Annual net nitrogen mineralization and litter flux in well-drained downy birch, Norway spruce and Scots pine forest ecosystems
Becker H., Aosaar J., Varik M., Morozov G., Aun K., Mander Ü., Soosaar K., Uri V. (2018). Annual net nitrogen mineralization and litter flux in well-drained downy birch, Norway spruce and Scots pine forest ecosystems. Silva Fennica vol. 52 no. 4 article id 10013. https://doi.org/10.14214/sf.10013
Highlights
- The net nitrogen mineralization (NNM) flux in drained peat soils depends largely on the C/N ratio and tree species
- The soil NNM process is affected by trees through organic litter input into soil
- Pine stand in low-fertility drained transitional bog is dominated by net ammonification
- Birch and spruce stands on the fertile drained peat soil with higher pH and N content are dominated by net nitrification.
Abstract
The main aim of the current study was to estimate the annual net nitrogen mineralization (NNM) flux in stands of different tree species growing on drained peatlands, as well as to clarify the effect of tree species, soil properties and litter on annual NNM dynamics. Three study sites were set up in May 2014: a downy birch (Betula pubescens Ehrh.) stand and a Norway spruce (Picea abies (L.) Karst.) stand in Oxalis full-drained swamp (ODS) and a Scots pine (Pinus sylvestris L.) stand in Myrtillus full-drained swamp (MDS). The NNM flux was estimated using the in situ method with incubated polyethylene bags. The highest value of NNM was found in stands that were growing on fertile ODS: 127.5 kg N ha–1 yr–1 and 87.7 kg N ha–1 yr–1, in the downy birch stand and in the Norway spruce stand, respectively. A significantly lower annual NNM flux (11.8 kg N ha–1 yr–1) occurred in the Scots pine stand growing in MDS. Nitrification was highest at fertile ODS sites and ammonification was the highest at the low fertility MDS site. For all study sites, positive correlation was found between soil temperature and NNM intensity. The difference in annual NNM between the downy birch stand and the Norway spruce stand growing on similar drained fertile peatlands was due to litter quality. The annual N input into the soil through leaf litter was the highest at the downy birch site where also the C/N ratio of litter was the lowest. The second highest N input into the soil was found in the spruce stand and the lowest in the pine stand.
Keywords
Pinus sylvestris;
Picea abies;
Betula pubescens;
drained peatland forests;
ammonification;
effect of tree species;
swamp;
nitrification;
transitional bog
-
Becker,
Chair of Silviculture and Forest Ecology, Institute of Forestry and Rural Engineering, Estonian University of Life Sciences, Friedrich Reinhold Kreutzwaldi 1, 51014 Tartu, Estonia
E-mail
hardo.becker@emu.ee

- Aosaar, Chair of Silviculture and Forest Ecology, Institute of Forestry and Rural Engineering, Estonian University of Life Sciences, Friedrich Reinhold Kreutzwaldi 1, 51014 Tartu, Estonia E-mail jyrgen.aosaar@emu.ee
- Varik, Chair of Silviculture and Forest Ecology, Institute of Forestry and Rural Engineering, Estonian University of Life Sciences, Friedrich Reinhold Kreutzwaldi 1, 51014 Tartu, Estonia E-mail mats.varik@emu.ee
- Morozov, Chair of Silviculture and Forest Ecology, Institute of Forestry and Rural Engineering, Estonian University of Life Sciences, Friedrich Reinhold Kreutzwaldi 1, 51014 Tartu, Estonia E-mail gunnar.morozov@emu.ee
- Aun, Chair of Silviculture and Forest Ecology, Institute of Forestry and Rural Engineering, Estonian University of Life Sciences, Friedrich Reinhold Kreutzwaldi 1, 51014 Tartu, Estonia E-mail kristiina.aun@emu.ee
- Mander, Department of Geography, Institute of Ecology & Earth Sciences, University of Tartu, Ülikooli 18, 50090 Tartu, Estonia E-mail ulo.mander@ut.ee
- Soosaar, Department of Geography, Institute of Ecology & Earth Sciences, University of Tartu, Ülikooli 18, 50090 Tartu, Estonia E-mail kaido.soosaar@ut.ee
- Uri, Chair of Silviculture and Forest Ecology, Institute of Forestry and Rural Engineering, Estonian University of Life Sciences, Friedrich Reinhold Kreutzwaldi 1, 51014 Tartu, Estonia E-mail veiko.uri@emu.ee
Received 12 June 2018 Accepted 18 September 2018 Published 27 September 2018
Views 152994
Available at https://doi.org/10.14214/sf.10013 | Download PDF

1 Introduction
Forest drainage is an important and widely used forest management practice for increasing site fertility and forest growth in excessively moist soils and peatlands in the boreal and hemiboreal zones. About 15 million ha of peat soils have been drained in the temperate and boreal regions for the purpose to improve forest site fertility (Paavilainen and Päivinen 1995). In Finland, over 5.5 million ha are under drained forests, of which 4.5 million ha grow on peatlands (Peltomaa 2007). In Estonia, the most intensive forest draining was carried out during 1970–1980 and the total area of drained forest formed over 500 000 ha (Yearbook of Forest 2016). Approximately 20% of Estonian forests grow on peatlands, from which 14% are drained (Yearbook of Forest 2016). These forests are diverse; a large share of them are managed regularly and some belong to protected forest areas. After drainage of peatlands, soil organic matter starts to decompose, which can also lead to the increased rate of greenhouse gas (GHG) emission. Several studies have reported intensive soil respiration in drained forests (Martikainen et al. 1993; Silvola et al. 1994; Ojanen et al. 2013). In the light of increased CO2 emissions and expected global warming, a number of studies have focused on carbon (C) emissions in drained forest (Silvola et al. 1994; Ojanen et al. 2013; Meyer et al. 2013; Birdsey and Pan 2015). However, since nitrogen (N) is strongly related to the C cycle, studies of the N cycle and N mineralization in drained forest ecosystems should be emphasized as well. Nitrogen is one of the mineral elements limiting forest growth in the boreal region (Luo et al. 2004). Thus N availability is an essential factor affecting also C accumulation by plants also, there is strong relationship between forest C and N cycling (Millard et al. 2007). Net nitrogen mineralization (NNM) is an essential flux in whole N cycle of boreal and temperate forests (Zak et al. 1990; Goodale and Aber 2001; Lovett et al. 2002; Uri et al. 2008), since most of the N utilized for plant production is produced by in situ mineralization of organic matter (Tate 1995).
The intensity of NNM depends on many factors: soil type, tree species, land use history etc. (Zak et al. 1990; Goodale and Aber 2001; Lovett et al. 2002; Uri et al. 2008). Also soil water content can influence the intensity of NNM (Raison et al. 1987; Stenger et al. 1995; Persson and Wiren 1995). Reduction in soil water content and improvement in soil aeration in drained peatlands also make conditions more favourable for NNM, which improves tree growth and influences ecosystem structure (Silins and Rothwell 1999). Wang et al. (2018) conclude that long term drainage increases nutrient availability and the vegetation changes in response to drainage. Some studies report that the N pool of drained forest can even be higher than the N pool of upland sites (Westman and Laiho 2003). Also a change in the leaching of N in the form of ammonium may be increased after drainage (Laine et al. 1995). When NNM studies mostly focus on mineral soils in different forest ecosystems (Connell et al. 1995; Goodale and Aber 2001; Andersson et al. 2002; Lõhmus et al. 2002; Pajuste and Frey 2003; Uri et al. 2008, 2011; Becker et al. 2015, 2016), then relevant studies focusing on organic soils are still scarce.
