Effects of environmental factors on the species richness, composition and community horizontal structure of vascular plants in Scots pine forests on fixed sand dunes
Tilk M., Tullus T., Ots K. (2017). Effects of environmental factors on the species richness, composition and community horizontal structure of vascular plants in Scots pine forests on fixed sand dunes. Silva Fennica vol. 51 no. 3 article id 6986. https://doi.org/10.14214/sf.6986
Highlights
- Factors affecting the species richness, composition and horizontal structure of vascular plants are related to dune topography, resulting in the differentiation of soils and therefore complexes of different microhabitats that are populated by various vascular plant species and causing vegetation zonation.
Abstract
Different environmental factors were studied to determine which factors influence the species richness, composition and structure of vascular plants in Pinus sylvestris L. forests in a fixed dune landscape in south-western Estonia. In addition to site topographic factors, different environmental parameters were investigated. Thirty-four vascular plant species were recorded in 232 quadrats. The most abundant species was Vaccinium vitis-idaea L., which was in 82.8% of quadrats, followed by Vaccinium myrtillus L. (74.1%), Melampyrum pratense L. (71.1%) and Deschampsia flexuosa (L.) Trin. (69.8%). The multiple response permutation procedure (MRPP) showed considerable differences in species composition at the bottoms of dunes compared with that on the slopes and at the tops of dunes. Indicator species analysis (ISA) determined species exhibited characteristics specific to zone: V. myrtillus had the highest indicator value at the bottoms of dunes; Calluna vulgaris L., at the tops. Soils were Haplic Podzols, and the presence of humus horizon depended on zone. Soil conditions on the dunes were variable and site specific, in general, soils at the bottoms of the dunes were more acidic and moist compared with those of the slopes and tops of the dunes, and the nutrient content decreased toward the dune tops. According to non-metric multidimensional scaling (NMDS) and linear mixed model analyses, species coverage, composition and richness were controlled by site-specific factors such as absolute height, location and aspect of the quadrat on the dune; soil nitrogen, potassium and phosphorus contents; soil pH and moisture; light conditions; and the thickness of the litter horizon.
Keywords
biodiversity;
inland dunes;
microhabitats;
soil conditions;
vegetation–site relationships
-
Tilk,
Department of Silviculture, Estonian University of Life Sciences, Kreutzwaldi 5, Tartu, Estonia, 51014; Tallinn Botanic Garden, Kloostrimetsa Road 52, Tallinn, Estonia, 11913
E-mail
Mari.Tilk@botaanikaaed.ee

- Tullus, Department of Silviculture, Estonian University of Life Sciences, Kreutzwaldi 5, Tartu, Estonia, 51014 E-mail Tea.Tullus@emu.ee
- Ots, Department of Silviculture, Estonian University of Life Sciences, Kreutzwaldi 5, Tartu, Estonia, 51014 E-mail Katri.Ots@emu.ee
Received 2 March 2017 Accepted 5 July 2017 Published 13 July 2017
Views 138804
Available at https://doi.org/10.14214/sf.6986 | Download PDF

1 Introduction
The wooded dunes of the boreal region are among the priority habitats according to the Council of European Union (1992). In Europe, wooded dunes are primarily sea dunes of the Atlantic Ocean, Mediterranean Sea, North Sea and Baltic Sea coasts. Fixed dune landscapes are usually characterised by specific topographical features that vary in aspect, slope angle and microclimate. Plant communities differ on slopes facing different cardinal directions; some species are generally confined to specific zones (Houston 2008; Mandre et al. 2008; Tilk et al. 2011). There have been numerous studies concerning coastal dunes and their vegetation, growth conditions and vegetation changes induced by disturbances (Kutiel et al. 1999; Kooijman and Besse 2002; Ensign et al. 2006; Isermann 2008; Brunbjerg et al. 2015; Ciccarelli 2015; Pinna et al. 2015). Topography, human influence, and soil conditions such as pH, nutrients and moisture are important factors that affect ground vegetation in dune areas (Isermann 2005; Ruocco et al. 2014; Sewerniak et al. 2017). In Estonia, Ilves (1966), Eltermann and Raukas (1966), Raukas (1968), Örd (1972, 1973) and Mandre et al. (2008) studied the aspects of fixed dune history and soils in the Rannametsa dune area. Vascular plant diversity in the same dune area has been studied by Mandre et al. (2006) and Tilk et al. (2011). However, these studies included data from one or two dunes and focussed on one slope of the dunes, but it is known that dune vegetation, soil conditions and even forest site type can differ drastically between slopes (Raukas 1968; Örd 1972). Research on dune habitats is vital. The current assessments of dune habitats indicate an unfavourable conservation status (European Commission 2015), and to acknowledge the temporal changes (like climate warming, atmospheric nitrogen deposition) and increasing recreational pressure on these areas, further actions should be implemented. However, valuable complex and statistically relevant information about fine-scale variations in ground vegetation communities and their relationships to soil resources and growth conditions on Pinus sylvestris L. forested inland dunes is still lacking. The objective of this paper is to provide new knowledge concerning ground vegetation in dune forests and to determine which environmental (light and soil conditions) and topographical factors affect the vascular plant species richness and composition of the ground vegetation and are therefore responsible for ground vegetation zonation. As such, the following hypothesis was formed:
• Dune forest ground vegetation forms different vegetation zones and patches along the dune profile primarily on higher dunes where growth conditions such as soil moisture and nutrient contents change more rapidly than on significantly lower dunes; therefore the absolute and relative heights of the dune are important topographical features that cause the zonation of vascular plant species.
2 Material and methods
2.1 Description of the study area
The studied dune system is situated in south-western Estonia. To investigate the ecosystems on dunes of different heights, four sampling sites were selected (Fig. 1). Dunes in the studied area were formed by Lake Ancylus and the Littorina Sea during the ice ages and are with different heights: site 1 absolute height 28 m and relative height 16 m; site 2 absolute height 33 m and relative height 21 m; site 3 absolute height 12 m and relative height 6 m; site 4 absolute height 10 m and relative height 6 m.
