Interpreting common garden studies to understand cueing mechanisms of spring leafing phenology in temperate and boreal tree species
Salk C. F. (2020). Interpreting common garden studies to understand cueing mechanisms of spring leafing phenology in temperate and boreal tree species. Silva Fennica vol. 54 no. 5 article id 10381. https://doi.org/10.14214/sf.10381
Abstract
Trees are particularly susceptible to climate change due to their long lives and slow dispersal. However, trees can adjust the timing of their growing season in response to weather conditions without evolutionary change or long-distance migration. This makes understanding phenological cueing mechanisms a critical task to forecast climate change impacts on forests. Because of slow data accumulation, unconventional and repurposed information is valuable in the study of phenology. Here, I develop and use a framework to interpret what phenological patterns among provenances of a species in a common garden reveal about their leafing cues, and potential climate change responses. Species whose high elevation/latitude provenances leaf first likely have little chilling requirement, or for latitude gradients only, a critical photoperiod cue met relatively early in the season. Species with low latitude/elevation origins leafing first have stronger controls against premature leafing; I argue that these species are likely less phenologically flexible in responding to climate change. Among published studies, the low to high order is predominant among frost-sensitive ring-porous species. Narrow-xylemed species show nearly all possible patterns, sometimes with strong contrasts even within genera for both conifers and angiosperms. Some also show complex patterns, indicating multiple mechanisms at work, and a few are largely undifferentiated across broad latitude gradients, suggesting phenotypic plasticity to a warmer climate. These results provide valuable evidence on which temperate and boreal tree species are most likely to adjust in place to climate change, and provide a framework for interpreting historic or newly-planted common garden studies of phenology.
Keywords
photoperiod;
bud break;
budburst;
chilling;
elevation gradients;
latitudinal gradients;
leaf flush;
reciprocal transplant experiments;
xylem anatomy
-
Salk,
Southern Swedish Forest Research Centre, Swedish University of Agricultural Sciences, P.O. Box 49, SE- 230 53 Alnarp, Sweden; Faculty of International Studies, Utsunomiya University, 350 Minemachi, Utsunomiya-shi, Tochigi 321-8505 Japan; Institute for Globally Distributed Open Research and Education (IGDORE)
E-mail
carl.salk@slu.se

Received 22 May 2020 Accepted 17 November 2020 Published 30 November 2020
Views 69513
Available at https://doi.org/10.14214/sf.10381 | Download PDF

1 Introduction
Accelerating environmental change is a particular threat to tree species whose slow dispersal and advanced age at reproductive maturity hinder migration and evolutionary adaptation (Jump and Peñuelas 2005). Organisms unable to evolve in place or move to a more favorable environment can only survive by tolerating or adjusting to new conditions via phenotypic plasticity (Nicotra et al. 2010). Shifts in phenology, or the timing of annual events, are a critical response of woody plants to a changing climate. The environmental cueing mechanisms of individual tree species’ phenology are still debated (Laube et al. 2014; Fu et al. 2019) impeding forecasts of vegetation responses to future climates. The gradual accumulation of once-a-year data points make creative approaches essential to understanding phenology. This paper analyzes what overlooked information about phenological cueing mechanisms can be gleaned from published common garden studies, and maps what combinations of phenological and other ecological traits occur in nature, particularly among factors like wood anatomy, successional status and biogeographic history.
Tree phenology affects important ecological processes. Trees’ annual carbon balance is highly sensitive to growing season length (Dragoni et al. 2011), which scales to ecosystem-level carbon fluxes (Richardson et al. 2009). Small changes in spring leafing dates have big consequences for understory plants that depend on early-spring light for most of their photosynthesis (Augspurger et al. 2005; Lopez et al. 2008). Insects, birds and other animals that eat young leaves, nectar or fruit can suffer if their phenological responses to a changing climate differ from the plants producing these resources (Visser and Both 2005; Mayor et al. 2017). Phenology also affects invasion ecology; exotic species with extended or flexible growing seasons perform better in deciduous forest understories (Fridley et al. 2012).
While tree phenology is simple to observe, its underlying cueing mechanisms are complex and unlikely to be fully understood using any single approach (Tang et al. 2016; Piao et al. 2019). Fortunately, several complementary methods can contribute to understanding of phenology cueing mechanisms and help improve forecasts of how vegetation will respond to future climates. These include long-term observational datasets (Thackeray et al. 2016), statistical modeling (Zhao et al. 2013), climate-change experiments (Chung et al. 2013), genetic and epigenetic studies (Kudoh 2016), common garden studies, and reciprocal transplant experiments which frequently incorporate common gardens (Vitasse et al. 2013). Common gardens can either look at many accessions of one species grown together (the focus of this study), or many species of disparate origin planted in a single location (Zohner and Renner 2014); cross-species common gardens are important to understand wide phenological patterns but are not considered here. These methods sometimes have apparently contradictory results about what cueing mechanisms trigger phenological responses (Vitasse and Basler 2013) and species’ sensitivity to increased temperatures (Wolkovich et al. 2012). In some cases, these apparent contradictions can be resolved by considering whether annual temperature averages or seasonal averages when plants are most phenolgically sensitive are used in analyses (Clark et al. 2014a, 2014b). Having a carefully considered framework for interpreting phenological studies is crucial.
Historical datasets, some extending back centuries, have been a recent focus of study into the drivers and potential future trajectory of phenology (Primack and Miller-Rushing 2012). Given the long timescales of phenological shifts, unconventional and repurposed historical datasets are too valuable to be ignored. Many publications, often in hard-to-find books or technical reports, include phenological observations from common gardens planted for other reasons, frequently tree improvement studies. Because phenology depends on both genotype and environment (Nienstaedt 1974; Keller et al. 2011), common gardens can yield difficult-to-obtain information about which cueing mechanisms affect a species’ phenology by eliminating environmental differences; observed variation is due to different cue requirements within the species. Studies using plant material collected along elevation gradients may be especially useful in untangling photoperiod and temperature cues that covary with latitude. These types of studies are a previously under-appreciated data source that could improve knowledge of phenological cueing mechanisms without the expense of climate-change experiments, the time commitment of long-term observational work, or the logistical complexity of spatially-dispersed data collection.
In this study, I develop a framework for interpreting what common garden studies say about species’ leafing phenology drivers and how this in turn can help us understand trees’ responses to climate change. This is done with a goal of understanding the diversity of how different ecological traits map onto phenological cues, not with the intent of quantitatively meta-analyzing mean values of these patterns. I focus mostly on what I call ‘cueing mechanisms’ (e.g. spring temperature, chilling, photoperiod) rather than ‘cues’ (e.g. the specific critical photoperiod or degree-day requirement of a plant) as these are more likely to be a shared feature within a species. Because of its fundamental importance for carbon balance, especially in deciduous species (Dragoni et al. 2011), this paper focuses on leafing phenology in temperate and boreal trees. I do not consider flowering phenology due to complex coevolution with pollinators and the time needed to grow fruits of different sizes. These findings probably apply to shrubs and woody vines as well, but I did not find studies of these life forms apart from a few dwarf shrubs (McDonough MacKenzie et al. 2018) whose phenology is closely tied to snow cover, so they are not considered here. Although within-species differentiation of phenological dates in a common garden is direct evidence only of evolutionary adaptation among populations, this differentiation can indicate what cues drive plants’ leafing phenology. Which cue(s) a species uses indicate how phenotypically plastic plants are likely to be in the face of climate change. For instance, a species relying on temperature cues will likely be more phenologically plastic to warmer temperatures than a species responding to photoperiod, a cue that won’t be affected by climate change.
This paper is organized as follows: Section 2 outlines how different environmental cues vary with latitude and elevation, and discusses the expected leafing order of species relying on those mechanisms in common gardens. Section 3 builds on Section 2, taking the opposite perspective of interpreting what phenological patterns observed in common gardens can say about the cueing mechanisms that influence leafing timing. Section 4 collects information on phenological patterns from published common garden studies and Section 5 relates this to species’ biogeographic history and ecological traits, particularly wood anatomy and successional status. Wood anatomy is particularly important for phenology; ring porous species which have some large diameter xylem conduct water more efficiently, but are at greater risk of drought- or frost-induced malfunction than trees with narrow xylem (Choat et al. 2011), hence their tendency for relatively late onset of spring activity (Lechowicz 1984). The studies summarized here show nearly all possible leafing date orders in common gardens, including non-linear patterns and near-simultaneous leafing among plants from very different climates. Much of this diversity makes sense when interpreted in context of the geographical patterns of cues and species’ traits, particularly wood anatomy and post-glacial migration patterns.