This study involved three main tree species that form most part of the stands growing in drained forest ecosystems in Estonia: Scots pine (Pinus sylvestris L.), Norway spruce (Picea abies (L.) Karst.) and downy birch (Betula pubescens Ehrh.). The main aim of the study was to estimate the annual NNM flux in different stands growing on drained peatlands and to clarify the effect of tree species, soil type and litter quality on annual NNM dynamics. We hypothesized that annual NNM is higher in deciduous (birch) stand than in coniferous (spruce) stand growing on similar organic soils.
2 Materials and methods
2.1 Study sites
Three study sites were set up in May 2014 in the Järvselja experimental forest district (58°16´N, 27°18´E) which is located in the eastern part of Estonia. The area belongs to the hemiboreal vegetation zone (Ahti et al. 1968), which is a transition zone from the temperate to the boreal climate. The Järvselja forest district has a long drainage history (the first drainage systems were set up at the end of the 19th century) and drained forests are widespread in this area. All study sites of different tree species are located on long-term drained forested peatlands (Table 1) and the average distance between the study sites was roughly 5 km. In Estonia, Oxalis full-drained swamp (ODS) and Myrtillus full-drained swamp (MDS) are the two main forest site types in peatland forest (Yearbook forest 2016). The ODS site type made up 14% and the MDS site type made up 6% of the Järvselja forest district (Korjus et al. 2015). All studied sites had been drained approximately 40–50 years earlier using open ditches. As a result of drainage, groundwater level was normally below 40 cm from surface during the growing season.
| Table 1. Main stand characteristics of the study sites. H – average stand height, D1.3 – average breast height diameter, BA – basal area. | |||||||
| Study site | Age (yr) | Stand area (ha) | N (trees ha–1) | H (m) | D1,3 (cm) | BA (m2 ha–1) | Volume (m3 ha–1) |
| Downy birch | 30 | 5.1 | 1660 | 15 | 14 | 24.5 | 193 |
| Norway spruce | 55 | 0.9 | 942 | 17 | 18,4 | 25 | 208 |
| Scotch pine | 65 | 1.3 | 620 | 23 | 22 | 20.1 | 218 |
At all study sites, a sample plot (20×25 m) was established and the main stand characteristics were measured (Table 1). The main tree species according to the study sites were downy birch, Norway spruce and Scots pine and all stands were naturally regenerated. However, in the spruce stand and in the pine stand, some birch trees were growing as secondary species whose basal area (BA) formed roughly 15% and 10% of total stand BA, respectively.
The soil of the study sites was classified according to World Reference Base for Soil Resources (FAO 2006) (Table 2). The region’s long term average precipitation is 650 mm, average temperature is 17 °C in July and –6.7 °C in January and the growing season usually lasts 175–180 days (Kupper et al. 2011). Soil pits were dug at all sites to estimate the soil type as well as its bulk density. The soils and the site types of the birch and spruce stands were similar and the site type was classified as ODS. The pine stand was growing on MDS.
| Table 2. Soil characteristics of the studied stands. C – organic carbon (%), N – Kjeldahl nitrogen (%), P – available (AL) phosphorus (mg kg–1), K 620 available (AL) potassium (mg kg–1), average concentrations are presented for the upper 10 cm soil layer (n = 5), World Reference Base for soil resources (WRB). View in new window/tab. |
2.2 Incubation method
The NNM experiment was performed by using the method with incubated polyethylene bags (Eno 1960; Uri et al. 2008; Becker et al. 2015, 2016). Polyethylene bags ensure permeability to gases (O2, CO2, N2, etc.), but prevent leaching and the input of soluble N, as well as the direct nitrogen uptake by plants. The dynamics of NNM was studied in the 0–10 cm soil layer at all three study sites from May 2014 to June 2015. At all sites, sampling and incubation were performed at an approximately monthly interval, which has been reported to be an optimal period for changes in the concentration of the mineral forms of N (Adams et al. 1989), and has also been applied in our earlier studies (Uri et al. 2003, 2008, 2011; Becker et al. 2015, 2016). At each sampling session, 24 samples for incubation were taken from all study sites by using a cylindrical soil corer (Ø 48 mm). The internal diameter of the inner part of the corer was 1.6 mm larger than the diameter of the cutting edge to avoid compression of the soil. The intact soil cores were packed in polyethylene bags with a thickness of 18 µm and incubated inside the same hole. Simultaneously with the incubation of a new sample, an adjacent initial sample was taken next to the incubated sample each time. Both the incubated and the initial samples from each sampling place were collected separately and gathered then by three in order to form eight composite samples from the incubated and initial samples, which were transported to the laboratory on the same day. Sampling was done monthly throughout the year, except when the soil was frozen. A more detailed description of the incubation methods has been published earlier in Uri et al. (2003, 2008) and Becker et al. (2015, 2016).
2.3 Litter collection
Aboveground litter was collected in 2015 using seven litter traps installed at each of the three study sites. In the downy birch stand the collecting area of a litter trap was 0.36 m2 and in the coniferous stands it was 0.53 m2. Litter was collected from the litter traps at an approximately monthly interval. However, in the autumn when litter fall in the birch stand was more intensive the interval was about two weeks. All collected litter was taken to the laboratory and dried to constant weight. After drying, litter was fractioned according to the tree species growing at the site and all fractions were weighed. As the decomposition of leaf and needle litter is faster compared to branch litter and affects net nitrogen mineralization the most, we considered only the leaf and needle fractions of litter.
2.4 Soil chemical analysis
The Tecator ASN 3313 was employed for testing the soil samples for nitrogen after Kjeldahl. Soil NO2–-N, NO3–-N and NH4+-N were determined by flow injection analysis with the Tecator ASN 65-32/84 and the Tecator ASN 65-31/84. Soil pH was determined in a 2.5:1 KCl soil (vol/wt) suspension. Available P and K were extracted with ammonium lactate (0.1 M NH4CH3CH(OH)COO– + 0.4M CH3COOH, pH 3.75). Available phosphorus in the extraction solution was determined by flow injection analysis with of the Tecator ASTN 9/84 and the content of available potassium was determined from the same solution by the flame photometric method. For determination of Corg content in the oven-dried samples, the combustion method (1150 °C) was applied using a varioMAX CNS elemental analyser (ELEMENTAR, Germany).
The samples of litter were analysed for total Kjeldahl nitrogen (N). The block digestion and steam distillation methods were used for testing the plant material (leaves and needles) for N concentration (Tecator AN 300).