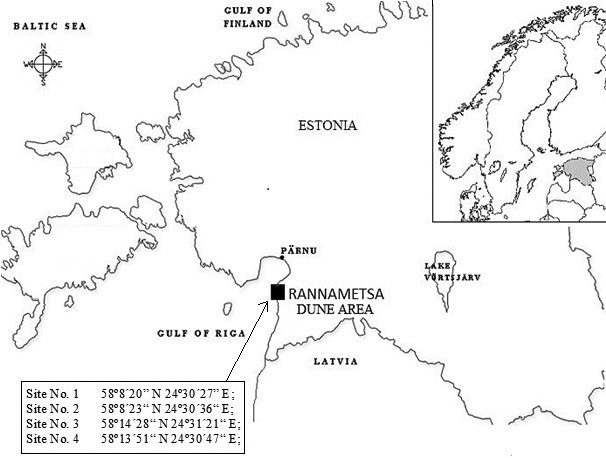
Fig. 1. Location of study area and study sites.
Lower dunes are situated in the Uulu-Võiste landscape protection area, where three priority habitats according to the European Commission (2013) are present: wooded dunes of the Atlantic, Continental and Boreal region (type 2180); Western Taïga (type 9010*); and Fennoscandian deciduous swamp woods (type 9080*). Higher dunes are located in the Luitemaa nature reserve, where fixed coastal dunes with herbaceous vegetation (grey dunes) (type 2130*); wooded dunes of the Atlantic, Continental and Boreal region (type 2180); and humid dune slacks (2190) are under protection.
2.2 Climate
According to the closest weather station of the Estonian Meteorological and Hydrological Institute in Pärnu, the main climate characteristics during the period of investigation in 2008 were as follows: average annual temperature of 7.6 °C, with a maximum average of 16.6 °C in July and a minimum average of –0.6 °C in January; total precipitation of 862 mm, with a maximum of 147.5 mm in August and a minimum of 17 mm in May. The relative humidity was 83%. The length of the thermal growing period (temperature greater than 5 °C) was 232 days (30.03.2008–17.11.2008). During the winter of 2007/2008, no permanent snow cover was recorded, but throughout the winter, there were 48 days with snow.
2.3 Methods
Field studies to analyse ground vegetation were carried out in 2008. Sample plots that formed continuous transects over the dunes were established on all four dunes. Transects started from the plain in front of the dune and extended over the top to the back plain in increments of 1 m. The descriptions of the plant communities were made in quadrats 1 × 1 m in size at different heights on the dunes; altogether, 232 quadrats were studied.
The transect at site 1 started from the southern plain of the dune and extended continuously over the top of the dune to the northern plain; altogether, 58 quadrats were analysed. The transect at site 2 extended from the western plain over the top to the eastern plain and encompassed 108 quadrats. At site 3, the sample plots (n = 32) extended from the southern plain to the northern plain, and at site 4, the transect of sample plots (n = 34) was directed from east to west.
Average characteristics for the studied stands were obtained from the State Forest Management Centre database and are presented in Table 1. Forest site type is in accordance with those of Lõhmus (2004).
| Table 1. Average characteristics of the investigated stands. | ||||||||||
| Site No. | Soil type | Forest site type | Composition of trees | No. of trees per hectare | Age, yr | Site quality index | Canopy cover | Height, m | Breast height diameter, cm | Density of understory |
| 1 | Haplic Podzol | Rhodococcum | 100 Ps | 149 | 180 | IV | 0.45 | 24 | 37 | Low |
| 2 | Haplic Podzol | Cladonia | 100 Ps | 191 | 190 | IV | 0.54 | 24 | 36 | Low |
| 3 | Haplic Podzol | Cladonia | 100 Ps | 98 | 200 | IV | 0.62 | 21 | 44 | Low |
| 4 | Haplic Podzol | Cladonia | 100 Ps | 143 | 210 | IV | 0.60 | 23 | 38 | Low |
| Ps = Pinus sylvestris | ||||||||||
The species composition of vascular plants in the quadrats was determined in May, July and September to record seasonal features of the vegetation and therefore the complete species composition (Kalda 1966; Masing 1979). The nomenclature follows the keybook of Estonian vascular plants (Leht 2010). Dominant species were determined based on abundance (Braun–Blanquet five-point cover scale). The total cover of the vascular plant layer, the total cover of the bryophyte and lichen layers and the cover of each vascular plant species in the quadrats were estimated visually using a scale of 1–100%.
Canopy cover as a measure of the percentage of forest floor covered by the vertical projection of tree canopies was estimated in every quadrat. To evaluate the amount of light transmitted through the canopy to the ground vegetation, below-canopy photosynthetically active radiation (PAR, μmol m–2 s–1) was measured (n = 10 per quadrat) with Decagon Devices AccuPAR (Model PAR-80) light-interception device at midday on 23.07.2008 (sunny, clear conditions). Because light is highly variable, measurements were performed during a short period (from 11.00 to 13.00) at all sites. Quadrats angles were measured to describe micro-topographical features using an inclinometer for every quadrat and values were classified according angle degree to five classes: 1 (1–10°); 2 (11–20°); 3 (21–30°); 4 (31–40°); and 5 (41–50°). Absolute altitudes were measured using a Garmin GPSMap 76CSx device and relative height was calculated using first quadrat of the transect as a zero.
Soil volumetric water content (VWC, %) was determined in every quadrat using a Field ScoutTM TDR 300 at a depth of 20 cm (n = 3 per quadrat) to describe soil water-holding capacity. Soil VWC data were collected in May, July and September when no rain had occurred for at least 3 days before measurements.
All soil types were classified according to the FAO (FAO-ISRIC-ISSS 1998). Soil samples from every quadrat were collected in July 2010 at a maximum depth of 20 cm to measure pH and electrical conductivity. The soil pH and electrical conductivity (EC; µS) were measured in soil: water mixtures (1:2.5 or 1:5) using a Eutech Instruments PC300 pH/conductivity meter. To analyse the nitrogen, phosphorus, potassium, calcium and magnesium contents, soil samples were collected; soil horizons were distinguished by bottoms, slopes and the tops of dunes (n = 3 at every location, 15 samples per dune) collected at a maximum depth of 20 cm, as most roots for majority of vascular plants are concentrated in the first 20 cm (Bednarek et al. 2005; Solon et al. 2007). The contents of total nitrogen, phosphorus, potassium, calcium and magnesium in the top soil samples were determined at the Laboratory of Plant Biochemistry of the Estonian University of Life Sciences. The soil samples were analysed for their extractable content of phosphorus (ammonium lactate; FIAStar 5000 (flow injection analyser)), potassium (ammonium lactate; flame photometric method), calcium (ammonium acetate; flame photometric method), magnesium (ammonium acetate; FIAStar 5000 (flow injection analyser)) and total nitrogen (copper catalyst; Kjeldahl method).
