Tetraploid production through zygotic chromosome doubling in Populus
Wang J., Shi L., Song S., Tian J., Kang X. (2013). Tetraploid production through zygotic chromosome doubling in Populus. Silva Fennica vol. 47 no. 2 article id 932. https://doi.org/10.14214/sf.932
Abstract
The most direct approach in breeding triploid Populus is crossing allotetraploids with diploids. However, the lack of allotetraploid Populus restricts application of this approach. In this investigation, zygotic chromosome doubling was induced with colchicine and high temperatures to produce ((Populus pseudo-simonii × P. nigra ‘Zheyin3#’) × (P. × beijingensis)) allotetraploids. We screened 6 and 25 tetraploid individual offspring from the colchicine and high-temperature treatments respectively, indicating that both colchicine and high temperature are effective for tetraploid production by zygotic chromosome doubling of Populus. Developmental characteristics of seed hairs in the ovaries were temporally associated with zygotic development, which was used to successfully guide the colchicine and high-temperature treatments. During certain stages of hair development, the efficiency of tetraploid production was significantly high. However, efficiency of production was not significantly influenced by other factors, i.e. colchicine concentration, temperature or duration of high-temperature treatment. Size and frequency of leaf stomata between tetraploid and diploid plants were significantly different, suggesting that this character can be altered via genomic increase in material. The allotetraploids produced in this investigation, having different genotypes, provide important parental germplasms for further triploid breeding.
Keywords
Populus;
colchicine;
high temperature;
seed hair;
tetraploid induction;
zygote
-
Wang,
National Engineering Laboratory for Tree Breeding; Key Laboratory of Genetics and Breeding in Forest Trees and Ornamental Plants, Ministry of Education; College of Biological Sciences and Technology, Beijing Forestry University, Beijing 100083, P.R. China
E-mail
wangjun@bjfu.edu.cn

- Shi, National Engineering Laboratory for Tree Breeding; Key Laboratory of Genetics and Breeding in Forest Trees and Ornamental Plants, Ministry of Education; College of Biological Sciences and Technology, Beijing Forestry University, Beijing 100083, P.R. China E-mail 7320932@qq.com
- Song, National Engineering Laboratory for Tree Breeding; Key Laboratory of Genetics and Breeding in Forest Trees and Ornamental Plants, Ministry of Education; College of Biological Sciences and Technology, Beijing Forestry University, Beijing 100083, P.R. China E-mail angela-song@foxmail.com
- Tian, National Engineering Laboratory for Tree Breeding; Key Laboratory of Genetics and Breeding in Forest Trees and Ornamental Plants, Ministry of Education; College of Biological Sciences and Technology, Beijing Forestry University, Beijing 100083, P.R. China E-mail 1215813245@qq.com
- Kang, National Engineering Laboratory for Tree Breeding; Key Laboratory of Genetics and Breeding in Forest Trees and Ornamental Plants, Ministry of Education; College of Biological Sciences and Technology, Beijing Forestry University, Beijing 100083, P.R. China E-mail kangxy@bjfu.edu.cn
Received 14 December 2012 Accepted 14 March 2013 Published 20 June 2013
Views 181124
Available at https://doi.org/10.14214/sf.932 | Download PDF

1 Introduction
The genus Populus L. is widely cultivated throughout the northern hemisphere, providing plenty of raw material for the wood industry. Recently, more attention has been paid to Populus species as a response to global climate change because of their biomass production and carbon fixation capabilities (Liberloo et al. 2006; Werner et al. 2012). In China, more than 7 million hectares of land is covered by poplars (Fang 2008), leading to a huge demand for varieties with good growth, stress resistance and timber quality. Although biotechnological techniques have been more and more frequently applied to improving poplar varieties (Yang et al. 2008; Lu and Hu 2011; Ye et al. 2011), cultivars are still produced through traditional breeding methods, such as hybridization and polyploid breeding (FAO 2008; FAO 2012).
