Birch sap exudation: influence of tree position in a forest stand on birch sap production, trunk wood anatomy and radial bending strength
Zajączkowska U., Kaczmarczyk K., Liana J. (2019). Birch sap exudation: influence of tree position in a forest stand on birch sap production, trunk wood anatomy and radial bending strength. Silva Fennica vol. 53 no. 2 article id 10048. https://doi.org/10.14214/sf.10048
Highlights
- Birch trees along the forest edge exude more xylem sap but less concentrated than the trees from the interior
- Radial bending strength of wood in birch trunk is higher in the trees from forest edge
- Trees exhibit higher bending strength in western side of the trunk, where the number of vessels and the wood potential conductivity index are smaller.
Abstract
It is commonly accepted that the period of early-spring xylem sap exudation marks a stage during which a positive pressure builds inside the tree trunks. This state changes when leaves appear, initiating water transport within the trunk. It is unknown, however, how the wood anatomical structure and its mechanical resistance influences the sap. We present the results of research on the relationship between exudation of sap from Betula pendula Roth trees from the interior of a forest stand and from its edge, and the anatomical structure of the trunk wood and its bending strength. During the period between March 21 and April 18, we performed five sets of measurements of sap exudation from trees at the edge of the stand and from the forest interior. The resulting radial wood samples were tested for bending strength using a fractometer. We tested the sap for electrolytic conductivity and sugars content. For the anatomical analysis of the wood, we determined the number of vessels per 1 mm2, average vessel lumen area and potential conductivity index. We found that the trees along the edge of the stand exude more sap, but it is less concentrated than the sap from the trees from the interior. Bending strength perpendicular to wood fibres is higher in the trees from the stand edge and in the western side of the trunk, where the number of vessels per 1 mm2 and conductivity index are smaller. Seemingly, this is the result of western winds, which are dominant in Poland.
Keywords
biomechanics;
wood anatomy;
forest edge;
xylem sap
-
Zajączkowska,
Department of Forest Botany, Faculty of Forestry, Warsaw University of Life Sciences, 159 Nowoursynowska St., 02-776 Warsaw, Poland
E-mail
urszula.zajaczkowska@wl.sggw.pl

- Kaczmarczyk, Department of Forest Botany, Faculty of Forestry, Warsaw University of Life Sciences, 159 Nowoursynowska St., 02-776 Warsaw, Poland E-mail karina.kaczmarczyk@wl.sggw.pl
- Liana, Department of Forest Botany, Faculty of Forestry, Warsaw University of Life Sciences, 159 Nowoursynowska St., 02-776 Warsaw, Poland E-mail janusz.liana@wl.sggw.pl
Received 23 September 2018 Accepted 7 May 2019 Published 27 May 2019
Views 84279
Available at https://doi.org/10.14214/sf.10048 | Download PDF

1 Introduction
In the spring, before the leaves develop, in many trees a physiological positive pressure system activates that can cause sap exudation when the trunk or branch is cut (Cirelli et al. 2008; Sevanto et al. 2012). This phenomenon has been a subject of interdisciplinary studies, with most works concerning Acer saccharum Marshall due to the unique character of its sap. However, few works have focused on the phenomenon in birch trees (Betula spp.), despite the extraction of sap from these trees being a tradition in eastern and north-eastern Europe (Tyree 1983; Sperry et al. 1988; Svanberg et al. 2012; Graf et al. 2015). Positive pressure within the trunk is the result of the interaction of various physiochemical phenomena. The most important of them seem to be the freezing and thawing of sap, the lowering of the osmotic potential of the sap, the freeing of osmotic-active elements to tracheal elements by living cells of rays or axial parenchyma, the lack of transpiration, and the root pressure (Johnson 1945; Zimmermann 1958; Wegner 2014; Charrier et al. 2014; Steppe et al. 2015). The atmospheric air temperature is an important environmental parameter that influences the metabolic level of living wood cells as well, and thus affects the osmotic pressure of water within the wood (Westhoff et al. 2008).
When the temperature gradient within the trunk is too high, the alignment of the various internal pressures within the stem may shift and the trunk might split deeply. This phenomenon is observed when, for example, ice forms within the trunk and tangential directional tension stress is generated, or conversely – during the thaw expansion – when daily environmental temperatures increase (Zweifel and Häsler 2000; Pearce 2001; Turcotte et al. 2009; De Swaef et al. 2015). This type of thermal split is more frequently seen in forest-edge trees, mainly due to uneven warming of the trunks. The forest edge is a very specific transitional zone between the environments. The trees in this zone grow partially immersed in a dense stand with increased humidity and with limited access to light, and partially in conditions of dispersed light, in a space with decreased humidity. This is manifested in asymmetrical tree crowns, eccentric piths, and the frequent occurrence of reaction wood inside the trunk (Timell 1986; Gardiner et al. 2014). The forest edge is thus an important zone in regard to the stability of the entire stand (Young and Hubbell 1991; Matlack 1994; Zajączkowska 2013; Tomczak et al. 2014).
So far, the idea of linking xylem sap properties with the wood strength and the anatomy concerning antagonistic consequences of increased osmotic pressure inside the stem during the early spring has not been studied. With respect to the wood resistance, it is especially interesting to compare the wood from silver birch trees from the edge and from the inside of the stand, as this is an especially light-demanding and pioneering species and thus often forms the forest edge. The aim of this work is to test the variation of early-spring sap exudation in mature birch trees growing both at the edge and inside a stand. The diversity of anatomical structures and the relative resistance of wood to bending, with respect to the side of the tree trunk from which it comes, were also investigated.