2.4 Data analysis
The species richness of vascular plants was estimated for every quadrat as the number of species present in the quadrat. Microsoft Excel 2010 was used to perform basic statistical analysis.
One-way ANOVA followed by post-hoc Tukey HSD test using Tukey-Kramer method was applied to determine significant differences between environmental characteristics at different locations on dunes (Vasavada 2016). Level of significance of ɑ = 0.05 was used. For the statistical analysis, transects on dunes were divided into three zones/locations: plain before the dune, slope and top, based on the relative height and the angle of the quadrat.
The effect of grouping factors on species composition was tested using the multiple response permutation procedure (MRPP) (Mielke et al. 1976). To correct the p-values for multiple comparisons using MRPP, a Bonferroni correction was applied. Indicator species analysis (ISA) (Dufrene and Legendre 1997) was conducted to specify indicator species for different zones. The statistical significance of indicator values was proven using the Monte Carlo simulation technique. MRPP and ISA were performed using PC-ORD Version 6 (McCune and Mefford 2011). Overlapping and individuality of species at different sites and zones on dunes were described using a Venn diagram, made using Venny (Oliveros 2015).
To study variation in vascular plant species composition and to determine its influencing factors, non-metric multidimensional scaling (NMDS) ordination was performed using the free statistical software R Version 3.2.3, with the community ecology package “Vegan”. A linear mixed model (with dune as a random factor) was applied to clarify the effects of environmental variables (light conditions, soil moisture, soil pH, macronutrient contents, litter horizon thickness, and the cover of the bryophyte and lichen layers) and location (zone, aspect and absolute height of the quadrat) on species richness and the total cover of vascular plants. Due to intercorrelation between canopy cover and PAR, canopy cover was left out of model. Q-Q plots and residual distributions were used to assess the normality of model residuals.
3 Results
3.1 Species composition
Thirty-four species of vascular plants belonging to 18 families were recorded in transects. The most represented family was the Poaceae with four species, followed by the Asteraceae (three species), Ericaceae (three species), Juncaceae (three species), Liliaceae (three species) and Vaccinaceae (three species).
There were six vascular plant species present at all sites: Calluna vulgaris L., Vaccinium myrtillus L., Melampyrum pratense L., Empetrum nigrum L., Festuca polesica Zapal. and Vaccinium vitis-idaea L.. Overlapping and individual species on different dunes are shown in Fig. 2.
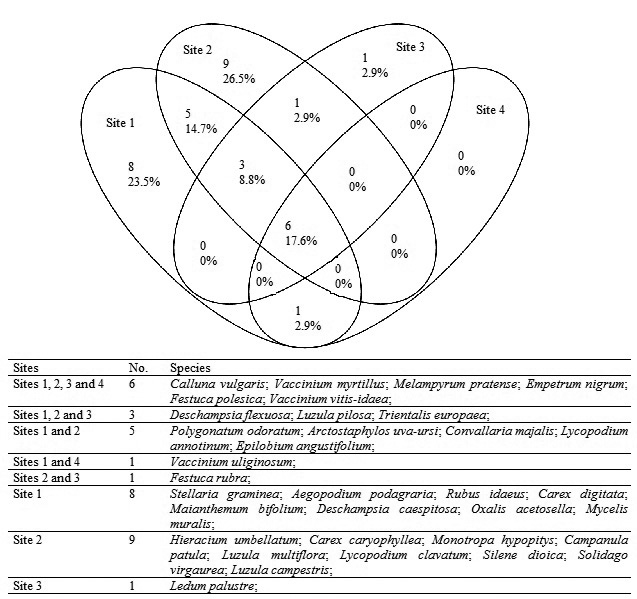
Fig. 2. Venn diagram showing the overlapping and individuality of vascular plant species at different sites.
At site 1, 23 species occurred with Deschampsia flexuosa (L.) Trin. in 100%, V. myrtillus in 89.7% and V. vitis-idaea in 86.2% of the quadrats. The highest number of species was recorded at site 2, where 24 vascular plant species were found; the most frequent vascular plant species recorded were D. flexuosa (85.2%), V. vitis-idaea (76.9%) and V. myrtillus (74.1%). At sites 3 and 4, 11 and 7 species of vascular plants were distinguished, respectively. Melampyrum pratense L. (84.4%), V. vitis-idaea (78.1%) and F. polesica (56.3%) were dominant at site 3, and V. vitis-idaea (100%), M. pratense (85.3%) and V. myrtillus (70.6%) were most prevalent at site 4. Vascular plant species were not found in the four quadrats on the upper part of the slope at site 2.
According to the results of the MRPP tests, almost all dunes differed statistically from each other, with the exception of the lower dunes 3 and 4, which did not significantly differ in vascular plant species composition (Table 2). As the MRPP results indicated considerable differences in species composition between dunes, subsequent tests to compare the effect of location on species composition were performed separately for each dune. The MRPP analyses revealed that species composition at the bottoms of all dunes differed significantly from species composition in the other zones (slope and top). However, a significant difference between slope and top was observed only on the highest dune (site 2). Overlapping species and species distribution between different zones are shown in Fig. 3.
| Table 2. The results of multiple response permutation procedure (MRPP) tests for the comparison of species composition on different dunes. | ||||||
| Test pair | Site 1 vs. Site 2 | Site 1 vs. Site 3 | Site 1 vs. Site 4 | Site 2 vs. Site 3 | Site 2 vs. Site 4 | Site 3 vs. Site 4 |
| p-value | <0.001 | <0.001 | <0.001 | 0.002 | <0.001 | 0.011 |
| Bold values are significant after the Bonferroni correction | ||||||
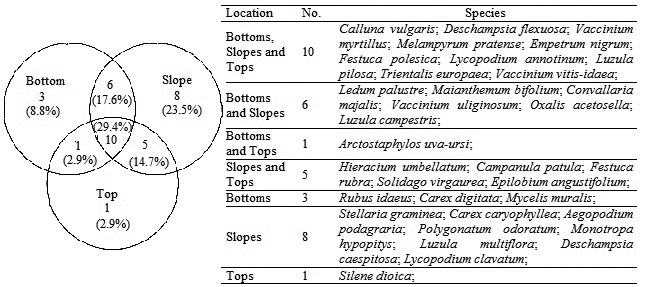
Fig. 3. Venn diagram showing the overlapping and individuality of vascular plant species at different locations on dunes.