2 Geographical patterns of environmental cues
Three types of mechanisms are well established to affect leaf-out timing in temperate and boreal trees: spring temperature, over-winter chilling, and photoperiod (Tang et al. 2016). A general ‘temperature’ mechanism might seem a sensible consolidation of chilling and spring temperature, but these two factors affect phenology in fundamentally different ways, so I discuss them separately. In addition, recent research suggests that increased absolute (but not relative) air humidity accelerates leafing (Laube et al. 2014; but see Zipf and Primack 2017). However, because warmer air has a higher water-holding capacity, this factor is closely correlated with temperature, especially in temperate regions during the spring (Laube et al. 2014), so I don’t consider absolute humidity further. However, possible experimental confusion of these factors should be kept in mind.
Spring temperature is the most straightforward of these three cueing mechanisms. It is not just a signal to plants that spring has begun, but is also a fundamental control on photosynthetic physiology (Leuning 2002). It is frequently quantified as a temperature sum, ‘degree-days’ or ‘degree-hours’ above a particular threshold (Bonhomme 2000). As computed degree-days are correlated (but not identical) regardless of the threshold used, determining the ‘correct’ threshold is tricky (Bonhomme 2000), and it might differ within a species’ range (see below). Light’s role in photosynthesis is probably not the main reason that photoperiod is a driver of phenology. Rather, photoperiod (Way and Montgomery 2015) along with temperature requirements and chilling signals (Cannell et al. 1985) help avoid premature leaf out during winter warm spells. However, the chilling requirement’s role may be limited as it can be met quite early in the winter, especially in cold temperate and boreal areas (Myking and Heide 1995). It is also important to note that night length rather than day length has been long recognized as the effective signal in many plant processes (Garner and Allard 1923), but I use the term ‘photoperiod’ for consistency with the bulk of the phenology literature. Beyond photoperiod, light quality factors such as twilight red to far red (R:FR) ratio and increased light in blue spectra can also affect leaf budburst timing (Brelsford et al. 2019).
The spring temperatures, chilling and light that plants experience in their environment all vary with latitude and elevation in predictable ways that I describe in the next paragraphs. Inferring phenological cueing mechanisms from common garden results (which is discussed in detail in Section 3) requires first describing what leafing patterns among plants of different elevation/latitude origin can be expected in species relying on different mechanisms. My goal here is to outline the different theoretical possibilities that are broadly consistent with the tradeoff between maximizing photosynthesis while reducing frost risk that temperate and boreal trees face when leafing, not to only review the ones for which there is some degree of published evidence. In addition, the strength of evidence for different cues is debated, even within individual studies (Carter et al. 2017). For these reasons, I don’t list specific references claiming examples of the particular explanations, although many of them are adduced among the common garden studies reviewed in Section 5.
Spring temperature -Temperature gradients are familiar, with cooler climates away from the Equator and toward higher elevations. However, these simple patterns may have complicated impacts on phenology. An individual plant requiring only a fixed degree-day exposure to burst leaf buds would become active later when grown at higher latitude or elevation. Locally-adapted thermal requirements are possible if a species spans a wide elevation or latitude, in which case plants from different origins would leaf at different times when grown in the same environment (Osada et al. 2018). In warm temperate regions, a plant with a small degree-day requirement would be more likely to begin growth prematurely and later suffer frost damage in a warm climate than in a colder climate. This implies that populations from lower latitudes could minimize frost damage by having bigger spring temperature exposure requirements. If so, they would have later leaf-out dates than individuals with higher-latitude origins when grown in a common garden. The possibility of geographically-variable threshold temperatures for degree-day accumulation is a further complication discussed in Section 3.
Chilling - The length and severity of winter both increase with latitude and elevation. However, how chilling makes plants more sensitive to warm temperatures is complex, likely species specific, and often poorly understood (Schwartz and Hanes 2010). Some species clearly respond to winter chilling (Nanninga et al. 2017), although even interpretation of single species’ patterns can be controversial, notably for Fagus sylvatica (Vitasse and Basler 2013). Two contrasting geographical patterns of chilling requirements seem plausible: (1) cold-origin populations have a bigger chilling requirement since they are exposed to so much cold over the winter, or (2) warm-origin populations have a bigger chilling requirement since, as mentioned above, warm temperatures are not a reliable indicator of the onset of spring in milder climates. Possibility (1) would lead to warmer-origin plants leafing out first in a common garden, whereas possibility (2) would cause the opposite pattern.
Light - In temperate and boreal regions, both photoperiod and light spectral properties (at both red to far red (R:FR) and blue wavelengths; Brelsford et al. 2019) change in geographically complicated ways during the period affecting leafing phenology. In the astronomical winter, higher-latitude locations have shorter days than places closer to the Equator in the same hemisphere. After the spring equinox the reverse is true, with lower latitude locations having shorter days (Fig. 1). These patterns are important. A hypothetical species with a critical photoperiod determining when it becomes sensitive to degree-day accumulation would show different transplant responses in a common garden depending on whether the critical day length is reached before or after the spring equinox. Plants with a critical photoperiod that occurs after the equinox would meet this requirement sooner if transplanted poleward. However, if its critical photoperiod were before the equinox, the same plant would meet its requirement later if transplanted poleward. Realistically, photoperiod probably does not work like flipping a switch when a critical threshold is met. A tradeoff with other cues is more likely, for example degree-day requirements that shrink as days become longer in the spring (Basler and Körner 2012), or photoperiod dependence that varies with the extent of chilling (Heide 1993).
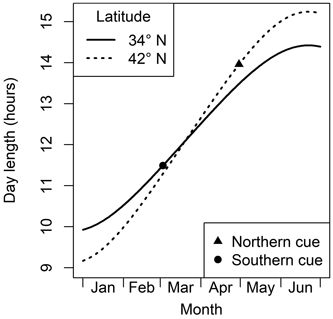
Fig. 1. An illustration of day length patterns in the northern and southern parts of the eastern deciduous region of North America. If a critical day length is shorter than on the spring equinox (the point where the two lines intersect), then moving a plant to the south means this requirement is met later, and vice versa. If the critical day length happens after the equinox, these patterns are switched. The triangle and circle denote day length when many trees are growing new leaves in the north and south, respectively (see text in Section 3).
To be an effective phenological cueing mechanism, photoperiod must be adapted to different times of the year, as appropriate to local climate (Way and Montgomery 2014). In a place like the northeastern USA where spring usually begins in April or May, a very long day would be a useful signal of spring (triangle in Fig. 1). In a milder climate such as the southeastern USA with spring beginning in early March, a relatively short critical day length would signal that it is safe to leaf out (circle in Fig. 1). The big difference between photoperiods at leafing in northern and southern locales means that a species with photoperiod dependency would need very strong latitudinal differentiation in response patterns. For a species with these adaptions to photoperiod, southern genotype trees would always reach their critical photoperiod before genotypes of northerly origin. If planted in a common garden, a south to north pattern of leaf budburst would be expected. In contrast to the complex pattern of day length with latitude, photoperiod does not change with elevation. However, because later flushing at higher elevations happens in a longer photoperiod, a similar but weaker pattern of cooler-site plants responding to longer photoperiods (i.e. later leafing in a common garden) would be expected in species using this cue.
The intertwined issues of R:FR ratio and twilight length are more complicated and less understood than photoperiod. Apart from dawn and dusk, daytime R:FR is remarkably constant at ground level, and is barely affected by clouds (Smith 1994). However, R:FR decreases when the sun is within 10° of the horizon. At higher latitudes, the sun spends more time near the horizon, although with complicated patterns in the yearly cycle; at middle latitudes, twilight length has two peaks, a higher peak at midsummer and a secondary peak around the winter solstice (Smith 1994). How these factors vary with elevation is less clear. As several atmospheric gasses absorb red and far-red light differently, R:FR can be expected to change as the atmosphere becomes thinner at higher elevations. I have not found any published measurements of R:FR at different elevations, but it should be kept in mind that this could have physiological effects on plants.