Since the first triploid Populus individual with fast-growth and extremely large leaves was discovered in Sweden (Müntzing 1936; Nilsson-Ehle 1936), polyploid breeding programs have played an important role in Populus improvement and variety selection. Some triploid poplar cultivars, demonstrating advantages in growth, biomass production and pulpwood properties, have been widely used in plantations (Weisgerber et al. 1980; Zhu et al. 1995; Zhang et al. 2004; Zhang et al. 2005). Therefore, production of triploids has been of concern for the last several decades. Many approaches can be used to produce triploid poplars, e.g. pollination with spontaneous or induced diploid (2n) pollen (Johnsson and Eklundh 1940; Seitz 1954; Mashkina et al. 1989a; Zhu et al. 1995; Kang et al. 2000) and hybridization with natural or artificial 2n female gametes (Li et al. 2008; Zhang et al. 2009; Wang et al. 2010; Wang et al. 2012a, b). However, hybridization of tetraploid parents should be the most direct approach because of 2n gamete production from normal meiosis of the allotetraploid. Einspahr (1984) produced some triploid hybrids with favorable growth and wood properties through pollination of diploid P. tremuloides by tetraploid P. tremula. Baumeister (1980) selected a superior triploid hybrid named Astria from the offspring of tetraploid P. tremula and diploid P. tremuloides. This particular cultivar has a narrow crown, fast growth and good resistance to rust disease. Furthermore, female tetraploid aspens were even planted in superior stands as parents in order to produce offspring in some regions of Northern Europe and the USA (Xu 1988). However, spontaneous tetraploid plants are rarely found in nature, restricting the application of this approach.
Tetraploids are usually induced via mitotic inhibition in vitro (Dhooghe et al. 2010). Seeds, shoot tips and calli of poplars have been used to induce somatic chromosome doubling (Mattila 1961; Pesina 1963; Ewald et al. 2009), but a number of mixoploids formed along with the tetraploids. A zygote, as a single cell, should be ideal material for tetraploid induction. However, reports on zygotic chromosome doubling are rare (Mashkina et al. 1989b), because zygotic development, which is located inside the ovule, is difficult to detect. In this investigation, a method for instant detection of zygotic development of ((P. pseudo-simonii × P. nigra ‘Zheyin3#’) × (P. × beijingensis)) was formulated to guide treatment; zygotic chromosome doubling was induced with colchicine and high temperature to produce allotetraploids.
2 Materials and Methods
2.1 Plant materials
Floral branches of the female parent, Populus pseudo-simonii × P. nigra ‘Zheyin3#’ (2n = 2x = 38), were collected from a plantation in Tongliao City (Inner Mongolia Autonomous Region, People’s Republic of China). Floral branches of the male parent, Populus × beijingensis (2n = 2x = 38), were collected at the campus of Beijing Forestry University. The branches were water-cultured to force floral development in a greenhouse (10–20 °C) for later use.
2.2 Cytological observation of zygote development
After pollination the catkins of the ‘Zheyin3#’ were collected from the branches. A total of 18 catkins were dissected to observe the development of seed hairs, which was photographically recorded by a stereo-microscope (Olympus SZX12) with an Olympus C5060 Wide Zoom camera. According to the characteristics of hair development presented in Table 1, the catkins were classified and fixed in a fixative (70% ethanol: acetic acid: 40% formaldehyde, 90:5:5). Eight μm paraffin sections were cut at and stained with iron hematoxylin.
| Table 1. Relationship between seed hair and embryo development in ((Populus pseudo simonii × P. nigra ‘Zheyin3#’) × (P. × beijingensis)) ovaries. | ||||||||
| Defined stages | Developmental characteristics of seed hair | Percentage of each developmental stage (%) | ||||||
| Developing embryo sac | Mature embryo sac and double fertilization | Hypnozygote | 2-celled embryo | 4-celled embryo | 8-celled embryo | >8-celled embryo | ||
| I | No hair forms | 83.72 (36) a) | 16.28 (7) | |||||
| II | Development of the hair initiates around the funiculus | 23.68 (9) | 63.16 (24) | 13.16 (5) | ||||
| III | The hair gradually elongates to cover the funiculus | 36.36 (16) | 56.82 (21) | 6.82 (3) | ||||
| IV | The hair begins to enclose the ovules | 44.44 (20) | 37.78 (17) | 15.56 (7) | 2.22 (1) | |||
| V | The hair encloses the ovules completely, but does not fill the ovary | 9.52 (4) | 26.19 (11) | 38.10 (16) | 21.43 (9) | 4.76 (2) | ||
| VI | The hair fills the ovary | 18.37 (9) | 34.69 (17) | 46.94 (23) | ||||
| a) Number of observed ovules. | ||||||||
2.3 Treatment with colchicine solution and high temperatures
Based on the characteristics of seed hair development, pollinated female catkins at different stages were selected for treatment. Both colchicine and high temperature were used to induce zygotic chromosome doubling. For the colchicine treatment, the catkins, which still adhered to branches, were immersed in 0.3% and 0.5% colchicine solution for 24 h. After treatment, the catkins were rinsed with tap water to clean the residual colchicine. For the high-temperature treatment, the whole branches with catkins were exposed to 39 °C, 41 °C and 43 °C for 2 h, 4 h and 6 h. After treatment, all branches were water-cultured in the greenhouse until seed collection. Untreated buds were defined as the control group.