ISA was also conducted separately for each dune, and the results showed characteristic species were causing zonation of the bottoms, slopes and tops (Table 3). Although indicator species for the bottoms and slopes were different for each dune, C. vulgaris was as an indicator species for tops on three dunes; Festuca rubra L., for two.
| Table 3. Vascular plant indicator species with indicator values (IV) for different zones (bottom, slope and top) on dunes according to the indicator species analysis (ISA). | |||||||||||
| Bottom | Slope | Top | |||||||||
| IV | p-value | IV | p-value | IV | p-value | ||||||
| Site 1 | Rubu ida | 28 | 0.011 | Empe nig | 36 | 0.016 | Call vul | 32 | 0.05 | ||
| Oxal ace | 25 | 0.041 | |||||||||
| Mela pra | 50 | 0.042 | |||||||||
| Site 2 | Empe nig | 53 | 0.0002 | Hier umb | 26 | 0.001 | |||||
| Fest pol | 50 | 0.0004 | Call vul | 50 | 0.001 | ||||||
| Conv maj | 13 | 0.035 | Mela pra | 52 | 0.002 | ||||||
| Soli vir | 26 | 0.002 | |||||||||
| Fest rub | 35 | 0.006 | |||||||||
| Trie eur | 21 | 0.021 | |||||||||
| Site 3 | Vacc myr | 73 | 0.0002 | Trie eur | 61 | 0.001 | Fest rub | 41 | 0.05 | ||
| Vacc vit | 69 | 0.0002 | |||||||||
| Ledu pal | 43 | 0.012 | |||||||||
| Site 4 | Vacc uli | 38 | 0.012 | Fest pol | 48 | 0.025 | Empe nig | 52 | 0.002 | ||
| Call vul | 59 | 0.008 | |||||||||
| Rubu ida = Rubus idaeus; Oxal ace = Oxalis acetosella; Mela pra = Melampyrum pratense; Empe nig = Empetrum nigrum; Fest pol = Festuca polesica; Conv maj = Convallaria majalis; Vacc myr = Vaccinium myrtillus; Vacc vit = Vaccinium vitis-idaea; Ledu pal = Ledum palustre; Vacc uli = Vaccinium uliginosum; Trie eur = Trientalis europaea; Call vul = Calluna vulgaris; Hier umb = Hieracium umbellatum; Soli vir = Solidago virgaurea; Fest rub = Festuca rubra | |||||||||||
3.2 Environmental variables
Soil horizons were distinguished through 20 cm. Site 1 represented a Rhodococcum forest site type, with Haplic Podzol and humus horizon (1–7.5 cm) soil at the bottoms and Haplic Podzol soil on the slopes and on top of the dune.
Site 2 belonged to a Cladonia forest site type, with Haplic Podzol and humus horizon (1–7 cm) soil at the bottoms and on the slopes of the dune and Haplic Podzol soil at the top of the dune.
Site 3 belonged to the Cladonia forest site type, with Haplic Podzol soil on the southern bottom slope and at the top of the dune, Haplic Podzol and humus horizon (1–15 cm) soil on the northern slope and Carbi-Saprihistic Podzol on the northern bottom.
Site 4 belonged to the Cladonia forest site type, with Haplic Podzol soil on the bottoms and eastern slope and at the top of the dune. The western slope soil type was a Haplic Podzol with a humus horizon (1–2 cm). Soil and light characteristics are presented in Table 4.
| Table 4. Average values of soil and light characteristics from different sites and locations on the dunes. View in new window/tab. |
Average soil moisture was 8.2% (VWC) but ranged from 0.7% to 43.9%. In general the driest quadrats were located at the tops of the dunes, whereas the moistest were at the bottoms; on the slopes, the volumetric water content was in between that of the tops and bottoms. The highest soil moisture value (43.9%) was recorded at site 3, where the northern bottom of dune a Carbi-Saprihistic Podzol was present. The lowest average soil moisture value (0.73%) was recorded on the upper part of the slope at site 2 in a quadrat where the vascular plant species cover was nearly zero. The soil volumetric water content was seasonally variable; significant differences in soil water content were recorded between spring, summer and autumn. Soil moisture was highest in autumn and was 55% higher than the vegetation period average. The driest period was spring, which was 44% lower than the vegetation period average, whereas the summer soil moisture was 11% lower than the vegetation period average. Seasonal features showed significant differences with respect to location and site (Table 4).
The average pHH2O was 4.3, with a minimum value of 3.4 and a maximum of 5.7 for all sites. At sites 2 and 3 the soil pH at the bottoms of the dunes was significantly lower compared with that of the slopes of the dunes (Table 4). No significant differences were observed at sites 1 and 4 regarding soil pH. Soil EC was used to obtain information about soil properties that induce plant growth. The average EC for dunes was 151.3 µS; the maximum value was 724.3 µS, and the minimum was 55.4 µS. According to the comparison analysis, the EC values were significantly higher at the bottoms of the dunes at sites 2 and 3 (Table 4). The average nitrogen content was 0.28%; the average at the tops of dunes was 0.17%, on the slopes was 0.20% and at the bottoms 0.46%. The total nitrogen content was significantly higher at the bottoms than on the slopes at site 2 and on the slopes and top at site 3; there were no significant differences recorded between locations at site 1 and 4 (Table 4). The average phosphorus content in the dune soils was 24.3 mg kg–1; the phosphorus content was 20.7 mg kg–1 at the tops of the dunes, 18.1 mg kg–1 on the slopes and 34.2 mg kg–1 at the bottoms. Phosphorus content showed significant differences between locations only at higher dunes (Table 4). The average potassium content was 68.6 mg kg–1, average Ca content was 244.2 mg kg–1 and average Mg 72.3 mg kg–1.
