Effects of clear-cutting and slash removal on soil water chemistry and forest-floor vegetation in a nutrient optimised Norway spruce stand
Hedwall P.-O., Grip H., Linder S., Lövdahl L., Nilsson U., Bergh J. (2013). Effects of clear-cutting and slash removal on soil water chemistry and forest-floor vegetation in a nutrient optimised Norway spruce stand. Silva Fennica vol. 47 no. 2 article id 933. https://doi.org/10.14214/sf.933
Abstract
Fertilisation with nutrient optimisation has in Sweden resulted in large increases in volume growth in young stands of Norway spruce. There are, however, environmental concerns about repeated fertilisation and one is the risk of nutrient leakage to ground water resources and aquatic ecosystems after clear-cutting of such forests. The present study followed soil-water chemistry in optimised fertilised stands after clear-cutting, as well as effects of harvest of slash on nutrient leakage. Parts of a 30-year-old stand of Norway spruce, which had been subject to a nutrient optimisation experiment for 17 years, were clear-cut. A split-plot design with whole-tree harvesting as the sub-plot treatment was applied. Lysimeters were installed and soil-water sampled at nine occasions during the following four years. No significant effects of fertilisation on nitrate leaching were found, while harvest of slash reduced the concentration of Ca, DOC, DON, K, Mg, ammonium and nitrate, as well as pH in the soil solution. While no effects of fertilisation could be seen on the soil water concentration of N, the results indicate an interaction between fertilisation and harvest of slash on the concentration of nitrate in the soil solution. The results indicate that forest-floor vegetation plays an important role in the retention of N after clear-cutting of fertilised forests.
Keywords
Picea abies;
logging residues;
forest fertilisation;
forest undergrowth;
N-retention;
nutrient leakage;
whole-tree harvest
-
Hedwall,
Southern Swedish Forest Research Centre, Swedish University of Agricultural Sciences (SLU), P.O. Box 49, SE-230 53 Alnarp, Sweden
E-mail
per-ola.hedwall@slu.se

- Grip, Department of Forest Ecology and Management, SLU, SE-901 83 Umeå, Sweden E-mail harald@grip2.se
- Linder, Southern Swedish Forest Research Centre, Swedish University of Agricultural Sciences (SLU), P.O. Box 49, SE-230 53 Alnarp, Sweden E-mail sune.linder@slu.se
- Lövdahl, Department of Forest Ecology and Management, SLU, SE-901 83 Umeå, Sweden E-mail ll@nn.se
- Nilsson, Southern Swedish Forest Research Centre, Swedish University of Agricultural Sciences (SLU), P.O. Box 49, SE-230 53 Alnarp, Sweden E-mail urban.nilsson@slu.se
- Bergh, Southern Swedish Forest Research Centre, Swedish University of Agricultural Sciences (SLU), P.O. Box 49, SE-230 53 Alnarp, Sweden E-mail johan.bergh@slu.se
Received 20 December 2012 Accepted 27 March 2013 Published 19 June 2013
Views 175971
Available at https://doi.org/10.14214/sf.933 | Download PDF

1 Introduction
An increasing demand for forest products and enhanced interest of mitigating climate change by using forest products to substitute fossil fuel and energy demanding building materials (e.g. cement, steel, aluminium), has recently lead to an enhanced focus on risks and benefits of intensive forestry (Nordin et al. 2011). Conventional forest fertilisation is usually applied as one to three applications of nitrogen (N) during the second half of the rotation period, which normally increases wood production by 13–20 m3 ha–1 during the following 8–10 years (Pettersson and Högbom 2004). Fertilisation with the principle of nutrient optimisation in young Norway spruce stands (Picea abies (L.) Karst.) has, however, shown a large potential to increase the production significantly more (Bergh et al. 1999; Bergh et al. 2005).
Boreal ecosystems are generally limited by N (Tamm 1991) and the N retention capacity is usually considerable. Even though a large fraction of the N applied in fertilisation is integrated into tree biomass about two thirds remain in the soil (Melin et al. 1983). One of the environmental impacts of forest fertilisation that has been debated vividly is the risk for nutrient leakage to ground water and aquatic ecosystems. There is an extensive amount of work done on the impact of traditional fertilisation on the environment in general, and especially on nutrient leakage (cf. Nohrstedt 2001), although with rather few studies on the effects of clear-cut on leakage in previously fertilised forests. Considerably less has been done on the effects of nutrient optimisation (see, however, Bergh et al. 2008) and the question of the effect of clear-cutting fertilised forests is therefore at the moment of great concern (e.g. Skogsstyrelsen 2007; Nordin et al. 2009).
There are a few studies of nutrient leakage after clear-cut in previous fertilisation experiments with large doses of N. On a relatively poor Scots pine (Pinus sylvestris L.) site in central Sweden clear-cutting enhanced nitrate leaching when the total amount of added N exceeded 1440 kg N ha–1 (Nohrstedt et al. 1994; Ring 1996). This increase in nitrate levels was first noticed five years after the clear-cut (Ring 1996) and lasted for at least another eight years (Ring 2004). In contrast, a few studies on richer Norway spruce sites found increasing nitrate concentrations in soil water instantly after clear-cut (Berdén et al. 1997; Smolander et al. 1998; Ring et al. 2003). On the least productive site among these studies, however, a significant effect on nitrate concentrations was only found when an amount of 1700 kg N ha–1 had been added (Berdén et al. 1997).
Harvest of slash (small woody residues, branches, and foliage) for bio-energy has become a common practise in Swedish forestry. Slash can, when having a high C/N-ratio, reduce the risk of leaching through immobilisation of N (Berg and Ekbohm 1983; Palviainen et al. 2004). Removal of slash in connection with clear-cutting has, however, been shown to reduce the risk of N-leakage, both in fertilised forests and those exposed to high anthropogenic N-deposition (Lundborg 1997). Besides that, removal of slash reduces the N storage in the forest ecosystem, it is also considered to influence N mineralisation through effects on the soil micro-climate (Roberts et al., 2005) and to obstruct the establishment of forest-floor vegetation (Fahey et al. 1991), which could reduce or prevent N leakage (Rosén and Lundmark-Thelin 1987).