After the catkins matured, seeds were collected and germinated in sterile soil. When the seedlings grew to approximately 5 cm in height, they were transferred into containers with nutritious soil to promote growth. The seedlings were transplanted to the field site at approximately 30 cm height.
2.4 Ploidy level determination
Flow cytometric analysis is a reliable method for ploidy level determination of Populus (Wang et al. 2012b), so seedlings were first analyzed by flow cytometry to screen for tetraploids. After flow cytometric analysis, stem tips of the tetraploids were used to count somatic chromosomes to provide cytological evidence. Both flow cytometric analysis and somatic chromosome counting were conducted according to Wang et al. (2012b).
2.5 Stomata characteristic measurement
Thirty-one randomly selected diploids of (P. pseudo-simonii × P. nigra ‘Zheyin3#’) × P. × beijingensis were used to compare with the induced tetraploids. For stomata analysis, the lower epidermis from the middle portion of the tenth mature leaf from the shoot tip was peeled off and placed on a slide. To determine stomatal size, 40 randomly selected stomata per plant were measured microscopically using an ocular micrometer. Fifteen randomly selected microscopic field areas per plant were counted to obtain stomatal frequency.
2.6 Statistical analysis
The data of tetraploid production was analyzed using the Chi-Square test. Stomatal size and frequency data were analyzed with t-tests. Stomatal frequency data were log-transformed prior to the t-tests. All statistical analyses were performed in SPSS (version 18.0).
3 Results
3.1 Double fertilization and embryo development
Double fertilization of the ‘Zheyin3#’ was porogamous and occurred between 84 and 144 h after pollination because of asynchronous development. Pollen tubes entered the embryo sac through the micropyle and released two sperms in one synergid cell (Figs. 1a and b). After the degeneration of the receptive synergid, one sperm moved close to the egg (Fig. 1c) and the other close to the central cell. Subsequently, the other synergid cell degenerated, which was followed by double fertilization. One sperm each fused with the egg and central cell (Fig. 1d), resulting in the formation of a diploid zygote (Fig. 1e) and a triploid primary endosperm nucleus. The zygote began the first mitotic division 2–3 days after fertilization, forming a 2-celled embryo (Fig. 1f). Following that, the embryo developed into 4-celled (Fig. 1g), 8-celled (Fig. 1h), globular (Fig. 1i), heart-shaped, torpedo-shaped and cotyledon-stage embryos successively, until maturation was reached approximately 30d after pollination.
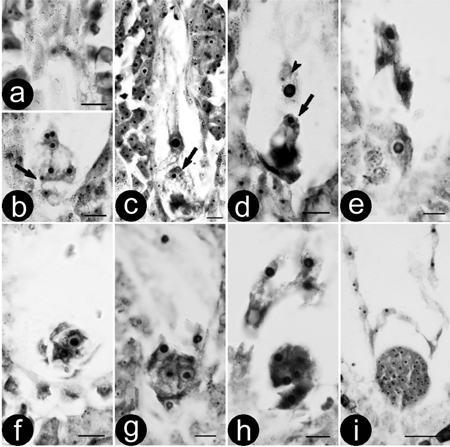
Fig. 1. Double fertilisation and zygote development of (P. pseudo-simonii × P. nigra ‘Zheyin3#’) × (P. × beijingensis). a-b) Serial sections showing a mature embryo sac and two sperms (arrow); c) Movement of the sperm, arrow showing a sperm is close to the egg; d) Double fertilisation, one sperm is fusing to the egg (arrow) and the other to central cell (arrow head); e) Zygote; f) Two-celled embryo; g) Four-celled embryo; h) Eight-celled embryo; i) Globular embryo. Bars are equal to 10 μm in a–h and 50 μm in i.
In the ovary, completion of double fertilization was accompanied by the initiation of seed hair growth around the funicle. The relationship between embryo development and developmental characteristics of the seed hair was established (Table 1), this can be used to guide treatment methods of tetraploid induction. Six stages corresponding to the characteristics of seed hair development were defined. In a stage I ovary, no hair formed around the funicles (Fig. 2a), corresponding to embryo sac development. A stage II ovary was characterized by the initiation of hair (Fig. 2b), corresponding to fertilization. In a stage III ovary, the hair elongated and covered the funiculus (Fig. 2c). Zygotes had formed in many ovules at this stage. Ovaries were considered stage IV once the hair had begun to enclose the ovules (Fig. 2d). More than half of the zygotes had developed into 2-celled embryos, some were 8-celled embryos. At stage V, hair enclosed the ovules completely, but did not fill the ovary (Fig. 2e). 90.48% of zygotes started their embryo development. When the hair filled the ovary (stage VI, Fig. 2f), all zygotes had developed into embryos with more than 4 cells.

