1. The location of study area (A) and sampling design of the sample plots per each study site (B).
Our study sites (Fig. 2) fall into the territory of the nature reserve Stikli Mires. All sampled stands are dominated by Scots pine (Pinus sylvestris) with an admixture of birch (Betula pendula Roth and Betula pubescens Ehrh.) and Norway spruce (Picea abies (L.) Karst.). Sample plots are established on nutrient-poor and mesic Vacciniosa and Vaccininioso–sphagnosa forest types (classification according to Bušs (1997) (Table 1)).

Fig. 2. Vegetation recovery after wildfire in Northern Latvia study sites dominated by Scots pine (Pinus sylvestris) (photo: Līga Liepa).
| Table 1. Main characteristics of studied burned stands (n = 7). | |||
| No. | Coordinates | Forest site type* | Stand age (2019) |
| 1 | 57.329 N; 22.272 E | Oxalidosa | 67 |
| 2 | 57.329 N; 22.271 E | Vaccinioso-sphagnosa | 67 |
| 3 | 57.327 N; 22.270 E | Vacciniosa | 157 |
| 4 | 57.327 N; 22.268 E | Vacciniosa | 157 |
| 5 | 57.326 N; 22.268 E | Vacciniosa | 64 |
| 6 | 57.326 N; 22.270 E | Vaccinioso-sphagnosa | 157 |
| 7 | 57.325 N; 22.267 E | Vacciniosa | 167 |
| * Forest types according to Bušs (1997). | |||
2.2 Data collection
The permission No. 3.15/454/2019-N from the Nature Conservation Agency was obtained for data collection inside the nature reserve. A total of seven stands, each about 3–5 ha, were selected as experimental study sites. Three stands were 64–67 years old (further – middle-aged stands) and four stands were 157–167 years old (further over-mature stands) (Table 1). In each stand 16 square-shaped permanent vegetation sampling plots (1 m2) were established. Sample plots were established sequentially in the direction of following compass (at 0°, 45°, 90°, 135°, 180°, 215°, 270° and 315° degree transects), but were moved aside positions if there osculated with downed logs or large stumps. For each direction transect two permanent sample plots were established, respectively 11–12 m and 23–24 m from the center point. In total 112 sample plots in the seven stands were surveyed in 2019, 2020, 2021 and 2022 during the vegetation season (July and August).
The vegetation projective cover (%) was recorded at three main vegetation layers – ground layer, herb layer and additionally for regenerating trees. The projective cover was measured for each layer separately. The projective cover (expressed in percentage of cover) was recorded for all species of vascular plants, dwarf shrubs, shrub, and tree species up to height 1.5 m in the field layer and that for mosses in the ground layer. The inventory of vegetation was performed by the same person each year to avoid alterations of the measurements. The sample of undefined species were collected and later identified in laboratory. The nomenclature of vascular plants follows Gavrilova and Šulcs (1999) and that for bryophytes Āboltiņa et al. (2015).
In each study site four separate, circular sample plots were established (radius 5.64 m, 100 m2 in total) at 0°, 90°, 180°, 270° where all tree seedling species were recorded in terms of projective cover (%).
Species trait characteristics of vascular plants were extracted from trait databases LEDA (Kleyer et al. 2008) and BIDS Ecoflora (Fitter and Peat 1994). We classified plants using trait characteristics into plant strategy groups (C-S-R) (Hodgson et al. 1999), Raunkiær life history forms (Cain 1950), dispersal agents of plant seeds and spores and habitat types by referencing them to the corresponding categories. Habitat types included boreal, meadow, nemoral, nitrophilous, pine-forest and water-swamp species. We also classified the plant species into the following traits: Sphagnum mosses, feather mosses, other bryophytes, hepatics, tree and shrub seedlings and saplings, dwarf shrubs, herbaceous plants, ferns, grasses, and sedges. We characterized the dominance of plant trait characteristics using projective coverage for each species.
Plant indicator values for environmental factors such as light, moisture, soil pH and temperature were extracted from Ellenberg indicator scales (Ellenberg et al. 1992). The indicator numbers were computed using projective coverage for each species. The occurrence of specialized fire-adapted plants was recorded in terms of projective coverage in sample plots.
2.3 Data analysis
We used Microsoft Excel for data collection, processing, production of charts and calculating average values. Shannon-Wiener Diversity Index (H) was calculated in Excel (Eq. 1) based on the projective cover of each species at each vegetation layer. To calculate H values, we combined all sample plots for middle-aged and over-mature stands at each year and calculated the relative proportions from the projected cover of each species.
![]()
where:
pi = proportion of total projective cover by species i (decimal).
S = number of species.
R 4.4.0. (R Core team 2023) was used for statistical analyses, packages Base R and ggplot2. The Shapiro-Wilk test was used to test the distribution analytically and quantile-quantile (Q-Q) comparison plots to assess distributions visually. We used Wilcoxon rank sum test with continuity correction to test for significant differences in projective cover between successive years and between projective cover values by forest age groups.
3 Results
3.1 Vegetation projective cover
Generally, the projective cover was the lowest for trees and quite similar between ground and herb layers. When middle-aged and over-mature stands were characterized side-by-side, we found the most pronounced disparities for trees, where much higher variance in projective cover was present in over-mature stands. At ground and herb layers the variance in projective cover increased with the years since fire, especially at the herb layer.
3.1.1 Ground layer species
A total of 15 different bryophyte species (listed in Supplementary file S1) were found during the inventory. The differences in projective cover varied by the stand age group and increased with a period after fire (Fig. 3). Four years after the wildfire, we found that coverage of ground layer species increased in over-mature stands, but in the fourth year of inventory ground layer coverage slightly decreased in middle-aged stands (Fig. 3).
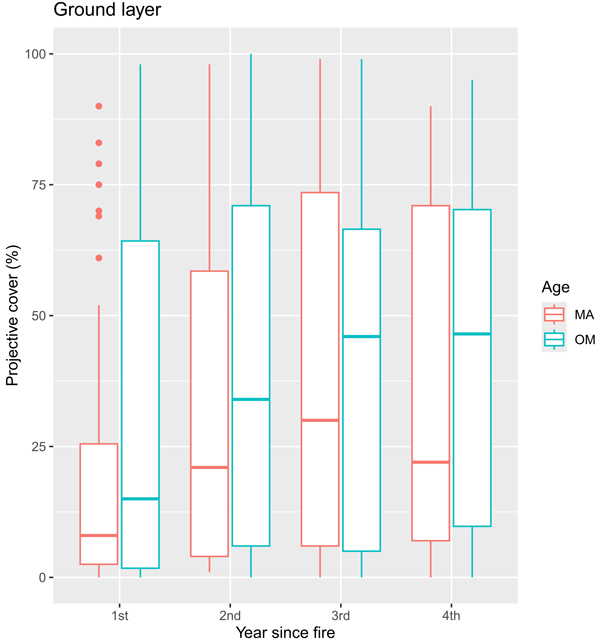
Fig. 3. Boxplots showing the median and the spread of the data with outliers of vegetation projective cover (%) for ground layer during four years after fire in sample plots (n = 112). MA – middle-aged stands, OM – over-mature stands.
At the ground layer we recorded similar patterns of vegetation recovery in middle-aged and over-mature stands (Fig. 4). Feather moss and Sphagnum comprised the smallest coverage fractions, followed by hepatics and other moss species. The latter category was the most dominant in terms of projective coverage, reaching 17–35% in middle-aged stands and 19–41% in over-mature stands, and had the highest rate of increase during the first two years (for middle-aged stands) and the first year (for over-mature stands).