2.5 Statistical analysis of the data
Normality of variables was checked using the Lilliefors and Shapiro-Wilk’s tests. For multiple comparisons of means, in case the assumptions were satisfied, the t-test was employed to compare the two group means. The correlation matrix was used to estimate relationships. In all cases the level of significance 0.05 was accepted. STATISTICA 7 (StatSoft, Inc., 2013) software was used in all cases.
3 Results
3.1 The soil nitrogen pool and the seasonal dynamics of soil mineral nitrogen
The total N pool for the upper 10 cm soil layer was higher in the birch and spruce stands growing on Drainic Eutric Histosols and it was estimated roughly at 5 t N ha–1. In the pine stand on Drainic Mesic Histosol, the total N pool for the upper 10 cm soil layer was almost 2 t ha–1 (Table 2). The average concentrations of mineral N for the different months were higher in the birch and spruce stands and were significantly lower in the well-drained Myrtillus-type pine stand (Fig. 1). There was no significant difference in annual (whole-year dataset) mineral N concentrations in the soil between the spruce and birch stands (t-test, p > 0.05). However, when comparing soil N concentrations in the different months for the birch and spruce stands, then they were significantly higher for the spruce stand in May 2014, November 2014 and May 2015 (t-test, p < 0.05) (Fig. 1).
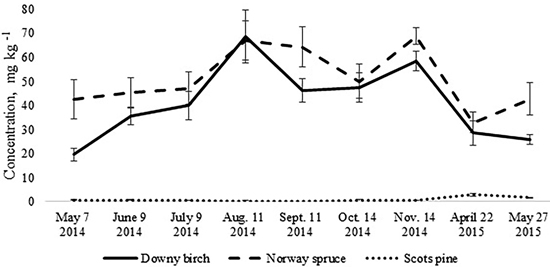
Fig. 1. Mean concentrations of soil mineral nitrogen in the upper 10 cm soil layer of birch, spruce and pine stand. Bars indicate the values of standard error, n=8 (24 replicates pooled by three).
The annual dynamics of soil mineral N concentrations was significantly different for the studied Oxalis and Myrtillus fully drained forest sites (Fig. 1). Both in the birch and spruce stands, soil mineral N concentration peaked in August and November, amounting almost to 70 mg kg–1 (Fig. 1). In the pine stand, the mineral N concentration varied between 0.1–2.8 mg kg–1 throughout the year and peaked in April when it was only up to 3 mg kg–1 (Fig. 2c). In the birch and spruce stands the highest share of soil mineral N was formed of NO3-N, while in the pine stand NH4-N was the dominating form of mineral N in the upper soil layer (Fig. 2). Also soil pH was significantly lower in the pine stand (Table 2). The pH of the incubated samples in the birch stand varied between 4.7 and 4.8, in the spruce stand between 4.3 and 4.5 and in the pine stand 2.5 and 2.8. There was no difference between the pH values of the initial and the incubated samples at all studied sites (t-test, p > 0.05). Although NH4-N was almost missing in the soil of the birch stand, it made up 30% of total mineral N in the spruce stand in the different months (Fig. 2).
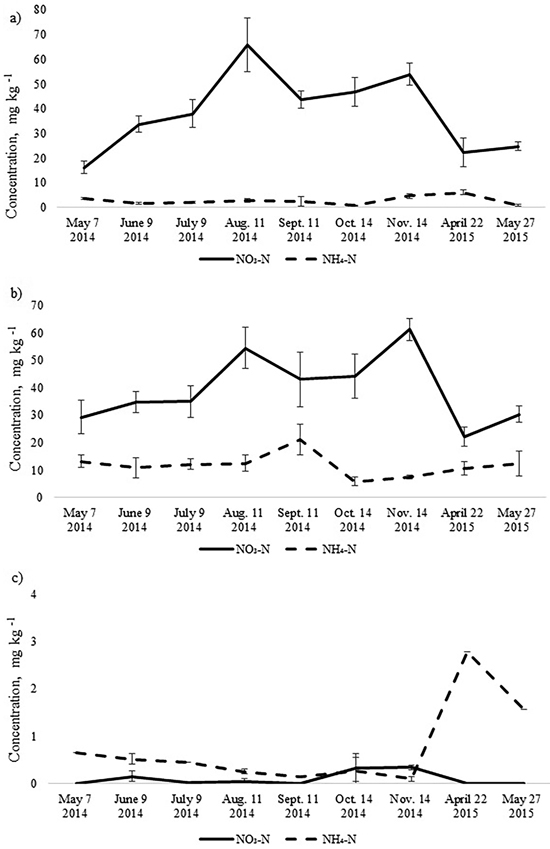
Fig. 2. Mean concentrations of soil NO3-N and NH4-N in the upper 10 cm soil layer of a) birch, b) spruce and c) pine stand. Bars indicate the values of standard error, n=8 (24 replicates pooled by three).
3.2 Annual net nitrogen mineralization
3.2.1 Birch and spruce stands
The intensity of annual NNM was the highest in the birch stand and the lowest in the pine stand (Fig. 3). In the birch stand a peak of NNM occurred in July 2014 (t-test, p < 0.05), when NNM intensity exceeded 120 mg N kg day–1, and another peak (109 mg N kg day–1) occurred in June 2015 (t-test, p < 0.05). The rate of net nitrification was very high in the birch stand whereas the rate of ammonification was even negative during seven months. There was positive correlation between NNM intensity and soil temperature for the birch stand (r = 0.78, p = 0.013). However, when we considered the net nitrification and the net ammonification processes separately, positive correlation occurred only between soil temperature and net nitrification (r = 0.80, p = 0.009). There was no correlation between soil pH and NNM intensity between soil moisture content and NNM intensity for the birch stand.
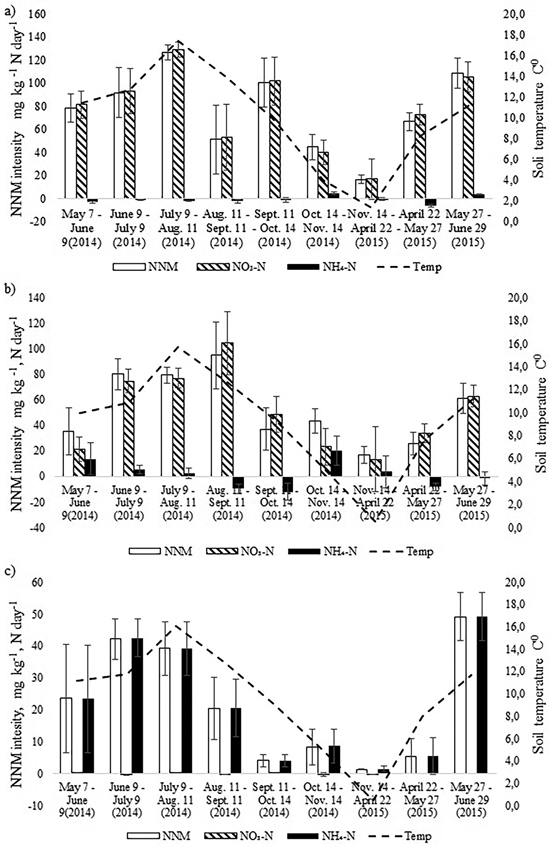
Fig. 3. Dynamics of monthly average soil temperature in the upper 10 cm soil layer and the dynamics of net nitrogen mineralization (NNM) intensity (mg kg–1 N day–1) in the a) birch, b) spruce and c) pine stand in 2014–2015. Bars indicate the values of standard error.