Fig. 2. Development of seed hairs in the ovaries: a) Stage I ovary without hair formation; b) Stage II ovary, indicating initiation of hairs around the funiculus; c) Stage III ovary, characterised by hair elongation gradually covering the funiculus; d) Stage IV ovary with hairs enclosing the ovules; e) Stage V ovary not filled by hairs, but completely enclosed ovules; f) Stage VI ovary filled by hairs.
3.2 Tetraploid production
Colchicine and high-temperature treatments produced 6 and 25 tetraploids (2n = 4x = 76, Fig. 3), respectively. No tetraploid was found in the control group, suggesting that hybridization between the ‘Zheyin3#’ and the P. × beijingensis rarely produces tetraploids.
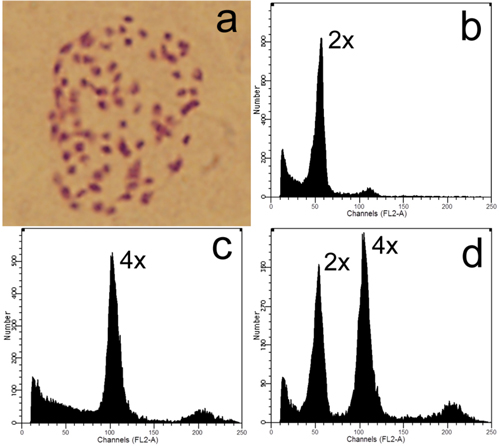
Fig. 3. Ploidy level detection of offspring derived from zygotic chromosome doubling: a) Somatic chromosome of a tetraploid (2n = 4x = 76); b–c) Flow cytometric analyses of a diploid and tetraploid, respectively; d) Histogram of flow cytometric analysis of nuclei mixture of young leaves from the former diploid and tetraploid plants.
While colchicine treatments induced tetraploid production (Table 2), the efficiency of tetraploid production differed among the treatments. The highest frequency of tetraploid induction was 3.13% in a treatment of 0.5% colchicine for 24 h at stage III. Although tetraploids were just induced at stages II and III, no significant differences were found by Chi-Square tests among the seed hair development stages (P = 0.603) and colchicine concentrations (P = 0.571).
| Table 2. Tetraploid production through colchicine-induced zygotic chromosome doubling in (P. pseudo-simonii × P. nigra ‘Zheyin3#’) × (P. × beijingensis) hybrids. | |||||||
| Stage of seed hair development | Concentration of colchicine solution (%) | Duration of treatment (h) | No. of catkins | No. of seeds | No. of seedlings | No. of tetraploids | Efficiency of tetraploid production (%) |
| II | 0.3 | 24 | 2 | 124 | 58 | 1 | 1.72 |
| 0.5 | 24 | 2 | 108 | 39 | 1 | 2.56 | |
| III | 0.3 | 24 | 3 | 248 | 174 | 2 | 1.15 |
| 0.5 | 24 | 2 | 104 | 64 | 2 | 3.13 | |
| IV | 0.3 | 24 | 2 | 36 | 6 | 0 | 0 |
| 0.5 | 24 | 2 | 78 | 28 | 0 | 0 | |
| V | 0.3 | 24 | 2 | 85 | 27 | 0 | 0 |
| 0.5 | 24 | 2 | 79 | 37 | 0 | 0 | |
| Control | 2 | 417 | 240 | 0 | 0 | ||
Table 3 presents the efficiency of tetraploid production through high-temperature-induced zygotic chromosome doubling. The highest rate of tetraploid production was up to 7.14%, which happened in the 43 °C for 2 h treatment at stage IV. A significant difference was found by Chi-Square test among the seed hair development stages (P = 0.017), indicating the importance of stage selection in tetraploid production. As far as tetraploid production goes, efficiency was highest during seed hair development stage IV, meaning it was more suitable for high-temperature-induced zygotic chromosome doubling than the other stages. There were no significant differences among different temperatures (P = 0.502) and durations of treatment (P = 0.899). However, many catkins were killed at 43 °C, causing no seed was collected in some treatments.