2 Methods
2.1 Study area
The study was carried out in the early spring of 2014 in a temperate-warm transitional climate zone in central-eastern Poland. The site has an average annual air temperature of 8 °C, a minimum average temperature in January of –3.7 °C, a maximum average temperature in July of 18.2 °C and an average annual precipitation of 500 mm. The average perennial air temperatures for March and April are 1.5 °C and 7.9 °C, respectively; the relevant data for average perennial precipitation are 25 and 33 mm. Poland is located in a changing winds zone, with the majority of winds hailing from the west (60%) (Błażejczyk 2006). A 60-year-old silver birch (Betula pendula Roth) stand was chosen for the study. It is located in the Drewnica Forestry Management District, GPS: 52°29´N, 21°23´E. The southern edge of the study stand is adjacent to a forest cultivation site and the northern sides of trunks of all trees at the forest edge were oriented to the inside of the forest. The trees for the research were chosen from an experimental plot located in the stand in a shape of rectangle with side dimensions 250×50 m with one of the longest sides adjacent to the forest edge. The remaining sides were located inside the birch forest stand. Average diameter at breast height of the trees used for the experiment was 36.0 (SD ± 2.1) cm.
2.2 Sap exudation measurement
On six different dates (March 21, March 28, April 4, April 11, April 18, and April 25, 2014), six trees were tested from the forest edge and six from the interior of the same stand, located at about 30–50 meters from the forest edge. At any given time, the distance between adjacent trees tested ranged from 20 to 50 meters. Exudation of sap was observed on the first five dates and on the last sampling date, April 25, no sap was exuded from trees, hence, this date is not included in further analyses. Each of the tested trees was drilled with a Pressler incremental drill at a height of 1.3 m. Four holes were made in each tree trunk, penetrating it to the pith. The drilling was started every time from the northern (N) side of the trunk and then successively from the southern (S), eastern (E), and western (W) sides. Each hole had a silicone tube fed through it, with the other end placed in a container located on the ground next to the tree trunk. This installation for sap collection was left next to the trees for 20 hours (Fig. 1a). Within an hour from the collection, the sap was analyzed in a laboratory. The measurement of sugars (summary glucose, fructose, and sucrose) was done using an optical refractometer (Carl Zeiss Jena 725202, T-VI-50, Germany), and the ionic content were measured with a conductivity (Siemens/cm) probe (Vernier Software and Technology, USA). At each date of the sample collection, bud bursting and emergence of young leaves was observed, and the results were as follows: March 21 – swollen buds, March 28 – swollen buds with broken scales, April 4 – broken scales with tips of leaves visible, April 11 – broken scales with small leaves with folded blades, April 18 – small partly unfolded leaves, April 25 – expanded young leaves. The temperature span during the measurement period was obtained from the Institute of Meteorology, Hydrology and Water Management in Warsaw (Table 1). The average data of air temperature for March and April 2014 was 6 °C and 10 °C, respectively and the relevant data for precipitation were 10 and 22 mm.
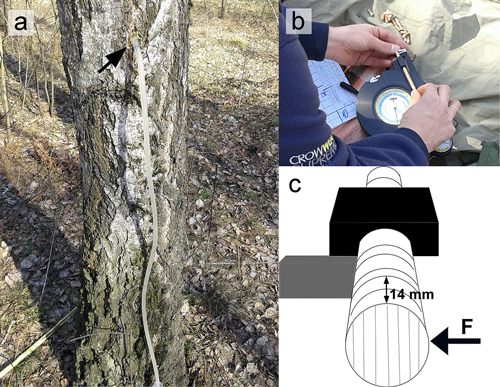
Fig. 1. Illustration of the birch sap exudation experiment. (a) Tree-trunk from the edge of the forest stand during sap exudation. (b) Mechanical tests using a fractometer. (c) Scheme of a bending strength measurement in a fractometer, consecutive 14 mm fragments of the core are broken across the fibres.
| Table 1. Daily temperature within the test period. | |||
| Temperature [°C] | |||
| Date | Avg. | Max. | Min. |
| March 21 | 13 | 22 | 4 |
| March 28 | 11 | 17 | 6 |
| April 4 | 7 | 12 | 2 |
| April 11 | 6 | 11 | 1 |
| April 18 | 6 | 9 | 3 |
2.3 Mechanical testing of wood
Immediately after each incremental core was made with the Pressler drill, the bending strength perpendicular to wood fibres was tested, using a Fractometer II (IML GmbH, Wiesloch, Germany) (Mattheck et al. 1995; Chiu et al. 2006; Barij et al. 2007). The core was placed into the device, aligned perpendicular to wood fibres, and broken with the bending lever (Fig. 1b, c). The maximum force necessary for the core break was recorded. The tests were carried out along the entire length of the incremental core at equal distances of 14 mm. The results from measurements of the incremental cores were averaged according to the side of the trunk from which they were taken, keeping the distinction between forest edge trees and those from the inside of the forest stand.
2.4 Anatomical studies of wood
At the end of October, six trees from the edge and six from the interior of the stand were cut down. Five cm thick discs were cut from the trunks at a height of 1.3 m, where the sap tests had been previously performed. From the discs, wood fragments with the dimensions of 2×10×5 cm were cut and boiled with glycerin (3:1). Next, using a sliding microtome, cross-section microscopic slices were made of each sample. Photographs of the slices were taken using an Olympus BX61 microscope and digitalized, and the images were analyzed in Wincell™ (Deslauriers et al. 2003). Anatomical measurements were performed on ten recently formed annual wood rings. The number of vessels per 1 mm2 of the cross-section area was determined, as was the average field of vessel lumen. With these parameters, the relationship between the vessel size and the number of vessels per 1 mm2 (S) and the vessel fraction (F) was calculated. F determines the area of the wood cross-section formed by the vessels. The higher the S value, the higher the participation of the wide vessels active in water transportation in the given area. Conversely, the lower the F indicator, the lesser the conductivity and, usually, the higher the wood mechanical resistance. The obtained parameters were then used to determine the potential conductivity index (PCI = F1.5×S0.5), used in wood conductivity measurements (Ks), where Ks α F1.5×S0.5 (Zanne et al. 2010; Zheng and Martinez-Cabrera 2013).