In the Nordic boreal forest late successional species (e.g. Vaccinium spp.), can dominate the pre-harvest forest-floor vegetation. Commonly, as a result of clear-cut of the forest the biomass of these species decreases (Palviainen et al. 2005a) and early successional species (e.g. graminoids) increase in abundance (Bergstedt and Milberg 2001; Palviainen et al. 2005b). The N-retention capacity of the forest-floor vegetation can, however, be expected to be reduced in forests with a closed canopy, because of low amounts of forest floor vegetation (Berdén 1994; Hedwall et al. 2010). Subsequently, there is an increased risk of N leakage when such forests are clear-cut since the forest-floor vegetation initially has a low capacity of N retention (Emmet et al. 1991).
The main objective of this study was to analyse post-harvest effects on soil water chemistry in nutrient optimised stands and the effect of slash and forest-floor vegetation on nutrient leakage. We tested the following hypotheses: (i) as an effect of fertilisation nutrient leakage increases after clear-cut, (ii) removal of slash reduces nutrient leakage in fertilised stands, although not to the level of non-fertilised stands, (iii) uptake of N in forest-floor vegetation significantly decreases N leakage and, (iv) slash hampers the development of forest-floor vegetation, and thus affects the leakage of N and other nutrient elements.
2 Material and methods
2.1 Site description
This study was performed in a long-term nutrient optimisation experiment in Asa, southern Sweden (57°08ʹN, 14°45ʹE, 225–250 m a.s.l.). The experiment was established in 1987, in a Norway spruce (Picea abies (L.) Karst.) stand planted in 1975 with two-year-old seedlings. The soil is a podsol formed in a stony shallow (< 1 m) glacial silt loam till overlaying granite bedrock situated above the highest postglacial shore line. The O horizon was 2.5 cm and the E horizon 5 cm. General constraint when the site was selected was that it should be representative for large parts of southern Sweden concerning climate and soil type. Other constraints were the need for a young, large, even aged and homogeneous spruce stand, with access to irrigation water and reasonable short distance to maintenance staff.
The growing season at the site is about 190 days, the mean air temperature during the growing season 11.5 °C, and the mean annual precipitation ca 700 mm (Bergh et al. 1999). At the start of the treatments in 1987, the stand density was ca. 2400 stems ha–1 and the site productivity was classified according to Hägglund and Lundmark (1977) as G32–G34 (top height in metres of the largest trees at age 100 years). The treatments included untreated controls, irrigation, and two nutrient optimisation treatments in a fully randomised design with four replicates consisting of 50 x 50 m plots (cf. Bergh et al. 1999). In the present study only untreated control plots (C) and one nutrient optimisation treatment (annual supply of a complete solid fertiliser) were included. From the start of the experiment in 1987 to 2003 a total of 1000 kg N ha–1 had been added, with other nutrients in proportion to N (cf. Linder 1995). During the same period the deposition of N was approximately 9 kg ha–1 yr–1 (SMHI 2009).
2.2 Experimental design
During the winter 2003/2004, two fertilised (F) and two control (C) plots were harvested. These adjacent plots were randomised in the original experimental design and were selected due to similar soil conditions and the opportunity to get a large clear-cut area with minimal edge effects. Before the clear-cut, the stands were measured and tree volume estimated for each plot. A split-plot design, with fertilisation as the main-plot treatment, with two replicates and removal of slash, with eight replicates as the sub-plot treatment, was applied in 25 x 25 m plots (cf. Fig. 1). The location of sub-plot treatments was randomised within main-plots. The harvest was either conventional (CH) where the slash was left on the plot or a whole-tree harvest (WTH). In the plots subjected to whole-tree harvest about 20% of the slash remained. At the time of harvest both the control and the fertilised stands had a closed canopy and therefore the biomass of forest-floor vegetation was close to negligible.
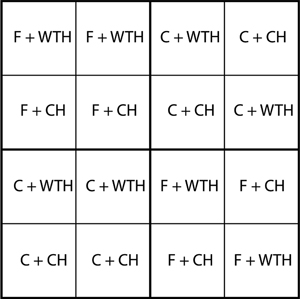
Fig. 1. Schematic map of the experiment showing the four main plots, 16 sub-plots, and four combinations of treatments: non-fertilised + conventional harvest (C + CH), non-fertilised + whole-tree harvest (C + WTH), fertilised + conventional harvest (F + CH), and fertilised + whole-tree harvest (F + WTH).
2.3 Soil-water sampling and analyses
In 1990, ceramic cup suction lysimeters (P80) were installed at 0.5 m depth in the mineral soil in the original treatments. These were replaced after the harvest by five new suction lysimeters in each subplot (a total of 80). Soil water was thereafter sampled at nine occasions from spring 2004 until autumn 2007. The soil water samples were pooled within subplot and sampling occasion and kept frozen until analysed.
Dissolved organic carbon (DOC) in the bulked soil solutions was analysed by means of a total organic carbon analyser (TOC-5000, Shimadzu, Tokyo, Japan). An ion chromatograph (Dionex model 4000i, Dionex Corporation, Sunnyvale, CA, USA) was used to determine nitrate and flow-injection analysis was used to determine ammonium and phosphate (Model 5012, Tecator, Höganäs, Sweden). Total N was determined after wet digestion by K2S2O8. Dissolved organic nitrogen (DON) was calculated as the difference between total N and inorganic-N. An ICP-MS (Elan 6100, PerkinElmer, Norwalk, CT, USA) was used to measure K, Mg, Ca and Al. In most cases, the analytical error was less than ±10% of reported values. The chemical analysis on soil water was done on unfiltered samples.
Daily water balance for both the intact control (C) and fertilised (F) stands, and the clear-cut treatments was simulated by the CoupModel (ver. 4; Jansson and Karlberg 2010), designed to simulate water, energy, carbon and nitrogen processes in an ecosystem. Depending on settings, the coupled model can be run in a number of ways. In the present case we concentrated on water properties and used measured stand height and leaf area index (LAI) to define surface properties in the Penman combination equation (Monteith 1965). Parameters describing the soil physical properties, such as hydraulic conductivity and soil moisture characteristics were derived from field soil sampling and laboratory analysis. The model was calibrated for the control treatment and a good agreement between measured and simulated soil water tensions during the period 1990–2003 was achieved. Because of similar amounts of forest-floor vegetation in the different clear-cut treatments identical surface properties were used for them. Monthly nitrate leaching was calculated as the product of modelled drainage and the soil water concentration measured closest in time.