| Table 3. Tetraploid production through high-temperature-induced zygotic chromosome doubling in (P. pseudo-simonii × P. nigra ‘Zheyin3#’) × (P. × beijingensis) hybrids. | |||||||
| Stage of seed hair development | Temperature (°C) | Duration of treatment (h) | No. of catkins | No. of seeds | No. of seedlings | No. of tetraploids | Efficiency of tetraploid production (%) |
| II | 39 | 2 | 2 | 132 | 74 | 0 | 0 |
| 4 | 3 | 172 | 84 | 0 | 0 | ||
| 6 | 2 | 153 | 72 | 1 | 1.39 | ||
| 41 | 2 | 3 | 217 | 112 | 0 | 0 | |
| 4 | 3 | 232 | 122 | 1 | 0.82 | ||
| 6 | 4 | 341 | 295 | 1 | 0.34 | ||
| 43 | 2 | 2 | 106 | 32 | 0 | 0 | |
| 4 | 2 | 24 | - | - | - | ||
| 6 | 2 | 17 | 2 | 0 | 0 | ||
| III | 39 | 2 | 3 | 208 | 137 | 3 | 2.19 |
| 4 | 3 | 254 | 142 | 2 | 1.41 | ||
| 6 | 2 | 131 | 81 | 2 | 2.47 | ||
| 41 | 2 | 3 | 219 | 120 | 0 | 0 | |
| 4 | 2 | 137 | 42 | 1 | 2.38 | ||
| 6 | 2 | 147 | 44 | 2 | 4.55 | ||
| 43 | 2 | 2 | 114 | 26 | 0 | 0 | |
| 4 | 2 | 13 | - | - | - | ||
| 6 | 2 | - | - | - | - | ||
| IV | 39 | 2 | 2 | 80 | 21 | 0 | 0 |
| 4 | 2 | 155 | 92 | 1 | 1.09 | ||
| 6 | 2 | 177 | 118 | 3 | 2.54 | ||
| 41 | 2 | 2 | 149 | 97 | 2 | 2.06 | |
| 4 | 2 | 73 | 23 | 1 | 4.35 | ||
| 6 | 2 | 74 | 36 | 0 | 0 | ||
| 43 | 2 | 2 | 101 | 27 | 2 | 7.41 | |
| 4 | 2 | 24 | - | - | - | ||
| 6 | 2 | - | - | - | - | ||
| V | 39 | 2 | 3 | 182 | 80 | 0 | 0 |
| 4 | 2 | 130 | 53 | 1 | 1.89 | ||
| 6 | 2 | 79 | 22 | 0 | 0 | ||
| 41 | 2 | 2 | 138 | 77 | 1 | 1.3 | |
| 4 | 2 | 83 | 22 | 0 | 0 | ||
| 6 | 2 | 105 | 50 | 0 | 0 | ||
| 43 | 2 | 2 | 125 | 86 | 1 | 1.16 | |
| 4 | 2 | 57 | 14 | 0 | 0 | ||
| 6 | 2 | - | - | - | - | ||
| Control | 2 | 476 | 256 | 0 | |||
3.3 Stomatal comparison between diploid and tetraploid plants
Stomatal characteristics of 31 individuals of each ploidy level, including length, width and frequency, were recorded and compared (Fig. 4). The range of variation for stomata length, width and frequency was large among individuals of the same ploidy level (Table 4), which indicated character segregation was common in the F1 generation. Although the ranges intersected slightly, t-tests revealed that all of the stomatal characteristics were significantly different between tetraploids and diploids (t = 14.350, P < 0.0001 in stomata length; t = 10.178, P < 0.0001 in stomata width; and t = 11.142, P < 0.0001 in stomatal frequency, respectively).
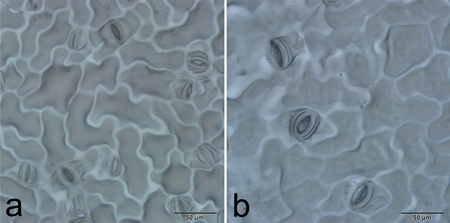
Fig. 4. Stomatal size and frequency of diploid (a) and tetraploid (b) plants.