2.5 Statistical analysis
Three-factor mixed model ANOVA was applied to test the differences between the means of the studied xylem sap characteristics. The tests were performed separately for each of the three xylem sap parameters: volume, electrolytic conductivity, and sugar content. In each case, the within-subjects factor was the sample position around the stem circumference (E vs S vs W vs N) and the between-subjects factors were: (i) the dates of samples collections (March 21 vs March 28 vs April 4 vs April 11 vs April 18) and (ii) the location of trees at the forest edge or in the forest interior (FE vs FI). The effects of following factors were tested: (1) main within-subjects factor of the sample position on the tree stem, (2) main between-subjects factor – the date of sample collection, (3) main between subject factor – the location of the tree in the forest, (4) interaction between the date of sample collection and the location of tree in the forest, (5) interaction between the sample position on the tree stem and the date of sample collection, (6) interaction between sample position on the tree stem and the location of the tree in the forest. The two-factor mixed model ANOVA was applied to test differences between wood bending strength and anatomical characteristics of the xylem. In this case, the within-subjects factor was the sample position around the stem circumference (E vs S vs W vs N) and the between-subjects factor was the location of the trees at the forest edge or in the forest interior (FE vs FI).
The standard error of mean (SEM) is presented in the figures as the variability parameter. Pearson’s correlation coefficient was used to examine the relations between sap characteristics.
3 Results
3.1 Sap exudation dynamics
Over the course of the study, the quantity of sap exuded from tree trunks at the forest edge (FE) was larger than from the trees in the forest interior (FI) (Fig. 2a). The highest total quantity of sap from the readings from all four sides of the tree trunk collectively was noted on March 28 and subsequent readings yielded lower total volumes of sap. This decrease was especially marked in the trees from the interior of the forest stand. On the final date of the measurements, April 25, no sap flow was detected from any tree at either the edge or within the stand and this date is not included in figures and tables. Among the trees at the forest edge, the highest sap flow yield was recovered from core holes made in the northern side of the tree trunk, which was turned to the inside of the forest (Fig. 2b); this was also the point where the drilling was started. Similarly, the lowest sap flow yield was recovered from the core holes made in the western side of the trees, which was the final point of the measurement.
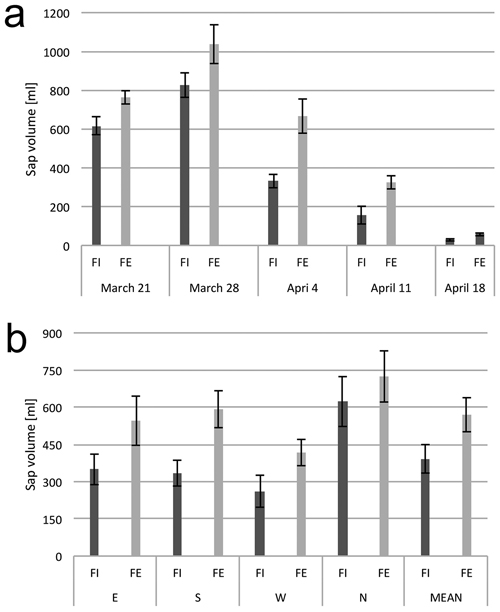
Fig. 2. Xylem sap flow from birch trunks. (a) Dynamics of sap flow from tree trunks from the interior (FI) and from the edge (FE) of the forest stand, at consecutive dates in the early spring. (b) Xylem sap flow from birch trunks from the interior (FI) and from the edge (FE) of the forest stand from the samples collected from four sides around the stem circumference (N, E, S and W). The means from 6 trees for (a) and from 30 trees sampled during the study period from March 21 to April 18 (b). Standard errors of means are indicated.
The values for electrolytic conductivity and sugars content of the wood sap varied over the study period. At each date, the conductivity of the sap collected from the trees on the forest edge was, on average, about 10–25% lower than that from the trees in the interior of the stand (Fig. 3a). On the date of the first measurement, March 21, the electrolytic conductivities had the lowest average values. During the three following weeks (until April 11), the conductivity value increased slightly and then decreased on the last noted day of sap flow on April 18 (Fig. 3b).
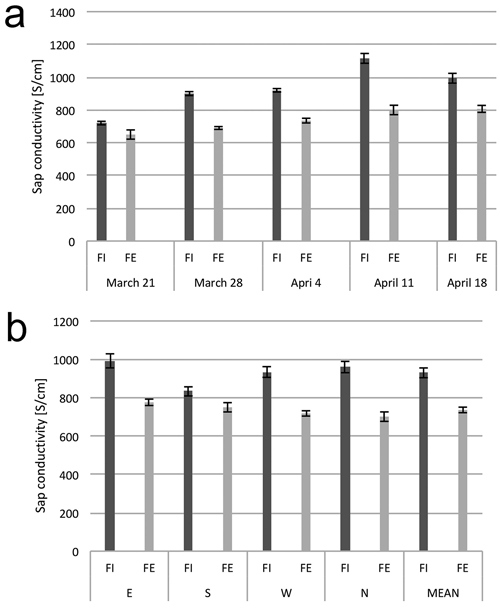
Fig. 3. Electrolytic conductivity of xylem sap. (a) Dynamics of electrolytic conductivity of the sap from birch trunks from the interior (FI) and from the edge (FE) of the forest stand, at consecutive dates in early spring. (b) Electrolytic conductivity of sap from birch trunks from the interior (FI) and from the edge (FE) of the forest stand from the samples collected from four sides around stem circumference (N, E, S and W). The means from 6 trees for (a) and from 30 trees sampled during the study period from March 21 to April 18 (b). Standard errors of means are indicated.