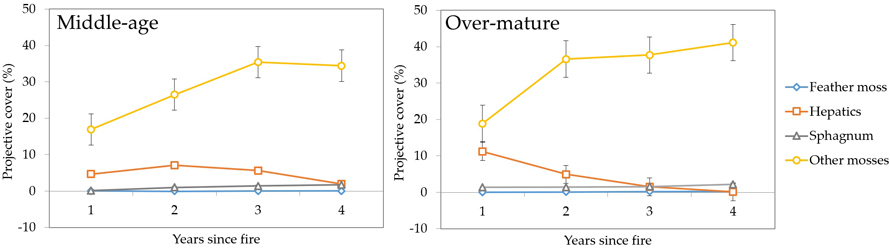
Fig. 4. Changes in ground layer vegetation projective cover (%) during four years after fire in sample plots (n = 112). Standard errors are shown.
Colonization and rapid coverage increase of pioneer moss species showed clear differences. The most abundant species just after wildfire in both age groups were other mosses (Pohlia nutans (Hedw.) Lindb., Funaria hygrometrica Hedw. and Ceratodon purpureus (Hedw.) Brid.) and hepatics (Marchantia polymorpha L.). Also, during the second year the colonization of Polytrichum spp. has increased in over-mature stands, which was still high even after four years.
3.1.2 Herb layer species
A total of 58 herb layer species were recorded during the period of inventory. Vegetation re-growth was primarily initiated by herbaceous plants, for instance, Chamaenerion angustifolium L. was commonly found. Both middle-aged and over-mature stands showed the coverage increases even after four years after the disturbance. Four years after the wildfire the average projective cover in over-mature stands was 81.5% whereas in middle-aged stands – 71%. In the period of four years the coverage increased by 41% in middle-aged stands and by 38% in over-mature stands (Fig. 5).
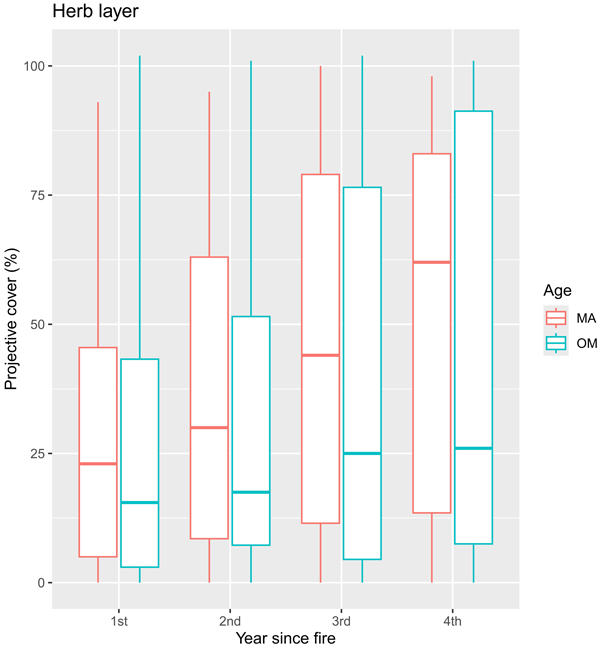
Fig. 5. Boxplots showing the median and the spread of the data with outliers of vegetation projective cover (%) for herb layer during four years after fire in sample plots (n = 112). MA – middle-aged stands, OM – over-mature stands.
In both middle-aged and over-mature stands (Fig. 6) herb layer was dominated by dwarf shrubs (up to 30% and 35% cover, respectively). Dwarf shrubs like trees and ferns showed steady increases in projected cover during the four years since the fire disturbance. The dominance of dwarf shrubs was more pronounced in middle-aged stands compared to over-mature ones. Middle-aged stands also had much higher grass cover (up to 12.6% vs. 1.7%) and lower tree cover (16.5% vs. 31.6%) compared to over-mature stands.
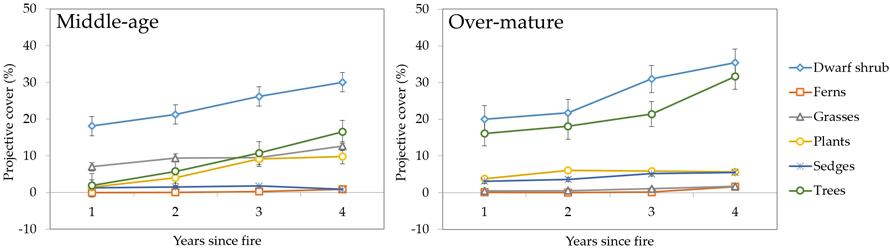
Fig. 6. Changes in herb layer vegetation projective cover (%) during four years after fire in sample plots (n = 112). Standard errors are shown.
In over-mature stands dwarf shrubs and trees made up combined 40–50% of projective cover (mostly Vaccinium myrtillus L., Vaccinium vitis-idaea L. and Vaccinium uliginosum L.). Only two groups (sedges and plants) showed a decline during the four years of observation, for example, sedges in middle-aged stands decreased in cover during the fourth year from 1.8% to 0.8% (Fig. 6).
3.1.3 Tree species regeneration
A total of 9 tree and shrub species were found in sampled plots. The projective coverage of the four most prominent tree species – Betula spp., Populus tremula L., Pinus sylvestris and Salix spp. is shown in Fig. 7.
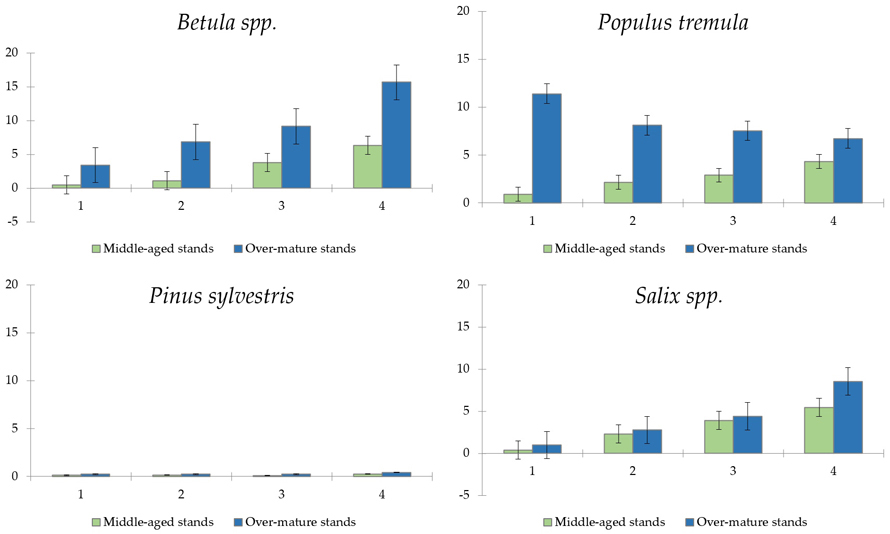
Fig. 7. Projective cover (%) by regenerating tree species in the middle-aged and over-mature stands (n = 112). Standard errors are shown.
Young Betula spp., and Populus tremula trees were the most dominant by projective cover, reaching over 15% and over 10%, respectively (reaching up to 1.5 m in height). The overall tree cover was notably lower in middle-aged stands compared to over-mature ones (Fig. 8). Interestingly, Betula spp. gained the highest projective cover over other species only during the fourth year (in a case of middle-aged stands) and third year (in over-mature stands).