| Table 4. Stomatal characteristics of diploid and tetraploid (P. pseudo-simonii × P. nigra ‘Zheyin3#’) × (P. × beijingensis) offspring. | ||||||
| Characters | Tetraploids | Diploids | t-value | P-value | ||
| Range | Mean ± SE | Range | Mean ± SE | |||
| Stomata length (μm) | 31.9–42.6 | 37.6 ± 0.4 | 24.3–33.2 | 28.9 ± 0.5 | 14.350** | <0.0001 |
| Stomata width (μm) | 18.0–23.5 | 20.4 ± 0.2 | 15.0–19.8 | 17.1 ± 0.2 | 10.178** | <0.0001 |
| Stomatal frequency (mm–2) | 57.7–180.6 | 113.6 ± 5.4 | 146.7–294.3 | 219.6 ± 7.7 | 11.142** | <0.0001 |
| ** Significant differences between diploids and tetraploids at α = 0.01 in t-test. | ||||||
4 Discussion
In plants, tetraploids can be induced by treating seeds, shoots, somatic embryos, calli and protoplasts with chemical mutagenic agents (Särkilahti and Valanne 1990; Kadota and Niimi 2002; Zeng et al. 2006; Ewald et al. 2009; Dhooghe et al. 2010; Kanchanapoom and Koarapatchaikul 2012). In Populus, Mattila (1961) treated seeds of P. tremula × P. tremuloides with 0.1% colchicine solution for 1 h before sowing, resulting in some tetraploid seedlings. Ewald et al. (2009) produced tetraploid poplar plants by inducing chromosome doubling using colchicine on the shoot tips in vitro. Nevertheless, because it is difficult to induce chromosome doubling for all cells in a multicellular tissue, mixoploids, which are unstable because of the asynchronous cell cycles of the two types of cells, were common (Mergen and Lester 1961; Nilanthi et al. 2009). In addition, all tetraploids from the same explant have the same genotype. In this investigation, chromosome doubling was induced in unicellular zygotes, avoiding mixoploid production and producing 31 tetraploids with different genotypes. This demonstrates, therefore, that the zygote is ideal material for tetraploidy germplasm production.
Both colchicine and high temperature are usually used to induce polyploids in plants (Randolph 1932; Särkilahti and Valanne 1990; Zhang et al. 2002; Dhooghe et al. 2010). In the genus Populus, colchicine has been used to successfully induce 2n pollen, 2n eggs and somatic chromosome doubling (Mattila 1961; Kang et al. 2000; Ewald et al. 2009; Wang et al. 2010), producing both triploids and tetraploids. Recently, Wang et al. (2012a, b) reported 2n female gamete induction with high-temperature treatment during megasporogenesis and embryo sac development, resulting in the production of a number of triploid plants. In the present study, both colchicine and high-temperature treatments produced tetraploid plants, suggesting that both are effective for zygotic chromosome doubling. The suitable stage of seed hair development for guiding the colchicine treatment (stage II and III) was earlier than that for the high-temperature treatment (stage III and IV), likely because of the slower transfer of liquid than temperature in the air-filled ovary (Wang et al. 2012b).
In Populus ovaries, seed hairs originate from epidermal cells of the funiculi. Previous studies found that fertilization was not necessary to hair cell initiation and that development of hair cells was not associated with seed development (Nagaraj 1952; Fechner 1972). However, a temporal relationship between seed hair development and zygotic development in the ovary was observed in the present study. Although the mechanism of hair cell formation is uncertain, the relationship still provides a viable and instant tool to guide effective treatments for zygotic chromosome doubling.
Stomatal characteristics, including size, frequency and chloroplast number, are commonly used in determining ploidy levels in plants (Ewald et al. 2009; Sun et al. 2009; Zhang et al. 2010). Ewald et al. (2009) reported chloroplast counting as an effective and reliable method for polyploid prescreening in Populus. Both stomatal size and frequency, in our investigation, exhibited highly significant differences between tetraploid and diploid plants, indicating that stomatal characteristics might be reliable enough for polyploid confirmation. Although chromosome counting is widely considered the most precise method for ploidy level detection, stomatal characteristics should be given preference for polyploid pre-screening of large numbers of plants because of its simple operation and high accuracy.
In China, some triploid cultivars of Populus, e.g. P. × canadensis ‘Zhonglin-46’, P. × canadensis ‘Sacrau 79’, P. × liaohenica, P. × langfangensis-3 and triploid clones of P. tomentosa, have been widely deployed in artificial plantations because of their good growth properties and pulpwood characteristics. In order to obtain triploid plants of Populus, unilateral sexual polyploidization via induced 2n male or female gametes has been widely studied (Mashkina et al. 1989a; Kang et al. 2000; Li et al. 2008; Wang et al. 2010; Wang et al. 2012a, b). More than 60% triploid production efficiency can be achieved via hybridization with induced 2n female gametes (Wang et al. 2010; Wang et al. 2012b). However, crossing allotetraploids with diploids is still the most direct approach. In our investigation, production of allotetraploids with different genotypes provided sufficient amounts of parental germplasm for further triploid breeding.
To date, reports on the use of Populus tetraploid clones in forest plantations are rare. However, tetraploid cultivars of other forest trees, such as Robinia pseudoacacia, have performed well in terms of wood production and foraging properties (Li and Jiang 2006), suggesting that selection of allotetraploid poplar with high heterosis is a possibility. Production of the 31 allotetraploid plants and development of the method for allotetraploid induction presented in this investigation provide valuable prospect for the selection of tetraploid cultivars in Populus.