In the spruce stand, likewise, net nitrification was the dominating process and net ammonification was negative during four months in the study period (Fig. 3). The NNM intensity peaked one month later than in the birch stand (in August), being as high as 100 mg N kg day–1 and another peak (61 mg N kg day–1) occurred in May (t-test, p < 0.05). There was also strong correlation between NNM intensity and soil temperature (r = 0.79, p = 0.012) as well as between NO3-N and soil temperature (r = 0.79, p = 0.011). The share of net ammonification in total NNM was larger compared with the birch stand, but like for the birch stand, there was no correlation between net ammonification and soil temperature or between pH and NNM intensity.
Annual cumulative NNM was significantly higher in the birch stand compared with the spruce stand (t-test, p = 0.045) (Table 3), being almost 130 kg ha–1, which made up 2.4% of the soil N pool of the upper 0–10 cm. In the spruce stand the annual NNM flux was 88 kg ha–1, which accounted for about 1.7% of the upper 0–10 cm soil N pool. At the same time, in both stands the share of net nitrification made up 95%. The share of net ammonification in total NNM in May and October was substantial in the spruce stand (Fig. 3). Yet because of the microbial immobilization of ammonium nitrogen in the other months, total annual net ammonification was still low.
| Table 3. Annual net nitrification (NO3-N), annual net ammonification (NH4-N), total net nitrogen mineralization (NNM) (kg ha–1) and the share of net nitrification (%) in the studied stands. | ||||
| Study site | NO3-N ha | NH4-N ha | NNM | Nitrification |
| Downy birch | 129.3 | –1.7 | 127.5 | 100.0 |
| Norway spruce | 83.3 | 4.4 | 87.7 | 94.9 |
| Scots pine | –0.1 | 11.8 | 11.8 | 0.0 |
3.2.2 Pine stand
The intensity of NNM in the pine stand had a different pattern form that of the birch and spruce stands which were growing on a more fertile drained peat soil. Overall, it was much lower with peaks in June in both 2014 and 2015. Only in June 2015 was it significantly higher than in the other months (t-test, p < 0.05) (Fig. 3). Net ammonification was the highest in the pine stand where nitrification was practically missing. Positive correlation between soil temperature and NNM intensity was found also for the pine stand (r = 0.74, p = 0.023), but there was no correlation between soil pH and NNM intensity. In all cases, NNM intensity was the lowest in the autumn months (October and November) and cumulative annual NNM was only 12 kg ha–1 (Table 3).
3.3 Nitrogen input into soil through litter fall
The annual litter flux in 2015 was around 4 t ha–1 at all study sites (Table 4). To calculate the N input into the soil through litter, only leaf litter and needle litter were taken into account as the corresponding fractions have the highest decomposing rate and form the main share of the annual litter flux. In the birch stand, leaf litter was 3.1 t ha–1 and contributed the most to the N input (36 kg N ha–1) into the soil among the study sites. In the spruce stand, the needles and the birch leaves produced 2.8 t ha–1 and their total N input into the soil through litter was 25 kg N ha–1 (Table 4). In the pine stand, the N input into the soil through litter was the smallest (10 kg N ha–1) and N concentrations in the leaves and needles were also the lowest.
| Table 4. Litter characteristics of the studied stands (n = 7). | ||||||||||
| Stand | Leaves (t ha–1) | Needles (t ha–1) | Branches (t ha–1) | Other (t ha–1) | Total | N concentration (%) | N input (kg ha–1) | C/N | ||
| Leaves | Needles | Leaves | Needles | |||||||
| Birch | 3.1 | 0.4 | 0.1 | 3.7 ± 0.18 | 1.14 | 35.7 | 42 | |||
| Spruce | 0.8 | 2.0 | 0.9 | 0.6 | 4.3 ± 0.16 | 1.11 | 0.80 | 24.9 | 59 | |
| Pine | 0.3 | 2.1 | 0.5 | 0.9 | 3.8 ± 0.15 | 0.96 | 0.34 | 10.0 | 157 | |
4 Discussion
4.1 Soil nitrogen pool and its dynamics
4.1.1 Birch and spruce
A large soil nitrogen (N) pool is typical of drained eutrophic swamp forests (Westman and Laiho 2003). In this study the total soil N pool in the 0–10 cm upper soil layer in the birch and spruce stands was about 5 t N ha–1 and in the soil layer up to 40 cm it even amounted to 20 t N ha–1 (Table 2). This exceeds to considerably the N storages reported for mineral soils of the boreal and hemiboreal forest ecosystems (Gundersen 1995; Uri et al. 2003, 2008; Becker et al. 2015, 2016). The large total soil N storage, inherent in organic soils, can be explained by presence of a thick peat layer and high N concentration of peat. However, a large soil N pool does not reflect the amount of N that is actually available for uptake by plants. According to Helmisaari (1995), typically only about 0.1–1% of the soil N pool is available for plants in the form of inorganic N in boreal forests. The corresponding share of 1–3.5% in the upper soil N pool has been found to be available as mineral N (Baldock and Nelson 2000; Uri et al. 2008; Becker et al. 2015). In this study the share of mineral N of the total N pool in the upper 0–10 cm soil layer was in a similar range and varied from 1.7% to 2.4% in the spruce and birch stands, respectively.
The annual dynamics of soil mineral N in the birch and spruce stands demonstrated a similar pattern. There was no difference between these stands in mineral N concentration in the upper 0–10 cm soil layer over the whole-year dataset (t-test, p > 0.05). In both stands, soil mineral N concentration peaked in August and November 2014. The increase of soil mineral N in autumn is a typical pattern which can be explained mainly by the ceased N uptake by plants and by the input of fresh organic matter through litter (Uri et al. 2003, 2008; Becker et al. 2015). Mineral N concentration in the soil was low in spring and increased up to autumn, which is in good accordance with the dynamics of NNM intensity. High soil mineral N concentration during the vegetation period (Fig. 1) is the result of favourable conditions that promote NNM.
4.1.2 Pine stand
As the pine stand was growing on a relatively poor drained oligotrophic peat soil, the content and dynamics of mineral N was different from those of the birch and spruce stands. The total soil N pool in the pine stand was significantly smaller, amounting to 1.9 t N ha–1 in the upper 10 cm soil layer. Also the content of soil mineral N was several times lower, which is mainly related to soil properties and site history. It is well known that the concentration of soil mineral N depends on the intensity of NNM and, on the other hand, on plants uptake. The low share of mineral N of the upper soil layer (0.6% of the total soil N pool) in the pine stand could be explained by low NNM intensity. Ammonium N was the prevailing form of mineral N in the pine stand, whereas nitrate N concentration was negligible in all months. The concentration of soil ammonium N varied between 0.45 and 2.8 mg kg–1 during the whole study period. This estimate can be considered low since it is roughly ten times as low as the corresponding estimate for a Rhodococcum pine stand in Estonia (Kurvits et al. 2004).