2.4 Vegetation and tree biomass analyses
To achieve an estimate of the maximum aboveground biomass of the forest-floor vegetation, a harvest was made in late summer (August) 2007, in four systematically distributed 0.25 m2 plots per subplot. The plant material was sorted into species/groups of species and weighed after drying (24 h, 65 °C). Material of Deschampsia flexuosa (L.) Trin. (wavy hair grass), Pteridium aquilinum (L.) Kuhn. (bracken fern), and Rubus idaeus L. (raspberry), which constituted the major part of the forest-floor vegetation, was sampled for analysis of N content. The material from R. idaeus was separated into stems and leaves before milling and chemical analysis. Nitrogen and carbon concentrations of the plant material was analysed using a NC Soil Analyzer (Thermo Electron Corporation). To estimate the total amount of N in the forest-floor vegetation, the average share of N in the plant material at subplot level, was multiplied with the dry mass of each vegetation fraction and summed up. Amount of N in the slash was estimated by applying allometric relationships for P. abies biomass components developed at the same experimental site (Albaugh et al. 2009) taking into account a later published erratum (Albaugh et al. 2012). The biomass of the slash fractions (branches and foliage), were multiplied with N concentrations of needles and branches from a harvest in 2003 (cf. Albaugh et al. 2009). The N-content of the biomass samples (pooled within treatment) was determined with a Flash EA 2000 (Thermo Fisher Scientific) connected to a Delta V (Thermo Fisher Scientific). An assumed C content of 50% was used in the calculations of C/N-ratios.
2.5 Statistical analyses
Treatment effects on concentrations of elements and compounds in the soil water, species composition, and N content in the forest-floor vegetation, were analysed by the univariate GLM procedure in SPSS (2008). The split-plot design was taken into account by including a random variable (blocking factor) with two levels in which fertilisation was nested. The degrees of freedom for each term and their error terms are presented in Table 1. To fulfil the assumptions the GLM of normal distribution and homoscedasticity, square-root or log transformation as well as weighted least squares were applied. Probably because of shallow soil-depth, one subplot in an untreated control plot gave extreme values for chemical properties of the soil-water. One observation from this plot (in 2005) was defined as an outlier (> 8 standard errors higher than the overall mean for nitrate in that occasion) and excluded from the analyses. Effects of fertilisation on total mass and N content of slash were tested by means of a One-Way ANOVA in SPSS (2008). To get a measure of the error in the estimate of total nitrate leakage during the four years following the clear cut the time component was first eliminated from all 16 transport time series (four treatments x four replicates) by subtracting the mean at each time point (= month) from each value at that time point. The standard deviation (sd) was then calculated for each treatment series (n = 196; 4 replicates x 49 months). Then a standard error was calculated by dividing sd by the square root of the number of concentration measurements (n = ca. 30). This value was considered to be the mean error at each time point and the final cumulative standard error was that value multiplied by number of months of the time series. To test for relationships between N leakage and forest-floor vegetation, Pearson correlation was applied with soil water concentration of nitrate in 2007, and as average for the whole period, total biomass of the forest-floor vegetation, and total amount of N in the vegetation. To analyse for influence of the treatments on the correlation between the different variables, partial correlation was applied with the control variables fertilisation and removal of slash. One subplot with an extremely low vegetation biomass/N-storage and high average concentration of nitrate in the soil water was defined a bivariate outlier by comparing the Mahalanobis distance with chi-square statistics (df 2, P = 0.05). A significance level of 0.1 was used in all analyses.
| Table 1. Degrees of freedom for the variables and their respective error terms included in the GLMs. | ||
| Source | df | |
| Intercept | Hypothesis | 1 |
| Error | 2 | |
| Fertilisation | Hypothesis | 1 |
| Error | 2 | |
| WTH | Hypothesis | 1 |
| Error | 10 | |
| Block(Fertilisation) | Hypothesis | 2 |
| Error | 10 | |
| Fertilisation * WTH | Hypothesis | 1 |
| Error | 10 | |
3 Results
Fertilisation increased nitrogen concentrations and content of both branches and foliage (Table 2). The C/N-ratios of the slash were lower in fertilised plots independent of slash fraction. The One-Way ANOVA showed that the amount of slash, N concentration and consequently, the total amount of N in the slash were significantly larger on fertilised than on non-fertilised plots (Table 2).
| Table 2. Estimated amount of biomass and N and C/N-ratio in branches, foliage, and total slash on fertilised and non-fertilised plots. Statistics from One-Way ANOVA. The N concentrations and C/N-ratios of branches and foliage were not testable due to absence of within group variation. | |||
| Control | Fertilised | P | |
| Branches (Mg ha–1) | 17 | 25 | 0.019 |
| N concentration in branches | 0.43 | 0.49 | |
| N in branches (Mg ha–1) | 0.07 | 0.12 | 0.012 |
| C/N in branches | 115 | 101 | - |
| Foliage (Mg ha–1) | 17 | 22 | 0.015 |
| N concentration in foliage | 1.23 | 1.30 | |
| N in foliage (Mg ha–1) | 0.21 | 0.30 | 0.007 |
| C/N in foliage | 41 | 36 | - |
| Slash (Mg ha–1) | 34 | 47 | 0.018 |
| Total N in slash (Mg ha–1) | 0.29 | 0.43 | 0.009 |
| N in slash (%) | 0.9 | 0.8 | 0.004 |
| C/N in slash | 60 | 55 | 0.003 |
In July 2004 the nitrate concentration in all treatments was < 0.2 mg NO3-N l–1 (Fig. 2). This could be compared with the background concentrations of soil water, for the period 2004–2007 (data not shown), in the adjacent non-logged fertilised (0.18 mg NO3-N l–1) and control (0.11 mg NO3-N l–1) plots, respectively. From July to October the concentrations increased and were in October 3.8 mg NO3-N l–1 in the fertilised treatments and 1.8 mg NO3-N l–1 in the control treatments. The concentrations continued to increase until the summer 2006, when maximum values of 6.6–14.9 mg NO3-N l–1 were reached, but decreased after that drastically and were between 0.7 and 3.0 mg NO3-N l–1 in October 2006 in all treatments (Fig. 2). The same general pattern was also valid for the treatments conventional harvest (CH) and whole-tree harvest (WTH), but the nitrate concentrations in WTH treated plots increased slower and to a lower maximum value of 8.3 mg NO3-N l–1 compared to CH treated plots where the maximum value was 12.4 mg NO3-N l–1.