The average PAR at midday in the fixed dune forests was 450 μmol m–2 s–1. Significant differences based on location on dune were revealed at site 1 (Table 4). Canopy cover values varied between 0.1 to 0.8 and the average value was 0.5. At site 1, the bottoms and slopes showed significant differences in canopy cover, at site 2 slopes and top showed significant differences and at site 4 all locations differed from each other (Table 4).
The results of the linear mixed model showed that vascular plant species richness in the quadrats was affected by zone, aspect of the quadrat, light conditions (PAR), average pH, average soil water content, and total nitrogen and potassium contents (Table 5). The same factors significantly affected the total cover of vascular plants, with the exception of light conditions and the aspect of the quadrat, which exhibited no significant effects. In addition, the total cover was affected by the absolute height of the quadrat and by the thickness of the litter horizon.
| Table 5. Significance (p-values, type 3 effects) of factors contributing to species richness and the coverage of vascular plants according to a linear mixed model. | |||
| Species richness of vascular plants | DF | F-value | p-value |
| Location on dune (zone) | 215.97 | 15.43 | <0.001 |
| Aspect of the quadrat | 7.87 | 16.67 | <0.001 |
| Absolute height | 183.53 | 0.17 | 0.685a |
| Cover of bryophyte and lichen layers | 215.42 | 0.003 | 0.960a |
| PAR | 215.18 | 15.46 | <0.001a |
| VWC | 216.36 | 26.54 | <0.001 |
| pH | 216.54 | 10.70 | 0.001 |
| Ntotal | 215.46 | 29.22 | <0.001a |
| P | 216.92 | 0.002 | 0.969 |
| K | 216.85 | 4.98 | 0.027 |
| O horizon | 215.50 | 1.97 | 0.162 |
| Total cover of vascular plant species | DF | F-value | p-value |
| Location on dune | 102.68 | 13.46 | <0.001 |
| Aspect of the quadrat | 3.29 | 1.53 | 0.356 |
| Absolute height | 2.31 | 22.06 | 0.032 |
| Cover of bryophyte and lichen layers | 199.17 | 1.86 | 0.174a |
| PAR | 216.78 | 0.04 | 0.844 |
| VWC | 205.64 | 22.96 | <0.001 |
| pH | 124.85 | 4.78 | 0.031a |
| Ntotal | 217.00 | 27.28 | <0.001a |
| P | 32.27 | 0.12 | 0.727 |
| K | 139.17 | 24.43 | <0.001 |
| O horizon | 174.46 | 6.61 | 0.011 |
| a – indicates a negative effect | |||
| PAR = below-canopy photosynthetically active radiation (μmol m–2 s–1); VWC = average soil volumetric water content (%); pH = average pHH2O; O = soil litter horizon thickness (cm); Ntotal = nitrogen content (%); P = phosphorus content (mg kg–1); K = potassium content (mg kg–1) | |||
The NMDS analysis of vascular plant species data resulted in a stress value of 0.208 (stress type 1). The NMDS analysis indicated that the most important factors (p ≤ 0.005) controlling vascular plant species composition in the dune forests included site; the absolute height of the sample plot; canopy cover; the aspect of the quadrat; total bryophyte and lichen cover; and soil nitrogen, potassium and phosphorus contents (Fig. 4 and Table 6).
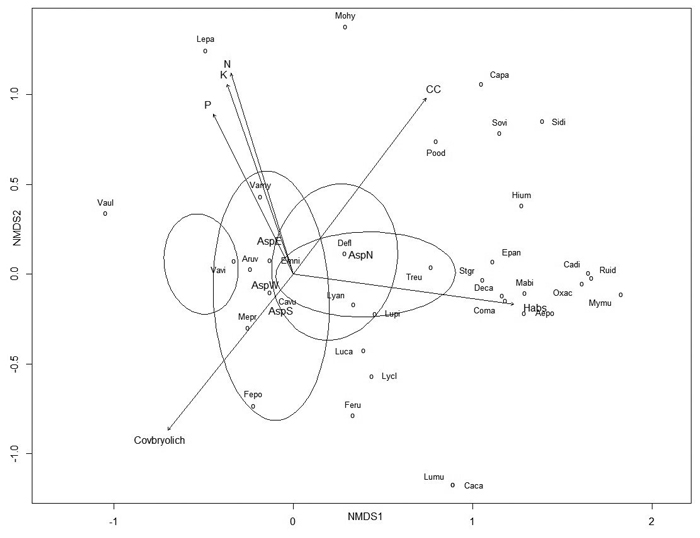
Fig. 4. Non-metric multidimensional scaling (NMDS) ordination graph with fitted environmental variables. Arrows represent environmental variables that were most significantly (p ≤ 0.005) related to ordination. View larger in new window/tab.