4.2 Annual NNM and litter N input
4.2.1 Birch and spruce stands
In this study N mineralization occurred in the upper 0–10 cm soil layer, which is the most active soil part. The upper soil layer is characterized by higher nutrient content, higher microbial biomass and activity as well as the largest fine root biomass (Lõhmus et al. 2006; Uri et al. 2009; Aosaar et al. 2013; Varik et al. 2013). Moreover, according to several studies, most of NNM occurs in the topsoil layer. Connell et al. (1995) concluded that 75–85% of NNM generally takes place in the upper 0–20 cm soil layer. Persson and Wirén (1995) have reported that on average 78% of NNM occurs in the 0–10 cm topsoil layer and the remaining 22%, in the 10–50 cm layer of the mineral soil.
The buried bag method used in situ is one of the most common methods for in situ NNM studies (Hanselman et al. 2004; Duran et al. 2012) and reflects the actual rate of N mineralization in the soil. Also the method with the covered cylinders may give similar results assuming that the diameter of the incubated soil core is sufficiently large (5 cm) (Hanselman et al. 2004; Duran et al. 2012).
NNM is mainly affected by soil temperature and pH (Tietema and Verstraten 1992) while the effect of soil moisture is not very clear (Uri et al. 2008; Becker et al. 2015, 2016). The effect of soil temperature on NNM intensity was revealed also in the present study, but the effects of pH and soil moisture on NNM were not detected. Although it has been reported that the concentration of soil nitrate N is largely affected by temperature and drainage while the concentration of soil ammonium N depends on soil moisture and precipitation rate (Glina et al. 2016), we did not find any positive correlation between NH4-N and soil moisture. At the same time, the nitrification process was more favoured in the birch stand than in the spruce stand despite the fact that both were growing on peat soils with similar properties and N content (Table 2). Nitrification was also the major process of N transformation in the spruce stand while ammonification accounted for roughly 5% of total NNM. In the birch stand, the rate of nitrification was 100% (Table 3). Lower nitrification in the spruce stand can partly be explained by lower soil pH (Table 1), which is evidently the result of more acid needle litter. The favourable range for nitrification is from pH 3.9 to 6.3 (Van Praag and Weissen 1973); low pH often restricts nitrification and almost no nitrification can be detected at pH < 4 (Persson and Wiren 1995). As in both stands the pH values were higher than 4, nitrification was a favoured process in both of them.
Also the annual cumulative NNM flux was different in the birch and spruce stands growing on fertile drained Drainic Eutric Histosols, which are nutrient rich and whose pH is relatively high. Annual NNM in the birch and spruce stands was estimated roughly at 130 kg N ha–1 yr–1 and 90 kg N ha–1 yr–1, respectively. This difference is likely due to the difference in the species composition of these stands. Several studies indicate that also the quantity and quality of soil organic matter have a significant effect on NNM intensity, the C/N ratio being one of the key factors (McClaugherty et al. 1985; Zak et al. 1990; Tietema et al.1993). According to literature data (Aber et al. 1989; Scott and Binkley 1997; Magill et al. 2000; Uri et al. 2011; Becker et al. 2015), the annual NNM in deciduous stands varies between 24 kg ha–1 yr–1 and 200 kg ha–1 yr–1 and can usually cover a major part of the annual N demand of these stands. An earlier study on mineral soil in Estonia found the annual N mineralization flux to be only 24 kg N ha–1 yr–1 in a spruce stand (Pajuste and Frey 2003). At a site with low fertility the C/N ratio for a spruce stand growing on Haplic Podzol was between 28–31 kg N ha–1 yr–1 (Pajuste and Frey 2003), which is much higher than the corresponding ratio in our the present study. In a spruce stand, after a clear cut for the purpose of stump harvesting, the annual NNM flux was as high as 200 kg N ha–1 yr–1 (Becker et al. 2016). In the present study the soil C/N ratio for the upper 0–10 cm layer in the birch and spruce stands was 15.3 and 12.3, respectively (Table 2). These figures are of the same magnitude as those found for fertile forest site types on mineral soils (Cools et al. 2014; Uri et al. 2014, 2015; Varik et al. 2015; Becker et al. 2015, 2016). In Estonian birch stands, aged 13 to 45 years and growing on fertile mineral soils, the soil C/N ratio for the A-horizon has been evaluated to range between 13.6 and 15.8 (Varik et al. 2015).
Soil nitrogen dynamics is strongly related to the carbon cycle (C). Both the downy birch stand (Uri et al. 2017) and spruce stand (unpublished data) were highly productive and acted as C sinks. In forests growing on drained organic soils, the high C uptake by trees can often compensate for intensive soil respiration (Minkkinen et al. 2002; Hargreaves et al. 2003) and in terms of productivity and more effective C sequestration, available soil N is crucial. Stand productivity depended on the co-effect of multiple factors; high annual NNM intensity is one factor which ensures vigorous growth of trees and hence high C accumulation. As a result of the long term drainage of swamps, there has emerged a specific fertile site type which is very favourable for forest management. However, in order to increase the C sequestration ability of stands, also tree species play an important role. Further, reforestation of these areas after clear-cut by more productive tree species like black alder (Alnus glutinosa (L.) Gaertn) or Norway spruce (P. abies) would be a reasonable option. Still, it should be noted that, despite their high productivity, spruce stands on organic soils may be sensitive to wind throw.
Annual NNM in forest soils has often two peaks, one at the beginning and the other one at the end of the growing season (Nadelhoffer et al. 1984; Uri et al. 2003). In the current study NNM mineralization in both stands peaked at the early in the vegetation period in both years (2014 and 2015). The second peak in the rate of NNM mineralization at the end of the vegetation period occurred in the birch stand but not in the spruce stand.
The negative net ammonification fluxes noted for the spruce stand in the four months of the study period (Fig. 3) can most probably be explained by N immobilization or by gaseous losses of denitrification (Maag and Vinther 1996). Negative available ammonium was oxidized into nitrate.
One characteristic that describes the influence of tree species on NNM is the difference in their litter quality. The difference in the annual NNM flux between the birch stand and the spruce stand was about 30% and the difference in the annual N fluxes that reached the soil through leaf litter and needle litter has the same range (Table 4). Our result about the annual N input into the soil through needle litter is in good accordance with earlier studies (Pajuste and Frey 2003) where the N input into the soil through spruce needles was estimated at 24.2 kg N ha–1 yr–1. The impact of tree species on N cycling in forest ecosystems resulted from different soil properties (Lovett et al. 2004). The decomposition rate of litter is strongly affected by leaf nitrogen content (Cotrufo et al. 1995). In this study N content was the highest in the birch leaves and the C/N ratio, which is an essential factor in terms of litter decomposition, was about one third smaller for the birch leaves compared with the spruce needles.