Sugar content was the highest in xylem sap samples collected on the first date, March 21, and then gradually decreased until the last date of xylem sap flow, April 18 (Fig. 4a). During the period of four weeks of xylem sap flow collection, the sugar content differed among the trees at the forest edge and in the interior of the forest; and slightly higher sugar concentrations were found in the sap from trees inside the stand (Fig. 4b).
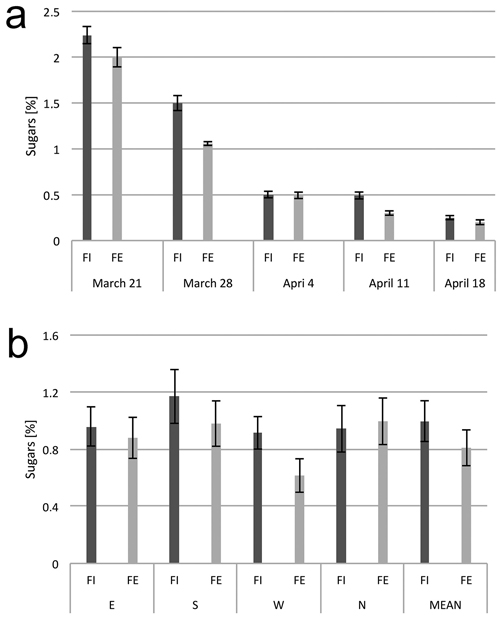
Fig. 4. Sugar content in the xylem sap. (a) Dynamics of sugar content in the sap from birch trunks from the interior (FI) and from the edge (FE) of the forest stand, at consecutive dates in the early spring. (b) Sugar content in the xylem sap from birch trunks from the interior (FI) and from the edge (FE) of the forest stand from the samples collected from four sides around the stem circumference (N, E, S and W). The means from 6 trees for (a) and from 30 trees sampled during the study period from March 21 to April 18 (b). Standard errors of means are indicated.
Results of the ANOVA test concerning significance of main and interaction factors are summarized in Table 2 in which the F and p values are shown for each of the tested xylem sap characteristics. All studied main within-subjects and between-subjects factors concerning three xylem sap characteristics seemed significant. It means that the xylem sap characteristics significantly differed depending on sample position around the tree trunk circumference, the date of the sample collection, and the location of the trees in the forest. Similar results were obtained when interaction between the sample position on the tree stem and the date of the sample collection or the location of the tree in the forest were tested. Analogous significant effects were observed for sap conductivity and sap sugars when interaction between the date of the sample collection and the location of the tree in the forest was tested. However, no significant effect of this interaction (p = 0.088) was found for the xylem sap volume.
| Table 2. Results of the three-factor mixed model ANOVA for testing differences between the means of xylem sap characteristics. ANOVA F and p values are shown for three tests performed separately for each of the three xylem sap parameters: volume, electrolytic conductivity and sugar content. | |||
| Type of effect | Source of variable | Xylem sap characteristics | ANOVA estimates F, p |
| Main within-subjects | Sample position on tree stem E vs S vs W vs N | Volume | F (3, 50) = 61.93; p < 0.001 |
| Conductivity | F (3, 50) = 15.26; p < 0.001 | ||
| Sugars | F (3, 50) = 25.83; p < 0.001 | ||
| Main inter-subjects | Date of sample collection 21.03 vs 28.03 vs 04.04 vs 11.04 vs 18.4 | Volume | F (4, 50) = 86.95; p < 0.001 |
| Conductivity | F (4, 50) = 48.12; p < 0.001 | ||
| Sugars | F (4, 50) = 386.39; p < 0.001 | ||
| Main inter-subjects | Location of tree in forest (FE vs FI) | Volume | F (1, 50) = 27.65; p < 0.001 |
| Conductivity | F (1, 50) = 207.42; p < 0.001 | ||
| Sugars | F (1, 50) = 26.89; p < 0.001 | ||
| Interaction | Date of sample collection × location of tree in forest | Volume | F (4, 50) = 2.15; p = 0.088 |
| Conductivity | F (4, 50) = 8.43; p < 0.001 | ||
| Sugars | F (4, 50) = 4.57; p = 0.003 | ||
| Interaction | Sample position on tree stem × date of sample collection | Volume | F (12, 50) = 42.19; p < 0.001 |
| Conductivity | F (12, 50) = 4.50; p < 0.001 | ||
| Sugars | F (12, 50) = 13.08; p < 0.001 | ||
| Interaction | Sample position on tree stem × location of tree in forest | Volume | F (3, 50) = 3.46; p = 0.018 |
| Conductivity | F (3, 50) = 15.18; p < 0.001 | ||
| Sugars | F (3, 50) = 12.25; p < 0.001 | ||
Pearson correlation for parameters of wood sap collected from 30 tree trunks over the full measurement period (March 21 – April 18) showed significant correlation coefficients (r) at p < 0.05 as follows: (i) between the sap volume and sap conductivity (r = –0.58, (ii) between the sap volume and sap sugars (r = 0.61), and (iii) between sap conductivity and sap sugar content (r = –0.34). Detailed data concerning Pearson correlation coefficients with respect to the sample position on the stem circumference are presented in Table 3.
| Table 3. Pearson-r correlation coefficients between xylem sap characteristics: volume, conductivity and sugar content in four sample positions (E, S, W, N) around stem circumference; * p < 0.050, **p < 0.001. View in new window/tab. |
3.2 Mechanical testing of wood
The results of measurements of 30 trees from the forest edge and 30 trees from the forest interior were averaged according to the side of the tree trunk and keeping distinction between the two locations in the forest (Fig. 5). When means across all measurements were compared, it was observed that the bending strength of tree trunks from the forest edge was higher as compared to those from inside the forest. Additionally, it was also noted that regardless of the location of the tree in the forest stand, the wood on the western side of the trunk had the largest average bending strength. The largest difference in resistance between the trees from inside the stand and the edge trees was noted in the wood samples from the north side of the trunk.