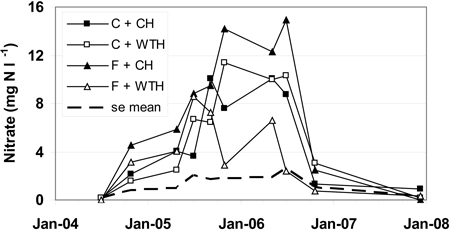
Fig. 2. Nitrate concentrations in soil water (mg NO3-N l–1). The abbreviations are: non-fertilised plots with conventional harvest (C + CH), non-fertilised plots with whole-tree harvest (C + WTH), fertilised plots with conventional harvest (F + CH), fertilised plots with whole-tree harvest (F + WTH), and average standard error (se mean).
For the study period the mean yearly precipitation was 730 mm, which was close to the long-term mean of about 700 mm yr–1. During the same period the drainage from the intact stands were 290 mm yr–1 for control and 250 mm yr–1 for fertilised stands, respectively. Drainage from clear-cut plots was substantially larger, 530 mm yr–1 (Table 3). Precipitation was high during the first year (860 mm for 2004) and the last year (840 mm for 2007), but exceptionally low in 2005 (490 mm), which influenced on drainage in a similar way (Table 3, Fig. 3). This resulted in a high weight for the low concentrations measured in the beginning and the end of the study period, but in a low weight for the high nitrate concentrations measured 2005. The nitrate leakage (Fig. 3) followed mainly the pattern of nitrate concentration (Fig. 2).
| Table 3. Precipitation (P), and total drainage in clear-cut (control and fertilised), and intact control (C), and fertilised plots (F), respectively (mm per year). | ||||
| P | Clear-cut | C | F | |
| 2004 | 860 | 620 | 400 | 350 |
| 2005 | 490 | 360 | 170 | 150 |
| 2006 | 730 | 450 | 240 | 210 |
| 2007 | 840 | 700 | 340 | 290 |
| Mean | 730 | 530 | 290 | 250 |
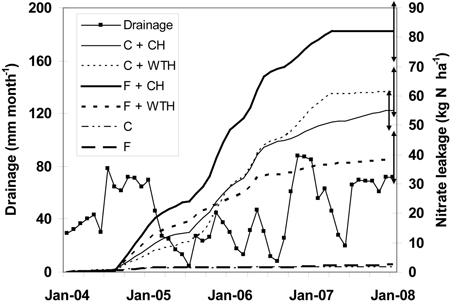
Fig. 3. Drainage (mm month–1) and cumulative nitrate leakage (kg N ha–1) at 0.5 m depth in the soil after clear-cutting in December 2003. The abbreviations are: non-fertilised plots with conventional harvest (C + CH), non-fertilised plots with whole-tree harvest (C + WTH), fertilised plots with conventional harvest (F + CH), fertilised plots with whole-tree harvest (F + WTH), fertilised (F), and control plots (C) that were not clear-cut in 1994. Double arrows to the right indicate final standard error for the respective series.
The cumulative nitrate leakage was higher from F treatments than from C treatments, and higher from CH treatments than from WTH treatments, respectively (Fig. 3). Then, from late 2005 the cumulative leakage from C + WTH increased faster than from F + WTH and C + CH and was next to largest from spring 2006. At the same time the cumulative leakage from F + WTH treated plots levelled off and was lowest from December 2005. The final cumulative nitrate leakages were 38 (F + WTH), 55 (C + CH), 61 (C + WTH), and 82 (F + CH) kg N ha–1, respectively (Fig. 3). For the same time period the leakages from adjacent non-harvested fertilised and control plots were 2.5 and 1.6 kg NO3-N ha–1, respectively (Fig. 3).
All analysed elements, except ammonium, showed a pattern over time similar to nitrate with a peak in concentration 1.5 to 2.5 years after the clear-cut (not shown). The concentrations of ammonium peaked earlier and then declined. Interactions between fertilisation and WTH were found on the concentrations of Al, Mg, ammonium, and nitrate (Table 4). In the case of Al, Mg, and nitrate the reduction in soil water concentrations by WTH was larger on fertilised plots or absent in unfertilised plots. Ammonium, on the other hand, showed a pattern where WTH only had a reductive effect in unfertilised plots. Significant effects of the interaction between the treatments were found also on pH (Table 4). It was only on fertilised plots that WTH had a positive effect on pH. A general pattern was that WTH in fertilised plots reduced soil water concentrations to levels comparable to unfertilised plots (Fig. 4). The nitrate concentrations were lower in fertilised plots with WTH and higher with CH than on control plots. As an effect of fertilisation an increase in soil water concentrations for many of the analysed elements was noticed, but significant effects were only found for K and Mg (Table 4). The concentrations of Ca, DOC, DON, K, Mg, ammonium, nitrate, and pH in soil water were all significantly affected by WTH, although these results should be interpreted with care due to the inclusion of the interaction in the statistical models.
| Table 4. Statistics for effects of fertilisation and whole-tree harvest (WTH) on the levels of aluminium, calcium, DOC (dissolved organic carbon), DON (dissolved organic nitrogen), potassium, magnesium, ammonium, nitrate, phosphate, and pH in soil water. Effects significant at the 0.1 level in bold. | |||
| Fertilisation | WTH | Fertilisation x WTH | |
| P | P | P | |
| Aluminium | 0.647 | 0.176 | 0.055 |
| Calcium | 0.698 | 0.060 | 0.557 |
| DOC | 0.251 | 0.057 | 0.488 |
| DON | 0.132 | 0.076 | 0.668 |
| Potassium | 0.001 | 0.015 | 0.258 |
| Magnesium | 0.051 | 0.019 | 0.054 |
| Ammonium | 0.419 | 0.016 | 0.057 |
| Nitrate | 0.821 | 0.007 | 0.012 |
| Phosphate | 0.538 | 0.957 | 0.239 |
| pH | 0.429 | 0.042 | 0.071 |
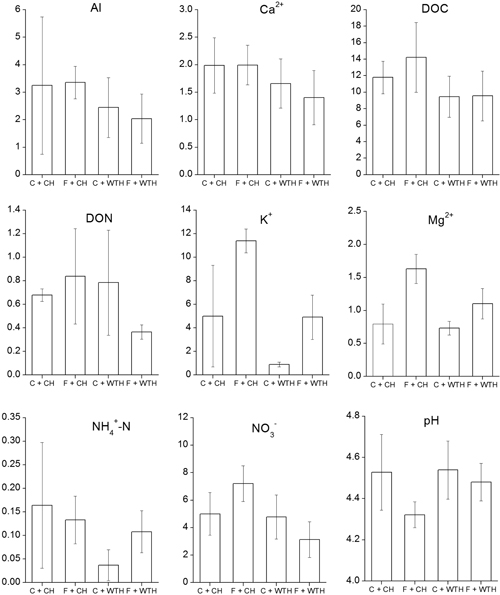
Fig. 4. Average soil water concentrations (mg l–1) of Al, Ca, DOC (dissolved organic carbon), DON (dissolved organic nitrogen), K, Mg, ammonium, nitrate, and pH, that were significant (P<0.1) in the GLM (Table 3). Bars represent from left non-fertilised plots with conventional harvest (C + CH), fertilised plots with conventional harvest (F + CH), non-fertilised plots with whole-tree harvest (C + WTH) and fertilised plots with whole-tree harvest (F + WTH).Error bars show ±2 standard errors.