4.2.2 Pine stand
In the studied pine stand, annual NNM was significantly lower than in the birch or spruce stand, being about 12 kg N ha–1 yr–1. This can be explained by lower site fertility, on the one hand, and by the quality of organic litter, on the other. The pine stand is growing in the MDS site type with Drainic Mesic Histosols, which are known as relatively nutrient poor soils but suitable for pine. In the present case the fertility of the soil is strongly affected by site history; MDS is the result of long term drainage of transitional (mesotrophic) bog. The fertility of such sites is mainly influenced by the previous drainage situation, but first and foremost, by the thickness of the peat layer. Drainage intensity also plays a crucial role; annual NNM tends to be lower in poorly drained soils than in well-drained soils (Ullah and Moore 2009). In the present case, the peat layer was quite thin, having attained a thickness of only up to 45 cm after the almost 50-year drainage period. Similar or even lower values of NNM in pine stands (6–24 kg ha–1 yr–1) have been reported earlier from Estonia (Pajuste and Frey 2003; Külla et al. 2004). In a study from Sweden, negative N mineralization occurred in pine stands growing on sandy soils (Vestgarden et al. 2003).
The share of net nitrification and net ammonification processes at the studied sites was different, which can be explained by soil fertility and reaction. It is known that low pH restricts nitrification and almost no nitrification can be detected at pH < 4 (Persson and Wiren 1995); in the studied pine stand average pH was 2.6. (Table 2). When in the birch and spruce stands nitrification was the dominating process, then in the pine stand ammonification was prevailing. The NNM flux in the pine stand was also strongly affected by litter quality. It was estimated that about 10 kg of N reached the soil annually through litterfall, which was about three times lower than in the birch and spruce stands. The NNM flux is clearly affected by the litter C/N ratio as for the pine stand the litter C/N ratio was the highest among the studied stands.
5 Conclusion
In the studied drained peatlands, the annual net nitrogen mineralization (NNM) flux was largely influenced by the post-drainage peatland type and the tree species of stand. The annual NNM flux was large in fertile full-drained eutrophic swamps; it was lower in the spruce stand (87.7 kg N ha–1 yr–1) than in the downy birch stand (127.5 kg N ha–1 yr–1); this difference was related to the quality of aboveground litter. Thus both N content and the litter C/N ratio are important indicators of litter affecting NNM.
The annual NNM flux in the pine stand growing in a full-drained transitional bog was significantly smaller (11.8 kg N ha–1 yr–1), which was due to the co-effect of the soil and the ree species. Myrtillus full-drained swamp has evolved from transitional bog after long-term intensive drainage, but soil fertility and especially soil N content in the peat soil of transitional bog are significantly lower compared with eutrophic swamp. Moreover, N content is much lower in the needle litter of Scots pine than in the birch leaves or spruce needles.
The nitrification process was limited by soil pH: nitrification was dominating in the fertile drained peat soil with higher pH and N content, whereas ammonification dominated in the pine stand in low-fertility drained transitional bog. The high NNM rate indicates that drained peat soils can be as fertile as mineral soils, while the NNM flux depends in turn largely on soil properties and on tree species.
Acknowledgements
The research project “Carbon and nitrogen cycle in drained forests” (2013–2016), based of which the current study was completed, was supported by the State Forest Management Centre of Estonia No 1-18/113. The research was also supported by the Ministry of Education and Research of Estonia (grants IUT21-04 and IUT2-16); and the EU through European Regional Development Fund project BioAtmos (3.2.0802.11-0043) and Centres of Excellence ENVIRON and EcolChange. Also by the project of the Estonian University of Life Sciences T170053MIMK as well as by the Environmental Investment Centre project No. 5725. We thank Mrs. Ester Jaigma for revising the English text of the manuscript.
References
Aber J.D., Nadelhoffer J.K., Steudler P., Melillo J.M. (1989). Nitrogen saturation in Northern forest ecosystems. BioScience 39(6): 378–386. https://doi.org/10.2307/1311067.
Adams M.A., Polglase P.J., Attiwill M.P., Weston C.J. (1989). In situ studies on nitrogen mineralization and uptake in forest soils: some comments on methodology. Soil Biology and Biochemistry 21(39): 423–429. https://doi.org/10.1016/0038-0717(89)90154-5.
Ahti T., Hämet-Ahti L., Jalas J. (1968). Vegetation zones and their sections in north-western Europe. Annales Botanici Fennici 5: 169–211.
Andersson P., Berggren D., Nilsson I. (2002). Indices for nitrogen status and nitrate leaching from Norway spruce (Picea abies (L.) Karst.) stands in Sweden. Forest Ecology and Management 157(1–3): 39–53. https://doi.org/10.1016/S0378-1127(00)00651-4.
Aosaar J., Varik M., Lõhmus K., Ostonen I., Becker H., Uri V. (2013). Long-term study of above- and below-ground biomass production in relation to nitrogen and carbon accumulation dynamics in a grey alder (Alnus incana (L.) Moench) plantation on former agricultural land. European Journal of Forest Research 132(5–6): 737–749. https://doi.org/10.1007/s10342-013-0706-1.
Baldock J.A., Nelson P.N. (2000). Soil organic matter. In: Sumner M.E. (eds.). Handbook of soil science. CRC Press LLC, Boca Raton, USA. B25-B84.
Becker H., Aosaar J., Varik M., Morozov G., Kanal A., Uri V. (2016). The effect of Norway spruce stump harvesting on net nitrogen mineralization and nutrient leaching. Forest Ecolology and Management 377: 150–160. https://doi.org/10.1016/j.foreco.2016.07.005.
Becker H., Uri V., Aosaar J., Varik M., Mander Ü., Soosaar K., Hansen R., Teemusk A., Morozov G., Kutti S., Lõhmus K. (2015). The effects of clear-cut on net nitrogen mineralization and nitrogen losses in a grey alder stand. Ecological Engineering 85: 237–246. https://doi.org/10.1016/j.ecoleng.2015.10.006.
Birdsey R., Pan Y. (2015). Trends in management of the world’s forests and impacts on carbon stocks. Forest Ecology and Management 355: 83–90. https://doi.org/10.1016/j.foreco.2015.04.031.
Cools N., Vesterdal L., De Vos B., Vanguelova E., Hansen K. (2014). Tree species is the major factor explaining C:N ratios in European forest soils. Forest Ecology and Management 311: 3–16. https://doi.org/10.1016/j.foreco.2013.06.047.
Connell M.R., Raison R.J., Khanna P.K. (1995). Nitrogen mineralization in relation to site history and soil properties in a range of Australian forest soils. Biology and Fertility of Soils 20(4): 213–220. https://doi.org/10.1007/BF00336080.
Cotrufo M.F., Ineson P., Roberts J.D. (1995). Decomposition of leaf litters with varying C-to-N ratios. Soil Biology and Biochemistry 27(9): 1219–1221. https://doi.org/10.1007/BF00336080.
Duran J., Morse J.L., Groffman P.M. (2012). Comparison of in situ methods to measure N mineralization rates in forest soils. Soil Biology and Biochemistry 46: 145–147. https://doi.org/10.1016/j.soilbio.2011.12.005.
Eno C.F. (1960). Nitrate production in the field by incubating the soil in polyethylenebags. In: Proceedings of the Soil Science Society of America 24(4): 277–279. https://doi.org/10.2136/sssaj1960.03615995002400040019x.