R:FR affects plants via phytochromes, the main photoreceptors active at long wavelengths. They switch form with exposure to different ratios of red and far red light (Smith 1995). This physiology affects phenology. Although light spectra are difficult to manipulate in outdoor experiments, light quality in both the red and blue spectral regions has clear impacts on leaf-out timing in at least some species (Brelsford et al. 2019). A plant requiring a critical twilight length would reach this cutoff earlier in the spring if transplanted poleward, and later if moved in the opposite direction (Smith 1994). However, like photoperiod, specific adaptations to twilight and R:FR should correspond to the time of year when leaves emerge. In a common garden, the expectation of southern phenotypes leafing before plants of northern origin if relying on photoperiod cues also applies to R:FR.
3 Interpreting common garden results
This section builds on the cue patterns described in Section 2, but takes the opposite perspective: what can geographical patterns of leaf-out timing in common gardens say about different species’ cueing mechanisms and how responsive they are likely to be to global change? As in Section 2, the goal is to outline the reasonable possibilities rather than documented or claimed evidence, so specific examples from the literature aren’t cited. This discussion is more complex than the previous section because different cuing variables may interact with one another (Heide 1993; Keller and Körner 2003), making interpretation of common garden results tricky.
For a given species, if trees of higher-latitude or -elevation origin leaf-out before those from lower areas in a common garden (hereafter called the H→L pattern), then at least one of the following is true:
1. Degree-day requirements or threshold temperatures decrease with latitude/elevation of origin.
2. The species has little or no requirement for chilling exposure, or this requirement decreases steeply with increasing latitude/elevation of origin.
If leafing follows the H→L pattern for latitude, but not elevation, there is a further possibility:
3. A critical photoperiod that is reached before the spring equinox.
The logic of the final item in this list is that when a tree is transplanted closer to the Equator, it would experience the critical night length (which would be <12 hours) sooner than at its origin. This can be seen as the difference between the solid and dashed lines in the left (winter) side of Fig. 1. All of these possibilities would suggest that safety mechanisms preventing early leaf budburst are relatively weak. A chilling-based mechanism is unlikely to be at work here, and any day length requirement would be met so early that it would still be meteorological winter in all except the lowest-latitude temperate forests. Thus, species with a H→L leafing pattern in common gardens are likely to be relatively phenotypically plastic in their phenological response to warmer temperatures.
If leafing happens in the opposite direction (low elevation/latitude origins come before higher origins, hereafter L→H), then cues can be expected to have one or more of the following properties:
1) Degree-day requirements or threshold temperatures increase substantially with elevation/latitude of origin.
2) There is a strong requirement for chilling exposure which increases with elevation/latitude.
3) Photoperiod lengths promoting faster leafing increase with elevation/latitude of origin (note that the converse of this possibility is not included in the H→L list above because earlier leafing in colder climates does not make sense from a frost avoidance perspective and has never to my knowledge been observed).
Only for latitudinal common gardens, there is one more possibility:
4) A critical photoperiod similar among all provenances that is met well after the spring equinox.
Explanations number 2–4 would generally indicate that species showing the L→H pattern have strong photoperiod or chilling requirements to prevent premature leafing. Only the first explanation for the L→H pattern would be consistent with relatively high phenotypic plasticity to variable weather; this pattern seems unlikely as it would mean extremely short growing seasons at higher elevation/latitude. Thus, L→H species are likely to advance their leaf-out dates less in response to a warmer climate than H→L species.
Leaf out in common gardens may also lack a one-to-one relationship between latitude or elevation origin and relative leafing date. The response could be u- or n-shaped, with the earliest or latest genotypes falling in the middle of the elevation or latitude gradient, or a more complicated pattern. This would point to a few possibilities about a species’ gradients in phenological adaptation. One is a non-linear adaptation to the broadly predictable decrease in temperature with elevation/latitude. A second is a combination of varied adaptation in more than one of the cueing mechanisms presented above. As a hypothetical example, a species could have decreasing photoperiod limitations and increasing chilling requirements with latitude. If there is little limitation by either cue at mid latitudes, then those origins would leaf before plants coming from more northerly or southerly locations (u-shaped response). If both photoperiod and chilling exert control over mid-latitude provenances, then an n-shaped response may result. A similar combination of cues might also result in all genotypes leafing out at the same time, although simultaneous leafing would be more simply explained by genotypes not having adaptive differentiation along the elevation/latitude gradient with respect to phenological cues.
4 Methods
The aim of this study is to map the diversity of spring phenological responses in common gardens, and relate this to species’ other ecological traits. Toward this goal, I synthesized results from published studies meeting several criteria. They should report vegetative phenology of temperate or boreal woody plants; the exact stage(s) of phenology evaluated varied somewhat among studies. The plants should be grown together in one or more locations (referred to as ‘common gardens’) with provenances of a species spanning an elevation of ≥400 m or latitude of ≥4° within its native range. The number of plant sources per species ranged from 2 to 148 (mean 23.4; median 10; when a study reported both the number of genotypes or half-sib groups and provenances, I used the lower provenance number; in a few studies not all provenances were planted in all gardens). While most of these studies report on a single garden, some are reciprocal transplant experiments or other configurations with multiple gardens. The phenology results should be reported in terms of these gradients. Studies were not used if phenology was not clearly reported or analyzed in terms of latitude or elevation as this prevents disentangling of photoperiod and chilling effects. For instance, results were not used if analyzed in terms of climate only (obscuring whether a latitude or elevation gradient was at play), or not clearly interpretable (for instance latitude/elevation predictor variables buried in an ordination analysis).
The list of studies was assembled in three ways. First, over several years, I opportunistically collected studies as I encountered them by chance (encountered when looking for other literature, Google Scholar searches, journal tables of contents, etc.), yielding 19 studies encompassing 24 species (some studies reported on multiple species and some species were studied in multiple papers). Then, on 18 April 2020, I searched Web of Science using the term “TS = ’Common garden’ AND TS = ’Phenolog*’” which yielded 467 results. After checking studies against the criteria listed above, this search yielded 21 new studies, in addition to finding only seven of the 19 previously encountered studies. This low yield was in part due to many of the original 19 studies being from sources not indexed in Web of Science such as conference proceedings or agricultural experiment station reports from the 1950s–1970s. Finally, the list of studies was extended by seven papers incidentally discovered while reviewing suitability of papers from the Web of Science search. All studies were manually checked to verify that they reported on unique experiments, and a few duplicates were discarded. An additional study meeting these criteria was published while this manuscript was in review. At the end of this process there were 47 studies covering 38 species, with some species represented in as many as six different studies for a total of 60 distinct species-study combinations (which I refer to as ‘cases’), of which one reported results in terms of both elevation and latitude (Table 1). Most studies were of saplings at least 1 year old; gardens with decades-old trees likely to be reproductively mature were rare (Table 1). Most plants were derived from seeds except for species that readily propagated vegetatively (e.g. Populus spp.; Table 1).
| Table 1. A summary of common garden studies synthesized in this paper. All encountered studies of temperate or boreal trees reporting leaf-out observations were included. ‘Leafing Pattern’ gives the order of leaf budburst: L→H means low latitude/elevation genotypes leaf out earliest, while H→L indicates high latitude/elevation genotypes leaf first. Species nomenclature and authorities follow the referenced publications except where noted. The ‘Range’ column gives the extreme lower and upper values of elevation or latitude and the difference between these values. Key to ‘Wood Anat.’ column abbreviations: DP = diffuse porous, RP = ring porous, NC = narrow coniferous. View in new window/tab. |
Common garden results were summarized by environmental gradient type (elevation vs. latitude), the order of leafing among plant origins in common gardens (H→L elevation/latitude, L→H, more complex patterns), ecological traits (wood anatomy, successional status) and taxonomic affinity. Tendency toward H→L vs. L→H leafing patterns as a function of wood anatomy and gradient type was analyzed using binomial tests implemented in R version 3.3.2 (R core team 2016). Wood anatomy was categorized as coniferous, diffuse porous, and ring porous. Because of the importance of vessel diameter to frost-induced embolism risk in spring (Hacke and Sperry 2001), any species having large vessels were assigned to the ring porous category regardless of the specific anatomical terminology applied to it in the literature. As the ultimate goal of this study is to synthesize and highlight the diversity of phenological responses as a function of tree species ecological and evolutionary traits, the combinations of traits observed in different studies is at least as important as statistical differences among functional groups. Thus, presentation of results in terms of what combinations are (and aren’t) observed is emphasized. For successional status, finding consistent classifications for species native to four different continents proved impossible, so this trait is not analyzed statistically.