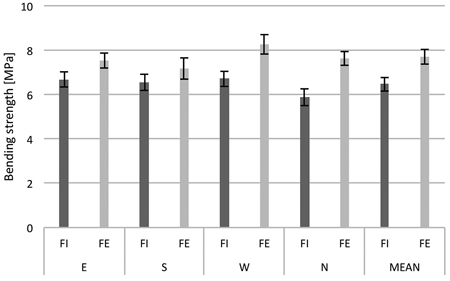
Fig. 5. Bending strength of birch trunk wood from the interior (FI) and from the edge (FE) of the forest stand; samples from four sides around the stem circumference (N, E, S and W). The means from 30 trees sampled during the study period (March 21 to April 18). Standard errors of means are indicated.
For more detailed statistical analysis of the biomechanical data, the two-factor mixed model ANOVA was applied to test the differences between wood bending strength characteristics. In this case, the within-subjects factor was the sample position around the stem circumference (E vs S vs W vs N) and the between-subjects factor was the location of trees at the forest edge or in the forest interior (FE vs FI) (Table 4). Analysis of variance showed a significance of both within-subjects and between-subjects main factors. This indicates that the samples of wood collected from the western side of the trunk (W) and from the trees located at the forest edge (FE) have significantly higher bending strength values than the samples from other sides of tree trunks and the trees from the forest interior (FI). No significant effect of interaction between the sample position around the stem circumference and the location of trees in the forest was found.
| Table 4. Results of the two-factor mixed model ANOVA for testing differences between the means of xylem structure characteristics. ANOVA F and p values are shown for four tests performed separately for each of the four xylem structure parameters: bending strength, average vessel lumen area, vessel number per 1 mm2 of wood cross-section and potential conductivity index (PCI). | |||
| Type of effect | Source of variable | Wood characteristics | NOVA estimates F, p |
| Main effect of within-subjects factors | Sample position on tree stem (E vs S vs W vs N) | Bending strength | F (3, 58) = 3.70; p = 0.013 |
| Vessel area | F (3, 10) = 0.22; p = 0.884 | ||
| Vessel number | F (3, 10) = 57.20; p < 0.001 | ||
| PCI | F (3, 10) = 6.20; p = 0.002 | ||
| Main effect of between subjects factors | Location of tree in forest (FE vs FI) | Bending strength | F (1, 58) = 7.18; p = 0.010 |
| Vessel area | F (1, 10) = 0.52; p = 0.487 | ||
| Vessel number | F (1, 10) = 33.36; p < 0.001 | ||
| PCI | F (1, 10) = 0.21; p = 0.654 | ||
| Interaction | Sample position on tree stem × location of tree in forest | Bending strength | F (3, 58) = 2.59; p = 0.054 |
| Vessel area | F (3, 10) = 0.06; p = 0.979 | ||
| Vessel number | F (3, 10) = 24.18; p < 0.001 | ||
| PCI | F (3, 10) = 1.14; p = 0.350 | ||
3.3 Anatomical studies
Anatomical analysis of the wood from the twelve trees (six trees from FI and six trees from FE) showed a visible heterogeneity of the wood structure in relation to the side of the trunk from which it was sampled. When means across all measurements were compared, it was observed that wood of the trees from the forest edge had a smaller number of vessels per 1 mm2 of cross-section area, a slightly lower potential conductivity index (PCI), and a slightly higher average vessel lumen area (Fig. 6). In all cases, the wood from the western side had a smaller number of vessels and a lower PCI. In addition, despite a relatively high variation, the average vessel lumen area was also the smallest in wood from this side of a tree trunk.
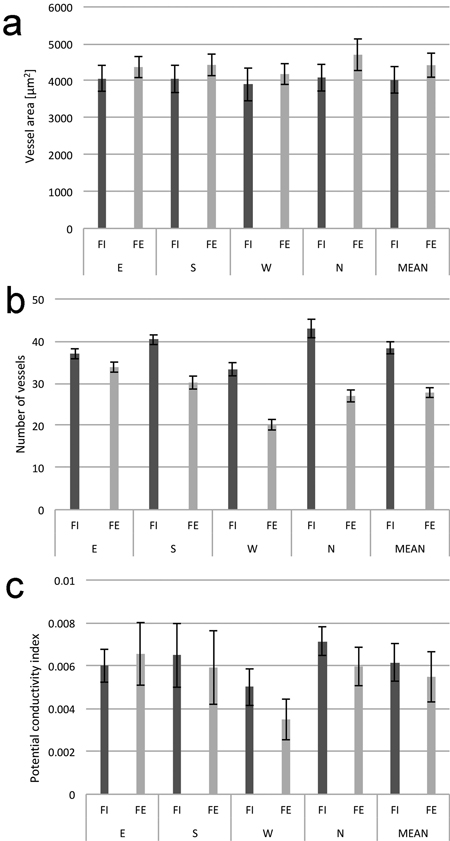
Fig. 6. Average vessel lumen area (a), number of vessels (b), and potential conductivity index (c) calculated for 1 mm2 of cross-sections area of wood in birch trunks from the interior (FI) and from the edge (FE) of the forest stand; samples from four sides around stem circumference (N, E, S and W). The means from 6 trees felled in October. Standard errors of means are indicated.