FAO (2006). World reference base for soil resources. A framework for International classification, correlation, and communication. Rome. 128 p.
Glina B., Bogacz A., Woźniczka P. (2016). Nitrogen mineralization in forestry-drained peatland soils in the Stołowe Mountains National Park (Central Sudetes Mts). Soil Science Annual 67(2): 64–72. https://doi.org/10.1515/ssa-2016-0009.
Goodale C.L., Aber J.D. (2001). The long term effects of land-use history on nitrogen cycling in northern hardwood forests. Ecological Applications 11: 253–267. https://doi.org/10.1890/1051-0761(2001)011%5B0253:TLTEOL%5D2.0.CO;2.
Gundersen P. (1995). Impacts of nitrogen deposition: scientific background. In: Forsius M., Kleemola S. (eds.). Fourth Annual Synoptic Report, Helsinki. p. 9–18.
Hanselman T.A., Graetz D.A., Obreza T.A. (2004). A comparison of in situ methods for measuring net nitrogen mineralization rates of organic soil amendments. Journal of Environmental Quality 33(3): 1098–1105. https://doi.org/10.2134/jeq2004.1098.
Hargreaves K.J., Milne R., Cannell M.G.R. (2003). Carbon balance of afforested peatland in Scotland. Forestry 76(3): 299–317. https://doi.org/10.1093/forestry/76.3.299.
Helmisaari H.-S. (1995). Nutrient cycling in Pinus sylvestris stand in eastern Finland. Plant and Soil 168(1): 327–336. https://doi.org/10.1007/BF00029345.
Helmisaari H.-S., Makkonen K., Kellomäki S., Valtonen E., Mälkonen E. (2002). Below- and above-ground biomass, production and nitrogen use in Scots pine stands in eastern Finland. Forest Ecology and Management 165(1–3): 317–326. https://doi.org/10.1016/S0378-1127(01)00648-X.
Korjus H., Põllumäe P., Kangur A. (2015). Why do we need a research and demonstration area of forest management planning at Järvselja? Forestry Studies 63: 151–159.
Kupper P., Sõber J., Sellin A., Lõhmus K., Tullus A., Räim O., Lubenets K., Tulva I. Uri V. Zobel M., Kull O., Sõber A. (2011). An experimental facility for free air humidity manipulation (FAHM) can alter water flux through deciduous tree canopy. Environmental and Experimental Botany 72(3): 432–438. https://doi.org/10.1016/j.envexpbot.2010.09.003.
Külla T., Lõhmus K., Kurvits V., Seemen H. (2004). In situ net nitrogen mineralisation in the organic layer under a middle-aged Rhodococcum Scots pine (Pinus sylvestris (L.) stand on podzol. Forestry Studies 40: 176–186.
Laine J., Vasander H., Sallantaus T. (1995). Ecological effects of peatland drainage for forestry. Environmental Review 3(3–4): 286–303. https://doi.org/10.1139/a95-015.
Lovett G.M., Weathers K.C., Arthur M.A. (2002). Control of nitrogen loss from forested watersheds by soil carbon:nitrogen ratio and tree species composition. Ecosystems 5(7): 712–718. https://doi.org/10.1007/s10021-002-0153-1.
Lovett G.M., Weathers K.C., Arthur M.A., Schultz J.C. (2004). Nitrogen cycling in a northern hardwood forest: do species matter? Biochemistry 67(3): 289–308. https://doi.org/10.1023/B:BIOG.0000015786.65466.f5.
Luo Y., Su B., Currie W.S., Dukes J.S., Finzi A., Hartwig A., Hungate B., McMurtrie R.E., Oren R., Parton W.J., Pataki D.E., Shaw M.R., Zak D.R., Field C.B. (2004). Progressive nitrogen limitation of ecosystem responses to rising atmospheric carbon dioxide. Bioscience 54(8): 731–739. https://doi.org/10.1641/0006-3568(2004)054%5B0731:PNLOER%5D2.0.CO;2.
Lõhmus K., Kuusemets V., Ivask M., Teiter S., Augustin J., Mander Ü. (2002). Budgets of nitrogen fluxes in riparian grey alder forests. Archive für Hydrobiologie 13(3–4): 321–332. https://doi.org/10.1127/lr/13/2002/321.
Lõhmus K., Truu M., Truu J., Ostonen I., Kaar E., Vares A., Uri V., Alama S., Kanal A. (2006). Functional diversity of culturable bacterial communities in the rhizosphere in relation to fine-root and soil parameters in alder stands on forest, abandoned agricultural, and oil-shale areas. Plant and Soil 283(1–2): 1–10. https://doi.org/10.1007/s11104-005-2509-8.
Maag M., Vinther F.P. (1996). Nitrous oxide emission by nitrification and denitrification in different soil types and at different soil moisture contents and temperatures. Applied Soil Ecology 4(1): 5–14. https://doi.org/10.1016/0929-1393(96)00106-0.
Magill A.H., Aber J.D., Berntso G.M., McDowell W.H., Nadelhoffer K.J., Melillo J.M., Staudler P. (2000). Long-term nitrogen additions and nitrogen saturation in two temperate forests. Ecosystems 3(3): 238–253. https://doi.org/10.1007/s100210000023.
Martikainen P.J., Nykänen H., Grill P., Silvola J. (1993). Effect of a lowered water table on nitrous oxide fluxes from northern peatlands. Nature 366: 51–53. https://doi.org/10.1038/366051a0.
McClaugherty C.A., Pastor J., Aber J.D., Melillo J.M. (1985). Forest litter decomposition in relation to soil nitrogen dynamics and litter quality. Ecology 66(1): 266–275. https://doi.org/10.2307/1941327.
Meyer A., Tarvainen L., Nousratpour A., Björk R.G., Ernfors M., Grelle A., Kasimir Klemedtsson Å., Lindroth A., Räntfors M., Rütting T., Wallin G., Weslien P., Klemedtsson L. (2013). A fertile peatland forest does not constitute a major greenhouse gas sink. Biogeosciences 10: 7739–7758. https://doi.org/10.5194/bg-10-7739-2013.
Millard P., Sommerkorn M., Grelet G.-A. (2007). Environmental change and carbon limitation in trees: a biochemical, ecophysiological and ecosystem appraisal. New Phytology 175(1): 11–28. https://doi.org/10.1111/j.1469-8137.2007.02079.x.
Minkkinen K., Korhonen R., Savolainen I., Laine J., (2002). Carbon balance and radiative forcing of Finnish peatlands 1900–2100 – the impact of forestry drainage. Global Change Biology 8(8): 785–799. https://doi.org/10.1046/j.1365-2486.2002.00504.x.
Nadelhoffer K.J., Aber J.D., Melillo J.M. (1984). Seasonal patterns of ammonium and nitrate uptake in nine temperate forest ecosystems. Plant and Soil 80(3): 321–335. https://doi.org/10.1007/BF02140039.
Ojanen P., Minkkinen K., Penttilä T. (2013). The current greenhouse gas impact of forestry-drained boreal peatlands. Forest Ecolology and Management 289: 201–208. https://doi.org/10.1016/j.foreco.2012.10.008.