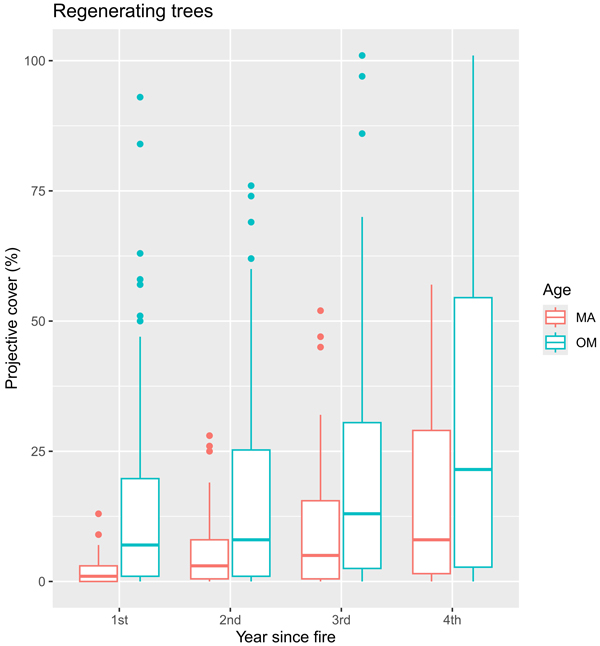
Fig. 8. Boxplots showing the median and the spread of the data with outliers of vegetation projective cover (%) for regenerating trees during four years after fire in sample plots (n = 112). MA – middle-aged stands, OM – over-mature stands.
All species of regenerating trees generally showed increasing trends, except for Populus tremula in over-mature stands, which showed steadily declining projective cover with every year (from 11.4% during the first year to 6.7% during the fourth year).
3.1.4 Ellenberg indicator values
Ellenberg indicator values were determined for analysed plant communities during the four years since the fire event (Fig. 9). Differences in temperature values between middle-aged and over-mature stands, which were notable during the first year after the fire, evened out during the third year. Ellenberg pH values, which were generally low overall, did not show much change or differences between stand age groups.
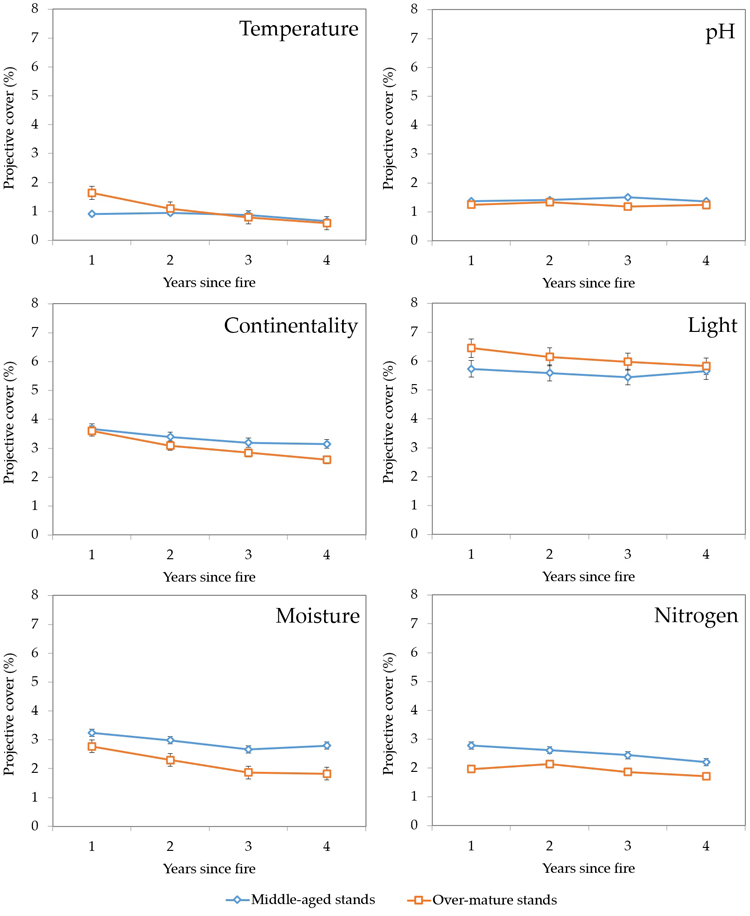
Fig. 9. Changes in average Ellenberg indicator values during four years after fire in sample plots (n = 112). Standard errors are shown.
Ellenberg continentality values were initially very similar, drifted apart during the four years since the fire event, with middle-aged stands having slightly higher continentality values. As expected, light indicator values were generally high, and the higher values in over-mature stands (6.5 vs. 5.7 in middle-aged stands) evened out with middle-aged stands during the fourth year. Ellenberg moisture and nitrogen values showed similar dynamics with generally declining trends and middle-aged stands having the higher values compared to over-mature stands.
3.2 Plant functional groups
3.2.1 CSR strategies
Plants corresponding to different types of life strategies (C-S-R model: competitors, stress-tolerators and ruderals) formed two distinctive groups (Fig. 10). One group showed rapidly increasing projective cover for C-S, C and C-R type species (middle-aged stands) or C-S and C type species (over-mature stands). The other contained the rest of the species corresponding to C-S-R and S type species and their overall dominance remained low. The biggest difference between middle-aged and over-mature stands was in the dominance of C-R species, which was negligible in over-mature stands.
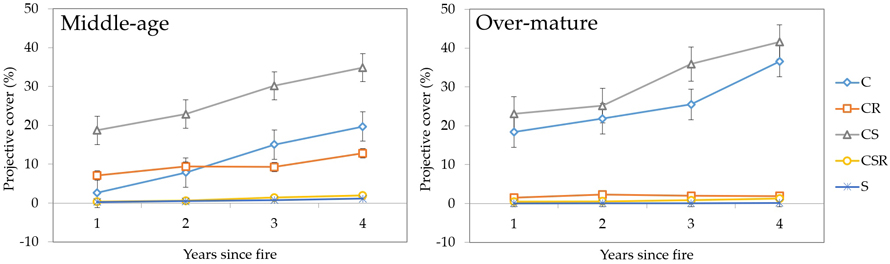
Fig. 10. Changes in vegetation projective cover (%) by C-S-R strategies during four years after fire in sample plots (n = 112). Standard errors are shown.
Our results show that as succession progresses, species dominance generally increases. It’s interesting that in middle-aged stands C type species overtook C-R type species after during the third year after fire event. S type species comprised the smallest projective coverage in both middle-aged and over-mature stands.
3.2.2 Raunkiær life forms
The dynamics of post-fire vegetation recovery, expressed in the form of life-forms (after Raunkiær) indicated the dominance of phanerophytes – for both middle-aged and over-mature stands (Fig. 11). Phanerophytes reached the cover of over 40% during the fourth year since fire event and also had the most rapid increase in projective cover among the life-forms in middle-aged stands. In over-mature stands, phanerophytes increased the projective cover more rapidly after the first year and reached the cover of 66% during the fourth year after fire.
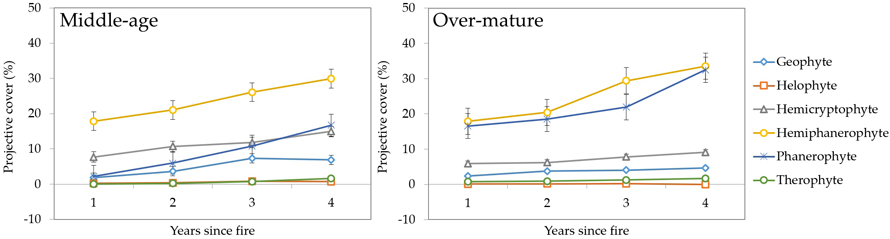
Fig. 11. Changes in vegetation projective cover (%) by Raunkiær life forms during four years after fire in sample plots (n = 112). Standard errors are shown.
The lowest projective cover (%) was found for therophytes like Melampyrum pratense L. in middle-aged stands and helophytes like Carex sylvatica Huds. in over-mature stands. Geophytes, for example Epilobium spp. in middle-aged stands were the only group which showed a decline during the fourth year since the fire.