The two-factor mixed model ANOVA was applied to test the differences between the anatomical characteristics of xylem. The analysis was performed separately for each of the three studied wood characteristics: vessel cross-section surface area, number of vessels per 1 mm2, and potential conductivity index (PCI). Results of the test are presented in Table 4. Analysis of variance revealed a significant effect of main within-subjects factor (the position of the sample on the stem circumference) in the case of the number of vessels and the potential conductivity index. The main between-subjects (the location of trees in the forest) factor appeared significant only with respect to the number of vessels. This indicates that the samples from the western sides of tree trunks have a significantly lower number of vessels per 1 mm2, and the trees from the forest edge are characterized by a lower value of the PCI index. Because of the high variability, no significant effect of main within-subjects and between-subjects factors was revealed with respect to the average cross-section vessel lumen area. A significant effect of interaction between the position of the sample on the stem circumference and the location of trees in the forest was found only when the number of vessels per 1 mm2 was tested.
4 Discussion
In the early spring, the water-transport system of trees is activated and the positive pressure builds in the trunks (Cirelli et al. 2008; Sevanto et al. 2012). Our results suggest that this pressure varies according to the tree’s position within a forest stand. Trees on the periphery of a stand had a higher internal pressure, as these trees produced larger quantities of sap than the trees inside the stand.
The maximum quantity of sap flow was observed on March 21 and 28 when daily air temperature reached the maxima. This period corresponded to the time of swelling and bursting of the leaf buds. Further stages of bud bursting and young leaves development were accompanied by the decrease in the xylem sap exudation, which ceased when young leaves with unfolded blades developed. This process was probably associated with decreasing xylem sap pressure due to the initiation of transpiration from the bursting buds and newly formed leaves. It is worth noting, however, that air temperature in the early spring of 2014 was higher than the average perennial values for March and April. It could have sped up the breaking of dormant period spring initiation developmental processes. Temperature has a considerable influence on the dynamics of sap flow from the tree trunks, along with the condensation of osmotic-active compounds (Merwin and Lyon 1909; Hacke and Sauter 1996; Westhoff et al. 2008). Here we found a negative correlation between the quantity of sap and its level of electrolytic conductivity, which is a measure of the content of diluted, dissociated compounds. Trees from the edge of the stand produced a larger output of sap, yet the sap collected had a lower concentration of electrolytes. Similarly, the content of sugars in the sap varied by sampling location and date, but overall the recorded values of 1–2% correlate with works by other authors (Kallio and Ahtonen 1987; Łuczaj et al. 2014). Interestingly, the sap sugar concentrations from the trees on the edge of the stand were slightly lower than the concentrations found in the sap from trees inside the stand.
The highest value was observed at the first measurement date (March 21) and at consecutive measurements dates, the sap had sequentially lower concentrations of sugars. This suggests a correlation between the activity of live cells within the wood and phloem (axial parenchyma, ray parenchyma, phloem cells) and their role in decreasing the osmotic potential of water in tracheal elements as one strategy of refilling embolized xylem (Zwieniecki et al. 2004; Nardini et al. 2011; Brown 2013). Light-demanding species dynamically react to increased quantities of light. For example, with increased light, the relative transpiration and assimilation of carbon per leaf area increases, as does the radial growth of phloem (Zhang et al. 2015). Thus, we hypothesize that trees growing on the edge of a forest stand could also have a higher living cell activity rate – despite having the same breast height diameter but a larger crown – thus releasing active osmotic compounds at the highest rate. After this initial high-positive pressure period, the pressure will decrease as a result of the initiation of transpiration from developing buds, as well as from water uptake from the soil, resulting in a decreased sap sugar concentration.
Wood mechanical properties depend mainly on its anatomical structure and the amount of bounded moisture, and these properties change very little at moisture contents above fibre saturation point (FSP) (Simpson and TenWolde 1999). In our experiment, the samples from living trees had a very high content of water, significantly above the FSP. Therefore, we may assume that the measured values of the bending strength in the samples of wood collected on the consecutive dates resulted from the variability of individual trees with respect to wood anatomical structure but not from the seasonal changes of the wood strength parameter. Thus, the relations between the xylem sap characteristics which show pronounced seasonal dynamics and the mechanical and anatomical properties of wood can be analyzed only when the average values of the characteristics from various dates for different sides of the tree trunks are compared. It is interesting to note that the results concerning anatomical characteristics of wood coincide with the quantity of flowing sap as well as with the wood resistance properties that are recorded from the various sides of the trunk. Regardless of the tree’s position in the forest stand, the wood on the western trunk side had a smaller (although statistically insignificant) average vessel lumen area and a lower number of vessels per 1 mm2 of cross-section area, which resulted in a lower potential conductivity index (PCI). This is probably connected to the western winds, which are dominant in Poland. Seemingly, the effects of these winds are reflected in the trunk anatomy and also the differential tension of the wood, that is higher on the western side. The trees from the forest edge exhibited this difference in anatomy more strongly than those from inside the stand. Dominant winds are considered to be one of the important factors causing the formation of tension wood which differs from the normal or opposite wood with respect to vessel and fibre anatomy as well as mechanical properties (Ruelle 2014; Ruelle and Thibaut 2014). It is interesting to note that although the edge of the forest stand was exposed to the south, the trees have the highest PCI values on the opposite (north) side, the lowest PCI values and the average vessel areas were not observed on the south side, but instead on the western sides of the trees. This was reflected in the results of the bending strength measurements in which, on average, the western side of the trunk was the most resistant. Barij et al. (2007) observed a negative correlation between topsoil water content around a tree and the wood compression strength (determined using a fractometer) with trees growing on a slope. Forest stand edges are also known to have a larger deficit of soil water than the area inside of a stand. This might have also influenced the mechanical characteristics of the wood. The present results illustrate that trees on the edge of the forest stands function in a different micro-climate and form an important physio-ecological boundary (Davies-Colley et al. 2000), which is physically expressed through their physiology and anatomical parameters.