The forest-floor vegetation was dominated by D. flexuosa and R. idaeus, which together constituted the major part of the biomass (Fig. 5). In one of the non-fertilised main-plots, however, P. aquilinum also contributed substantially. There were no significant treatment effects on the total biomass of the forest-floor vegetation. There was, however, a significant change in species composition as an effect of WTH. The amount of biomass showed significant treatment response to WTH for D. flexuosa (P = 0.002) and R. idaeus (P = 0.015) as well as other species (P = 0.034). The analysis for vegetation cover gave similar results for both D. flexuosa (P < 0.001) and R. idaeus (P = 0.011). The amount of D. flexuosa was larger while that of R. idaeus was smaller on plots with WTH (Fig. 5). Fertilisation did not affect the biomass of the analysed species/groups of species. The analysis of vegetation cover did, however, reveal a negative effect of fertilisation on D. flexuosa (P = 0.027) and an interaction between the two treatments (P = 0.080).
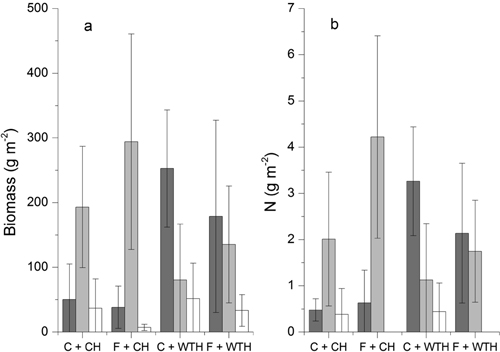
Fig. 5. a) Mean dry weight (g m–2) for Deschampsia flexuosa (dark grey), Rubus idaeus (light grey), and other species (white), b) Mean amount of N (g m–2) for D. flexuosa (dark grey), R. idaeus (light grey), and Pteridium aquilinum (white) in non-fertilised plots with conventional harvest (C + CH), fertilised plots with conventional harvest (F + CH), non-fertilised plots with whole-tree harvest (C + WTH), and fertilised plots with whole-tree harvest (F + WTH). Error bars show ±2 standard errors.
The average concentrations of N in plant material was 1.92% ±0.14(SD) and 0.66% ±0.09 for leaves and stems of R. idaeus, respectively, and 1.47% ±0.32 for leaves of D. flexuosa. The N concentrations of the plant material did not show any significant treatment effects. Neither were there any effects of the individual treatments on the total amount of N in the vegetation although the interaction between treatments were significant (P = 0.029). The average amounts of N in the plant material were 2.9 (C + CH), 4.8 (C + WTH), 4.9 (F + CH), and 3.9 (F + WTH) g N m–2. In 2007, there was no significant correlation (r = –0.254; p = 0.402) between the nitrate concentration in the soil water and the total amount of N in the vegetation (Table 5). However, the mean soil water concentration of nitrate over the whole period of measurements was positively correlated (r = 0.546; p = 0.035) with the total amount of N in the biomass of the forest-floor vegetation. Testing for impact of the treatments on the relation by partial correlation improved the correlation between soil water nitrate and biomass N (r = 0.697; p = 0.008). The total amount of N in the biomass of the forest-floor vegetation was well correlated (r = 0.798; p < 0.001) with the biomass (Table 5). There were, on the other hand, no significant correlation between soil water nitrate and biomass.
| Table 5. Pearson correlations between concentration of nitrate in the soil water (for 2007 and mean value for the whole period of measurement), weight of the biomass of the forest-floor vegetation, amount of N in the biomass of the forest-floor vegetation as continuous variables, and whole-tree harvest (WTH) and fertilisation as binomial variables. Partial correlation between concentration of nitrate in the soil water, weight of the biomass of the forest-floor vegetation, and amount of N in the biomass of the forest-floor vegetation with WTH and fertilisation as control variables. Correlations significant at the 0.1 level in bold. | |||||
| Correlation | 2007 | Mean | Biomass | ||
| Pearson | Biomass | r | –0.189 | 0.224 | 1.000 |
| p | 0.535 | 0.423 | . | ||
| Biomass – N | r | –0.254 | 0.546 | 0.798 | |
| p | 0.402 | 0.035 | <0.001 | ||
| WTH | r | –0.108 | –0.478 | 0.232 | |
| p | 0.724 | 0.072 | 0.405 | ||
| Fertilisation | r | –0.256 | –0.034 | 0.201 | |
| p | 0.398 | 0.906 | 0.472 | ||
| Partial | Biomass – weight | r | –0.187 | 0.399 | 1.000 |
| p | 0.582 | 0.177 | . | ||
| Biomass – N | r | –0.229 | 0.697 | 0.794 | |
| p | 0.499 | 0.008 | 0.001 | ||
The N mass balance of the soil compartment during the four years after clear-cutting was dominated by input from foliage in connection with the harvest (Table 6). The total input was 170 kg N ha–1 higher in the F + CH- than in the C + CH-treatment. Total input of N to the WTH treatments were similar and about half of that in the C + CH-treatment. For the output from the soil uptake in vegetation was largest for C + WTH- and F + CH-treatments and amounted to about 120 kg N ha–1 during four years. In the C + CH and F + WTH treatments the forest-floor vegetation extracted 73 and 98 kg N ha–1, respectively. Leakage in the form of nitrate varied between 38 (F + WTH) and 82 (F + CH) kg N ha–1 (Table 6). The increase in N storage was largest for the F + CH-treatment (325 kg N ha–1) and a small decrease was found for the C + WTH-treatment (–17 kg N ha–1) (Table 6).