5 Results
The surveyed studies reported on common gardens with plant origins spanning elevation ranges of 440 to 1775 m (mean: 1047 m; 28 cases) or latitude ranges of 4 to 22.41° (mean 12.9°; 33 cases). All but one case reported observations from three or fewer growing seasons; the longest reported 5 years of observations (Liang 2019). Four species (Fagus sylvatica, Pinus sylvestris, Populus trichocarpa, Quercus petraea) were studied along both elevation and latitude gradients (Table 1). Every possible combination of geographical leafing pattern, gradient type and xylem anatomy was seen in at least one case (Table 1; Fig. 2). Some species leafed out from low to high (L→H) latitude, while in others high latitude plants begin first (H→L). Both the L→H and H→L patterns are seen with respect to elevation as well. Other cases showed no clear pattern along latitude or elevation gradients. Five cases showed virtually the same leaf budburst date for all origins, even across genotypes from a broad range of climates (Table 1; Fig. 2) in the following species: Fagus sylvatica, Populus tremula, Populus balsamifera, Populus deltoides, Fagus sylvatica, and Pinus ponderosa (Table 1). When a species was studied in more than one garden, the results were usually in agreement. Weak disagreement, meaning some studies showed a clear pattern while others showed a complicated or inconclusive result along the same type of gradient, was seen in six species. Acer pseudoplatanus elevation gradients showed a L→H pattern in one study and no pattern in another study (Table 1). Fagus sylvatica followed a H→L latitudinal pattern in in one study, but little differentiation in another case (Table 1); this species always showed a H→L pattern in four elevational studies (Table 1). Pinus ponderosa followed L→H elevational ordering (although with small but significant differentiation) in one study and no differentiation in another study (Table 1). Populus balsamifera, and Populus deltoides both showed H→L latitude patterns in one case each and both exhibited a non-linear or undifferentiated patterns in an another case (Table 1). Populus trichocarpa leafed from L→H latitudes in one study, but showed no pattern in another (Table 1). Quercus petraea showed a L→H elevation pattern in two studies but had no pattern in an additional study (Table 1), while consistently showing a L→H pattern in latitudinal studies. Only for Picea glauca were clearly opposite patterns seen in different studies (Table 1); both were latitudinal studies, although the study showing the H→L had four times the latitudinal width and a simpler to interpret analysis than the study finding the L→H pattern. In addition to these differences among studies, one case of Fraxinus americana found a reversal of its usual L→H pattern in a minority of study years (Table 1).
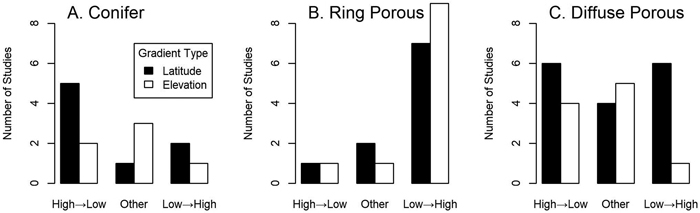
Fig. 2. The relative frequency of reviewed studies showing different leafing patterns in (A) conifers, (B) ring-porous angiosperms, and (C) diffuse-porous angiosperms. All angiosperm species with any large vessels (see Table 1) were included in the ring porous category. High→Low means leaf out happened in order from highest to lowest elevation/latitude and Low→High indicates the opposite; the Other category includes all other patterns including nonlinear, undifferentiated and no pattern at all (see Table 1). Only for ring porous species did binomial tests reveal statistical differences below or near traditional cutoffs between the High→Low and Low→High categories for latitude (p = 0.070) and elevation (p = 0.021).
Leaf budburst trends among diffuse-porous angiosperms showed no obvious relationship with successional status. Among light-demanding species, both H→L and L→H leafing order were seen in common gardens. For example, Liriodendron tulipifera displayed L→H leaf out, while Betula alleghaniensis followed the opposite H→L pattern (Table 1). Similarly, more shade-tolerant species showed both the H→L (e.g. Fagus sylvatica) and L→H (e.g. Acer pseudoplatanus) patterns when planted in common gardens (Table 1).
For 16 of 21 cases of ring-porous species (including semi ring-porous species and species with less-organized large vessels) low latitude and elevation genotypes consistently grew leaves before plants from colder origins in common gardens (Fig. 2; Table 1). Three cases showed no or irregular patterns, and only Laburnum alpinum and one study of the semi-ring porous Populus deltoides showed a clear H→L pattern. These deviations from random (assuming the H→L and L→H patterns to be equally likely) had moderate statistical strength for both elevation (p = 0.021) and latitude (p = 0.070; binomial tests). Species with narrow xylem (conifers and diffuse-porous angiosperms) did not show the consistent phenological trends seen among ring-porous species (Fig. 2). Narrow-xylemed species showing the L→H (10 cases) and H→L (17 cases) patterns were common, with no statistical evidence of a trend in either direction (p ≥ 0.289; binomial tests for elevation and latitude; Fig. 2). Of the 15 species that at least in some cases showed no pattern, nonlinear patterns or near-simultaneous leaf out, 12 were diffuse porous or conifers (Table 1; p = 0.286; binomial test with a 65% expectation reflecting the relative abundance of narrow-xylemed species among the cases reviewed). The exceptions were the semi-ring porous Frangula alnus, one of two cases of Populus deltoides (also semi-ring porous), and one case out of six of Quercus petraea.
Some patterns within taxonomic groups are visible in these common garden studies. Conifer species with clear results showed the H→L leafing order, although there are not enough cases to clearly demonstrate a non-random trend (binomial test, 3 successes on 10 trials, p = 0.344) and these results are not significantly different from diffuse-porous angiosperms (χ2 test, χ2df=1 = 0.0283, p = 0.867). The only conifer unambiguously showing L→H leafing order was Picea abies, although as noted above for both Picea glauca and Pinus ponderosa one of two cases also showed this pattern. However, being closely-related does not guarantee that species behave similarly; within genera having narrow xylem, contrasting leaf-out order was observed for both gymnosperms (e.g. Picea abies vs. Picea sitchensis) and angiosperms (Acer saccharum vs. Acer pseudoplatanus and Betula alleghaniensis vs. Betula papyrifera; Table 1). Among ring-porous and other wide-vesseled species, congeners never showed clearly opposite patterns.
6 Discussion
These common garden studies shed light on the diversity of temperate and boreal trees’ phenological mechanisms. Published studies were found showing four different geographical patterns of leafing in common gardens: (1) the coldest origins (high elevation/latitude) leafing out first (H→L), (2) the warmest origins (low elevation/latitude) beginning first (L→H), (3) nonlinear patterns with mid-gradient plants starting growth before or after those from ends of ranges, and (4) geographically-broad synchrony. As discussed in Section 3, these evolutionary patterns are informative of how strong species’ controls against premature leafing are. Species showing the L→H ordering of leafing among origins in a common garden are likely to be less phenotypically plastic than H→L ordered species, with implications for how much these species can shift their phenology to adjust to climate change. Although some consistency is seen among species in common garden patterns within ecological or taxonomic groups, the diversity of observed responses reinforces that not all species will respond phenolgically to a warmer climate in the same way.