Acknowledgements
We would like to acknowledge the generous assistance of the personnel of the Drewnica Forestry Management District in collecting the plant material. We also want to thank Dr. Hubert Lachowicz, Faculty of Forestry, Warsaw University of Life Sciences, for sharing the refractometer for the sap sugar content analysis.
References
Barij N.N., Stokes A.A., Bogaard T.T., Van Beek R.R. (2007). Does growing on a slope affect tree xylem structure and water relations? Tree Physiology 27(5): 757–764. https://doi.org/10.1093/treephys/27.5.757.
Błażejczyk K. (2006). Climate and bioclimate of Poland. In: Degórski M. (ed.). Natural and human environment of Poland. A geographical overview. Polish Academy of Sciences Institute of Geography and Spatial Organization, Polish Geographical Society, Warsaw. p. 31–48.
Brown H.R. (2013). The theory of the rise of sap in trees: some historical and conceptual remarks. Physics in Perspective 15(3): 320–358. https://doi.org/10.1007/s00016-013-0117-1.
Charrier G., Charra-Vaskou K., Kasuga J., Cochard H., Mayr S., Ameglio T. (2014). Freeze-thaw stress: effects of temperature on hydraulic conductivity and ultrasonic activity in ten woody angiosperms. Plant Physiology 164: 992–998. https://doi.org/10.1104/pp.113.228403.
Chiu C.M., Wang S.Y., Lin C.J., Yang T.H., Jane M.C.H. (2006). Application of the fractometer for crushing strength: juvenile-mature wood demarcation in Taiwania (Taiwania cryptomerioids). Journal of Wood Science 52(1): 9–14. https://doi.org/10.1007/s10086-005-0723-x.
Cirelli D., Jagels R., Tyree M.T. (2008). Toward an improved model of maple sap exudation: the location and role of osmotic barriers in sugar maple, butternut and white birch. Tree Physiology 28(8): 1145–1155. https://doi.org/10.1093/treephys/28.8.1145.
Davies-Colley R.J., Payne G.W., Van Elswijk M. (2000). Microclimate gradients across a forest edge. New Zealand Journal of Ecology 24(2): 111–121. https://www.jstor.org/stable/24054666.
De Swaef T., De Schepper V., Vandegehuchte M.W., Steppe K. (2015). Stem diameter variations as a versatile research tool in ecophysiology. Tree Physiology 35(10): 1047–1061. https://doi.org/10.1093/treephys/tpv080.
Deslauriers A., Morin H., Begin Y. (2003). Cellular phenology of annual ring formation of Abies balsamea in the Quebec boreal forest. Canadian Journal of Forest Research 33(2): 190–200. https://doi.org/10.1139/x02-178.
Gardiner B., Flatman T., Thibaut B. (2014). Commercial implications of reaction wood and the influence of forest management. In: Gardiner B., Barnett J., Saranpää J.P. , Gril J. (eds.). The biology of reaction wood. Springer-Verlag, Berlin, Heidelberg, Germany. p. 249–274. https://doi.org/10.1007/978-3-642-10814-3_9.
Graf I., Ceseri M., Stockie J.M. (2015). Multiscale model of a freeze-thaw process for tree sap exudation. Journal of the Royal Society Interface 12(111): 20150665. https://doi.org/10.1098/rsif.2015.0665.
Hacke U., Sauter J.J. (1996). Xylem dysfunction during winter and recovery of hydraulic conductivity in diffuse-porous and ring-porous trees. Oecologia 105(4): 435–439. https://doi.org/10.1007/BF00330005.
Johnson L.P.V. (1945). Physiological studies on sap flow in the sugar maple, Acer saccharum Marsh. The Canadian Journal of Research 23c(6): 192–197. https://doi.org/10.1139/cjr45c-016.
Kallio H., Ahtonen S. (1987). Seasonal variations of the sugars in birch sap. Food Chemistry 25(4): 293–304. https://doi.org/10.1016/0308-8146(87)90016-1.
Łuczaj Ł., Bilek M. Stawarczyk K. (2014). Sugar content in the sap of birches, hornbeams and maples in southeastern Poland. Central European Journal of Biology 9(4): 410–416. https://doi.org/10.2478/s11535-013-0284-8.
Matlack G.R. (1994). Vegetation dynamics of the forest edge--trends in space and successional time. Journal of Ecology 82(1): 113–123. https://doi.org/10.2307/2261391.
Mattheck C.G., Breloer H., Bethge K.A., Albrecht W.A., Zipse A. (1995). Use of the fractometer to determine the strength of wood with incipient decay. Journal of Arboriculture 21: 105–112.
Merwin H.E., Lyon H. (1909). Sap pressure in the birch stem. Botanical Gazette 48(6): 442–458. https://doi.org/10.1086/330065.
Nardini A., Gullo Lo M.A., Salleo S. (2011). Refilling embolized xylem conduits: is it a matter of phloem unloading? Plant Science 180(4): 604–611. https://doi.org/10.1016/j.plantsci.2010.12.011.
Pearce R. (2001) Plant freezing and damage. Annals of Botany 87(4): 417–424. https://doi.org/10.1006/anbo.2000.1352.