| Table 6. Relationships between species composition (non-metric multidimensional scaling (NMDS) ordination, Fig. 4) and environmental variables on dunes. Bold values are variables presented as arrows in Fig. 4. | |||
| Variable | r2 | p-value | Level of significance |
| Habs | 0.2541 | 0.001 | *** |
| CC | 0.2486 | 0.001 | *** |
| Covbryolich | 0.2054 | 0.001 | *** |
| Site | 0.2949 | 0.001 | *** |
| Ntotal | 0.2259 | 0.002 | ** |
| Asp | 0.1552 | 0.003 | ** |
| K | 0.2067 | 0.004 | ** |
| P | 0.1634 | 0.005 | ** |
| Mg | 0.1826 | 0.007 | ** |
| VWCaver | 0.1553 | 0.008 | ** |
| pH | 0.1248 | 0.022 | * |
| Ca | 0.1180 | 0.030 | * |
| Hrel | 0.0942 | 0.062 | . |
| EC | 0.0809 | 0.073 | . |
| Degr | 0.0622 | 0.158 | |
| Loc | 0.0497 | 0.225 | |
| O | 0.0150 | 0.655 | |
| A | 0.0103 | 0.744 | |
| PAR | 0.0026 | 0.914 | |
| Significance codes: 0 ‘***’ 0.001 ‘**’ 0.01 ‘*’ 0.05 ‘.’ 0.1 ‘ ’ 1 | |||
| Habs = absolute height of the quadrat location (m); CC = average canopy cover; Covbryolich = total coverage of bryophytes and lichens (%); Ntotal = average total nitrogen content (%); Asp = aspect of the quadrat; K = average potassium content (mg kg–1); P = average phosphorus content (mg kg–1); Mg = average magnesium content (mg kg–1); VWCaver = average soil water content (%); pH = average soil pHH2O; Ca = average soil calcium content (mg kg–1); Hrel = relative height (m); EC = electrical conductivity (µS); Degr = degree of inclination or ascent; Loc = location on dune (bottom, slope, top); O = average litter horizon thickness (cm); A = average humus horizon thickness (cm); PAR = average photosynthetically active radiation (μmol m–2 s–1) | |||
4 Discussion
The most represented family on the studied dunes was the Poaceae with four species, followed by the Asteraceae with three species. These results are similar to those of Ruocco et al. (2014), who reported that the same families were most abundant on Mediterranean dunes. According to the MRPP test, ground vegetation zonation was prevalent on the studied dunes, with indicator species present for all zones. The zonation of ground vegetation is characteristic for dunes (Isermann 2005; Mandre et al. 2006; Tilk et al. 2011). V. myrtillus had the highest indicator value for the bottoms of the dunes and C. vulgaris for the tops. Only three species were determined to be indicators for the slopes of the dunes. This is because the species composition on the slopes involved species from bottoms and tops; therefore, the slopes can be considered transition zones.
The determination of factors that control the distribution patterns of plant communities remains a central goal in ecology studies. Species composition and community structure in dune forests is regulated by a variety of environmental factors. Based on the NMDS analysis, the most significant factors influencing species composition in the studied dunes were related to the site, such as absolute height, aspect and canopy cover of the quadrat, and its soil fertility, such as soil total nitrogen, potassium and phosphorus content. The most relevant (p < 0.001) environmental variables influencing vascular plant species richness according to the model were quadrat location and aspect on the dune, the amount of PAR, soil volumetric water and total nitrogen content. This finding was in accordance with that of Pausas (1994), who concluded that main species richness determinants in forests are solar radiation, altitude, soil nitrogen content and soil moisture.
In dune areas, topography plays an important role in soil development and therefore affects the variability and distribution of vegetation (Jenny 1941; Sewerniak et al. 2017). Slope aspect also has strong effects on vegetation in large-scale relief forms, but slope aspect also is an important factor of inland dunes. North-facing slopes receive less solar radiation; these slopes have higher moisture contents, lower temperature and higher fertility (Huang et al. 2015; Sewerniak 2016). Both the model and the NMDS analysis confirmed that the aspect of the quadrat and the direction of transect had major influences on vascular plant species richness and composition, whereas vascular plant species cover was unaffected by the aspect of the slope and quadrat. Just as the aspect of the dune slope is important, the quadrat location on the dune is also highly important. Our results showed that species richness and cover were affected by location. Low-situated soils on the bottoms of dunes are characterised by much higher fertility and soil water content compared with those of the slopes and tops of the dunes, which are usually dry and infertile; similar conclusions were made by Örd (1972), Sewerniak (2016) and Sewerniak et al. (2017). The dominant soil type of the studied dunes was a Haplic Podzol, which is usually acidic, with a pH in the range of 4–5; the activity of microfauna is disrupted, which interferes with plant nutrition, especially the nitrogen supply to plants (Lõhmus 2004). Soil type and slope position affect soil fertility and therefore affect ground vegetation abundance, composition and diversity (Hart and Chen 2006; Tilk et al. 2011). The average pH of the studied dunes was 4.3, which is slightly lower than a pH value of 5.0 reported by Mandre et al. (2006) in the same dune area. In our study, the soil pH varied by nearly two units, possibly due to the relatively low vegetation cover and the rather high diversity of vegetation, as suggested by Isermann (2005). Soil reaction significantly influences the mineral nutrition of plants by directly or indirectly altering the availability of mineral nutrients to plants (Klõšeiko 2003; Marschner 2012). Our model results showed that an increase in soil pH resulted in higher numbers of vascular plant species but negatively affected vascular plant species coverage. Similar conclusions were reported by Grime (1973), who reported that the maximum numbers of species in unmanaged grassland occur at a soil pH of 6.1–6.5; only a few species are adapted to exploit highly acidic soils. Also, according to the NMDS, species composition moderately depended on soil pH. One method for obtaining information about soil nutritional status is to evaluate EC; in the current study, significant differences were between the tops and bottoms and between the slopes and bottoms of dunes at sites 2 and 3; sites 1 and 4 showed no significant differences concerning soil EC based on location. However, according to the NMDS analysis, electrical conductivity may be irrelevant.
The well-known pattern that species richness peaks at intermediate levels and starts to decline at higher nutrient levels (Grime 1979) has also been demonstrated in dune areas (Lichter 1998). The results of the current study also confirm this statement: according to the model, higher total nitrogen content significantly reduces species richness. In addition, the NMDS analysis indicated that nitrogen was a statistically important factor for modelling vascular plant species composition on dunes. In contrast to the effect of nitrogen, the concentrations of potassium positively affected vascular plant species richness and coverage according to model. The NMDS also indicated potassium and phosphorus as variables that significantly affect species composition, whereas the effects of calcium and magnesium were less significant in shaping species composition, although calcium is often among the most important exchangeable cations in the growth substrate and can affect the availability of other nutrients (Pausas and Austin 2001).
Light is the most important environmental factor affecting ground vegetation (Reich et al. 2012; Márialigeti et al. 2016). The results of current study showed that more light reduced the number of vascular plant species in dune forests. We can assume that on dunes, ground vegetation is stressed, and an increase in one stress factor decreases the number of different vascular plant species and may increase the occurrence of specific cryptogams (Košuthová et al. 2015). Separating the effect of light from the availability of water to vascular plants is complicated, as solar radiation affects both soil temperature and soil moisture, which, in turn, affect soil chemical properties (Bravo et al. 2011). Our model indicated that while increased light reduced species richness, the increase in volumetric water content increased species richness and also positively affected the cover of vascular plants. Dunes are presumed to be highly limited by water stress because of their sandy texture (Martinez and Psuty 2004). High topographical heterogeneity of dunes leads to the formation of microhabitats with different soil water conditions. In this respect, we should not underestimate the interaction between soil water content and ground vegetation, as dense ground vegetation can affect soil moisture content, which was also reported by both Liu et al. (2010) and Zheng et al. (2015). According to our results, in the spring, soil moisture was statistically lower compared to summer and autumn measurements. Ground vegetation in the spring is sparse and therefore unable to contain moisture in the soil, but in the summer and autumn, when vegetation is thicker and in full growth, soil moisture is influenced by ground vegetation coverage.
