Ips acuminatus kills pines in southern Finland
Siitonen J. (2014). Ips acuminatus kills pines in southern Finland. Silva Fennica vol. 48 no. 4 article id 1145. https://doi.org/10.14214/sf.1145
Highlights
- Recently dead pines colonized by Ips acuminatus were frequently found in southern Finland, in a region where the species was thought to be absent
- Colonized trees were typically large (average DBH 30 cm), located at open spots in pine-dominated stands, often forming groups of several trees
- The damages may be a consequence of dry and hot summers during the 2000s.
Abstract
Recently dead Scots pines (Pinus sylvestris L.) apparently killed by Ips acuminatus (Gyllenhal) were observed in Sipoo, southern Finland, in summer 2013. This record was unexpected and in contradiction with what is currently known about the distribution and aggressiveness of the species in Finland. The aim of this study was to survey a larger area in Uusimaa region, to find out whether I. acuminatus occurs frequently in recently dead pines, and whether inhabited trees share some common tree- or site-level characteristics. Galleries of I. acuminatus were found in most of the studied trees. A total of 96 inhabited trees were found in 21 separate sites. Colonized pines were typically large (average DBH 30 ± 9 cm) trees located in relatively open pine-dominated heathland stands at half-open, sun-exposed spots. The whole upper part of the trunk with thin bark was usually occupied. Galleries of Tomicus piniperda L. or T. minor Hartig occurred only in few cases in the same trees, indicating that the trees had died later in the summer. Galleries of the jewel beetle Phaenops cyanea F. were found in 13 trees. Trees colonized by I. acuminatus often occurred as small groups, with generally 1–12 trees (average 3 trees), but in one exceptional group there were no less than 35 trees. It is possible that the hot and dry summers during the 2000s have increased the susceptibility of pines to insect damage, and have contributed to a population growth of I. acuminatus.
Keywords
tree mortality;
Scots pine;
Ips acuminatus;
Phaenops cyanea;
drought
Received 14 March 2014 Accepted 19 September 2014 Published 20 November 2014
Views 71548
Available at https://doi.org/10.14214/sf.1145 | Download PDF

1 Introduction
Ips acuminatus (Gyllenhal) is a bark beetle species (Curculionidae: Scolytinae) which lives mainly on Scots pine (Pinus sylvestris L.). The beetle has a wide distribution ranging from northern Spain to northern Fennoscandia in Europe, and through Siberia to China, Japan and Korea in the east (Wood and Bright 1992; Bright and Skidmore 2002). It breeds under the thin, scaly bark of pine in the upper parts of trunks and in thick branches. The species can breed in weakened or dead standing trees, in fallen trees, in cut bolts with thin bark and in logging residues.
Ips acuminatus has traditionally been treated in forest entomological textbooks as a timber pest that can cause economic damage by transmitting blue-stain fungi to fresh logs and pulpwood of pine. The beetle carries its own blue-stain fungus species, Ophiostoma clavatum Mathiesen-Käärik (Villari et al. 2013), which causes rapid and strong staining of the wood. However, I. acuminatus has not been considered as an aggressive, tree-killing bark beetle species in older forest entomological literature in Europe (e.g. Saalas 1949; Bakke 1968; Schwenke 1974).
Since 1950s, I. acuminatus started to decline in southern Finland and disappeared from large regions. Previously the species occurred throughout the country from southernmost coastal areas to the northern timberline of pine (Lekander et al. 1977). The southern border of distribution was surveyed in detail in the beginning of 1980s, and at that time the southern range limit ran approximately from Vaasa (in the midway along the Gulf of Bothnia) to Imatra in southeastern Finland (Puukko 1981). Some sporadic observations of breeding individuals have been made later in southern Finland (Heliövaara and Puukko 1986; Eero Helve 2012, pers. comm.). Ips acuminatus has declined in southern Sweden in much the same way as in Finland (Lindelöw 2010), but the reasons for the decline are unknown. It has been hypothesized that, starting from 1950s, the intensification of forestry and increasing logging may have favored some bark beetle species and disfavored others. Species thriving in managed forests include e.g. Tomicus piniperda L. and T. minor Hartig, which breed in pine and swarm earlier than I. acuminatus. These species could colonize most of the breeding material suitable for I. acuminatus, such as weakened and recently dead trees, and thus displace it (Puukko 1981; Heliövaara and Puukko 1986).