With only one clear exception, ring-porous species followed the L→H pattern; plants with warmer origins consistently leaf before conspecific trees of colder origin. This suggests these species have strong controls against early leafing such as high spring temperature exposure requirements, strong chilling requirements, or a critical photoperiod met relatively late in the spring, either alone or in combination. All of these features would lead plants to respond conservatively to warmer spring temperatures, consistent with ring-porous species’ other frost-avoiding strategies like leafing out later than sympatric diffuse-porous species (Lechowicz 1984). These patterns suggest that ring-porous species, most of which grow in mid- to late-successional forests, will benefit less from longer frost-free growing seasons in a warming climate. This would have consequences for the biodiversity of trees and wildlife that depends on the nuts produced by many ring-porous species. However, if frost probabilities continue to increase during vegetative growth onset (Augspurger 2013), conservative frost-avoidant strategies may still be beneficial. And further, it is important to ephasize that in spite of the statistical evidence that the L→H pattern is more common in ring-porous species, it is important to keep in mind the wide array of patterns seen in these and other species when preparing ecological forecasts and management plans.
Some leafing patterns were observed in common gardens with respect to species’ functional ecological properties, but most such trends are not particularly strong, so should not be extrapolated to other species in most cases. About a third of diffuse porous species leafed out in the H→L direction. This pattern can be explained by relatively small chilling requirements or critical photoperiods, consistent with an opportunistic use of warm weather periods (Körner and Basler 2010), or by decreasing degree-day requirements with increased elevation/latitude. Although some narrow-xylemed species did not follow this pattern, those that do may enjoy an advantage in a uniformly warmer climate due to a longer photosynthetic season, either throughout their ranges for chilling/photoperiod limited species, or in the upper elevation/latitude ranges of degree-day limited species. However, this benefit could be offset by late-spring frosts whose frequency is increasing in a warmer and more variable climate (Augspurger 2013). Species’ successional status had little evident relationship to leafing order along elevation/latitude origins in common gardens; both the H→L and L→H patterns were common among early and late successional species. This is significant because while early-successional species have been predicted to be more phenologically plastic to warmer temperatures (Körner and Basler 2010), it should not be assumed that all species in this functional group, even ones with similar xylem anatomy, will respond in the same way.
Species’ evolutionary affinities showed no clear relationship with leafing patterns. Both H→L and L→H patterns were seen among angiosperms and to a lesser extent gymnosperms. In some cases both patterns were seen within a single genus, for instance Acer and Picea. Some genera show consistent phenological patterns, for instance all Aesculus L. species I am aware of are among the first species to leaf out where they grow (Lechozicz 1984; Defila and Clot 2001; Augspurger et al. 2005). Broader phylogenetic studies also demonstrate evolutionary conservatism with regard to phenological cues (Davies et al. 2013). In spite of these examples, it is probably not appropriate to assume that a species will show similar phenological responses to a warming climate as do congeners, particularly among diffuse-porous trees; management of forests in the face of climate change requires species-specific data.
The species showing virtually simultaneous leaf out among provenances may be explained by a relatively short time available to evolve differentiation in this trait. Plant material from such cases came mostly from areas covered by ice during the Pleistocene (e.g. Sweden, Ontario, Poland). The plant populations currently found in those areas must have quickly and recently migrated to their current locations, so may not have had time to evolve differentiated responses to their current climate and light environments. Alternatively, these species may not benefit evolutionarily from local adaptation of phenological cues. Some species’ leafing synchrony in common gardens suggest that some trees can survive and grow in many different conditions despite having seemingly no local adaption to phenological cues. A particularly extreme example is Populus tremula collected over 10° of latitude in Sweden (Luquez et al. 2008), across huge gradients of temperature and light seasonality. Examples like these give hope that existing trees of at least some species will be able to phenologically adjust to substantial climate change.
Cases showing complex and non-linear trends may have multiple non-exclusive explanations. The possibility of more than one cue affecting the species’ leafing phenology was discussed in Section 3. Also possible is evolutionary adaptation to microclimatic anomalies where broad geographical trends toward colder temperatures are locally reversed. Such reversals can be seen in the St. Lawrence valley and to a lesser extent the Great Lakes region of North America (USDA undated). One case showing this pattern (Populus balsamifera; Farmer et al. 1993) does have accessions from the western edge of the Great Lakes region. Along elevational gradients, complicated trends could arise from microclimatic effects due to differences in topographic position (valley bottom vs. ridge top) or water bodies. Six of the 11 cases showing no or complicated patterns were elevational studies of European species from the Alps or Pyrenees, so these possibilities should be considered.
This study has demonstrated the value of existing, but in many cases obscure or overlooked studies of within-species differentiation of leaf budburst cues in temperate and boreal trees. The value of common garden phenology studies is similar to herbarium specimens and records from amateur naturalists in that it is an existing source of information that gives insight into an important but slow and difficult to study process. However, the encountered studies overwhelmingly focused on saplings <5 years old, leading to a possible developmental bias in these results. Studies that tracked and reported results of young plants for many years were rare enough that the impact and direction of any such bias is not possible to evaluate. Thus, continuing studies of newly planted gardens and rediscovered old common gardens would both be valuable targets of future phenological research. The high number of studies detected outside of the Web of Science search suggests that there are more studies to be found. However, the diversity of common garden leafing patterns found within many functional (e.g. diffuse porous) and ecological (successional status) groups mean detection of additional studies is unlikely to elucidate new generalizable patterns among these traits. These results should help guide the management of forests and reforestation programs, particularly in forecasting which species may be at increased risk of frost damage under climate change and selecting plants from origins more likely to have suitable phenological adaptions to a warmer climate.
Acknowledgements
I would like to thank Carol K. Augspurger, Dylan O. Burge and two anonymous reviewers for their constructive feedback on this paper.
References
Alberto F., Bouffier L., Louvet J.M., Lamy J.B., Delzon S., Kremer A. (2011). Adaptive responses for seed and leaf phenology in natural populations of sessile oak along an altitudinal gradient. Journal of Evolutionary Biology 24(7): 1442–1454. https://doi.org/10.1111/j.1420-9101.2011.02277.x.
Augspurger C.K. (2013). Reconstructing patterns of temperature, phenology, and frost damage over 124 years: spring damage risk is increasing. Ecology 94(1): 41–50. https://doi.org/10.1890/12-0200.1.
Augspurger C.K., Cheeseman J.M., Salk C.F. (2005). Light gains and physiological capacity of understorey woody plants during phenological avoidance of canopy shade. Functional Ecology 19(4): 537–546. https://doi.org/10.1111/j.1365-2435.2005.01027.x.
Basler D., Körner C. (2012). Photoperiod sensitivity of bud burst in 14 temperate forest tree species. Agricultural and Forest Meteorology 165: 73–81. https://doi.org/10.1016/j.agrformet.2012.06.001.
Bayramzadeh V., Funada R., Kubo T. (2008). Relationships between vessel element anatomy and physiological as well as morphological traits of leaves in Fagus crenata seedlings originating from different provenances. Trees 22: 217–224. https://doi.org/10.1007/s00468-007-0178-3.
Bey C.F. (1972). Leaf flush in black walnut at several midwest locations. Proceedings of the 19th Northeastern Forest Tree Improvement Conference. p. 47–51.
Blum B.M. (1988). Variation in the phenology of bud flushing in white and red spruce. Canadian Journal of Forest Research 18(3): 315–319. https://doi.org/10.1139/x88-048.
Bonhomme R. (2000). Bases and limits to using ‘degree.day’ units. European Journal of Agronomy 13(1): 1–10. https://doi.org/10.1016/S1161-0301(00)00058-7.
Brelsford C.C., Nybakken L., Kotilainen T.K., Robson T.M. (2019). The influence of spectral composition on spring and autumn phenology in trees. Tree Physiology 39(6): 925–950. https://doi.org/10.1093/treephys/tpz026.
Burley J. (1966). Genetic variation in seedling development of Sitka spruce Picea sitchensis (Bong.) Carr. Forestry 39(1): 68–94. https://doi.org/10.1093/forestry/39.1.68.
Butnor J.R., Verrico B.M., Johnsen K.H., Maier C.A., Vankus V., Keller S.R. (2019). Phenotypic variation in climate-associated traits of red spruce (Picea rubens Sarg.) along elevation gradients in the southern Appalachian Mountains. Castanea 84(2): 128–143. https://doi.org/10.2179/0008-7475.84.2.128.
Cannell M.G.R., Murray M.B., Sheppard L.J. (1985). Frost avoidance by selection for late budburst in Picea sitchensis. Journal of Applied Ecology 22(3): 931–941. https://doi.org/10.2307/2403241.