According to our hypothesis, ground vegetation forms different vegetation zones and patches; species composition at the bottoms of dunes differed significantly from that of other locations, not only on the higher dunes but also on the lower dunes. However, between slopes and tops, no significant differences were recorded; only at the highest site 2 there was significant difference in species composition. Therefore, we can conclude that complex growth conditions at the bottoms of the dunes are markedly different compared with those on the slopes and at the tops of dunes even on relatively low dunes, resulting in substantially different vascular plant species richness and composition.
In conclusion, factors affecting vascular plant species richness, composition and horizontal structure are related to dune topography, leading to the differentiation of soils and therefore complexes of different microhabitats that are populated by various vascular plant species.
Acknowledgements
This work was supported by institutional research funding IUT21-4 of the Estonian Ministry of Education and Research.
References
Bednarek R., Dziadowiec H., Pokojska U., Prusinkiewicz Z. (2005). Badania ekologiczno-gleboznawcze. [Soil-Ecological Research]. PWN, Warzawa. 340 p. [In Polish].
Bravo F., Lucà M., Mercurio R., Sidari M., Muscolo A. (2011). Soil and forest productivity: a case study from Stone pine (Pinus pinea L.) stands in Calabria (southern Italy). iForest 4: 25–30.
Brunbjerg A.K., Jørgensen G.P., Nielsen K.M., Pedersen M.L., Svenning J.-C., Ejrnæs R. (2015). Disturbance in dry coastal dunes in Denmark promotes diversity of plants and arthtopods. Biological Conservation 182: 243–253. https://doi.org/10.1016/j.biocon.2014.12.013.
Ciccarelli D. (2015). Mediterranean coastal dune vegetation: are disturbance and stress the key selective forces that drive the psammophilous succession. Estuarine, Coastal and Shelf Science 165: 247–253. https://doi.org/10.1016/j.ecss.2015.05.023.
Council of the European Union (1992). Council Directive 92/43/EEC on the conservation of natural habitats and of wild fauna and flora. http://eur-lex.europa.eu/legal-content/EN/LSU/?uri=CELEX:31992L0043. [Cited 12 Jan 2017].
Dufrene M., Legendre P. (1997). Species assemblages and indicator species: the need for a flexible asymmetrical approach. Ecological Monographs 67(3): 345–366. https://doi.org/10.2307/2963459.
Eltermann G., Raukas A. (1966). Eesti luidetest. [About Estonian dunes]. Eesti Loodus 1: 12–18.
Ensign K.L., Webb E.A., Longstaffe F.J. (2006). Microenvironmental and seasonal variations in soil water content of the unsaturated zone of a sand dune system at Pinery Provincial Park, Ontario, Canada. Geoderma 136(3–4): 788–802. https://doi.org/10.1016/j.geoderma.2006.06.009.
European Commission (2013). Interpretation manual of European Union habitats – EUR28. http://ec.europa.eu/environment/nature/legislation/habitatsdirective/docs/Int_Manual_EU28.pdf. [Cited 12 Jan 2017].
European Commission (2015). The state of nature in the EU. http://ec.europa.eu/environment/nature/pdf/state_of_nature_en.pdf. [Cited 12 April 2017].
FAO- ISRIC- ISSS (1998). World reference base for soil resources. 84 World Soil Resources Reports, Rome.
Grime J.P. (1973). Competitive exclusion in herbaceous vegetation. Nature 242: 344–347. https://doi.org/10.1038/242344a0.
Grime J.P. (1979). Plant Strategies and Vegetation Processes. J Wiley & Sons, Chichester. 222 p.
Hart S.A., Chen H.Y. (2006). Understory vegetation dynamics of North American boreal forests. Critical Reviews in Plant Science 25(4): 381–397. https://doi.org/10.1080/07352680600819286.
Houston J. (2008). Management of Natura 2000 habitats. 2130 *Fixed coastal dunes with herbaceous vegetation (´grey dunes´). European Commission. European Communities, Liverpool.
Huang Y.M., Liu D., An S.S. (2015). Effects of slope aspect on soil nitrogen and microbial properties in the Chinese Loess region. Catena 125: 135–145. https://doi.org/10.1016/j.catena.2014.09.010.
Ilves A. (1966). Luidete taimkattest ja metsastamisest. [About the vegetation and afforestation of dunes]. Eesti Loodus 1: 19–23.
Isermann M. (2005). Soil pH and species diversity in coastal dunes. Plant Ecology 178(1): 111–120. https://doi.org/10.1007/s11258-004-2558-8.
Isermann M. (2008). Expansion of Rosa rugosa and Hippophae rhamnoides in coastal grey dunes: effects at different spatial scales. Flora 203(4): 273–280. https://doi.org/10.1016/j.flora.2007.03.009.
Jenny H. (1941). Factors of soil formation. McGraw Hill, New York. 281 p.
Kalda A. (1966). Välibotaanika. [Field Botany]. Tartu Riiklik Ülikool, Tartu. 186 p.
Klõšeiko J. (2003). Carbohydrate metabolism of conifers in alkalised growth conditions. Dissertation, Estonian Agricultural University. 183 p.
Košuthovà A., Svitkovà I., Pišùt I., Senko D., Valachovic M., Zaniewski P.T., Hàjek M. (2015). Climatic gradients within temperate Europe and small-scale species composition of lichen-rich dry acidophilous Scots pine forests. Fungal Ecology 14: 8–23. https://doi.org/10.1016/j.funeco.2014.10.005.
Kooijman A.M., Besse M. (2002). The higher availability of N and P in lime-poor than in lime-rich coastal dunes in the Netherlands. Journal of Ecology 90: 394–403.