During the last decades, tree mortality caused by I. acuminatus seems to have increased in central and southern Europe. In the beginning of the 2000s, a review of bark- and wood-boring insects assessed I. acuminatus as belonging among the ten most important wood-boring pest species in Europe, and it was scored as particularly damaging in Germany, Slovakia, Switzerland, Romania and Spain (Grégoire and Evans 2004). Recently, unprecedented outbreaks have been observed in Swiss and Italian Alps (Wermelinger et al. 2008; Colombari et al. 2013).
Exceptional drought in summers 2003 and 2006 caused local mortality of trees over a large area in southern Finland. Mortality of trees was particularly frequent in pine-dominated stands growing on rocky outcrops, and trees have often remained unharvested in these sparsely wooded, economically marginal areas. Since 2008, I have observed old galleries belonging to I. acuminatus on sparsely wooded rocks in several places in the municipalities of Helsinki, Vantaa, Sipoo and Porvoo. In all these cases, galleries were found on barkless trees that had died already several years earlier, presumably because of drought.
In spring 2013, I investigated the cause of death of recently dead pines in Sipoo. Several large trees at a forest edge bordering a farmyard had died during the previous two summers, and part of the dead trees had already been removed. It turned out that the remaining three pines were full of galleries of I. acuminatus in the upper parts of the trunks, and the beetle had clearly contributed to the death of these trees. The occurrence of I. acuminatus as a primary mortality factor of pines in southern Finland was unexpected and in contradiction with what is currently known about the distribution and aggressiveness of the species in Finland. The aim of this study was to survey a larger area in Uusimaa region in southern Finland, to find out whether I. acuminatus occurs frequently in recently dead pines, and whether colonized trees share some common tree- or site-level characteristics.
2 Material and methods
The material was gathered in Uusimaa region during the field season 2013. When recently dead pines were detected e.g. along roadsides they were investigated for the wood-boring species occurring in them, and for the possible causes of death. All trees belonging to the same group (with a distance < 20 m from each other) were studied. The coordinates of each site were measured using a GPS device, and the site type, stand openness (open, half-open, half-shaded, shaded), and topography (flat, top of a hill or a rock, slope, direction of the slope) were assessed. The diameter at breast height of each dead tree was measured, and the height and time since death were estimated. The most abundant bark beetle species and other wood-boring insect species were identified based on their galleries by removing some bark from the base of the trunk. Galleries located higher up in the trunk were identified by the aid of binoculars, or using pieces of bark that had fallen to the ground.
In trees inhabited by I. acuminatus from which most of the bark of the upper trunk had already come off (in Scots pine, this usually happens already during the second year after the death), the height of the lowermost and the uppermost galleries was estimated in meters, and the proportion of trunk surface between these points occupied by the galleries (below: cover) in 10% classes was estimated.
3 Results
Galleries of I. acuminatus were found in most of the trees studied. The species was detected at 21 different sites out of the 23 studied sites. The westernmost site was in Karkkila (about 60°29´N, 24°22´E), and the easternmost in Pernaja (about 60°29´N, 25°53´E) with a distance of about 90 km from each other. In south-north direction, the southernmost sites were located at the coastline while the northernmost ones were about 60 km inland. Galleries were observed in a total of 96 trees (Figs. 1A and B). In most cases, I. acuminatus appeared to be the proximate or at least contributing mortality factor of the trees. The whole upper part of the trunk with thin bark had usually been colonized, and the cover of I. acuminatus galleries within the colonized part of the trunk was 90–100% (n = 35 trees). In addition to I. acuminatus, galleries of Tomicus piniperda or T. minor occurred only in few cases in the same trees, which indicated that the trees had died later in the summer, only after the swarming of Tomicus species. Galleries and sometimes larvae of the jewel beetle species Phaenops cyanea F. were found in 13 trees inhabited by I. acuminatus. In these trees, P. cyanea had attacked the lower trunk with thick bark, and the species had very likely contributed to the death of the trees. Galleries always occurred only on the southern side of the trunk.