Acknowledgements
We thank the Forestry Research Institute of Tongliao City, the Inner Mongolia Autonomous Region, People’s Republic of China, for collecting the plant material and for additional help. This work was supported by grants from the National Natural Science Foundation of China (grant number 31000306) and a Foundation for the Author of National Excellent Doctoral Dissertation of PR China (grant number 201267).
References
Baumeister G. (1980). Beispiele der polyploidie-Züchtung. Allgemeine Forestzeitschrift 35: 697–699.
Dhooghe E., Van Laere K., Eeckhaut T., Leus L., Van Huylenbroeck J. (2010). Mitotic chromosome doubling of plant tissues in vitro. Plant Cell, Tissue and Organ Culture 104: 359–373.
Einspahr D.W. (1984). Production and utilization of triploid hybrid aspen. Iowa State Journal of Research 58: 401–409.
Ewald D., Ulrich K., Naujoks G., Schröder M.B. (2009). Induction of tetraploid poplar and black locust plants using colchicine: chloroplast number as an early marker for selecting polyploids in vitro. Plant Cell, Tissue and Organ Culture 99: 353–357.
Fang S.Z. (2008). Silviculture of poplar plantation in China: a review. Chinese Journal of Applied Ecology 19: 2308–2316.
FAO. (2008). Synthesis of country progress reports. Food and Agriculture Organization of the United Nations: the 23rd session of the International Poplar Commission, Beijing.
FAO. (2012). Synthesis of country progress reports. Food and Agriculture Organization of the United Nations: the 24rd session of the International Poplar Commission, Dehradun.
Fechner G.H. (1972). Development of the pistillate flower of Populus tremuloides following controlled pollination. Canadian Journal of Botany 50: 2503–2509.
Johnsson H., Eklundh C. (1940). Colchicine treatment as a method in breeding hardwood species. Svensk Papperstidning 43: 373–377.
Kadota M., Niimi Y. (2002). In vitro induction of tetraploid plants from a diploid Japanese pear cultivar (Pyrus pyrifolia N. cv. Hosui). Plant Cell Reports 21: 282–286.
Kanchanapoom K., Koarapatchaikul K. (2012). In vitro induction of tetraploid plants from callus cultures of diploid bananas (Musa acuminata, AA group) ‘Kluai Leb Mu Nang’ and ‘Kluai Sa’. Euphytica 183: 111–117.
Kang X.Y., Zhu Z.T., Zhang Z.Y. (2000). Breeding of triploids by the reciprocal crossing of Populus alba × P. glandulosa and P. tomentosa × P. bolleana. Journal of Beijing Forestry University 22: 8–11.
Li Y., Jiang J. (2006). Research progress of feed tetraploid black locust. Pratacultural Science 23: 41–46.
Li Y.H., Kang X.Y., Wang S.D., Zhang Z.H., Chen H.W. (2008). Triploid induction in Populus alba × P. glandulosa by chromosome doubling of female gametes. Silvae Genetica 57: 37–40.
Liberloo M., Calfapietra C., Lukac M., Godbold D., Luo Z.B., Polle A., Hoosbeek M.R., Kull O., Marek M., Raines C., Rubino M., Taylor G., Scarascia-Mugnozza G., Ceulemans R. (2006). Woody biomass production during the second rotation of a bio-energy Populus plantation increases in a future high CO2 world. Global Change Biology 12: 1094–1106.
Lu M.Z., Hu J.J. (2011). A brief overview of field testing and commercial application of transgenic trees in China. BMC Proceedings 5: O63.
Mashkina O.S., Burdaeva L.M., Belozerova M.M., V’yunova L.N. (1989a). Method of obtaining diploid pollen of woody species. Lesovedenie 1: 19–25.
Mashkina O.S., Burdaeva L.M., V’yunova L.N. (1989b). Experimental mutagenesis and polyploidy in breeding forest trees. Lesnaya Genetika, Lesnaya genetika, selektsiya i fiziologiya drevesnykh rasteniĭ, Voronezh, 25–30 sentyabrya, 1989: Materialy Mezhdunarodnogo simpoziuma. p. 136–137. http://www.cabdirect.org/abstracts/19901696883.html. [Cited 8 Feb 2013].
Mattila R.E. (1961). On the production of the tetraploid hybrid aspen by colchicines treatment. Hereditas 47: 631–640.
Mergen F., Lester D.T. (1961). Colchicine induced polyploidy in Abies. Forest Science 7: 314–319.
Müntzing A. (1936). The chromosomes of a giant Populus tremula. Hereditas 21: 383–393.