3.2.3 Ecological plant groups
Grouped into plant ecological groups, post-fire vegetation dynamics highlighted boreal species as the most distinct and dominant type (Fig. 12), especially Vaccinium myrtillus and Populus tremula. For the rest of the groups, the increase in projective cover was more modest and differences between age groups – more pronounced. For instance, meadow species like Molinia caerulea (L.) Moench had the second-highest cover in middle-aged stands but for over-mature stands it was species belonging to water-swamp group. The remaining groups showed similar dominance in both middle-aged and over-mature stands.
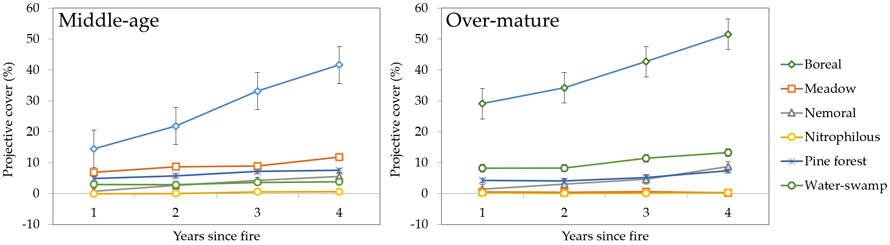
Fig. 12. Changes in vegetation projective cover (%) by plant ecological groups during four years after fire in sample plots (n = 112). Standard errors are shown.
3.2.4 Seed dispersal agents
In the dynamics of post-fire vegetation recovery, seed dispersal strategies for plants are an important factor (Fig. 13). Our results showed that both in middle-aged and over-mature stands wind (Betula spp., Chamaenerion angustifolium and others) and birds (Vaccinium myrtillus and others) are the major seed dispersal agents, combining for 67% and 80% of respective projective cover of corresponding plants. The cover of other types of plants remained negligible.
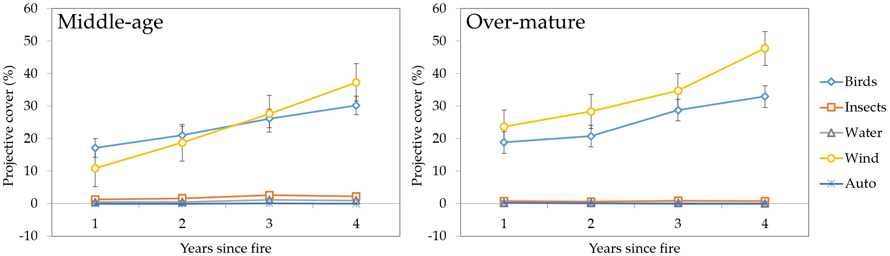
Fig. 13. Changes in vegetation projective cover (%) by seed dispersal agents during four years after fire in sample plots (n = 112). Standard errors are shown.
3.2.5 Fire-adapted plants
We recorded the dominance of fire-adapted species Marchantia polymorpha, Polytrichum spp., Funaria hygrometrica and Chamaenerion angustifolium (Fig. 14). From these species Polytrichum spp. exhibited the most rapid increase in projected cover, reaching 29% in middle-aged stands and 37% in over-mature stands. Two other species – Marchantia polymorpha and Funaria hygrometrica showed declining dominance with their projective cover dropping to almost zero during the fourth year since the fire event. The dominance of Chamaenerion angustifolium stable during the studied period.
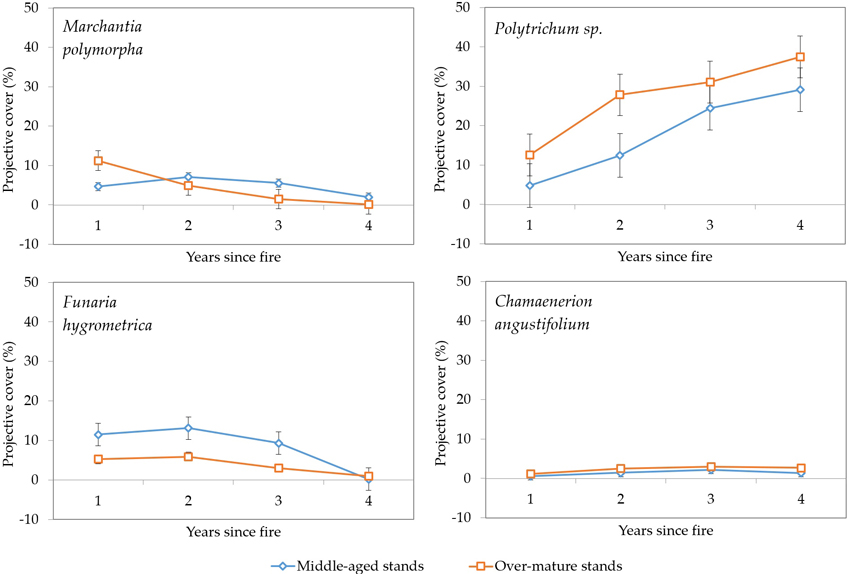
Fig. 14. Changes in vegetation projective cover (%) of four fire-adapted species during four years after fire in sample plots (n = 112). Standard errors are shown.
3.3 Species diversity
Species diversity during the vegetation recovery (expressed in Shannon-Wiener index (H) values based on projective cover (Table 2) showed different types of dynamics at each vegetation layer. In middle-aged stands ground layer species diversity peaked in 2 years after the fire (2020), reaching H of 1.31 and subsequently declining to 0.83 in the 4th year (2022). However, in over-mature stands species diversity started to decline from the first year since fire (H = 1.50 in 2019) and the overall H values were higher compared to middle-aged stands.
| Table 2. Species diversity (Shannon-Wiener index H) and standard errors in sample plots (n = 112). | ||||
| Year since fire | ||||
| 1 | 2 | 3 | 4 | |
| Ground layer | ||||
| Middle-aged stands | 1.20 ± 0.09 | 1.31 ± 0.08 | 1.22 ± 0.08 | 0.83 ± 0.08 |
| Over-mature stands | 1.50 ± 0.07 | 1.45 ± 0.06 | 1.23 ± 0.05 | 1.03 ± 0.05 |
| Herb layer | ||||
| Middle-aged stands | 2.00 ± 0.06 | 2.25 ± 0.08 | 2.57 ± 0.10 | 2.56 ± 0.08 |
| Over-mature stands | 2.41 ± 0.08 | 2.50 ± 0.09 | 2.47 ± 0.08 | 2.58 ± 0.07 |
| Tree species regeneration | ||||
| Middle-aged stands | 1.42 ± 0.17 | 1.42 ± 0.11 | 1.44 ± 0.14 | 1.45 ± 0.14 |
| Over-mature stands | 0.99 ± 0.09 | 1.41 ± 0.14 | 1.39 ± 10.14 | 1.53 ± 0.15 |
At herb layer H values were the highest among all sample plots (H > 2.00). At this vegetation layer species diversity increased with each year, both in middle-aged and over-mature stands (with the exception of over-mature stands in year 3), reaching very similar values during 4th year since fire (H = 2.56 and H = 2.58, respectively. Tree species regeneration showed that H values were quite stable and the first year since fire in over-mature stands was the exception with relatively low species diversity of H = 0.99 due to the pronounced dominance of young Populus tremula trees.