Carter J.M., Orive M.E., Gerhart L.M., Stern J.H., Marchin R.M., Nagel J., Ward J.K. (2017). Warmest extreme year in US history alters thermal requirements for tree phenology. Oecologia 183(4): 1197–1210. https://doi.org/10.1007/s00442-017-3838-z.
Chmura D.J., Rożkowski R. (2002). Variability of beech provenances in spring and autumn phenology. Silvae Genetica 51: 123–127.
Choat B., Medek D.E., Stuart S.A., Pasquet‐Kok J., Egerton J.J., Salari H., Sack L., Ball M.C. (2011). Xylem traits mediate a trade‐off between resistance to freeze–thaw‐induced embolism and photosynthetic capacity in overwintering evergreens. New Phytologist 191(4): 996–1005. https://doi.org/10.1111/j.1469-8137.2011.03772.x.
Chmura D.J., Rożkowski R., Chałupka W. (2012). Growth and phenology variation in progeny of Scots pine seed orchards and commercial seed stands. European Journal of Forest Research 131: 1229–1243. https://doi.org/10.1007/s10342-012-0594-9.
Christensen-Dalsgaard K.K., Tyree M.T., Mussone P.G. (2011). Surface tension phenomena in the xylem sap of three diffuse porous temperate tree species. Tree Physiology 31(4): 361–368. https://doi.org/10.1093/treephys/tpr018.
Chung H., Muraoka H., Nakamura M., Han S., Muller O., Son Y. (2013). Experimental warming studies on tree species and forest ecosystems: a literature review. Journal of Plant Research 126: 447–460. https://doi.org/10.1007/s10265-013-0565-3.
Clark J.S., Salk C.F., Melillo J.L., Mohan J.M. (2014a). Tree phenology responses to winter chilling, spring warming, at north and south range limits. Functional Ecology 28(6): 1344–1355. https://doi.org/10.1111/1365-2435.12309.
Clark J.S., Mohan J.M., Melillo J.L., Salk C.F. (2014b). The seasonal timing of warming that controls onset of the growing season. Global Change Biology 20(4): 1136–1145. https://doi.org/10.1111/gcb.12420.
Clausen K.E., Garrett P.W. (1969). Progress in birch genetics and tree improvement. In: Doolittle W.T., Bruns P.E. (eds.). Birch symposium proceedings, 1969 August 19–21, Durham, NH. US Department of Agriculture, Forest Service, Northeastern Forest Experiment Station, Upper Darby, PA. p. 86–94.
Davies T.J., Wolkovich E.M., Kraft N.J., Salamin N., Allen J.M., Ault T.R., Betancourt J.L., Bolmgren K., Cleland E.E., Cook B.I., Crimmins T.M. (2013). Phylogenetic conservatism in plant phenology. Journal of Ecology 101(6): 1520–1530. https://doi.org/10.1111/1365-2745.12154.
Deans J.D., Harvey F.J. (1995). Phenologies of sixteen European provenances of sessile oak growing in Scotland. Forestry: An International Journal of Forest Research 68(3): 265–274. https://doi.org/10.1093/forestry/68.3.265.
Defila C., Clot B. (2001). Phytophenological trends in Switzerland. International Journal of Biometeorology 45: 203–207. https://doi.org/10.1007/s004840100101.
Dettmann S., Pérez C.A., Thomas F.M. (2013). Xylem anatomy and calculated hydraulic conductance of four Nothofagus species with contrasting distribution in South-Central Chile. Trees 27: 685–696. https://doi.org/10.1007/s00468-012-0824-2.
Dhar A., Balliet N., Hawkins C.D., Carlson M.R., Berger V.G., Mahoney R. (2015). Bud flush phenology and nursery carryover effect of paper birch provenances. iForest-Biogeosciences and Forestry 8(6): 809. https://doi.org/10.3832/ifor1367-008.
Díaz-Sala C., Cabezas J.A., de Simón B.F., Abarca D., Guevara M.Á., de Miguel M., Cadahía E., Aranda I., Cervera M.T. (2013). The uniqueness of conifers. In: Poltronieri P., Burbulis N., Fogher C. (eds.). From plant genomics to plant biotechnology. Woodhead Publishing, Cambridge, UK. p. 67–96. https://doi.org/10.1533/9781908818478.67.
Dixit A., Kolb T. (2020). Variation in seedling budburst phenology and structural traits among southwestern ponderosa pine provenances. Canadian Journal of Forest Research 50(9): 872–879. https://doi.org/10.1139/cjfr-2019-0333.
Dragoni D., Schmid H.P., Wayson C.A., Potter H., Grimmond C.S.B., Randolph J.C. (2011). Evidence of increased net ecosystem productivity associated with a longer vegetated season in a deciduous forest in south‐central Indiana, USA. Global Change Biology 17(2): 886–897. https://doi.org/10.1111/j.1365-2486.2010.02281.x.
Duboscq-Carra V.G., Arias-Rios J.A., El Mujtar V.A., Marchelli P., Pastorino M.J. (2020). Differentiation in phenology among and within natural populations of a South American Nothofagus revealed by a two-year evaluation in a common garden trial. Forest Ecology and Management 460 article 117858. https://doi.org/10.1016/j.foreco.2019.117858.
Ducousso A., Guyon J.P., Kremer A. (1996). Latitudinal and altitudinal variation of bud burst in western populations of sessile oak (Quercus petraea (Matt) Liebl). Annales des Sciences Forestieres 53(2–3): 775–782. https://doi.org/10.1051/forest:19960253.
Evert R.F. (2006). Esau’s plant anatomy: meristems, cells, and tissues of the plant body: their structure, function, and development. John Wiley and Sons, Hoboken, New Jersey. 600 p. https://doi.org/10.1002/0470047380.
Farmer R.E. (1993). Latitudinal variation in height and phenology of balsam poplar. Silvae Genetica 42: 148–153.
Farmer R.E., Russell T.E., Krinard R.M. (1967). Sixth-year results from a yellow-poplar provenance test. Proceedings of the 9th Southern Conference on Forest Tree Improvement. p. 65–68.
Frank A., Pluess A.R., Howe G.T., Sperisen C., Heiri C. (2017). Quantitative genetic differentiation and phenotypic plasticity of European beech in a heterogeneous landscape: indications for past climate adaptation. Perspectives in Plant Ecology, Evolution and Systematics 26: 1–13. https://doi.org/10.1016/j.ppees.2017.02.001.
Fridley J.D. (2012). Extended leaf phenology and the autumn niche in deciduous forest invasions. Nature 485: 359–362. https://doi.org/10.1038/nature11056.
Friedman J.M., Roelle J.E., Cade B.S. (2011). Genetic and environmental influences on leaf phenology and cold hardiness of native and introduced riparian trees. International Journal of Biometeorology 55(6): 775–787. https://doi.org/10.1007/s00484-011-0494-6.
Fu Y.H., Zhang X., Piao S., Hao F., Geng X., Vitasse Y., Zohner C., Peñuelas J., Janssens I.A. (2019). Daylength helps temperate deciduous trees to leaf‐out at the optimal time. Global Change Biology 25(7): 2410–2418. https://doi.org/10.1111/gcb.14633.
Garner W.W., Allard H.A. (1923). Further studies in photoperiodism: the response of the plant to relative length of day and night. US Government Printing Office.
Hacke U.G., Sperry J.S. (2001). Functional and ecological xylem anatomy. Perspectives in Plant Ecology, Evolution and Systematics 4(2): 97–115. https://doi.org/10.1078/1433-8319-00017.
Hacke U.G., Sperry J.S., Pockman W.T., Davis S.D., McCulloh K.A. (2001). Trends in wood density and structure are linked to prevention of xylem implosion by negative pressure. Oecologia 126: 457–461. https://doi.org/10.1007/s004420100628.
Heide O.M. (1993). Dormancy release in beech buds (Fagus sylvatica) requires both chilling and long days. Physiologia Plantarum 89(1): 187–191. https://doi.org/10.1111/j.1399-3054.1993.tb01804.x.
Jump A.S., Peñuelas J. (2005). Running to stand still: adaptation and the response of plants to rapid climate change. Ecology Letters 8(9): 1010–1020. https://doi.org/10.1111/j.1461-0248.2005.00796.x.