Nagaraj M. (1952). Floral morphology of Populus deltoides and P. tremuloides. Botanical Gazette 114: 222–243.
Nilanthi D., Chen X.L., Zhao F.C., Yang Y.S., Wu H. (2009). Induction of tetraploids from petiole explants through colchicine treatments in Echinacea purpurea L. Journal of Biomedicine and Biotechnology 2009: 343485. doi:10.1155/2009/343485.
Nilsson-Ehle H. (1936). Note regarding the gigas form of Populus tremula found in nature. Hereditas 21: 372–382.
Pesina K. (1963). Experimental induction of polyploidy in poplars. Preslia 35: 101–109.
Randolph L.F. (1932). Some effects of high temperature on polyploidy and other variations in maize. Proceedings of the National Academy of Sciences, USA 18: 222–229.
Särkilahti E., Valanne T. (1990). Induced polyploidy in Betula. Silva Fennica 24: 227–234.
Seitz F.W. (1954). The occurrence of triploids after self-pollination of anomalous androgynous flowers of a grey poplar. Zeitschrift für Forstgenetik und Forstpflanzenzüchtung 3: 1–6.
Sun Q.R., Sun H.S., Li L.G., Bell R.L. (2009). In vitro colchicine-induced polyploid plantlet production and regeneration from leaf explants of the diploid pear (Pyrus communis L.) cultivar ‘Fertility’. Journal of Horticultural Science & Biotechnology 84: 548–552.
Wang J., Kang X., Li D., Chen H., Zhang P. (2010). Induction of diploid eggs with colchicine during embryo sac development in Populus. Silvae Genetica 59: 40–48.
Wang J., Kang X.Y., Li D.L. (2012a). High temperature-induced triploid production during embryo sac development in Populus. Silvae Genetica 61: 85–93.
Wang J., Li D., Kang X. (2012b). Induction of unreduced megaspores with high temperature during megasporogenesis in Populus. Annals of Forest Science 69: 59–67.
Weisgerber H., Rau H.M., Gartner E.J., Baumeister G., Kohnert H., Karner L. (1980). 25 years of forest tree breeding in Hessen. Allgemeine Forstzeitschrift 26: 665–712.
Werner C., Haas E., Grote R., Gauder M., Graeff-Hönninger S., Claupein W., Butterbach-Bahl K. (2012). Biomass production potential from Populus short rotation systems in Romania. GCB Bioenergy 4: 642–653.
Xu W.Y. (1988). Poplar. Heilongjiang People’s Press, Harbin.
Yang L., Sun Y., Xie L. (2008). Reviews on transgenic poplar and biosafety evaluation. Molecular Plant Breeding 6: 123–127.
Ye X., Busov V., Zhao N., Meilan R., McDonnell L.M., Coleman H.D., Mansfield S.D., Chen F., Li Y., Cheng Z.M. (2011). Transgenic Populus trees for forest products, bioenergy, and functional genomics. Critical Reviews in Plant Sciences 30: 415–434.
Zeng S., Chen C., Hong L., Liu J., Deng X. (2006). In vitro induction, regeneration and analysis of autotetraploids derived from protoplasts and callus treated with colchicine in Citrus. Plant Cell, Tissue and Organ Culture 87: 85–93.
Zhang S., Qi L., Chen C., Li X., Song W., Chen R., Han S. (2004). A report of triploid Populus of the section Aigeiros. Silvae Genetica 53: 69–75.
Zhang S.G., Chen C.B., Han S.Y., Li X.L., Ren J.Z., Zhou Y.Q., Song W.Q., Chen R.Y., Qi L.W. (2005). Chromosome numbers of some Populus taxa from China. Acta Phytotaxonomica Sinica 43: 539–544.
Zhang X.Z., Liu G.J., Yan L.Y., Zhao Y.B., Chang R.F., Wu L.P. (2002). Creating triploid germplasm via induced 2n pollen in Capsicum L. Journal of Horticultural Science & Biotechnology 78: 84–88.
Zhang J.F., Wei Z.Z., Li D., Li B.L. (2009). Using SSR markers to study the mechanism of 2n pollen formation in Populus × euramericana (Dode) Guinier and P. × popularis. Annals of Forest Science 66: 53–62.
Zhang Q.Y., Luo F.X., Liu L., Guo F.C. (2010). In vitro induction of tetraploids in crape myrtle (Lagerstroemia indica L.). Plant Cell, Tissue and Organ Culture 101: 41–47.
Zhu Z.T., Lin H.B., Kang X.Y. (1995). Studies on allotriploid breeding of Populus tomentosa B301 clones. Scientia Silvae Sinicae 31: 499–505.
Total of 44 references