3.4 Differences in projective coverage between years and age groups
Post-fire vegetation recovery showed the most pronounced differences in projective cover after the first year since fire and during each successive year differences became less statistically significant (Table 3). Projective cover at the ground layer increased significantly (p < 0.05) after the first year since fire event, just as in herb layer and for regenerating trees, where significance level was lower (p < 0.1). After the second year, only changes at the herb layer were significantly different (p < 0.1) and after the third year – only regenerating trees (p < 0.1).
| Table 3. Vegetation projective cover (%) p-values and significance levels for by layer between successive years since fire event (n = 434). | |||
| Vegetation layer | 1st vs. 2nd year | 2nd vs. 3rd year | 3rd vs. 4th year |
| Ground | 0.003** | 0.542ns | 0.969ns |
| Herb | 0.088* | 0.099* | 0.292ns |
| Tree species regeneration | 0.064* | 0.143ns | 0.083* |
| *** p < 0.001; ** p < 0.05; * p < 0.1; ns – not significant. | |||
When tested for differences in projective cover by age groups, only regenerating trees showed statistically significant differences (Table 4). The significance between projective cover values declined gradually with every year and was not significant during the fourth year since the fire event.
| Table 4. p-values and significance levels for vegetation projective cover (%) by age groups (middle-aged and over-mature stands) during the four years since fire event (n = 434). | ||||
| Vegetation layer | 1st year | 2nd year | 3rd year | 4th year |
| Ground | 0.465ns | 0.267ns | 0.683ns | 0.301ns |
| Herb | 0.607ns | 0.393ns | 0.282ns | 0.493ns |
| Tree species regeneration | 0.000*** | 0.011** | 0.035** | 0.049** |
| *** p < 0.001; ** p < 0.05; * p < 0.1; ns – not significant. | ||||
4 Discussion
Our study indicates different early successional patterns in protected middle-aged and over-mature forests. Initial projective cover of vegetation was generally higher in over-mature stands. The natural regeneration of tree seedlings was still high in burned over-mature stands dominated by Betula spp., Populus tremula and Salix spp. with Pinus sylvestris in the smallest proportion. This is quite characteristic to the region, as the spatial distribution of forest fires coincides with the distribution of Pinus sylvestris-dominated forests (Donis et al. 2014).
In protected forests, logging is primarily limited according to the protection regime. Salvage logging for ecological restoration (sensu Pyke et al. 2010; Müller et al. 2019) was not utilized here after the wildfire and did not influence our established sample plots. Salvage logging was done selectively in some adjacent areas to create suitable and functioning firebreak lines for public safety purposes in nearby villages. The access to the area during the period of wildfire was also limited.
This, however, is related to the issue of underdeveloped forest management infrastructure in Latvian protected forests, especially remote from population centers like in Stikli Mires. Missing forest roads and even firebreaks in this region delayed the extinguishing efforts and thus relatively large area was burnt.
4.1 Patterns of vegetation recovery
The early development of certain species and communities developed rapidly after the fire disturbance. The re-vegetation immediately after the wildfire was dominated by grasses and dwarf shrubs and also bryophytes, for instance Marchantia polymorpha. Also, some studies on boreal post-fire vegetation re-growth pointed out similar observations (Gongalsky and Persson 2013; Jean et al. 2019). The composition of species at herb and shrub layers is characterized as defining the vegetation recovery trajectories since these elements are the most sensitive to altered environmental conditions (Khapugin et al. 2016).
During the first year after fire the rate of colonization at the ground layer was the highest compared to herb and tree layers, notably by Marchantia polymorpha, Pohlia nutans, Funaria hygrometrica and Ceratodon purpureus. Additionally, the projective cover at tree layer was significantly higher compared to middle-aged stands. Mosses at the ground layer have the largest species turnover after wildfires, since they do not have underground propagules (Markham and Essery 2015). Although we do not have vegetation data before fire disturbance, we found rapid increases in the dominance of 8 moss species after fire, both in middle-aged and over-mature stands.
We found steady increases in species diversity at herb layer for both middle-aged and over-mature stands, which is typical in boreal and hemiboreal forests after fire disturbance (Marozas et al. 2007; Ruokolainen and Salo 2009) and similar to the effect of clear-cutting in terms of species richness (Pykälä 2004). This is explained by rapid colonization by Chamaenerion angustifolium, Epilobium spp. and others – this short-lived domination immediately after the fire was also noted by Ananyev et al. (2022). Over the four years after the fire disturbance dwarf shrubs retained the dominance in terms of projective cover and showed rapid increases in both middle-aged and over-mature stands.
Commonly, the invasion of species is also observed in burned areas, for instance, the abundance of Chamaenerion angustifolium, Taraxacum spp., Senecio spp. was high in studied forest stands. It is also known that some individuals might sprout from old subsoil seeds (Kellman 1970). In this study, we found Rumex acetosa L., Luzula pilosa (L.) Willd., Cirsium arvense (L.) Scop. and Salix spp. species. Also, the occurrence of Calluna vulgaris seedlings were observed in the second, third and fourth year after wildfire. This might be because the viability of seeds was initiated by heating the soil surface the humus layer containing the seeds.
Several studies (Laiviņš et al. 2019; Dawe et al. 2022) have found that three years after fire disturbance marks the stabilization of dwarf shrubs in particular. However, we did not observe this, however, the overall slower increase in dwarf shrub cover in over-mature stands after year 3 might be a sign of this. Specific dwarf shrubs, for instance Vaccinium vitis-idaea and Vaccinium uliginosum steadily increased their dominance over the entire study period – from average 12% and 15% in 2019 (respectively) to average 17% and 25% in 2022.
As expected, tree species regeneration was dominated by Betula spp., Populus tremula but Pinus sylvestris occupying a fraction of the total projective cover. This is corroborated by results from boreal Russia (Ananyev et al. 2022). In addition, Populus tremula marked a difference between recovery trajectories in over-mature stands, where species projective cover declined – in contrast to middle-aged stands and all other regenerating tree species.
During the four years of vegetation recovery, changes in environmental variables, expressed in Ellenberg indicator values, showed small but important changes over time – overall decrease in light demand, continentality and moisture. These results highlighted notable differences between middle-aged and over-mature stands, for instance, in over-mature stands continental and moisture-demanding plants declined notably but light-demanding plants had slightly higher dominance compared to middle-aged stands.
4.2 Plant functional groups
In addition to species dominance, expressed in terms of projective cover, plant functional traits provided important findings on early-successional developments in post-fire vegetation recovery. As expected, light-demanding species dominated during the first four years, and the increase of Vaccinium vitis-idaea coverage in middle-aged stands is related to greater light availability at the ground layer after the ground was exposed. This fact was also detected in earlier study (Lindholm and Vasander 1987). It is known that forest plant community reaches their greatest diversity of mixture of light-demanding and shade-tolerant species in the early development stage of forests (Valladares et al. 2016; Adie and Lawes 2023). Our results showed signs of this with the steady decline in the dominance of light-demanding species over the studied 4-year period.
The dominance of competitor (C) and competitor-stress tolerator (C-S) species was expected during the early stages of succession (Petrokas and Manton 2023). The rapid expansion contrasted with the stable cover of C-S-R and S species, especially in over-mature stands. In the case of middle-aged stands, C-R species were also dominant during the first year after fire disturbance but had less increase in projective cover compared to C species.
Low-intensity fires favor species which reproduce vegetatively, but moderately strong fires promote species with wind dispersal mode (Boucher et al. 2014). We indeed found a dominance of Populus tremula in the first couple years after the fire, however, its cover declined and was overtaken by Betula spp. and Salix spp. These species are typical post-fire colonizers in boreal and hemiboreal forests (Zyryanova et al. 2010).
4.3 Succession of forest plant communities
The differences between forest site type and forest plant association type must be considered while different local and regional landscape characteristics also play an important role. In our study, the Myrtillus type of forests visibly showed the re-vegetation occurrence of species which are basically found in forests on peat soils, for instance, Ledum palustre L., Vaccinium uligonosum, Eriophorum spp., Sphagnum spp. and others. These species are quite resilient to surface fires due to their adaptations like the extensive rhizome and favorable conditions after fire. Also, site microrelief depression provides specific conditions for above-mentioned species.