| Table 6. Soil compartment nitrogen mass balance (kg N ha–1) for the first four years following clear-cutting | ||||
| C + CH | C + WTH | F + CH | F + WTH | |
| Input to soil | ||||
| Atmospheric deposition | 36 | 36 | 36 | 36 |
| Slash – branches | 70 | 14 | 120 | 24 |
| Slash – foliage | 210 | 42 | 300 | 60 |
| Vegetation litter a) | 44 | 72 | 74 | 59 |
| Total input | 360 | 164 | 530 | 179 |
| Output from soil | ||||
| Uptake in vegetation a) | 73 | 120 | 123 | 98 |
| Leakage | 55 | 61 | 82 | 38 |
| Total output | 128 | 181 | 205 | 136 |
| Change in storage | 232 | –17 | 325 | 43 |
| a) The maximum vegetation biomass each year was assumed to increase linearly from 0 in 2003 to measured amounts in 2007 and to form litter the year following its production. Thus the vegetation litter includes biomass produced during 2004 to 2006 and uptake in vegetation includes biomass produced during 2004 to 2007. The C/N ratio of vegetation biomass was assumed constant. | ||||
4 Discussion
A considerable share of the applied N in the fertilised plots is accumulated in the aboveground biomass and can therefore be removed by harvest. As an effect of fertilisation the increase in both amount of slash and N content in the branches and foliage lead to an approximate 50% increase in N-load. Simultaneously, the C/N ratio of the slash was lower in fertilised plots.
Slash can initially, depending on N concentration, act both as a source and sink of N. Berg and Ekbohm (1983) showed that net mineralisation of N in P. sylvestris litter was dependent on C/N and that net mineralisation took place at ratios below 63. In the present study the C/N ratios of the slash from fertilised and non-fertilised plots were 60 and 55, respectively, implying a potential shift from source to sink as an effect of fertilisation. Further support of this interpretation is that WTH did not cause any change in the soil water nitrate concentration in non-fertilised plots.
In contrast to removal of slash, fertilisation had only a significant effect on leakage of K and Mg. Thus, we could not find any support for our first hypothesis that nutrient leakage increases after clear-cut as an effect of fertilisation. This may be the result of low statistical power caused by a low number of replications for fertilisation (n = 2) in relation to WTH (n = 8). This is a problem which the present study has in common with many other related studies, where the original purpose of the experiment has been extended by adding new treatments. Generalisations from this experiment should, since it was carried out on only one site, be done with caution (Hurlbert 1984). Leakage of nitrate decreased with WTH, but only in fertilised plots. This is partly in line with our second hypothesis, however, WTH in fertilised plots tended to decrease nitrate to levels lower than in non-fertilised plots.
In accordance with results of earlier studies of fertilised (Berdén et al. 1997; Ring et al. 2003) and non-fertilised (Staaf and Olsson 1994) stands, the soil water nitrate concentration increased almost instantly after the forest was clear-cut. Similarly to Ring et al. (2003) the levels rapidly decreased after a peak 1–2 years after the forest was cut, and approached pre-cut levels at the end of the third summer. Most of the analysed elements showed a pattern over time similar to nitrate, but ammonium peaked early and then rapidly decreased. This is probably the result of increased nitrification after the first year. A few studies of N-leakage after clear-cut of fertilised forest show results diverging from the present study (Nohrstedt et al. 1994; Ring 1996; Ring 2004). In these studies increased N concentration in the soil water, caused by clear-cut and fertilisation, occurred first a few years after the forest was harvested and only at a high fertiliser dose. These studies, however, originate from one experiment on a relatively poor site in a harsh climate with low deposition of anthropogenic N. The site used in the present study has been exposed to long-term deposition of anthropogenic N. Long-term measurements of N leakage from control plots in present experiment indicate that N from deposition to a large extent is retained within the system (Grip 2006). This N is accumulated in soil organic matter (Tietema et al. 1998) and later partly released when the forest is clear-cut, which is an effect of increased degradation of organic matter and relaxed competition from trees and their mycorrhizal symbionts.
Two species, D. flexuosa and R. idaeus, dominated the forest-floor vegetation independent of treatment. D. flexuosa is usually known to increase as an effect of increased nutrient availability (e.g. Strengbom and Nordin 2008). In this study, however, D. flexuosa decreased as an effect of fertilisation, which is most likely an effect of competition from R. idaeus, which is an even more nitrophilous species than D. flexuosa (Ellenberg et al. 2001).
In the WTH-treatments D. flexuosa dominated in comparison with R. idaeus and other species. The negative effect of CH on D. flexuosa is supported by results of Olsson and Staaf (1995) and Åström et al. (2005), who found that D. flexuosa and other graminoids were suppressed by slash. Both species can penetrate moderately thick layers of slash, but R. idaeus has a competitive advantage by its tall stature. Even though there was a change in species dominance as an effect of the treatments, there was no significant effect on total biomass of the forest-floor vegetation, which is contrary to our fourth hypothesis, that slash should hamper the development of forest-floor vegetation and thereby impact the N retention capacity negatively. The lack of treatment effects is supported by Örlander et al. (1996) who did not find any significant effect of slash on the vegetation biomass. No significant effects of treatment on the N concentration or the total N content of the plant material was found. This is contradictory to other studies, which have reported significant effects of fertilisation on the N content of D. flexuosa (Quist et al. 1999; Hedwall et al. 2010), which is reported to be long lasting, even after relatively low doses of supplied N (Strengbom and Nordin 2008). Högbom and Högberg (1991) found that, on a site in southern Sweden, exposed to large amounts of anthropogenic N, the N concentration of D. flexuosa leaves were enhanced only in spring and autumn. To obtain an estimate of maximum biomass, the forest-floor vegetation was sampled in late summer, which may have caused the lack of treatment effects on N concentrations.
The biomass of the forest floor vegetation did not respond to increased levels of nitrate in the soil water. The total amount of N in the vegetation biomass was, however, positively correlated with the soil water nitrate content. The lack of correlation between biomass and soil water nitrate, and the significant correlation between biomass N and nitrate indicates that even though the vegetation did not respond to nitrate by increased growth, it had the ability to increase the total content of N and potentially decrease the soil water concentrations. Thus we have found some support of our third hypothesis.