Keller F., Körner C. (2003). The role of photoperiodism in alpine plant development. Arctic, Antarctic, and Alpine Research 35(3): 361–368. https://doi.org/10.1657/1523-0430(2003)035[0361:TROPIA]2.0.CO;2.
Keller S.R., Soolanayakanahally R.Y., Guy R.D., Silim S.N., Olson M.S., Tiffin P. (2011). Climate-driven local adaptation of ecophysiology and phenology in balsam poplar, Populus balsamifera L. (Salicaceae). American Journal of Botany 98(1): 99–108. https://doi.org/10.3732/ajb.1000317.
Körner C., Basler D. (2010). Phenology under global warming. Science 327(5972): 1461–1462. https://doi.org/10.1126/science.1186473.
Kriebel H.B. (1957). Patterns of genetic variation in sugar maple. Ohio Agricultural Experiment Station Research Bulletin 791: 1–56.
Kudoh H. (2016). Molecular phenology in plants: in natura systems biology for the comprehensive understanding of seasonal responses under natural environments. New Phytologist 210(2): 399–412. https://doi.org/10.1111/nph.13733.
Laube J., Sparks T.H., Estrella N., Höfler J., Ankerst D.P., Menzel A. (2014). Chilling outweighs photoperiod in preventing precocious spring development. Global Change Biology 20(1): 170–182. https://doi.org/10.1111/gcb.12360.
Lechowicz M.J. (1984). Why do temperate deciduous trees leaf out at different times? Adaptation and ecology of forest communities. The American Naturalist 124(6): 821–842. https://doi.org/10.1086/284319.
Leuning R. (2002). Temperature dependence of two parameters in a photosynthesis model. Plant, Cell and Environment 25(9): 1205–1210. https://doi.org/10.1046/j.1365-3040.2002.00898.x.
Li P., Beaulieu J., Bousquet J. (1997). Genetic structure and patterns of genetic variation among populations in eastern white spruce (Picea glauca). Canadian Journal of Forest Research 27(2): 189–198. https://doi.org/10.1139/x96-159.
Liang L. (2019). Geographic variations in spring and autumn phenology of white ash in a common garden. Physical Geography: 36(6): 489–509. https://doi.org/10.1080/02723646.2015.1123538.
Liepe K. (1993). Growth-chamber trial on frost hardiness and field trial on flushing of sessile oak (Quercus petraea Liebl). Annales des Sciences Forestières 50: 208–214. https://doi.org/10.1051/forest:19930719.
Lopez O.R., Farris‐Lopez K., Montgomery R.A., Givnish T.J. (2008). Leaf phenology in relation to canopy closure in southern Appalachian trees. American Journal of Botany 95(11): 1395–1407. https://doi.org/10.3732/ajb.0800104.
Luquez V., Hall D., Albrectsen B.R., Karlsson J., Ingvarsson P., Jansson S. (2008). Natural phenological variation in aspen (Populus tremula): the SwAsp collection. Tree Genetics and Genomes 4: 279–292. https://doi.org/10.1007/s11295-007-0108-y.
Martínez-Berdeja A., Hamilton J.A., Bontemps A., Schmitt J., Wright J.W. (2019). Evidence for population differentiation among Jeffrey and Ponderosa pines in survival, growth and phenology. Forest Ecology and Management 434: 40–48. https://doi.org/10.1016/j.foreco.2018.12.009.
Masiokas M., Villalba R. (2004). Climatic significance of intra-annual bands in the wood of Nothofagus pumilio in southern Patagonia. Trees 18: 696–704. https://doi.org/10.1007/s00468-004-0355-6.
Mayor S.J., Guralnick R.P., Tingley M.W., Otegui J., Withey J.C., Elmendorf S.C., Andrew M.E., Leyk S., Pearse I.S., Schneider D.C. (2017). Increasing phenological asynchrony between spring green-up and arrival of migratory birds. Scientific Reports 7 article 1902. https://doi.org/10.1038/s41598-017-02045-z.
McBride J.R., Norberg E.A., Bertenshaw J.L., Kloss S., Mossadegh A. (1997). Genetic variation in shoot growth, phenology, and mineral accumulation of northern and central Sierra Nevada foothill populations of blue oak. In: Pillsbury N.H., Verner J., Tietje W.D. (technical coordinators). Proceedings of a symposium on oak woodlands: ecology, management, and urban interface issues; 19–22 March 1996; San Luis Obispo, CA. General Technical Report PSW-GTR-160. Pacific Southwest Research Station, Forest Service, US Department of Agriculture, Albany, CA. p. 117–126.
McDonough Mackenzie C., Primack R.B., Miller-Rushing A.J. (2018). Local environment, not local adaptation, drives leaf-out phenology in common gardens along an elevational gradient in Acadia National Park, Maine. American Journal of Botany 105(6): 1–10. https://doi.org/10.1002/ajb2.1108.
McGee C.E. (1974). Elevation of seed sources and planting sites affects phenology and development of red oak seedlings. Forest Science 20: 160–164.
McKown A.D., Guy R.D., Klápště J., Geraldes A., Friedmann M., Cronk Q.C., El‐Kassaby Y.A., Mansfield S.D., Douglas C.J. (2014). Geographical and environmental gradients shape phenotypic trait variation and genetic structure in Populus trichocarpa. New Phytologist 201(4): 1263–1276. https://doi.org/10.1111/nph.12601.
McMillan C., Winstead J.E. (1976). Adaptive differentiation in Liquidambar styraciflua L. from eastern United-States and northeastern Mexico under uniform environmental-conditions. Botanical Gazette 137(4): 361–367. https://doi.org/10.1086/336885.
Mimura M., Aitken S.N. (2007). Adaptive gradients and isolation-by-distance with postglacial migration in Picea sitchensis. Heredity 99: 224–232. https://doi.org/10.1038/sj.hdy.6800987.
Myking T., Heide O.M. (1995). Dormancy release and chilling requirement of buds of latitudinal ecotypes of Betula pendula and B. pubescens. Tree Physiology 15(11): 697–704. https://doi.org/10.1093/treephys/15.11.697.
Nanninga C., Buyarski C.R., Pretorius A.M., Montgomery R.A. (2017). Increased exposure to chilling advances the time to budburst in North American tree species. Tree Physiology 37(12): 1727–1738. https://doi.org/10.1093/treephys/tpx136.
Nicotra A.B., Atkin O.K., Bonser S.P., Davidson A.M., Finnegan E.J., Mathesius U., Poot P., Purugganan M.D., Richards C.L., Valladares F., van Kleunen M. (2010). Plant phenotypic plasticity in a changing climate. Trends in Plant Science 15(12): 684–692. https://doi.org/10.1016/j.tplants.2010.09.008.
Nienstaedt H. (1974). Genetic variation in some phenological characteristics of forest trees. In: Leith H. (ed.). Phenology and seasonality modeling. Springer, Berlin. p. 389–400. https://doi.org/10.1007/978-3-642-51863-8_33.
Osada N., Murase K., Tsuji K., Sawada H., Nunokawa K., Tsukahara M., Hiura T. (2018). Genetic differentiation in the timing of budburst in Fagus crenata in relation to temperature and photoperiod. International Journal of Biometeorology 62(9): 1763–1776. https://doi.org/10.1007/s00484-018-1579-2.
Oubida R.W., Gantulga D., Zhang M., Zhou L., Bawa R., Holliday J.A. (2015). Partitioning of multivariate phenotypes using regression trees reveals complex patterns of adaptation to climate across the range of black cottonwood (Populus trichocarpa). Frontiers in Plant Science 6 article 181. https://doi.org/10.3389/fpls.2015.00181.
Pearse I.S., Baty J.H., Herrmann D., Sage R., Koenig W.D. (2015). Leaf phenology mediates provenance differences in herbivore populations on valley oaks in a common garden. Ecological Entomology 40(5): 525–531. https://doi.org/10.1111/een.12219.
Piao S., Liu Q., Chen A., Janssens I.A., Fu Y., Dai J., Liu L., Lian X., Shen M., Zhu X. (2019). Plant phenology and global climate change: current progresses and challenges. Global Change Biology 25(6): 1922–1940. https://doi.org/10.1111/gcb.14619.