Surviving trees remain and also affect the recovery of vegetation (Adámek et al. 2016). Fire legacies arising from past fire disturbance influence not just vegetation recovery trajectories for decades to come (Jonstone et al. 2016; Dawe et al. 2022) but also the resilience of communities to the impacts of wildfires in the future (Day et al. 2022). Such forms of “ecological memory” (Franklin et al. 2000), in addition to site conditions and species diversity, must be considered in forest management planning (Day et al. 2022).
Obviously, the first four years of post-disturbance succession cannot provide a full picture of vegetation recovery, since full recovery can take even 100 years or longer (Marozas et al. 2007). However, as stressed earlier in the text, the first few years are critically important in forming future trajectories for the diversity and resilience of forest species and communities. We found important indications that can guide ecological restoration efforts and the management of protected forests in the long term, for example, the persistence of Vaccinum cover and the importance of ground layer in post-fire regeneration in general. However, using fire as a tool for ecological restoration is controversial since many factors must be considered (Pyke et al. 2010; Hamman et al. 2011) to reach the desired outcome. Our study demonstrated that short-term vegetation recovery in oligotrophic boreal forests results in the dominance of Betula spp. and Vaccinium myrtillus, especially in over-mature forests.
5 Conclusions
We observed early successional vegetation recovery after fire disturbance. At herb and tree layers we found the highest variance of projective cover both for middle-aged and over-mature stands. During the fourth year we observed declines in projective cover for some bryophyte species and dwarf shrubs in middle-aged stands. Regenerating tree species were dominated by Betula spp. and Populus tremula. The dominance of fire-adapted species declined rapidly after the fire with the exception of moss Polytrichum spp. Overall, hemiboreal over-mature stands demonstrated higher vegetation cover and more rapid rate of initial colonization compared to middle-aged stands. We also found the highest statistical significance of year-to-year projective cover after the first year since fire, but significance decreased with time. Our study contributes to the better understanding of short-term dynamics of vegetation recovery after fire disturbances in hemiboreal forests and its findings can help to plan ecological restoration efforts.
Authors’ contributions
Conceptualization – L.L., Ā.J., E.D.; methodology – L.L., Ā.J.; data collection and analysis – L.L., Z.R. and Ā.J.; writing – original draft preparation – L.L., Z.R. and Ā.J.; writing – review and editing – L.L., E.D., O.M., Z.R. and Ā.J. All authors have read and agreed to the published version of the manuscript.
Acknowledgements
This study was financially supported in year of 2019 and 2020 by the ERDF Post-doctoral Research Support Program (project Nr.1.1.1.2/16/I/001) research application “Balancing ecological interests with increasing demands for natural resources in production forests.” (Nr.1.1.1.2/VIAA/2/18/294) to L. Liepa. Data from 2021 and 2022 was collected with the support of JCS Latvia’s State Forests research programme “Effect of climate change on forestry and associated risks” (agreement No. 5-5.9.1_007p_101_21_78). We thank the Nature Conservation Agency of Latvia for the permission to collect data inside the nature reserve.
Declaration of openness of research materials, data, and code
Datset with the results is supplied with the paper.
Conflicts of interest
The authors declare no conflicts of interest.
References
Āboliņa A, Piterāns A, Bambe B (2015) Lichens and bryophytes in Latvia. Checklist. Latvian State Forest Research Institute Silava, Daugavpils University Academic Publisher “Saule”. [in Latvian].
Adámek M, Hadincová V, Wild J (2016) Long-term effect of wildfires on temperate Pinus sylvestris forests: vegetation dynamics and ecosystem resilience. For Ecol Manag 380: 285–295. https://doi.org/10.1016/j.foreco.2016.08.051.
Adie H, Lawes MJ (2023) Solutions to fire and shade: resprouting, growing tall and the origin of Eurasian temperate broadleaved forest. Biol Rev 98: 643–61. https://doi.org/10.1111/brv.12923.
Ahti T, Hämet-Ahti L, Jalas J (1968) Vegetation zones and their sections in northwestern Europe. Ann Bot Fenn 5: 169–211.
Ananyev VA, Timofeeva VV, Kryshen AM, Pekkoev AN, Kostina EE, Ruokolainen AV, Moshnikov SA, Medvedeva MV, Polevoi AV, Humala AE (2022) Fire severity controls successional pathways in a fire-affected spruce forest in Eastern Fennoscandia. Forests 13, article id 1775. https://doi.org/10.3390/f13111775.
Angelstam PK (1998) Maintaining and restoring biodiversity in European boreal forests by developing natural disturbance regimes. J Veget Sci 9: 593–602. https://doi.org/10.2307/3237275.
Bessie WC, Johnson EA (1995) The relative importance of fuels and weather on fire behavior in subalpine forests. Ecology 76: 747–762. https://doi.org/10.2307/1939341.
Bond WJ, Keeley JE (2005) Fire as a global ‘herbivore’: the ecology and evolution of flammable ecosystems. Trends Ecol Evol 20: 387–394. https://doi.org/10.1016/j.tree.2005.04.025.
Briede A (2024) Klimats Latvijā. Nacionālā Enciklopēdija. https://enciklopedija.lv/skirklis/26052-klimats-Latvij%C4%81. Accessed 8 December 2024.
Brown CD, Johnstone JF (2012) Once burned, twice shy: repeat fires reduce seed availability and alter substrate constraints on Picea mariana regeneration. For Ecol Manag 266: 34–41. https://doi.org/10.1016/j.foreco.2011.11.006.
Bušs K (1997) Forest ecosystem classification in Latvia. Proc Latv Acad Sci B: Nat Exact Appl Sci 51: 204–218.
Cain SA (1950) Life-forms and phytoclimate. Botan Rev 16: 1–32. https://doi.org/10.1007/BF02879783.
Central Statistics Bureau (2023) MEU010. Causes of Forest Fires 2013 – 2023. https://data.stat.gov.lv/pxweb/lv/OSP_PUB/START__NOZ__ME__MEU/MEU010. Accessed 12 May 2024.
Clarke PJ, Keith DA, Vincent BE, Letten AD (2015) Post‐grazing and post‐fire vegetation dynamics: long‐term changes in mountain bogs reveal community resilience. J Veg Sci 26: 278–290. https://doi.org/10.1111/jvs.12239.
Dawe DA, Parisien MA, Van Dongen A, Whitman E (2022) Initial succession after wildfire in dry boreal forests of northwestern North America. Plant Ecol 223: 789–809. https://doi.org/10.1007/s11258-022-01237-6.
Doerr SH, Santín C (2016) Global trends in wildfire and its impacts: perceptions versus realities in a changing world. Philos Trans R Soc Lond B Biol Sci 371, article id 20150345. https://doi.org/10.1098/rstb.2015.0345.
Donis J, Kitenberga M, Šņepsts G, Matisons R, Zariņš J, Jansons Ā (2017) The forest fire regime in Latvia during 1922–2014. Silva Fenn 51, article id 7746. https://doi.org/10.14214/sf.7746.
Driscoll DA, Lindenmayer DB, Bennett AF, Bode M, Bradstock RA, Cary GJ, Clarke MF, Dexter N, Fensham R, Friend G, Gill M (2010) Fire management for biodiversity conservation: key research questions and our capacity to answer them. Biol Cons 143: 1928–1939. https://doi.org/10.1016/j.biocon.2010.05.026.
Drobyshev I, Niklasson M, Linderholm HW (2012). Forest fire activity in Sweden: climatic controls and geographical patterns in 20th century. Agr Forest Meteorol 154–155: 174–186. https://doi.org/10.1016/j.agrformet.2011.11.002.