Fig. 1. A group of four large pines in rocky terrain colonized by Ips acuminatus and (one of the trees) Phaenops cyanea second summer after their death (A). Section of upper trunk entirely covered by I. acuminatus galleries (B). In trees where the bark has fallen off, it is easy to ascertain the presence of I. acuminatus based on its characteristic galleries using binoculars or a digital camera with a sufficient zoom (here about 15x).
The diameter of trees colonized by I. acuminatus varied between 8 and 49 cm, and the average diameter (± SD) was 30 ± 9 cm (Fig. 2). Site types ranged from herb-rich heathland forest (Oxalis-Myrtillus type) to rocky subxeric heathland forest (Vaccinium type). However, the majority of the colonized pines were old and large trees in relatively open, pine-dominated stands. It was also typical that the trees were located in open or half-open spots on hillocks and ridges, at forest edges, in rocky terrain, or they were seed trees left in regeneration areas. At least the upper part of each tree – where the galleries of I. acuminatus were located – was always exposed to direct sunlight. In addition to the dead standing trees, galleries were found in one fallen seed-tree pine.
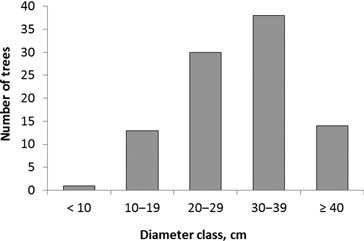
Fig. 2. Diameter distribution of pines (n = 96) colonized by Ips acuminatus.
Trees colonized by I. acuminatus often occurred as small groups, in which trees with different sizes had died at the same time during the same summer (as deduced by the degree of needle and bark loss, and by the composition of the associated insect assemblage). This also indicated that this species was the primary cause of death of the trees. Ips acuminatus possesses an aggregation pheromone. Individuals that manage to successfully attack a weakened tree release the pheromone, which will lead to the aggregation of many individuals to the same tree, and which can induce attacking also neighboring trees − with the result that trees will die in groups. The number of trees killed by I. acuminatus in one group was generally 1−12 trees (on the average 3 trees), but in one exceptional group there were no less than 35 dead trees (Fig. 3). In this group, all the trees had died during the summer 2013. Pines of all sizes had died at the site: the diameter of dead trees varied from 11 cm to 35 cm. The youngest dead trees were about 50-year-old and the oldest ones about 90-year-old. The site was the southern end of a narrow esker surrounded by fields from three sides.
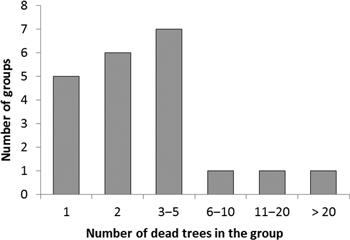
Fig. 3. Frequency distribution of tree group sizes (no. of adjacent dead trees forming a group) colonized by Ips acuminatus.
4 Discussion
The results of this study showed that mortality of pines that can be attributed to I. acuminatus is widespread within the surveyed area in southern Finland. This could mean either that such damages are a new phenomenon, or that pines killed by I. acuminatus have actually occurred all the time but remained unnoticed. Lack of damages would thus merely indicate lack of observation, and ‘new’ damages could be explained by the observation specifically directed to the species.
Unfortunately, is not possible to resolve this question indisputably. The survey by Puukko (1981) concerning the southern range limit of I. acuminatus during the 1980s was based on surveying pulpwood piles or logging residues in recently clear-cut areas, and on galleries of the focal species found in these substrates. However, in dead standing trees that were surveyed in this study, the galleries of I. acuminatus are located in the upper parts of trunks, usually at a height of over 5 metres, and binoculars are needed to identify the galleries from the ground. This means that in dead standing trees the galleries will easily remain undetected. On the other hand, I. acuminatus also breeds in fallen pines and logging residues with thin bark, from which the species should be as easy to find as before.