Paavilainen E., Päivänen J. (1995). Peatland forestry: ecology and principles Ecological studies 111. Springer, Berlin. https://doi.org/10.1007/978-3-662-03125-4.
Pajuste K., Frey J. (2003). Nitrogen mineralisation in podzol soils under boreal Scots pine and Norway spruce stands. Plant and Soil 257(1): 237–247. https://doi.org/10.1023/A:1026222831694.
Peltomaa R. (2007). Drainage of forests in Finland. Irrigation and Drainage 56(1): 151–159. https://doi.org/10.1002/ird.334.
Persson T., Wirén A. (1995). Nitrogen mineralization and potential nitrification at different depths in acid forest soils. Plant and Soil 168(1): 55–65. https://doi.org/10.1007/BF00029313.
Raison R.J., Conell M.J., Khanna P.K. (1987). Methodology for studying fluxes of soil mineral- N in situ. Soil Biology and Biochemistry 19(5): 521–530. https://doi.org/10.1016/0038-0717(87)90094-0.
Scott A.N., Binkley D. (1997). Foliage litter quality and annual net N mineralization: comparison across North American forest sites. Oecologia 111(2): 151–159. https://doi.org/10.1007/s004420050219.
Silins U., Rothwell R.L. (1999). Spatial patterns of aerobic limit depth and oxygen diffusion rate at two peatlands drained for forestry in Alberta. Canadian Journal of Forest Research 29(1): 53–61. https://doi.org/10.1139/x98-179.
Silvola J., Alm J., Ahlholm U., Nykanen H., Martikainen P.J. (1996). CO2 fluxes from peat in boreal mires under varying temperature and moisture conditions. Journal of Ecology 84(2): 219–228. https://doi.org/10.2307/2261357.
Stenger R., Priesack E., Beese F. (1995). Rates of net nitrogen mineralization in disturbed and undisturbed soils. Plant and Soil 171(2): 323–332. https://doi.org/10.1007/BF00010288.
Tate R.L. (1995). Soil microbiology. John Wiley & Sons, Inc, New York. 398 p.
Tietema A., Riemer L., Verstraten J.M., van der Maas M.P., van Wijk A.J., van Voorthuyzen I. (1993). Nitrogen cycling in acid forest soils subject to increased atmospheric input. Forest Ecology and Management 57(1–4): 29–44. https://doi.org/10.1016/0378-1127(93)90160-O.
Tietema A., Verstraten J.M. (1992). Nitrate cycling in an acid forest ecosystem in the Netherlands under increased atmospheric nitrogen input. Biogeochemistry 15: 21–46.
Ullah S., Moore T.R. (2009). Soil drainage and vegetation controls of nitrogen transformation rates in forest soils, southern Quebec. Journal of Geophysical Research: Biogeosciences 114: G01014. https://doi.org/10.1029/2008JG000824.
Uri V., Aosaar J., Varik M., Becker H., Kukumägi M., Ligi K., Pärn L., Kanal A. (2015). Biomass resource and environmental effects of Norway Spruce (Picea abies): an Estonian case study. Forest Ecology and Management 335: 207–215. https://doi.org/10.1016/j.foreco.2014.10.003.
Uri V., Aosaar J., Varik M., Becker H., Ligi K., Padari A., Kanal A., Lõhmus K. (2014). The dynamics of biomass production, carbon and nitrogen accumulation in grey alder (Alnus incana (L.) Moench) chronosequence stands in Estonia. Forest Ecology and Management 327: 106–117. https://doi.org/10.1016/j.foreco.2014.04.040.
Uri V., Lõhmus K., Kiviste A., Aosaar J. (2009). The dynamics of biomass production in relation to foliar and root traits in a grey alder (Alnus incana (L.) Moench) plantation on abandoned agricultural land. Forestry 82(1): 61–74. https://doi.org/10.1093/forestry/cpn040.
Uri V., Lõhmus K., Kund M., Tullus H. (2008). The effect of land use on net nitrogen mineralization on abandoned agricultural land: silver birch stand versus grassland. Forest Ecolology and Management 255(1): 226–233. https://doi.org/10.1016/j.foreco.2007.09.019.
Uri V., Lõhmus K., Mander Ü., Ostonen I., Aosaar J., Maddisson M., Helmisaari H.-S., Augustin J. (2011). Long-term effects on nitrogen budget of a short-rotation grey alder (Alnus incana (L.) Moench) forest in abandoned agricultural land. Ecological Engineering 37(6): 920–930. https://doi.org/10.1016/j.ecoleng.2011.01.016.
Uri V., Lõhmus K., Tullus H. (2003). Annual net nitrogen mineralization in a grey alder (Alnus incana (L.) Moench) plantation on abandoned agricultural land. Forest Ecology and Management 184(1–3): 167–176. https://doi.org/10.1016/S0378-1127(03)00210-X.
Uri V., Kukumägi M., Aosaar J., Varik M., Becker H., Morozov G., Karoles K. (2017). Ecosystems carbon budgets of differently aged downy birch stands growing on well-drained peatlands. Forest Ecology and Management 399: 82–93. https://doi.org/10.1016/j.foreco.2017.05.023.
Van Praag H.J., Weissen F. (1973). Elements of a functional definition of oligotrophic humus based on the nitrogen nutrition of forest stands. Journal of Applied Ecology 10(2): 569–583. https://doi.org/10.2307/2402302.
Varik M., Aosaar J., Ostonen I., Lõhmus K., Uri V. (2013). Carbon and nitrogen accumulation in belowground tree biomass in a chronosequence of silver birch stands. Forest Ecology and Management 302: 62–70. https://doi.org/10.1016/j.foreco.2013.03.033.
Varik M., Kukumägi M., Aosaar J., Becker H., Ostonen I., Lõhmus K., Uri V. (2015). Carbon budgets in fertile silver birch (Betula pendula Roth) chronosequence stands. Ecological Engineering 77: 284–296. https://doi.org/10.1016/j.ecoleng.2015.01.041.
Vestgarden L.S., Selle L.T., Stuanes A.O. (2003). In situ soil nitrogen mineralization in a Scots pine (Pinus sylvestris L.) stand: effects of increased nitrogen input. Forest Ecology and Management 176(1–3): 205–216. https://doi.org/10.1016/S0378-1127(02)00275-X.
Wang M., Talbot J., Moore T.R. (2018) Drainage and fertilization effects on nutrient availability in and ombrotrophic peatland. Sceiance of the Total Environment 621: 1255–1263. https://doi.org/10.1016/j.scitotenv.2017.10.103.
Westman C.J., Laiho R. (2003). Nutrient dynamics of drained peatland forests. Biogeochemistry 63(3): 269–298. https://doi.org/10.1023/A:1023348806857.
Yearbook Forest 2016 (2017). Aastaraamat Mets 2016. Keskkonnateabe Keskus, Tartu. [In Estonian].
Zak D.R., Grigal D.R., Gleeson S., Tilman D. (1990). Carbon and nitrogen cycling during old-field succession: constraints on plant and microbial biomass. Biochemistry 11(2): 111–129. https://doi.org/10.1007/BF00002062.
Total of 68 references.