Ellenberg H, Weber HE, Düll R, Wirth V, Werner W, Paulißen D (1992) Zeigerwerte von Pflanzen in Mitteleuropa (2nd edition). Scr Geobot 18: 1–258.
Erdős L (2014) Post-fire regeneration of a forest-steppe: vegetation status 20 years after the fire event. Tiscia 40: 11–15.
Fernández-Guisuraga JM, Fernandes PM, Tárrega R, Beltrán-Marcos D, Calvo L (2023) Vegetation recovery drivers at short-term after fire are plant community-dependent in mediterranean burned landscapes. For Ecol Manag 539, article id 121034. https://doi.org/10.1016/j.foreco.2023.121034.
Fitter AH, Peat HJ (1994) The ecological flora database. J Ecol 82: 415–425. https://doi.org/10.2307/2261309.
Franklin JF, Lindenmayer D, MacMahon JA, McKee A, Magnuson J, Perry DA, Waide R, Foster D (2000) Threads of continuity. Conservation in Practice 1: 8–17. https://doi.org/10.1111/j.1526-4629.2000.tb00155.x.
Gajendiran K, Kandasamy S, Narayanan M (2024) Influences of wildfire on the forest ecosystem and climate change: a comprehensive study. Environ Res 240, article id 117537. https://doi.org/10.1016/j.envres.2023.117537.
Gavrilova G, Šulcs V (1999) Flora of Latvian vascular plants. List of taxa. Institute of Biology of University of Latvia, Riga, Latvia.
Gongalsky KB, Persson T (2013) Recovery of soil macrofauna after wildfires in boreal forests. Soil Biol Biochem 57: 182–191. https://doi.org/10.1016/j.soilbio.2012.07.005.
Granström A (2001) Fire management for biodiversity in the European boreal forest. Scand J For Res 16: 62–69. https://doi.org/10.1080/028275801300090627.
Hamman ST, Dunwiddie PW, Nuckols JL, McKinley M (2011) Fire as a restoration tool in Pacific Northwest prairies and oak woodlands: challenges, successes, and future directions. Northwest Science 85: 317–328. https://doi.org/10.3955/046.085.0218.
Hart SJ, Henkelman J, McLoughlin PD, Nielsen SE, Truchon‐Savard A, Johnstone JF (2019) Examining forest resilience to changing fire frequency in a fire‐prone region of boreal forest. Glob Chang Biol 25: 869–884. https://doi.org/10.1111/gcb.14550.
Hislop S, Jones S, Soto‐Berelov M, Skidmore A, Haywood A, Nguyen TH (2019) High fire disturbance in forests leads to longer recovery, but varies by forest type. Remote Sens Ecol Conserv 5: 376–388. https://doi.org/10.1002/rse2.113.
Hodgson JG, Wilson PJ, Hunt R, Grime JP, Thompson K (1999) Allocating CSR plant functional types: a soft approach to a hard problem. Oikos 85: 282–294. https://doi.org/10.2307/3546494.
Jean M, Lafleur B, Fenton NJ, Paré D, Bergeron Y (2019) Influence of fire and harvest severity on understory plant communities. For Ecol Manag 436: 88–104. https://doi.org/10.1016/j.foreco.2019.01.004.
Jõgiste K, Korjus H, Stanturf JA, Frelich LE, Baders E, Donis J, Jansons A, Kangur A, Köster K, Laarmann D, Maaten T, Marozas V, Metslaid M, Nigul K, Polyachenko O, Randveer T, Vodde F (2017) Hemiboreal forest: natural disturbances and the importance of ecosystem legacies to management. Ecosphere 8, article id e01706. https://doi.org/10.1002/ecs2.1706.
Johnson EA, Miyanishi K, Weir JM (1998) Wildfires in the western Canadian boreal forest: landscape patterns and ecosystem management. J Veget Sci 9: 603–610. https://doi.org/10.2307/3237276.
Johnson EA, Miyanishi K, Bridge SR (2001) Wildfire regime in the boreal forest and the idea of suppression and fuel buildup. Cons Biol 15: 1554–1557. https://doi.org/10.1046/j.1523-1739.2001.01005.x.
Johnstone JF, Allen CD, Franklin JF, Frelich LE, Harvey BJ, Higuera PE, Mack MC, Meentemeyer RK, Metz MR, Perry GLW, Schoennagel T, Turner MG (2016) Changing disturbance regimes, ecological memory, and forest resilience. Front Ecol Environ 14: 369–378. https://doi.org/10.1002/fee.1311.
Karavani A, Boer MM, Baudena M, Colinas C, Díaz‐Sierra R, Pemán J, de Luis M, Enríquez‐de‐Salamanca Á, Resco de Dios V (2018) Fire‐induced deforestation in drought‐prone Mediterranean forests: drivers and unknowns from leaves to communities. Ecol Monogr 88: 141–169. https://doi.org/10.1002/ecm.1285.
Kellman MC (1970) The viable seed content of some forest soil in coastal British Columbia. Can J Botan 48: 1383–1385. https://doi.org/10.1139/b70-209.
Khapugin AA, Vargot EV, Chugunov GG (2016) Vegetation recovery in fire-damaged forests: a case study at the southern boundary of the taiga zone. For Stud 64: 39–50. https://doi.org/10.1515/fsmu-2016-0003.
Kibler CL, Parkinson AM, Peterson SH, Roberts DA, D’Antonio CM, Meerdink SK, Sweeney SH (2019) Monitoring post-fire recovery of chaparral and conifer species using field surveys and Landsat time series. Remote Sens 11, article id 2963. https://doi.org/10.3390/rs11242963.
Kitenberga M, Elferts D, Adamovics A, Katrevics J, Donis J, Baders E, Jansons A (2020) Effect of salvage logging and forest type on the post-fire regeneration of Scots pine in hemiboreal forests. New For 51: 1069–1085. https://doi.org/10.1007/s11056-020-09775-5.
Kleyer M, Bekker RM, Knevel IC, Bakker JP, Thompson K, Sonnenschein M, Poschlod P, Van Groenendael JM, Klimeš L, Klimešová J, Klotz SR (2008) The LEDA Traitbase: a database of life‐history traits of the Northwest European flora. J Ecol 96: 1266–1274. https://doi.org/10.1111/j.1365-2745.2008.01430.x.
Krebs P, Pezzatti GB, Mazzoleni S, Talbot LM, Conedera M (2010) Fire regime: history and definition of a key concept in disturbance ecology. Theory Biosci 129: 53–69. https://doi.org/10.1007/s12064-010-0082-z.
Kuuluvainen T (2002) Introduction. Disturbance dynamics in boreal forests: defining the ecological basis of restoration and management of biodiversity. Silva Fenn 36: 5–11. https://doi.org/10.14214/sf.547.
Kuuluvainen T, Angelstam P, Frelich L, Jõgiste K, Koivula M, Kubota Y, Lafleur B, Macdonald E (2021) Natural disturbance-based forest management: moving beyond retention and continuous-cover forestry. Front For Glob Change 4, article id 629020. https://doi.org/10.3389/ffgc.2021.629020.
Laivins M, Kalvite Z, Klavins I, Kaupe D, Matisone I, Karklina I, Smits A (2019) Post-fire dynamics in a mesotrophic pine forest: the third year after fire. Latvijas Vegetacija 29: 5–32. (in Latvian). https://mail.silava.lv/images/articles/Latvijas-Vegetacija/2019-29/2019-LatVeg-29.pdf.
Lindholm T, Vasander H (1987) Vegetation and stand development of mesic forest after prescribed burning. Silva Fenn 21: 259–278. https://doi.org/10.14214/sf.a15475.