Ruelle B., Thibaut B. (2014). Physical and mechanical properties of reaction wood. In: Gardiner B., Barnett J., Saranpää J.P., Gril J. (eds.). The biology of reaction wood. The biology of reaction wood. Springer-Verlag, Berlin- Heidelberg. p. 171–200.
Ruelle J. (2014). Morphology, anatomy and ultrastructure of reaction wood. In: Gardiner B., Barnett J., Saranpää J.P., Gril J. (eds.). The biology of reaction wood. The biology of reaction wood. Springer-Verlag, Berlin-Heidelberg. p. 13–35. https://doi.org/10.1007/978-3-642-10814-3_2.
Sevanto S., Holbrook N., Ball M.C. (2012). Freeze/thaw-induced embolism probability of critical bubble formation depends on speed of ice formation. Frontiers in Plant Science 3.107. https://doi.org/10.3389/fpls.2012.00107.
Simpson W., TenWolde A. (1999). Physical properties and moisture relations of wood. Wood handbook : wood as an engineering material. Forest Products Laboratory, General technical report FPL-GTR-113, Madison, WI, USA. Chapter 3. p. 3.1–3.24.
Sperry J.S., Donnelly J.R., Tyree M.T. (1988). Seasonal occurrence of xylem embolism in sugar maple (Acer saccharum). American Journal of Botany 75(8): 1212–1218. https://doi.org/10.2307/2444104.
Steppe K., Sterck F., Deslauriers A. (2015). Diel growth dynamics in tree stems: linking anatomy and ecophysiology. Trends in Plant Science 20(6): 335–343. https://doi.org/10.1016/j.tplants.2015.03.015.
Svanberg I., Sõukand R., Łuczaj Ł., Kalle R., Zyryanova O., Dénes A., Papp N., Nedelcheva A., Šeškauskaitė D., Kołodziejska-Degórska I., Kolosova V.l. (2012). Uses of tree saps in northern and eastern parts of Europe. Acta Societatis Botanicorum Poloniae 81(4): 343–357. https://doi.org/10.5586/asbp.2012.036.
Timell T.E. (1986). Compression wood in gymnosperms. Vol 3. Springer-Verlag, Berlin-New York. 2150 p.
Tomczak A., Jelonek T., Pazdrowski W. (2014). Charakterystyka wybranych cech morfologicznych drzew w dojrzałych drzewostanach sosnowych eksponowanych na działanie wiatru.[Characteristics of selected morphological traits of trees in mature Scots pine stands exposed to wind]. Sylwan 158: 183–191.
Turcotte A., Morin H., Krause C., Deslauries A., Thibeault-Martel M. (2009). The timing of spring rehydration and its relation with the onset of wood formation in black spruce. Agricultural and Forest Meteorology 149(9): 1403–1409. https://doi.org/10.1016/j.agrformet.2009.03.010.
Tyree M.T. (1983.) Maple sap uptake, exudation, and pressure changes correlated with freezing exotherms and thawing endotherms. Plant Physiology 73: 277–285. https://doi.org/10.1104/pp.73.2.277.
Wegner L.H. (2014). Root pressure and beyond: energetically uphill water transport into xylem vessels? Journal of Experimental Botany 65(2): 381–393. https://doi.org//10.1093/jxb/ert391.
Westhoff M., Schneider H., Zimmermann D., Mimietz S., Stinzing A., Wegner L.H., Kaiser W., Krohne G., Shirley St., Jakob P., Bamberg E., Bentrup F.-W., Zimmermann U. (2008). The mechanisms of refilling of xylem conduits and bleeding of tall birch during spring. Plant Biology 10(5): 604–623. https://doi.org/10.1111/j.1438-8677.2008.00062.x.
Young T. Hubbell S.P. (1991). Crown asymmetry, treefalls, and repeat disturbance of broad-leaved forest gaps. Ecology 72(4): 1464–1471. https://doi.org/10.2307/1941119.
Zajączkowska U. (2013). Architektura drzewa w aspekcie biomechaniki i działania auksyny. [Tree architecture in the context of biomechanics and auxin activity]. Sylwan 157: 453–457.
Zanne A.E., Westoby M., Falster D.S., Ackerly D.D., Loarie S.R., Arnold S.E.J., Coomes D.A. (2010). Angiosperm wood structure: global patterns in vessel anatomy and their relation to wood density and potential conductivity. American Journal of Botany 97(2): 207–215. https://doi.org/10.3732/ajb.0900178.
Zhang L., Copini P., Weemstra M., Sterck F. (2015). Functional ratios among leaf, xylem and phloem areas in branches change with shade tolerance, but not with local light conditions, across temperate tree species. New Phytologist 209(4): 1566–1575. https://doi.org/10.1111/nph.13731.
Zheng J., Martinez-Cabrera H.I. (2013). Wood anatomical correlates with theoretical conductivity and wood density across China: evolutionary evidence of the functional differentiation of axial and radial parenchyma. Annals of Botany 112(5): 927–935. https://doi.org/10.1093/aob/mct153.
Zimmermann M.H. (1958). Translocation of organic substances in the phloem of trees. In: Thimann K.V. (ed.). The physiology of forest trees. The Ronald Press Co., New York. p. 381–340.
Zweifel R., Häsler R. (2000). Frost-induced reversible shrinkage of bark of mature subalpine conifers. Agricultural and Forest Meteorology 102(4): 213–222. https://doi.org/10.1016/S0168-1923(00)00135-0.
Zwieniecki M.A., Melcher P.J., Feild T.S., Holbrook N.M (2004). A potential role for xylem-phloem interactions in the hydraulic architecture of trees: effects of phloem girdling on xylem hydraulic conductance. Tree Physiology 24(8): 911–917. https://doi.org/10.1093/treephys/24.8.911.
Total of 41 references.