In any case, damages caused by I. acuminatus have recently increased elsewhere in Europe, and they have been attributed to raising summer temperatures and drought. In Italian Alps, an outbreak started in 2005, probably triggered by the record summer drought in Europe in 2003 (Colombari et al. 2013). In Swiss Alps, increasing mortality of pines at least partly caused by I. acuminatus (Wermelinger et al. 2008), started already in the 1990s and peaked following the drought summers in 1998 and 2003 (Dobbertin et al. 2007). Moreover, the long-term (since the beginning of 1900s) forest reports recording pine mortality and its causes showed that beetle-related mortality strikingly coincided with periods of above-average summer temperatures and had considerably increased since mid-1990s (Dobbertin et al. 2007).
The jewel beetle P. cyanea has never been reported to contribute to tree death in Finland before. In central Europe (e.g. in Poland) it is considered as an important pest of pine (Grégoire and Evans 2004), and it is capable of attacking trees that are only moderately weakened (Wermelinger et al. 2008). Damages caused by P. cyanea are particularly connected to dry and hot summers (Wermelinger et al. 2008).
Drought stresses trees and predisposes them to insect attack in several different ways simultaneously (Bréda et al. 2006; McDowell et al. 2008, 2011). Drought is not only connected to precipitation but to the interaction between precipitation and temperature. Drought stress occurs in trees when evapotranspiration from leaves or needles exceeds the water-taking capacity of roots. Two different mechanisms can cause mortality during drought. Hydraulic failure can occur when reduced soil water availability is combined with high transpiration. This can lead to air-filled sections in the water-conducting vessels, which in turn cuts off water transport. Another mechanism is carbon starvation caused by the closure of stomata to prevent desiccation. However, stomatal closure reduces the uptake of carbon dioxide and therefore photosynthetic activity. The reduced production of sugar from photosynthesis while respiration continues leads to carbon starvation and reduced defensive capability against pathogenic fungi and insects. Reduced tree growth and increased mortality following a drought period can continue for many years (Bigler et al. 2006), probably as a consequence of both water transport dysfunction and depletion of plant carbon reserves (Bréda et al. 2006). Trees suffering from drought stress are vulnerable to pathogenic fungi and insect pests, because the water potential in sapwood has decreased (which induces the growth of e.g. blue-stain fungi carried by bark beetles), or because the production of resin and other compounds used for defense has been reduced. On the other hand, warm temperatures have direct effects on the population dynamics of insect pest by accelerating their development and reproduction.
In southern coastal Finland, the worst drought damages during the 2000s occurred in summer 2002. The record drought summer of 2003 in central Europe was ordinary in southern Finland as regards precipitation, but the number of heat days with the temperature ≥ 25 °C was almost twice as high as during the reference period (see: Finnish Meteorological Institute, http://en.ilmatieteenlaitos.fi/weather-in-recent-years). Also summer 2006 was exceptionally dry in whole Finland. Summer 2010 was exceptionally hot, with daily temperatures continuously exceeding 25 °C during a period of six weeks in July–August in southern Finland.
It is probable that the hot and dry summers during the 2000s have increased the susceptibility of pines to insect damage, and may have contributed to a population growth of I. acuminatus in southern Finland. Based on the present survey, it is not possible to estimate how large an area in southern Finland has been affected and how significant a factor the species is in increasing mortality of pines. As average summer temperatures are expected to rise, this may mean that I. acuminatus becomes established as a ‘new’ pest of pine in southern Finland. Further surveys and monitoring of the species are obviously needed.
References
Bakke A. (1968). Ecological studies on bark beetles (Coleoptera: Scolytidae) associated with Scots pine (Pinus sylvestris L.) in Norway with particular reference to the influence of temperature. Meddelelser fra det Norske skogforsøksvesen 21: 443–602.
Bigler C., Bräker O.U., Bugmann H., Dobbertin M., Rigling A. (2006). Drought as an inciting mortality factor in Scots pine stands of the Valais, Switzerland. Ecosystems 9: 330–343. http://dx.doi.org/10.1007/s10021-005-0126-2.
Bréda N., Huc R., Granier A., Dreyer E. (2006). Temperate forest trees and stands under severe drought: a review of ecophysiological responses, adaptation processes and long-term consequences. Annals of Forest Science 63: 625–644. http://dx.doi.org/10.1051/forest:2006042.