Premoli A.C., Raffaele E., Mathiasen P. (2007) Morphological and phenological differences in Nothofagus pumilio from contrasting elevations: evidence from a common garden. Austral Ecology 32(5): 515–523. https://doi.org/10.1111/j.1442-9993.2007.01720.x.
Primack R.B. Miller-Rushing A.J. (2012). Uncovering, collecting, and analyzing records to investigate the ecological impacts of climate change: a template from Thoreau’s Concord. BioScience 62(2): 170–181. https://doi.org/10.1525/bio.2012.62.2.10.
Putnam R.C., Reich P.B. (2017). Climate and competition affect growth and survival of transplanted sugar maple seedlings along a 1700‐km gradient. Ecological Monographs 87(1): 130–157. https://doi.org/10.1002/ecm.1237.
R Core Team (2016). R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/.
Richardson A.D., Hollinger D.Y., Dail D.B., Lee J.T., Munger J.W., O’keefe J. (2009). Influence of spring phenology on seasonal and annual carbon balance in two contrasting New England forests. Tree Physiology 29(3): 321–331. https://doi.org/10.1093/treephys/tpn040.
Salmela M.J., Cavers S., Cottrell J.E., Iason G.R., Ennos R.A. (2011). Seasonal patterns of photochemical capacity and spring phenology reveal genetic differentiation among native Scots pine (Pinus sylvestris L.) populations in Scotland. Forest Ecology and Management 262(6): 1020–1029. https://doi.org/10.1016/j.foreco.2011.05.037.
Schmitt D.M., Webb C.D. (1971). Georgia sycamore seed sources in Mississippi plantings: site adaptability a key factor. Proceedings of the eleventh conference on southern forest tree improvement.
Schoch W., Heller I., Schweingruber F.H., Kienast F. (2004). Wood anatomy of central European species. http://www.woodanatomy.ch/. [Cited 4 May 2020].
Schwartz M.D., Hanes J.M. (2010). Continental‐scale phenology: warming and chilling. International Journal of Climatology 30(11): 1595–1598. https://doi.org/10.1002/joc.2014.
Silvestro R., Rossi S., Zhang S., Froment I., Huang J.G., Saracino A. (2019). From phenology to forest management: ecotypes selection can avoid early or late frosts, but not both. Forest Ecology and Management 436: 21–26. https://doi.org/10.1016/j.foreco.2019.01.005.
Smith H. (1994). Sensing the light environment: the functions of the phytochrome family. In: Kendrick R.E., Kronenberg G.H.M. (eds.). Photomorphogenesis in plants. Kluwer Academic Publishers, Dordrecht. p. 170–181. https://doi.org/10.1007/978-94-011-1884-2_15.
Smith H. (1995). Physiological and ecological function within the Phytochrome family. Annual Review of Plant Physiology and Plant Molecular Biology 46: 289–315. https://doi.org/10.1146/annurev.pp.46.060195.001445.
Søgaard G., Johnsen O., Nilsen J., Junttila O. (2008). Climatic control of bud burst in young seedlings of nine provenances of Norway spruce. Tree Physiology 28(2): 311–320. https://doi.org/10.1093/treephys/28.2.311.
Soolanayakanahally R.Y., Guy R.D., Silim S.N., Song M. (2013). Timing of photoperiodic competency causes phenological mismatch in balsam poplar (Populus balsamifera L.). Plant, Cell and Environment 36(1): 116–127. https://doi.org/10.1111/j.1365-3040.2012.02560.x.
Sparks J.P., Campbell G.S., Black R.A. (2000). Liquid water content of wood tissue at temperatures below 0°C. Canadian Journal of Forest Research 30(4): 624–630. https://doi.org/10.1139/x99-241.
Taeger S., Sparks T.H., Menzel A. (2015). Effects of temperature and drought manipulations on seedlings of Scots pine provenances. Plant Biology 17(2): 361–372. https://doi.org/10.1111/plb.12245.
Tang J., Körner C., Muraoka H., Piao S., Shen M., Thackeray S.J., Yang X. (2016). Emerging opportunities and challenges in phenology: a review. Ecosphere 7(8) article e01436. https://doi.org/10.1002/ecs2.1436.
Thackeray S.J., Henrys P.A., Hemming D., Bell J.R., Botham M.S., Burthe S., Helaouet P., Johns D.G., Jones I.D., Leech D.I., Mackay E.B. (2016). Phenological sensitivity to climate across taxa and trophic levels. Nature 535: 241–245. https://doi.org/10.1038/nature18608.
Vander Mijnsbrugge K., Turcsán A., Michiels B. (2016). Population differentiation and phenotypic plasticity in temperature response of bud burst in Frangula alnus provenances of different latitude. Plant Systematics and Evolution 302(3): 257–264. https://doi.org/10.1007/s00606-015-1258-2.
Visser M.E., Both C. (2005). Shifts in phenology due to global climate change: the need for a yardstick. Proceedings of the Royal Society B: Biological Sciences 272(1581): 2561–2569. https://doi.org/10.1098/rspb.2005.3356.
Vitasse Y., Basler D. (2013). What role for photoperiod in the bud burst phenology of European beech. European Journal of Forest Research 132: 1–8. https://doi.org/10.1007/s10342-012-0661-2.
Vitasse Y., Delzon S., Bresson C.C., Michalet R., Kremer A. (2009). Altitudinal differentiation in growth and phenology among populations of temperate-zone tree species growing in a common garden. Canadian Journal of Forest Research 39(7): 1259–1269. https://doi.org/10.1139/X09-054.
Vitasse Y., Bresson C.C., Kremer A., Michalet R., Delzon S. (2010). Quantifying phenological plasticity to temperature in two temperate tree species. Functional Ecology 24(6): 1211–1218. https://doi.org/10.1111/j.1365-2435.2010.01748.x.
Vitasse Y., Hoch G., Randin C.F., Lenz A., Kollas C., Scheepens J.F., Körner C. (2013). Elevational adaptation and plasticity in seedling phenology of temperate deciduous tree species. Oecologia 171: 663–678. https://doi.org/10.1007/s00442-012-2580-9.
USDA (undated). USDA plant hardiness zone map. https://planthardiness.ars.usda.gov/PHZMWeb/Images/northamerica.jpg. [Cited 24 Sept. 2020].
Ware I.M., Van Nuland M.E., Schweitzer J.A., Yang Z., Schadt C.W., Sidak‐Loftis L.C., Stone N.E, Busch J.D., Wagner D.M., Bailey J.K. (2019). Climate‐driven reduction of genetic variation in plant phenology alters soil communities and nutrient pools. Global Change Biology 25(4): 1514–1528. https://doi.org/10.1111/gcb.14553.
Way D.A., Montgomery R.A. (2015). Photoperiod constraints on tree phenology, performance and migration in a warming world. Plant Cell and Environment 38(9): 1725–1736. https://doi.org/10.1111/pce.12431.
Wolkovich E.M., Cook B.I., Allen J.M., Crimmins T.M., Betancourt J.L., Travers S.E., Pau S., Regetz J., Davies T.J., Kraft N.J., Ault T.R. (2012). Warming experiments underpredict plant phenological responses to climate change. Nature 485: 494–497. https://doi.org/10.1038/nature11014.
Ying C.C., Bagley W.T. (1976). Genetic-Variation of Eastern Cottonwood in an Eastern Nebraska Provenance Study. Silvae Genetica 25: 67–73.
Zhao M., Peng C., Xiang W., Deng X., Tian D., Zhou X., Yu G., He H., Zhao Z. (2013). Plant phenological modeling and its application in global climate change research: overview and future challenges. Environmental Reviews 21(1): 1–14. https://doi.org/10.1139/er-2012-0036.
Zipf L., Primack R.B. (2017). Humidity does not appear to trigger leaf out in woody plants. International Journal of Biometeorology 61: 2213–2216. https://doi.org/10.1007/s00484-017-1428-8.
Zohner C.M., Renner S.S. (2014). Common garden comparison of the leaf‐out phenology of woody species from different native climates, combined with herbarium records, forecasts long‐term change. Ecology Letters 17(8): 1016–1025. https://doi.org/10.1111/ele.12308.
Total of 105 references.