Kutiel P., Zhevelev H., Harrison R. (1999). The effect of recreational impacts on soil and vegetation of stabilised Coastal Dunes in the Sharon Park, Israel. Ocean & Coastal Management 42(12): 1041–1060. https://doi.org/10.1016/S0964-5691(99)00060-5.
Leht M. (2010). Eesti taimede määraja. [Keybook of Estonian vascular plants]. Eesti Loodusfoto, Tartu. 447 p.
Lichter J. (1998). Primary succession and forest development on coastal Lake Michigan sand dunes. Ecological Monographs 68(4): 487–510. https://doi.org/10.2307/2657151.
Liu Z., Notaro M., Gallimore R. (2010). Indirect vegetation-soil moisture feedback with application to Holocene North Africa climate. Global Change Biology 16: 1733–1743.
Lõhmus E. (2004). Eesti metsakasvukohatüübid. [Estonian forest site types]. Eesti Loodusfoto, Tartu. 80 p.
Mandre M., Pärn H., Tilk M., Kõresaar P., Kõresaar K., Kösta H. (2006). Diversity of soil conditions and plant cover of Scots pine forest on coastal dunes in Southwest Estonia. Forestry Studies 44: 42–51.
Mandre M., Tilk M., Kõresaar P. (2008). Chemical characteristics of soils in Scots pine forests of Cladina and Vaccinium vitis-idaea site types on coastal dunes of Baltic Sea. Forestry Studies 49: 5–12. https://doi.org/10.2478/v10132-011-0058-x.
Manual for integrated monitoring (1993). Programme phase 1993–1996. Environmental report 5. Environment Data Centre National Board of Waters and the Environment, Helsinki.
Márialigeti S., Tinya F., Bidló A., Ódor P. (2016). Environmental drivers of the composition and diversity of the herb layer in mixed temperate forests in Hungary. Plant Ecology 217(5): 549–563. https://doi.org/10.1007/s11258-016-0599-4.
Marschner H. (2012). Mineral nutrition of higher plants. Third Edition. Elsevier Academic Press, London. 651 p.
Martinez M.L., Psuty N.P. (2004). Coastal dunes: ecology and conservation. Springer-Verlag, Berlin. 388 p. https://doi.org/10.1007/978-3-540-74002-5.
Masing V. (1979). Botaanika III. [Botany III]. Valgus, Tallinn. 414 p.
McCune B., Mefford M.J. (2011). PC-ORD Multivariate analysis of ecological data. Version 6. MjM Software, Oregon.
Mielke P.W., Berry K.J., Johnson E.S. (1976). Multi-response permutation procedures for a priori classications. Communications in Statistics - Theory and Methods 5(14): 1409–1424. https://doi.org/10.1080/03610927608827451.
Oliveros J. (2015). Venny. An interactive tool for comparing lists with Venn’s diagrams. http://bioinfogp.cnb.csic.es/tools/venny/index.html. [Cited 12 Jan 2017].
Örd A. (1972). Edela-Eesti luitemetsade mullastikust. [On soils of dune forests in South-western Estonia]. Metsanduslikud Uurimused 9: 207–221.
Örd A. (1973). Edela-Eesti luitemännikute tootlikkusest ja kasvukäigust. [On the productivity and growth process of dune pine-woods in south-western Estonia]. Metsanduslikud uurimused 10: 144–167.
Pausas J.G. (1994). Species richness patterns in the understorey of Pyrenean Pinus sylvestris forest. Journal of vegetation Science 5: 517–524.
Pausas J.G., Austin M.P. (2001). Patterns of plant species richness in relation to different environments: an appraisal. Journal of Vegetation Science 12: 153–166.
Pinna M.S., Cogoni D., Fenu G., Bacchetta G. (2015). The conservation status and anthropogenic impacts assessments of Mediterranean coastal dunes. Estuarine, Coastal and Shelf Science 167: 25–31. https://doi.org/10.1016/j.ecss.2015.07.002.
Raukas A. (1968). Eesti luiteliivade koostisest ja kihilisusest. [Composition and stratification of sand on dunes in Estonia]. In: Kildema K. (ed.). Eesti geograafiaseltsi aastaraamat 1966. Valgus, Tallinn. p. 72–87.
Reich P.B., Frelich L.E., Voldseth R.A., Bakken P., Adair E.C. (2012). Understorey diversity in southern boreal forests is regulated by productivity and its indirect impacts on resource availability and heterogeneity. Journal of Ecology 100: 539–545.
Ruocco M., Bertoni D., Sarti G., Ciccarelli D. (2014). Mediterranean coastal dune systems: Which abiotic factors have the most influence on plant communities? Estuarine, Coastal and Shelf Science 149: 213–222. https://doi.org/10.1016/j.ecss.2014.08.019.
Sewerniak P. (2016). Differences in early dynamics and effects of slope aspect between naturally regenerated and planted Pinus sylvestris woodland on inland dunes in Poland. iForest 9: 875–882.
Sewerniak P., Jankowski M., Dąbrowski M. (2017). Effect of topography and deforestation on regular variation of soils on inland dunes in the Toruń Basin (N Poland). Catena 149: 318–330. https://doi.org/10.1016/j.catena.2016.10.008.
Solon J., Degòrski M., Roo-Zielińska E. (2007). Vegetation response to a topographical-soil gradient. Catena 71(2): 309–320. https://doi.org/10.1016/j.catena.2007.01.006.
Tilk M., Mandre M., Klõšeiko J., Kõresaar P. (2011). Ground vegetation under natural stress conditions in Scots pine forests on fixed sand dunes in southwest Estonia. Journal of Forest Research 16(3): 223–227. https://doi.org/10.1007/s10310-011-0282-5.
Vasavada N. (2016). One-way ANOVA (ANalysisOf VAriance) with post-hoc Tukey HSD (Honestly Significant Difference) Test Calculator for comparing multiple treatments. http://astatsa.com/OneWay_Anova_with_TukeyHSD/. [Cited 26 June 2017].
Zheng H., Gao J., Teng Y., Feng C., Tian M. (2015). Temporal variations in soil moisture for three typical vegetation types in inner Mongolia, Northern China. PLoS ONE 10(3): e0118964. https://doi.org/10.1371/journal.pone.0118964.
Total of 53 references.