The estimated nitrogen budget gave a clear indication of the most important fluxes where the largest input to the soil-fraction was from slash. This forest was clear-cut at the age of 30 years, which is considerably less than the normal rotation periods in southern Sweden and about 10 years less than the estimated rotation period in this type of management with optimised fertilisation. The amount of slash and thereby potential input of N to the soil-fraction in conventional harvest were, thus, probably underestimated in this study. The uptake of N by forest-floor vegetation, which was 50–100 % more than the accumulated leakage, seems to be an important path for mineralised nitrogen and may be one reason for the limited leakage. Additionally, the importance of the forest-floor vegetation is underestimated by this study since nitrogen storage in roots and rhizomes was not measured. Results of Palviainen et al. (2005a) indicate that the belowground biomass of the forest floor vegetation may be as large as the aboveground parts.
5 Conclusions
The pre-harvest optimised fertilisation carried out on this site did not cause any significant increase in N leakage after clear-cut, which is in contrast to our expectations (hypothesis 1). This could be an effect of a high background deposition of anthropogenic N and scarce forest floor vegetation which caused enhanced leakage also in non-fertilised plots. We expected whole-tree harvest in fertilised stands to reduce N leakage, although not to the levels of the unfertilised control (hypothesis 2). Our results indicate that N leakage were reduced even further, to below the levels of non-fertilised plots. We expected the total biomass of the forest floor vegetation to be negatively affected by slash, resulting in a reduced N storage capacity (hypothesis 4). This was, however, not the case as the biomass of the vegetation was unaffected by whole-tree harvest. On the other hand the vegetation responded on enhanced levels of nitrate in the soil water with larger amounts of N in the biomass, which did not prevent, but potentially reduced N leakage. This result, together with a considerable output of N by the vegetation shown in the nitrogen budget, gives some support to our third hypothesis, that N uptake in vegetation significantly reduces leakage. Our results imply that WTH could be considered when clear-cutting fertilised forests or forests exposed to high levels of anthropogenic N deposition. Before large scale implementation, however, also knowledge from other soil types is needed, as well as an assessment of the effects on other soil properties and flora and fauna connected to thin woody debris.
Acknowledgements
We thank two anonymous reviewers for their valuable comments on an earlier version of this paper. This study was part of the ”Fiberskog” programme (1997–2005), which was sponsored by SLU and Swedish forest companies. We would like to thank the staff at Asa Experimental Forest for assistance when establishing the experiment and being responsible for a major part of sampling and measurements.
References
Albaugh T.J., Bergh J., Lundmark T., Nilsson U., Stape J.L., Allen H.L., Linder S. (2009). Do biological expansion factors adequately estimate stand-scale aboveground component biomass for Norway spruce? Forest Ecology and Management 258: 2628–2637.
Albaugh T.J., Bergh J., Lundmark T., Nilsson U., Stape J.L., Allen H.L., Linder S. (2012). Erratum to Forest Ecology and Management 258: 2628–2637.
Åström M., Dynesius M., Hylander K., Nilsson C. (2005). Effects of slash harvest on bryophytes and vascular plants in southern boreal forest clear-cuts. Journal of Applied Ecology 42: 1194–1202.
Berdén M. (1994). Ion leaching and soil acidification in a forest haplic podsol: effects of nitrogen application and clear-cutting. Dissertation. Department of Ecology and Environmental Research, Swedish University of Agricultural Sciences, Report 73.
Berdén M., Nilsson S.I., Nyman P. (1997). Ion leaching before and after clear-cutting in a Norway spruce stand – effects of long-term application of ammonium nitrate and superphosphate. Water, Air and Soil Pollution 93: 1–26.
Berg B., Ekbohm G. (1983). Nitrogen immobilization in decomposing needle litter at variable carbon: nitrogen ratios. Ecology 64: 63–67.
Bergh J., Linder S., Lundmark T., Elfving B. (1999). The effect of water and nutrient availability on the productivity of Norway spruce in northern and southern Sweden. Forest Ecology and Management 119: 51–62.
Bergh J., Linder S., Bergström J. (2005). Potential production of Norway spruce in Sweden. Forest Ecology and Management 204: 1–10.
Bergh J., Nilsson U., Grip H., Hedwall P.-O., Lundmark T. (2008). Effects of frequency of fertilisation on production, foliar chemistry and nutrient leaching in young Norway spruce stands in Sweden. Silva Fennica 42: 721–733.
Bergstedt J., Milberg P. (2001). The impact of logging intensity on field-layer vegetation in Swedish boreal forests. Forest Ecology and Management 154: 105–115.
Ellenberg H., Weber H.E., Düll R., Wirth V., Werner W. (2001). Zeigerwerte von Pflanzen in Mitteleuropa. Scripta Geobotanica, vol. 18, third edition. Verlag Erich Goltze, Göttingen. [In German with English summary].
Emmett B.A., Anderson J.M., Hornung M. (1991). The controls on dissolved nitrogen losses following two intensities of harvesting in a Sitka spruce forest (N. Wales). Forest Ecology and Management 41: 65–80.
Fahey T.J., Hill M.O., Stevens P.A., Hornung M., Rowland P. (1991). Nutrient accumulation in vegetation following conventional and whole-tree harvest of Sitka spruce plantations in North Wales. Forestry 64: 271–288.
Grip H. (2006). Miljöeffekter av intensivodling. Effekter på näringsläckage. In: Bergh J., Oleskog G. (eds.). Slutrapport för Fiberskogsprogrammet. Sveriges lantbruksuniversitet, Institutionen för sydsvensk skogsvetenskap, Arbetsrapport 27. p. 38–60. ISBN 91-576-7161-3. [In Swedish].
Hägglund B., Lundmark J.-E. (1977). Site index estimation by means of site properties – Scots pine and Norway spruce in Sweden. Studia Forestalia Suecica 138. 33 p.
Hedwall P.-O., Nordin A., Brunet J., Bergh J. (2010). Compositional changes of forest-floor vegetation in young stands of Norway spruce as an effect of repeated fertilisation. Forest Ecology and Management 259: 2418–2425.
Högbom L., Högberg P. (1991). Nitrate nutrition of Deschampsia flexuosa (L.) Trin. in relation to nitrogen deposition in Sweden. Oecologia 87: 488–494.