Lydersen JM, Collins BM, Miller JD, Fry DL, Stephens SL (2016) Relating fire-caused change in forest structure to remotely sensed estimates of fire severity. Fire Ecol 12: 99–116. https://doi.org/10.4996/fireecology.1203099.
Markham J, Essery E (2015) Stand-and plot-level changes in a boreal forest understory community following wildfire. Plant Ecol Divers 8: 585–590. https://doi.org/10.1080/17550874.2015.1049234.
Marozas V, Racinskas J, Bartkevicius E (2007) Dynamics of ground vegetation after surface fires in hemiboreal Pinus sylvestris forests. For Ecol Manag 250: 47–55. https://doi.org/10.1016/j.foreco.2007.03.008.
Marozas V, Armolaitis K, Aleinikovienė J (2013) Changes of ground vegetation, soil chemical properties and microbiota following the surface fires in Scots pine forests. J Environ Eng Landsc 21: 67–75. https://doi.org/10.3846/16486897.2012.663087.
Meng R, Dennison PE, D’Antonio CM, Moritz MA (2014) Remote sensing analysis of vegetation recovery following short-interval fires in southern California shrublands. PloS One 9, article id e110637. https://doi.org/10.1371/journal.pone.0110637.
Müller J, Noss RF, Thorn S, Bässler C, Leverkus AB, Lindenmayer D (2019) Increasing disturbance demands new policies to conserve intact forest. Cons Lett 12, article id e12449. https://doi.org/10.1111/conl.12449.
Noss RF, Lindenmayer DB (2006) The ecological effects of salvage logging after natural disturbance. Cons Biol 20: 946–948. https://doi.org/10.1111/j.1523-1739.2006.00498.x.
Orumaa A, Köster K, Tullus A, Tullus T, Metslaid M (2022) Forest fires have long-term effects on the composition of vascular plants and bryophytes in Scots pine forests of hemiboreal Estonia. Silva Fenn 56, article id 10598. https://doi.org/10.14214/sf.10598.
Parro K, Metslaid M, Renel G, Sims A, Stanturf JA, Jõgiste K, Köster K (2015) Impact of postfire management on forest regeneration in a managed hemiboreal forest, Estonia. Can J For Res 45: 1192–1197. https://doi.org/10.1139/cjfr-2014-0514.
Pausas JG, Keeley JE (2019) Wildfires as an ecosystem service. Front Ecol Environ 17: 289–295. https://doi.org/10.1002/fee.2044.
Peeler JL and Smithwick EA (2021) Interactions between landscape and local factors inform spatial action planning in post-fire forest environments. Landsc Ecol 36: 3523–3537. https://doi.org/10.1007/s10980-021-01325-4.
Petrokas R, Manton M (2023) Adaptive Relationships in Hemi-Boreal Forests: Tree Species Responses to Competition, Stress, and Disturbance. Plants 12, article id 3256. https://doi.org/10.3390/plants12183256.
Purina L, Straupe I, Liepa L, Libiete Z, Zadina M, Jansons A (2016) Long-term influence of large forest fire on ground vegetation. In: ‘Research for Rural Development’ 18–20 May 2016, Latvia University of Agriculture, Jelgava, pp 28–33.
Pykälä J (2004) Immediate increase in plant species richness after clear‐cutting of boreal herb‐rich forests. Appl Veg Sci 7: 29–34. https://doi.org/10.1111/j.1654-109X.2004.tb00592.x.
Pyke DA, Brooks ML, D’Antonio C (2010) Fire as a restoration tool: a decision framework for predicting the control or enhancement of plants using fire. Restor Ecol 18: 274–284. https://doi.org/10.1111/j.1526-100X.2010.00658.x.
R Core Team (2023) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/.
Ruokolainen L, Salo K (2009) The effect of fire intensity on vegetation succession on a sub-xeric heath during ten years after wildfire. Ann Botan Fenn 46: 30–42. https://doi.org/10.5735/085.046.0103.
Ryan KC (2002) Dynamic interactions between forest structure and fire behavior in boreal ecosystems. Silva Fenn 36: 13–39. https://doi.org/10.14214/sf.548.
Schimmel J, Granstrom A (1996) Fire severity and vegetation response in the Boreal Swedish forest. Ecology 77: 1436–1450. https://doi.org/10.2307/2265541.
Seidl R, Fernandes PM, Fonseca TF, Gillet F, Jönsson AM, Merganičová K, Netherer S, Arpaci A, Bontemps J-D, Bugmann H, González-Olabarria JR, Lasch P, Meredieu C, Moreira F, Schelhaas M-J, Mohren F (2011) Modelling natural disturbances in forest ecosystems: a review. Ecol Model 222: 903–924. https://doi.org/10.1016/j.ecolmodel.2010.09.040.
State Forest Service (2021) Fire protection in forests. https://www.vmd.gov.lv/lv/ugunsapsardziba. (in Latvian).
Tepley AJ, Thomann E, Veblen TT, Perry GL, Holz A, Paritsis J, Kitzberger T, Anderson‐Teixeira KJ (2018) Influences of fire–vegetation feedbacks and post‐fire recovery rates on forest landscape vulnerability to altered fire regimes. J Ecology 106: 1925–1940. https://doi.org/10.1111/1365-2745.12950.
Thompson DK, Parisien MA, Morin J, Millard K, Larsen CP, Simpson BN (2017) Fuel accumulation in a high-frequency boreal wildfire regime: from wetland to upland. Can J Forest Res 47: 957–964. https://doi.org/10.1139/cjfr-2016-0475.
Tingley MW, Ruiz-Gutiérrez V, Wilkerson RL, Howell CA, Siegel RB (2016) Pyrodiversity promotes avian diversity over the decade following forest fire. Proc R Soc B Biol Sci 283, article id 20161703. https://doi.org/10.1098/rspb.2016.1703.
Valladares F, Laanisto L, Niinemets Ü, Zavala MA (2016) Shedding light on shade: ecological perspectives of understorey plant life. Pl Ecol Divers 9: 237–251. https://doi.org/10.1080/17550874.2016.1210262.
Vilà‐Cabrera A, Rodrigo A, Martínez‐Vilalta J, Retana J (2012) Lack of regeneration and climatic vulnerability to fire of Scots pine may induce vegetation shifts at the southern edge of its distribution. J Biogeo 39: 488–496. https://doi.org/10.1111/j.1365-2699.2011.02615.x.
Wallenius T (2011). Major decline in fires in coniferous forests – reconstructing the phenomenon and seeking for the cause. Silva Fenn 45: 139–155. https://doi.org/10.14214/sf.36.
Wang GG, Kemball KJ (2005) Effects of fire severity on early development of understory vegetation. Can J Forest Res 35: 254–262. https://doi.org/10.1139/x04-177.
Wang W, Wu W, Guo F, Wang G (2022) Fire regime and management in Canada’s protected areas. Int J Geoheritage Parks 10: 240–251. https://doi.org/10.1016/j.ijgeop.2022.04.003.
Yin J, He B, Fan C, Chen R (2024) Fire has become a major disturbance agent in the forests of Southwest China. Ecol Ind 160, article id 111885. https://doi.org/10.1016/j.ecolind.2024.111885.
Zyryanova OA, Abaimov AP, Bugaenko TN, Bugaenko NN (2010) Recovery of forest vegetation after fire disturbance. In: Osawa A, Zyryanova O, Matsuura Y, Kajimoto T, Wein R (eds) Permafrost ecosystems: Siberian larch forests. Ecological Studies 209. Springer, Dordrecht, pp 83–96. https://doi.org/10.1007/978-1-4020-9693-8_5.
Total of 79 references.
Send to email