Bright D., Skidmore R.E. (2002). A catalog of Scolytidae and Platypodidae (Coleoptera), Supplement 2 (1995–1999). National Research Council Press, Ottawa. 523 p.
Colombari F., Schroeder M., Battisti A., Faccoli M. (2013). Spatio-temporal dynamics of Ips acuminatus outbreak and implications for management. Agricultural and Forest Entomology 15: 34−42. http://dx.doi.org/10.1111/j.1461-9563.2012.00589.x.
Dobbertin M., Wermelinger B., Bigler C., Bürgi M., Carron M., Foster B., Gimmi U., Rigling M. (2007). Linking increasing drought stress to Scots pine mortality and bark beetle infestations. The Scientific World Journal 7: 231–239. http://dx.doi.org/10.1100/tsw.2007.58.
Finnish Meteorological Institute. http://en.ilmatieteenlaitos.fi/weather-in-recent-years.
Grégoire J.-C., Evans H. (2004). Damage and control of BAWBILT organisms, an overview. In: Lieutier F., Day K.R., Battisti A., Grégoire J.-C., Evans H. (eds.). Bark and wood boring insects in living trees in Europe, a synthesis. Kluwer, Dordrecht. p. 19−37. http://dx.doi.org/10.1007/978-1-4020-2241-8_4.
Heliövaara K., Puukko K. (1986). Note on Ips acuminatus (Coleoptera, Scolytidae) on the south coast of Finland. Notulae Entomologicae 66: 179.
Lekander B., Bejer-Petersen B., Kangas E., Bakke A. (1977). The distribution of bark beetles in the Nordic countries. Acta Entomologica Fennica 32: 1–37.
Lindelöw Å. (2010). Aktuellt om svenska barkborrar (Coleoptera: Curculionidae, Scolytinae). Entomologisk Tidskrift 131: 97–104.
McDowell N., Pockman W.T., Allen C.D., Breshears D.D., Cobb N., Kolb T., Sperry J., West A., Williams D., Yepez E.A. (2008). Mechanisms of plant survival and mortality during drought: why some plants survive while others succumb to drought? New Phytologist 178: 719–739. http://dx.doi.org/10.1111/j.1469-8137.2008.02436.x.
McDowell N.G., Beerling D.J., Breshers D.D., Fisher R.A., Raffa K.F., Stitt M. (2011). The interdependence of mechanisms underlying climate-driven vegetation mortality. Trends in Ecology and Evolution 26: 523–532. http://dx.doi.org/10.1016/j.tree.2011.06.003.
Puukko K. (1981). Okakaarnakuoriaisen, Ips acuminatus Gyll. (Coleoptera, Scolytidae) levinneisyyden nykyinen eteläraja Suomessa. Silva Fennica 15: 222–227. http://dx.doi.org/10.14214/sf.a15060.
Saalas U. (1949). Suomen metsähyönteiset. Werner Söderström Oy, Porvoo. 719 s.
Schwenke W. (1974). Die Forstschädlinge Europas. Band 2, Käfer. Parey. 500 p.
Villari C., Tomlinson J.A., Battisti A., Boonham N., Capretti P., Faccoli M. (2013). Use of loop-mediated isothermal amplification for detection of Ophiostoma clavatum, the primary blue stain fungus associated with Ips acuminatus. Applied and Environmental Microbiology 79: 2527–2533. http://dx.doi.org/10.1128/AEM.03612-12.
Wermelinger B., Rigling A., Schneider Mathis D., Dobbertin M. (2008). Assessing the role of bark- and wood-boring insects in the decline of Scots pine (Pinus sylvestris) in the Swiss Rhone valley. Ecological Entomology 33: 239−249. http://dx.doi.org/10.1111/j.1365-2311.2007.00960.x.
Wood S.L., Bright D.E. (1992). A catalog of Scolytidae and Platypodidae (Coleoptera), Part 2: Taxonomic index. Great Basin Naturalist Memoirs 13. 1553 p.
Total of 19 references