Hurlbert S.H. (1984). Pseudoreplication and the design of ecological field experiments. Ecological Monographs 54: 187–211.
Jansson P.-E., Karlberg L. (2010). Coupled heat and mass transfer model for soil-plant-atmosphere systems. Royal Institute of Technology, Stockholm. 454 p. http://www.lwr.kth.se/Vara%20Datorprogram/CoupModel/coupmanual.pdf.
Linder S. (1995). Foliar analysis for detecting and correcting nutrient imbalances in Norway spruce. Ecological Bulletins (Copenhagen) 44: 178–190.
Lundborg A. (1997). Reducing the nitrogen load: whole-tree harvesting. Ambio 26: 387–393.
Melin J., Nömmik H., Lohm U., Flower-Ellis J.G.K. (1983). Fertilizer nitrogen budget in a Scots pine ecosystem attained by using root-isolated plots and 15N tracer technique. Plant and Soil 74: 249–263.
Monteith J.L. (1965). Evaporation and environment. In: Fogg G.E. (ed.). The state and movement of water in living organisms. 19th Symp. Soc. Exp. Biol., Cambridge. The Company of Biologists. p. 205–234.
Nohrstedt H.-Ö. (2001). Response of coniferous forest ecosystems on mineral soils to nutrient additions: a review of Swedish experiences. Scandinavian Journal of Forest Research 16: 555–573.
Nohrstedt H.-Ö., Ring E., Klemedtsson L., Nilsson Å. (1994). Nitrogen losses and soil water acidity after clear-felling of fertilized experimental plots in a Pinus sylvestris stand. Forest Ecology and Management 66: 69–86.
Nordin A., Bergström A.-K., Granberg G., Grip H., Gustafsson D., Gärdenäs A., Hyvönen-Olsson R., Jansson P.-E., Laudon H., Nilsson M.B., Svensson M., Öquist M. (2009). Effekter av ett intensivare skogsbruk på skogslandskapets mark, vatten och växthusgaser. Delrapport från regeringsuppdrag Jo 2008/1885. [In Swedish].
Nordin A., Larsson S., Moen J., Linder S. (2011). Science for trade-offs between conflicting interests in future forests. Forests 2: 631–636.
Olsson B.A., Staaf H. (1995). Influence of harvesting intensity of logging residues on ground vegetation in coniferous forests. Journal of Applied Ecology 32: 640–654.
Örlander G., Nilsson U., Hällgren J.-E. (1996). Competition for water and nutrients between ground vegetation and planted Picea abies. New Zealand Journal of Forest Science 26: 99–117.
Palviainen M., Finér L., Kurka A.-M., Mannerkoski H., Piirainen S., Starr M. (2004). Decomposition and nutrient release from logging residues after clear-cutting of mixed boreal forest. Plant and Soil 263: 53–67.
Palviainen M., Finér L., Mannerkoski H., Piirainen S., Starr M. (2005a). Changes in the above- and below-ground biomass and nutrient pools of ground vegetation after clear-cutting of a mixed boreal forest. Plant and Soil 275: 157–167.
Palviainen M., Finér L., Mannerkoski H., Piirainen S., Starr M. (2005b). Responses of ground vegetation species to clear-cutting in a boreal forest: aboveground biomass and nutrient contents during the first 7 years. Ecological Research 20: 652–660.
Pettersson F., Högbom L. (2004). Long-term growth effects following forest nitrogen fertilization in Pinus sylvestris and Picea abies stands in Sweden. Scandinavian Journal of Forest Research 19: 339–374.
Quist M.E., Näsholm T., Lindeberg J., Johannisson C., Högbom L., Högberg P. (1999). Responses of a nitrogen-saturated forest to a sharp decrease in nitrogen input. Journal of Environmental Quality 28: 1970–1977.
Ring E. (1996). Effects of previous N fertilizations on soil-water pH and N concentrations after clear-felling and soil scarification at a Pinus sylvestris site. Scandinavian Journal of Forest Research 11: 7–16.
Ring E. (2004). Experimental N fertilization of Scots pine: effects on soil-solution chemistry 8 years after final felling. Forest Ecology and Management 188: 91–99.
Ring E., Bergholm J., Olsson B.A., Jansson G. (2003). Urea fertilizations of a Norway spruce stand: effects on nitrogen in soil water and field-layer vegetation after final felling. Canadian Journal of Forest Research 33: 375–384.
Roberts S.D., Harrington C.A., Terry T.A. (2005). Harvest residue and competing vegetation affect soil moisture, soil temperature, N availability, and Douglas-fir seedling growth. Forest Ecology and Management 205: 333–350.
Rosén K., Lundmark-Thelin A. (1987). Increased nitrogen leaching under piles of slash – a consequence of modern forest harvesting techniques. Scandinavian Journal of Forest Research 2: 21–29.
Skogsstyrelsen. (2007). Kvävegödsling av skogsmark. Meddelande 2:2007. Skogsstyrelsens förlag, Jönköping, Sweden. 33 p. ISSN 1100-0295. [In Swedish].
SMHI. (2009). National mapping of atmospheric chemical data for Swedish environmental monitoring on behalf of Swedish EPA. http://www.smhi.se.
Smolander A., Priha O., Paavolainen L., Steer J., Mälkönen E. (1998). Nitrogen and carbon transformations before and after clear-cutting in repeatedly N-fertilised and limed forest soil. Soil Biology and Biochemistry 30: 477–490.
SPSS. (2008). SPSS 17.0.0. SPSS Inc.
Staaf H., Olsson B.A. (1994). Effects of slash removal and stump harvesting on soil water chemistry in a clear cutting in SW Sweden. Scandinavian Journal of Forest Research 9: 305–310.
Strengbom J., Nordin A. (2008). Commercial forest fertilization causes long-term residual effects in ground vegetation of boreal forests. Forest Ecology and Management 256: 2175–2181.
Tamm C.O. (1991). Nitrogen in terrestrial ecosystems. Questions of productivity, vegetational change and ecosystem stability. Ecological Studies 81. Springer-Verlag. 115 p.
Tietema A., Emmett B.A., Gundersen P., Kjønaas O.J., Koopmans C.J. (1998). The fate of 15N-labelled nitrogen deposition in coniferous forest ecosystems. Forest Ecology and Management 101: 19–27.
Total of 47 references

